ABSTRACT
Background
Monounsaturated fatty acids (MUFAs) improve blood lipid profiles in intervention studies, but prospective evidence with regard to MUFA intake and coronary heart disease (CHD) risk is limited and controversial.
Objective
We investigated the associations of cis MUFA intake from plant (MUFA-P) and animal (MUFA-A) sources with CHD risk separately among 63,442 women from the Nurses’ Health Study (1990–2012) and 29,942 men from the Health Professionals Follow-Up Study (1990–2012).
Design
Intakes of MUFA-Ps and MUFA-As were calculated by using validated food-frequency questionnaires collected every 4 y. Incident nonfatal myocardial infarction and fatal CHD cases (n = 4419) were confirmed by medical record review.
Results
During follow-up, MUFA-Ps and MUFA-As contributed 5.8–7.9% and 4.2–5.4% of energy on average, respectively. When MUFA-Ps were modeled to isocalorically replace other macronutrients, HRs (95% CIs) of CHD were 0.83 (0.68, 1.00) for saturated fatty acids (SFAs; 5% of energy), 0.86 (0.76, 0.97) for refined carbohydrates (5% of energy), and 0.80 (0.70, 0.91) for trans fats (2% of energy) (P = 0.05, 0.01, and 0.001, respectively). For MUFA-As, corresponding HRs (95% CIs) for the same isocaloric substitutions were 1.04 (0.79, 1.38) for SFAs, 1.11 (0.91, 1.35) for refined carbohydrates, and 0.88 (0.77, 1.01) for trans fats (P = 0.76, 0.31, and 0.08, respectively). Given the common food sources of SFAs and MUFA-As (Spearman correlation coefficients of 0.81–0.83 between these groups of fatty acids), we further estimated CHD risk when the sum of MUFA-As and SFAs (5% of energy) was replaced by MUFA-Ps, and found that the HR was 0.81 (95% CI: 0.73, 0.90; P < 0.001) for this replacement.
Conclusions
The largely different associations of MUFA-Ps and MUFA-As with CHD risk suggest that plant-based foods are the preferable sources of MUFAs for CHD prevention. These findings are observational and warrant confirmation in intervention settings. This study was registered at clinicaltrials.gov as NCT00005152 and NCT00005182.
Keywords: monounsaturated fat, saturated fat, plant fat, animal fat, coronary heart disease
INTRODUCTION
Dietary MUFAs consist of oleic acid (cis-9-octadecenoic acid, >90%) and other minor MUFAs [e.g., palmitoleic acid (cis-9-hexadecenoic acid)], which collectively contribute to 12% of total energy intake in the US diet (1, 2). In clinical trials, cis MUFAs improve lipid profiles and other cardiovascular disease (CVD) risk factors, including hypertension and central obesity (3–5). A Mediterranean diet high in MUFAs also reduced CVD risk in a large intervention study (6).
Prospective cohort studies linking MUFA intake with CVD risk have been limited and inconclusive (7–15). A recent meta-analysis reported no association between MUFA intake and coronary heart disease (CHD) risk but noticed significant between-study heterogeneities (7). Findings from early investigations may not be entirely comparable to each other, because other macronutrients with various health effects were not simultaneously considered in the analysis. In particular, PUFAs and carbohydrates from whole grains have been associated with a lower CHD risk, whereas SFAs, trans fats, and refined carbohydrates may exert the opposite effects (8, 14). Therefore, potential findings could be obscured due to confounding by these constituents. In addition, associations for MUFAs may also depend on other macronutrients specified in isocaloric analyses.
Unlike PUFAs that are primarily from plant-based sources, dietary MUFAs can come from various plant-based (e.g., vegetable oils and nuts) and animal-based sources (e.g., red meats and high-fat dairy products) that have quite differential health effects (15–17). In particular, animal foods, as a major source of MUFAs, also contain high amounts of SFAs, which may confound the associations of total MUFAs and CHD risk (16, 17). It was proposed that separating MUFAs from different food sources may help to clarify the role of MUFAs in CHD risk (15–17). In 2 large prospective cohorts of US men and women, we differentiated MUFAs from plant sources (MUFA-Ps) and MUFAs from animal sources (MUFA-As) and examined the hypothesis that MUFA-P intake is associated with a lower risk of CHD, whereas MUFA-A intake is not. Moreover, we estimated CHD risk for substituting MUFAs for SFAs, trans fats, and refined carbohydrates, macronutrients that should be replaced with healthier alternatives per the current dietary guidelines.
METHODS
Study population
In 1976, a total of 121,700 female nurses aged 30–55 y were enrolled in the Nurses’ Health Study (NHS) (18). In 1986, a total of 51,529 male health professionals aged 40–75 y were enrolled in the Health Professionals Follow-Up Study (HPFS) (19). For both cohorts, participants reported information on medical history, lifestyle, potential risk factors, and disease diagnosis at baseline and every 2 y by using a self-administered questionnaire. The study protocol was approved by the institutional review boards of the Brigham and Women's Hospital and the Harvard School of Public Health. The completion of the self-administered questionnaires was considered to imply informed consent. The follow-up rates (active and death follow-up) up to 2012 were 94.8% in the NHS and 96.4% in the HPFS. This study was registered at clinicaltrials.gov as NCT00005152 and NCT00005182.
We used 1990 as the baseline of 2 cohorts when olive oil consumption was first asked in the food-frequency questionnaires (FFQs). Of the 80,332 women and 38,842 men who completed the 1990 FFQ, participants were excluded if they reported physician-diagnosed cancer, diabetes, or CVD at study baseline; reported implausible energy intake (<600 or >3500 kcal/d in the NHS; <800 or >4200 kcal/d in the HPFS); answered the baseline questionnaires only; or had missing age, which left 63,442 women and 29,942 men in the final analysis (Supplemental Figure 1).
Ascertainment of diet
Participants were mailed validated FFQs with >130 items every 4 y to assess and update their habitual diet. The questionnaires inquired how often, on average, participants had consumed specific foods and the types of fats, oils, and margarines used during cooking and at the table. Nutrient intake was calculated on the basis of the USDA and Harvard University food-composition databases, which have been updated over time to include new food items and to reflect changes in food composition (20). Specifically, we repeatedly (5 times from 1991 to 2011) selected commonly consumed foods on the basis of market share or cohort pilot data, including specific brands and types of margarines, and analyzed their fatty acid composition to account for changes over time. Fatty acid composition was measured with the use of gas chromatography coupled with Flame ionization detector in the Nutritional Biomarker Laboratory at Harvard TH Chan School of Public Health. The nutrient database separated trans MUFAs from cis isomers, which were the main exposures in the current study. MUFA-As were the sum of cis MUFAs from animal foods, such as animal fats for cooking, dairy products, eggs, poultry, processed and unprocessed red meats, and fish; cis MUFA-Ps were calculated on the basis of plant foods, such as vegetable cooking oils, breads and cereals, fruit, vegetables, legumes, nuts, and seeds. For mixed food items, ingredients were identified according to the manufacturer's product labels or recipes for home-prepared items. We derived refined carbohydrates as the sum of added sugar and carbohydrates from refined grains and subtracted refined carbohydrates from total carbohydrates to estimate unrefined carbohydrate intake.
To better represent long-term diet, the cumulative average intake was calculated as the average of all previous dietary assessment up to the end of each 4-y follow-up interval (i.e., we used 1990 dietary data for the 1990–1994 follow-up period; the average of 1990 and 1994 data for the 1994–1998 period; the average of 1990, 1994, and 1998 data for the 1998–2002 period and so on) (21). To reduce the possibility of reverse causation bias, we stopped updating diet after participants reported a diagnosis of diabetes, stroke, or cancer. Missing values of food and nutrient intakes during follow-up were replaced with cumulative averages of previous assessments. The validity of FFQ assessments of dietary fats has been shown in multiple validation studies (22–24). In the most recent validation study in NHS participants, de-attenuated Spearman correlation coefficients (rs) of energy-adjusted nutrient intake assessments by FFQs compared with multiple 7-d dietary records were 0.58 (P < 0.001) for total cis MUFAs and 0.65 (P < 0.001) for oleic acid (25).
Ascertainment of CHD
Total CHD included nonfatal myocardial infarction (MI) and fatal CHD (26). To ascertain nonfatal MI, we first obtained permission for access to medical records of participants who reported having physician-diagnosed heart disease in follow-up questionnaires. Medical records were reviewed by study physicians who were blinded to exposure status. Nonfatal MI was confirmed by using the WHO criteria of typical symptoms plus either elevated enzymes or diagnostic electrocardiography changes (27). MIs that required hospital admission and for which confirmatory information was obtained by phone interview or letter, but for which no medical records were available, were classified as probable. Deaths were identified by reports from next of kin and US postal authorities, or by searching the National Death Index. More than 98% of deaths can be identified with the use of these approaches (26). Fatal CHD was confirmed by a review of hospital records or autopsy reports if CHD was listed as the underlying cause of death and if evidence of previous CHD was available from medical records. Probable fatal CHD was assigned to cases with CHD listed as the underlying cause of death on the death certificate but when no medical records concerning the death were available and no previous report of CHD was indicated. For the main analysis, we included both confirmed and probable cases to maximize statistical power.
Statistical analysis
Macronutrients were analyzed as percentages of energy by dividing energy from the specific macronutrient by total energy intake. Because MUFA-P and MUFA-A consumption changed over time (Figure 1), we present population characteristics according to MUFA intake quintiles at the midpoint of follow-up (1998). The cross-sectional correlations among dietary fats were also based on 1998 data. Person-years of follow-up for each participant were calculated from the return date of baseline questionnaires to the date when participants were diagnosed with CHD, the date of death, or the end of follow-up, whichever came first. Of note, by using these strategies for identifying deaths, we were able to identify and ascertain cardiovascular deaths or mortality due to other causes during follow-up, which do not rely on self-reports. HRs and 95% CIs of incident CHD were estimated by using time-dependent Cox proportional hazards regression models in each cohort with follow-up duration as the time scale. Regression models were stratified jointly by age in months and calendar year to better control for their confounding and possible interactions. In multivariate-adjusted models, we further adjusted for some established and potential demographic and lifestyle risk factors of CHD, including ethnicity, smoking status, alcohol intake, family history of MI, family history of diabetes, menopausal status and postmenopausal hormone use (NHS only), physical activity, current aspirin use, multivitamin use, baseline hypertension, baseline hypercholesterolemia, BMI, total energy intake, and intakes of fruit and vegetables. We further adjusted for SFA intake to control for confounding. A test for linear trend was performed by modeling median values of MUFAs in each category as a continuous variable. The proportional hazards assumption was tested by fitting a model that included interaction terms between MUFAs and duration of follow-up and by using a likelihood ratio test. The assumption was not violated (P > 0.05 for all tests). We further explicitly estimated CHD risk when SFAs were replaced by MUFA-Ps in an energy-density model that included total calorie intake and total fats, PUFAs, trans fats, and MUFA-As. By leaving SFAs out of the model, regression coefficients for MUFA-Ps can be interpreted as the estimated effect of isocalorically substituting MUFA-Ps for SFAs while holding total energy, total fats, and other fat intakes constant. Similar isocaloric substitution analyses were conducted for MUFA-As, as well as for substituting MUFAs for trans fats or refined carbohydrates. Given the common food sources of MUFA-As and SFAs, we also estimated CHD risk by replacing the sum of MUFA-As and SFAs with MUFA-Ps. Analyses were conducted in the 2 cohorts separately, and then results were pooled with a fixed-effects model when P values for heterogeneity were >0.05.
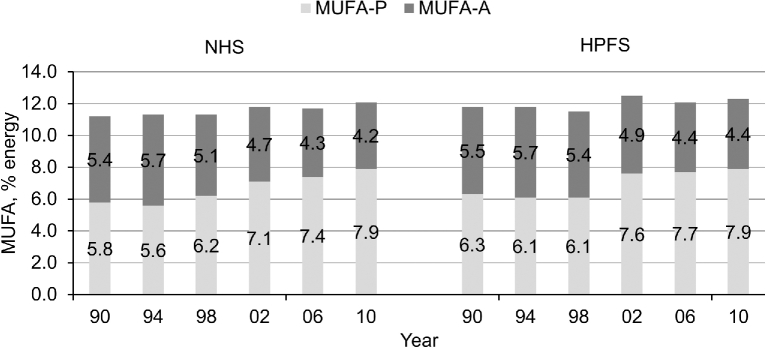
FIGURE 1.
Trends in age-adjusted MUFA-P and MUFA-A intakes as percentages of energy during follow-up. n = 63,442; 62,729; 60,930; 58,435; 55,167; and 50,962 from 1984 to 2010 in the NHS, and n = 29,942; 29,131; 27,501; 25,589; 23,396; and 22,272 from 1986 to 2010 in the HPFS. HPFS, Health Professionals Follow-Up Study; MUFA-A, MUFA from animal sources; MUFA-P, MUFA from plant sources; NHS, Nurses’ Health Study.
We performed several sensitivity analyses to examine the robustness of findings: 1) controlling for baseline BMI instead of updated BMI, because weight change could be an intermediate factor between fat intake and CHD; 2) adjusting for hypertension, hypercholesterolemia, and diabetes diagnosed during follow-up; or 3) excluding probable CHD cases. Statistical analyses were performed by using SAS 9.4 (SAS Institute). All P values were 2-sided, with significance defined as P < 0.05.
RESULTS
Participant characteristics according to percentages of energy from MUFAs are presented in Table 1. Women with a higher MUFA-P intake were younger and more likely to use postmenopausal hormones, whereas men with higher MUFA-P intake were more likely to be white. In both cohorts, those with higher MUFA-P intake were more likely to use multivitamins and aspirin. Participants with high MUFA-A intakes were younger, less physically active, heavier, and more likely to smoke, have a family history of diabetes, or use postmenopausal hormones and less likely to have a family history of MI or use multivitamins and aspirin. For dietary factors, individuals with higher MUFA-P or MUFA-A intake also consumed higher total fats, PUFAs, SFAs, and trans fats, but lower proteins and unrefined carbohydrates. Those with higher MUFA-Ps had a higher alcohol intake, whereas the opposite was true for those with higher MUFA-As.
TABLE 1.
Age-standardized characteristics according to MUFA intake at the midpoint of follow-up (1998)1
| NHS | HPFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q1 | Q2 | Q3 | Q4 | Q5 | |
| MUFA-Ps | ||||||||||
| n | 11,687 | 11,687 | 11,688 | 11,687 | 11,687 | 5117 | 5118 | 5118 | 5518 | 5518 |
| MUFA-Ps, % of energy | 3.73 ± 0.61 | 4.98 ± 0.27 | 5.88 ± 0.26 | 6.91 ± 0.35 | 9.24 ± 1.72 | 4.01 ± 0.68 | 5.35 ± 0.27 | 6.22 ± 0.25 | 7.18 ± 0.32 | 9.23 ± 1.50 |
| MUFA-As, % of energy | 5.45 ± 1.78 | 5.50 ± 1.58 | 5.46 ± 1.52 | 5.32 ± 1.47 | 4.94 ± 1.55 | 5.62 ± 2.10 | 5.70 ± 1.81 | 5.62 ± 1.75 | 5.45 ± 1.66 | 4.97 ± 1.70 |
| Age, y | 68.8 ± 7.1 | 67.8 ± 7.2 | 67.3 ± 7.1 | 67.1 ± 7.1 | 66.9 ± 6.9 | 68.0 ± 8.9 | 67.1 ± 8.9 | 66.5 ± 8.7 | 66.9 ± 8.8 | 67.4 ± 8.6 |
| Whites, % | 97 | 98 | 98 | 98 | 98 | 94 | 96 | 96 | 96 | 97 |
| Alcohol intake, g/d | 5.8 ± 11.5 | 5.7 ± 10.3 | 5.6 ± 10.1 | 6.3 ± 10.4 | 7.2 ± 11.2 | 13.3 ± 17.4 | 12.4 ± 15.8 | 12.6 ± 15.8 | 12.6 ± 14.9 | 12.5 ± 16.1 |
| Current smoking, % | 8 | 8 | 8 | 8 | 7 | 3 | 3 | 3 | 4 | 4 |
| Physical activity, METs/wk | 18.5 ± 24.7 | 17.4 ± 21.3 | 16.8 ± 21.0 | 17.1 ± 21.1 | 18.2 ± 21.5 | 35.9 ± 40.9 | 36.4 ± 40.8 | 35.5 ± 42.4 | 35.4 ± 40.5 | 36.0 ± 40.4 |
| BMI, kg/m2 | 26.7 ± 5.4 | 26.6 ± 5.2 | 26.6 ± 5.3 | 26.6 ± 5.3 | 26.1 ± 5.1 | 23.3 ± 8.9 | 24.2 ± 7.9 | 24.1 ± 7.9 | 24.3 ± 7.5 | 23.9 ± 7.9 |
| Family history of MI, % | 37 | 37 | 37 | 37 | 36 | 32 | 30 | 30 | 31 | 31 |
| Family history of diabetes, % | 25 | 26 | 24 | 25 | 24 | 21 | 21 | 21 | 21 | 20 |
| Multivitamin use,% | 60 | 62 | 63 | 63 | 62 | 54 | 58 | 58 | 58 | 58 |
| Current use of aspirin, % | 32 | 33 | 34 | 35 | 33 | 44 | 47 | 47 | 47 | 46 |
| Any use of postmenopausal hormones, % | 67 | 71 | 71 | 72 | 72 | NA | NA | NA | NA | NA |
| Total energy, kcal | 1556 ± 505 | 1655 ± 521 | 1718 ± 529 | 1774 ± 550 | 1803 ± 563 | 1868 ± 586 | 1989 ± 616 | 2052 ± 613 | 2118 ± 649 | 2152 ± 673 |
| Total fats, % of energy | 24.3 ± 4.9 | 27.2 ± 4.3 | 28.9 ± 4.2 | 30.4 ± 4.2 | 32.9 ± 4.8 | 24.8 ± 5.6 | 27.9 ± 4.7 | 29.5 ± 4.6 | 30.9 ± 4.4 | 33.2 ± 4.9 |
| PUFAs, % of energy | 4.59 ± 0.83 | 5.25 ± 0.82 | 5.67 ± 0.91 | 6.05 ± 1.04 | 6.57 ± 1.38 | 4.75 ± 0.87 | 5.41 ± 0.83 | 5.79 ± 0.86 | 6.18 ± 0.93 | 6.89 ± 1.34 |
| SFAs, % of energy | 9.11 ± 2.45 | 9.73 ± 2.20 | 10.06 ± 2.14 | 10.25 ± 2.14 | 10.33 ± 2.26 | 9.02 ± 2.74 | 9.77 ± 2.37 | 10.01 ± 2.29 | 10.16 ± 2.18 | 10.23 ± 2.25 |
| trans Fats, % of energy | 1.21 ± 0.35 | 1.46 ± 0.39 | 1.60 ± 0.44 | 1.68 ± 0.51 | 1.66 ± 0.63 | 1.22 ± 0.41 | 1.50 ± 0.44 | 1.65 ± 0.49 | 1.75 ± 0.56 | 1.78 ± 0.70 |
| Protein, % of energy | 19.4 ± 3.0 | 18.5 ± 2.5 | 18.0 ± 2.4 | 17.5 ± 2.3 | 16.8 ± 2.4 | 19.1 ± 3.0 | 18.2 ± 2.4 | 17.7 ± 2.4 | 17.2 ± 2.3 | 16.5 ± 2.4 |
| Unrefined carbohydrates, % of energy | 34.5 ± 7.8 | 31.9 ± 6.7 | 30.5 ± 6.4 | 29.2 ± 6.3 | 28.0 ± 6.6 | 32.9 ± 8.4 | 30.5 ± 7.1 | 29.2 ± 6.6 | 28.2 ± 6.4 | 27.2 ± 6.7 |
| MUFA-As | ||||||||||
| n | 11,687 | 11,687 | 11,688 | 11,687 | 11,687 | 5117 | 5118 | 5118 | 5518 | 5518 |
| MUFA-Ps, % of energy | 6.49 ± 2.49 | 6.26 ± 2.10 | 6.18 ± 1.92 | 6.07 ± 1.84 | 5.76 ± 1.78 | 6.68 ± 2.39 | 6.54 ± 1.92 | 6.48 ± 1.80 | 6.32 ± 1.68 | 5.97 ± 1.65 |
| MUFA-As, % of energy | 3.23 ± 0.67 | 4.46 ± 0.24 | 5.26 ± 0.22 | 6.08 ± 0.27 | 7.64 ± 1.00 | 3.05 ± 0.75 | 4.48 ± 0.28 | 5.39 ± 0.25 | 6.33 ± 0.32 | 8.13 ± 1.13 |
| Age, y | 69.3 ± 7.1 | 68.1 ± 7.1 | 67.4 ± 7.1 | 66.8 ± 7.0 | 66.3 ± 6.9 | 67.7 ± 8.9 | 67.4 ± 8.9 | 67.1 ± 8.9 | 66.8 ± 8.7 | 66.9 ± 8.7 |
| Whites, % | 97 | 98 | 98 | 98 | 98 | 95 | 96 | 96 | 96 | 96 |
| Alcohol intake, g/d | 6.2 ± 10.8 | 6.6 ± 11.2 | 6.3 ± 10.8 | 6.2 ± 10.8 | 5.4 ± 10.2 | 12.4 ± 16.4 | 13.6 ± 16.5 | 13.5 ± 16.7 | 12.7 ± 15.7 | 11.3 ± 14.6 |
| Current smoking, % | 4 | 5 | 7 | 9 | 13 | 1 | 3 | 4 | 4 | 6 |
| Physical activity, METs/wk | 23.5 ± 26.1 | 19.1 ± 21.6 | 17.2 ± 22.4 | 15.4 ± 19.4 | 13.4 ± 18.5 | 43.2 ± 45.7 | 38.6 ± 39.4 | 34.3 ± 39.1 | 32.3 ± 38.1 | 31.0 ± 41.7 |
| BMI, kg/m2 | 25.0 ± 4.5 | 26.0 ± 4.7 | 26.6 ± 5.0 | 27.2 ± 5.4 | 27.8 ± 5.9 | 22.9 ± 7.2 | 23.8 ± 7.5 | 24.5 ± 7.5 | 24.4 ± 8.3 | 24.2 ± 9.4 |
| Family history of MI, % | 38 | 37 | 37 | 37 | 36 | 35 | 32 | 30 | 31 | 27 |
| Family history of diabetes, % | 24 | 24 | 24 | 25 | 26 | 20 | 21 | 21 | 21 | 21 |
| Multivitamin use, % | 68 | 66 | 63 | 60 | 54 | 63 | 61 | 58 | 54 | 49 |
| Current use of aspirin, % | 35 | 35 | 34 | 33 | 30 | 50 | 49 | 48 | 44 | 41 |
| Any use of postmenopausal hormones, % | 72 | 73 | 72 | 70 | 66 | NA | NA | NA | NA | NA |
| Total energy, kcal | 1691 ± 543 | 1716 ± 539 | 1720 ± 538 | 1714 ± 544 | 1673 ± 547 | 1986 ± 625 | 2021 ± 622 | 2044 ± 634 | 2066 ± 633 | 2064 ± 664 |
| Total fats, % of energy | 23.7 ± 4.6 | 26.7 ± 4.0 | 28.7 ± 3.8 | 30.7 ± 3.8 | 33.9 ± 4.1 | 23.6 ± 4.9 | 27.1 ± 4.0 | 29.3 ± 3.8 | 31.4 ± 3.6 | 34.9 ± 4.0 |
| PUFAs, % of energy | 5.38 ± 1.33 | 5.50 ± 1.18 | 5.65 ± 1.16 | 5.75 ± 1.15 | 5.84 ± 1.21 | 5.59 ± 1.44 | 5.72 ± 1.16 | 5.82 ± 1.14 | 5.91 ± 1.13 | 5.97 ± 1.15 |
| SFAs, % of energy | 7.33 ± 1.43 | 8.87 ± 1.25 | 9.84 ± 1.24 | 10.85 ± 1.32 | 12.60 ± 1.83 | 7.00 ± 1.51 | 8.74 ± 1.23 | 9.84 ± 1.24 | 10.89 ± 1.30 | 12.73 ± 1.80 |
| trans Fats, % of energy | 1.16 ± 0.44 | 1.41 ± 0.44 | 1.54 ± 0.44 | 1.67 ± 0.45 | 1.81 ± 0.46 | 1.15 ± 0.49 | 1.46 ± 0.48 | 1.62 ± 0.49 | 1.77 ± 0.51 | 1.91 ± 0.52 |
| Protein, % of energy | 17.2 ± 2.7 | 17.8 ± 2.6 | 18.0 ± 2.5 | 18.2 ± 2.6 | 18.9 ± 2.7 | 16.8 ± 2.7 | 17.5 ± 2.5 | 17.6 ± 2.5 | 17.9 ± 2.5 | 18.8 ± 2.6 |
| Unrefined carbohydrates, % of energy | 36.6 ± 7.2 | 32.7 ± 6.2 | 30.7 ± 5.7 | 28.6 ± 5.5 | 25.5 ± 5.3 | 36.0 ± 7.8 | 31.5 ± 6.1 | 29.2 ± 5.8 | 27.1 ± 5.4 | 24.1 ± 5.2 |
1Values are means ± SDs unless otherwise indicated and are standardized to the age distribution of the study population. HPFS, Health Professionals Follow-Up Study; MET, metabolic equivalent task; MI, myocardial infarction; MUFA-A, MUFA from animal sources; MUFA-P, MUFA from plant sources; NA, not applicable; NHS, Nurses’ Health Study; Q, quintile.
Olive oil was the largest contributor of MUFA-P intake (15–17% of total consumption; Supplemental Table 1), followed by nuts (e.g., peanuts, peanut butter, and other nuts), salad dressing, fried foods (e.g., French fries and fried foods cooked away from home), baked products (chocolate chip cookies and pies), margarines, and chocolate. MUFA-As mainly came from red and processed meats (beef and pork). Dairy products, butter, poultry, eggs, and fish were other major sources of MUFA-As. In both cohorts, mean MUFA-P intake increased from 5.8–6.3% to 7.9% of energy during the follow-up, whereas MUFA-As decreased from 5.4–5.5% to 4.2–4.4% of energy (Figure 1).
Intakes of MUFA-Ps and MUFA-As were weakly correlated (rs between −0.08 and −0.14; Table 2). PUFA intake was moderately correlated with MUFA-P intake (rs ≥ 0.59, P < 0.001) but weakly with MUFA-A intake (rs ≤ 0.18, P < 0.001), whereas SFA intake was highly correlated with MUFA-A intake (rs ≥ 0.81, P < 0.001) but not with MUFA-P intake (rs ≤ 0.21, P < 0.001). Both MUFA-P and MUFA-A intakes were moderately correlated with trans fat intake (rs between 0.35 and 0.47, P < 0.001).
TABLE 2.
Correlations between dietary fats at the midpoint of follow-up (1998)1
| NHS | HPFS | |||
|---|---|---|---|---|
| MUFA-Ps | MUFA-As | MUFA-Ps | MUFA-As | |
| MUFA-As | −0.08 | −0.14 | ||
| Total MUFAs | 0.68 | 0.61 | 0.63 | 0.61 |
| SFAs | 0.21 | 0.83 | 0.20 | 0.81 |
| PUFAs | 0.59 | 0.18 | 0.66 | 0.04 |
| trans Fats | 0.35 | 0.47 | 0.47 | 0.39 |
1Values are Spearman correlation coefficients. All P < 0.001. HPFS, Health Professionals Follow-Up Study; MUFA-A, MUFA from animal sources; MUFA-P, MUFA from plant sources; NHS, Nurses’ Health Study.
A positive association between total MUFAs and CHD risk was observed in age- and multivariate-adjusted models that included other demographic characteristics, lifestyle, BMI, and total energy (Table 3). This association was largely attenuated after further controlling for SFAs (HR: 0.95; 95% CI: 0.83, 1.08; P-trend = 0.47). Similarly, MUFA-P intake was associated with CHD risk in the multivariate-adjusted model after SFAs were adjusted for: the HR comparing the highest with the lowest quintiles of MUFA-P intakes was 0.93 (95% CI: 0.85, 1.03; P-trend = 0.04). In contrast, MUFA-As were significantly associated with a higher CHD risk with multivariate adjustments of demographic characteristics, lifestyle, BMI, and total energy [HR (95% CI) comparing the highest to the lowest quintile: 1.30 (1.17, 1.44); P-trend < 0.001], which was slightly attenuated to 1.23 (1.06, 1.42; P-trend = 0.002) after further controlling for SFA intake. Further adjusting for PUFAs did not change the associations, and HRs (95% CIs) comparing extreme quintiles (high compared with low) were 1.04 (0.91, 1.18) for total MUFAs, 0.90 (0.80, 1.01) for MUFA-Ps, and 1.23 (1.06, 1.43) for MUFA-As (P-trend = 0.53, 0.02, and 0.001, respectively).
TABLE 3.
Associations between MUFA intake and CHD risk1
| Quintile of MUFA intake, % of energy | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-trend | |
| Total MUFAs | ||||||
| NHS | ||||||
| Median (range), % | 8.7 (0.5–9.9) | 10.5 (9.6–11.4) | 11.7 (11.0–12.7) | 13.0 (12.2–14.2) | 14.9 (13.6–45.3) | |
| Cases/person-years | 393/254,654 | 380/255,118 | 422/255,036 | 367/254,931 | 410/254,721 | |
| Model 1 | 1 | 1.06 (0.92, 1.23) | 1.26 (1.10, 1.45) | 1.14 (0.99, 1.32) | 1.34 (1.17, 1.55) | <0.001 |
| Model 2 | 1 | 1.04 (0.90, 1.19) | 1.18 (1.02, 1.36) | 1.02 (0.88, 1.18) | 1.11 (0.96, 1.30) | 0.24 |
| Model 3 | 1 | 0.99 (0.85, 1.15) | 1.10 (0.94, 1.29) | 0.93 (0.78, 1.10) | 0.98 (0.81, 1.19) | 0.67 |
| HPFS | ||||||
| Median (range), % | 8.8 (1.3–10.1) | 10.8 (9.9–11.5) | 12.1 (11.3–12.8) | 13.3 (12.6–14.4) | 15.3 (14.0–35.1) | |
| Cases/person-years | 483/117,823 | 433/118,019 | 497/117,920 | 472/117,798 | 562/117,667 | |
| Model 1 | 1 | 0.88 (0.77, 1.00) | 1.08 (0.95, 1.22) | 1.04 (0.92, 1.18) | 1.22 (1.08, 1.38) | <0.001 |
| Model 2 | 1 | 0.88 (0.77, 1.00) | 1.06 (0.93, 1.21) | 1.00 (0.87, 1.14) | 1.10 (0.96, 1.26) | 0.06 |
| Model 3 | 1 | 0.82 (0.71, 0.94) | 0.95 (0.83, 1.10) | 0.86 (0.74, 1.01) | 0.90 (0.76, 1.07) | 0.46 |
| Pooled2 | ||||||
| Model 1 | 1 | 0.96 (0.87, 1.05) | 1.16 (1.05, 1.27) | 1.08 (0.99, 1.19) | 1.27 (1.16, 1.39) | <0.001 |
| Model 2 | 1 | 0.95 (0.86, 1.05) | 1.12 (1.02, 1.24) | 1.02 (0.92, 1.13) | 1.12 (1.01, 1.24) | 0.01 |
| Model 3 | 1 | 0.90 (0.81, 0.99) | 1.02 (0.92, 1.14) | 0.90 (0.80, 1.01) | 0.95 (0.83, 1.08) | 0.47 |
| MUFA-Ps | ||||||
| NHS | ||||||
| Median (range), % | 3.7 (0.4–4.8) | 4.9 (4.2–5.7) | 5.8 (5.1–6.7) | 6.8 (6.0–8.0) | 8.6 (7.2–43.9) | |
| Cases/person-years | 437/253,782 | 422/254,769 | 388/255,040 | 371/255,201 | 354/255,667 | |
| Model 1 | 1 | 1.01 (0.89, 1.16) | 0.97 (0.84, 1.11) | 0.94 (0.82, 1.08) | 0.90 (0.78, 1.04) | 0.08 |
| Model 2 | 1 | 1.02 (0.89, 1.17) | 0.97 (0.84, 1.11) | 0.93 (0.80, 1.07) | 0.91 (0.79, 1.05) | 0.09 |
| Model 3 | 1 | 1.01 (0.89, 1.16) | 0.95 (0.83, 1.09) | 0.91 (0.79, 1.05) | 0.89 (0.77, 1.03) | 0.04 |
| HPFS | ||||||
| Median (range), % | 4.0 (0.3–5.0) | 5.2 (4.5–6.0) | 6.1 (5.5–6.9) | 7.1 (6.4–8.0) | 8.8 (7.6–32.4) | |
| Cases/person-years | 560/117,331 | 507/117,821 | 467/118,035 | 429/117,983 | 484/118,057 | |
| Model 1 | 1 | 0.98 (0.86, 1.10) | 0.94 (0.83, 1.07) | 0.87 (0.77, 0.99) | 0.94 (0.83, 1.07) | 0.15 |
| Model 2 | 1 | 1.02 (0.90, 1.15) | 0.98 (0.86, 1.11) | 0.91 (0.80, 1.03) | 0.97 (0.86, 1.10) | 0.32 |
| Model 3 | 1 | 1.00 (0.88, 1.13) | 0.96 (0.85, 1.09) | 0.89 (0.78, 1.01) | 0.95 (0.84, 1.08) | 0.22 |
| Pooled2 | ||||||
| Model 1 | 1 | 0.99 (0.91, 1.09) | 0.95 (0.87, 1.05) | 0.90 (0.82, 0.99) | 0.92 (0.84, 1.01) | 0.03 |
| Model 2 | 1 | 1.03 (0.94, 1.12) | 0.98 (0.89, 1.08) | 0.93 (0.84, 1.02) | 0.95 (0.87, 1.05) | 0.10 |
| Model 3 | 1 | 1.01 (0.92, 1.11) | 0.96 (0.88, 1.06) | 0.91 (0.82, 1.00) | 0.93 (0.85, 1.03) | 0.04 |
| MUFA-As | ||||||
| NHS | ||||||
| Median (range), % | 3.4 (0.02–4.2) | 4.5 (3.8–5.1) | 5.3 (4.6–5.9) | 6.1 (5.3–7.0) | 7.5 (6.2–22.6) | |
| Cases/person-years | 348/255,276 | 369/255,152 | 421/255,195 | 400/254,908 | 434/253,928 | |
| Model 1 | 1 | 1.17 (1.01, 1.35) | 1.41 (1.22, 1.63) | 1.40 (1.21, 1.62) | 1.60 (1.39, 1.85) | <0.001 |
| Model 2 | 1 | 1.12 (0.97, 1.30) | 1.31 (1.13, 1.52) | 1.23 (1.06, 1.43) | 1.26 (1.08, 1.47) | 0.004 |
| Model 3 | 1 | 1.11 (0.95, 1.29) | 1.29 (1.09, 1.51) | 1.20 (1.00, 1.43) | 1.21 (0.97, 1.49) | 0.11 |
| HPFS | ||||||
| Median (range), % | 3.2 (0.01–4.0) | 4.5 (3.9–5.1) | 5.4 (4.8–6.0) | 6.4 (5.7–7.2) | 8.0 (6.7–20.8) | |
| Cases/person-years | 436/118,120 | 425/118,116 | 476/117,944 | 508/117,679 | 602/117,368 | |
| Model 1 | 1 | 1.02 (0.89, 1.17) | 1.19 (1.04, 1.35) | 1.33 (1.17, 1.51) | 1.49 (1.32, 1.69) | <0.001 |
| Model 2 | 1 | 1.00 (0.87, 1.15) | 1.15 (1.00, 1.31) | 1.25 (1.10, 1.44) | 1.31 (1.15, 1.51) | <0.001 |
| Model 3 | 1 | 0.99 (0.86, 1.14) | 1.12 (0.97, 1.31) | 1.22 (1.04, 1.44) | 1.26 (1.04, 1.54) | 0.004 |
| Pooled2 | ||||||
| Model 1 | 1 | 1.08 (0.98, 1.20) | 1.28 (1.16, 1.41) | 1.36 (1.24, 1.50) | 1.54 (1.40, 1.69) | <0.001 |
| Model 2 | 1 | 1.06 (0.96, 1.17) | 1.23 (1.11, 1.35) | 1.25 (1.13, 1.38) | 1.30 (1.17, 1.44) | <0.001 |
| Model 3 | 1 | 1.04 (0.94, 1.16) | 1.19 (1.07, 1.33) | 1.21 (1.07, 1.36) | 1.23 (1.06, 1.42) | 0.002 |
1HRs (95% CIs) were calculated by using a Cox proportional hazards model. Model 1 adjusted for age; model 2 further adjusted for ethnicity (white or other ethnicity), smoking status [never, former, current (1–14, 15–24, or ≥25 cigarettes/d), or missing], alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/d in women; 0, 0.1–4.9, 5.0–29.9, and ≥30.0 in men; or missing), family history of myocardial infarction (yes or no), family history of diabetes (yes or no), menopausal status and postmenopausal hormone use [premenopause, postmenopause (never, former, or current hormone use), or missing for women], physical activity (<3, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 metabolic equivalent tasks/wk or missing), current aspirin use (yes or no), multivitamin use (yes or no), baseline hypertension, baseline hypercholesterolemia, BMI (kg/m2; <23, 23–24.9, 25–29.9, 30–34.9, or ≥35 or missing), total energy intake (kilocalories per day), and intake of fruit and vegetables (in quintiles) based on model 1; model 3 further adjusted for energy from SFAs based on model 2. CHD, coronary heart disease; HPFS, Health Professionals Follow-Up Study; MUFA-A, MUFA from animal sources; MUFA-P, MUFA from plant sources; NHS, Nurses’ Health Study.
2Study estimates from 2 cohorts were pooled by using a fixed-effects model.
Results for substitution analysis are presented in Table 4. For MUFA-Ps, pooled HRs (95% CIs) of CHD were 0.83 (0.68, 1.00) for replacing 5% of energy from SFAs, 0.86 (0.76, 0.97) for replacing 5% of energy from refined carbohydrates, and 0.80 (0.70, 0.91) for replacing 2% of energy from trans fats (P = 0.05, 0.01, and 0.001, respectively). The same replacement with MUFA-As was not significantly associated with CHD risk, and HRs (95% CIs) were 1.04 (0.79, 1.38) for SFAs, 1.11 (0.91, 1.35) for refined carbohydrates, and 0.88 (0.77, 1.01) for trans fats (P = 0.76, 0.31, and 0.08, respectively). CHD risk was 24% lower when 5% of energy from MUFA-As was isocalorically replaced with MUFA-Ps (HR: 0.76; 95% CI: 0.65, 0.88; P < 0.001), and 19% lower when the sum of SFAs and MUFA-As (5% of energy) was isocalorically replaced with MUFA-Ps (HR: 0.81; 95% CI: 0.73, 0.90; P < 0.001). Findings from the substitution analyses were not materially changed when baseline BMI was adjusted for (Supplemental Table 2); when hypertension, hypercholesterolemia, and diabetes diagnosed during follow-up were adjusted for (Supplemental Table 3); or when probable CHD cases were excluded (Supplemental Table 4).
TABLE 4.
HRs (95% CIs) of CHD risk for replacing other fats with MUFAs1
| CHD risk | ||||||
|---|---|---|---|---|---|---|
| NHS | P | HPFS | P | Pooled2 | P | |
| MUFA-Ps replacing | ||||||
| SFAs (5% of energy) | 0.77 (0.58, 1.01) | 0.06 | 0.90 (0.69, 1.19) | 0.47 | 0.83 (0.68, 1.00) | 0.05 |
| Refined carbohydrates (5% of energy) | 0.85 (0.71, 1.01) | 0.06 | 0.87 (0.73, 1.03) | 0.11 | 0.86 (0.76, 0.97) | 0.01 |
| trans Fats (2% of energy) | 0.76 (0.63, 0.93) | 0.006 | 0.84 (0.70, 1.01) | 0.07 | 0.80 (0.70, 0.91) | 0.001 |
| MUFA-As replacing | ||||||
| SFAs (5% of energy) | 0.95 (0.63, 1.44) | 0.82 | 1.17 (0.80, 1.72) | 0.42 | 1.04 (0.79, 1.38) | 0.76 |
| Refined carbohydrates (5% of energy) | 1.01 (0.75, 1.35) | 0.97 | 1.21 (0.93, 1.57) | 0.15 | 1.11 (0.91, 1.35) | 0.31 |
| trans Fats (2% of energy) | 0.85 (0.69, 1.04) | 0.11 | 0.93 (0.77, 1.13) | 0.48 | 0.88 (0.77, 1.01) | 0.08 |
| MUFA-Ps replacing | ||||||
| MUFA-As (5% of energy) | 0.73 (0.58, 0.92) | 0.007 | 0.77 (0.63, 0.93) | 0.007 | 0.76 (0.65, 0.88) | <0.001 |
| SFAs+MUFA-As (5% of energy) | 0.79 (0.68, 0.92) | 0.002 | 0.82 (0.71, 0.94) | 0.005 | 0.81 (0.73, 0.90) | <0.001 |
1Values were calculated by using a Cox proportional hazards model, after adjusting for age, ethnicity (white or other ethnicity), smoking status [never, former, current (1–14, 15–24, or ≥25 cigarettes/d), or missing], alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/d in women; 0, 0.1–4.9, 5.0–29.9, and ≥30.0 in men; or missing), family history of myocardial infarction (yes or no), family history of diabetes (yes or no), menopausal status and postmenopausal hormone use [premenopause, postmenopause (never, former, or current hormone use), or missing for women], physical activity (<3, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 metabolic equivalent tasks/wk or missing), current aspirin use (yes or no), multivitamin use (yes or no), baseline hypertension, baseline hypercholesterolemia, BMI (kg/m2; <23, 23–24.9, 25–29.9, 30–34.9, or ≥35 or missing), total energy intake (kilocalories per day), and intakes of fruit and vegetables (in quintiles). For refined-carbohydrate substitution, the model was further adjusted for energy from protein, whole-grain carbohydrates, trans fats, PUFAs, and SFAs; for trans fat substitution, the model was further adjusted for total fats, PUFAs, and SFAs; for SFA substitution, the model was further adjusted for total fats, trans fats, and PUFAs. All MUFA-P models were further adjusted for MUFA-As, and vice versa. CHD, coronary heart disease; HPFS, Health Professionals Follow-Up Study; MUFA-A, MUFA from animal sources; MUFA-P, MUFA from plant sources; NHS, Nurses’ Health Study.
2Study estimates from 2 cohorts were pooled by using a fixed-effects model.
DISCUSSION
In 2 large prospective studies of US men and women, MUFA-Ps were correlated with PUFA intake, whereas MUFA-As were highly correlated with SFA intake. CHD risk was significantly lower when SFAs, refined carbohydrates, or trans fats were isocalorically replaced by MUFA-Ps but not by MUFA-As. Furthermore, replacement of MUFA-As and SFAs together with MUFA-Ps was significantly associated with a lower CHD risk. To our knowledge, this is the first prospective cohort study that considered MUFAs from plant and animal sources separately and examined their independent associations with CHD risk.
The health effects of a macronutrient may depend on the composition of other macronutrients when total calories remain constant. In our cohorts, total cis MUFAs were not inversely associated with CHD risk without specifying a reference macronutrient, but when MUFAs were examined to substitute for SFAs or refined carbohydrates in isocaloric models, lower CHD risks were consistently observed (8–10). Similarly, substituting MUFAs for SFAs, trans fats, and carbohydrates has been associated with a lower CVD risk in other studies. (11, 12). However, 2 studies reported a higher CHD risk when SFAs were replaced by MUFAs (14, 15), which could be partly attributed to the use of a single dietary assessment at baseline for long-term follow-up (15), as well as the inclusion of trans isomers in the estimates of total MUFA intake (28). Results from the recent Prospective Urban Rural Epidemiology (PURE) Study reported an inverse association of total MUFA intake with total mortality but no association with CHD was observed. It is difficult to compare our findings with those of the PURE study, which was conducted in many regions that may have drastically different food sources of MUFAs (29). In contrast to the inconsistent findings with regard to total MUFAs from mixed food sources, most studies showed that olive oil was inversely associated with CVD risk, suggesting that dietary source might be critical to the health benefit of MUFAs (16). We extended previous research by explicitly separating total cis MUFAs between plant and animal origins and found largely differential associations of 2 MUFA fractions with CHD risk.
Our findings of an inverse association between MUFA-P intake and CHD risk are in line with intervention studies that primarily examined effects of MUFAs from plant-based sources (e.g., olive oil, high-oleic-acid sunflower oil, canola oil, and nuts) on cardiovascular risk factors. For example, by pooling published data from 91 clinical trials, Mensink (3) concluded that substituting MUFAs for SFAs or carbohydrates was associated with lower circulating concentrations of LDL cholesterol, triglycerides, and apolipoprotein B and a lower total-to–HDL-cholesterol ratio. Another meta-analysis showed that replacing SFAs or carbohydrates with MUFAs reduced glycated hemoglobin and the HOMA-IR (4). Most of these intervention studies compared MUFAs with a specific macronutrient while keeping other macronutrients constant; therefore, findings were not explained by other constituents, such as PUFAs, in plant-based sources (3, 4).
In our study, MUFA-Ps and PUFAs were moderately correlated, and we observed a lower CHD risk for replacing SFAs, refined carbohydrates, or trans fats with MUFA-Ps after PUFA intake was controlled for. However, we cannot exclude the possibility that other beneficial nutrients or constituents rich in plant-based foods, such as dietary fiber, antioxidants (e.g., polyphenols, vitamin E), and minerals (e.g., magnesium) (30–32), may still partially account for the associations for MUFA-Ps due to residual confounding. On the other hand, current trials examined fats from the same plant sources with varying MUFA contents and showed that improvements in blood lipids and obesity were explained solely by the different MUFA contents in the comparison oils (33–35).
Last, synergistic effects of MUFAs with other nutrients in plant-based foods may also play a role in the beneficial associations between MUFA-P intake and CHD risk. For example, in animal models, vitamin E supplementation increased body vitamin E concentrations and suppressed lipid oxidation more efficiently when MUFA consumption was high (36). Apparently, more research is warranted to explore such interactions between MUFAs and other dietary constituents.
The remarkably high correlations between MUFA-As and SFAs (r > 0.8) reflect their overlapping food sources (21), such as red meats and dairy products (37). In this regard, SFA intake can be a major confounder for associations of MUFA-As. We found that the positive association between total MUFAs and CHD risk was abolished after further adjusting for SFAs. The strong correlations between MUFA-As and SFAs make it difficult to evaluate their independent associations with CHD risk by using statistical modeling. We have previously shown that intakes of individual SFAs are highly correlated and that replacing total SFA intake with unsaturated fatty acids or whole grains is more practical than specifically replacing individual SFAs (10). The same may hold true for MUFA-As and SFAs because of their strong correlations, and we observed a robust lower CHD risk when the sum of these was replaced by MUFA-Ps.
Of note, margarines, fried foods, and baked products contributed to MUFA-Ps in our study. Because partially hydrogenated vegetable oils were once widely used in these foods, MUFA-P intake and trans fat intake were moderately correlated. In recent years, oils high in MUFAs (e.g., >70% of total fats) are increasingly used to replace trans fats by the food industry (38). Because these foods are important sources of dietary fat in Americans (39), potential impacts of such replacement on cardiovascular health warrant investigations in future studies.
Strengths of our study include large sample sizes, long follow-up duration, and repeated measurements of dietary exposures and confounding factors. As one major limitation, dietary data from FFQs may inevitably contain measurement errors. However, because diet was assessed before disease ascertainment, measurement errors are independent of the outcome and will tend to attenuate true associations. We also used cumulative averages of diet to reduce short-term variability and represent long-term diet, and stopped updating diet after the development of certain major chronic diseases to minimize the impact of reverse causation bias. Second, individuals with higher plant-based food intake might be more health conscious and have a higher socioeconomic status, which may confound the associations of MUFAs. However, the NHS and HPFS participants are exclusively health professionals, thus relatively homogeneous in terms of socioeconomic status, educational attainment, and access to health care, which may help to alleviate confounding by these factors. Third, the ethnic and socioeconomic homogeneity of the study population may limit the generalizability of findings to other populations. Fourth, caution must be taken when interpreting associations as causality in observational studies.
In conclusion, we found largely different correlations of MUFAs from plant sources and animal sources with PUFAs and SFAs. Significantly lower CHD risk was observed when SFAs, trans fats, or refined carbohydrates were replaced by MUFA-Ps but not MUFA-As. Our findings support a beneficial role of MUFAs in long-term CHD prevention, when plant-based foods such as vegetable oils, nuts, and related products are the primary sources.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—FBH, AJW, PLZ, WCW, FBH, and QS: designed the study; GZ, YL, and QS: provided statistical expertise; GZ: wrote the manuscript, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: conducted the research, contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content, and read and approved the final manuscript. GZ is supported by a postdoctoral fellowship funded by Unilever R&D; AJW, MA, and PLZ are employees of Unilever R&D (Unilever is a producer of food consumer products); FBH has received research support from the California Walnut Commission and Metagenics. The remaining authors had nothing to disclose. The sponsors had no role in the study design; collection, analysis, or interpretation of data; writing of the report; or in the decision to submit the article for publication.
Notes
Supported by research grants UM1 CA186107, R01 HL034594, R01 HL35464, R01 HL60712, and UM1 CA167552 from the NIH. GZ is supported by a postdoctoral fellowship funded by Unilever R&D; FBH has received research support from the California Walnut Commission and Metagenics; QS was supported by ES021372 and ES022981 sponsored by the National Institute of Environmental Health Sciences.
Supplemental Figure 1 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CHD, coronary heart disease; CVD, cardiovascular disease; FFQ, food-frequency questionnaire; HPFS, Health Professionals Follow-Up Study; MI, myocardial infarction; MUFA-A, MUFA from animal sources; MUFA-P, MUFA from plant sources; NHS, Nurses’ Health Study.
REFERENCES
- 1. USDA, Agricultural Research Service; Beltsville Human Nutrition Research Center, Food Surveys Research Group; US Department of Health and Human Services, CDC, National Center for Health Statistics What we eat in America, NHANES 2013–2014 data: nutrient intakes: from food and beverages [Internet]. 2016[cited 2017 Jan 20]. Available from: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/. [Google Scholar]
- 2. Joris PJ, Mensink RP. Role of cis-monounsaturated fatty acids in the prevention of coronary heart disease. Curr Atheroscler Rep 2016;18:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mensink RP. Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. Geneva (Switzerland): WHO; 2016. [Google Scholar]
- 4. Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med 2016;13:e1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011;46:209–28. [DOI] [PubMed] [Google Scholar]
- 6. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 7. Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160:398–406. [DOI] [PubMed] [Google Scholar]
- 8. Li Y, Hruby A, Bernstein AM, Ley SH, Wang DD, Chiuve SE, Sampson L, Rexrode KM, Rimm EB, Willett WC, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J Am Coll Cardiol 2015;66:1538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, Willett WC, Hu FB. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med 2016;176:1134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zong G, Li Y, Wanders AJ, Alssema M, Zock PL, Willett WC, Hu FB, Sun Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ 2016;355:i5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simila ME, Kontto JP, Mannisto S, Valsta LM, Virtamo J. Glycaemic index, carbohydrate substitution for fat and risk of CHD in men. Br J Nutr 2013;110:1704–11. [DOI] [PubMed] [Google Scholar]
- 12. Guasch-Ferre M, Babio N, Martinez-Gonzalez MA, Corella D, Ros E, Martin-Pelaez S, Estruch R, Aros F, Gomez-Gracia E, Fiol M, et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr 2015;102:1563–73. [DOI] [PubMed] [Google Scholar]
- 13. Campmans-Kuijpers MJ, Sluijs I, Nothlings U, Freisling H, Overvad K, Boeing H, Masala G, Panico S, Tumino R, Sieri S, et al. The association of substituting carbohydrates with total fat and different types of fatty acids with mortality and weight change among diabetes patients. Clin Nutr 2016;35:1096–102. [DOI] [PubMed] [Google Scholar]
- 14. Jakobsen MU, OʼReilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89:1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Praagman J, Beulens JW, Alssema M, Zock PL, Wanders AJ, Sluijs I, van der Schouw YT. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European Prospective Investigation into Cancer and Nutrition–Netherlands cohort. Am J Clin Nutr 2016;103:356–65. [DOI] [PubMed] [Google Scholar]
- 16. Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis 2014;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Degirolamo C, Rudel LL. Dietary monounsaturated fatty acids appear not to provide cardioprotection. Curr Atheroscler Rep 2010;12:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7. [DOI] [PubMed] [Google Scholar]
- 19. van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–24. [DOI] [PubMed] [Google Scholar]
- 20. Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ 2014;348:g3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr 1999;70:1001–8. [DOI] [PubMed] [Google Scholar]
- 22. Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 23. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 24. Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr 1998;67:25–30. [DOI] [PubMed] [Google Scholar]
- 25. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiuve SE, Sampson L, Willett WC. The association between a nutritional quality index and risk of chronic disease. Am J Prev Med 2011;40:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rose GA. Cardiovascular survey methods. 2nd ed.Geneva (Switzerland): WHO; 1982. [Google Scholar]
- 28. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schunemann H, Beyene J, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015;351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050–2062. [DOI] [PubMed] [Google Scholar]
- 30. Kwok CS, Umar S, Myint PK, Mamas MA, Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: a systematic review and meta-analysis. Int J Cardiol 2014;176:680–6. [DOI] [PubMed] [Google Scholar]
- 31. Huang T, Yang B, Zheng J, Li G, Wahlqvist ML, Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: a meta-analysis and systematic review. Ann Nutr Metab 2012;60:233–40. [DOI] [PubMed] [Google Scholar]
- 32. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med 2016;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lichtenstein AH, Matthan NR, Jalbert SM, Resteghini NA, Schaefer EJ, Ausman LM. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr 2006;84:497–504. [DOI] [PubMed] [Google Scholar]
- 34. Liu X, Kris-Etherton PM, West SG, Lamarche B, Jenkins DJ, Fleming JA, McCrea CE, Pu S, Couture P, Connelly PW, et al. Effects of canola and high-oleic-acid canola oils on abdominal fat mass in individuals with central obesity. Obesity (Silver Spring) 2016;24:2261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones PJ, MacKay DS, Senanayake VK, Pu S, Jenkins DJ, Connelly PW, Lamarche B, Couture P, Kris-Etherton PM, West SG, et al. High-oleic canola oil consumption enriches LDL particle cholesteryl oleate content and reduces LDL proteoglycan binding in humans. Atherosclerosis 2015;238:231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lopez-Bote CJ, Isabel B, Ruiz J, Daza A. Effect of vitamin E supplementation and partial substitution of poly- with mono-unsaturated fatty acids in pig diets on muscle, and microsome extract alpha-tocopherol concentration and lipid oxidation. Arch Tierernahr 2003;57:11–25. [DOI] [PubMed] [Google Scholar]
- 37. USDA, Agricultural Research Service Fat and fatty acid content of selected foods containing trans-fatty acids [Internet]. June 13th, 2007. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Classics/trans_fa.pdf. [Google Scholar]
- 38. Huth PJ, Fulgoni VL III, Larson BT. A systematic review of high-oleic vegetable oil substitutions for other fats and oils on cardiovascular disease risk factors: implications for novel high-oleic soybean oils. Adv Nutr 2015;6:674–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O’Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Food sources of energy and nutrients among adults in the US: NHANES 2003–2006. Nutrients 2012;4:2097–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.