Abstract
Importance
Maternal overweight, which often results in cesarean delivery, is a strong risk factor for child overweight. Little is known about the joint contribution of birth mode and microbiota in the infant gut to the association between maternal prepregnancy overweight and child overweight.
Objective
To investigate the association of birth mode with microbiota in the infant gut, and whether this mediates the association between maternal and child overweight.
Design, Setting, and Participants
An observational study was conducted of 935 full-term infants born between January 1, 2009, and December 31, 2012, in the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort. Maternal prepregnancy body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared using height and weight data taken from medical records or maternal report. Infant gut microbiota were profiled with 16S ribosomal RNA sequencing in fecal samples collected at a mean (SD) age of 3.7 (1.0) months. At ages 1 and 3 years, BMI z scores adjusted for age and sex were generated according to World Health Organization criteria. Statistical analysis was conducted from January 29 to June 15, 2017.
Exposures
Mothers of normal weight (BMI, 18.5-24.9) and overweight or obese (BMI, ≥25.0) mothers.
Main Outcome and Measures
Risk of overweight and obesity (>97th percentile BMI z scores) among children at ages 1 and 3 years.
Results
Of the 935 mother-infant pairs in the study (mean [SD] age, 32.5 [4.5] years) 382 (40.9%) were overweight, 69 of 926 infants (7.5%) were overweight at age 1 year, and 90 of 866 infants (10.4%) were overweight at age 3 years. Compared with being born vaginally to a mother of normal weight, infants born vaginally to overweight or obese mothers were 3 times more likely to become overweight at age 1 year (adjusted odds ratio [OR], 3.33; 95% CI, 1.49-7.41), while cesarean-delivered infants of overweight mothers had a 5-fold risk of overweight at age 1 year (adjusted OR, 5.02; 95% CI, 2.04-12.38). Similar risks were also observed at age 3 years. Multiple mediator path modeling revealed that birth mode and infant gut microbiota (Firmicutes species richness, especially of the Lachnospiraceae family) sequentially mediated the association between maternal prepregnancy overweight and childhood overweight at ages 1 and 3 years. Bacterial genera belonging to the Lachnospiraceae family were more abundant in infants of overweight mothers; however, the participating genera of Lachnospiraceae differed between infants delivered vaginally and those delivered via cesarean birth.
Conclusions and Relevance
This study found evidence of a novel sequential mediator pathway involving birth mode and Firmicutes species richness (especially higher abundance of Lachnospiraceae) for the intergenerational transmission of overweight.
This cohort study examines whether birth mode and microbiota in the infant gut mediate the association between maternal and child overweight.
Key Points
Question
Do birth mode and microbiota in the infant gut mediate the association between maternal prepregnancy overweight and childhood overweight?
Findings
In this cohort study of 935 mother-infant pairs, both vaginally and cesarean-delivered infants born to overweight and obese mothers were at greater risk of being overweight at ages 1 and 3 years compared with infants born vaginally to a mother of normal weight. Birth mode and microbiota in the infant gut (especially Lachnospiraceae) act sequentially to mediate the association between maternal prepregnancy overweight and child overweight.
Meaning
Together, birth mode and Lachnospiraceae family microbiota in the infant gut sequentially mediate the intergenerational association of overweight, underscoring their potential contributions in developing child overweight and obesity.
Introduction
Childhood obesity is a global health concern. More than 20% of preschool-aged children in Canada are overweight or obese (OWOB). Also on the rise is maternal OWOB during pregnancy and associated higher rates of birth by cesarean delivery. Furthermore, carrying OWOB into pregnancy presents, at minimum, a 2-fold greater risk of obesity in offspring. Some of this excess risk can be attributed to cesarean delivery, as children delivered by this method are 30% more likely to develop OWOB compared with those delivered vaginally. However, while the epidemiologic evidence is strong that prenatal maternal obesity predisposes newborns to develop OWOB, the mechanism behind this intergenerational association has not, to our knowledge, been delineated by known genetic or lifestyle factors shared between mothers and their offspring.
Mother-to-newborn transfer of obesogenic microbes has been put forward as a biological pathway for the intergenerational transmission of OWOB. Substantial changes to the intestinal microbiota of women are observed as pregnancy progresses to parturition. The weight status of women entering pregnancy can modify this process. Compared with their normal-weight counterparts, women with a high prepregnancy body mass index (BMI) have elevated levels of Bacteroides in their third trimester. Because maternal microbiota are the primary source for the first inoculation of newborn infants and microbiota have been implicated in adipogenesis, it is conceivable that prepregnancy weight and the maternal intestinal microbiome influence microbial assembly in the infant gut, as well as OWOB outcomes.
Several studies have investigated maternal OWOB-associated changes in microbiota of the infant gut. Prepregnancy overweight has been found to affect microbial composition in infant stool, manifested as increases in Bacteroides species, at the ages of 1 month, 6 months, and 2 years. Microbial compositional changes associated with maternal OWOB are more evident in newborns after vaginal rather than cesarean delivery, and, for the latter, reductions in Bacteroides are seen. No gut dysbiosis in relation to maternal OWOB has been found in later infancy at ages 9 and 18 months. Although cesarean delivery alters the structure of gut microbiota during early life, it remains to be determined whether birth mode plays a role in maternal OWOB-related changes to the microbiota of the infant gut and subsequent risk for OWOB. To address this gap in knowledge, we determined the joint associations of maternal prepregnancy OWOB and birth mode with microbial composition of the infant gut and microbiota interactions at ages 3 to 4 months, and with OWOB outcomes at ages 1 and 3 years. Second, we assessed whether microbiota of the infant gut mediated the association between maternal OWOB and child OWOB, and whether the mediation was dependent on birth mode.
Methods
Study Design
This study involved a subsample of 935 Canadian full-term infants from the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort (http://www.childstudy.ca). Mothers of studied infants were enrolled during pregnancy between January 1, 2009, and December 31, 2012. Maternal BMI was calculated as weight in kilograms divided by height in meters squared using measured height and self-reported prepregnancy weight or estimated from measured weight at 1 year post partum. Validation against prenatal records showed that prepregnancy weight was slightly underestimated by maternal recall (mean difference, –1.0 kg; 95% CI, –1.5 to –0.4 kg) and slightly overestimated by measured weight at 1 year post partum (mean difference, 1.3 kg; 95% CI, 0.5-2.2 kg). Maternal BMI was classified as normal weight (BMI, 18.5-24.9 [553 mothers]) and overweight and obese (BMI, ≥25.0 [382 mothers]). Child OWOB was defined as BMI z scores greater than the 97th percentile according to World Health Organization criteria; scores were generated from weight and height measured at ages 1 and 3 years. Data on covariates were obtained from hospital records (birth mode and intrapartum antibiotic prophylaxis [IAP]) or standardized questionnaires (maternal race/ethnicity, maternal asthma or allergy status, smoking during pregnancy, infant sex, infant direct antibiotic exposure by age 1 year, breastfeeding status before 3 months, siblingship, and pet ownership). The Human Research Ethics boards at the University of Alberta, University of Manitoba, and University of British Columbia approved this study. Written informed consent was obtained from parents at enrollment.
Fecal Microbiota Analysis
Fecal samples of infants were collected at a mean (SD) age of 3.7 (1.0) months using a standard protocol during a planned home visit. Methods of sample collection, DNA extraction and amplification, 16S ribosomal RNA sequencing, and taxonomic classification have been previously described (eAppendix in the Supplement).
Statistical Analysis
Statistical analysis was conducted from January 29 to June 15, 2017. The association between maternal OWOB and cesarean vs vaginal delivery, or a 4-category variable for birth mode (vaginal without IAP, vaginal with IAP, scheduled cesarean delivery with IAP, and emergency cesarean delivery with IAP), was determined by binary and multinomial logistic regression, adjusting for location, infant sex, maternal race/ethnicity, maternal prenatal asthma, and maternal smoking during pregnancy. Logistic regression models were performed after covariate adjustment to test associations between maternal weight status (OWOB vs normal weight) or joint categories of maternal OWOB and birth mode with childhood OWOB (at ages 1 and 3 years) and microbiota of the infant gut (overall Chao1, Chao1, and Shannon indices of Firmicutes).
The association of maternal OWOB with microbiota of the infant gut (richness, α and β diversity, and relative abundance of taxa) was examined by a nonparametric Kruskal-Wallis test followed by post hoc comparison using the Dunn test. As evaluated by others, the coexistence of gut microbiota was measured as the Firmicutes to Bacteroidetes and Enterobacteriaceae to Bacteroidaceae ratios. Regression models of gut microbial measures (higher vs lower values based on a median cutoff) were performed against maternal OWOB and birth mode. Mediation analysis was conducted using the Hayes PROCESS macro in SPSS, version 23.0 (SPSS Inc), to permit evaluation of sequential mediators for the maternal-child OWOB association. A multiple mediator path model was evaluated to examine indirect associations of the 4-category variable of birth mode (mediator 1) and α diversity or relative abundance (categorized into tertiles) of microbiota of the infant gut at the family level (mediator 2). Bootstrapping, a nonparametric resampling procedure (5000 bootstrap resamples), was used to generate 95% CIs in mediation models. Microbial interaction networks by maternal-child OWOB group were built using the Spearman correlation coefficient (ρ > 0.3 or <–0.3) and visualized with Cytoscape, version 3.5.1. To identify discriminative biomarkers for maternal OWOB, the linear discriminant analysis effect size (LEfSE) was determined with a linear discriminant analysis log score cutoff of 2.
Results
In this population-based cohort of 935 mother-infant pairs, 382 mothers (40.9%) were OWOB, 69 of 926 infants (7.5%) became OWOB at 1 year, and 90 of 866 infants (10.4%) became OWOB at 3 years (Table). Childhood weight status was associated with maternal weight status. Significant differences in the prevalence of childhood OWOB at age 1 or 3 years were found according to study location, infant sex, maternal prenatal asthma, maternal smoking during pregnancy, and direct exposure of infants to antibiotics. Prepregnancy rates of OWOB were higher in women who gave birth by cesarean delivery and who partially breastfed or did not breastfeed their infants.
Table. Population Characteristics and Their Associations With Maternal and Childhood OWOB.
| Characteristic | Maternal OWOB (n = 382/935 [40.9%]) | P Value | Childhood OWOB at 1 y (n = 69/926 [7.5%]) | P Value | Childhood OWOB at 3 y (n = 90/866 [10.4%]) | P Value |
|---|---|---|---|---|---|---|
| Location, No. (%) | ||||||
| Vancouver, British Columbia | 94/323 (29.1) | <.001 | 21/326 (6.4) | .19 | 16/304 (5.3) | .001 |
| Edmonton, Alberta | 150/324 (46.3) | 31/323 (9.6) | 42/284 (14.8) | |||
| Winnipeg, Manitoba | 138/288 (47.9) | 17/277 (6.1) | 32 /278(11.5) | |||
| Birth mode, No. (%) | ||||||
| Vaginal, IAP− | 177/480 (36.9) | .06 | 28/473 (5.9) | .07 | 36/441 (8.2) | .10 |
| Vaginal, IAP+ | 90/208 (43.3) | 15/206 (7.3) | 26/202 (12.9) | |||
| Cesarean, scheduled | 48/100 (48.0) | 7/102 (6.9) | 10/91 (11.0) | |||
| Cesarean, emergency | 59/127 (46.5) | 16/125 (12.8) | 17/113 (15.0) | |||
| Infant sex, No. (%) | ||||||
| Male | 202/496 (40.7) | .86 | 46/493 (9.3) | .02 | 54/455 (11.9) | .13 |
| Female | 178/431 (41.3) | 22/425 (5.2) | 35/403 (8.7) | |||
| Maternal race/ethnicity, No. (%) | ||||||
| White | 303/706 (42.9) | .01 | 53/699 (7.6) | .51 | 67/653 (10.3) | .15 |
| Asian | 40/98 (40.8) | 8/96 (8.3) | 14/89 (15.7) | |||
| Other | 36/125 (28.8) | 6/124 (4.8) | 9/120 (7.5) | |||
| Maternal prenatal asthma, No. (%) | ||||||
| No | 342/854 (40.0) | .07 | 58/840 (6.9) | .07 | 77/790 (9.7) | .04 |
| Yes | 39/77 (50.6) | 10/81 (12.3) | 13/74 (17.6) | |||
| Maternal prenatal allergy, No. (%) | ||||||
| No | 137/342 (40.1) | .64 | 23/338 (6.8) | .71 | 30/314 (9.6) | .47 |
| Yes | 236/567 (41.6) | 42/563 (7.5) | 59/530 (11.1) | |||
| Maternal smoking during pregnancy, No. (%) | ||||||
| No | 353/875 (40.3) | .03 | 58/866 (6.7) | .005 | 85/817 (10.4) | .24 |
| Yes | 21/36 (58.3) | 7/37 (18.9) | 5/29 (17.2) | |||
| Siblings, No. (%) | ||||||
| No | 175/445 (39.3) | .27 | 35/449 (7.8) | .70 | 49/426 (11.5) | .32 |
| Yes | 195/454 (43.0) | 31/439 (7.1) | 38/405 (9.4) | |||
| Direct antibiotic exposure (0-12 mo) | ||||||
| No | 228/565 (40.3) | .09 | 39/557 (7.0) | .14 | 48/522 (9.2) | .006 |
| Yes | 78/163 (47.9) | 18/167 (10.8) | 27/155 (17.4) | |||
| Breastfeeding status (0-3 mo), No. (%) | ||||||
| No | 86/154 (55.8) | <.001 | 17/153 (11.1) | .12 | 20/141 (14.2) | .06 |
| Partial | 127/279 (45.5) | 22/282 (7.8) | 32/258 (12.4) | |||
| Exclusive | 169/497 (34.0) | 30/487 (6.2) | 38/464 (8.2) | |||
| Household pet exposure, No. (%) | ||||||
| No | 135/394 (34.3) | .001 | 24/393 (6.1) | .40 | 39/363 (10.7) | .28 |
| Only prenatal | 31/67 (46.3) | 4/67 (6.0) | 3/59 (5.1) | |||
| Both prenatal and postnatal | 189/405 (46.7) | 34/401 (8.5) | 46/383 (12.0) | |||
| Maternal prepregnancy weight status, No. (%) | ||||||
| Normal | NA | NA | 22/522 (4.2) | <.001 | 24/507 (4.7) | <.001 |
| OWOB | NA | 45/371 (12.1) | 63/328 (19.2) |
Abbreviations: IAP−, no intrapartum antibiotic prophylaxis; IAP+, intrapartum antibiotic prophylaxis; NA, not applicable; OWOB, overweight or obese.
Association Between Maternal OWOB and Cesarean Delivery
Almost half of mothers delivering by cesarean delivery had prepregnancy OWOB (Table). After covariate adjustment, maternal OWOB was associated with a 1.50 (95% CI, 1.10-2.07) times greater odds of cesarean delivery. A similar association was also observed for maternal BMI z score (eFigure 1A in the Supplement). Overweight or obese mothers were more likely to undergo scheduled cesarean delivery or to have an emergency cesarean delivery than to deliver vaginally without IAP (eFigure 1B in the Supplement).
Impact of Maternal OWOB on Childhood OWOB at Ages 1 and 3 Years
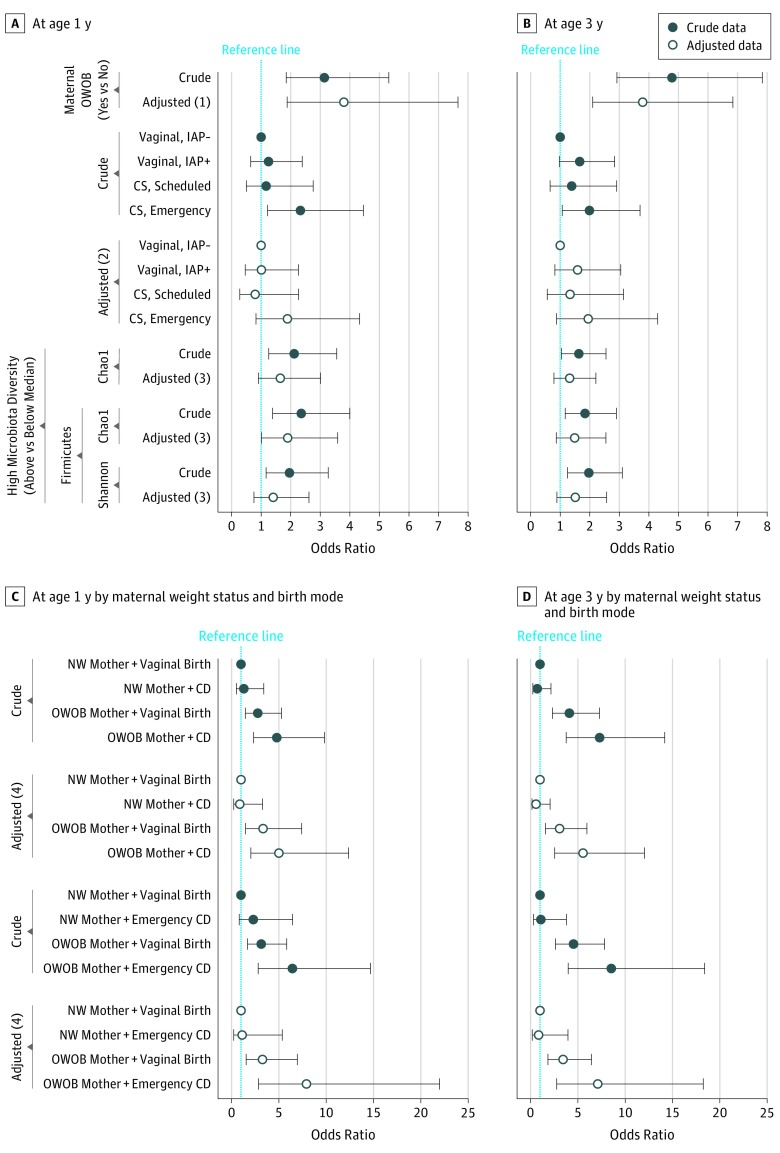
As seen in Figure 1A and B, infants born to OWOB mothers were more likely to become OWOB at ages 1 year (adjusted odds ratio [OR], 3.80; 95% CI, 1.88-7.66) and 3 years (adjusted OR, 3.79; 95% CI, 2.10-6.84). This association was also significant with the maternal BMI z score (eTable 1 in the Supplement). Compared with vaginal delivery without IAP (reference group), emergency cesarean delivery was associated with a 2-fold greater odds of childhood OWOB at ages 1 and 3 years, but not after covariate adjustment (Figure 1A and B and eTable 1 in the Supplement). Joint associations of maternal weight status and birth mode with childhood OWOB were observed after covariate adjustment (Figure 1C and D). Compared with infants delivered vaginally to normal-weight mothers without IAP (reference group), vaginally delivered infants of OWOB mothers had approximately 3 times greater odds of OWOB at ages 1 year (3.33; 95% CI, 1.49-7.41) and 3 years (3.07; 95% CI, 1.58-5.96), whereas cesarean-delivered infants of OWOB mothers had approximately 5 times greater odds of OWOB at ages 1 year (5.02; 95% CI, 2.04-12.38) and 3 years (5.55; 95% CI, 2.55-12.04). The joint association with maternal OWOB was more prominent for emergency cesarean delivery than scheduled cesarean delivery (Figure 1C and D and eTable 1 in the Supplement).
Figure 1. Forest Plots Portraying Odds Ratios and 95% CIs of Factors Associated With Childhood Overweight or Obesity (OWOB).
A, Odds ratios at age 1 year for childhood OWOB in association with maternal weight status, birth mode, and infant gut microbiota. B, Odds ratios at age 3 years for childhood OWOB in association with maternal weight status, birth mode, and infant gut microbiota. C, Joint associations at age 1 year of maternal weight status and birth mode. D, Joint associations at age 3 years of maternal weight status and birth mode. Adjusted (1): Adjusted for location, birth mode, infant sex, socioeconomic status (SES), maternal race/ethnicity, maternal prenatal asthma, maternal prenatal smoking, breastfeeding status, oral antibiotic use (0-12 months), and pet exposure. Adjusted (2): Adjusted for location, infant sex, SES, maternal weight status, maternal race/ethnicity, maternal prenatal asthma, maternal prenatal smoking, breastfeeding status, oral antibiotic use (0-12 months), and pet exposure. Adjusted (3): Adjusted for location, birth mode, infant sex, maternal weight status, maternal race/ethnicity, maternal prenatal asthma, maternal prenatal smoking, breastfeeding status, oral antibiotic use (0-12 months), pet exposure, and age at fecal sampling. Adjusted (4): Adjusted for location, infant sex, SES, maternal race/ethnicity, maternal prenatal asthma, maternal prenatal smoking, breastfeeding status, oral antibiotic use (0-12 months), and pet exposure. Error barrs indicate 95% CIs. CD indicates cesarean delivery; IAP−, no intrapartum antibiotic prophylaxis; IAP+, intrapartum antibiotic prophylaxis; NW, normal-weight; and Ref, reference. The dotted lines indicate an odds ratio of 1.
Association of Maternal OWOB With Microbiota of the Infant Gut
Microbiota β diversity differed by maternal weight status (pseudo F = 3.14; P = .001 for unweighted UniFrac; pseudo F = 2.45; P = .06 for weighted UniFrac). Infants born to OWOB mothers had greater species richness (Chao1) in their gut microbiota, especially within the Firmicutes phyla; the diversity of the Firmicutes phyla was also higher (eTable 2 in the Supplement). Associations with higher Chao1 richness were independent of birth mode (eTable 3 in the Supplement). The Proteobacterial families Enterobacteriaceae and Pasteurellaceae were less abundant in infants born to OWOB vs normal-weight mothers, whereas 4 bacterial families (Coriobacteriaceae, Erysipelotrichaceae, Lachnospiraceae, and Ruminococcaceae) belonging to Actinobacteria and Firmicutes were more abundant (eTable 2 in the Supplement). Only maternal OWOB-associated changes in the Enterobacteriaceae to Bacteroidaceae ratio remained statistically significant after covariate adjustment (eTable 4 in the Supplement). Our LEfSe analysis indicated that several genera, including those of the Lachnospiraceae family, were significantly higher in infants born to OWOB mothers (eFigure 2 in the Supplement).
Joint Associations of Maternal OWOB and Birth Mode With Microbiota of the Infant Gut
Infants of OWOB mothers born via emergency cesarean delivery were twice more likely than vaginally born infants of normal-weight mothers to have higher Firmicutes richness (eTable 5 in the Supplement). Jointly, maternal OWOB and emergency cesarean delivery were significantly associated with a higher abundance of fecal Lachnospiraceae in infants (adjusted OR, 2.02; 95% CI, 1.06-3.87) and of several other microbiota at the family level. Few family-level differences in gut microbiota were found after maternal OWOB and vaginal birth.
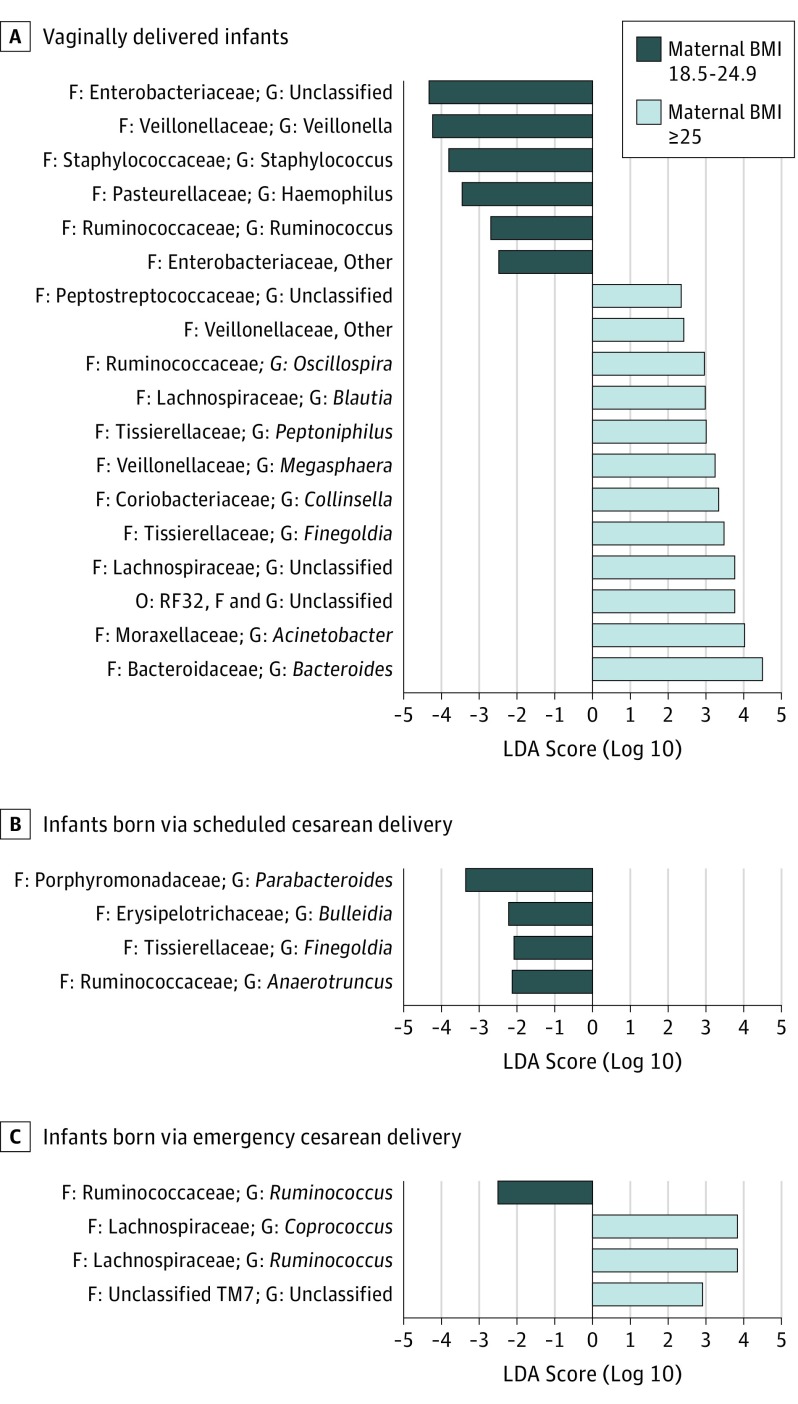
Among vaginally born infants (Figure 2), the LEfSe analysis revealed that infants born to OWOB vs normal-weight mothers had a statistically higher abundance of several genera (eg, Bacteroides, Megasphaera, Blautia, and Oscillospira) but reduced abundance of others (eg, Haemophilus and Veillonella). After emergency cesarean delivery, genera Coprococcus and Ruminococcus of the Lachnospiraceae family were more abundant in infants born to OWOB mothers than normal-weight mothers.
Figure 2. Differences in Relative Abundance of Bacterial Taxa in Microbiota of the Infant Gut Jointly Stratified by Delivery Mode and Maternal Prepregnancy Weight Status.
Linear discriminant analysis (LDA) scores provided for differential taxon abundance between normal-weight mothers (BMI, 18.5-24.9 [dark blue]) and overweight mothers (BMI, ≥25 [light blue]). A, Vaginally delivered infants. B, Infants born via scheduled cesarean delivery. C, Infants born via emergency cesarean delivery. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); F, family; G, genus; and O, order.
Sequential Mediators in the Intergenerational Transmission of OWOB
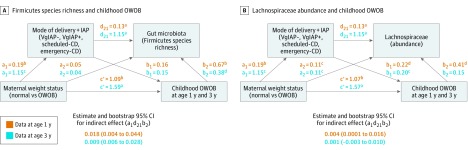
The sequential mediation model (Figure 3A) showed direct associations (path c′) of prepregnancy OWOB with childhood OWOB and indirect associations (path a1d21b2) with the sequential mediators, birth mode, and Firmicutes richness of the microbiota of the infant gut. Maternal OWOB was associated with cesarean delivery (path a1) and cesarean delivery enriched Firmicutes richness (path d21). A positive direct association of Firmicutes species richness with childhood OWOB was also observed (path b2). Similar results were found for sequential mediation by birth mode and total species richness (eFigure 3 in the Supplement). The abundance of Lachnospiraceae in microbiota of the infant gut had a direct association with child OWOB at age 1 year (Figure 3B, path b2: β = 0.41; P < .05). Sequential mediation involving birth mode and Lachnospriaceae abundance was identified in the pathway between maternal OWOB and child OWOB at age 1 year (path a1d21b2; β = 0.004; 95% CI, 0.0001-0.0156). Direct and indirect associations between fecal Lachnospiraceae and OWOB at age 3 years were borderline. Other microbiota at the family level, found to be associated with maternal OWOB or cesarean delivery, were not statistically significant mediators of the maternal and child OWOB association.
Figure 3. Sequential Mediation Models of Associations Between Maternal Weight Status, Modes of Delivery, and Microbiota of the Infant Gut.
A, Firmicutes species richness and childhood overweight or obesity (OWOB). B, Lachnospiraceae abundance and childhood OWOB. CD indicates cesarean delivery; IAP, intrapartum antibiotic prophylaxis; VgIAP–, vaginal delivery without IAP; and VgIAP+, vaginal delivery with IAP.
aP < .001.
bP < .01.
cP < .10.
dP < .05.
Microbiota Interaction Networks Associated With Child OWOB
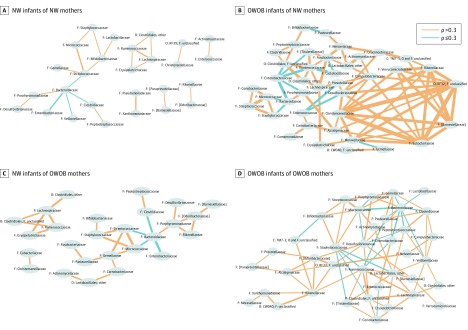
Compared with normal-weight infants, complex microbiota interaction networks involving more microbiota taxa were identified for OWOB children at ages 1 and 3 years regardless of their maternal weight status. However, among OWOB children born to OWOB mothers, the family Lachnospiraceae became more abundant with increasing levels of several other families or orders, including the Lactobacillales (lactic acid bacteria), Ruminococcaceae, and Veillonellaceae. Few of the same interactions for the family Lachnospiraceae were seen among OWOB children after a normal-weight pregnancy, and notably absent was a positive correlation with Lactobacillales (Figure 4 and eFigure 4 in the Supplement).
Figure 4. Microbiota Interaction Networks for Groups of Overweight or Obese (OWOB) Phenotype Association Between Mothers and Offspring at Age 1 Year.
A, Normal-weight (NW) infants of normal-weight mothers. B, OWOB infants of normal-weight mothers. C, Normal-weight infants of OWOB mothers. D, OWOB infants of OWOB mothers. C indicates class; F, family; and O, order. Connector line thickness represents the value of the Spearman correlation coefficient (ρ), and brackets around the family name represent the proposed taxonomy by the Greengenes database.
Discussion
In our general population birth cohort of 935 infants, those born to OWOB mothers were more likely to develop OWOB at ages 1 and 3 years, and the magnitude of the risk varied by birth mode. Compared with infants born vaginally after a normal-weight pregnancy, those born vaginally to OWOB mothers were 3.33 times more likely to become OWOB at age 1 year (95% CI, 1.49-7.41), whereas cesarean-delivered infants of OWOB mothers had a 5.02-fold risk of OWOB (95% CI, 2.04-12.38), when adjusted for other covariates. Infants born by emergency cesarean delivery to OWOB mothers were at highest risk for OWOB. Similar associations were found for OWOB at age 3 years. In keeping with the thesis of intergenerational transmission of obesogenic microbes, we found that enrichment of infant gut microbiota with the family Lachnospiraceae at ages 3 to 4 months mediated the association between maternal OWOB and child OWOB through a birth mode pathway. A mediation association for richness of total microbial species was also observed for child OWOB; this association also depended on birth mode, such that several microbial species (eg, Bacteroides) were more abundant in infants born vaginally to OWOB women. Our findings are consistent with those of other reports of overweight risk in preschool children following prepregnancy overweight and they extend our knowledge of causal pathways.
Among several emerging theories, the development of OWOB has been attributed to greater energy harvest from short-chain fatty acids produced by gut microbes when the abundance of Firmicutes exceeds that of the Bacteroidetes phylum. This thesis has found support in some, but not all, studies of obese children. We observed higher species richness and diversity within the Firmicutes phylum in infants born vaginally or by cesarean delivery to OWOB mothers, as well as evidence of mediation with this phylum. Within the Firmicutes phylum, experimental evidence is accumulating that the family Lachnospiraceae promotes adiposity, inflammation in body fat, and the development of diabetes. We found indirect or mediating associations for infant fecal abundance of Lachnospiraceae in the association between maternal OWOB and child OWOB after vaginal or cesarean delivery. Within the Lachnospiraceae, genus Blautia was elevated among infants born vaginally to OWOB mothers, whereas abundance of Coprococcus and Ruminococcus was higher after prepregnancy OWOB in cesarean-delivered infants. We also found a direct association between infant fecal abundance of Lachnospiraceae and OWOB that was potentially initiated by postnatal factors affecting levels of these microbiota.
By including birth mode in a sequential mediation model, our study was able to determine whether mediating associations of gut microbiota were influenced by the birth process. With one exception, birth mode has not been accounted for in published studies of maternal overweight and microbiota of the child or infant gut. When newborns were stratified by birth mode, Mueller et al found higher fecal levels of Bacteroides only after vaginal birth in those born to mothers with prepregnancy OWOB. That study evaluated the composition of meconium soon after birth at a time when Lachnospiraceae are much less abundant. In agreement with the premise of the study by Mueller et al, but with results on the gut microbiota at 3 to 4 months after birth, our study points to birth mode as a major pathway for the association between maternal prepregnancy and child OWOB that involves Lachnospiraceae microbiota.
In addition to enrichment with Lachnospiraceae species, our biomarker analysis tool revealed greater prominence of genera belonging to Acinetobacter, Bacteroides, Collinsella, Megasphaera, Finegoldia, Peptoniphilus, and the Peptostreptococaceae family at ages 3 to 4 months in infants born vaginally to OWOB women. Most of these genera have been linked to obesity in adults; the short-chain fatty acid metabolites they produce promote gluconeogenesis and fat storage. In the network analysis we undertook in this study, multiple correlations between the abundance of individual taxa, including Lachnospiraceae, were apparent in OWOB children. Riva et al also reported greater microbial taxon involvement with complex interaction networks in obese children. In our study, the abundance of Lachnospiraceae in OWOB children born to OWOB mothers increased with higher levels of several microbiota, notably of lactic acid bacteria, a correlation that was not seen in OWOB children born to normal-weight mothers. Hence, Lachnospiraceae microbes, which are known users of lactate, may be essential to microbial interactions that initiate the OWOB phenotype in offspring, but the metabolic pathway may differ according to maternal weight status and the presence of other gut microbiota and their metabolites, such as lactate.
Because cesarean delivery is a key determinant of early gut dysbiosis in the infant, namely, delayed colonization of Bacteroides, it is important to understand its association with risk of OWOB. Congruent with other cohorts, we found associations between maternal and child OWOB to be of greater magnitude for cesarean delivery than for vaginal delivery. As many have shown, maternal prepregnancy OWOB increased the likelihood of cesarean delivery in our study; this association was driven by emergency cesarean delivery. We observed many more perturbations to microbiota of the infant gut at the family level with maternal OWOB after cesarean delivery than after vaginal delivery; however, aside from Lachnospiraceae, these perturbations did not mediate the association between maternal and child OWOB. At the genus level, Coprococcus was associated with maternal prepregnancy OWOB in cesarean-delivered infants. A member of the Lachnospiraceae family, Coprococcus is more abundant in the gut of obese individuals, including OWOB women during early pregnancy.
Strengths and Limitations
Our study has several strengths, including the application of high-throughput deep sequencing to profile gut microbiota in a birth cohort, with a representative and large sample size. We pioneered tests of sequential mediation involving birth mode and infant gut microbial diversity. On the other hand, 16S ribosomal RNA sequencing in our study may have underrepresented organisms such as the bifidobacteria. Because the association of breastfeeding and antibiotic exposure with microbiota of the infant gut and childhood OWOB was reported previously, we adjusted for those covariates; further study is needed to examine the roles of these covariates in the sequential mediator pathway proposed in this study. The role of maternal microbiota (gut, vaginal, and skin) and of gut microbiota in older children in the intergenerational transmission of OWOB deserves further attention.
Conclusions
Both maternal weight status and cesarean delivery shape early-life gut microbial development and the weight outcome of offspring, for which a mediation role for gut microbiota has been posited. In this large prospective cohort, we found evidence of a novel sequential mediator pathway involving birth mode and Firmicutes species richness of microbiota of the infant gut, namely, higher abundance of Lachnospiraceae for the intergenerational transmission of OWOB. The mediation association was evident in vaginal and cesarean delivery, but vaginal birth yielded a lower risk for child OWOB than did cesarean delivery.
eAppendix. Detailed Methods
eFigure 1. Odds Ratios for (A) Cesarean Delivery and (B) Various Birth Modes, in Relation With Infants Born to OWOB Mothers
eFigure 2. Linear Discriminant Analysis (LDA) Scores for Differentially Abundant of Bacterial Taxa due to Maternal Weight Status (P < .05)
eFigure 3. A Sequential Mediation Model of Associations Between Maternal Weight Status, Modes of Delivery, Infant Gut Microbiota (Total Species Richness), and Childhood OWOB
eFigure 4. Microbiota Interaction Networks for Groups of OWOB Phenotype Relationship Between Mothers and Offspring at Age 3 Years
eTable 1. Odds Ratios for Childhood OWOB at Age 1 and 3 in Relation to Maternal Weight Status, Birth Modes, and Infant Gut Microbiota
eTable 2. Significant Microbiota Measurements in Infant Gut at 3-4 Months, According to Maternal Weight Status (P < .05)
eTable 3. Significant Microbiota Measurements in Infant Gut at 3-4 Months, According to Joint Effects of Maternal Prepregnancy OWOB and Emergency CS (P < .05)
eTable 4. Crude and Adjusted Likelihoods of Infant Gut Microbiota Measurements at 3-4 Months in Relation to Maternal OWOB
eTable 5. Crude and Adjusted Likelihoods of Infant Gut Microbiota Measurements at 3-4 Months in Relation to Maternal Prepregnancy OWOB and Emergency Cesarean Delivery
References
- 1.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92(5):1257-1264. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Twells LK, Newhook LA. Obesity prevalence estimates in a Canadian regional population of preschool children using variant growth references. BMC Pediatr. 2011;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491-497. [DOI] [PubMed] [Google Scholar]
- 6.Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4(12):1025-1036. [DOI] [PubMed] [Google Scholar]
- 7.Mueller NT, Mao G, Bennet WL, et al. Does vaginal delivery mitigate or strengthen the intergenerational association of overweight and obesity? findings from the Boston Birth Cohort. Int J Obes (Lond). 2017;41(4):497-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu SY, Kim SY, Schmid CH, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007;8(5):385-394. [DOI] [PubMed] [Google Scholar]
- 9.Whitaker KL, Jarvis MJ, Beeken RJ, Boniface D, Wardle J. Comparing maternal and paternal intergenerational transmission of obesity risk in a large population-based sample. Am J Clin Nutr. 2010;91(6):1560-1567. [DOI] [PubMed] [Google Scholar]
- 10.Murrin CM, Kelly GE, Tremblay RE, Kelleher CC. Body mass index and height over three generations: evidence from the Lifeways cross-generational cohort study. BMC Public Health. 2012;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes (Lond). 2013;37(7):893-899. [DOI] [PubMed] [Google Scholar]
- 12.Llewellyn CH, Trzaskowski M, Plomin R, Wardle J. Finding the missing heritability in pediatric obesity: the contribution of genome-wide complex trait analysis. Int J Obes (Lond). 2013;37(11):1506-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galley JD, Bailey M, Kamp Dush C, Schoppe-Sullivan S, Christian LM. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS One. 2014;9(11):e113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuriel-Ohayon M, Neuman H, Koren O. Microbial changes during pregnancy, birth, and infancy. Front Microbiol. 2016;7:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88(4):894-899. [DOI] [PubMed] [Google Scholar]
- 17.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070-11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718-15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozyrskyj AL, Kalu R, Koleva PT, Bridgman SL. Fetal programming of overweight through the microbiome: boys are disproportionately affected. J Dev Orig Health Dis. 2016;7(1):25-34. [DOI] [PubMed] [Google Scholar]
- 21.Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr. 2010;92(5):1023-1030. [DOI] [PubMed] [Google Scholar]
- 22.Mueller NT, Shin H, Pizoni A, et al. Birth mode–dependent association between pre-pregnancy maternal weight status and the neonatal intestinal microbiome. Sci Rep. 2016;6:23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laursen MF, Andersen LB, Michaelsen KF, et al. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. mSphere. 2016;1(1):e00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azad MB, Konya T, Maughan H, et al. ; CHILD Study Investigators . Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185(5):385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971-11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbarao P, Anand SS, Becker AB, et al. ; CHILD Study investigators . The Canadian Healthy Infant Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax. 2015;70(10):998-1000. [DOI] [PubMed] [Google Scholar]
- 27.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76-85. [DOI] [PubMed] [Google Scholar]
- 28.Tun HM, Konya T, Takaro TK, et al. ; CHILD Study Investigators . Exposure to household furry pets influences the gut microbiota of infant at 3-4 months following various birth scenarios. Microbiome. 2017;5(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azad MB, Konya T, Guttman DS, et al. ; CHILD Study Investigators . Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45(3):632-643. [DOI] [PubMed] [Google Scholar]
- 30.Mariat D, Firmesse O, Levenez F, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408-420. doi: 10.1080/03637750903310360 [DOI] [Google Scholar]
- 32.Hayes AF, Preacher KJ. Quantifying and testing indirect effects in simple mediation models when the constituent paths are nonlinear. Multivariate Behav Res. 2010;45(4):627-660. [DOI] [PubMed] [Google Scholar]
- 33.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizzi C, Cole TJ, Richiardi L, dos-Santos-Silva I, Corvalan C, De Stavola B. Prenatal influences on size, velocity and tempo of infant growth: findings from three contemporary cohorts. PLoS One. 2014;9(2):e90291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins SS, Cole TJ, Law C; Millennium Cohort Study Child Health Group . An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK Millennium Cohort Study. J Epidemiol Community Health. 2009;63(2):147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes Rev. 2017;18(1):18-31. [DOI] [PubMed] [Google Scholar]
- 38.Riva A, Borgo F, Lassandro C, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol. 2017;19(1):95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koleva PT, Bridgman SL, Kozyrskyj AL. The infant gut microbiome: evidence for obesity risk and dietary intervention. Nutrients. 2015;7(4):2237-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravussin Y, Koren O, Spor A, et al. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring). 2012;20(4):738-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kameyama K, Itoh K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014;29(4):427-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poroyko VA, Carreras A, Khalyfa A, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. 2016;6:35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanislawski MA, Dabelea D, Wagner BD, Sontag MK, Lozupone CA, Eggesbø M. Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome. 2017;5(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiu CM, Huang WC, Weng SL, et al. Systematic analysis of the association between gut flora and obesity through high-throughput sequencing and bioinformatics approaches. Biomed Res Int. 2014;2014:906168. doi: 10.1155/2014/906168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasai C, Sugimoto K, Moritani I, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588(22):4223-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azad MB, Konya T, Persaud RR, et al. ; CHILD Study Investigators . Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983-993. [DOI] [PubMed] [Google Scholar]
- 50.Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol. 2016;16(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller NT, Whyatt R, Hoepner L, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond). 2015;39(4):665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernardi JR, Pinheiro TV, Mueller NT, et al. Cesarean delivery and metabolic risk factors in young adults: a Brazilian birth cohort study. Am J Clin Nutr. 2015;102(2):295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M; SPRING Trial Group . Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes. 2016;65(8):2214-2223. [DOI] [PubMed] [Google Scholar]
- 54.Korpela K, Salonen A, Virta LJ, Kekkonen RA, de Vos WM. Association of early-life antibiotic use and protective effects of breastfeeding: role of the intestinal microbiota. JAMA Pediatr. 2016;170(8):750-757. [DOI] [PubMed] [Google Scholar]
- 55.Paolella G, Vajro P. Childhood obesity, breastfeeding, intestinal microbiota, and early exposure to antibiotics: what is the link? JAMA Pediatr. 2016;170(8):735-737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Detailed Methods
eFigure 1. Odds Ratios for (A) Cesarean Delivery and (B) Various Birth Modes, in Relation With Infants Born to OWOB Mothers
eFigure 2. Linear Discriminant Analysis (LDA) Scores for Differentially Abundant of Bacterial Taxa due to Maternal Weight Status (P < .05)
eFigure 3. A Sequential Mediation Model of Associations Between Maternal Weight Status, Modes of Delivery, Infant Gut Microbiota (Total Species Richness), and Childhood OWOB
eFigure 4. Microbiota Interaction Networks for Groups of OWOB Phenotype Relationship Between Mothers and Offspring at Age 3 Years
eTable 1. Odds Ratios for Childhood OWOB at Age 1 and 3 in Relation to Maternal Weight Status, Birth Modes, and Infant Gut Microbiota
eTable 2. Significant Microbiota Measurements in Infant Gut at 3-4 Months, According to Maternal Weight Status (P < .05)
eTable 3. Significant Microbiota Measurements in Infant Gut at 3-4 Months, According to Joint Effects of Maternal Prepregnancy OWOB and Emergency CS (P < .05)
eTable 4. Crude and Adjusted Likelihoods of Infant Gut Microbiota Measurements at 3-4 Months in Relation to Maternal OWOB
eTable 5. Crude and Adjusted Likelihoods of Infant Gut Microbiota Measurements at 3-4 Months in Relation to Maternal Prepregnancy OWOB and Emergency Cesarean Delivery