Abstract
Prion diseases are associated with the conversion of the cellular prion protein (PrPC), a glycoprotein expressed at the surface of a wide variety of cell types, into a misfolded conformer (the scrapie form of PrP, or PrPSc) that accumulates in brain tissues of affected individuals. PrPSc is a self-catalytic protein assembly capable of recruiting native conformers of PrPC, and causing their rearrangement into new PrPSc molecules. Several previous attempts to identify therapeutic agents against prion diseases have targeted PrPSc, and a number of compounds have shown potent anti-prion effects in experimental models. Unfortunately, so far, none of these molecules has successfully been translated into effective therapies for prion diseases. Moreover, mounting evidence suggests that PrPSc might be a difficult pharmacological target because of its poorly defined structure, heterogeneous composition, and ability to generate different structural conformers (known as prion strains) that can elude pharmacological intervention. In the last decade, a less intuitive strategy to overcome all these problems has emerged: targeting PrPC, the common substrate of any prion strain replication. This alternative approach possesses several technical and theoretical advantages, including the possibility of providing therapeutic effects also for other neurodegenerative disorders, based on recent observations indicating a role for PrPC in delivering neurotoxic signals of different misfolded proteins. Here, we provide an overview of compounds claimed to exert anti-prion effects by directly binding to PrPC, discussing pharmacological properties and therapeutic potentials of each chemical class.
Keywords: cellular prion protein, prion diseases, PrP ligands, pharmacological chaperones
1. Introduction
With few exceptions, proteins evolved their biological function in parallel with the ability to remain soluble under physiological conditions. However, in several pathological situations, specific proteins lose their native fold and acquire a different tertiary and quaternary conformation, clustering into aberrant aggregates. This phenomenon, known as protein misfolding, lays at the root of a wide variety of human diseases, such as neurodegenerative disorders, in which protein aggregation occurs in the brain [1]. Examples include common disorders such as Alzheimer’s and Parkinson’s diseases, or rarer disorders such as amyotrophic lateral sclerosis and prion diseases. Despite the fact that the pathological protein component is different in each neurodegenerative disorder, compelling evidence coming from genetic, biophysical and biochemical studies indicate that misfolded proteins are toxic to neurons. In fact, they often expose regions that are normally buried in the native state, leading to aggregation and aberrant interaction with cellular components such as membranes, proteins, or other macromolecules. These events may negatively affect neuronal homeostasis, for example, by blocking axonal transport, damaging synaptic endings or sequestering essential proteins, ultimately leading to cell death [2]. Possible strategies for tackling protein aggregation include breaking-up aggregates, increasing their degradation, or blocking their formation by stabilizing the native conformation of the monomeric protein precursors. While the first two have largely been explored in the past, the latter is a relatively new concept, and may possibly provide theoretical and technical advantages. For example, although detailed information about the structure of protein aggregates is rarely available, the three-dimensional organization of the monomeric precursors is often well characterized. A particularly meaningful example is represented by prion diseases. These disorders have the peculiarity of manifesting in a sporadic, inherited or transmissible fashion, and are associated with the conformational conversion of the cellular prion protein (PrPC), a glycoprotein of uncertain function anchored to the outer surface of the plasma membrane, into a misfolded isoform (called PrPSc) that accumulates in the central nervous system of affected organisms [3]. PrPSc is a proteinaceous infectious particle (prion), capable of multiplying by directly recruiting native conformers of PrPC, and causing their conformational rearrangement into new PrPSc molecules [4].
The vast majority of experimental strategies aimed at identifying therapeutics for human prion diseases has so far targeted PrPSc, the most direct, pathologically-relevant form of PrP [5]. However, the structure of PrPSc is poorly defined, and this form is also likely to be heterogeneous in composition and conformation. In fact, one of the most puzzling aspects of prion diseases is the phenomenon of prion strains [6]. It is believed that distinct conformations of PrPSc may explain the unusually wide spectrum of biochemical, neuropathological and clinical features that characterize prion diseases [7]. Prion strains are of particular relevance for the treatment of prion diseases, as their appearance may cause the acquisition of drug resistance to therapeutic treatments [8,9]. Indeed, a number of previously discovered anti-prion compounds have been shown to act in a strain-specific fashion, a property that severely limits their therapeutic potentials [10,11,12].
A possible, perhaps less intuitive strategy to overcome these limitations could be to target PrPC, the common substrate of any prion strain replication. The structure of PrPC is known at atomic level resolution, thanks to multiple previous reports employing nuclear magnetic resonance (NMR) or X-ray crystallography [13,14,15]. This provides a convenient ground to carry out rational drug design campaigns. Moreover, from a theoretical standpoint, a molecule binding to PrPC with sufficiently high-affinity might in principle stabilize its folding by reducing the Gibbs free energy. Consequently, the activation energy (∆G) required for the unfolding process will increase proportionally, with the result that the rate of formation of any PrPSc strain will be kinetically and thermodynamically disfavored. Small molecules acting with such mechanisms are known as pharmacological chaperones. Interestingly, two or more ligands with independent binding sites on PrPC could synergize to completely block the formation of any unfolded form, since the relationship between ∆G and the stability constant of a folded polypeptide chain is exponential. In light of these conclusions, PrPC appears as a convenient molecular target for tackling prion propagation [16]. Is this protein also the right pharmacological target for preventing prion diseases? It is widely agreed that PrPC plays a crucial role in the pathogenesis of prion diseases not only by virtue of its ability to serve as substrate for generation of PrPSc. In fact, it has been reported that genetically depleting neuronal PrPC in mice with established prion infection reverses neuronal loss and progression of clinical signs, despite the continuous production of infectious PrPSc by surrounding astrocytes [17]. Similarly, the absence of endogenous PrPC renders host brain tissue resistant to the toxic effects of PrPSc emanating from implanted graft tissue [18]. These data indicate that other toxic species, rather than fully aggregated PrPSc, are responsible for the pathology of prion diseases. This conclusion is consistent with a number of previous reports underscoring the distinction between prion infectivity and prion toxicity [19,20,21,22]. In particular, recent experiments indicate that accumulation of infectivity and neurodegeneration proceed in distinct chronological and mechanistic phases [23]. While infectivity accumulates relatively rapidly, and requires only a minimum expression of PrPC, neurodegeneration takes much longer and is directly dependent on the amount of PrPC expressed in the brain. Taken together, these lines of evidence suggest that an unknown PrP conformer, either “on” or “off” pathway to PrPSc, could be the pathological form in prion diseases. These data provide a possible explanation for the evidence that, with few exceptions [12,24], none of the anti-prion compounds identified so far has shown a substantial effect in vivo. In fact, these molecules could disfavor PrPSc accumulation without hampering the neurotoxicity originating from other toxic conformers. Conversely, stabilizing the folded state of PrPC has the potential to block not only PrPSc formation and propagation, but also the appearance of any putative toxic conformer. Another potential advantage of targeting PrPC arises from recent observations indicating that PrPC may exert a toxicity-transducing activity upon binding to PrPSc, as well as to various disease-associated, misfolded oligomeric assemblies, such as those formed by the amyloid β (Aβ) peptide, or by the protein alpha-synuclein, linked to Alzheimer’s and Parkinson’s diseases, respectively [25,26,27,28,29]. Importantly, mice depleted for PrP expression develop normally, with subtle phenotypic changes appearing only later in life, thus suggesting that pharmacological decrease of PrPC function could produce little side effects. This conclusion is also supported by the recent identification of loss-of-function PrP alleles in healthy subjects [30]. Overall, these data support the potential value of targeting PrPC, as this approach may provide therapeutic benefits not only for prion diseases, but possibly also for other neurodegenerative disorders. In this manuscript, we review the main chemical classes reported to act against prion replication in a PrPC-directed fashion, focusing our discussion on molecules for which binding constant (KD), structural information and anti-prion half-maximal effective concentration (EC50) have experimentally been determined (Figure 1).
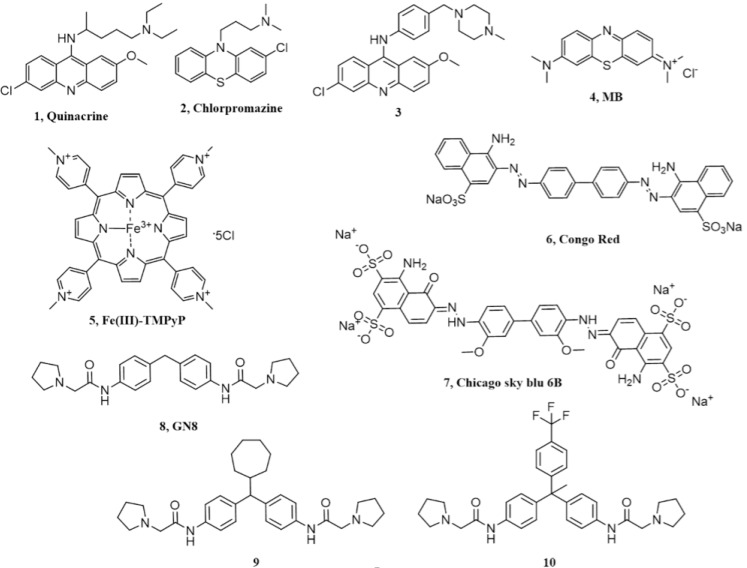
Figure 1.
Chemical structures of the different compounds claimed to directly bind PrPC.
2. Acridine and Phenothiazine Derivatives
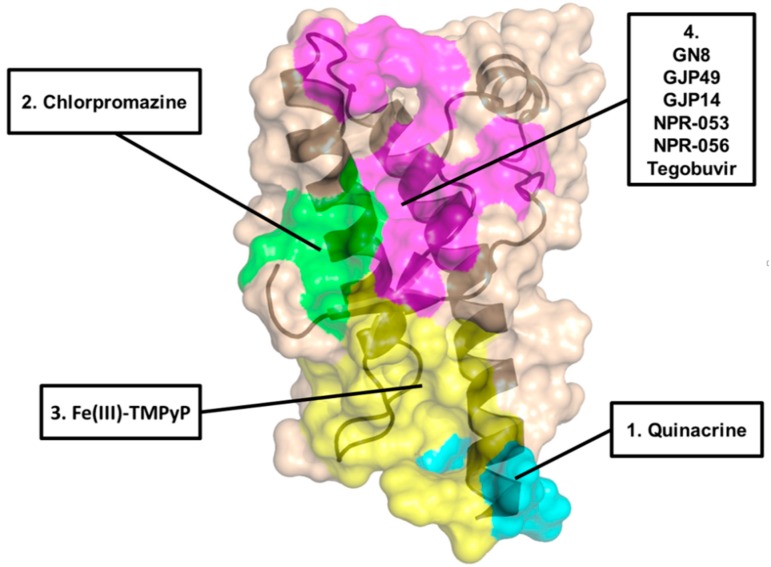
Tricyclic derivatives of acridines (compound 1 in Figure 1, quinacrine) and phenothiazines like chlorpromazine (compound 2 in Figure 1) were initially reported to be promising candidates for the treatment of prion diseases [31,32]. Indeed, these drugs have already been used in humans for many years, and are known to cross the blood–brain barrier, thus giving hope to their repurposing for prion diseases. The antimalarial agent quinacrine and the antipsychotic drug chlorpromazine showed inhibition of PrPSc formation in prion-infected N2a cells, with EC50 values of ~0.3 µM and ~3 µM, respectively. The acridine derivative quinacrine deserves particular attention, as it showed better potency in cell cultures, and was tested in human trials for prion diseases (more extensively than chlorpromazine, which was tested only in combination with the antimalarial agent). Quinacrine enantiomers showed stereoselectivity against prions, with the (S)-quinacrine exhibiting superior activity in eradicating PrPSc from cells [33]. Unfortunately, despite the promising in vitro profile, no beneficial effects were observed in vivo, using prion-infected rodent models of prion disease [34,35]. In addition to animal models, the activity and safety of quinacrine was assessed in clinical trials in human Creutzfeldt–Jakob disease (CJD) patients, but no effects were observed either on survival at the two-month time point or on the clinical course of the disease [36,37]. Pharmacokinetic studies unveiled that free quinacrine concentration in the brain reached only ~1 µM, which is a lower value than the cellular EC50 observed in vitro [11,38]. These results highlighted the difficulty of translating results obtained by in vitro or cell-based methods to the clinical context. The lack of clinical efficacy of quinacrine against CJD was mainly attributed to metabolic instability, scarce accumulation of the drug into the brain due to active efflux by P-glycoprotein (P-gp) and the formation of drug resistant prion strains [11]. Original studies suggested that the anti-prion activity of quinacrine was directly connected to its ability to modify the lysosomal environment, causing improved clearance of PrPSc [31]. However, later studies reported that quinacrine binds to the globular domain of human recombinant PrP (residues 121–230), as observed by NMR spectroscopy. Tyr225, Tyr226, and Gln227 of helix 3 (H3) were identified as key residues in such ligand–protein interaction (region 1 in Figure 2) [39]. Of note, these experiments were conducted at very high concentrations, and the obtained dissociation constant of quinacrine (KD = 4.6 mM) was about four orders of magnitude higher than its cellular EC50 value (required to clear PrPSc from prion-infected cells in vitro). Similar data (KD ~ 1 mM) were obtained in another study where quinacrine binding to recombinant human PrP was analyzed by surface plasmon resonance (SPR) [40], although other SPR studies reported the ability of quinacrine to bind human recombinant PrP with a KD of 15 µM [41]. In another report, dynamic light scattering studies and circular dichroism (CD) measurements suggested that quinacrine binding induces a conformational change in PrP, disfavouring PrPSc formation [42]. It is worth noting that the potential of quinacrine as a prion inhibitor has stimulated great interest in the 9-aminoacridine family as therapeutic candidates for prion diseases, and intensive research efforts have been spent on the synthesis, biological evaluation and structure–activity relationship (SAR) studies of quinacrine derivatives [43,44,45,46]. In particular, the nature of the aliphatic side-chain on 9-amino group of the tricyclic scaffold was found to be one key feature for enhancing binding affinity to PrP, PAMPA permeability and inhibition of PrPSc accumulation. As an example, a quinacrine derivative (compound 3 in Figure 1) showed improved anti-prion activity, as compared to the parent compound, across different prion-infected murine cell models (ScN2a, N167, F3). In addition, this compound exhibited stronger binding affinity by SPR, and seemed to be a weaker substrate for P-gp [46]. However, more recent SPR- and NMR-based studies have highlighted a non-specific binding interaction of quinacrine to PrPC, reiterating the original observation that its mode of action involves PrP-independent mechanisms [47,48].
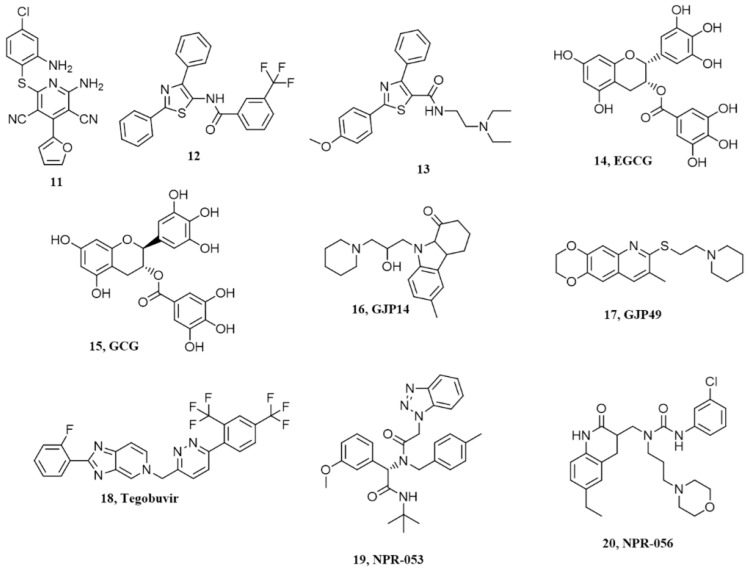
Figure 2.
Visualization of the proposed binding regions for the different PrPC ligands (indicated).
Similarly to quinacrine, the direct binding of phenothiazine derivative chlorpromazine to PrPC was originally investigated by NMR [39] and SPR [41], showing a weaker interaction with recombinant PrP, as compared to quinacrine. A subsequent study based on NMR and X-ray crystallography (PDB ID 4MA8) reported a precise binding site of phenothiazines on PrPC, located in a hydrophobic pocket formed by helix-2 (H2) and the two anti-parallel β-sheets (S1 and S2; region 2 in Figure 2) [49]. The data also indicated that an unexpected intramolecular reorganization of the N-terminal, unstructured tail of PrPC around the C-terminal domain, through the formation of a hydrophobic anchor, directly suggesting a mechanism by which phenothiazines may act as pharmacological chaperone of PrPC. Unfortunately, the study did not provide an affinity value for the binding of phenothiazines to PrPC. Such value was instead precisely defined in the following report, employing SPR and dynamic mass redistribution (DMR) [50]. The results confirmed original observations indicating a weak interaction of chlorpromazine to PrPC, with an estimated KD higher than 400 µM, compatible with data collected in the original study [49], which employed millimolar concentrations of chlorpromazine to carry out NMR and X-ray crystallography experiments. A KD value in the high micromolar concentration range is incompatible with the reported anti-prion effects of chlorpromazine in cells, indicating that its mode of action is independent from direct PrP binding [50]. Moreover, chlorpromazine also failed to inhibit prion replication in vitro (by the protein misfolding cyclic amplification reaction, PMCA), as instead it would be expected for a pharmacological chaperone of PrPC. Interestingly, the same study reported compelling evidence indicating that the mechanism of action underlying the anti-prion effect of chlorpromazine is related to the previously known ability of the compound to inhibit clathrin-mediated endocytosis, leading to decreased levels of PrPC at the cell surface. Consistent with this conclusion, two inhibitors of dynamins, proteins involved in the regulation of the scission of membrane vesicles, and recently reported to be targeted by chlorpromazine [51], mimicked PrPC-relocalizing effects, and blocked the replication of two different prion strains in cell cultures [50]. An additional recent work provided evidence for a chlorpromazine-induced redistribution of PrPSc from the endocytic-recycling pathway to the lysosomal compartment, an effect that could be the direct consequence of the relocalization of PrPC from the cell surface [52].
Methylene Blue (MB, compound 4 in Figure 1), a phenothiazine derivative, has been shown to affect the kinetics of PrP oligomerization by binding to a surface cleft on PrPC [53]. Using size exclusion chromatography, static light scattering, differential scanning calorimetry and transmission electron microscopy, the authors studied the influence of methylene blue on the oligomerization and fibrillation of human, ovine and murine recombinant PrP, observing a decrease in oligomerization kinetics and overall levels. NMR experiments mapped MB binding sites in a surface cleft delimited by residues belonging to S1-H1 and H2-H3 loops, and H1, H2 and H3 helices (residues Asn146, Asn156, Tyr160, Lys188, Thr191, Val192, Thr194, Thr195, Gln215). Of note, MB has been investigated as potential therapeutic agent in other proteinopathies [54,55,56,57], which is consistent with the number of potential applications that have been tested for this compound, likely reflecting its ability to engage non-specific interactions with a broad range of proteins.
3. Cyclic Tetrapyrroles
Cyclic tetrapyrroles, planar aromatic ring systems coordinating metal ions and bearing pendants of different chemical nature, were originally found to be effective in prion-infected cells, and later claimed to act by directly binding to PrPC [58,59]. In particular, by employing isothermal titration calorimetry (ITC), the cationic tetrapyrrole Fe(III)-TMPyP (compound 5 in Figure 1) was shown to bind human recombinant PrP in the C-terminal, globular domain (KD = 4.52 µM), which was consistent with its cellular EC50 of 1.6 µM in cells (as tested in rocky mountain laboratory, RML-infected PK1 cells) and the range of concentrations (1–11 µM) active in the protein-misfolding cyclic amplification (PMCA) reaction [48]. NMR studies allowed the identification of the binding site of Fe(III)-TMPyP on human PrP, with key interacting residues clustered at the C terminus of H3 and in the loop between residues 160 and 180 (region 3 in Figure 2). Importantly, Fe(III)-TMPyP, or highly similar porphyrins, also showed the ability to inhibit the cytotoxic activity of a mutant PrP carrying a deletion in the central region (Δ105–125), abrogated the PrPC-mediated synaptotoxic effects of Aβ oligomers in primary hippocampal neurons, and significantly prolonged survival time in prion-infected mice [60,61]. Unfortunately, the therapeutic potentials of porphyrins like Fe(III)-TMPyP is dampened by their poor pharmacokinetic properties, such as possible non-specific interactions with plasma proteins, and unlikelihood to cross the blood–brain barrier [62]. However, as assayed by in vitro and cell-based tests, these compounds appear as the most effective pharmacological chaperones of PrPC, and have already been employed to gain insights into the physiological activity of PrPC, and its functional connection to neurodegenerative pathways. Performing extensive pharmacokinetic profiling of this class of molecules, coupled to chemical optimization efforts and/or innovative ways of delivery to the central nervous system, could provide effective therapeutic strategies for prion diseases, and possibly other neurodegenerative disorders linked to the toxicity-transducing activity of PrPC.
4. Diazo Dyes
The diazo dye Congo red (compound 6 in Figure 1) was found to possess anti-prion activity in cells and in vivo, using scrapie-infected golden Syrian hamsters [63,64,65,66,67]. In particular, Congo red prevented the formation and accumulation of PrPSc in neuroblastoma cells with an EC50 of about 0.015 µM. The binding of Congo red to human recombinant PrP was investigated by SPR, and showed a KD value of 1.6 µM [40]. However, other studies reported that, in physiological conditions, the molecule binds non-specifically to PrPC as an aggregated polyanion [47]. Congo Red itself has a number of shortfalls, such as non-specific interactions with various macromolecules, self-polymerization, toxicity and poor permeability through BBB. For this reason, several Congo red derivatives were designed and synthesized to improve the pharmacological profile of the compound, and a number of analogues showed anti-prion effects at nanomolar concentrations, even though no information about their possible interaction with PrPC was reported [68,69,70].
5. Chicago Sky Blue 6B
This molecule emerged from a screen of 1200 approved drugs and pharmacological tool compounds (Prestwick Chemical Library) based on a fluorescence polarization (FP) assay, and aimed at identifying compounds capable of inhibiting the binding of Aβ oligomers to PrPC [71]. Chicago Sky Blue 6B (compound 7 in Figure 1) was identified as the best-ranked candidate, with EC50 values of 0.41 µM and 19.7 µM in FP and ELISA assays, respectively. ITC experiments confirmed that Chicago Sky Blue 6B is able to interact with human recombinant PrP, with a KD value of 0.55 µM. Importantly, the compound did not bind a PrP construct containing only residues 119–231, indicating that its binding site lies within the N-terminal, unstructured tail of the protein. Since Aβ oligomers are known to bind PrP in the same region, the data suggested that Chicago Sky Blue 6B may act by a mechanism of direct competition. Of note, Chicago Sky Blue 6B also showed anti-prion effects in RML-infected N2a cells, with EC50 values in the low micromolar range, and in absence of evident cytotoxicity. At the time this manuscript was prepared, no other studies have employed Chicago Sky Blue 6B in the context of prion diseases.
6. Diphenylmethane Derivatives
A compound known as GN8 (compound 8 in Figure 1) emerged from an in silico, dynamics-based drug screen of ~320,000 compounds aimed at directly identifying pharmacological chaperones for PrPC [72]. In vitro validation studies estimated the affinity of GN8 for recombinant, mouse PrP in the low micromolar range (KD ~ 5 µM). Heteronuclear NMR and molecular modeling mapped the PrP binding region of GN8 at the C-terminal domain, particularly involving residues N159 and E196 (region 4 in Figure 2). Furthermore, the authors employed CD in a thermal-denaturation assay to confirm that the binding of GN8 stabilizes the PrPC conformation significantly (ΔΔH = 6.7 kcal/mol). Biological validation showed that GN8 efficiently inhibits prion replication in cells, with an estimated EC50 of ~1.35 µM. Importantly, GN8 was also found to prolong the survival of prion-infected mice, thus confirming the effective anti-prion activity of this molecule. Subsequent studies focused on the synthesis and evaluation of anti-prion effects for a series of GN8 analogues with the main objective of generating a SAR profile [73]. Two derivatives (compounds 9 and 10 in Figure 1) were found to be approximately three times more potent than the parent compound, with EC50 values around 0.5 µM, in absence of detectable toxicity. CD-coupled thermal-denaturation assays indicated that one of these molecules significantly stabilized recombinant PrP, with a degree of stabilization by this ligand approximately doubled, as compared with that of GN8 (ΔΔH = 14.2 kcal/mol). Binding was also confirmed by SPR. According to these data, GN8 and its derivatives appear as promising pharmacological chaperones of PrPC. However, it is worth noting that two subsequent studies failed to confirm binding of GN8 to mouse or human recombinant PrP, using a battery of biophysical techniques [48,60]. Such experimental discrepancy is currently unresolved.
7. Pyridine Dicarbonitriles
Four pyridine dicarbonitrile analogues, originally identified as anti-prion compounds in prion-infected cells [74], were later tested for their direct interaction with PrPC using SPR [41]. One derivative (compound 11 in Figure 1) showed anti-prion activity (EC50 values ~ 20 µM) and detectable binding to recombinant PrP. This observation justified the following efforts to generate small libraries of pyridine dicarbonitrile derivatives, which were then tested by SPR for binding to PrPC, and in cellular assays to evaluate anti-prion activity [75,76]. Unexpectedly, no direct correlation was observed between binding to PrPC and anti-prion efficacy, with the most potent anti-prion pyridine dicarbonitrile showing either weak or no binding to PrPC. Collectively, these data suggested that pyridine dicarbonitrile likely inhibit prion replication in a PrPC-independent fashion.
8. Diarylthiazoles
The same team originally involved in the study on the dicarbonitrile derivatives also reported the synthesis and screening of 2,4-diarylthiazole-based compounds as potential anti-prion agents [77,78]. The authors stated that original 2,4-diarylthiazole scaffold was identified as a PrP ligand through a virtual screening campaign, although details of such screening were not described. SPR was then employed to test the binding of several derivatives to mouse or human recombinant PrP. Only one compound (compound 12 in Figure 1) showed a high-affinity interaction with PrP. All the molecules were also tested in prion-infected SMB cells, but once again no correlation was found between PrP binding and anti-prion activity in cells. A second series of reverse amide 2,4-diarylthiazole-based anti-prion compounds was later reported in a following study. The molecules were first tested for prion inhibition in SMB cells and then evaluated for binding to recombinant PrP, as assayed by SPR. Among the compounds active in cells, one derivative (compound 13 in Figure 1) (EC50 = 4 µM) also showed affinity for PrP, although a careful evaluation of SPR data suggested the possibility of a non-specific interaction. Overall, these studies highlighted a general lack of correlation between anti-prion activity and PrP binding for 2,4-diarylthiazole-based compounds, suggesting that other PrP-independent modes of action account for the anti-prion effects of this chemical class.
9. Natural Polyphenols
In search of small molecules able to interfere with prion propagation, another study screened a collection of natural compounds with proven activity against amyloid formation in vitro [79,80]. The major polyphenols component of green tea, i.e., epigallocatechin gallate (EGCG, compound 14 in Figure 1) and its stereoisomer gallocatechin gallate (GCG, compound 15 in Figure 1), showed anti-prion activity in prion-infected N2a cells. The direct interaction of EGCG with recombinant PrP (residues 90–232) was experimentally tested by ITC, showing a strong affinity (KD = 0.13 µM) and a remarkable stabilization effect (ΔH of −43 KJ). Further experiments on the effect of EGCG binding revealed an unexpected destabilization effect of the compound on the native conformation of PrPC, inducing its rapid transition into detergent-insoluble species, which were rapidly degraded intracellularly. The authors also observed that the anti-prion activity depended on the gallate side chain and the three hydroxyl groups of the trihydroxyphenyl side chain. Unfortunately, a subsequent study characterized the binding properties of EGCG to PrPC by SPR and NMR, concluding that the compound binds to the protein in a non-specific fashion [47]. These results dampened the enthusiasm for the treatment of prion diseases with EGCG-like polyphenols.
10. Miscellanea
Several structurally diverse compounds identified by virtual screening campaigns on the proposed binding pocket for GN8 have been claimed to be specific PrPC ligands, capable of acting as chemical chaperones. In 2009, a virtual screening study led to the selection of 205 commercially available compounds to be evaluated for their effects on the PrPC conversion process [81]. Ex vivo-experiments identified 24 non-cytotoxic molecules that significantly inhibited prion replication in GT-FK cells, at a concentration of 10 µM. To further elucidate their mechanism of action, the authors measured the binding affinity for recombinant PrP by SPR, and then compared anti-prion activity in cells with affinity values. Eleven compounds were classified as PrP-directed anti-prion compounds; for example, for a molecule named GJP14 (compound 16 in Figure 1), the authors reported an EC50 = 8.54 µM [82]. Compounds GJP14 and GJ49 (compound 17 in Figure 1) were further characterized for their binding properties by SPR and NMR (for example, for GJ49 KD = 50.8 µM), showing a ligand-binding pocket in the C-terminal, globular domain of PrPC (region 4 in Figure 2) [47].
A related study performed a 3D pharmacophore-based virtual screen of an in-house chemical library, and selected 37 potential anti-prion compounds to be assessed by cell-based and SPR-based assays [83]. The results identified a molecule named BMD42-29 (a benoxazole derivative whose structure was not disclosed) as the best hit among the screened molecules, with an EC50 value against prion replication in cells in the low micromolar range (<5 µM). Of note, in prion-infected N2a cells, the compound did not produce a marked reduction in total PrP levels. SPR experiments revealed that BMD42-29 had strong binding affinity to PrPC (KD = 21.5 µM), with kinetic rates characterized by rapid association and slow dissociation constants. The predicted binding mode of BMD42-29 was located in the same pocket of GN8, and was characterized by two hydrogen bonds with Asn159 and Glu196, and hydrophobic interactions with Leu130 and Arg156. The author concluded that BMD42-29 may act by stabilizing PrPC, thus inhibiting its pathological conformational change to PrPSc. In 2016, another group built a platform called “NAGARA”, aimed at unifying docking simulation, molecular dynamics and quantum chemistry to perform large-scale screening of commercially available compounds [84]. One hundred hits predicted in silico to bind PrPC were subjected to cell-based validation to evaluate anti-prion effects. Tegobuvir (previously known as an anti-hepatitis C agent, compound 18 in Figure 1) emerged as one of the most promising candidates, with an estimated EC50 of 1.7 µM, as assayed in immortalized neuronal mouse cells persistently infected with the human Fukuoka-1 prion strain. The molecule also showed detectable binding to PrPC in the low micromolar range (KD = 19 µM, region 4 in Figure 2). In the same year, by coupling docking simulations of a large virtual library (~200K compounds) and binding interaction analyses, another group reported the identification of 96 novel small molecules capable of binding PrPC in the same pocket of GN8 [85]. The ability of the in silico-predicted hits to target PrPC was evaluated by SPR and thermal shift assay (TSA), whereas their anti-prion effects were estimated using persistently infected cells and animal models of prion diseases. Compounds NPR-053 (compound 19 in Figure 1) and NPR-056 (compound 20 in Figure 1) emerged as the most promising candidates, in light of their ability to reduce PrPSc levels in cultured cells, with EC50 values of 7.68 µM and 3.72 µM, respectively. Both SPR and TSA provided evidence for a direct binding of both compounds to PrPC (region 4 in Figure 2), with NPR-053 inducing the strongest stabilization effect on PrPC native folding (ΔTm = 2.69 °C). All of these compounds represent promising candidate pharmacological chaperones for PrPC, although further experimental validation is needed before considering them as promising therapeutic agents for prion diseases.
11. Conclusions
Mounting evidence indicates that the accumulation of PrPSc alone could not account for the wide spectrum of neurotoxic events occurring in prion diseases. Instead, an unexpected role for PrPC as toxicity–transducer receptor for PrPSc and other disease-associated misfolded oligomeric assemblies, such as Aβ and alpha-synuclein, has raised great interest for targeting this protein pharmacologically. In this manuscript, we reviewed previous efforts to identify PrPC-directed compounds, taking into account limitations and reproducibility of each experimental attempt. A number of chemical scaffolds, identified by combining computational methods with biochemical, biophysical and cell-based assays, have been claimed to exert anti-prion effects by targeting PrPC (Table 1). Some of these molecules, such as the cationic tetrapyrrole Fe(III)-TMPyP, provide a proof-of-principle for targeting PrPC pharmacologically. Others, such as chlorpromazine, reveal unexpected mechanisms to counteract prion replication by lowering cell surface PrPC. However, the vast majority of compounds show inconsistencies between affinity for PrPC and biologically-active concentrations, low binding specificity, and/or lack of reproducibility. At the moment, none of these molecules appear as immediate candidates for clinical testing in the near future. Moreover, the great deal of negative data eventually provide further support to the notion that the vast majority of anti-prion molecules identified so far exert their activity through unknown targets, or by altering the homeostasis of PrPC, rather than binding the protein directly. What could be the reason for such a lack of success in identifying small ligands of PrPC? We believe the answer to this question may lie in a few, non-mutually exclusive possibilities. First, the screening techniques employed so far (e.g., in silico approaches coupled to biophysical assays) could have been inadequate for effectively identifying PrPC-directed molecules. Moreover, most of the approaches reviewed in this manuscript relied on recombinant PrP for testing the binding of small molecules, while physiological, post-translational modifications of the protein (sugar and lipid moieties) may heavily influence ligand binding. It is also possible that a single PrPC ligand will never be truly effective in preventing prion replication, since its stabilization effect on PrPC folding could be counteracted by the strong affinity of PrPSc for its substrate. In this scenario, testing the combination of two or three ligands binding PrPC in distinct pockets may produce the expected anti-prion effects. Ultimately, it is also possible that PrPC simply lies among the proteins that can be classified as “undraggable”. We like to believe that the latter conclusion will soon be refuted by direct experimental evidence.
Table 1.
Summary of main chemical scaffolds reported to exert anti-prion effects by directly targeting PrPC.
| Chemical Scaffold | Compound (Figure 1) | KD * | EC50 ** | Effect In Vivo *** | Conclusions |
|---|---|---|---|---|---|
| Acridine derivatives | 1 | ~1mM | ~0.3 µM | Not significant | Primary effects are PrP-independent |
| Phenothiazine derivatives | 2 | >400 µM | ~3 µM | Not significant | Likely acting by inducing PrPC re-localization from the cell surface |
| Tetrapyrroles | 5 | 4.52 µM | 1.6 µM | Prolongation of survival time in prion-infected mice | Low specificity and possible poor pharmacokinetics |
| Diazo dyes | 6 | 1.6 µM | 0.015 µM | Not available | Low specificity |
| Chicago sky blue 6B | 7 | 0.55 µM | Low µM | Not available | Need confirmation |
| Diphenylmethane derivatives | 8 | 5 µM | 1.35 µM | Prolongation of survival time in prion-infected mice | PrPC binding not reproduced in some study |
| Pyridine Dicarbonitriles | 11 | ~20µM | 18.6 µM | Not available | No correlation between anti-prion activity and binding to PrPC |
| Diarylthiazoles | 13 | 3.8 µM | 4 µM | Not available | No correlation between anti-prion activity and binding to PrPC |
| Natural polyphenols | 14 | 0.13 µM | - | Not available | Possible non-specific interaction with PrPC |
| Miscellanea | 17 | 50.8 µM | Not available | Not available | Need confirmation |
| 20 | 19 µM | 3.72 µM | Not available | Need confirmation |
* Reported affinity for PrPC; ** Anti-prion activity measured in cell cultures; *** Tested in prion-infected rodent models and/or human patients.
Acknowledgments
The study was supported by a Young Investigator Award from the Italian Ministry of Health (GR-2010-2312769), and a grant from the CJD Foundation. EB is an Assistant Telethon Scientist at the Dulbecco Telethon Institute (TCP14009, Fondazione Telethon, Italy).
Author Contributions
M.L.B. and E.B. conceived the main aspects of the review, M.L.B., N.I., S.B., V.C. and E.B. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. M.L.B. and E.B. are co-founders of Sibylla Biotech (www.sibyllabiotech.it), a startup company focused on developing new therapeutics for neurodegenerative disorders, including prion diseases.
References
- 1.Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 2.Bucciantini M., Giannoni E., Chiti F., Baroni F., Formigli L., Zurdo J., Taddei N., Ramponi G., Dobson C.M., Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner S.B. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 2013;47:601–623. doi: 10.1146/annurev-genet-110711-155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prusiner S.B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 5.Giles K., Olson S.H., Prusiner S.B. Developing therapeutics for prp prion diseases. Cold Spring Harbor Perspect. Med. 2017;7:a023747. doi: 10.1101/cshperspect.a023747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baskakov I.V. The many shades of prion strain adaptation. Prion. 2014;8:27836. doi: 10.4161/pri.27836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinosa J.C., Nonno R., Di Bari M., Aguilar-Calvo P., Pirisinu L., Fernandez-Borges N., Vanni I., Vaccari G., Marin-Moreno A., Frassanito P., et al. PrPc governs susceptibility to prion strains in bank vole, while other host factors modulate strain features. J. Virol. 2016;90:10660–10669. doi: 10.1128/JVI.01592-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collinge J. Medicine. Prion strain mutation and selection. Science. 2010;328:1111–1112. doi: 10.1126/science.1190815. [DOI] [PubMed] [Google Scholar]
- 9.Li J., Browning S., Mahal S.P., Oelschlegel A.M., Weissmann C. Darwinian evolution of prions in cell culture. Science. 2010;327:869–872. doi: 10.1126/science.1183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim V.L. Prion disease: Chemotherapeutic strategies. Infect. Disord. Drug Targets. 2012;12:144–160. doi: 10.2174/187152612800100161. [DOI] [PubMed] [Google Scholar]
- 11.Ghaemmaghami S., Ahn M., Lessard P., Giles K., Legname G., DeArmond S.J., Prusiner S.B. Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog. 2009;5:e1000673. doi: 10.1371/journal.ppat.1000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles K., Berry D.B., Condello C., Hawley R.C., Gallardo-Godoy A., Bryant C., Oehler A., Elepano M., Bhardwaj S., Patel S., et al. Different 2-aminothiazole therapeutics produce distinct patterns of scrapie prion neuropathology in mouse brains. J. Pharmacol. Exp. Ther. 2015;355:2–12. doi: 10.1124/jpet.115.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riek R., Hornemann S., Wider G., Billeter M., Glockshuber R., Wüthrich K. NMR structure of the mouse prion protein domain PrP(121–231) Nature. 1996;382:180–182. doi: 10.1038/382180a0. [DOI] [PubMed] [Google Scholar]
- 14.Zahn R., Liu A., Luhrs T., Riek R., von Schroetter C., Lopez Garcia F., Billeter M., Calzolai L., Wider G., Wüthrich K. NMR solution structure of the human prion protein. Proc. Natl. Acad. Sci. USA. 2000;97:145–150. doi: 10.1073/pnas.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonyuk S.V., Trevitt C.R., Strange R.W., Jackson G.S., Sangar D., Batchelor M., Cooper S., Fraser C., Jones S., Georgiou T., et al. Crystal structure of human prion protein bound to a therapeutic antibody. Proc. Natl. Acad. Sci. USA. 2009;106:2554–2558. doi: 10.1073/pnas.0809170106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicoll A.J., Collinge J. Preventing prion pathogenicity by targeting the cellular prion protein. Infect. Disord. Drug Targets. 2009;9:48–57. doi: 10.2174/1871526510909010048. [DOI] [PubMed] [Google Scholar]
- 17.Mallucci G.R., White M.D., Farmer M., Dickinson A., Khatun H., Powell A.D., Brandner S., Jefferys J.G., Collinge J. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron. 2007;53:325–335. doi: 10.1016/j.neuron.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Brandner S., Isenmann S., Raeber A., Fischer M., Sailer A., Kobayashi Y., Marino S., Weissmann C., Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 19.Biasini E., Medrano A.Z., Thellung S., Chiesa R., Harris D.A. Multiple biochemical similarities between infectious and non-infectious aggregates of a prion protein carrying an octapeptide insertion. J. Neurochem. 2008;104:1293–1308. doi: 10.1111/j.1471-4159.2007.05082.x. [DOI] [PubMed] [Google Scholar]
- 20.Biasini E., Seegulam M.E., Patti B.N., Solforosi L., Medrano A.Z., Christensen H.M., Senatore A., Chiesa R., Williamson R.A., Harris D.A. Non-infectious aggregates of the prion protein react with several PrPsc-directed antibodies. J. Neurochem. 2008;105:2190–2204. doi: 10.1111/j.1471-4159.2008.05306.x. [DOI] [PubMed] [Google Scholar]
- 21.Chiesa R., Harris D.A. Prion diseases: What is the neurotoxic molecule? Neurobiol. Dis. 2001;8:743–763. doi: 10.1006/nbdi.2001.0433. [DOI] [PubMed] [Google Scholar]
- 22.Aguzzi A., Falsig J. Prion propagation, toxicity and degradation. Nat. Neurosci. 2012;15:936–939. doi: 10.1038/nn.3120. [DOI] [PubMed] [Google Scholar]
- 23.Sandberg M.K., Al-Doujaily H., Sharps B., Clarke A.R., Collinge J. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature. 2011;470:540–542. doi: 10.1038/nature09768. [DOI] [PubMed] [Google Scholar]
- 24.Wagner J., Ryazanov S., Leonov A., Levin J., Shi S., Schmidt F., Prix C., Pan-Montojo F., Bertsch U., Mitteregger-Kretzschmar G., et al. ANLE138B: A novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol. 2013;125:795–813. doi: 10.1007/s00401-013-1114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elezgarai S.R., Biasini E. Common therapeutic strategies for prion and alzheimer’s diseases. Biol. Chem. 2016;397:1115–1124. doi: 10.1515/hsz-2016-0190. [DOI] [PubMed] [Google Scholar]
- 26.Lauren J., Gimbel D.A., Nygaard H.B., Gilbert J.W., Strittmatter S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biasini E., Harris D.A. Targeting the cellular prion protein to treat neurodegeneration. Future Med. Chem. 2012;4:1655–1658. doi: 10.4155/fmc.12.114. [DOI] [PubMed] [Google Scholar]
- 28.Biasini E., Turnbaugh J.A., Unterberger U., Harris D.A. Prion protein at the crossroads of physiology and disease. Trends Neurosci. 2012;35:92–103. doi: 10.1016/j.tins.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira D.G., Temido-Ferreira M., Miranda H.V., Batalha V.L., Coelho J.E., Szego E.M., Marques-Morgado I., Vaz S.H., Rhee J.S., Schmitz M., et al. Alpha-synuclein interacts with PrP(c) to induce cognitive impairment through mGluR5 and NMDAR2B. Nat. Neurosci. 2017;20:1569–1579. doi: 10.1038/nn.4648. [DOI] [PubMed] [Google Scholar]
- 30.Minikel E.V., Vallabh S.M., Lek M., Estrada K., Samocha K.E., Sathirapongsasuti J.F., McLean C.Y., Tung J.Y., Yu L.P., Gambetti P., et al. Quantifying prion disease penetrance using large population control cohorts. Sci. Transl. Med. 2016;20:322–323. doi: 10.1126/scitranslmed.aad5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doh-Ura K., Iwaki T., Caughey B. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J. Virol. 2000;74:4894–4897. doi: 10.1128/JVI.74.10.4894-4897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korth C., May B.C., Cohen F.E., Prusiner S.B. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. USA. 2001;98:9836–9841. doi: 10.1073/pnas.161274798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryou C., Legname G., Peretz D., Craig J.C., Baldwin M.A., Prusiner S.B. Differential inhibition of prion propagation by enantiomers of quinacrine. Lab. Investig. 2003;83:837–843. doi: 10.1097/01.LAB.0000074919.08232.A2. [DOI] [PubMed] [Google Scholar]
- 34.Collins S.J., Lewis V., Brazier M., Hill A.F., Fletcher A., Masters C.L. Quinacrine does not prolong survival in a murine Creutzfeldt-JaKob disease model. Ann. Neurol. 2002;52:503–506. doi: 10.1002/ana.10336. [DOI] [PubMed] [Google Scholar]
- 35.Barret A., Tagliavini F., Forloni G., Bate C., Salmona M., Colombo L., De Luigi A., Limido L., Suardi S., Rossi G., et al. Evaluation of quinacrine treatment for prion diseases. J. Virol. 2003;77:8462–8469. doi: 10.1128/JVI.77.15.8462-8469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collinge J., Gorham M., Hudson F., Kennedy A., Keogh G., Pal S., Rossor M., Rudge P., Siddique D., Spyer M., et al. Safety and efficacy of quinacrine in human prion disease (prion-1 study): A patient-preference trial. Lancet Neurol. 2009;8:334–344. doi: 10.1016/S1474-4422(09)70049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geschwind M.D., Kuo A.L., Wong K.S., Haman A., Devereux G., Raudabaugh B.J., Johnson D.Y., Torres-Chae C.C., Finley R., Garcia P., et al. Quinacrine treatment trial for sporadic Creutzfeldt-JaKob disease. Neurology. 2013;81:2015–2023. doi: 10.1212/WNL.0b013e3182a9f3b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn M., Ghaemmaghami S., Huang Y., Phuan P.W., May B.C., Giles K., DeArmond S.J., Prusiner S.B. Pharmacokinetics of quinacrine efflux from mouse brain via the P-glycoprotein efflux transporter. PLoS ONE. 2012;7:e39112. doi: 10.1371/journal.pone.0039112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogtherr M., Grimme S., Elshorst B., Jacobs D.M., Fiebig K., Griesinger C., Zahn R. Antimalarial drug quinacrine binds to C-terminal helix of cellular prion protein. J. Med. Chem. 2003;46:3563–3564. doi: 10.1021/jm034093h. [DOI] [PubMed] [Google Scholar]
- 40.Kawatake S., Nishimura Y., Sakaguchi S., Iwaki T., Doh-ura K. Surface plasmon resonance analysis for the screening of anti-prion compounds. Biol. Pharm. Bull. 2006;29:927–932. doi: 10.1248/bpb.29.927. [DOI] [PubMed] [Google Scholar]
- 41.Touil F., Pratt S., Mutter R., Chen B. Screening a library of potential prion therapeutics against cellular prion proteins and insights into their mode of biological activities by surface plasmon resonance. J. Pharm. Biomed. Anal. 2006;40:822–832. doi: 10.1016/j.jpba.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Georgieva D., Schwark D., von Bergen M., Redecke L., Genov N., Betzel C. Interactions of recombinant prions with compounds of therapeutical significance. Biochem. Biophys. Res. Commun. 2006;344:463–470. doi: 10.1016/j.bbrc.2006.03.135. [DOI] [PubMed] [Google Scholar]
- 43.Cope H., Mutter R., Heal W., Pascoe C., Brown P., Pratt S., Chen B. Synthesis and SAR study of acridine, 2-methylquinoline and 2-phenylquinazoline analogues as anti-prion agents. Eur. J. Med. Chem. 2006;41:1124–1143. doi: 10.1016/j.ejmech.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y., Okochi H., May B.C., Legname G., Prusiner S.B., Benet L.Z., Guglielmo B.J., Lin E.T. Quinacrine is mainly metabolized to mono-desethyl quinacrine by CYP3A4/5 and its brain accumulation is limited by P-glycoprotein. Drug Metab. Dispos. 2006;34:1136–1144. doi: 10.1124/dmd.105.008664. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen T., Sakasegawa Y., Doh-Ura K., Go M.L. Anti-prion activities and drug-like potential of functionalized quinacrine analogs with basic phenyl residues at the 9-amino position. Eur. J. Med. Chem. 2011;46:2917–2929. doi: 10.1016/j.ejmech.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen T.H., Lee C.Y., Teruya K., Ong W.Y., Doh-ura K., Go M.L. Antiprion activity of functionalized 9-aminoacridines related to quinacrine. Bioorg. Med. Chem. 2008;16:6737–6746. doi: 10.1016/j.bmc.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 47.Kamatari Y.O., Hayano Y., Yamaguchi K., Hosokawa-Muto J., Kuwata K. Characterizing antiprion compounds based on their binding properties to prion proteins: Implications as medical chaperones. Protein Sci. 2013;22:22–34. doi: 10.1002/pro.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicoll A.J., Trevitt C.R., Tattum M.H., Risse E., Quarterman E., Ibarra A.A., Wright C., Jackson G.S., Sessions R.B., Farrow M., et al. Pharmacological chaperone for the structured domain of human prion protein. Proc. Natl. Acad. Sci. USA. 2010;107:17610–17615. doi: 10.1073/pnas.1009062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baral P.K., Swayampakula M., Rout M.K., Kav N.N., Spyracopoulos L., Aguzzi A., James M.N. Structural basis of prion inhibition by phenothiazine compounds. Structure. 2014;22:291–303. doi: 10.1016/j.str.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Stincardini C., Massignan T., Biggi S., Elezgarai S.R., Sangiovanni V., Vanni I., Pancher M., Adami V., Moreno J., Stravalaci M., et al. An antipsychotic drug exerts anti-prion effects by altering the localization of the cellular prion protein. PLoS ONE. 2017;12:e0182589. doi: 10.1371/journal.pone.0182589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniel J.A., Chau N., Abdel-Hamid M.K., Hu L., von Kleist L., Whiting A., Krishnan S., Maamary P., Joseph S.R., Simpson F., et al. Phenothiazine-derived antipsychotic drugs inhibit dynamin and clathrin-mediated endocytosis. Traffic. 2015;16:635–654. doi: 10.1111/tra.12272. [DOI] [PubMed] [Google Scholar]
- 52.Yamasaki T., Suzuki A., Hasebe R., Horiuchi M. Comparison of the anti-prion mechanism of four different anti-prion compounds, anti-PrP monoclonal antibody 44B1, pentosan polysulfate, chlorpromazine, and u18666a, in prion-infected mouse neuroblastoma cells. PLoS ONE. 2014;9:e106516. doi: 10.1371/journal.pone.0106516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavaliere P., Torrent J., Prigent S., Granata V., Pauwels K., Pastore A., Rezaei H., Zagari A. Binding of methylene blue to a surface cleft inhibits the oligomerization and fibrillization of prion protein. Biochim. Biophys. Acta. 2013;1832:20–28. doi: 10.1016/j.bbadis.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Mori T., Koyama N., Segawa T., Maeda M., Maruyama N., Kinoshita N., Hou H., Tan J., Town T. Methylene blue modulates beta-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice. J. Biol. Chem. 2014;289:30303–30317. doi: 10.1074/jbc.M114.568212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sontag E.M., Lotz G.P., Agrawal N., Tran A., Aron R., Yang G., Necula M., Lau A., Finkbeiner S., Glabe C., et al. Methylene blue modulates huntingtin aggregation intermediates and is protective in huntington’s disease models. J. Neurosci. 2012;32:11109–11119. doi: 10.1523/JNEUROSCI.0895-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wischik C.M., Edwards P.C., Lai R.Y., Roth M., Harrington C.R. Selective inhibition of alzheimer disease-like tau aggregation by phenothiazines. Proc. Natl. Acad. Sci. USA. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamashita M., Nonaka T., Arai T., Kametani F., Buchman V.L., Ninkina N., Bachurin S.O., Akiyama H., Goedert M., Hasegawa M. Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models. FEBS Lett. 2009;583:2419–2424. doi: 10.1016/j.febslet.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 58.Caughey W.S., Raymond L.D., Horiuchi M., Caughey B. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA. 1998;95:12117–12122. doi: 10.1073/pnas.95.21.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Priola S.A., Raines A., Caughey W.S. Porphyrin and phthalocyanine antiscrapie compounds. Science. 2000;287:1503–1506. doi: 10.1126/science.287.5457.1503. [DOI] [PubMed] [Google Scholar]
- 60.Massignan T., Cimini S., Stincardini C., Cerovic M., Vanni I., Elezgarai S.R., Moreno J., Stravalaci M., Negro A., Sangiovanni V., et al. A cationic tetrapyrrole inhibits toxic activities of the cellular prion protein. Sci. Rep. 2016;6:23180. doi: 10.1038/srep23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kocisko D.A., Caughey W.S., Race R.E., Roper G., Caughey B., Morrey J.D. A porphyrin increases survival time of mice after intracerebral prion infection. Antimicrob. Agents Chemother. 2006;50:759–761. doi: 10.1128/AAC.50.2.759-761.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajora M.A., Lou J.W.H., Zheng G. Advancing porphyrin’s biomedical utility via supramolecular chemistry. Chem. Soc. Rev. 2017;46:6433–6469. doi: 10.1039/C7CS00525C. [DOI] [PubMed] [Google Scholar]
- 63.Caspi S., Halimi M., Yanai A., Sasson S.B., Taraboulos A., Gabizon R. The anti-prion activity of congo red. Putative mechanism. J. Biol. Chem. 1998;273:3484–3489. doi: 10.1074/jbc.273.6.3484. [DOI] [PubMed] [Google Scholar]
- 64.Milhavet O., Mange A., Casanova D., Lehmann S. Effect of congo red on wild-type and mutated prion proteins in cultured cells. J. Neurochem. 2000;74:222–230. doi: 10.1046/j.1471-4159.2000.0740222.x. [DOI] [PubMed] [Google Scholar]
- 65.Caughey B., Brown K., Raymond G.J., Katzenstein G.E., Thresher W. Binding of the protease-sensitive form of prp (prion protein) to sulfated glycosaminoglycan and congo red [corrected] J. Virol. 1994;68:2135–2141. doi: 10.1128/jvi.68.4.2135-2141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caughey B., Ernst D., Race R.E. Congo red inhibition of scrapie agent replication. J. Virol. 1993;67:6270–6272. doi: 10.1128/jvi.67.10.6270-6272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ingrosso L., Ladogana A., Pocchiari M. Congo red prolongs the incubation period in scrapie-infected hamsters. J. Virol. 1995;69:506–508. doi: 10.1128/jvi.69.1.506-508.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rudyk H., Knaggs M.H., Vasiljevic S., Hope J., Birkett C., Gilbert I.H. Synthesis and evaluation of analogues of congo red as potential compounds against transmissible spongiform encephalopathies. Eur. J. Med. Chem. 2003;38:567–579. doi: 10.1016/S0223-5234(03)00081-3. [DOI] [PubMed] [Google Scholar]
- 69.Rudyk H., Vasiljevic S., Hennion R.M., Birkett C.R., Hope J., Gilbert I.H. Screening congo red and its analogues for their ability to prevent the formation of PrP-res in scrapie-infected cells. J. Gen. Virol. 2000;81:1155–1164. doi: 10.1099/0022-1317-81-4-1155. [DOI] [PubMed] [Google Scholar]
- 70.Sellarajah S., Lekishvili T., Bowring C., Thompsett A.R., Rudyk H., Birkett C.R., Brown D.R., Gilbert I.H. Synthesis of analogues of congo red and evaluation of their anti-prion activity. J. Med. Chem. 2004;47:5515–5534. doi: 10.1021/jm049922t. [DOI] [PubMed] [Google Scholar]
- 71.Risse E., Nicoll A.J., Taylor W.A., Wright D., Badoni M., Yang X., Farrow M.A., Collinge J. Identification of a compound that disrupts binding of amyloid-beta to the prion protein using a novel fluorescence-based assay. J. Biol. Chem. 2015;290:17020–17028. doi: 10.1074/jbc.M115.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuwata K., Nishida N., Matsumoto T., Kamatari Y.O., Hosokawa-Muto J., Kodama K., Nakamura H.K., Kimura K., Kawasaki M., Takakura Y., et al. Hot spots in prion protein for pathogenic conversion. Proc. Natl. Acad. Sci. USA. 2007;104:11921–11926. doi: 10.1073/pnas.0702671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kimura T., Hosokawa-Muto J., Kamatari Y.O., Kuwata K. Synthesis of GN8 derivatives and evaluation of their antiprion activity in TSE-infected cells. Bioorg. Med. Chem. Lett. 2011;21:1502–1507. doi: 10.1016/j.bmcl.2010.12.132. [DOI] [PubMed] [Google Scholar]
- 74.Perrier V., Wallace A.C., Kaneko K., Safar J., Prusiner S.B., Cohen F.E. Mimicking dominant negative inhibition of prion replication through structure-based drug design. Proc. Natl. Acad. Sci. USA. 2000;97:6073–6078. doi: 10.1073/pnas.97.11.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo K., Mutter R., Heal W., Reddy T.R., Cope H., Pratt S., Thompson M.J., Chen B. Synthesis and evaluation of a focused library of pyridine dicarbonitriles against prion disease. Eur. J. Med. Chem. 2008;43:93–106. doi: 10.1016/j.ejmech.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 76.Reddy T.R., Mutter R., Heal W., Guo K., Gillet V.J., Pratt S., Chen B. Library design, synthesis, and screening: Pyridine dicarbonitriles as potential prion disease therapeutics. J. Med. Chem. 2006;49:607–615. doi: 10.1021/jm050610f. [DOI] [PubMed] [Google Scholar]
- 77.Heal W., Thompson M.J., Mutter R., Cope H., Louth J.C., Chen B. Library synthesis and screening: 2,4-diphenylthiazoles and 2,4-diphenyloxazoles as potential novel prion disease therapeutics. J. Med. Chem. 2007;50:1347–1353. doi: 10.1021/jm0612719. [DOI] [PubMed] [Google Scholar]
- 78.Thompson M.J., Louth J.C., Greenwood G.K., Sorrell F.J., Knight S.G., Adams N.B., Chen B. Improved 2,4-diarylthiazole-based antiprion agents: Switching the sense of the amide group at C5 leads to an increase in potency. ChemMedChem. 2010;5:1476–1488. doi: 10.1002/cmdc.201000217. [DOI] [PubMed] [Google Scholar]
- 79.Porat Y., Abramowitz A., Gazit E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 80.Rambold A.S., Miesbauer M., Olschewski D., Seidel R., Riemer C., Smale L., Brumm L., Levy M., Gazit E., Oesterhelt D., et al. Green tea extracts interfere with the stress-protective activity of PrP and the formation of PrP. J. Neurochem. 2008;107:218–229. doi: 10.1111/j.1471-4159.2008.05611.x. [DOI] [PubMed] [Google Scholar]
- 81.Hosokawa-Muto J., Kamatari Y.O., Nakamura H.K., Kuwata K. Variety of antiprion compounds discovered through an in silico screen based on cellular-form prion protein structure: Correlation between antiprion activity and binding affinity. Antimicrob. Agents Chemother. 2009;53:765–771. doi: 10.1128/AAC.01112-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimura T., Hosokawa-Muto J., Asami K., Murai T., Kuwata K. Synthesis of 9-substituted 2,3,4,9-tetrahydro-1H-carbazole derivatives and evaluation of their anti-prion activity in tse-infected cells. Eur. J. Med. Chem. 2011;46:5675–5679. doi: 10.1016/j.ejmech.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 83.Hyeon J.W., Choi J., Kim S.Y., Govindaraj R.G., Jam Hwang K., Lee Y.S., An S.S., Lee M.K., Joung J.Y., No K.T., et al. Discovery of novel anti-prion compounds using in silico and in vitro approaches. Sci. Rep. 2015;5:14944. doi: 10.1038/srep14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma B., Yamaguchi K., Fukuoka M., Kuwata K. Logical design of anti-prion agents using nagara. Biochem. Biophys. Res. Commun. 2016;469:930–935. doi: 10.1016/j.bbrc.2015.12.106. [DOI] [PubMed] [Google Scholar]
- 85.Ishibashi D., Nakagaki T., Ishikawa T., Atarashi R., Watanabe K., Cruz F.A., Hamada T., Nishida N. Structure-based drug discovery for prion disease using a novel binding simulation. EBioMedicine. 2016;9:238–249. doi: 10.1016/j.ebiom.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]