SUMMARY
The strategic approach for preventing congenital toxoplasmosis is strictly related to the incidence of primary T. gondii infection during pregnancy in a studied population. Early postnatal diagnosis by mass testing of newborns is an option in areas where obligatory serological screening in pregnant women has not been implemented but it requires sensitive immunodiagnostic methods followed by a good confirmatory analysis. The aims of the regional neonatal screening programme were (i) analysis of the prevalence of congenital T. gondii infection at birth in the West Poland Province, (ii) determination of the value of the serological examination of filter-paper blood specimens collected at birth for the diagnosis of congenital toxoplasmosis, and (iii) evaluation of the duration of T. gondii-specific immunoglobulin A and immunoglobulin M antibodies in infants’ sera.
The neonates born in the obstetric clinics of the University Gynaecology-Obstetrics Hospital in Poznan (Poland) and in the maternity wards of the 10 main district hospitals from the West Poland region were systematically screened for congenital T. gondii infection. Peripheral blood from newborns was collected by a non-invasive heel-stick puncture during the first 3 days of life, absorbed onto Guthrie cards and analysed for anti-T. gondii specific IgM (1996-1998) or both IgA and IgM antibodies (1998-2000) by non-commercial immunocapture ELISAs. When the screening result was positive, the diagnosis of congenital infection was confirmed by testing serum samples from the suspected neonate and the mother using a Western blot IgM-IgG comparative immunological profile analysis and traditional serological techniques (ELISA, ISAGA) for anti-Toxoplasma IgA, IgM and IgG specific antibodies.
From June 1996 to April 2000, 45,169 filter-paper specimens from liveborn neonates were screened: 27,516 samples were tested for specific IgM and the next 17,653 Guthrie cards were analysed by the combined IgA/IgM assay. The prevalence of anti-Toxoplasma IgM in filter-paper eluates at birth was 1 per 2,117 liveborn neonates (0.47/1000) or 1 per 1,185 infants (0.84/1000) born to seronegative women with a potential risk of primary T. gondii infection during pregnancy. For the joint detection of IgA and IgM, these values significantly increased to 1 per 929 neonates (1.08/1000) or 1 per 520 pregnancies at risk (1.92/1000) respectively, comparing to the seropositivity rate of 43.7% in a pregnant women population in the studied area. In newborns untreated prenatally, the diagnostic sensitivity of the IgM ELISA using neonatal Guthrie cards was not more than 86.7% and that of the combined IgA/IgM ELISA was 95%; the diagnostic specificity of the both methods was calculated to be 99.9%. Congenital T. gondii infection was finally diagnosed in 35 neonates, mostly asymptomatic at birth.
Conclusions: (i) The neonatal screening for anti-Toxoplasma IgA and/or IgM antibodies is a good sensitivity method for an early postnatal diagnosis of congenital toxoplasmosis in newborns untreated prenatally. (ii) In the absence of obligatory nation-wide screening during pregnancy followed by an early prenatal treatment, this valuable technique may be considered a preventive option in areas of a high annual number of births associated with a high seroprevalence of T. gondii infection.
Key words: congenital toxoplasmosis, Toxoplasma gondii, neonatal screening, Guthrie cards, filter-paper, dried blood spots, specific IgA and IgM antibodies, early postnatal diagnosis
INTRODUCTION
The strategic approach for preventing clinically overt form of congenital toxoplasmosis in foetuses and neonates is strictly related to the incidence of primary Toxoplasma infection during pregnancy in a given geographic area, resulting from the absence of protective specific IgG antibodies in women in a childbearing age group, a local risk of transplacental transmission of the parasite, and the Toxoplasma strain patogenecity. There are four available strategies to prevent congenital T. gondii infection: (i) health education concerning sanitary-dietary recommendations how to avoid Toxoplasma infection during pregnancy, (ii) preconceptional serological screening and then testing of seronegative pregnant women for Toxoplasma-specific IgG, followed by prenatal diagnosis with materno-foetal treatment of suspected and infected cases (1,2), (iii) non-invasive perinatal screening for specific IgM using cord blood samples (3), and (iv) mass neonatal screening based on peripheral blood collected on filter-papers (Guthrie cards) at birth (4-7). Regular serological testing of women who were seronegative before pregnancy or at the beginning of the first trimester of gestation was introduced as mandatory in Austria and France in 1970-ties, and actually it is widely recommended in majority of European countries by National Health Services or research/university centres (8-12). Combination of preconceptional screening with health education seems to be the most reasonable preventive method but it may be difficult to motivate non-pregnant women for testing. An application of simple dietary-hygienic measures during pregnancy may reduce the potential risk of primary T. gondii infection up to 60%. It is very difficult to evaluate which of preventive strategies is the most effective, economically and psychologically acceptable for screened population, considering the lowest risk of maternal anxiety during pregnancy, and possible false diagnosis.
So far, the incidence of congenital toxoplasmosis in a Polish population was evaluated on a number of registered deaths in severely affected cases and selected symptomatic infections with typical clinical signs shown at routine paediatric examination (13-15), which constitute a low percentage of all infected foetuses (16, 17). Majority of congenital Toxoplasma infections left undiagnosed or they were supradiagnosed (18). Moreover, indirect epidemiological evaluation of the prevalence of congenital T. gondii infection in Poland based on a simple comparison with some other populations having similar hygienic and dietary habits seems to be not sufficient because of proven differences in a patogenecity of T. gondii strains from various geographic areas or another sources of infection (19), as well as a widely used prevention of spiramycine in pregnant women in West Europen countries (20).
In 1990-ties, perinatal screening based on a detection of specific IgM antibody in cord blood using the commercial ImmunoSorbent Agglutination Assay (ISAGA, bioMerieux, France) showed only false positive results in 7 cases of 2,200 newborns born to seropositive mothers in 4 selected obstetrics wards in the West Poland Province, and no typical clinical signs of congenital toxoplasmosis were found in 4,311 successively born neonates (21). The screening results suggested that the incidence of congenital toxoplasmosis in Poland is lower than previously suspected and it may be probably less than 1 per 1000 children born alive (21,22). For this reason, the implementation of the nation-wide serological screening of Polish pregnant women was not accepted by the National Health Services (15), however serological testing is widely proposed to pregnant women and in individual suspected cases is recommended by gynaecologists as covered by social care units.
Because of the lack of obligatory screening of pregnant women for primary T. gondii infection in Poland, the Poznan University Centre was practiced the regional screening programme for congenital toxoplasmosis in neonates born in the West Poland area since 1996, in order to determine an actual epidemiological status of congenital toxoplasmosis in Poland, to evaluate a risk of primary infection in a pregnant women population, and to increase an early postnatal detection of congenital Toxoplasma infection in the Poznan Province. The aims of the regional study were (i) to determine the prevalence of congenital T. gondii infection at birth in the West Poland Province, (ii) to evaluate diagnostic parameters of the serological examination of filter-paper blood specimens collected at birth for the diagnosis of congenital toxoplasmosis, (iii) to determine the duration of T. gondii-specific immunoglobulin A and immunoglobulin M antibodies in infants’ sera for a practical detection of the congenital infection, and (iv) to evaluate the efficacy of early postnatal treatment.
Serological screening of newborns based on a detection of Toxoplasma-specific IgM in dried blood samples associated with testing of metabolic errors and other genetic disorders was used in Massachusetts, New Hampshire (USA), Denmark, and Sweden – in countries with a low risk of the primary infection during pregnancy and a relatively low annual number of births (5,7,23). The last years, the nation-wide serological screening of neonates based on commercially available IgM assay was implemented in Brazil, when clinical sequelae of congenital infection are more severe, (24, 25). Detection of Toxoplasma-specific IgA in filter-paper specimens from Polish neonates collected at birth, using a new non-commercial combined IgA/IgM ELISA was applied for the first time (26).
MATERIALS AND METHODS
All neonates born between 1996 and 2000 in the obstetric clinics of the University Hospital of Gynaecology and Obstetrics in Poznan and in maternity wards of the 10 main district hospitals from the West Poland Province (about 12,000 births per year; 130 km around Poznan) were systematically screened for congenital T. gondii infection. Peripheral blood absorbed onto separate phenylketonuria cards (Guthrie cards) (Schleicher and Schuell, Dassel, Germany) using a low invasive heel-stick method was collected during the first week of life, dried shortly at room temperature, and kept at +4 °C before being delivered to the laboratory by an ordinary mail or a messenger twice a week. The capillary blood was initially obtained from the same heel puncture as performed for phenylketonuria and congenital hypothyroidism routine tests. Later on, considering a possibility of an early disappearance of specific IgM and IgA antibodies, the samples were collected from a separate puncture during the children’s stay at neonatal wards, between 1 and 3 days of life, i.e. earlier than for other obligatory neonatal tests. Consecutively born neonates were only analysed, and some district hospitals sending filter-papers which were sporadically collected in individual cases suspected of intrauterine infections were excluded from the study. Neonatal Guthrie cards included identification data (name of the mother, sex of the neonate, address of accommodation), date of sampling, some most important obstetric information (pregnancy age, delivery mode, date of delivery, birth weight), and in the laboratory they were marked with a subsequent number of serological analysis and a hospital code (Fig. 1). All the samples were tested during a few days after being delivered to the laboratory. All filter-paper cards were kept at +4 °C before testing. After analysis, the samples were packed into plastic bags with a dessicant pad and stored at -20 °C for 2 years for an eventual verification.
Fig. 1.

A filter-paper card used for neonatal screening for congenital toxoplasmosis in Poland. Explanations: name of the hospital, name of the mother, age of pregnancy, date of birth, date of sampling, sex of the neonate, birth weight, address of domicile.
Advantages of the filter-paper method as the only ethically accepted procedure for mass neonatal screening were: (i) low invasiveness of the heel blood puncture, (ii) identification data placed directly on the analysed sample (low risk of potential missing data), (iii) easy storage and transport of dried blood samples (room temperature), (iv) long persistence of antibodies, and (v) anti-bacterial protection (minimal risk of contamination).
The 3.2-mm filter-paper spots were screened for Toxoplasma-specific immunoglobulin M (June 1996 - October 1998) or both - immunoglobulin A and immunoglobulin M antibodies (December 1998 - April 2000) using a non-commercial immunocapture ELISAs. The tests were based on polystyrene microwells coated at home with rabbit anti-human IgM or IgA and IgM antibodies (DAKO, Glostrup, Denmark), sonicated Tx12 T. gondii tachyzoites antigen from in vitro culture of RH strain on VERO cells, and they used monoclonal anti-SAG1 (P30) antibody (Statens Seruminstitut, Copenhagen, Denmark). The bound anti-Toxoplasma antibodies from filter-paper eluates were revealed by an addition of rabbit anti-mouse immunoglobulins secondary antibody labelled with alkaline phosphatase (DAKO, Glostrup, Denmark), and the reaction was then developed in a presence of p-nitrophenyl phosphate dissolved in diethanolamine/MgCl2 buffer as the enzyme substrate (Sigma) (7,26,27). The new combined IgA/IgM ELISA was adapted for a joint detection of actively synthesized neonatal IgA and IgM using a single determination procedure. The technique of a high sensitivity allowed to detect more difficult cases with low antibody levels that were weakly positive or even borderline in separate traditional assays for analysis of individual antibody classes IgA either IgM (26).
All IgA and/or IgM positive or equivocal eluates from filter-papers were re-tested twice using double determination analysis, and additionally tested for IgG antibodies. If the IgA and/or IgM-positive screening result was confirmed and the IgG test based on filter-paper spots was positive, a serum sample from the newborn and the mother was required for a verification. The Toxoplasma infection was finally confirmed by testing serum samples from the suspected infant and the mother by conventional serology for IgA, IgM and IgG specific antibodies (ELISA, BIO-RAD; ELFA, VIDAS TOXO IgM and IgG, and ISAGA PLUS IgA/ IgM, bioMerieux, France), and the IgM-IgG Western blot comparative immunological profile analysis (LDBIO Diagnostics, Lyon, France) after the second week of life. The congenital infection was defined by the presence of specific IgM and/or IgA antibodies in infant’s peripheral blood after the 10th day of age, and/or by the detection of actively synthesized IgG antibody of different antigenic specificity than that of the mother shown by immunoblotting. Follow-up serological testing for anti-T. gondii IgA, IgM and IgG serum antibodies was repeated every 2 months during the first year of life, then every 4 months after the treatment withdrawal in early childhood, and twice a year during the school period, together with clinical examinations (Fig. 2).
Fig. 2.
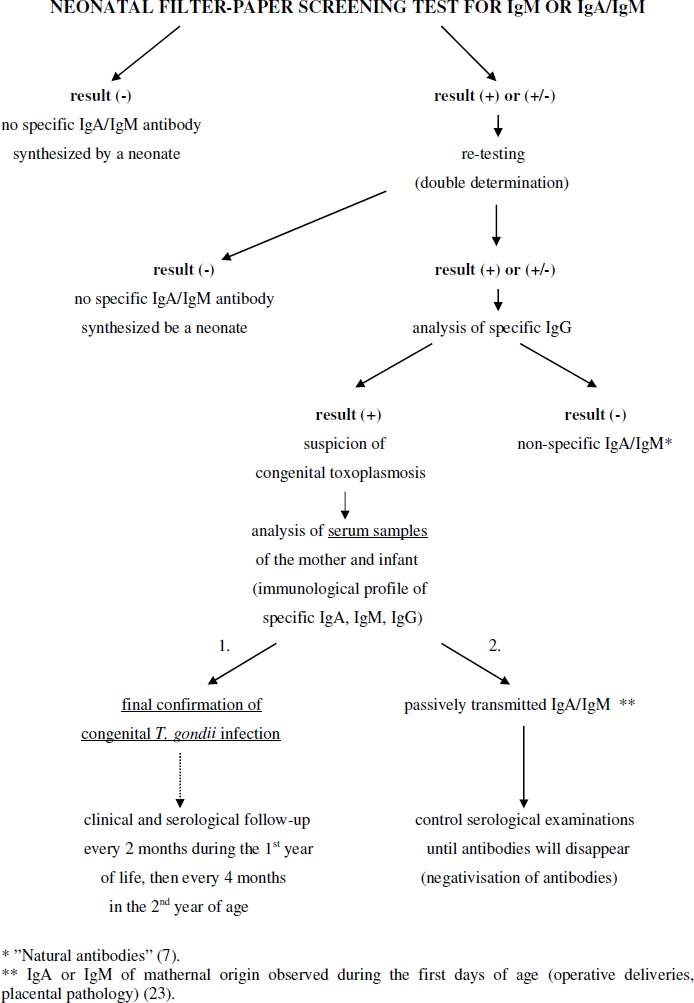
Diagnostic algorithm of the regional screening programme of newborns for congenital toxoplasmosis in the Poznan Province of Poland.
The paediatric follow-up, including an assessment of psychomotor and growth development with a routine neurological examination was performed soon after birth and again every 2 months during the first year of life, every 4 months after anti-parasitic treatment was stopped in early childhood, and then twice a year in the school period. Transfontanel ultrasonography of the brain was repeated, when feasible, every 2 months in the 1st year. The general ophthalmic examination, followed by direct and indirect ophthalmoscopy after the pupils dilatation, was usually done before 4 weeks of age and repeated every 3 months, and at each immunological rebound. Clinical hearing investigation followed by audiometry with the ECHO-SCREEN apparatus (Fisher-Zoth GmbH, Wilhelmsburg, Germany) and cranial radiography done in two projections (saggital and sutural) were performed during the neonatal period. The imaging investigation was completed by the brain computerized tomography scan near the 12 months of age.
Children with subclinical Toxoplasma infection or some non-specific signs at birth, were treated with pyrimethamine (1 mg/kg body weight/day) plus sulphadiazine (100 mg/kg body weight/day) completed with folinic acid (5 mg 3 times per week) for 4 weeks alternated with spiramycin (375,000 IU/kg body weight/day) for another 4 weeks. For infants with a clinically overt form of congenital toxoplasmosis, the continuous therapy with pyrimethamine, sulphadiazine and folinic acid (Lederfolat) was prescribed for 12 to 24 months, according to the severity of infection and immunological status of the patient. Leucocytes and thrombocytes counts were controlled twice a month during the periods of pyrimethamine/sulphadiazine administration. Infants with neurological abnormalities were managed by a regular rehabilitation therapy using Vojta’s or Bobath’s methods.
Maternal IgG antibody is transferred to the foetus, so the seroprevalence of Toxoplasma-specific IgG antibody in neonates born in 1998-2000 was therefore regarded as equivalent to the seropositivity rate in the pregnant women population at delivery in the studied area. Anti-Toxoplasma antibodies were measured by the reference direct agglutination assay (Toxo-Screen DA, bioMerieux, Marcy-l’Etoile, France), with some modifications adapted to filter-papers, which was positive from 4 IU/ml. Briefly, three filter-paper discs of 3.2 mm in diameter were punched into polystyrene microwell and eluted overnight at +4 °C in phosphate buffered saline (PBS), pH 7.2, and then transferred to a new round-bottomed microtitration plate. Fifty microliters of Toxoplasma antigen suspension in borate albumin buffered saline (BABS), pH 8.95 were applied to each well, and the reaction was read after 5 hours of incubation at room temperature.
RESULTS
Between June 1996 and April 2000, 45,169 filter-paper samples from neonates born alive in the study area were successively tested: 27,516 neonatal Guthrie cards were analysed for specific IgM (June 1996 – October 1998) and the next 17,653 filter-papers were tested by the new combined IgA/IgM ELISA (December 1998 – April 2000) (Fig. 3A and B). During the regional screening programme, 32 infants (18 males and 14 females, all single births) were identified by serological screening alone as congenitally infected: 13 filter-paper cards were positive in IgM ELISA screening (7 males and 6 females), and 19 neonates were positive for Toxoplasma-specific IgA and/or IgM antibodies (11 males and 8 females). In 1996-1998, the prevalence of Toxoplasma-specific IgM in newborns was at least 1 per 2,117 liveborn children (0.47/1,000) or 1 per 1,192 neonates (0.84/1,000) born to seronegative women being at risk of primary T. gondii infection during pregnancy. In 1998-2000, after introduction of combined IgA/IgM ELISA screening, the prevalence of Toxoplasma-specific IgA and/or IgM in filter-paper specimens at birth was at least 1 per 929 liveborn infants (1.08/1,000) or 1 per 523 deliveries (1.9/1,000) of non-immune mothers being at risk of infection, when compared with the mean seropositivity rate in the Polish pregnant women population at delivery in 1998-2000 of 43.7% (26,27).
Fig. 3.
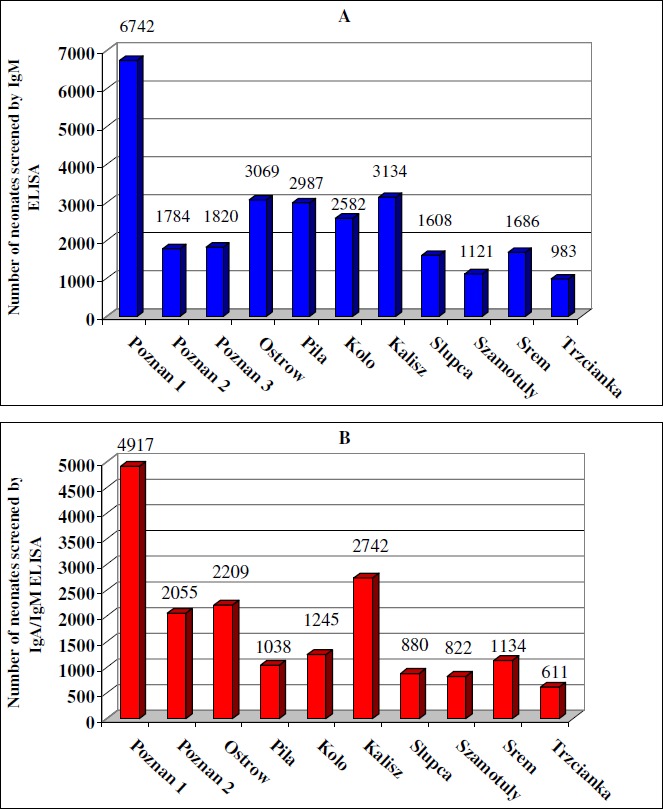
Number of neonates screened for congenital toxoplasmosis in particular hospitals in the West Poland Province: (A) ▪ for the presence of specific IgM (pilot screening), (B) ▪ for the presence of specific IgA and/or IgM antibodies (combined screening).
Additionally, 3 infected children from the study area were identified to be congenitally infected (1 male and 2 females, all singletons). One premature infant who was IgA and IgM – negative at birth, was born to a seroconverted mother who was already treated during pregnancy. The child began to synthesize her own IgM of other antigenic specificity than that of the mother at 28 days of life as shown by the Western blot technique. In two other IgM-negative newborns, blood was taken late at 5th and 8th day after birth, respectively. They were diagnosed from typical clinical signs of a classic triad (retinochoroiditis, ventricular dilatation, intracranial calcification), and both children had high levels of specific immunoglobulin G at 6 weeks and 11 months, respectively. A retrospective detection of specific IgA and IgM antibodies in filter-paper eluates collected at birth by a more sensitive ISAGA technique, and strongly elevated titres of IgG antibody (> 240 IU/ml) were specific markers of congenital Toxoplasma infection in these infants. Including the three cases with a delayed synthesis of IgM (n=1) or retarded neonatal sampling (n=2), who were not diagnosed by serological screening but born in the study area, the birth prevalence of congenital toxoplasmosis in the Poznan region was at least 1 per 1,834 live births (0.55/1,000) or 1 per 1,033 infants born to susceptible women with a potential risk of the infection (0.97/1,000) in the years 1996-1998, and at least 1 per 883 children born alive (1.13/1,000) or 1 per 497 deliveries at risk (2.0/1,000) in the years 1998-2000 (26,27).
The false positive rate of the IgM assay was 1.5 per 10,000 determinations (4 of 27,516) and that of the combined IgA/IgM ELISA was 2.3 per 10,000 examined neonatal samples (4 of 17,653), so the diagnostic specificity of the both methods was calculated to be 99.9%. The false reactivity was related to “naturally occurring “ IgM or IgA antibodies (n=4), blood transfusion before sampling (n=1) or resulted from a contamination with IgM or IgA antibodies from maternal blood in operative deliveries (caesarean section) (n=3).
Of 13 infected neonates recognized on a basis of the IgM screening test, IgM and IgA specific antibodies were both found by ISAGA technique at a dilution of 1/100 in 12 filter-paper samples taken shortly after birth; one case was strongly positive for IgM and equivocal for IgAantibody (27).
Of 19 congenitally infected newborns with a presence of specific IgA and/or IgM in filter-paper eluates at birth, anti-Toxoplasma IgA and IgM antibodies were detected in commercial assays in 12 patients, 3 cases were only IgM-positive when tested by traditional techniques, and the remaining 3 children were confirmed by comparative immunological profiles analysis alone (Westernblotting) using serum samples from the mother and infected infant (26).
Combined treatment with pyrimethamine plus sulphadiazine with or without alternation of spiramycin was initiated between the 1st day to 11 weeks of life (mean 4 weeks), and continued for 12-24 months according to a clinical course and severity of Toxoplasma infection (mean 13.7 months). Nine infants received the contineuse therapy with pyrimethamine plus sulphadiazine, and the remaining 16 children were treated with the less intensive tretment regimen. No important side-effects giving indications to the treatment interruption were seen. Mild skin rash probably related to antiparasitic chemotherapy was observed in 2 infants, and transitory crystalluria occurred in 5 cases; one of them had hyperuricaemia and a family predisposition to renal lithiasis.
Twenty-four of 35 infected infants (68.6%) presented a subclinical form of congenital T. gondii infection during the neonatal period before treatment and during the clinical follow-up period of 6.9 to 10.5 years (mean 8.4 years) or they demonstrated some non-specific symptoms or signs at birth, related to prematurity or adaptative respiratory disorders. The clinically overt form of toxoplasmosis, mild or moderate, occurred in 7 cases; in 2 other infants co-existing patent cytomegalovirus infection was diagnosed. Only one of the 35 children developed severe sequelae of congenital infection during the pre- and neonatal period with generalized parasitaemia and profound central nervous system damage, leading to the death at 12 months of age. In another child, who presented some neurological signs at birth, non-febrile seizures confirmed by a generalized paroxysmal activity in electroencephalography without Toxoplasma-specific signs in brain imaging examinations were demonstrated at the age of 6.2 years. New pathognomonic signs of congenital infection were diagnosed in 2 postnatally treated cases (small intracranial calcifications or peripheral chorioretinal scar) at the age of 2.5 and 7.7 years, respectively.
Two other postnatal deaths occurred in children with subclinical form of Toxoplasma infection: one of severe staphylococcal sepsis at 3 months in a child from extremely bad socio-economic conditions, and another one from pulmonary dysplasia at 11 months in a prematurely born infant.
Toxoplasma-specific IgG showed decreasing titres in all the infants during administration of anti-parasitic treatment. In 4 prematurely born infants, a transient negativisation of specific antibodies was observed at 2-10 months of the age (mean 7.3 months) during intensive therapy. In 22 infants, asymptomatic serological rebounds of IgG (n=4), IgG and IgA (n=17) or IgA alone (n=1) occurred firstly at 16-35 months of life (mean 24.5 months) or between 2-48 months (mean 8.7 months) after the end of initial pyrimethamine/sulphadiazine therapy. During the follow-up period, 16 children had multiple serological recurrences (range 2-5, mean 2.6 rebounds). Patients with eye and/or brain lesions had no more serological reactivations (mean 1.8), than other children with the asymptomatic infection (mean 2.1). After 6 years of life, serological rebounds were detected less frequently (26.1%) in comparison to the early infancy and pre-school period (91.3%). In one patient, a rising titre of specific IgG antibody was observed 8 weeks before a primary attack of non-febrile seizures confirmed by electroencephalography, but intracranial calcifications were not found in brain computerized tomography scans performed at the age of 10 and 76 months.
Epidemiological investigation on Toxoplasma seropositivity in newborns or pregnant women population at delivery was performed using neonatal filter-papers tested for specific immunoglobulin G. Altogether, 2684 filter-paper samples from neonates born alive were analysed at birth or 2,656 gravidae, adjusting to the 28 multiple pregnancies (1.05%): 905 samples from consecutive births in October 1998, 864 specimens from children born in August 1999, and 915 samples collected in March 2000. The proportion of samples collected at maternity departments from the urban (n=1423) and rural (n=1261) areas inhabitants was comparable (1:1.1). The mean seropositivity rate of 43.7% (range 41.5- 45.6%) was the same for the newborns and pregnant women, and it declined slightly during the study period (P = 0.06). Twin pregnancies were positive in 46.4% (13 of 28). The population of seronegative pregnant women susceptible to Toxoplasma infection was increased from the early 1990-ties, when 58.9% of gravidae were immune to T. gondii. The seroprevalence of Toxoplasma of 49.0% in women from rural areas was significantly higher when compared with the seropositivity rate of 34.4% in urban agglomerations (P < 0.00001). Similarly, the Poznan inhabitants were less frequently positive for T. gondii, than people living in other localities (P < 0.00001).
Thirty-five mothers of congenitally infected children aged of 18 to 40 years (mean 25.2 years) were qualified to the epidemiological analysis; 4 of them had medically oriented professions (paediatrician, nurse, electrocardiographist, dentist), and 15 mothers had delivered more than one child (42.9%). The proportion of women living in rural (n=9) and urban (n=26) areas was 1:3. Six women achieved a high level of university education with diploma and 24 completed college or professional school. The potential risk factors of Toxoplasma infection were known in 31 mothers: eating or testing raw pork was the main source of infection in 24 women, 8 mothers lived in a household with kittens or cats, 4 persons used to eat frequently unwashed fruit or vegetables; in 15 of them more than one source of infection was revealed. In only 3 of 35 infected women (8.6%) professional contact with soil, raw meat or animals was reported.
DISCUSSION
Infection with Toxoplasma gondii when acquired during pregnancy or in the periconceptional period can cause intrauterine damage with unpredictable clinical sequelae for the foetus, newborn infant or older child. Because almost 90% of congenitally infected children are asymptomatic at birth or they present some non-specific and reversible symptoms in neonatal period, the infection may pass undiagnosed until childhood, adolescence or even early adult life, when secondary eye lesions and neurodevelopmental disorders are its most common late consequences (5,7). Long-term antiparasitic treatment administrated during the first year of life (or longer) can reduce a risk of chorioretinal lesions within 20 years of age from more than 85% to 5-8% (28). Secondary neurological symptoms were not observed in children intensively treated in the neonatal period and in early infancy (29), and a reduction or resolution of intracranial calcification in brain computerized tomography scans was documented during postnatal treatment (30). Furthermore, impaired psychomotor development may be improved by early anti-parasitic chemotherapy starting from the neonatal period (29,31). Actually, postnatal treatment of newborns and infants seems to be significantly effective and protective against a late apparition of clinical sequelae in older children and adolescents (29-31).
The presence of Toxoplasma-specific IgA or IgM antibodies in peripheral blood of a neonate after the first week of life is considered a definite marker of congenital infection (32). However, diagnostic sensitivity of different serological techniques varies widely among authors, and the diagnostic accuracy of Toxoplasma IgM and IgA assays in infants is a source of constant discussions (33-37). The study of Lebech et al. (2001) documented that neonatal screening based on a detection of specific IgM alone is able to diagnose more than 75% of infected infants who not received antenatal therapy, but testing of newborns for Toxoplasma-specific IgA may pick up an additional 5–10% of infected infants (7). These neonates are mostly asymptomatic or they present some non-typical clinical symptoms related to prematurity or some problems with an adaptation to extra-uterine life, and they can be not diagnosed on a routine paediatric examination at birth. Naessens and colleagues (1999) have found IgM antibody in neonatal blood in 85% of congenitally infected children born to untreated mothers but only in 25% of infants who were treated prenatally. Similarly, the sensitivity of diagnosing IgA in untreated and treated infants’ groups was 80 and 57%, respectively, although the impact of maternal-foetal treatment on the suppression of newborn’s immunological response has not been significantly proven (38). Couvreur (1993) reported that specific IgM is not detected by the reference ImmunoSorbent Agglutination Assay (ISAGA) in 30% of infants at birth but the discordance rises up to 82%, if their mothers received the pyrimethamine-sulphadiazine therapy during pregnancy (28). The study of Remington and colleagues (2001) reported that only 25% of infected children were IgM-positive at birth, but these observations were all performed in France where prenatal treatment is applied, which may reduce the IgM response in the Toxoplasma infected foetus (17). A non-satisfactory detection of specific IgM antibody at birth in France was caused by prenatal treatment which could modify specific immunological response and diminish a probability to detect anti-Toxoplasma IgM antibody in newborns and infants (7). The European multicentre study showed that a combination of comparative immunological profiles analysis by ELIFA (Enzyme-Linked ImmunoFiltration Assay) with classic serological assays like ELISA or ISAGA can increase diagnostic sensitivity to detect anti-Toxoplasma IgM and IgA up to 96.2% (20).
As antenatal treatment following prenatal diagnosis is sporadically prescribed in Poland, the implementation of mass neonatal screening for congenital toxoplasmosis seems to be fully justified. It is difficult to evaluate which of preventive methods for congenital toxoplasmosis is the most efficacious, economically profitable and ethically acceptable in a particular stage of the life like pregnancy or the neonatal period. The suggestions exist, that national serological screening for pregnant women widely recommended in France and Austria since 1970-ties is not so much effective as previously suspected (data from the European Research Network on Congenital Toxoplasmosis study group). Moreover, screening during pregnancy is not able to detect T. gondii infections acquired the last weeks before delivery, when a potential risk of transplacental transmission is the highest and exceeds 90% (39). For this reason, some reference centres in France decided to carry on regional screening in newborns for a detection of specific IgM (H. Dumon, personal correspondence). So far, widely proposed health education is practiced in Great Britain, because there are no justified arguments for mass testing of pregnant women.
Anti-parasitic chemotherapy is believed to reduce the risk of sequelae and should be initiated as soon as the infection is diagnosed, but the impact of treatment during pregnancy on materno-foetal transmission or development of new ocular and brain lesions is still doubtful and controversial (40-43), so screening of neonates followed by intensive long-term postnatal treatment for 12-24 months seems to be more justified. Detection of anti-T. gondii IgA and IgM in the early neonatal period is still the most practical and certain method to diagnose congenital toxoplasmosis, and a treatment delay of 1-2 months has no significant influence on a potential risk of clinical signs in the neonate or infant (44).
The last years, the number of centres in which screening of neonates for Toxoplasma-specific IgM is recommended is permanently increasing. The Boston University screening of 635,000 neonates born in Massachusetts and New Hampshire detected specific IgM in filter-paper samples collected at birth in only 52 patients (0.8 per 10,000 liveborn neonates), and the next 50 Guthrie cards gave false positive results (5). Similar work performed by The Danish Toxoplasma Study Group, combining a detection of seroconversion during pregnancy with analysis of neonatal IgM in filter-papers, identified 12 cases of congenital toxoplasmosis of 41,500 examined pregnancies (3 per 10,000 deliveries) (7). The pilot study was a reason for the implementation of obligatory nation-wide serological screening for all Danish neonates since the 1st January of 1999 (23). Detection of anti-Toxoplasma IgM in neonatal Guthrie cards using a commercially available assay was also introduced in Sweden, confirming congenital infection in 4 cases of 27,000 examined neonates (1.5/1,000) (45). The national neonatal screening programme in Brazil detected 47 infected neonates of 140,914 live births (1 per 3,000 neonates) with a higher risk of clinically overt form with ocular lesions (24,25). Combined neonatal screening programme detecting not only IgM but both antibody classes which do not cross the placenta - IgA and IgM in a single determination procedure, was firstly introduced by the Poznan University of Medical Sciences in Poland.
In Poland, where a level of Toxoplasma-seropositivity in a pregnant women population is elevated (22,26), use of obligatory serological screening during pregnancy, like in Austria and France, should create some economical problems because of a high cost of repeated serological testing. In a screened population with a high Toxoplasma seroprevalence, in a fact the first serological test is usually performed between 8 and 13 weeks of gestation, (11), a differentiation between acute infection with a potential risk for a foetus and previously acquired inactive form of infection without any risk for offspring may be diagnostically difficult, and it requires numerous re-testing, invasive prenatal diagnosis, unnecessary treatment, and anxiety during pregnancy with a potential risk of its interruption. Advantages of neonatal screening using filter-papers are: low invasiveness of the method, easy identification, storage and transport conditions of the samples, and lower cost when compared with monthly serological testing of seronegative pregnant women. A technical cost of a single laboratory analysis for IgA and IgM antibodies using a non-commercial ELISA assay was about 2 Euro, so a cost to detect one congenitally infected case in a studied Polish population was not more than 2,000 Euro, and 800,000 Euro annually for neonatal testing of all children born in Poland.
According to generally accepted criteria, only primary Toxoplasma infection during pregnancy constitutes a potential risk of the congenital infection, which justifies efficiency of serological testing of pregnant women. However, in some sporadic cases, T. gondii infection acquired before pregnancy of re-infection in a seropositive mother may be also a risk for a foetus. In such rare cases, serological screening of neonates remains the only possible and practical method to identify congenital T. gondii infection (46-50). Similarly, primary Toxoplasma infection acquired a few days/weeks before delivery cannot be recognized by routine serological screening for pregnant women (39,51).
Incidence of congenital toxoplasmosis in the West Poland Province of at least 1 per 883 children born alive (1.13/1,000) or 1 per 497 deliveries at risk (2.0/1,000), according to the percentage of seropositivity in a studied population, (26) seems to sufficiently justify the needs for some nation-wide preventive measures for congenital toxoplasmosis in Poland. The best strategy for a prevention and control of congenital T. gondii infections remains still unknown. The neonatal tests for a detection of Toxoplasma-specific IgM or IgA & IgM antibodies showed a good diagnostic sensitivity in a high seroprevalence population of newborns and successfully fulfil WHO criteria for screening programmes in newborns.
CONCLUSIONS
Serological screening of neonates for anti-Toxoplasma IgA and/or IgM antibodies in filter-paper samples collected at birth, followed by intensive postnatal treatment is a valuable and a good sensitivity preventive method for a detection of asymptomatic children with congenital toxoplasmosis, who were not treated prenatally.
Mass neonatal testing for congenital toxoplasmosis seems to be strongly justified in areas of a high annual number of births associated with a high seroprevalence of T. gondii infection, where national screening for pregnant women has not been implemented.
ACKNOWLEDGEMENTS
The author is indebted to Associate Professor Eskild Petersen from the Department of Infectious Diseases in the Aarhus University Hospital and the former chief of the Department of Mycobacteria and Parasitic Infections in Statens Seruminstitut in Copenhagen (Denmark) for his encouragement, professional advice and technical support. The directors of the neonatal and obstetric wards in the Poznan Province are acknowledged for their generous cooperation and skilful care for the children.
This work was supported by the State Research Committee in Warsaw, Poland (grants No. 4PO5E 08413 and 4PO5E 10017).
REFERENCES
- 1.Aspock H., Pollak A. Prevention of prenatal toxoplasmosis by serological screening of pregnant women in Austria. Scand. J. Infect. Dis. 1992; Supl. 84: 32-37. [PubMed] [Google Scholar]
- 2.Garin J.P. Toxoplasmose: aspects nouveaux dans la surveillance de la femme enceinte et du nouveau-ne. Feuill. Biol. 1988; 29: 21-30. [Google Scholar]
- 3.Berger R., Merkel S., Rudin C. Toxoplasmosis in pregnancy: findings from umbilical cord blood screening in 30,000 newborn infants. Schweiz. Med. Wochenschr. 1995; 125: 1168-1173. [PubMed] [Google Scholar]
- 4.Eaton R.B., Petersen E., Seppanen H., Tuuminen T. Multicenter evaluation of a fluorometric enzyme immunocapture assay to detect Toxoplasma-specific immunoglobulin M in dried blood filter paper specimens from newborns. J. Clin. Microbiol. 1996; 34: 3147-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerina N.G., Hsu H.W., Meissner H.C., Maguire J.H., Lynfield R., Stechenberg B., Abroms I., Pasternack M.S., Hoff R., Eaton R.B., Grady G.F. and the New England Regional Toxoplasma Working Group. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. N. Engl. J. Med. 1994; 330: 1859-1863. [DOI] [PubMed] [Google Scholar]
- 6.Lebech M, Petersen E. Detection by enzyme immunosorbent assay of Toxoplasma gondii IgG antibodies in dried blood spots on PKU-filter paper from newborns. Scand. J. Infect. Dis. 1995; 27: 259-263. [DOI] [PubMed] [Google Scholar]
- 7.Lebech M., Andersen O., Christensen N.C., Hertel J., Nielsen H.E., Peitersen B., Rechnitzer C., Larsen S.O., Norgaard-Pedersen B., Petersen E. and the Danish Congenital Toxoplasmosis Study Group. Feasibility of neonatal screening for Toxoplasma infection in the absence of prenatal treatment. Lancet 1999; 353: 1834-1837. [DOI] [PubMed] [Google Scholar]
- 8.Buffolano W., Gilbert R., Holland F.J., Fratta D., Palumbo F., Ades A.E. Risk factors for recent Toxoplasma infection in pregnant women in Naples. Epidemiol. Infect. 1996; 116: 347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evengard B., Lilja G., Capraru T., Malm G., Kussofsky E., Oman H.M., Forsgren M. A retrospective study of seroconversion against Toxoplasma gondii during 3000 pregnancies in Stockholm. Scand. J. Infect. Dis. 1999; 31: 127-129. [DOI] [PubMed] [Google Scholar]
- 10.Hohlfeld P. Toxoplasmosis in pregnancy: arguments in favor of systematic screening in Switzerland. Arch. Gynecol. Obstet. 1995; 256: 165-169. [PubMed] [Google Scholar]
- 11.Jenum P.A., Stray-Pedersen B., Gundersen A.G. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 1997; 35: 1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenum P.A., Stray-Pedersen B., Melby K.K., Kapperud G., Whitelaw A., Eskild A., Eng J. Incidence of Toxoplasma gondii infection in 35,940 pregnant women in Norway and pregnancy outcome for infected women. J. Clin. Microbiol. 1998; 36: 2900-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dzbenski T.H., Kopaczowa G. Remarks on the epidemiology and prevention of toxoplasmosis [Polish]. Przeg. Epid. 1984; 38: 235-241. [PubMed] [Google Scholar]
- 14.Milewska-Bobula B., Lipka B., Kassur-Siemienska B., Dunin-Wasowicz D., Idzik M., Bauer A., Marcinski P. Late sequelae of congenital toxoplasmosis in children observed from 1997 to 1999 in infant clinic of the Children’s Memorial Health Institute, Poland. Abstracts of the European Conference on Congenital Toxoplasmosis, Vienna, June 29-July 1, 2000. [Google Scholar]
- 15.Pawlowski Z.S. Control of congenital toxoplasmosis in Poland [Polish]. Wiad. Parazytol. 1993; 39: 331-338. [PubMed] [Google Scholar]
- 16.Desmonts G., Couvreur J. Toxoplasmose congenitale. Étude prospective de l’issue de la grossesse chez 542 femmes atteintes de toxoplasmose acquise en cours de gestation. Sem. Hop. Paris 1986; 62: 1418-1422. [PubMed] [Google Scholar]
- 17.Remington J.S., McLeod R., Thulliez P., Desmonts D. Toxoplasmosis. Infectious Diseases of the Fetus and the Newborn Infant. Remington J.S., Klein J.O., Philadelphia, W.B. Saunders Company, Vth ed., 2001: 205-346. [Google Scholar]
- 18.Dzbenski T.H., Reizer A. Toxoplasmosis in Poland in the years 1986-1989 [Polish]. Przeg. Epid. 1991; 45: 117-120. [PubMed] [Google Scholar]
- 19.Ambroise-Thomas P., Okay T. Variations inter et intra-cepales de pathogenicite chez Toxoplasma gondii. Consequences cliniques et epidemiologiques. Bull. Acad. Natle. Med. 1993; 177: 1411-1421. [PubMed] [Google Scholar]
- 20.Pinon J.M., Dumon H., Chemla C., Franck J., Petersen E., Lebech M., Zufferey J., Bessieres M.H., Marty P., Holliman R., Johnson J., Luyasu V., Lecolier B., Guy E., Joynson D.H.M., Decoster A., Enders G., Pelloux H., Candolfi E. Strategy for diagnosis of congenital toxoplasmosis: evaluation of methods comparing mothers and newborns and standard methods for postnatal detection of immunoglobulin G, M, and A antibodies. J. Clin. Microbiol. 2001; 39: 2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawlowski Z.S., Mrozewicz B., Kacprzak E., Pisarski T., Rybakowski L., Tomaszewski S., Swiecicka-Konarska T., Rokossowski H., Moczko J. Congenital toxoplasmosis in the Poznan Province [Polish]. Gin. Pol. 1994; 65: 409-412. [PubMed] [Google Scholar]
- 22.Pawlowski Z.S. Clinical epidemiology of toxoplasmosis in the Poznan Province [Polish]. Klin. Perin. Gin. 1995; Supl. XI: 5-11. [Google Scholar]
- 23.Petersen E, Eaton R. Control of congenital infection with Toxoplasma gondii by neonatal screening based on detection of specific immunoglobulin M antibodies eluted from phenylketonuria filter paper blood-spot samples. Acta Paediatr. 1999; 432 (Supl.): 36-39. [DOI] [PubMed] [Google Scholar]
- 24.Neto E.C., Anele E., Rubim R., Brites A., Schulte J., Becker D., Tuuminen T. High prevalence of congenital toxoplasmosis in Brazil estimated in a 3-year prospective neonatal screening study. Int. J. Epidemiol. 2000; 29: 941-947. [DOI] [PubMed] [Google Scholar]
- 25.Carvalheiro C.G., Mussi-Pinhata M.M., Yamamoto A.Y., De Souza C.B., Maciel L.M. Incidence of congenital toxoplasmosis estimated by neonatal screening: relevance of diagnostic confirmation in asymptomatic newborn infants. Epidemiol. Infect. 2005; 133: 485-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul M., Petersen E., Szczapa J. Prevalence of congenital Toxoplasma gondii infection among newborns from the Poznań region of Poland: validation of a new combined enzyme immunoassay for Toxoplasma gondii-specific immunoglobulin A and immunoglobulin M antibodies. J. Clin. Microbiol. 2001; 39: 1912-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul M., Petersen E., Pawlowski Z.S., Szczapa J. Neonatal screening for congenital toxoplasmosis in the Poznań region of Poland by analysis of Toxoplasma gondii-specific IgM antibodies eluted from filter-paper blood spots. Pediatr. Infect. Dis. J. 2000; 19: 30-36. [DOI] [PubMed] [Google Scholar]
- 28.Couvreur J. Toxoplasmose congenitale: prise on charge et devenir. Med. Mal. Infect. 1993; 23: 176-182. [Google Scholar]
- 29.McAuley J., Boyer K.M., Patel D., Mets M., Swisher C., Roizen N., Wolters C., Stein L., Stein M., Schey W., Remington J., Meier P., Johnson D., Heydeman P., Holfels E., Withers S., Mack D., Brown C., Patton D., McLeod R. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago collaborative treatment trial. Clin. Infect. Dis. 1994; 18: 38-72. [DOI] [PubMed] [Google Scholar]
- 30.Patel D.V., Holfels E.M., Vogel N.P., Boyer K.M., Mets M.B., Swisher C.N., Roizen N.J., Stein L.K., Stein M.A., Hopkins J., Withers S.E., Mack D.G., Luciano R.A., Meier P., Remington J.S., McLeod R.L. Resolution of intracranial calcifications in infants with treated congenital toxoplasmosis. Radiology 1996; 199: 433-440. [DOI] [PubMed] [Google Scholar]
- 31.Roizen N., Swisher C.N., Stein M.A., Hopkins J., Boyer M.M., Holfels E., Mets M.B., Stein L., Patel D., Meier P., Withers S., Remington J., Mack D., Heydemann P.T., Patton D., McLeod R. Neurologic and developmental outcome in treated congenital toxoplasmosis. Pediatrics 1995; 95: 11-20. [PubMed] [Google Scholar]
- 32.Lebech M., Joynson D.H.M., Seitz H.M., Thulliez P., Gilbert R.E., Dutton G.N., Ovlisen B., Petersen E. Classification system and case definitions of Toxoplasma gondii infection in immunocompetent pregnant women and their congenitally infected offspring. J. Clin. Microbiol. Inf. Dis. 1996; 15: 799-804. [DOI] [PubMed] [Google Scholar]
- 33.Bessieres M.H., Roques C., Berrebi A., Barre V., Cazaux M., Sequela J.P. IgA antibody response during acquired and congenital toxoplasmosis. J. Clin. Pathol. 1992; 45: 605-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decoster A., Darcy F., Caron A., Vinatier D., Houze de l’Aulnoit D., Vittu G., Niel G., Heyer F., Lecolier B., Delcroix M., Monnier J.C., Duhamel M., Capron A. Anti-P30 IgA antibodies as prenatal markers of congenital Toxoplasma infection. Clin. Exp. Immunol. 1992; 87: 210-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffy K.T., Wharton P.J., Johnson J.D., New L., Holliman R.E. Assessment of immunoglobulin-M immunosorbent agglutination assay (ISAGA) for detecting Toxoplasma specific IgM. J. Clin. Pathol. 1989; 42: 1291-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fricker-Hidalgo H., Pelloux H., Bost M., Goullier-Fleuret A., Ambroise-Thomas P. Congenital toxoplasmosis: contribution of postnatal biological follow-up. Presse Mèd. 1996; 25: 1868-1872. [PubMed] [Google Scholar]
- 37.Thulliez P., Dutriat P., Saulnier M., Vernes A., Desmonts G. Évaluation de trois reactifs de detection par immunocapture des IgM spécifiques de la toxoplasmose. Rev. Fr. Lab. 1988; 169: 25-31. [Google Scholar]
- 38.Naessens A., Jenum P.A., Pollak A., Decoster A., Lappalainen M., Villena I., Lebech M., Stray-Pedersen B., Hayde M., Pinon J.M., Petersen E., Foulon W. Diagnosis of congenital toxoplasmosis in the neonatal period: a multicenter evaluation. J. Pediatr. 1999; 145: 714-719. [DOI] [PubMed] [Google Scholar]
- 39.Marx-Chemla C., Puygauthier-Toubas D., Foudrinier F., Dorangeon P.H., Leulier J., Quereux C., Leroux B., Pinon J.M. La surveillance immunologique d’une femme enceinte seronegative pour la toxoplasmose doit-elle s’arreter a l’accouchement? Presse Med. 1990; 19: 367-368. [PubMed] [Google Scholar]
- 40.Foulon W., Villena I., Stray-Pedersen B., Decoster A., Lappalainen M., Pinon J.M., Jenum P.A., Hedman K., Naessens A. Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children’s sequelae at age 1 year. Am. J. Obstet. Gynecol. 1999; 180: 410-415. [DOI] [PubMed] [Google Scholar]
- 41.Wallon M., Liou C., Garner P., Peyron F. Congenital toxoplasmosis: systematic review of evidence of efficacy of treatment in pregnancy. B.M.J. 1999; 318: 1511-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The SYROCOT Study Group: Thiebaut R., Leproust S., Chene G., Gilbert R., Prusa A., Hayde M., Pollak A., Wallon M., Peyron F., Romand S., Thulliez P., Buffolano W., Romano A., Franck J., Dumon H., Bastion P., Issert E., Bessieres M.H., Ferret N., Marty P., Chemla C., Villena I., Pelloux H., Fricker-Hidalgo H., Bost-Bru C., Semprini E., Savasi V., Paul M., Malm G., Evengard B., Petersen E., Schmidt D., Kortbeek T., Logar J., Szenasi S., Stray-Pedersen B., Jenum P., Lappalainen M., Lago E., Neto E., Bahia-Oliveira I., Eaton R., Hsu H.W., Gomez-Marin J. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet 2007; 369: 115-122. [DOI] [PubMed] [Google Scholar]
- 43.Gras L., Wallon M., Polak A., Cortina-Borja M., Evengard B., Hayde M., Petersen E., Gilbert R. for the European Multicentre Study on Congenital Toxoplasmosis. Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: A cohort study in 13 European centres. Acta Paediatr. 2005; 94: 1721-1731. [DOI] [PubMed] [Google Scholar]
- 44.Wallon M., Dunn D., Slimani D., Girault V., Gay-Andrieu F., Peyron F. Diagnosis of congenital toxoplasmosis at birth: what is the value of testing for IgM and IgA? Eur. J. Pediatr. 1999; 158: 645-649. [DOI] [PubMed] [Google Scholar]
- 45.Malm G., Tear F.K., Wiklund S., Engman M.L., Ivarsson S.A., Petersson K., Evengard B. Three children with congenital toxoplasmosis: early report from a Swedish prospective screening study. Acta Paediatr. 1999; 88: 667-670. [DOI] [PubMed] [Google Scholar]
- 46.Desmonts G., Couvreur J., Thullier P. Toxoplasmose congenitale. Cinq cas de transmission a l’enfant d’une infection maternelle anterieure a la grossesse. Presse Med. 1990; 19: 1445-1449. [PubMed] [Google Scholar]
- 47.Fortier B., Pinto-Sousa M.I., Ajana F. Toxoplasma gondii: 2 cas de recontamination sur terrain immune. Presse Med. 1991; 20: 2109. [PubMed] [Google Scholar]
- 48.Gavinet M.F., Robert F., Firtion G., Delouvrier E., Hennequin C., Maurin J.R., Tourte-Schaefer C., Dupouy-Camet J. Congenital toxoplasmosis due to maternal reinfection during pregnancy. J. Clin. Microbiol. 1997; 35: 1276-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hennequin C., Dureau P., N’Guyen L., Thulliez P., Gagelin B., Dufier J.L. Congenital toxoplasmosis acquired from an immune woman. Pediatr. Infect. Dis. J. 1997; 16: 75-77. [DOI] [PubMed] [Google Scholar]
- 50.Hohlfeld P., Daffos F., Thulliez P., Aufrant C., Couvreur J., MacAleese J., Descombey D., Forestier F. Fetal toxoplasmosis: outcome of pregnancy and infant follow-up after in utero treatment. J. Pediatr. 1989; 115: 765-769. [DOI] [PubMed] [Google Scholar]
- 51.Luyasu V., Bauraind O., Bernard P., Bietlot Y., Brasseur C., Englebert J., Fiasse L., Givron O., Longueville E., Melard J.L., Michel M., Wacquez M. Congenital toxoplasmosis and seroconversion in last weeks of pregnancy – clinical observations. Acta Clin. Belgica 1997; 52: 381-387. [DOI] [PubMed] [Google Scholar]


