Summary
Background
Prostate cancer that progresses after enzalutamide treatment is poorly responsive to further antiandrogen therapy, and paradoxically, rapid cycling between high and low serum testosterone concentrations (bipolar androgen therapy [BAT]) in this setting might induce tumour responses. We aimed to evaluate BAT in patients with metastatic castration-resistant prostate cancer that progressed after enzalutamide.
Methods
We did this single-centre, open-label, phase 2, multicohort study in the USA. We included patients aged 18 years or older who had histologically confirmed and radiographically documented metastatic castration-resistant prostate cancer, with no more than two previous second-line hormonal therapies, and a castrate concentration of testosterone. Patients were asymptomatic, with Eastern Cooperative Oncology Group performance status of 0–2, and did not have high-risk lesions for tumour flare (eg, >5 sites of visceral disease or bone lesions with impending fracture). For the cohort reported here, we required patients to have had progression on enzalutamide with a continued prostate-specific antigen (PSA) rise after enzalutamide treatment discontinuation. Patients received BAT, which consisted of intramuscular testosterone cipionate 400 mg every 28 days until progression and continued luteinising hormone-releasing hormone agonist therapy. Upon progression after BAT, men were rechallenged with oral enzalutamide 160 mg daily. The co-primary endpoints were investigator-assessed 50% decline in PSA concentration from baseline (PSA50) for BAT (for all patients who received at least one dose) and for enzalutamide rechallenge (based on intention-to-treat analysis). These data represent the final analysis for the post-enzalutamide cohort, while two additional cohorts (post-abiraterone and newly castration-resistant prostate cancer) are ongoing. The trial is registered with ClinicalTrials.gov, number NCT02090114.
Findings
Between Aug 28, 2014, and May 18, 2016, we accrued 30 eligible patients and treated them with BAT. Nine (30%; 95% CI 15–49; p<0·0001) of 30 patients achieved a PSA50 to BAT. 29 patients completed BAT and 21 proceeded to enzalutamide rechallenge, of whom 15 (52%; 95% CI 33–71; p<0·0001) achieved a PSA50 response. During BAT, the only grade 3–4 adverse event occurring in more than one patient was hypertension (three [10%] patients). Other grade 3 or worse adverse events occurring during BAT in one [3%] patient each were pulmonary embolism, myocardial infarction, urinary obstruction, gallstone, and sepsis. During enzalutamide retreatment, no grade 3–4 toxicities occurred in more than one patient. No treatment-related deaths were reported during either BAT or enzalutamide retreatment.
Interpretation
BAT is a safe therapy that resulted in responses in asymptomatic men with metastatic castration-resistant prostate cancer and also resensitisation to enzalutamide in most patients undergoing rechallenge. Further studies with BAT are needed to define the potential clinical role for BAT in the management of metastatic castration-resistant prostate cancer and the optimal strategy for sequencing between androgen and antiandrogen therapies in metastatic castration-resistant prostate cancer to maximise therapeutic benefit to patients.
Introduction
Metastatic castration-resistant prostate cancer remains dependent on androgen receptor signalling for growth.1 Molecularly, this dependence is characterised by nearly universal upregulation of androgen receptor expression.2–4 Enzalutamide, an androgen receptor antagonist, was developed to inhibit androgen receptor signalling despite upregulated androgen receptor concentrations in patients with metastatic castration-resistant prostate cancer.5 Enzalutamide is effective in inducing tumour responses and produces a survival benefit in these patients.6,7 Clinically, metastatic castration-resistant prostate cancer that has progressed after enzalutamide treatment is minimally responsive to further therapy that inhibits androgen receptor signalling. This resistance is often mediated by a reactivation of the androgen receptor-signalling programme, often through androgen receptor overexpression, gene amplification, mutations, and expression of ligand-independent androgen receptor splice variants.8,9 Alternate approaches beyond androgen receptor-signalling inhibition are needed to produce clinical benefit in multidrug-resistant, metastatic castration-resistant prostate cancer.10
Although the adaptive overexpression of the androgen receptor by metastatic castration-resistant prostate cancer cells induces resistance to androgen receptor antagonists, this overexpression also represents a therapeutic vulnerability. In the setting of overexpressed androgen receptor, the administration of sufficient testosterone to achieve supraphysiological serum concentrations has paradoxically been shown to result in prostate cancer cell death and tumour regression in preclinical models.11,12 After an initial response to supraphysiological testosterone, castration-resistant prostate cancer cells can adaptively downregulate androgen receptors to allow for growth in an environment replete with testosterone.13 Theoretically, rapidly varying the androgen concentrations between the extremes of supraphysiological and near-castrate, a strategy termed bipolar androgen therapy (BAT),14 provides insufficient time for castration-resistant prostate cancer cells to adaptively regulate androgen receptor concentrations in response to androgen concentrations in the microenvironment. Therefore, castration-resistant prostate cancer cells with high androgen receptor concentrations are vulnerable to cell death following exposure to high androgen concentrations, and the rapidly varying androgen concentrations might prevent readaptation and resistance.14,15 The possible mechanisms through which BAT might operate include disruption of the process of DNA relicensing required for cell division and introduction of double-strand DNA breaks that could induce genomic instability.16,17 On the basis of these preclinical mechanistic studies, we did a pilot study18 testing BAT in combination with etoposide for three cycles (28 days each) followed by BAT monotherapy in patients with metastatic castration-resistant prostate cancer who were responding. While the combination with chemotherapy showed toxicity, BAT monotherapy was well tolerated, and radiographic and biochemical responses were seen with and without etoposide. After treatment with BAT was completed, most patients went on to benefit from further androgen receptor-directed therapy, despite being heavily pretreated.
Given the encouraging outcomes of this pilot study with BAT, we sought to test BAT in patients with metastatic castration-resistant prostate cancer who previously progressed on androgen receptor-directed therapies, including enzalutamide. We hypothesised that BAT, when used after enzalutamide, would therapeutically exploit adaptive androgen receptor upregulation resulting in tumour regression. We further hypothesised that patients progressing on BAT would have restored sensitivity to enzalutamide as the result of suppression of secondary resistance mechanisms (eg, androgen receptor splice variants or androgen receptor overexpression). To test these hypotheses, we did a phase 2 trial of BAT and subsequent enzalutamide rechallenge in a cohort of patients with asymptomatic metastatic castration-resistant prostate cancer after progression on enzalutamide.
Methods
Study design and participants
We did this multicohort, single-centre, open-label, phase 2 trial at Johns Hopkins University, Baltimore, MD, USA, to test BAT in cohorts of patients progressing on hormonal therapies, with the intention for each cohort to be analysed individually (protocol available in the appendix). For the post-enzalutamide cohort, we included patients aged 18 years or older if they had metastatic castration-resistant prostate cancer (disease progression despite a castrate concentration of testosterone [<50 ng/dL]). Evidence of metastatic disease by CT scan or nuclear medicine bone scan was required, but measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) was not a prerequisite for enrolment. Additionally, men were required to have radiographic or prostate-specific antigen (PSA) progression on enzalutamide, and a continuing PSA concentration rise during the 4 week washout from enzalutamide. We allowed up to two previous second-line hormonal therapies for metastatic castration-resistant prostate cancer (beyond first-generation antiandrogens and ketoconazole). We permitted previous docetaxel for castration-sensitive disease, radium-223, and sipuleucel-T. We also required patients to be asymptomatic or minimally symptomatic from their disease, and have an Eastern Cooperative Oncology Group performance status of 0–2. Adequate marrow and organ function was required for eligibility (ie, creatinine <2·5 × upper limit of normal [ULN]; bilirubin, aspartate aminotransferase, and alanine ainotransferase <2·5 × ULN; absolute neutrophil count ≥1500/μL, platelets ≥100 000 per μL, and haemoglobin ≥9 g/L). No minimum estimated life expectancy was required.
Given the potential for flare after testosterone initiation, we excluded patients if they had cancer-related pain requiring the use of opioid analgesics, the presence of more than five sites of visceral disease, urinary obstruction requiring catheterisation, or other lesions deemed high risk (such as femoral or spinal metastases with impending pathological fracture or cord compression). Additional exclusion criteria were active uncontrolled infection (HIV, hepatitis B, or hepatitis C), haematocrit of more than 50%, thromboembolic event in the past 2 years if not on systemic anticoagulation, myocardial infarction in the past year, uncontrolled heart failure, untreated severe obstructive sleep apnoea, or previous history of seizures while on enzalutamide. Previous treatment with docetaxel or cabazitaxel for metastatic castration-resistant prostate cancer was not allowed. This study was supervised by the Institutional Review Board at Johns Hopkins. All accrued patients provided written informed consent. Two additional cohorts (patients progressing after abiraterone treatment and patients with newly castration-resistant prostate cancer) are ongoing.
Procedures
After verification of eligibility and informed consent procedures, we treated patients with BAT, consisting of intramuscular testosterone cipionate 400 mg on day 1 of an every 28 day cycle while continuing luteinising hormone-releasing hormone agonist therapy. No stopping point was predefined and therapy could continue indefinitely. We did not modify testosterone doses. We assessed patients at each cycle for toxicity and with standard laboratory measures including complete blood count, comprehensive metabolic panel, and PSA. Staging CT and nuclear medicine bone scans were done every three cycles. The first response assessment did not occur until after three cycles. Patients who had a consistent rise of PSA concentration after three cycles stopped BAT if PSA was more than 25% higher than the baseline. We allowed patients who had an initial decrease in PSA with a subsequent rise of more than 25% from nadir to remain on BAT until a PSA increase over baseline. Patients with clinical or radiographic progression at any time stopped BAT. Patients with a treatment delay of longer than 2 weeks due to toxicity stopped treatment.
Patients discontinuing BAT proceeded to a 28 day washout period to allow testosterone concentrations to return to the castrate range. The PSA concentration after washout was considered baseline for purposes of evaluation of enzalutamide response. Treatment proceeded with oral enzalutamide 160 mg daily, with dose adjustments allowed per the US Food and Drug Administration’s label. During treatment with enzalutamide, we clinically assessed patients at each cycle for toxicity with standard laboratory measures including PSA, and radiographic assessments occurred every three cycles. First response assessment occurred after three cycles, and patients with an increase in PSA concentration of more than 25% above baseline discontinued enzalutamide and completed the study. Patients with an initial decrease in PSA concentration with a subsequent rise of more than 25% from nadir were allowed to remain on enzalutamide until a PSA increase over baseline. Radiographic or clinical progression at any time required patients to stop enzalutamide. Patients were allowed to stay on the treatment indefinitely. PSA progression was defined by Prostate Cancer Working Group 2 (PCWG2) criteria.19 Clinical or radiographic progression was defined by RECIST 1.120 (soft tissue lesions) and PCWG2 (clinical and bone lesions). Objective responses for patients with measurable disease were defined using RECIST 1.1 criteria.
Clinical investigations were done at baseline and every three cycles, and included complete blood counts, serum hormone concentrations (total testosterone, free testosterone, dihydrotestosterone [DHT], oestradiol, dehydroepiandrosterone [DHEA], DHEA-sulphate [DHEA-S], and sex hormone-binding globulin [SHBG]), and metabolic parameters (fasting glucose, glycated haemoglobin A1c [HbA1c], fasting insulin, C-telopeptides, osteocalcin, HDL, LDL, triglycerides, and C-reactive protein). We collected all laboratory measures on the first day of each cycle, which corresponds to the testosterone nadir. We report safety and tolerability as incidence and severity of adverse events according to National Cancer Institute Common Terminology Criteria for Adverse Events 4.0, monitored each cycle.
Quality of life (QOL) questionnaires were completed at baseline and every three cycles and included RAND Short Form-36 (RANDSF-36), Functional Assessment of Chronic Illness Therapy-Fatigue Subscale (FACIT-Fatigue), Brief Pain Inventory (BPI), International Index of Erectile Function (IIEF), and Positive and Negative Affect Schedule Short Form (PANAS-SF). Study staff administered the QOL questionnaires during treatment visits. RANDSF-36 assessed multiple health domains, and each answer to the 36 questions was assigned a value from 0 to 100 (higher score indicates better), as previously described,21 and we present a global mean score. The FACIT-Fatigue subscale consisted of 13 questions assigned a value from 0 to 4 (higher score indicates better), and we present a sum score.22 The BPI queries pain severity and interference with activities on a scale from 0 to 10 (higher score indicates worse); we report mean scores for both.23 The IIEF queries erectile and orgasmic function, sexual desire, and sexual satisfaction with 15 questions on a scale from 0 or 1 to 5 (higher score indicates better); we report sum scores.24 The PANAS-SF consists of 20 questions, ten each for domains of positive (higher score indicates better) and negative (higher score indicates worse) affect, each on a scale of 1 to 5.25 Both positive and negative affect questions were independently summed and reported. We did not follow up patients completing study treatments subsequently for QOL measures; thus QOL measures are reported only for patients actively on study treatment.
Blood samples for analysis of androgen receptor-full length (AR-FL) and androgen receptor splice variant 7 (AR-V7) mRNA in circulating tumour cells (CTCs) were drawn at baseline, after three cycles of BAT, and after three cycles of enzalutamide retreatment. AR-FL and AR-V7 transcript analyses were done and reported as previously described.8 Briefly, we isolated CTCs with antibody-conjugated magnetic beads, according to the Qiagen AdnaTest ProstateCancerSelect protocol (Germantown, MD, USA). We determined CTC-positivity according to Qiagen AdnaTest ProstateCancerDetect protocol, and determined AR-FL and AR-V7 copy numbers by quantitative RT-PCR using custom primers.8 Any amount of AR-FL was thus defined as CTC-positive, and any amount of AR-V7 was defined as CTC-positive and AR-V7-positive.
Outcomes
The co-primary endpoints for the study were investigator-assessed 50% declines in PSA concentration from baseline (PSA50) for BAT treatment and for enzalutamide treatment. We defined the PSA50 endpoints using the baseline value at initiation of BAT or enzalutamide, respectively, and therefore, patients with a 50% decline at any time while on treatment had met the endpoint.
Secondary endpoints were safety and tolerability, investigator-assessed PSA progression-free survival (from initiation of BAT or enzalutamide to the time of measured increase in PSA of at least 2 ng/dL and 25% from nadir values, confirmed with a second measurement at least 4 weeks later), investigator-assessed clinical or radiographic progression-free survival (from initiation of BAT or enzalutamide to the time of unequivocal clinical progression or radiographic progression), investigator-assessed objective response in measurable lesions, effect of BAT and enzalutamide on metabolic studies and QOL measures (at baseline, after three cycles of BAT, and after three cycles of enzalutamide), and time to initiation of cytotoxic chemotherapy (from study initiation [treatment with BAT] to either last known follow-up [resulting in censored for purposes of calculation] or receipt of chemotherapy). AR-FL and AR-V7 analyses were exploratory.
Statistical analysis
The null hypothesis for both co-primary endpoints was a PSA50 of 5%, with alternative hypotheses of PSA50 of 20% for BAT and 25% for enzalutamide. We required 30 patients for 83% power for BAT and 90% power for enzalutamide, with an overall one-sided type I error of 0·1 that was split equally between the tests for the two endpoints (0·05 each). We included all patients receiving at least one dose of study therapy in the primary analysis for BAT, and we counted patients achieving a PSA50 at any timepoint during the study period as responders. For the enzalutamide primary endpoint, we did an intention-to-treat analysis regardless of whether patients proceeded to enzalutamide treatment, unless they remained on BAT. We calculated the CIs for objective responses as 95% exact binomial CIs.
One interim analysis for futility of enzalutamide rechallenge was done after PSA response data to enzalutamide rechallenge was available for the first nine patients. The study proceeded to completion based on the pre-established futility rule of at least one responder among the first nine patients.
We analysed all time-to-event endpoints with the Kaplan-Meier method. We evaluated changes in QOL scores and metabolic parameters before and after treatment by paired-sample t tests, with a two-sided p value of less than 0·05 threshold for significance, using complete case analysis to account for missingness of data. Summary statistics and plots were based upon all observed data for each timepoint. These data represent the final analysis for the post-enzalutamide cohort.
We did analyses with R statistical package (version 3.4.0). This trial is registered with ClinicalTrials. gov, number NCT02090114.
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We accrued 30 eligible patients from Aug 28, 2014, to May 18, 2016 (table 1). All patients had previous enzalutamide therapy and 13 had both previous abiraterone and enzalutamide therapy. All enrolled patients were refractory to previous enzalutamide therapy, and 14 (47%) of 30 patients had previously achieved a PSA50 response to enzalutamide with a median time on enzalutamide of 8 months (range 1–39).
Table 1.
Baseline characteristics for enrolled patients
| Patients (n=30) | |
|---|---|
| Age (years) | 74 (50–89) |
|
| |
| Race or ethnicity | |
| White | 27 (90%) |
| Asian | 2 (7%) |
| African American | 1 (3%) |
|
| |
| ECOG PS | |
| 0 | 22 (73%) |
| 1 | 8 (27%) |
|
| |
| Gleason grade | |
| 6 | 3 (10%) |
| 7 | 6 (20%) |
| 8–10 | 20 (67%) |
| Not available | 1 (3%) |
|
| |
| PSA (ng/mL) | 39·8 (2·4–245·3) |
|
| |
| Testosterone <50 ng/dL | 30 (100%) |
|
| |
| Haemoglobin (g/dL) | 12·8 (9·1–15·1) |
|
| |
| Alkaline phosphatase | |
| Within normal limits | 28 (93%) |
| >Upper limit of normal | 2 (7%) |
|
| |
| Albumin (g/dL) | 4·3 (3·6–4·9) |
|
| |
| Metastatic disease | |
| Soft tissue only | 9 (30%) |
| Bone only | 16 (53%) |
| Bone and soft tissue | 5 (17%) |
| RECIST evaluable | 12 (40%) |
|
| |
| Previous therapy | |
| Enzalutamide | 30 (100%) |
| Abiraterone | 13 (43%) |
Data are median (range) or n (%). ECOG PS=Eastern Cooperative Oncology Group performance status. RECIST=Response Evaluation Criteria in Solid Tumors.
Patients were treated with BAT for a median of six cycles (range 1–26). All patients received at least one cycle of BAT. Nine (30%; 95% CI 15–49) of 30 patients achieved a PSA50 to BAT (p<0·0001; figure 1A). 21 (70%) patients who completed BAT proceeded to enzalutamide treatment. Of the remaining nine patients, two had severe adverse events and were removed from the study (including one death assessed as not related to BAT), five were removed from the study by treating physician preference, one withdrew, and one remained on BAT at the time of analysis. The intention-to-treat population for evaluation of enzalutamide was thus the 29 patients that completed BAT, excluding the one long-term responder to BAT, because this patient could potentially be rechallenged with enzalutamide in the future. For enzalutamide rechallenge, 15 (52%; 95% CI 33–71) patients achieved a PSA50 (p<0·0001; figure 2A).
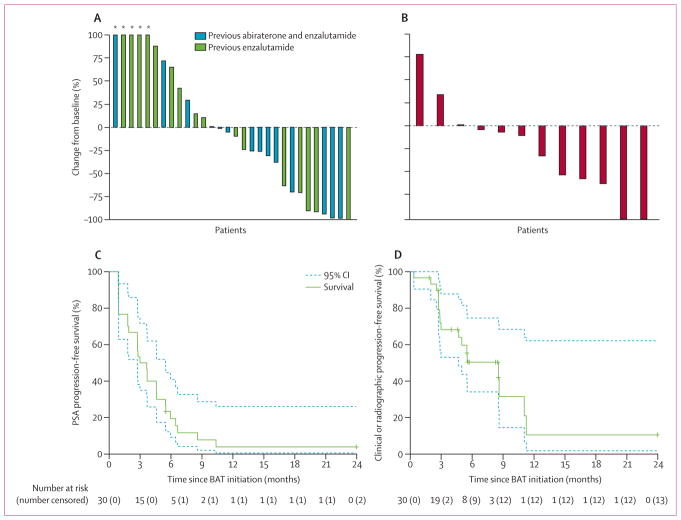
Figure 1. Responses to BAT.
Responses characterised by best PSA response (A), best radiographic response in target lesions by RECIST 1.1 (B), PSA progression-free survival defined by PCWG2 (C), and clinical or radiographic progression-free survival defined by PCWG2 and RECIST 1.1 (D). Survival curves (solid lines) are bracketed by 95% CIs (dashed lines). 12 patients with RECIST-evaluable lesions were included in the best radiographic response analysis. BAT=bipolar androgen therapy. PSA=prostate-specific antigen. RECIST=Response Evaluation Criteria in Solid Tumors. PCWG2=Prostate Cancer Working Group 2. *Values truncated at 100%.
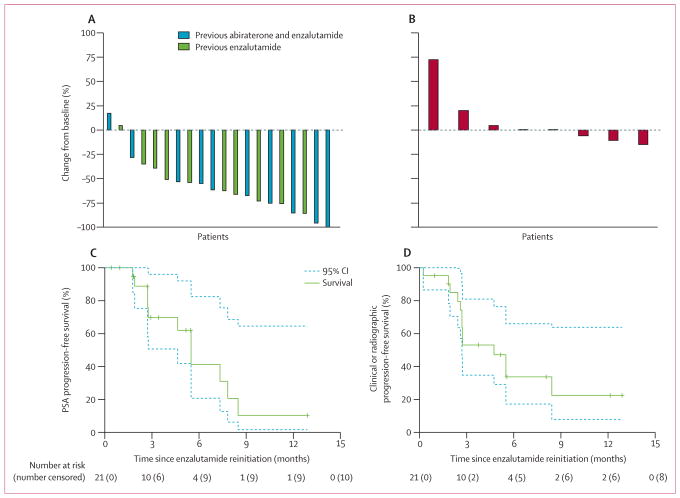
Figure 2. Responses to enzalutamide.
Responses characterised by best PSA response among patients with at least one follow-up PSA on enzalutamide (A), best radiographic response in target lesions by RECIST 1.1 (B), PSA progression-free survival defined by PCWG2 (C), and clinical or radiographic progression-free survival defined by PCWG2 and RECIST 1.1 (D). Survival curves (solid lines) are bracketed by 95% CIs (dashed lines). 20 patients who proceeded to enzalutamide treatment and had at least one follow-up PSA on enzalutamide were included in the best PSA response analysis. Eight patients with RECIST-evaluable lesions were included in the best radiographic response analysis. BAT=bipolar androgen therapy. PSA=prostate-specific antigen. RECIST=Response Evaluation Criteria in Solid Tumors. PCWG2=Prostate Cancer Working Group 2.
The median follow-up for patients on BAT was 4·9 months (IQR 2·8–7·6). Among 12 (40%) patients with RECIST-evaluable lesions, six (50%; 95% CI 21–79) had a partial or complete response (figure 1B). The remaining 18 (60%) patients were not RECIST-evaluable due to non-measurable bone-only metastatic disease (n=16) or lack of follow-up radiographic imaging (n=2). 28 (93%) of 30 patients had PSA progression events on BAT, leading to a median PSA progression-free survival of 3·3 months (95% CI 2·7–5·5; figure 1C). 17 (57%) of 30 patients had a clinical or radiographic progression event on BAT, and the median clinical or radiographic progression-free survival was 8·6 months (95% CI 4·7–not reached; figure 1D). After the 28-day washout period before restarting enzalutamide, 14 (70%) of 20 patients with measured testosterone values had castrate testosterone (<50 ng/dL) and the remainder had near castrate concentrations (median 30 ng/dL, range <20–133 ng/dL). 15 (68%) of 22 patients with PSA measured before and after the washout period had continued increases in PSA concentrations (appendix p 1).
The median follow-up after enzalutamide rechallenge was 2·7 months (IQR 2·4–5·5). 12 patients had RECIST-evaluable disease for BAT; however, four of these patients did not proceed to receive enzalutamide (one was a long-term responder to BAT and three terminated study participation after BAT); therefore, eight patients were evaluable for radiographic response (by RECIST) to enzalutamide. None (95% CI 0–37) of these patients had a radiographic response to enzalutamide (figure 2B). 11 (52%) of 21 patients rechallenged with enzalutamide had PSA progression events, and the median PSA progression-free survival was 5·5 months (95% CI 4·6–not reached; figure 2C). 13 (62%) of 21 patients rechallenged with enzalutamide had clinical or radiographic progression events and the median clinical or radiographic progression-free survival was 4·7 months (95% CI 2·7–not reached; figure 2D). Four patients remained on enzalutamide at the time of final analysis, all of whom had met the co-primary endpoint of PSA50 response to enzalutamide.
14 (47%) of 30 patients were known to have proceeded to cytotoxic chemotherapy, and the median time to initiation of docetaxel chemotherapy for patients was 20·4 months (95% CI 11·3–not reached).
Adverse events reported during BAT treatment are in table 2. The most common grade 3–4 adverse event was hypertension (three [10%] patients). During the study, no dose-limiting toxicities occurred and no patients required dose adjustment on BAT. Three serious (grade 3–4) adverse events were potentially attributable to testosterone (pulmonary embolism, non-ST segment elevation myocardial infarction, and urinary obstruction; table 2). The pulmonary embolism was diagnosed as an incidental finding on CT scan after the sixth cycle of BAT. The non-ST segment elevation myocardial infarction required percutaneous coronary intervention and occurred during the second cycle of BAT. The episode of urinary obstruction occurred during the first cycle of BAT, which is concerning for a flare phenomenon. Two patients had transient pain flares after initiation of BAT, characterised by onset of pain within hours of treatment that resolved within days (data not shown). One of these patients received further cycles of therapy without recurrence and achieved a PSA50 response, and the other patient had recurrent flare each cycle that was managed with opioid analgesics, thus allowing him to stay on study. Two patients discontinued BAT because of adverse events, including one non-treatment-related patient death (severe sepsis with multiorgan dysfunction syndrome) and one myocardial infarction. No treatment-related deaths occurred.
Table 2.
Adverse events for BAT
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|
| Musculoskeletal pain* | 12 (40%) | 0 | 0 | 0 |
| Increased haemoglobin (>ULN) | 11 (37%) | 0 | 0 | 0 |
| Hypertension | 4 (13%) | 3 (10%) | 0 | 0 |
| Anaemia | 5 (17%) | 0 | 0 | 0 |
| Breast tenderness | 5 (17%) | 0 | 0 | 0 |
| Rash | 5 (17%) | 0 | 0 | 0 |
| Fatigue | 5 (17%) | 0 | 0 | 0 |
| Nausea | 5 (17%) | 0 | 0 | 0 |
| Gynaecomastia | 4 (13%) | 0 | 0 | 0 |
| Hot flashes | 4 (13%) | 0 | 0 | 0 |
| Pruritis | 4 (13%) | 0 | 0 | 0 |
| Abdominal pain | 3 (10%) | 0 | 0 | 0 |
| Oedema | 3 (10%) | 0 | 0 | 0 |
| Fall | 3 (10%) | 0 | 0 | 0 |
| Sinusitis | 3 (10%) | 0 | 0 | 0 |
| Urinary obstruction | 0 | 0 | 1 (3%) | 0 |
| Gallstone | 0 | 1 (3%) | 0 | 0 |
| Sepsis | 0 | 0 | 0 | 1 (3%) |
| Non-ST segment elevation myocardial infarction | 0 | 1 (3%) | 0 | 0 |
| Pulmonary embolism | 0 | 1 (3%) | 0 | 0 |
Data are n (%) for 30 patients. Those adverse events occurring in 10% or more of the patients are listed for grades 1–2, and all events are listed for grades 3–5. BAT=bipolar androgen therapy. ULN=upper limit of normal.
Musculoskeletal pain not deemed to be tumour flare or clinical progression.
Low-grade adverse events occurring during enzalutamide treatment were consistent with previous clinical experience (appendix p 2). No patient deaths happened during the enzalutamide treatment portion of the study. Five serious adverse events occurred on enzalutamide treatment in three patients (abdominal pain, pancreatitis, paresthesias, gastrointestinal bleed, and non-ST segment elevation myocardial infarction), and none of the serious adverse events were assessed as probably, likely, or definitely related to study treatment (appendix p 2).
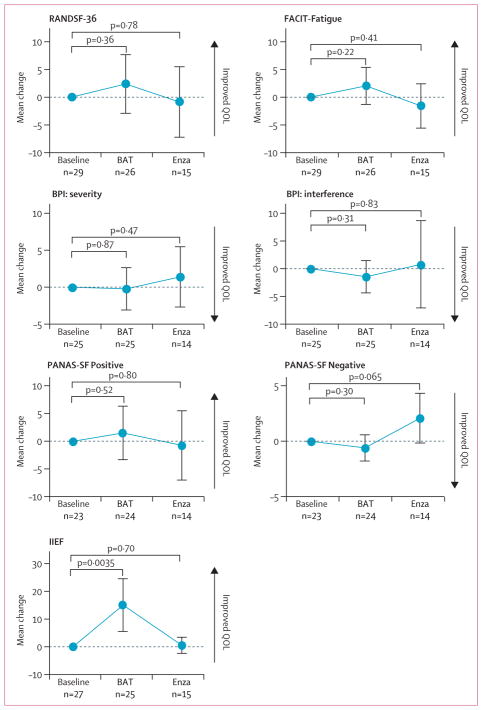
29 patients completed at least one QOL instrument at baseline, 26 completed at least one after three cycles of BAT, and 15 completed at least one after three cycles of enzalutamide treatment. Overall QOL (RANDSF-36), fatigue (FACIT-Fatigue), the degree of pain severity or interference (BPI), and positive and negative affect (PANAS-SF) after three cycles of BAT did not differ compared with baseline and after three cycles of enzalutamide (figure 3). Patients had significantly improved sum IIEF scores on BAT compared with baseline scores (figure 3).
Figure 3. Quality of life metrics.
Data are mean change from baseline after three cycles of BAT or three cycles of enzalutamide. Error bars are 95% CIs. RANDSF-36=RAND Short Form-36 item. FACIT-Fatigue=Functional Assessment of Chronic Illness Therapy-Fatigue Subscale. BPI=Brief Pain Inventory. PANAS-SF=Positive and Negative Affect Schedule Short Form. IIEF=International Index of Erectile Function. BAT=bipolar androgen therapy. Enza=enzalutamide. QOL=quality of life.
After three cycles of BAT, nadir median total testosterone concentrations increased from less than 20 ng/dL (range <20–47·7) to 207·5 ng/dL (65–2339; p<0·0001), with corresponding increases in free testosterone (from 0·7 pg/mL [0–10] to 25·2 pg/mL [3·3–358·2]; p<0·0001), DHT (from <5 ng/dL [<5–5] to 12 ng/dL [7–81]; p<0·0001), and DHEA-S (from 32 μg/dL [6–207] to 58 μg/dL [3–268]; p=0·068; appendix pp 3–4). Concentrations of total (p=0·59) and free testosterone (p=0·69), DHT (p>0·99), and DHEA-S (p=0·092) after three cycles of enzalutamide were similar to baseline (appendix pp 3–4). DHEA was similar to baseline after BAT (p=0·36) and after enzalutamide (p=0·11; appendix pp 3–4). Oestradiol significantly increased after BAT (p=0·0013) and after enzalutamide (p=0·042) compared with baseline (appendix pp 3–4). SHBG was significantly decreased after BAT (p=0·0006) but significantly increased after enzalutamide (p=0·0059) compared with baseline (appendix pp 3–4). Haemoglobin was significantly increased from baseline after BAT (p=0·0074) whereas enzalutamide was similar to baseline (p=0·84; appendix pp 3–4). Glycaemic tolerance, as measured by fasting glucose and HbA1c, significantly improved on BAT compared with baseline (p=0·032 and p=0·0066, respectively) whereas enzalutamide was similar to baseline (p=0·53 and p=0·10, respectively; appendix pp 3–4). HDL, LDL, and triglycerides were all significantly lower on BAT than baseline (p=0·0014, p=0·043, and p=0·031, respectively) whereas enzalutamide was similar to baseline (p=0·83, p=0·080, and p=0·24, respectively; appendix pp 3–4). Concentrations of C-telopeptides did not change from baseline on BAT (p=0·72) or enzalutamide (p=0·25) whereas osteocalcin was increased from baseline on BAT (p<0·0001), but not enzalutamide (p=0·62; appendix pp 3–4). Fasting insulin and C-reactive protein were not changed from baseline after either BAT (p=0·85 and p=0·65, respectively) or enzalutamide (p=0·95 and p=0·58, respectively, appendix pp 3–4).
All patients had samples available for CTC-based analysis of androgen receptor mRNA status (table 3). Responses to BAT were achieved in five (31%) of 16 patients who were baseline CTC-negative, three (27%) of 11 patients who were baseline CTC-positive and AR-V7-negative, and one (33%) of three patients who were baseline CTC-positive and AR-V7-positive (table 3). Responses to enzalutamide by intention-to-treat analysis were achieved in nine (60%) patients who were baseline CTC-negative, six (55%) who were baseline CTC-positive and AR-V7-negative, and no patients who were baseline CTC-positive and AR-V7-positive (table 3).
Table 3.
CTC-based measurements of mRNA copy numbers for AR-FL and AR-V7
| Baseline CTC | Post-BAT cycle 3 CTC | Post-enzalutamide cycle 3 CTC | BAT response | Enzalutamide response | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| AR-FL copy number | AR-V7 copy number | AR-FL copy number | AR-V7 copy number | AR-FL copy number | AR-V7 copy number | |||
| Baseline CTC-negative | ||||||||
|
| ||||||||
| 1 | Negative | Negative | Negative | Negative | Negative | Negative | Yes | No |
| 4 | Negative | Negative | Negative | Negative | Negative | Negative | Yes | Yes |
| 27 | Negative | Negative | Negative | Negative | NA | NA | Yes | NR |
| 28 | Negative | Negative | Negative | Negative | NA | NA | Yes | NR |
| 40 | Negative | Negative | Negative | Negative | 1 | 0 | Yes | Yes |
| 7 | Negative | Negative | Negative | Negative | Negative | Negative | No | Yes |
| 9 | Negative | Negative | Negative | Negative | Negative | Negative | No | Yes |
| 24 | Negative | Negative | Negative | Negative | Negative | Negative | No | Yes |
| 34 | Negative | Negative | NA | NA | NA | NA | No | NR |
| 38 | Negative | Negative | Negative | Negative | NA | NA | No | No |
| 39 | Negative | Negative | Negative | Negative | 3 | 0 | No | Yes |
| 47 | Negative | Negative | NA | NA | NA | NA | No | NR |
| 51 | Negative | Negative | Negative | Negative | NA | NA | No | Yes |
| 53 | Negative | Negative | 7 | 0 | Negative | Negative | No | Yes |
| 54 | Negative | Negative | NA | NA | NA | NA | No | Yes |
| 57 | Negative | Negative | 1 | 0 | NA | NA | No | NR |
|
| ||||||||
| Baseline CTC-positive and AR-V7-negative | ||||||||
|
| ||||||||
| 18 | 1 | 0 | Negative | Negative | 2 | 0 | Yes | Yes |
| 42 | 7 | 0 | Negative | Negative | NA | NA | Yes | NR |
| 31 | 25 | 0 | Negative | Negative | Negative | Negative | Yes | Yes |
| 2 | 1 | 0 | Negative | Negative | NA | NA | No | No |
| 19 | 1 | 0 | Negative | Negative | NA | NA | No | Yes |
| 43 | 2 | 0 | Negative | Negative | Negative | Negative | No | Yes |
| 46 | 2 | 0 | NA | NA | NA | NA | No | NR |
| 48 | 3 | 0 | 1 | 0 | 8 | 0 | No | Yes |
| 49 | 4 | 0 | NA | NA | NA | NA | No | NR |
| 55 | 7 | 0 | 5 | 0 | 118 | 3 | No | Yes |
| 50 | 40 | 0 | 57 | 0 | NA | NA | No | NR |
|
| ||||||||
| Baseline CTC-positive and AR-V7-positive | ||||||||
|
| ||||||||
| 5 | 43 | 6 | 2 | 0 | 154 | 96 | Yes | No |
| 44 | 7 | 14 | 24 | 2 | NA | NA | No | No |
| 17 | 16 | 4 | Negative | Negative | 285 | 44 | No | No |
Individual patient data for each patient ID number are listed. Responders to BAT and enzalutamide are denoted. CTC=circulating tumour cells. AR-FL=androgen receptor full length. AR-V7=androgen receptor splice variant 7. BAT=bipolar androgen therapy. NR=not rechallenged. NA=not available.
Discussion
Our study met its co-primary endpoints of showing PSA50 responses to BAT after progression on enzalutamide and following enzalutamide rechallenge. The evaluation of PSA response in patients receiving BAT is complicated by the fact that PSA is an androgen-responsive gene. Importantly, in addition to PSA responses, radiographic responses to BAT occurred in 50% of patients with RECIST-evaluable disease and the clinical or radiographic progression-free survival was nearly 9 months. These data are promising considering published series26–29 have shown evidence of cross-resistance between abiraterone and enzalutamide, with objective responses of 0–11% and progression-free survival of 3–4 months in patients receiving abiraterone or enzalutamide after progressing on the alternate drug. Furthermore, our results support the hypothesis that an alternative strategy of therapeutically targeting the androgen receptor with BAT in the setting of progression on second-line androgen receptor-signalling inhibitors might be beneficial to patients.
Adaptive upregulation of androgen receptor signalling by prostate cancer cells in response to continuous exposure to the low testosterone microenvironment produced by androgen deprivation therapy is a key factor in the development and progression of resistance to androgen ablative therapies, such as castration and antiandrogens. High androgen receptor expression produces a therapeutic vulnerability that might sensitise castration-resistant prostate cancer cells to supraphysiological concentrations of testosterone. BAT is designed to take advantage of and disrupt castration-resistant prostate cancer cells’ ability to adaptively upregulate or downregulate androgen receptor expression in response to testosterone concentrations in the tumour microenvironment. A high PSA50 response following enzalutamide rechallenge supports the ability of BAT to resensitise metastatic castration-resistant prostate cancer cells to androgen receptor-signalling inhibitors. This resensitisation to enzalutamide supports the hypothesis that BAT might modulate adaptive resistance mechanisms (eg, androgen receptor overexpression) that arise following androgen receptor-signalling pathway antagonism. However, the duration of response to enzalutamide was short in most patients, potentially reflecting a rapid readoption of a resistant phenotype. In future studies, potential strategies to address the transient resensitisation could include continually alternating between therapies to delay time to progression and maximise benefit to patients.
Testosterone was well tolerated in this study, and the side-effect profile was as expected for testosterone-replacement therapy. Other hormonal effects, including hot flashes, breast tenderness, and gynaecomastia, could have been the result of the fluctuating concentrations of testosterone and oestrogen through the BAT cycles. Despite the careful selection criteria intended to minimise risk for testosterone-induced pain flare, two patients had a post-BAT flare. Furthermore, low-grade musculoskeletal pain, not clearly related to prostate cancer disease burden, was observed during the trial. Serious (grade 3) cardiovascular events occurred during the trial, which included a myocardial infarction and pulmonary embolism on BAT. The metabolic parameters did not indicate a pattern of increased cardiovascular risk based upon the changes in inflammatory markers, lipid profile, or glucose metabolism. Although haemoglobin was increased above the upper limit of normal in 37% of patients on BAT, neither of the patients who had cardiovascular events while on BAT had increases in haemoglobin concentrations. Therefore, further close monitoring of cardiovascular risk during future trials of BAT is warranted. Nonetheless, this trial helps to show that study of BAT might be reasonably expanded to a broader population of patients with metastatic castration-resistant prostate cancer.
Some patients might have enrolled on this trial in the hope of mitigating the side-effects of androgen deprivation therapy. A fifth of patients had restored erectile function on BAT based upon paired IIEF evaluations—a finding consistent with the QOL changes observed with a modified version of BAT tested in the hormone-sensitive setting.30 Other QOL metrics did not show any differences from baseline, and larger followup studies will further explore QOL changes during BAT versus androgen receptor-directed therapy in this patient population. Metabolic parameters including glycaemic control and lipid profile appeared to be improved on BAT compared with baseline.
No clear association between baseline CTC-derived androgen receptor status and response was observed. The proposed mechanism for BAT requires high androgen receptor concentrations to be present for efficacy. Baseline androgen receptor mRNA copy numbers were likely to be indicative of overall disease burden and not necessarily representative of tumour-concentration androgen receptor expression, and the baseline status was not associated with response. However, the overall decreased AR-FL and AR-V7 concentrations in CTCs after three cycles of BAT is consistent with the hypothesis that BAT results in downregulation of androgen receptor expression or amplification. Patients who were CTC-negative after three cycles of BAT were most likely to be PSA responders. The subsequent increase in AR-FL and AR-V7 concentrations after enzalutamide rechallenge is also consistent with androgen receptor locus upregulation in response to androgen receptor antagonism. However, without concurrent tissue-based assessments, whether other mechanisms account for these changes, such as stabilisation of the tumour microenvironment leading to decreased tumour cell shedding, is not known. Importantly, presence of AR-V7 did not preclude response to BAT, although no patient who was CTC-positive and AR-V7-positive responded to enzalutamide retreatment. This study did not incorporate tissue-based assessments of androgen receptor expression nor genomic analysis. Future tissue-based assessment might yield a biomarker of response, by either identifying tissue-based androgen receptor overexpression or genetic alterations that potentially confer susceptibility to BAT, such as DNA repair defects.31
Limitations of the study included its single-institution, single-cohort analysis with strict entry criteria designed to mitigate against risks of adverse events. Randomisation is needed to define the QOL and metabolic differences between treatments, as sequential comparison is confounded by different times of follow-up on study, frequent removal of patients from study before completion of 3 months of enzalutamide, and symptoms related to disease progression.
In conclusion, for asymptomatic patients with progression after enzalutamide, BAT induces clinical responses and subsequent resensitisation to enzalutamide. Studies in progress, including a randomised trial comparing BAT to enzalutamide in patients progressing on abiraterone,32 will further define a potential clinical role for BAT in the management of metastatic castration-resistant prostate cancer as well as the clinical features of patients with the highest chance for benefit.
Supplementary Material
Research in context.
Evidence before this study
We did a systematic PubMed review on Sept 30, 2017, which included all articles to date with no language restrictions, using the terms “supraphysiologic testosterone prostate cancer”, which identified 16 results. The concept of using high concentrations of hormones as treatment for hormone-driven cancers was first described by Charles Huggins in 1964. At the time of study conception and initiation, only two previous studies described the use of physiological doses of testosterone as therapy for castration-resistant prostate cancer. Preclinical and pilot trial results from our institution with supraphysiological testosterone supported the hypotheses tested in this study. However, no data have been published regarding the efficacy of cyclic parenteral supraphysiological testosterone for castration-resistant prostate cancer and no trials have been done prospectively investigating the efficacy of enzalutamide rechallenge after bipolar androgen therapy (BAT) or any other intervening therapy. Evidence has shown that adaptive upregulation of androgen receptor concentrations, via upregulation of expression, gene amplification, and splice variant expression, contributed to resistance to androgen receptor-signalling inhibitors in prostate cancer. Multiple clinical reports describe diminishing clinical response with sequential use of androgen receptor-signalling inhibitors, such as abiraterone and enzalutamide. This cross-resistance to androgen ablative therapies has emerged as a major clinical problem.
Added value of this study
To our knowledge, this study is the first prospective trial to investigate the activity and safety of administration of pharmacological doses of testosterone to rapidly cycle between the extremes of supraphysiological and near-castrate serum testosterone concentrations (ie, BAT) in patients with metastatic castration-resistant prostate cancer after progression on enzalutamide. Although paradoxical, the results of this trial suggest that BAT, as an androgen receptor-directed agonist therapy, can result in clinical responses, while transiently resensitising most patients to further enzalutamide therapy. Furthermore, BAT could be administered with a reasonable safety and tolerability profile. CTC-based analysis of AR-FL and AR-V7 mRNA expression showed that the profile of responders appears distinct from previous studies associating AR-FL and AR-V7 with poor response.
Implications of all the available evidence
The results of this trial suggest a role for BAT in sequence with androgen ablative therapies as part of hormonal treatment for prostate cancer aimed at disrupting androgen receptor signalling within prostate cancer cells. With a paucity of data regarding best sequencing of androgen receptor-directed therapies in patients, the efficacy of BAT and transient resensitisation to enzalutamide serve as a basis for development of sequential or combinatorial approaches to improve outcomes for patients with metastatic castration-resistant prostate cancer.
Acknowledgments
Funding: National Institutes of Health and National Cancer Institute.
This trial was funding by National Institutes of Health and National Cancer Institute grant number RO1CA184012.
Footnotes
Contributors
HW, MTS, CGD, MAC, CJP, ESA, MAE, and SRD designed the study. BAT, RS, IR, AB, AS, MD, VS, CFP, JLS, MTS, CGD, MAC, CJP, ESA, MAE, and SRD collected data. BAT, HW, BL, CL, JL, and SRD analysed the data. BAT and SRD wrote the manuscript. All authors had access to the data, edited, and approved the final manuscript.
Declaration of interests
BAT reports grants from National Institutes of Health (NIH) and National Cancer Institute (NCI) and from Conquer Cancer Foundation, during the conduct of the study; and has a patent for Polymers for Functional Particles, System for targeted delivery of therapeutic agents with royalties paid to Pfizer, Selecta Biosciences, and Bind Therapeutics. CL reports a patent, C13162, with royalties paid to Tokai and Qiagen, and has a patent, C13084, pending. JL reports grants and personal fees from Astellas, Sanofi, and Gilead, personal fees from Janssen Oncology and Sun Pharma, and grants from Mirati and Orion, outside the submitted work, and has a patent, C10305, with royalties paid to A&G, Tokai, and Qiagen, a patent, C13126, with royalties paid to Tokai and Qiagen, and has a patent, C13084, pending. MAC reports personal fees from Pfizer, Astellas, Merck, and Abbvie, outside the submitted work. SRD reports grants from NIH and NCI, DOD Prostate Cancer Research Program, and One-in-Six Foundation, during the conduct of the study; and other from Medicenna, outside the submitted work. All other authors declare no competing interests.
References
- 1.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 2.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–06. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 4.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–55. [PubMed] [Google Scholar]
- 5.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 8.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell-free dna: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21:2315–24. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- 10.Antonarakis ES, Armstrong AJ, Dehm SM, Luo J. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis. 2016;19:231–41. doi: 10.1038/pcan.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umekita Y, Hiipakka RA, Kokontis JM, Liao S. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc Natl Acad Sci USA. 1996;93:11802–07. doi: 10.1073/pnas.93.21.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhau HY, Chang SM, Chen BQ, et al. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci USA. 1996;93:15152–57. doi: 10.1073/pnas.93.26.15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuu CP, Hiipakka RA, Fukuchi J, Kokontis JM, Liao S. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005;65:2082–84. doi: 10.1158/0008-5472.CAN-04-3992. [DOI] [PubMed] [Google Scholar]
- 14.Denmeade SR, Isaacs JT. Bipolar androgen therapy: the rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate. 2010;70:1600–07. doi: 10.1002/pros.21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaacs JT, D’Antonio JM, Chen S, et al. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate. 2012;72:1491–505. doi: 10.1002/pros.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42:668–75. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litvinov IV, Vander Griend DJ, Antony L, et al. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci USA. 2006;103:15085–90. doi: 10.1073/pnas.0603057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweizer MT, Antonarakis ES, Wang H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Sci Transl Med. 2015;7:269ra2. doi: 10.1126/scitranslmed.3010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1. 0. Health Econ. 1993;2:217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 22.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 23.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 24.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 25.Thompson ER. Development and validation of an internationally reliable short-form of the Positive and Negative Affect Schedule (PANAS) J Cross Cult Psychol. 2007;38:227–42. [Google Scholar]
- 26.Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol. 2015;67:23–29. doi: 10.1016/j.eururo.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 27.Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24:1807–12. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 28.Noonan KL, North S, Bitting RL, Armstrong AJ, Ellard SL, Chi KN. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–07. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 29.Smith MR, Saad F, Rathkopf DE, et al. Clinical outcomes from androgen signaling-directed therapy after treatment with abiraterone acetate and prednisone in patients with metastatic castration-resistant prostate cancer: post hoc analysis of COU-AA-302. Eur Urol. 2017;72:10–13. doi: 10.1016/j.eururo.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Schweizer MT, Wang H, Luber B, et al. Bipolar androgen therapy for men with androgen ablation naive prostate cancer: results from the phase II BATMAN study. Prostate. 2016;76:1218–26. doi: 10.1002/pros.23209. [DOI] [PubMed] [Google Scholar]
- 31.Teply BA, Kachhap S, Eisenberger MA, Denmeade SR. Extreme response to high-dose testosterone in BRCA2- and ATM-mutated prostate cancer. Eur Urol. 2017;71:499. doi: 10.1016/j.eururo.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teply BA, Antonarakis ES, Carducci MA, et al. A randomized phase II study comparing bipolar androgen therapy vs. enzalutamide in asymptomatic men with castration resistant metastatic prostate cancer: the TRANSFORMER trial. Proc Am Soc Clin Oncol. 2015;33(suppl 15) doi: 10.1200/JCO.20.02759. TPS5079 (abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.