This case-series study examines the course of appearance of cardiac abnormalities in patients with Hutchinson-Gilford progeria syndrome to identify meaningful cardiac end points to use in future clinical trials.
Key Points
Question
What is the natural history of cardiac disease in children with Hutchinson-Gilford progeria syndrome, an ultrarare premature aging disorder?
Findings
In this case-series study of 27 patients with Hutchinson-Gilford progeria syndrome, diastolic dysfunction was the most prevalent cardiac abnormality, appearing early in life with age-related decline, a finding mirroring those seen with normal aging. Other abnormalities, such as valve dysfunction and left ventricular hypertrophy, were less prevalent and appeared during the second decade of life.
Meaning
Diastolic dysfunction was the most prevalent cardiac abnormality in patients with Hutchinson-Gilford progeria syndrome and may be a potential end point in future clinical trials.
Abstract
Importance
Hutchinson-Gilford progeria syndrome (HGPS) is an ultrarare disorder associated with premature death due to cardiovascular events during the second decade of life. However, because of its rarity (107 identified living patients), the natural history of cardiac disease remains uncharacterized. Therefore, meaningful cardiac end points for clinical trials have been difficult to establish.
Objective
To examine the course of appearance of cardiac abnormalities in patients with HGPS to identify meaningful cardiac end points for use in future clinical trials.
Design, Setting, and Participants
In this prospective, cross-sectional, observational study, 27 consecutive patients with clinically and genetically confirmed classic HGPS were evaluated at a single center for 1 visit from July 1, 2014, through February 29, 2016, before initiation of treatment.
Exposure
Classic HGPS.
Main Outcomes and Measures
Echocardiography was used to assess ventricular and valve function using standard techniques. Diastolic left ventricular (LV) function was assessed using tissue Doppler imaging. Previously published normative data were used to adjust findings to age and body size.
Results
This study included 27 patients (median age, 5.6 years; age range, 2-17 years; 15 [56%] male). Among echocardiographic indicators, LV diastolic dysfunction, defined as a tissue Doppler septal or lateral early velocity z score less than −2, was the most prevalent abnormality, seen in 16 patients (59%). Diastolic dysfunction was seen in all age groups, and its prevalence increased with age, mirroring findings seen during normal aging. Indicators of LV diastolic function were more abnormal in older patients. The z scores for lateral and septal early velocities were lower (r = −0.77, P < .001; and r = −0.66, P < .001, respectively), whereas those for the ratio of early mitral inflow velocity to early diastolic tissue Doppler myocardial velocity were higher (r = 0.80, P < .001; and r = 0.72, P < .001, respectively) in older patients. Other echocardiographic findings, including LV hypertrophy, LV systolic dysfunction, and valve disease, were less prevalent in the first decade and were seen more frequently in the second decade.
Conclusions and Relevance
In this largest-to-date cohort of patients with HGPS, LV diastolic dysfunction was the most prevalent echocardiographic abnormality and its prevalence increased with aging. Echocardiographic indicators of LV diastolic function may be useful end points in future clinical trials in this patient population.
Introduction
Hutchinson-Gilford progeria syndrome (HGPS) is an ultrarare, sporadic, autosomal dominant, segmental, premature aging disease with a prevalence of 1 in 20 million. The disorder occurs because of aberrant splicing of the LMNA gene (OMIM 150330) resulting in accumulation of a permanently farnesylated, uncleaved lamin A isoform called progerin. Accumulation of progerin causes abnormalities in nuclear morphologic features and function as well as cellular stiffening. Clinical manifestations include extreme short stature, low body weight, total alopecia, lipodystrophy, sclerodermoid skin, decreased joint mobility, skeletal dysplasia, and strokes. Premature death usually occurs in the second decade of life after premature atherosclerosis leads to myocardial infarction. Cardiovascular findings at autopsy include loss of vascular smooth muscle cells in large and small arteries with replacement by fibrous tissue, thickening and calcification of the aortic and mitral valves, and interstitial myocardial fibrosis and infarction. However, because of the rarity of the condition (107 known living patients worldwide), limited information is available regarding the natural history of cardiovascular abnormalities. Therefore, meaningful cardiac end points for clinical trials have been difficult to establish. Echocardiography is a widely available noninvasive technique that can accurately identify anatomical and functional cardiac abnormalities in children. We performed a cross-sectional study of the largest-to-date cohort of children with HGPS to examine the prevalence and natural history of cardiac abnormalities to identify indicators that may serve as end points in future clinical trials.
Methods
Patients
Patients with clinically and genetically confirmed c.1824 C>T, p. Gly608Gly classic HGPS who enrolled in a clinical trial at Boston Children’s Hospital were evaluated prospectively at a single visit from July 1, 2014, through February 29, 2016. All reported data were obtained at the baseline visit, before initiation of treatment with the protein farnesylation inhibitor lonafarnib. All patients had not previously received treatment at evaluation. Patients with nonclassic forms of progeria were excluded. Written informed consent was obtained from parents, and all data were deidentified. The study was approved by Boston Children’s Hospital’s Committee on Clinical Investigation.
Echocardiography
Transthoracic echocardiography was performed using commercial scanners (iE33, Philips Healthcare) and standard imaging techniques recommended by the American Society of Echocardiography. Left ventricular (LV) volumes and mass were quantified using the area-length method as recommended by the American Society of Echocardiography. Body surface area (BSA) was calculated using the Haycock formula, and LV volume and mass were adjusted to the BSA by calculating z scores using previously published normative data from the echocardiography laboratory at Boston Children’s Hospital.
Diastolic Function
Mitral valve inflow velocity during early (E) and late (A) diastole were measured using pulsed wave Doppler imaging, and the E/A velocity ratio was calculated. Pulsed wave tissue Doppler evaluation of LV diastolic function was performed by measuring the lateral and septal early (E′) and late (A′) diastolic myocardial velocities. The E:E′ and E′:A′ velocity ratios were calculated using standard guidelines and were adjusted for age by using published normative data from our echocardiography laboratory to calculate z scores for each value. Patients with a septal or lateral E′ velocity z score less than −2 were classified as having LV diastolic dysfunction.
Valve Abnormalities
The mitral valve and aortic valves were assessed from apical 4-chamber and parasternal long- and short-axis views using standard techniques. A qualitative assessment for valve calcification was performed based on increased echogenicity visible from multiple imaging planes. Mitral and aortic regurgitation were graded semiquantitatively as mild, moderate, or severe, as previously described. Patients with a mitral valve mean inflow pressure gradient greater than 3 mm Hg measured using continuous wave Doppler imaging were classified as having mitral stenosis. Aortic stenosis was defined as a maximum instantaneous pressure gradient greater than 15 mm Hg measured using continuous wave Doppler imaging.
Electrocardiography
Twelve-lead electrocardiography was performed using a standard technique. Heart rate z scores were calculated using normative data from our laboratory. Electrocardiographic intervals were corrected for heart rate using the Bazett formula.
Blood Pressure and Carotid-Femoral Pulse Wave Velocity Measurement
Blood pressure (BP) was recorded in both arms after 5 minutes of rest with the patient seated with feet flat on the floor using a standard auscultation technique and size-appropriate cuffs. The BP measurements were taken twice in the arm, with the higher initial systolic BP reading and the mean of the 3 measurements in this arm used for final analysis. To account for the severe growth restriction in this patient population, height-age was substituted for chronologic age while calculating age- and sex-specific BP percentiles. As previously described, height-age was determined by calculating the median age in the general population of a child with the height of each patient with HGPS using the US Centers for Disease Control and Prevention sex-specific pediatric growth charts (http://www.cdc.gov/growthcharts/cdc_charts.htm). Similar to a prior report of patients with HGPS, for height-age calculations, segmental whole-body length was used instead of standing height, because joint contractures inherent to HGPS can result in an underestimation of true height. Patients who satisfied standard pediatric criteria (for patients <18 years of age, systolic and/or diastolic BP≥95th percentile for age, sex, and height-age) were classified as being hypertensive. Carotid-femoral pulse wave velocity (CFPWV) was determined using a previously described ultrasound technique.
Statistical Analysis
The statistical significance of differences between continuously distributed indicators was assessed using the Wilcoxon rank sum test. Associations between outcome and indicator variables were examined using simple linear regression and multivariable linear regression to adjust for potential confounders. The statistical significance level was set at P < .05 (2-sided). Statistical analysis was performed using SAS software, version 9.4 or higher (SAS Institute Inc).
Results
Study Patients
Characteristics of the 27 study patients (median age, 5.6 years; age range, 2-17 years; 15 [56%] male) are given in Table 1. Patients were evaluated before entry into an open-label drug trial of protein farnesylation inhibitors. Of 29 screened patients, 2 were excluded: one was ineligible for therapy because of liver dysfunction, and the other was excluded because of technical reasons. As previously reported, patients demonstrated severe growth failure with severe reduction in weight-for-age, height-for-age, and weight-for-height z scores and a reduced body mass index (BMI), findings suggesting that their body composition included a higher proportion of lean body mass compared with healthy individuals. Systemic hypertension was common, as previously reported. With use of height-age to calculate BP percentiles, 13 of 26 patients (50%) were hypertensive.
Table 1. Characteristics of the Study Patients.
| Characteristic | Findinga |
|---|---|
| Age, y | 5.6 (2.1 to 17.5) |
| Male, No. (%) | 15 (56) |
| Weight, kg | 10.9 (7.2 to 22.7) |
| Weight-for-age z score | −7.8 (−43.7 to −1.7) |
| Height, cm | 93.6 (70.6 to 133) |
| Height-for-age z score | −4.7 (−9.3 to −1.5) |
| BSA, m2 | 0.5 (0.4 to 0.9) |
| BMI | 12.8 (9.4 to 18.7) |
| Weight-for-height z score | −4 (−18.7 to 1) |
| Ideal body weight, kg | 14 (8.6 to 21.2) |
| BSA using ideal body weight, m2 | 0.6 (0.4 to 0.9) |
| Heart rate, /min | 106 (85 to 174) |
| SBP, mm Hg | 101 (62 to 124) |
| SBP percentileb | 90.5 (14.9 to 99.9) |
| DBP, mm Hg | 66 (43 to 85) |
| DBP percentileb | 88.5 (8 to 99.8) |
| Hypertension, No. (%) (n = 26)c | 13 (50) |
| Continent of origin, No. (%) (n = 27) | |
| Asia | 10 (37) |
| South America | 7 (26) |
| North America | 6 (22) |
| Africa | 2 (7) |
| Europe | 1 (4) |
| Australia | 1 (4) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; BSA, body surface area; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Data are presented as median (range) unless otherwise indicated.
The SBP and DBP percentiles were calculated using height-age instead of chronological age as previously described.
Accurate data were not available for 1 of the patients.
Metabolic profiles were similar to those in a prior report. As seen in the eTable in the Supplement, 13 patients (48%) had hyperinsulinemia, and 9 (33%) were insulin resistant. Lipid abnormalities were notable for abnormally low high-density lipoprotein cholesterol level in 17 (63%).
LV Size, Systolic Function, and Mass
Echocardiographic indicators of LV size, function, and mass are summarized in Table 2. Compared with normative data from our laboratory, z scores for LV end-diastolic volume, end-systolic volume, mass, mass to volume ratio, and ejection fraction were elevated. Because of the unusual body habitus, characterized by low BMI and weight-for-height z scores, we repeated these analyses using ideal body weight instead of actual body weight to calculate the BSA. We found that these adjusted z scores for LV size and mass were closer to normal values. Systolic dysfunction, defined as an LV ejection fraction less than 55%, was not seen in any patient. Left ventricular hypertrophy (LVH), defined as both LV mass and LV mass-to-volume z scores greater than 2, was present in 7 patients (25%). The presence of LVH was associated with older age at evaluation but not with sex, systemic hypertension, or CFPWV.
Table 2. Left Ventricular Size, Systolic Function, and Massa.
| Variable | Median (Range) | P Value vs Normative Data |
|---|---|---|
| EDV/BSA ratio | 63.4 (43.6 to 79.8) | NA |
| EDV z score | 0.6 (−1.1 to 2.5) | .009 |
| EDV z score using ideal body weight | −0.5 (−2.5 to 2.7) | .04 |
| ESV:BSA ratio | 19.2 (13.8 to 28.5) | NA |
| ESV z score | 0.5 (−2.1 to 2.3) | .02 |
| ESV z score using ideal body weight | −0.4 (−1.9 to 2.1) | .20 |
| Ejection fraction, % | 0.7 (0.6 to 0.8) | NA |
| Mass:BSA ratio | 54.9 (40.6 to 98.9) | NA |
| Mass z score | 0.8 (−0.5 to 3.6) | <.001 |
| Mass z score using ideal body weight | −0.2 (−1.2 to 2.6) | .57 |
| Mass to volume ratio | 0.9 (0.7 to 1.6) | NA |
Abbreviations: BSA, body surface area; EDV, end-diastolic volume; ESV, end-systolic volume; NA, not applicable.
The z scores were calculated using previously published normative data from the echocardiography laboratory at Boston Children’s Hospital. The P values were calculated by comparing median z scores against the expected population mean value of 0.
LV Diastolic Function
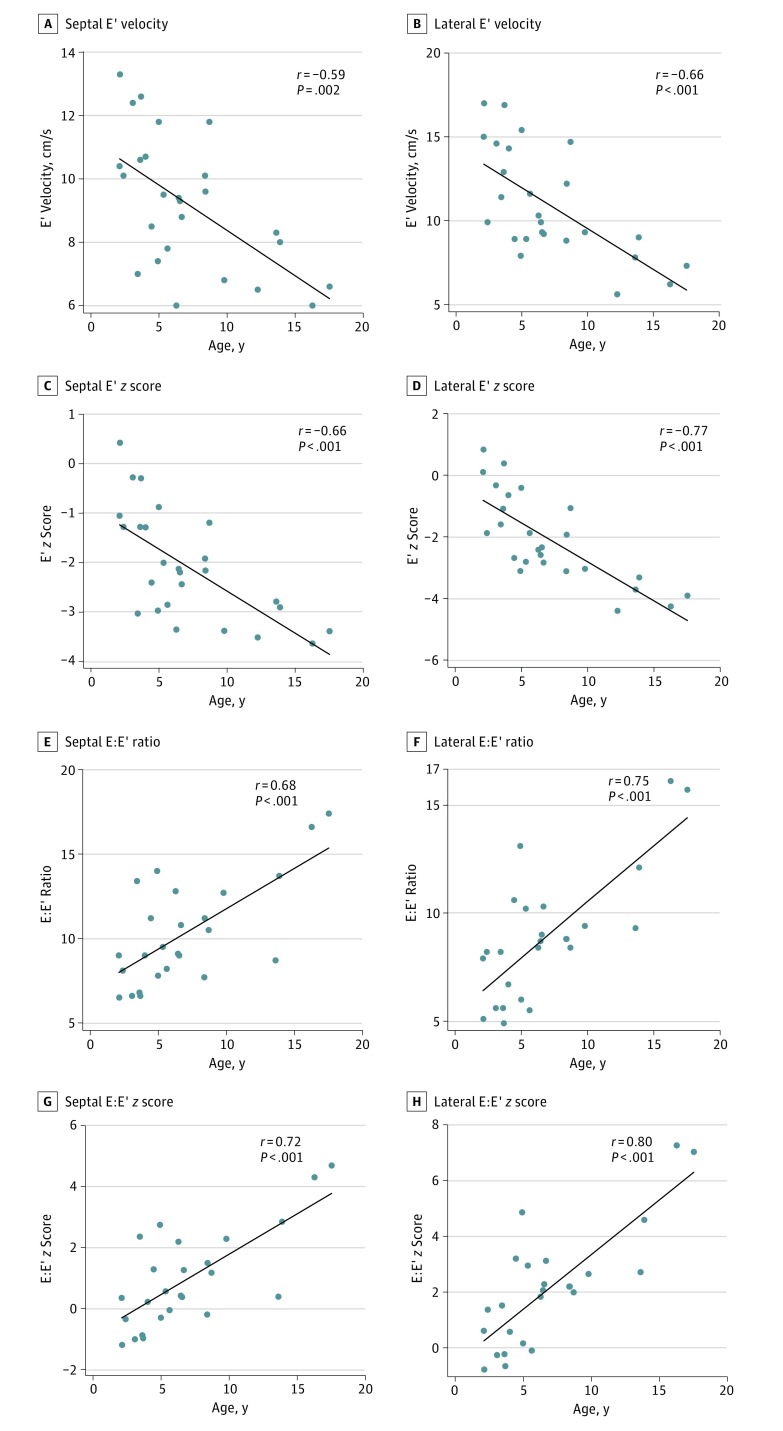
As indicated in Table 3, all echocardiographic indicators of diastolic LV function were abnormal compared with normative values from the echocardiography laboratory at Boston Children’s Hospital. Left ventricular diastolic function indicators are insensitive to body composition; therefore, these z scores were not recalculated using ideal body weight. Left ventricular diastolic dysfunction, defined as a tissue Doppler septal or lateral E′ velocity z score less than 2, was present in 16 patients (59%), and its prevalence steadily increased with age (Table 4). As seen in the Figure, LV diastolic function indicators were worse in older patients. Specifically, z scores for lateral and septal E’ velocities were lower (r = −0.77, P < .001; and r = −0.66, P < .001, respectively), whereas those for the E:E′ ratio were higher in older patients (r = 0.80, P < .001; and r = 0.72, P < .001, respectively). Diastolic indicators were not significantly different between hypertensive and normotensive patients.
Table 3. Left Ventricular Diastolic Function Indicatorsa.
| Indicator | Median (Range) | P Value vs Normative Data |
|---|---|---|
| Mitral inflow velocities | ||
| E:A ratio | 1.2 (0.7 to 2.7) | NA |
| E:A ratio z score | −1.4 (−2 to 1.2) | <.001 |
| Tissue Doppler velocities | ||
| Septal E′, cm/s | 9.3 (6.0 to 13.3) | NA |
| Septal E′ z score | −1.8 (−3.6 to 1.3) | <.001 |
| E:septal E′ ratio | 9.1 (6.5 to 17.4) | NA |
| E:septal E′ z score | 1 (−1 to 6.3) | <.001 |
| Septal E′:A′ ratio | 1.2 (0.8 to 3.5) | NA |
| Septal E′:A′ z score | −1.6 (−2.5 to 1.9) | <.001 |
| Lateral E′, cm/s | 9.9 (5.6 to 17) | NA |
| Lateral E′ z score | −2.4 (−4.4 to 0.8) | <.001 |
| E:lateral E′ ratio | 8.7 (4.9 to 16.1) | NA |
| E:lateral E′ z score | 2.1 (−0.8 to 7.3) | <.001 |
| Lateral E′:A′ ratio | 1.4 (0.6 to 4.6) | NA |
| Lateral E′:A′ z score | −1.7 (−2.5 to 2.4) | <.001 |
Abbreviations: A′, mitral late diastolic tissue Doppler myocardial velocity; E, mitral early diastolic Doppler inflow velocity; E′, mitral early diastolic tissue Doppler myocardial velocity; NA, not applicable.
The z scores were calculated using previously published normative data from the echocardiography laboratory at Boston Children’s Hospital. The P values were calculated by comparing median z scores against the expected population mean value of 0.
Table 4. Prevalence of Cardiac Abnormalities by Age.
| Abnormality | No. (%) of Patients by Age Quartiles, y | |||
|---|---|---|---|---|
| 2.1-3.6 (n = 6) | 3.7-5.9 (n = 7) | 6.0-9.2 (n = 7) | >9.2 (n = 7) | |
| LV abnormalities | ||||
| Diastolic dysfunctiona | 1 (17) | 3 (43) | 6 (86) | 6 (86) |
| Systolic dysfunction (LVEF<55%) | 0 | 0 | 0 | 0 |
| LVH (mass and mass/volume z scores >2) | 0 | 0 | 1 (14) | 6 (86) |
| Mitral valve abnormalities | ||||
| Stenosis (mean gradient >3 mm Hg) | 0 | 0 | 0 (10) | 2 (29) |
| Regurgitation (more than mild) | 0 | 0 | 0 | 0 |
| Annular or chordal calcification | 0 | 0 | 3 (43) | 5 (71) |
| Aortic valve abnormalities | ||||
| Stenosis (MIG>15 mm Hg) | 0 | 0 | 0 | 1 (14) |
| Regurgitation (more than mild) | 0 | 0 | 0 | 0 |
| Annular calcification | 0 | 0 | 1 (14) | 3 (43) |
| ECG abnormalities | 0 | 0 | 0 | 2 (29) |
| Cardiac history and symptoms | ||||
| Congestive heart failure | 0 | 0 | 0 | 0 |
| Dyspnea | 0 | 0 | 0 | 0 |
| Chest pain | 0 | 0 | 0 | 0 |
| Cardiac arrhythmias | 0 | 0 | 0 | 0 |
| Myocardial infarction | 0 | 0 | 0 | 0 |
Abbreviations: ECG, electrocardiogram; EF, ejection fraction; LV, left ventricle; LVH, left ventricular hypertrophy; MIG, maximum Doppler instantaneous gradient.
Left ventricular diastolic dysfunction was defined as septal or lateral E′ velocity z score less than −2.
Figure. Association Between Tissue Doppler Indicators of Diastolic Left Ventricular Function and Age.
In this cross-sectional analysis, each data point represents a unique patient. E indicates early mitral inflow velocity; E’, early diastolic tissue Doppler myocardial velocity.
Mitral Valve Abnormalities
As indicated in Table 4, the most common mitral valve abnormalities were annular and chordal calcification, occurring in roughly one-fourth of patients. Significant mitral stenosis and regurgitation were less common. As indicated in Table 4, the prevalence of mitral valve abnormalities was higher in older patients.
Aortic Valve Abnormalities
As indicated in Table 4, annular calcification was the most common abnormality, occurring in more than one-third of patients. Valvar aortic stenosis and regurgitation were less common. As seen in Table 4, the prevalence of aortic valve abnormalities was higher in older patients.
Aortic Root Wall Brightness
Assessed qualitatively, an unusually increased echo brightness of the aortic root wall was noted in 19 of the 27 patients (70%). Patients with an abnormally bright aortic root had lower lateral and septal E’ velocity z scores and higher lateral and septal E:E′ velocity z scores. Aortic root brightness was not associated with age.
Electrocardiography
The results of 12-lead electrocardiography were normal in 25 patients (93%). One 12-year-old patient’s electrocardiogram showed LVH, biatrial enlargement, and right axis deviation. Echocardiography revealed aortic and mitral stenosis and LVH. A 16-year-old patient’s electrocardiogram showed LVH with strain. Echocardiography showed LVH.
Among electrocardiographic measurements, heart rate was elevated for age (median z score, 1.8; range, 0.17-3.07), but the z score did not vary with age. The PR, QRS, and QT intervals (all corrected for heart rate) did not vary with age.
Carotid-Femoral Pulse Wave Velocity
As reported previously, CFPWV was elevated compared with prior published normative values in children (median, 9.1 m/s; range, 7.3-13.0 m/s). A higher CFPWV was associated with a lower lateral E′ z score (r = −0.41, P = .05). No associations were found between CFPWV and other indicators of diastolic LV function or with LVH.
Cardiac History and Symptoms
As indicated in Table 4, all patients were asymptomatic, and none had documented cardiac arrhythmia, congestive heart failure, or myocardial infarction.
Discussion
In this cohort of patients with HGPS, representing approximately 25% of the world’s population with HGPS, we found that LV diastolic dysfunction was the most prevalent echocardiographic abnormality, seen in all age groups, but its prevalence increased with age. Other cardiac abnormalities, including mitral and aortic valve stenosis or regurgitation and LVH, were less common and seen only during the second decade of life.
Although cardiovascular disease is the most frequent cause of death in patients with HGPS, studying the course of cardiac abnormalities in HGPS has been difficult because of the rarity of the syndrome (prevalence of 1 in 20 million living individuals). Until recently, description of the phenotype has relied on the review of isolated case reports. A more recent prospective report included 15 patients, most during the first decade of life, but did not include assessment of diastolic LV function. Our study includes the largest-to-date cohort that underwent comprehensive echocardiographic evaluation to assess for abnormalities in LV systolic and diastolic function, LVH, and LV valve abnormalities.
LV Size, Systolic Function, and Mass
The BSA-adjusted end-diastolic and end-systolic LV volumes were elevated in patients with HGPS compared with normative data from our laboratory. Left ventricular volume measurements in patients with HGPS have not been previously reported. It has been previously reported that cardiac output and LV volumes in healthy children are linearly related to BSA. These associations assume a normal body composition and BMI. Patients with HGPS have a markedly abnormal body composition, with reduced subcutaneous and visceral fat and low BMI values. As a result, the proportion of lean body mass to less metabolically active fatty tissue in patients with HGPS is higher compared with that in healthy children. Therefore, it may be expected that BSA-corrected cardiac output would be higher in patients with HGPS and could be responsible for the higher than normal LV volume z scores in our study. The confounding effect of body composition is supported by our finding that when ideal body weight was used to calculate BSA, LV volume z scores were similar to normative values. However, LV mass z scores remained significantly higher than normative values despite this correction, suggesting that elevated LV mass observed in patients with HGPS is independent of body composition abnormalities.
No patients had LV systolic dysfunction. The LV ejection fraction was on average higher than normal in our cohort, consistent with a prior report.
LV Diastolic Function
In contrast to normal systolic function in most patients, LV diastolic dysfunction was highly prevalent, with a higher prevalence in older patients. Although this finding is not surprising in the context of a premature aging disorder, it had not been previously reported; therefore, the course and severity of abnormalities were not known. The largest previous report of patients with HGPS did not include assessment of diastolic function. The inverse association between age and diastolic tissue Doppler velocities observed in our cohort mirrors the changes seen during the normal process of aging. However, the pathogenesis of LV diastolic dysfunction in HGPS remains unknown. Systemic hypertension is known to be associated with diastolic LV dysfunction. In our cohort, although hypertension and diastolic dysfunction were common, they were not significantly associated. It could be speculated that diffuse myocardial fibrosis, endocardial fibrosis, or subclinical coronary ischemia may play a role in the pathogenesis of cardiac disease in HGPS. Prior pathologic case reports found myocardial interstitial fibrosis and endocardial thickening, both of which could contribute to diastolic dysfunction. In the future, quantification of myocardial extracellular volume fraction by cardiac magnetic resonance may help evaluate the role of diffuse fibrosis in the etiology of diastolic dysfunction. The identification of LV diastolic dysfunction as an early abnormality in patients with HGPS raises the possibility of its use as an noninvasive marker to assess progression of disease and response to therapy in future studies.
Mitral and Aortic Valve Abnormalities
The most common valve abnormalities were calcification involving the mitral valve annulus and chords and the aortic valve annulus. These findings were most prevalent in the second decade of life. Similarly, valve dysfunction, including stenosis or regurgitation, was late and seen only in the second decade of life. These findings are similar to those reported by Merideth et al in a smaller cohort.
Electrocardiography
Electrocardiographic abnormalities were rare in our cohort and were seen only during the second decade of life. In contrast to a prior report, we did not find significant repolarization abnormalities. In the prior report, several patients with repolarization abnormalities had T-wave inversion in the right precordial leads, which is a normal finding in children. Misclassification of this normal finding likely led to an overestimation of abnormalities. It is also possible that some of the older patients in the prior report with ST-T–wave abnormalities had hemodynamically significant valve disease, resulting in pressure and/or volume overload of the left ventricle, contributing to the echocardiographic abnormalities. In our cohort, the prevalence of hemodynamically significant valve disease was low. Furthermore, in contrast to the bradycardia and PR, QRS, and QTc prolongation reported in progeroid mice, the patients in our cohort had higher than average heart rates, normal electrocardiographic intervals, and no documented arrhythmia. Given the discrepancy between preclinical and clinical data, this area deserves further investigation.
Cardiac History and Symptoms
Despite a high prevalence of diastolic dysfunction and a small but significant number of patients with mitral and/or aortic valve abnormalities, no patients reported cardiac symptoms, congestive heart failure, documented arrhythmias, or myocardial infarction. However, further longitudinal research is needed to determine whether some patients develop symptoms with further progression of valve disease or with development of coronary artery disease.
Other Cardiac Abnormalities
We subjectively found an unusually high level of echo brightness of the aortic root wall in a subset of patients with HGPS compared with pediatric and adult patients without HGPS. In our cohort, patients with HGPS with increased wall brightness were more likely to have features of diastolic LV dysfunction. The significance of this observation is unclear but may relate to pathologic findings that describe prominent thickening of the aortic adventitial layer. Increased carotid artery echo brightness demonstrated by ultrasonography in HGPS also suggests a more diffuse vascular pathologic finding. Future studies that use quantitative methods to assess aortic root wall brightness and correlation with pathologic observations may provide greater insight into the significance of this finding.
As previously shown, aortic stiffness assessed using CFPWV measurements was increased in this HGPS cohort. We found an association between higher CFPWV and lateral E’ velocity z score but not with other indicators of diastolic LV function. Although the association is relatively weak, it is consistent with a previously reported association between progressive ventricular and arterial stiffening during the process of normal aging. This association requires further investigation during longitudinal follow-up of these patients.
Limitations
Several limitations of the current study are worth considering. Although we report data on the largest-to-date cohort of patients with HGPS (representing approximately 25% of the world’s population with HGPS), our analyses remain limited by the small sample size inherent in the rarity of this condition. However, because of the small number of patients worldwide, larger studies may not be feasible to conduct. The cross-sectional study design has inherent limitations. In the future, we plan to report longitudinal data from these patients to address these limitations. Because of radiation and procedural risks, our study did not include cardiac catheterization to assess coronary artery stenosis or calcification. The assessment of diastolic dysfunction was based on tissue Doppler techniques, and invasive confirmation of abnormal diastolic compliance was not possible because of ethical considerations. However, the use of tissue Doppler echocardiography for assessment of LV diastolic function is well established. We did not measure other indicators of diastolic function, such as strain and left atrial size, because of the lack of adequate normative data.
Conclusions
In this largest-to-date cohort of patients with HGPS, LV diastolic function was the most prevalent cardiac abnormality, appearing early and affecting almost all patients older than 6 years. Other cardiac abnormalities, including valve abnormalities, were less common and appeared during the second decade of life. Echocardiographic indicators of LV diastolic dysfunction may be useful end points in future clinical trials.
eTable. Lipid Profile and Glucose Metabolism
References
- 1.Gordon LB. PRF by the numbers. 2013. http://www.progeriaresearch.org/prf-by-the-numbers.html. Accessed April 13, 2017.
- 2.De Sandre-Giovannoli A, Bernard R, Cau P, et al. . Lamin A truncation in Hutchinson-Gilford progeria. Science. 2003;300(5628):2055. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson M, Brown WT, Gordon LB, et al. . Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103(27):10271-10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman RD, Shumaker DK, Erdos MR, et al. . Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2004;101(24):8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006;140(23):2603-2624. [DOI] [PubMed] [Google Scholar]
- 7.Stehbens WE, Wakefield SJ, Gilbert-Barness E, Olson RE, Ackerman J. Histological and ultrastructural features of atherosclerosis in progeria. Cardiovasc Pathol. 1999;8(1):29-39. [DOI] [PubMed] [Google Scholar]
- 8.Olive M, Harten I, Mitchell R, et al. . Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30(11):2301-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker PB, Baba N, Boesel CP. Cardiovascular abnormalities in progeria: case report and review of the literature. Arch Pathol Lab Med. 1981;105(7):384-386. [PubMed] [Google Scholar]
- 10.Merideth MA, Gordon LB, Clauss S, et al. . Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358(6):592-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutgesell HP, Huhta JC, Latson LA, Huffines D, McNamara DG. Accuracy of two-dimensional echocardiography in the diagnosis of congenital heart disease. Am J Cardiol. 1985;55(5):514-518. [DOI] [PubMed] [Google Scholar]
- 12.Tworetzky W, McElhinney DB, Brook MM, Reddy VM, Hanley FL, Silverman NH. Echocardiographic diagnosis alone for the complete repair of major congenital heart defects. J Am Coll Cardiol. 1999;33(1):228-233. [DOI] [PubMed] [Google Scholar]
- 13.Lopez L, Colan SD, Frommelt PC, et al. . Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23(5):465-495. [DOI] [PubMed] [Google Scholar]
- 14.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. ; American Society of Echocardiography . Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777-802. [DOI] [PubMed] [Google Scholar]
- 15.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93(1):62-66. [DOI] [PubMed] [Google Scholar]
- 16.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol (1985). 2005;99(2):445-457. [DOI] [PubMed] [Google Scholar]
- 17.Lai WW, Mertens LL, Cohen MS, Geva T, eds. Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult. 2nd ed Hoboken, NJ: Wiley-Blackwell; 2016. [Google Scholar]
- 18.Strigl S, Hardy R, Glickstein JS, et al. . Tissue Doppler-derived diastolic myocardial velocities are abnormal in pediatric cardiac transplant recipients in the absence of endomyocardial rejection. Pediatr Cardiol. 2008;29(4):749-754. [DOI] [PubMed] [Google Scholar]
- 19.Prakash A, Lacro RV, Sleeper LA, et al. . Challenges in echocardiographic assessment of mitral regurgitation in children after repair of atrioventricular septal defect. Pediatr Cardiol. 2012;33(2):205-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazett HC. The time relations of the blood-pressure changes after excision of the adrenal glands, with some observations on blood volume changes. J Physiol. 1920;53(5):320-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2)(Suppl 4th Report):555-576. [PubMed] [Google Scholar]
- 22.Gerhard-Herman M, Smoot LB, Wake N, et al. . Mechanisms of premature vascular aging in children with Hutchinson-Gilford progeria syndrome. Hypertension. 2012;59(1):92-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon CM, Gordon LB, Snyder BD, et al. . Hutchinson-Gilford progeria is a skeletal dysplasia. J Bone Miner Res. 2011;26(7):1670-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15(5):426-444. [DOI] [PubMed] [Google Scholar]
- 25.Gordon LB, Kleinman ME, Miller DT, et al. . Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2012;109(41):16666-16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonetti GD, Eisenberger U, Bergmann IP, Frey FJ, Mohaupt MG. Pulse contour analysis: a valid assessment of central arterial stiffness in children? Pediatr Nephrol. 2008;23(3):439-444. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-David E, García-Fernández MA, Ledesma MJ, et al. . Age-related intramyocardial patterns in healthy subjects evaluated with Doppler tissue imaging. Eur J Echocardiogr. 2005;6(3):175-185. [DOI] [PubMed] [Google Scholar]
- 28.Chang WT, Chen JS, Hung YK, Tsai WC, Juang JN, Liu PY. Characterization of aging-associated cardiac diastolic dysfunction. PLoS One. 2014;9(5):e97455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flett AS, Hayward MP, Ashworth MT, et al. . Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122(2):138-144. [DOI] [PubMed] [Google Scholar]
- 30.Riesenkampff E, Messroghli DR, Redington AN, Grosse-Wortmann L. Myocardial T1 mapping in pediatric and congenital heart disease. Circ Cardiovasc Imaging. 2015;8(2):e002504. [DOI] [PubMed] [Google Scholar]
- 31.Sado DM, Flett AS, Banypersad SM, et al. . Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98(19):1436-1441. [DOI] [PubMed] [Google Scholar]
- 32.Rivera-Torres J, Calvo CJ, Llach A, et al. . Cardiac electrical defects in progeroid mice and Hutchinson-Gilford progeria syndrome patients with nuclear lamina alterations. Proc Natl Acad Sci U S A. 2016;113(46):E7250-E7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell V, Sigurdsson S, Westenberg JJ, et al. . Relations between aortic stiffness and left ventricular structure and function in older participants in the Age, Gene/Environment Susceptibility–Reykjavik Study. Circ Cardiovasc Imaging. 2015;8(4):e003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Lipid Profile and Glucose Metabolism