Key Points
Questions
Is the internal diameter z score associated with time-dependent coronary events in patients with Kawasaki disease with coronary artery aneurysms?
Findings
This cohort study surveyed 1006 patients with Kawasaki disease younger than 19 years who received a coronary angiography and found that the 10-year event-free survival rate for coronary events was 100%, 94%, and 52% in men and 100%, 100%, and 75% in women for small, medium, and large aneurysms, respectively. Large aneurysms, male sex, and resistance to intravenous immunoglobulin therapy were associated with coronary events.
Meaning
Careful management is essential for men and treatment-resistant patients with large coronary artery aneurysms based on the internal diameter z score.
Abstract
Importance
Few studies with sufficient statistical power have shown the association of the z score of the coronary arterial internal diameter with coronary events (CE) in patients with Kawasaki disease (KD) with coronary artery aneurysms (CAA).
Objective
To clarify the association of the z score with time-dependent CE occurrence in patients with KD with CAA.
Design, Setting, and Participants
This multicenter, collaborative retrospective cohort study of 44 participating institutions included 1006 patients with KD younger than 19 years who received a coronary angiography between 1992 and 2011.
Main Outcomes and Measures
The time-dependent occurrence of CE, including thrombosis, stenosis, obstruction, acute ischemic events, and coronary interventions, was analyzed for small (z score, <5), medium (z score, ≥5 to <10; actual internal diameter, <8 mm), and large (z score, ≥10 or ≥8 mm) CAA by the Kaplan-Meier method. The Cox proportional hazard regression model was used to identify risk factors for CE after adjusting for age, sex, size, morphology, number of CAA, resistance to initial intravenous immunoglobulin (IVIG) therapy, and antithrombotic medications.
Results
Of 1006 patients, 714 (71%) were male, 341 (34%) received a diagnosis before age 1 year, 501 (50%) received a diagnosis between age 1 and 5 years, and 157 (16%) received a diagnosis at age 5 years or older. The 10-year event-free survival rate for CE was 100%, 94%, and 52% in men (P < .001) and 100%, 100%, and 75% in women (P < .001) for small, medium, and large CAA, respectively. The CE-free rate was 100%, 96%, and 79% in patients who were not resistant to IVIG therapy (P < .001) and 100%, 96%, and 51% in patients who were resistant to IVIG therapy (P < .001), respectively. Cox regression analysis revealed that large CAA (hazard ratio, 8.9; 95% CI, 5.1–15.4), male sex (hazard ratio, 2.8; 95% CI, 1.7–4.8), and resistance to IVIG therapy (hazard ratio, 2.2; 95% CI, 1.4–3.6) were significantly associated with CE.
Conclusions and Relevance
Classification using the internal diameter z score is useful for assessing the severity of CAA in relation to the time-dependent occurrence of CE and associated factors in patients with KD. Careful management of CE is necessary for all patients with KD with CAA, especially men and IVIG-resistant patients with a large CAA.
This study demonstrates the use of the z score of the coronary arterial internal diameter for classifying the severity of coronary artery aneurysms in association with the occurrence of coronary events and major adverse cardiac events in patients with Kawasaki disease.
Introduction
In developed countries, Kawasaki disease (KD) is the most common cause of pediatric acquired heart disease with coronary artery aneurysms (CAA). Patients with KD with a large CAA often experience coronary events (CE), such as thrombosis, stenosis, and obstruction, leading to major adverse cardiac events (MACE), such as unstable angina pectoris or myocardial infarction and even death. Antithrombotic medical treatments and cardiac interventions are performed to prevent or treat these acute ischemic events.
The actual internal diameter of the CAA has been used in clinical management to predict CE,1,2 but the conventional criteria are flawed because of the lack of adjustment for body size and may lead to the underdiagnosis of the severity of CAA,3,4 especially in younger individuals. Several groups5,6,7 have proposed equations to calculate the z score of the coronary arterial internal diameter adjusted for body surface area. Thus, the current guidelines of the American Heart Association4 have adopted a new classification based on the z score; however, to our knowledge there are few studies with sufficient statistical power to provide a reliable account of the association between CE and severity classification based on the z score of the internal diameter. In a recent report, Friedman et al8 found the incidence of these events, including complete occlusion, myocardial infarction, coronary artery bypass graft, percutaneous coronary intervention, cardiac death, ventricular tachycardia, and orthotopic heart transplant, to be high and the regression rate to be low in 90 cases of large CAA as measured by the z score of the internal diameter, whereas the number of patients with MACE was comparatively small at 24. In addition, it is unclear whether the occurrence of CE is related to other factors, such as age, sex, morphology of CAA, severity in the acute phase, and medical interventions. The aim of this study was to clarify the association of the z score of the coronary arterial internal diameter with the time-dependent occurrence of CE and other related factors in patients with KD with CAA using a large-scale cohort in Japan.
Methods
Study Design
We performed a retrospective medical record review in a multicenter cohort study conducted in Japan with 44 participating institutions from July 2012 to December 2015. Patients with consecutive KD (age <19 years) who received a coronary angiography, including cardiac catheterization, coronary multidetector computed tomography, or magnetic resonance coronary angiography, between January 1992 and December 2011 were included. Patients were excluded if the coronary arterial internal diameter had not been determined by echocardiography in the acute phase or if other severe disorders affecting the coronary artery lesions or prognosis, such as familial hypercholesterolemia, cardiomyopathy, or severe congenital heart disease, were present. At each institute, patients with KD were examined and treated based on the guidelines of the Japanese Society of Cardiology1 and the Japanese Society of Pediatric Cardiology and Cardiac Surgery.9 Because this was a retrospective observational study, informed consent was waived based on the judgment of the central ethics board at Tokyo Metropolitan Children’s Medical Center.
This study was conducted in accordance with the principles of the Declaration of Helsinki and the ethical guidelines issued by the Ministry of Health, Labour, and Welfare, Japan, and was approved by a central ethics board at Tokyo Metropolitan Children’s Medical Center (approval number, H23–105). This study has been registered with the University Hospital Medical Information Network clinical trials registry (UMIN000010606).
Variables
We longitudinally studied patient data on demographics, medical interventions, coronary arterial internal diameter and morphology, cardiac interventions, and cardiac outcomes from the time of the first diagnosis to the last visit. Demographic data included sex, age at KD diagnosis, weight and height at the time of KD diagnosis, the date of the last visit, and mortality. Age at KD diagnosis was divided into 3 categories: (1) younger than 1 year, (2) 1 to 4 years, and (3) 5 years or older. Medical interventions included anti-inflammatory treatments (intravenous immunoglobulin [IVIG], prednisolone or pulsed methylprednisolone, ulinastatin, infliximab, cyclosporine, or plasma exchange) during the acute phase, antiplatelet medicines in the acute or chronic phase, and warfarin treatment during the chronic phase. We did not collect data on the dosage or use of medications. The acute phase was defined as occurring within 90 days of illness onset, and the chronic phase was defined as a period of illness lasting 91 days or longer. A patient who required any treatment following the initial IVIG treatment was considered to have been resistant to the initial IVIG treatment.
We collected data on the maximum coronary arterial internal diameter of the right coronary artery, left main coronary artery, left anterior descending artery, and left circumflex coronary artery by echocardiography during the acute phase. Echocardiographic studies were done 3 times or more within 2 weeks after diagnosis. The configuration and number of CAA were assessed by the initial coronary angiography rather than by echocardiography. Coronary artery aneurysm configuration was divided into the saccular type, in which the transverse dimension is greater than half of the longitudinal dimension, and the fusiform type, including the tubular subtype, in which the transverse dimension is half of the longitudinal dimension or less.10 If multiple CAA were present in multiple arteries, the definitions were used for assessing the largest CAA and the number in the corresponding artery.
In this study, CE was defined as the presence of coronary artery thrombosis, stenosis of 75% or greater according to the American Heart Association classification, obstruction, acute ischemic events, or coronary interventions, while MACE was defined as acute ischemic events or coronary interventions. A coronary thrombosis was diagnosed by echocardiography or coronary angiography results, and stenosis and obstruction were diagnosed on the basis of the results of each coronary angiography in the follow-up interval. Acute ischemic events included unstable angina pectoris, myocardial infarction, and cardiac-related death. Coronary interventions included percutaneous transluminal coronary recanalization for coronary thrombosis, percutaneous coronary intervention (balloon dilation, stent implantation, or rotablator), and coronary artery bypass grafting. Coronary artery aneurysms were defined in accordance with the report of the Japanese Ministry of Health and Welfare11 as having an actual internal diameter of 3 mm or more in a child younger than 5 years or 4 mm or more in a child 5 years or older, with the internal diameter of the segment being at least 1.5 times greater than that of an adjacent segment, or the luminal contour being clearly irregular.
Severity Classifications of CAA
The maximum coronary arterial internal diameter in each artery obtained by echocardiography, but not by coronary angiography, in the acute phase was converted to a z score using a model derived by the lambda-mu-sigma method7 based on data from healthy Japanese children. For the severity classification of CAA, 3 groups were formed based on 5 increments in the z score of the CAA internal diameter for the following parameters3,4: small, z score of less than 5; medium, z score of 5 to 10 and an actual internal diameter of less than 8 mm; and large, z score of 10 or more or an actual internal diameter of 8 or more mm. Patients with an unavailable z score and an actual diameter of less than 8 mm were defined as unclassifiable. Furthermore, we evaluated the severity classification based on less than 4 mm, 4 to 6 mm, 6 to 8 mm, and 8 or more mm of the actual internal diameter by reference to the guidelines1 of the Japanese Society of Cardiology.
Statistical Analysis
Baseline characteristics were described for all of the participants using the severity classification by median and interquartile range (IQR) for continuous variables and frequency and proportion for categorical variables. For all participants, the cumulative proportion of CE was estimated by the Kaplan-Meier method for severity classification, and comparisons were made with the log-rank test. The Cox proportional hazards regression model was used to identify factors associated with CE by calculating the hazard ratios (HRs) with a 95% confidence interval while simultaneously controlling for confounding variables, including age, sex, size, morphology, and number of CAA, and resistance to initial IVIG in the acute phase with or without use of warfarin. We also performed a similar analysis for the occurrence of MACE. A 2-sided P < .05 was considered statistically significant. All statistical analyses were carried out using SPSS, version 23.0 (IBM Corp).
Results
Patient Characteristics and Kinds of Treatments
During the study period, 1033 patients at 44 institutions received a coronary angiography. Of these patients, 27 were excluded because the echocardiographic measurements of the coronary arterial internal diameter in the acute phase were unavailable. There were no patients with other exclusion criteria, including severe disorders affecting the coronary artery lesions or prognosis. We thus enrolled 1006 patients (714 [71%] male and 292 [29%] female) in this study. The median age at KD diagnosis was 1.8 years (range, 0.07-15.7 years; IQR, 0.6-3.8 years) with a 6.4-year, median follow-up period (range, 0.04-22.5 years; IQR, 2.8-11.1 years). The first coronary angiography was performed via cardiac catheterization for all patients except 3, for whom enhanced computed tomography was performed. The patients underwent these procedures at 0.0 to 14.7 years after KD onset (median, 0.4 years; IQR, 0.2-1.4 years), and 799 patients (79%) underwent their first coronary angiography within 2 years of KD onset.
For the initial treatment, IVIG was administered to 882 of 1006 patients (88%); of these, 42 (5%) received a steroid treatment, 36 (4%) a protease inhibitor (ulinastatin or gabexate mesilate), and 3 (0.3%) infliximab in combination with IVIG. We did not obtain data on the timing of the initial IVIG administration. In total, 510 patients (58%) received additional treatment; IVIG was administered to 442 patients (50%), steroids to 209 (24%), a protease inhibitor to 112 (13%), cyclosporine to 17 (2%), infliximab to 25 (3%), and plasma exchange to 16 (2%).
Table 1 shows the patient characteristics, including the configuration and number of CAA, resistance to initial IVIG treatment, warfarin use, and the number of patients with CE and MACE by small, medium, and large CAA sizes based on the largest z score among all arteries. Coronary events and MACE did not occur in patients with small CAA, but were observed in 20 (5%) and 8 (2%) of the patients with medium CAA, and in 82 (35%) and 45 (19%) of the patients with large CAA, respectively.
Table 1. Patient Characteristics According to Classification of Coronary Artery Aneurysms.
| No. (%) | |||||
|---|---|---|---|---|---|
| Total | Small | Medium | Large | Unclassifiablea | |
| Total No. of patients | 1006 | 134 | 425 | 273 | 174 |
| Male | 714 (71) | 102 (76) | 312 (73) | 174 (64) | 126 (72) |
| Age at diagnosis, y | |||||
| <1 | 341 (34) | 21 (16) | 149 (35) | 101 (37) | 70 (47) |
| ≥1-<5 | 501 (50) | 78 (58) | 225 (53) | 118 (44) | 80 (41) |
| ≥5 | 157 (16) | 35 (26) | 51 (12) | 52 (19) | 19 (11) |
| Property of CAA | |||||
| Configuration | |||||
| Regression | 630 (63) | 109 (81) | 273 (65) | 88 (33) | 160 (94) |
| Dilation | 94 (9) | 17 (13) | 49 (12) | 23 (9) | 5 (3) |
| Fusiform | 126 (13) | 0 (0) | 43 (10) | 77 (29) | 6 (4) |
| Saccular | 148 (15) | 8 (6) | 58 (14) | 82 (30) | 0 (0) |
| No. | |||||
| Single | 231 (23) | 19 (14) | 99 (23) | 79 (29) | 34 (20) |
| Multiple | 243 (24) | 13 (10) | 86 (20) | 127 (47) | 17 (10) |
| Treatment | |||||
| Resistant to initial IVIG | 510 (51) | 58 (44) | 214 (51) | 177 (65) | 61 (37) |
| Use of warfarin | 219 (22) | 1 (1) | 58 (14) | 149 (55) | 11 (6) |
| Coronary imaging findings | |||||
| Thrombosis | 47 (5) | 0 (0) | 3 (1) | 42 (15) | 2 (1) |
| Stenosis | 92 (9) | 0 (0) | 13 (3) | 64 (23) | 15 (9) |
| Obstruction | 49 (5) | 0 (0) | 3 (1) | 42 (16) | 4 (2) |
| Ischemic heart diseases | |||||
| Unstable angina | 14 (1) | 0 (0) | 1 (0.2) | 11 (4) | 2 (1) |
| Myocardial infarction | 21 (2) | 0 (0) | 1 (0.2) | 19 (7) | 1 (1) |
| Cardiac death | 5 (0.5) | 0 (0) | 0 (0) | 5 (2) | 0 (0) |
| Coronary interventions | |||||
| PCI | 23 (2) | 0 (0) | 3 (1) | 18 (7) | 2 (1) |
| PTCR | 28 (3) | 0 (0) | 2 (0.5) | 25 (9) | 1 (1) |
| CABG | 20 (2) | 0 (0) | 1 (0.2) | 18 (7) | 1 (1) |
| End points | |||||
| Coronary eventsb | 127 (13) | 0 (0) | 17 (4) | 93 (34) | 17 (10) |
| Major adverse cardiac eventsc | 59 (6) | 0 (0) | 7 (2) | 50 (18) | 2 (1) |
Abbreviations: CAA, coronary artery aneurysm; CABG, coronary artery bypass grafting; IVIG, intravenous immunoglobulin; PCI, percutaneous coronary intervention; PTCR, percutaneous transluminal coronary recanalization.
Patients with unavailable z score of the coronary arterial internal diameter and the actual diameter of less than 8 mm were defined as unclassifiable.
Coronary events include coronary imaging findings, ischemic heart diseases, and coronary interventions.
Major cardiac adverse events include ischemic heart diseases and coronary interventions.
Time-Dependent Occurrence of CE and MACE
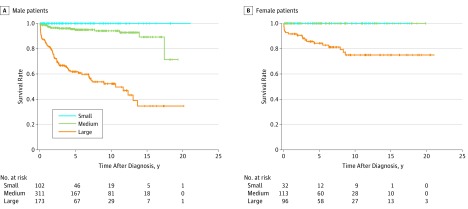
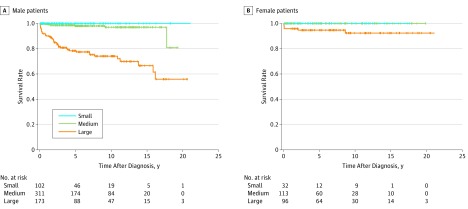
In total, the 10-year event-free survival rate was 100%, 96%, and 61% for CE (P < .001) and 100%, 98%, and 81% for MACE (P < .001) for small, medium, and large CAA, respectively. Figure 1 shows the Kaplan-Meier curves for severity classification based on the z score of the CAA internal diameter in relation to CE in male and female patients. The 10-year event-free survival rate for CE was 100%, 94%, and 52% in male (P < .001), and 100%, 100%, and 75% in female patients (P < .001), respectively. Figure 2 shows the Kaplan-Meier curves for MACE. The 10-year event-free survival rate for MACE was 100%, 97%, and 74% in male (P < .001), and 100%, 100%, and 92% in female patients (P < .001), respectively. Similar results showing sex differences were found using the severity classification in the actual internal diameter (eFigures 1 and 2 in the Supplement).
Figure 1. Kaplan-Meier Survival Curves for Coronary Events in the Classification by the Internal Diameter z Score of Coronary Artery Aneurysms in Male and Female Patients.
A, Coronary event–free survival rates for male patients. B, Coronary event–free survival rates for female patients. The survival curves were significantly different among the 3 groups (P < .001, log rank test of equality). Small, internal diameter z score of coronary artery aneurysms of less than 5; medium, z score of 5 to 10 and actual internal diameter of less than 8 mm; and large, z score of 10 or more or actual internal diameter of 8 mm or more.
Figure 2. Kaplan-Meier Survival Curves for Major Adverse Cardiac Events in the Classification by the Internal Diameter z Score of Coronary Artery Aneurysms in Male and Female Patients.
A, Major adverse cardiac event–free survival rates for male patients. B, Major adverse cardiac event–free survival rates for female patients. The survival curves were significantly different among the 3 groups (P < .001, log rank test of equality). Small, internal diameter z score of coronary artery aneurysms of less than 5; medium, z score of 5 to 10 and actual internal diameter of less than 8 mm; and large, z score of 10 or more or actual internal diameter of 8 mm or more.
Furthermore, the rate of CE was 100%, 96%, and 79% (P < .001) in patients who were not resistant to the initial IVIG treatment (P < .001) and 100%, 96%, and 51% in patients who were resistant to the initial IVIG treatment (P < .001) for small, medium, and large CAA, respectively; the rate of MACE was 100%, 97%, and 90% (P = .01) in patients who were not resistant to the initial IVIG therapy and 100%, 98%, and 76% (P < .001) in patients who were resistant to the initial IVIG therapy, respectively.
Risk Factors Associated With CE and MACE
We compared the risk of CE and MACE using the Cox proportional hazards regression model for the medium and large CAA groups because there were no events in the small CAA group. Table 2 shows the unadjusted and adjusted risk estimates for CE and MACE. In the unadjusted analysis, the statistically significant associated factors were large CAA, male sex, saccular segment morphology, multiple CAA, and resistance to initial IVIG treatment for both CE and MACE in addition to an age at diagnosis of 5 years or older for MACE. A multivariable analysis revealed that large CAA, male sex, and resistance to initial IVIG were significantly associated with an increased risk of both CE and MACE in addition to an age at diagnosis of 5 years or younger for MACE.
Table 2. Cox Regression Analysis for Coronary Events and Major Adverse Cardiac Events.
| Event | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Coronary Events | ||||
| Large CAA | 9.3 (5.4-15.9) | <.001 | 8.9 (5.1-15.4) | <.001 |
| Male | 2.4 (1.4-4.0) | .001 | 2.8 (1.7-4.8) | <.001 |
| Age at diagnosis vs ≥1-<5 y | ||||
| <1 | 0.8 (0.5-1.3) | .47 | 0.8 (0.5-1.3) | .45 |
| ≥5 | 1.4 (0.9-2.4) | .16 | 1.1 (0.7-1.8) | .72 |
| Saccular CAA | 2.0 (1.3-2.9) | .001 | 1.1 (0.7-1.7) | .61 |
| Multiple CAA | 2.6 (1.7-3.8) | <.001 | 1.2 (0.8-1.8) | .39 |
| Resistant to initial IVIG | 3.0 (1.9-4.9) | <.001 | 2.2 (1.4-3.6) | .001 |
| Major Adverse Cardiac Events | ||||
| Large CAA | 12.3 (5.3-28.9) | <.001 | 11.5 (4.8-27.6) | <.001 |
| Male | 4.6 (1.8-11.6) | .001 | 5.2 (2.0-13.1) | .001 |
| Age at diagnosis vs ≥1-<5 y | ||||
| <1 | 1.3 (0.7-2.5) | .38 | 1.5 (0.8-2.9) | .21 |
| ≥5 | 2.6 (1.3-5.0) | .01 | 2.3 (1.2-4.6) | .02 |
| Saccular CAA | 2.2 (1.3-3.9) | .003 | 1.3 (0.7-2.2) | .41 |
| Multiple CAA | 2.5 (1.5-4.4) | .001 | 1.0 (0.6-1.8) | .92 |
| Resistant to initial IVIG | 4.0 (2.0-8.0) | <.001 | 3.1 (1.5-6.3) | .002 |
Abbreviations: CAA, coronary artery aneurysm; IVIG, intravenous immunoglobulin.
When the use of warfarin was included in the explanatory variables, it was significantly associated with CE (hazard ratio [HR], 5.4; 95% CI, 3.6-8.2; P < .001) and MACE (HR, 8.1; 95% CI, 4.3-15.1; P < .001). However, the association with warfarin use was not significant if the events were confined to the thrombotic variety, including thrombosis, obstruction, percutaneous transluminal coronary recanalization, myocardial infarction, and cardiac-related death (HR, 1.4; 95% CI, 0.8-2.5; P = .32). Bilateral CAA was not significantly associated with CE or MACE in a multivariable Cox regression analysis (CE: HR, 1.9; 95% CI, 0.8-4.8; P = .18; MACE: HR, 1.6; 95% CI, 0.5-5.2; P = .47).
Discussion
This study demonstrated the use of the z score of the coronary arterial internal diameter for classifying the severity of CAA in relation to the time-dependent occurrence of CE and MACE using a large-scale cohort of more than 1000 patients with KD with CAA. While patients with KD with a large CAA had a higher risk of CE and MACE, those with a small CAA experienced no such events. It should be stressed that male sex and resistance to initial IVIG were associated with a higher occurrence of CE and MACE.
The event-free survival rate for CE and MACE was lowest for patients with a large CAA as measured by the z score of the internal diameter. Luminal myofibroblastic proliferation, as well as the layering of the mural thrombus, can reduce the internal lumen of a large CAA.12 Together with reduced shear stress and a disturbed flow pattern,13 it can result in progressive coronary stenosis or even complete occlusion. On the other hand, the prognosis of patients with a small CAA may be good because the event-free survival rate was 100% for CE and MACE. However, longer-term follow-up studies are needed to define the risk of later complications.
Male sex constituted a higher risk for CE and MACE among patients with KD, suggesting that sex interacted qualitatively with the z score of the coronary arterial internal diameter. The event-free survival rate was lower among male patients and higher among female patients for medium to large CAA; notably, female patients with a medium CAA had fewer risks. We speculate that damage to the vascular wall of the coronary artery caused by inflammation in KD may be more severe in male than female patients, with resulting differences in the incidence of CE. Alternatively, the sex differences in coronary heart disease among adults could explain the sex differences seen in KD, although the contributing factors are complex and may include the role of estrogen, lifestyle, and behavior patterns.14,15 Our findings are supported by another report16 that demonstrated that male patients, but not female patients, with cardiac sequelae due to KD had a higher mortality rate than the general population. That is to say, male patients with KD were at risk of contracting KD and developing CAA1,17 and generally had a poorer prognosis compared with female patients.
Intravenous immunoglobulin resistance was also found to be significantly associated with both CE and MACE. We suspect that the damage to the vascular wall due to inflammation may be more severe in IVIG-resistant patients than in IVIG-nonresistant patients; consequently, more treatment-resistant patients experienced CE and MACE. Friedman et al8 suggested that early IVIG treatment and anti-inflammatory medications as adjuncts to IVIG within 10 days of fever onset may improve the outcome of CAA. We agree with these findings; however, we were unable to demonstrate whether the dose and timing of IVIG administration and the kinds of additional treatment affected the outcomes in our study due to confounding factors and the paucity of data.
Limitations
Our study has some limitations, mostly due to its retrospective design. First, because the timing of the angiography was not standardized across participants, data pertaining to the CAA, such as their configuration and number, may not have accurately reflected the patients’ conditions when an echocardiogram was used to measure the internal diameter. Second, patients who died before receiving an angiography were not included in this study; hence, the incidence of MACE might have been underestimated, especially in those with a large CAA. Third, the treatment protocol for the short-term and long-term cases varied at each institution. Because 2 g/kg of IVIG is not given to all patients with KD in Japan and because data on the timing of administration were not available in this study, the effect of resistance to IVIG may not have been correctly evaluated. Fourth, our findings, derived from Japanese participants, may not be applicable to other racial/ethnic groups.
Conclusions
Assessing CAA severity using the z score of the internal diameter in patients with KD was significantly related to the time-dependent occurrence of CE and MACE. Male sex and resistance to initial IVIG were significantly associated with CE and MACE. We expect that assessing the z score of the CAA internal diameter will be useful not only for preventing acute ischemic events, but also for planning new international randomized clinical trials in the future because it allows meaningful comparisons to be made among different racial/ethnic groups.18 Furthermore, other factors, including sex and IVIG resistance, should be considered in the clinical management of CAA.
eFigure 1. Kaplan-Meier survival curves for coronary events classified by the actual internal diameter of coronary artery aneurysms in male and female patients
eFigure 2. Kaplan-Meier survival curves for major severe cardiac events classified by the actual internal diameter of coronary artery aneurysms in male and female patients
References
- 1.Ogawa S, Ayusawa M, Fukazawa R, et al. ; JCS Joint Working Group . Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013). Digest version. Circ J. 2014;78(10):-. [DOI] [PubMed] [Google Scholar]
- 2.Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Child. 2014;99(1):74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manlhiot C, Millar K, Golding F, McCrindle BW. Improved classification of coronary artery abnormalities based only on coronary artery z-scores after Kawasaki disease. Pediatr Cardiol. 2010;31(2):242-249. [DOI] [PubMed] [Google Scholar]
- 4.McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927-e999. [DOI] [PubMed] [Google Scholar]
- 5.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133(2):254-258. [DOI] [PubMed] [Google Scholar]
- 6.McCrindle BW, Li JS, Minich LL, et al. ; Pediatric Heart Network Investigators . Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116(2):174-179. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Fuse S, Sakamoto N, et al. ; Z score Project Investigators . A new Z-Score curve of the coronary arterial internal diameter using the lambda-mu-sigma method in a pediatric population. J Am Soc Echocardiogr. 2016;29(8):794-801.e29. [DOI] [PubMed] [Google Scholar]
- 8.Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. . Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saji T, Ayusawa M, Miura M, et al. ; Research Committee of the Japanese Society of Pediatric Cardiology; Cardiac Surgery Committee for Development of Guidelines for Medical Treatment of Acute Kawasaki Disease . Guidelines for medical treatment of acute Kawasaki disease: report of the Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery (2012 revised version). Pediatr Int. 2014;56(2):135-158. [DOI] [PubMed] [Google Scholar]
- 10.Onouchi Z, Shimazu S, Kiyosawa N, Takamatsu T, Hamaoka K. Aneurysms of the coronary arteries in Kawasaki disease. An angiographic study of 30 cases. Circulation. 1982;66(1):6-13. [DOI] [PubMed] [Google Scholar]
- 11.Research Committee on Kawasaki Disease Report of Subcommittee on Standardization of Diagnostic Criteria and Reporting of Coronary Artery Lesions in Kawasaki Disease. Tokyo, Japan: Ministry of Health and Welfare; 1984. (in Japanese) [Google Scholar]
- 12.Orenstein JM, Shulman ST, Fox LM, et al. . Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS One. 2012;7(6):e38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkubo T, Fukazawa R, Ikegami E, Ogawa S. Reduced shear stress and disturbed flow may lead to coronary aneurysm and thrombus formations. Pediatr Int. 2007;49(1):1-7. [DOI] [PubMed] [Google Scholar]
- 14.Barrett-Connor E. Sex differences in coronary heart disease. why are women so superior? the 1995 Ancel Keys Lecture. Circulation. 1997;95(1):252-264. [DOI] [PubMed] [Google Scholar]
- 15.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura Y, Aso E, Yashiro M, et al. . Mortality among Japanese with a history of Kawasaki disease: results at the end of 2009. J Epidemiol. 2013;23(6):429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin MT, Sun LC, Wu ET, Wang JK, Lue HC, Wu MH. Acute and late coronary outcomes in 1073 patients with Kawasaki disease with and without intravenous γ-immunoglobulin therapy. Arch Dis Child. 2015;100(6):542-547. [DOI] [PubMed] [Google Scholar]
- 18.Ogata S, Tremoulet AH, Sato Y, et al. . Coronary artery outcomes among children with Kawasaki disease in the United States and Japan. Int J Cardiol. 2013;168(4):3825-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Kaplan-Meier survival curves for coronary events classified by the actual internal diameter of coronary artery aneurysms in male and female patients
eFigure 2. Kaplan-Meier survival curves for major severe cardiac events classified by the actual internal diameter of coronary artery aneurysms in male and female patients