Abstract
Although estimated glomerular filtration rate (eGFR) and albuminuria are well established biomarkers of diabetic kidney disease (DKD), additional biomarkers are needed, especially for the early stages of the disease when both albuminuria and eGFR may still be in the normal range and are less helpful for identifying those at risk of progression. Traditional biomarker studies for early DKD are challenging because of a lack of good early clinical end-points, and most rely on changes in existing imprecise biomarkers to assess the value of new biomarkers. There are well characterized changes in kidney structure, however, that are highly correlated with kidney function, always precede the clinical findings of DKD and, at pre-clinical stages, predict DKD progression. These structural parameters may thus serve as clinically useful end-points for identifying new biomarkers of early DKD. In addition, investigators are analyzing tissue transcriptomic data to identify pathways involved in early DKD which may have associated candidate biomarkers measurable in blood or urine, and differentially expressed microRNAs and epigenetic modifications in kidney tissue are beginning to yield important observations which may be useful in identifying new clinically useful biomarkers. This review examines the emerging literature on the use of kidney tissue in biomarker discovery in DKD.
Keywords: biomarkers, kidney biopsy, diabetic kidney disease, morphometry, imaging
INTRODUCTION
Diabetic kidney disease (DKD) has largely been a clinical diagnosis based on the presence of proteinuria and impaired kidney function in the setting of diabetes1. As such, kidney biopsies are not a routine part of management for DKD. However, kidney tissue has been invaluable for determining the structural changes underlying DKD and showing how these structural changes relate to clinical findings. Glomerular basement membrane (GBM) width in normoalbuminuric people with type 1 diabetes, for example, predicts development of microalbuminuria, proteinuria, ESRD, and cardiovascular death2–4. Moreover, in multivariate piece-wise regression models, glomerular structural parameters classically associated with DKD, including increased GBM width, increased mesangial fractional volume, and reduced glomerular filtration surface correlate strongly with albuminuria and with renal functional changes throughout much of the clinical natural history of DKD, and renal interstitial changes are responsible for loss of kidney function in the later stages of the disease5–7.
The principal biomarkers presently used to predict DKD progression are albuminuria and estimated glomerular filtration rate (eGFR). However, not all cases of classical DKD are accompanied by increases in albuminuria8–11, which reduces the value of this biomarker, particularly in early DKD. Moreover, so called ‘persistent microalbuminuria’ defined as two of three consecutive urine samples in the microalbuminuria range, often spontaneously normalizes or stabilizes, limiting the value of this nonetheless useful biomarker2,12–14. Thus, it is important to develop additional biomarkers to supplement albuminuria14. The search for new biomarkers of DKD has centered primarily on identifying analytes in urine and blood that improve prediction of later established end-points, including ESRD, a GFR loss of >40%, or death. There is also an urgent need to identify biomarkers of earlier stages of DKD when advances in treatment may have the greatest chance of attenuating disease progression, yet this is a time when eGFR is often still in the normal range and before the onset of strongly predictive levels of albuminuria.
Much of what is written here is predicated on the simple but very important notion that the earlier preclinical lesions of diabetic nephropathy are the necessary precursors of the later more severe lesions that are the underpinning of the loss of GFR leading to ESRD. There are several ways in which kidney biopsy tissue may advance DKD biomarker discovery. The structural lesions associated with DKD can be reproducibly quantified and always precede changes in kidney function. As such, they may be used as end-points in biomarker studies. Searches are presently underway for proteins, peptides, or metabolites in the blood or urine that are reliably associated with earlier structural damage or predict changes in kidney structure and would therefore be useful biomarkers of early tissue injury and subsequent clinical progression of DKD. Such markers are likely interconnected in large networks which may be perturbed in the presence of disease, leading to changes in their concentrations in biological specimens such as urine or blood. Gene expression profiles derived from diseased kidney tissue may reflect these dynamic molecular perturbations underlying diabetic kidney structural injury. Their differential expression relative to healthy tissues or to tissues from people with diabetes who have very slow or no development of these lesions, will likely lead to the identification of candidate biomarkers for progression and/or protection from DKD as well as to new treatment targets15,16.
Changes in expression patterns of microRNAs (miRNAs) in kidney tissue may provide another means to identify key pathogenetic or protective processes underlying DKD risk and could be used to monitor development and progression of DKD17,18. In addition to being detectable in tissue, miRNAs can also be detected in blood and urine, raising the prospect that miRNA concentrations in these easily accessible bio-specimens might reflect the underlying tissue changes and therefore be clinically useful biomarkers for DKD19,20.
Epigenetic factors may also impact gene expression21,22, and differences in gene methylation and histone modification in kidney tissue from affected and unaffected individuals may provide insight into genes and pathway modifications involved in DKD which may identify new biomarkers. As with miRNAs, differential gene methylation and histone modifications may themselves be useful biomarkers, as gene methylation and histone modification patterns can be identified in other, more accessible cells, including peripheral blood cells, which may reflect changes in the underlying kidney tissue23.
This review examines the emerging role of kidney biopsies in biomarker research and considers potential future developments. Research kidney biopsies are the most suitable source of tissue for these types of studies, as clinically indicated kidney biopsies are typically performed to identify nondiabetic kidney disease in the setting of diabetes24, so they are not as useful when attempting to predict progression of DKD.
What is DKD?
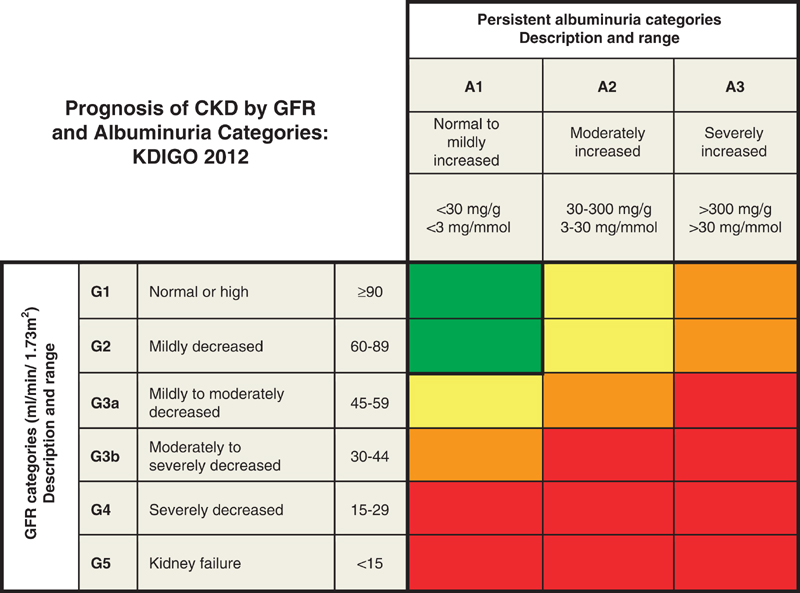
The Kidney Disease Improving Global Outcomes group (KDIGO) defines chronic kidney disease (CKD) as “abnormalities of kidney structure or function, present for greater than three months with implications for health”25. They further sub-divide CKD based on underlying cause, range of GFR, and degree of albuminuria26. Figure 1 illustrates the current stages of CKD along with their relationship to the risk of CKD progression to kidney failure, cardiovascular morbidity, and death. KDIGO proposes that the terms “microalbuminuria” and “macroalbuminuria” be replaced with “moderately increased” and “severely increased” albuminuria to better fit the distinction in degree of albumin loss.
Figure 1.
Prognosis of CKD by GFR and albuminuria category. Green, low risk (if no other markers of kidney disease, no CKD); Yellow, moderately increased risk; Orange, high risk; Red, very high risk. CKD, chronic kidney disease; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes. Reprinted with permission from Definition and classification of CKD Kidney Int Suppl (2011). 2013 Jan;3(1):19–6225.
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) defines DKD as the presence of elevated urine albumin excretion associated with progressive decline in GFR, increased systolic blood pressure, and high risk of kidney failure in people with diabetes27. Kidney biopsy is not routinely used to confirm the diagnosis of DKD, unless there is reason to believe that another kidney disease is involved, so it is not part of routine clinical management in most diabetic patients. Ascertaining DKD based solely on clinical assessment, however, inhibits biomarker discovery and the pathogenetic insights available from kidney tissue. This issue is particularly relevant in type 2 diabetes, where CKD is more often due to nondiabetic causes than in type 1 diabetes28. In type 1 diabetes, biopsy studies have confirmed that DKD without albuminuria is associated with the traditional structural changes29, whereas this is less well established in type 2 diabetes30,31.
In the early stages of DKD, GFR may be normal or even higher than normal (hyperfiltration). Hyperfiltration is seen in both type 1 and type 2 diabetes, though estimates of prevalence based on measured GFR vary markedly from as low as 6% to as high as 73%32. Hyperfiltration is associated with an increased risk of moderately elevated albuminuria33,34, but at present there are limited data examining its effect on later stages of DKD32. Nevertheless, because hyperfiltration is a common feature of early DKD, a person with a GFR above 90 ml/min may already have experienced a substantive decline in GFR. In non-linear (piece-wise) analyses of relationships between measured GFR and diabetic glomerulopathy parameters in people with type 1 diabetes, the point at which further reductions in GFR become strongly associated with these parameters is at 99 ml/min/1.73 m2 35. In such settings, where the CKD level would at most be stage 1 or 2, it is necessary to consider rate of change in GFR, albuminuria, and other evidence of kidney damage for diagnosis of DKD. As treatment may modify both GFR and albuminuria, previous degrees of albuminuria should be considered. Moreover, the diagnosis of DKD might be doubtful, despite the combination of albuminuria and reduced GFR, in the absence of diabetic retinopathy, when there are signs of other systemic diseases, or when heavy proteinuria, rapidly falling GFR, or refractory hypertension are present1.
While eGFR and albuminuria remain useful biomarkers of DKD, there are times when they are not sufficient. For instance, DKD may occur in the absence of sustained albuminuria8–11. Moreover, these markers are clearly not sufficient when trying to assess early DKD, when eGFR is still in the normal range and before the onset of elevated albuminuria.
Biomarkers of kidney structure
While there are clear associations between structure and function at modest levels of GFR decline in DKD, these relationships are more ambiguous at earlier stages when albumin may not have increased appreciably and when GFR is maintained. Thus, biomarkers that are associated with kidney structure, which provides unequivocal evidence of early kidney damage36,37, may be of greatest use clinically for identifying people at highest risk for early DKD progression. This review includes studies of protein biomarkers in blood and urine that reflect underlying structural lesions (Table 1). Some of the studies examine cross-sectional associations between structural measures and biomarkers and others look prospectively at whether the putative biomarkers predict changes in structure.
Table 1.
Biomarkers and Kidney Morphometric Studies in Diabetes
| Biomarker | Sample type | Study design |
Diabetes type |
Population | Morphometric associations |
Reference |
|---|---|---|---|---|---|---|
|
| ||||||
| Advanced Glycation End Products | Plasma/Urine | Prospective | Type 1 | NHDNS |
|
Beisswenger et al,200546 |
| Beisswenger et al,201348 | ||||||
| Beisswenger et al,201449 | ||||||
|
| ||||||
| Serum | Cross-sectional | Type 2 | American Indians |
|
Beisswenger et al,200546 | |
| Saulnier et al, 201647 | ||||||
|
| ||||||
| Beta-2 Microglobulin | Urine | Cross-sectional | Type 2 | Japanese |
|
Mise et al, 201674 |
|
| ||||||
| Bradykinin | Plasma | Cross-sectional | Type 1 | RASS |
|
Wheelock et al, 201762 |
|
| ||||||
| Plasma | Prospective | Type 1 | RASS |
|
Wheelock et al 201762 | |
|
| ||||||
| Monocyte Chemo-attractant Protein 1 | Urine | Cross-sectional | Type 1 | RASS |
|
Fufaa et al, 201643 |
|
| ||||||
| Urine | Prospective | Type 1 | RASS |
|
Fufaa et al, 201643 | |
|
| ||||||
| N-Acetyl-β-d-Glucosaminidase | Urine | Cross-sectional | Type 2 | Japanese |
|
Mise et al, 201674 |
|
| ||||||
| Tumor Necrosis Factor Receptor 1 | Serum | Cross-sectional | Type 2 | American Indians |
|
Pavkov et al, 201642 |
|
| ||||||
| Tumor Necrosis Factor Receptor 2 | Serum | Cross-sectional | Type 2 | American Indians |
|
Pavkov et al, 201642 |
|
| ||||||
| White blood cell fractions | Blood | Cross-sectional | Type 2 | American Indians |
|
Wheelock et al 201766 |
Morphometric measures are shown in bold where there is a statistically significant association with the biomarker. Positive associations are indicated by an up arrow and negative associations by a down arrow – both arrows are shown when variation in findings is observed for different sub-types of biomarker.
Association seen only in women.
Abbreviations used: NHDNS – natural history diabetic nephropathy study; RASS – renin angiotensin system study; %GS – percentage of global glomerular sclerosis; VG – mean glomerular volume; Vv(Int/cortex) – cortical interstitial fractional volume; Vv(Mes/glom) – mesangial fractional volume per glomerulus; (Sv(PGBM/glom) – surface density of the peripheral glomerular basement membrane per glomerulus; TFS/glom – total filtration surface per glomerulus; GBM width – glomerular basement membrane width; FPW – foot process width; % ECF – percentage of endothelial cell fenestrations; IFTA – interstitial fibrosis and tubular atrophy.
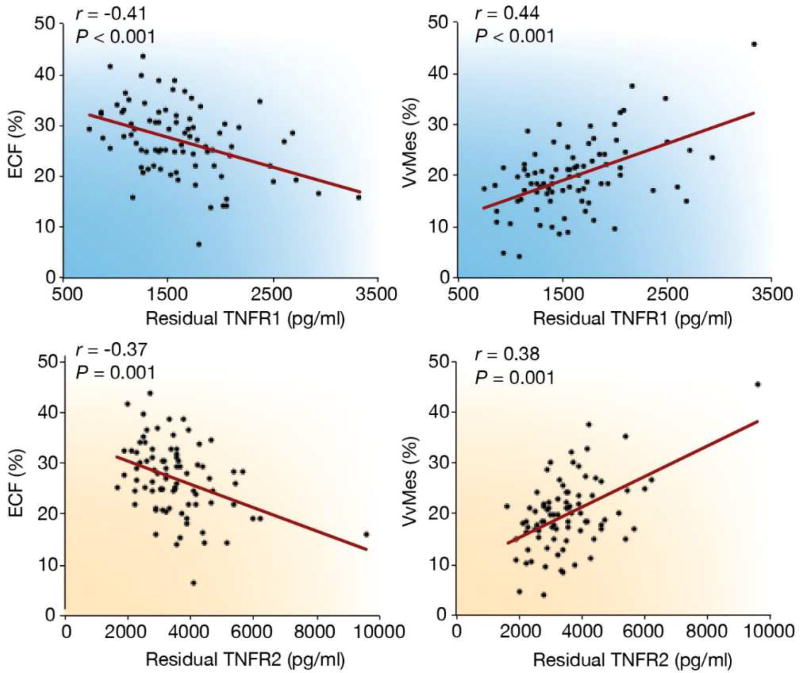
Serum Tumor Necrosis Factor Receptor 1 (TNFR1) and 2 (TNFR2) are promising proinflammatory biomarkers for DKD progression and are associated with worsening albuminuria38, declining GFR39,40, onset of ESRD, and death41. Elevated serum TNFR1 and TNFR2 concentrations were also associated with kidney structural parameters of classical diabetic glomerulopathy in American Indians with type 2 diabetes, most of whom had preserved GFR and no albuminuria42. The strongest associations were with greater mesangial expansion and lower percentage of endothelial cell fenestrations, which were statistically significant after adjustment for clinical measures, including albuminuria and GFR (Figure 2). Both of these structural lesions are strong predictors of progressive DKD in this population43. Although the mechanism underlying the relationship between the TNFRs and progressive DKD are presently unknown, in animal models, TNFR1 is expressed in normal glomerular endothelium, whereas TNFR2 is only expressed in the glomerulus in disease44. The pro-inflammatory actions of TNF are mediated by TNFR1 activation, a process that is partially regulated by TNFR245.
Figure 2.
Partial regression residual plot of the associations between tumor necrosis factor receptors (TNFRs), percent of normal endothelial cell fenestration (ECF), and mesangial fractional volume (VvMes). The residuals were computed from regressing each of the following variables: age, sex, diabetes duration, hemoglobin A1c, body mass index, and mean arterial pressure. Exclusion of the single outlier did not change the significance of associations between 2 morphometric variables. Reprinted with permission from Pavkov ME, Weil EJ, Fufaa GD, et al. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int. 2016;89:226–234. Copyright © International Society of Nephrology. Reprinted with permission from Looker HC and Nelson RG. Reading the tree leaves – how to enrich clinical trials of diabetic kidney disease Kidney Int. 2017;92:23–2595.
Advanced glycation endproducts (AGEs) are potential mediators by which hyperglycemia causes DKD. Several highly reactive dicarbonyl-derived AGEs are associated with progressive DKD defined by declining GFR46,47, and several studies now demonstrate strong associations between these AGEs and the structural determinants of that progression. In a cohort of people with type 1 diabetes and no evidence of DKD (i.e. no albuminuria and preserved GFR), three plasma AGEs – methylglyoxal hydroimidazolone (MGHI), carboxymethyl lysine (CML) and carboxy ethyl lysine (CEL) – positively predicted increase in GBM width over 5 years, and these associations were independent of diabetes duration and HbA1c48,49. This observation is important because increased GBM width predicts advanced DKD in type 1 diabetes4. In a type 2 diabetes cohort with predominantly early DKD (normal or moderately elevated albuminuria and preserved GFR), serum AGEs were associated cross-sectionally with classical diabetic glomerulopathy lesions, with MGHI, CEL and CML all associated with greater mesangial volume. CML and glyoxal-hydroimidazolone were also associated with higher cortical interstitial fractional volume, a parameter which is known to increase early in the natural history of kidney structural changes in type 1 diabetes50. AGEs are generated in the kidney and filtered by the glomerulus, and in DKD they accumulate in the glomerular interstitium and the capillary walls51 where they induce synthesis of extracellular matrix via induction of connective tissue growth factor52.
Monocyte Chemoattractant Protein 1 (MCP-1) is a cytokine that regulates migration and infiltration of monocytes in inflammation and as such is also a potential inflammatory biomarker. Urine MCP-1 is associated with rapid decline of eGFR in individuals with type 1 diabetes and normoalbuminuria53 as well as GFR decline in individuals with type 2 diabetes and advanced kidney disease (macroalbuminuria and ≥stage 3 CKD)54,55. A kidney biopsy study recently found that urine MCP-1 is associated cross-sectionally with cortical interstitial fractional volume in individuals with type 1 diabetes and normoalbuminuria. It also predicts change in GBM width and increased cortical interstitial fractional volume over 5-years in the same cohort, though the latter was only observed in women43.
Bradykinin has a direct renoprotective effect on kidney function, however there is also evidence that bradykinin can promote kidney damage in diabetes in some settings56–61. In a cross-sectional study62, plasma bradykinin and related peptides were modestly associated with kidney structural preservation in people with type 1 diabetes who were normoalbuminuric, normotensive, and had normal to high GFR. Higher bradykinin concentrations were associated with higher glomerular volume and total filtration surface area, and lower cortical interstitial fractional volume. In longitudinal analyses of the same cohort, bradykinin concentrations were associated with preservation of surface density of the peripheral glomerular basement membrane62. These changes were probably facilitated by the bradykinin 2 receptor, which mediates most of the physiological actions of bradykinin56. An inverse association appears to emerge between bradykinin and its related peptides and progression of DKD in more advanced disease, perhaps as activation of the bradykinin 1 receptor becomes more prominent and stimulates inflammatory changes and fibrosis58,59.
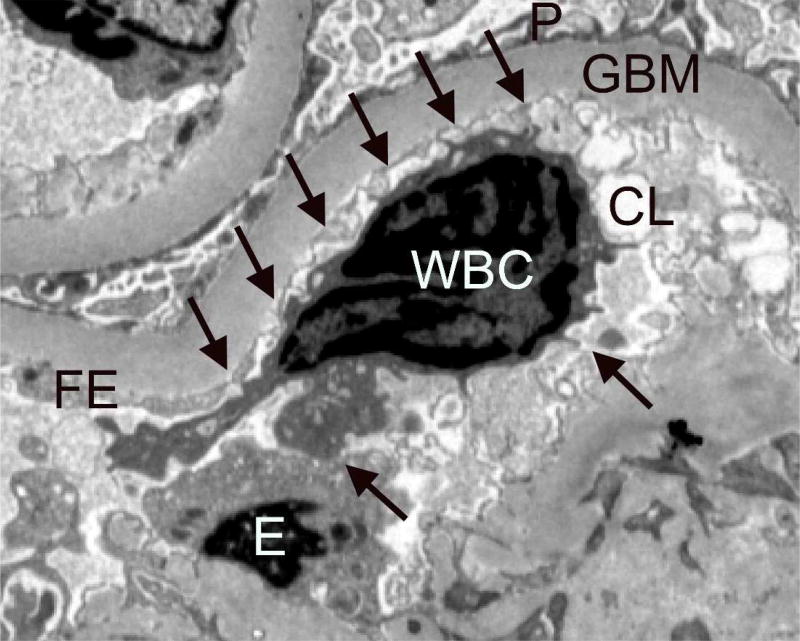
White blood cell counts and fractions are biomarkers of inflammation, which are linked to diabetes and its vascular complications63–65, including a greater risk of ≥40% loss of GFR in a European type 2 diabetes cohort66. These findings are thought to be related, at least in part, to activation of white cells by advanced glycation end-products. These activated cells in turn produce pro-inflammatory cytokines that promote local tissue damage67–69. A recent study in Pima Indians with type 2 diabetes found that lower lymphocyte and higher eosinophil and neutrophil fractions are also associated with early kidney structural damage, including increased GBM width and loss of glomerular endothelial cell fenestrations66. Examination of the kidney tissue revealed a higher proportion of WBC’s in the peripheral glomerular capillaries of participants with more advanced structural lesions, with frequent projections from the white cells touching endothelial cells within the glomerular capillary, suggesting signaling between these cells (Figure 3).
Figure 3.
Peripheral glomerular capillaries from a Pima Indian with type 2 diabetes. Arrows point to projections extending from the WBC to the adjacent endothelium within the glomerular capillary lumen. Transmission electron microscopy, ×3,000. WBC, white blood cell; GBM, glomerular basement membrane; P, podocyte foot processes; E, endothelial cell body; FE, fenestrated endothelium; CL, capillary lumen. Reprinted with permission from Wheelock KM, Saulnier PJ, Tanamas SK et al. White blood cell fractions correlate with lesions of diabetic kidney disease and predict loss of kidney function in type 2 diabetes. Nephrol Dial Transplant doi: 10.1093/ndt/gfx23166.
Beta-2 microglobulin is a filtration biomarker70 and in urine it is also a biomarker of tubulointerstitial injury71 as is N-Acetyl-β-d-Glucosaminidase (NAG)72. Both are candidate biomarkers for DKD progression, and in urine both are associated with albuminuria in diabetes73,74. Beta-2 microglobulin is also a key part of a urine proteomic panel used for distinguishing biopsy proven DKD from CKD not due to diabetes75. In a European study of eGFR loss among people with type 2 diabetes and established DKD (≥stage 3 CKD), serum beta-2 microglobulin predicts loss of >40% eGFR39, ESRD, and mortality39,76, but only marginally improves prediction when GFR and albuminuria are included in the models39,70. In type 2 diabetes, higher urine concentrations of both markers are associated cross-sectionally with more advanced structural injury, including increased GBM width, mesangial expansion, and greater cortical interstitial fibrosis, but not with clinical DKD progression during follow-up77. These associations may not reflect a specific causal mechanism involving these proteins, but rather an increase in their urine concentration in response to tubular damage, predominantly affecting the proximal tubule, that accompanies progressive DKD.
The studies described above have successfully linked early structural damage in the kidneys to clinical DKD and to subsequent clinical progression of DKD, providing proof-of-concept for this approach to biomarker assessment. Further work in this area may include studies of other promising biomarkers of DKD, such as plasma kidney injury molecule-139,78,79, and may eventually lead to the establishment of a biomarker panel that will identify those at greatest risk of early DKD progression.
Gene transcription and DKD
In addition to identifying biomarkers that are associated with structural measures in kidney biopsy tissue, kidney tissue can also be used to examine the transcriptome. This allows not only greater insight into the pathologic processes underlying structural changes by identifying genes and pathways which are differentially expressed at various stages of DKD, but can also identify novel biomarker candidates. An extensive evaluation of the kidney transcriptome in CKD was undertaken by researchers across four CKD cohorts15. The study identified six genes which were differentially transcribed in CKD versus living kidney donors and were associated with eGFR in an initial sample set from the European Renal cDNA Bank (ERCB). One of the genes, epidermal growth factor (EGF), was also primarily expressed in the kidney, thus substantially increasing the likelihood that urine EGF concentrations were related to kidney sources of the protein. In analyses of samples from the Nephrotic Syndrome Study Network (NEPTUNE) and the Peking University First Hospital immunoglobulin A nephropathy cohort (PKU-IgAN), urine EGF was associated with eGFR at the time of biopsy, and was associated with risk for declining GFR in longitudinal analyses.
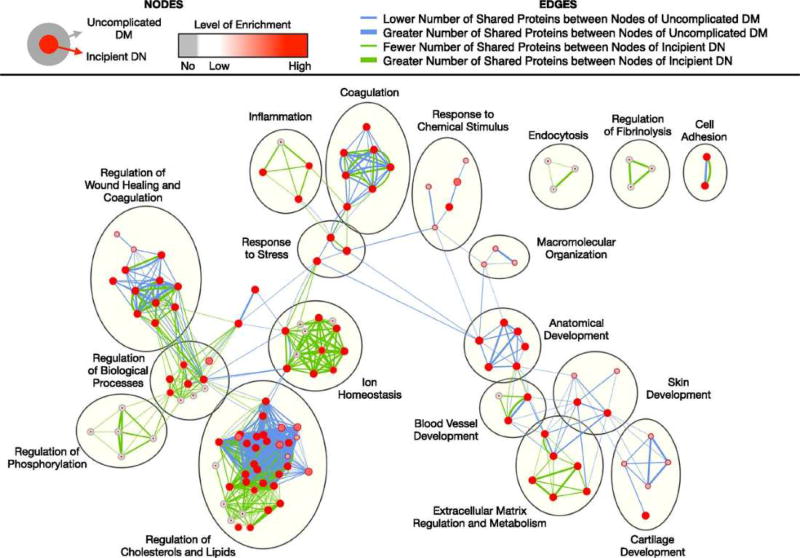
In a study that included data from 31 previously published studies of DKD in type 1 and type 2 diabetes in which urine protein and peptide biomarkers were measured, the results of urine proteomic studies and bioinformatic data, including pathway data and protein-protein interactions for biomarkers known to be expressed in the kidney, were combined to identify the underlying pathological processes in DKD16. The human protein atlas was used to identify tissue sources within the kidney (e.g. glomerulus, proximal tubule, distal tubule) which expressed each of the urine biomarkers identified in the proteomic analyses. By looking at results from different clinical stages, the investigators identified temporal patterns in pathways involved with DKD – i.e. biomarkers relating to fibrosis at the earliest stages, and biomarkers relating to inflammation and wound healing in established DKD16. An example of their findings is shown in Figure 4, which displays a schematic of which biological processes are enriched in early DKD compared to uncomplicated diabetes. The figure illustrates many biological processes which are enriched in early DKD compared to uncomplicated diabetes. The figure illustrates many biological processes across many pathways, including lipid regulation, wound healing, coagulation, and extra-cellular matrix regulation, all of which may have associated informative biomarkers measurable in blood or urine. A limitation of this review paper is the uncertainty of these relationships with the underlying kidney pathology, which was not available in most cases.
Figure 4.
Comparison of enriched biological processes in uncomplicated diabetes and incipient diabetic nephropathy. Significantly enriched biological processes were identified for each stage using Biological Networks Gene Ontology with Benjamini and Hochberg multiple testing correction (P<0.05) and then run on Enrichment Map with Jaccard coefficient of 0.5 (P value cut-off = 0.001; false discovery rate Q-value cut-off = 0.05). Each node represents an enriched biological process. Red node colors correspond to high enrichment, whereas gray node colors correspond to no enrichment. As shown in the figure legend, the outer circle color corresponds to the level of enrichment in uncomplicated diabetes, whereas the inner circle color corresponds to that in incipient diabetic nephropathy. Edge thickness denotes the number of overlapping markers between two connected nodes within uncomplicated diabetes (blue) and within incipient diabetic nephropathy (green). DM, diabetes mellitus; DN, diabetic nephropathy. Reprinted with permission from Van JAD, Scholey JW, Konvalinka. J Am Soc Nephrol. 2017 Apr;28(4):1050–106116.
Kidney miRNA and biomarkers
One of the mechanisms through which gene transcription is modified is via miRNAs, which themselves may have a role both as potential biomarkers and as targets for intervention in DKD18. A recent study looked in detail at kidney miRNA expression for a variety of causes of CKD, including DKD, in both the glomerular and the tubular compartments compared to tissue from healthy living donors80. Differentially expressed miRNAs were identified in both compartments. Some of these miRNAs were differentially expressed in several types of CKD, whereas others were unique to DKD. In general, there was greater overlap in miRNAs across diseases in the proximal tubules compared to the glomerulus, suggesting that proximal tubular changes may be a common pathway, whereas glomerular changes are more disease specific. MiRNAs can also be measured in blood and urine, and a variety of miRNA have been identified in both blood and urine that may play a role as biomarkers in DKD19,20,81,82. In type 1 diabetes, a set of urine miRNAs were differentially expressed in individuals with albuminuria compared to those without albuminuria20. One of the miRNAs, hsa-miRNA-214, was also over-expressed in the glomeruli in cases of DKD80. As techniques for measuring miRNAs in clinical samples improve, more developments in the potential use of miRNAs as biomarkers are expected18. Identification of informative miRNA markers of DKD in kidney tissue followed by a search for these markers in blood or urine may significantly enhance biomarker discovery in the future.
Gene methylation and DKD
Gene methylation is another mechanism for modifying gene expression. Structure and methylation patterns in kidney tissue from people with CKD (due to DKD or hypertension) were compared with kidney tissue from healthy transplant donors. The investigators confirmed the presence of structural lesions among their CKD samples, including increased fibrosis, tubular atrophy, and mesangial expansion, compared to the healthy controls. They also found many genes with differential methylation pattern in kidney tubules83. Most of the differentially methylated areas had lower levels of methylation in the CKD cases than in controls. The most commonly affected sites for differential methylation were enhancer regions and introns. This was unexpected as usually the promoter regions are the principal sites affected by differential methylation, where promoter hypermethylation is associated with a reduction in gene transcription84. Some of the genes affected have a role in fibrosis pathways (such as Tumor Growth Factor beta), while others are associated with kidney development. Differential methylation in DNA extracted from blood can vary with DKD as well. In a study of patients with type 2 diabetes, methylation of the methylenetetrahydrofolate reductase gene was lower in those with macroalbuminuria than in those with normoalbuminuria85, and methylation of two candidate DKD genes, TIMP-2 and AKR1B1, was negatively correlated with albuminuria86. Relationships between methylation patterns in kidney tissue and in blood are presently under investigation and may yield clinically useful biomarkers in the future.
Are there alternatives to tissue?
Kidney biopsy tissue is important for observing the detailed structural changes in DKD, and this may be especially important in understanding kidney functional alterations in type 2 diabetes. However, there are also developments in imaging that may allow us to assess structural changes non-invasively by making use of advances in magnetic resonance imaging (MRI)87,88. These approaches include the use of diffusion-weighted MRI, which measures the flow of water to determine degrees of fibrosis89,90; blood oxygenation level-dependent (BOLD) MRI which assesses the degree of hypoxia in tissue90,91; and MRI elastography (MRE) which measures tissue stiffness due to fibrosis92,93. The advantage of imaging to assess structure is not only that it is non-invasive, but that it also allows assessment of the whole kidney, whereas a biopsy can only show structure in one area of the kidney and kidney fibrosis is not uniform throughout the kidney. However, these imaging methods are in the early stages of development, and further work is needed before they are ready for clinical use94. Moreover, access to kidney tissue will be important for confirming the validity of these imaging methods as biomarkers of DKD.
Conclusions
Kidney tissue is a valuable resource for biomarker discovery. It may be most beneficial for identifying clinically useful biomarkers for the earliest stages of DKD where our currently available biomarkers are least effective. Identifying biomarkers that are linked to the earliest specific and non-specific structural changes seen in DKD may allow more precise risk prediction, pathogenetic insights, and earlier interventions which may preserve kidney function and delay or prevent the onset of ESRD. Thus, in addition to identifying biomarkers, this work may also lead to new therapeutic interventions and the means to assess the efficacy of those interventions. Advances in imaging may replace the need for tissue in the future, but for the time being access to kidney tissue remains a powerful but underutilized tool for biomarker discovery.
Clinical Summary.
Changes in kidney structure precede the clinical findings of DKD and provide useful endpoints for biomarker discovery in early DKD.
Examination of differentially expressed genes, microRNAs, and epigenetic modifications in kidney tissue may also yield clinically useful biomarkers.
Advances in imaging may replace the need for kidney tissue in the future, but for the time being access to kidney tissue remains a powerful but underutilized tool for biomarker discovery.
Acknowledgments
Support: This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare they have no relevant financial interests.
References
- 1.National Kidney Foundation. KDOQI clinical practice guidelines for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M. The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes. 2005;54(7):2164–2171. doi: 10.2337/diabetes.54.7.2164. [DOI] [PubMed] [Google Scholar]
- 3.Perrin NE, Torbjornsdotter T, Jaremko GA, Berg UB. Risk markers of future microalbuminuria and hypertension based on clinical and morphological parameters in young type 1 diabetes patients. Pediatr Diabetes. 2010;11(5):305–313. doi: 10.1111/j.1399-5448.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- 4.Caramori ML, Parks A, Mauer M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol. 2013;24(7):1175–1181. doi: 10.1681/ASN.2012070739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bader R, Bader H, Grund KE, Mackensen-Haen S, Christ H, Bohle A. Structure and function of the kidney in diabetic glomerulosclerosis. Correlations between morphological and functional parameters. Pathol Res Pract. 1980;167(2–4):204–216. doi: 10.1016/S0344-0338(80)80051-3. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56(5):1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 7.Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol. 2002;17(1):247–252. doi: 10.14670/HH-17.247. [DOI] [PubMed] [Google Scholar]
- 8.Molitch ME, Steffes M, Sun W, et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010;33(7):1536–1543. doi: 10.2337/dc09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832–1839. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 10.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69(11):2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 11.Thomas MC, Macisaac RJ, Jerums G, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11) Diabetes Care. 2009;32(8):1497–1502. doi: 10.2337/dc08-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348(23):2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 13.Forsblom CM, Groop PH, Ekstrand A, Groop LC. Predictive value of microalbuminuria in patients with insulin-dependent diabetes of long duration. BMJ. 1992;305(6861):1051–1053. doi: 10.1136/bmj.305.6861.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caramori ML, Fioretto P, Mauer M. Enhancing the predictive value of urinary albumin for diabetic nephropathy. J Am Soc Nephrol. 2006;17(2):339–352. doi: 10.1681/ASN.2005101075. [DOI] [PubMed] [Google Scholar]
- 15.Ju W, Nair V, Smith S, et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7(316):316ra193. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van JA, Scholey JW, Konvalinka A. Insights into diabetic kidney disease using urinary proteomics and bioinformatics. J Am Soc Nephrol. 2017;28(4):1050–1061. doi: 10.1681/ASN.2016091018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung ACK. microRNAs in Diabetic Kidney Disease. In: Santulli G, editor. microRNA: Medical Evidence: From Molecular Biology to Clinical Practice. Cham: Springer International Publishing; 2015. pp. 253–269. [Google Scholar]
- 18.Simpson K, Wonnacott A, Fraser DJ, Bowen T. MicroRNAs in diabetic nephropathy: from biomarkers to therapy. Curr Diab Rep. 2016;16(3):35. doi: 10.1007/s11892-016-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argyropoulos C, Wang K, Bernardo J, et al. Urinary microRNA profiling predicts the development of microalbuminuria in patients with type 1 diabetes. J Clin Med. 2015;4(7):1498–1517. doi: 10.3390/jcm4071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argyropoulos C, Wang K, McClarty S, et al. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PloS One. 2013;8(1):e54662. doi: 10.1371/journal.pone.0054662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caramori ML, Kim Y, Natarajan R, et al. Differential response to high glucose in skin fibroblasts of monozygotic twins discordant for type 1 diabetes. J Clin Endocrinol Metab. 2015;100(6):E883–889. doi: 10.1210/jc.2014-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caramori ML, Kim Y, Goldfine AB, et al. Differential gene expression in diabetic nephropathy in individuals with type 1 diabetes. J Clin Endocrinol Metab. 2015;100(6):E876–882. doi: 10.1210/jc.2014-4465. [DOI] [PubMed] [Google Scholar]
- 23.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–455. doi: 10.1007/s00125-014-3462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzucco G, Bertani T, Fortunato M, et al. Different patterns of renal damage in type 2 diabetes mellitus: a multicentric study on 393 biopsies. Am J Kidney Dis. 2002;39(4):713–720. doi: 10.1053/ajkd.2002.31988. [DOI] [PubMed] [Google Scholar]
- 25.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Chapter 1: Definition and classification of CKD. Kidney Int Suppl. 2013;3(1):19–62. [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl. 2013;3(1):63–72. doi: 10.1038/kisup.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Bell S, Fletcher EH, Brady I, et al. End-stage renal disease and survival in people with diabetes: a national database linkage study. QJM. 2015;108(2):127–134. doi: 10.1093/qjmed/hcu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52(4):1036–1040. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 30.Porrini E, Ruggenenti P, Mogensen CE, et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3(5):382–391. doi: 10.1016/S2213-8587(15)00094-7. [DOI] [PubMed] [Google Scholar]
- 31.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99(2):342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonneijck L, Muskiet MH, Smits MM, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017;28(4):1023–1039. doi: 10.1681/ASN.2016060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52(4):691–697. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 34.Ruggenenti P, Porrini EL, Gaspari F, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35(10):2061–2068. doi: 10.2337/dc11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauer M, Caramori ML, Fioretto P, Najafian B. Glomerular structural-functional relationship models of diabetic nephropathy are robust in type 1 diabetic patients. Nephrol Dial Transplant. 2015;30(6):918–923. doi: 10.1093/ndt/gfu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drummond K, Mauer M. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes. 2002;51(5):1580–1587. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 38.Krolewski AS, Niewczas MA, Skupien J, et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care. 2014;37(1):226–234. doi: 10.2337/dc13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Looker HC, Colombo M, Hess S, et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 2015;88(4):888–896. doi: 10.1038/ki.2015.199. [DOI] [PubMed] [Google Scholar]
- 40.Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23(3):516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavkov ME, Nelson RG, Knowler WC, Cheng Y, Krolewski AS, Niewczas MA. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015;87(4):812–819. doi: 10.1038/ki.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavkov ME, Weil EJ, Fufaa GD, et al. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int. 2016;89(1):226–234. doi: 10.1038/ki.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fufaa GD, Weil EJ, Lemley KV, et al. Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol. 2016;11(2):254–261. doi: 10.2215/CJN.05760515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vielhauer V, Stavrakis G, Mayadas TN. Renal cell-expressed TNF receptor 2, not receptor 1, is essential for the development of glomerulonephritis. J Clin Invest. 2005;115(5):1199–1209. doi: 10.1172/JCI23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 46.Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes. 2005;54(11):3274–3281. doi: 10.2337/diabetes.54.11.3274. [DOI] [PubMed] [Google Scholar]
- 47.Saulnier PJ, Wheelock KM, Howell S, et al. Advanced glycation end products predict loss of renal function and correlate with lesions of diabetic kidney disease in American Indians with type 2 diabetes. Diabetes. 2016;65(12):3744–3753. doi: 10.2337/db16-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care. 2013;36(10):3234–3239. doi: 10.2337/dc12-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beisswenger PJ, Howell SK, Russell G, Miller ME, Rich SS, Mauer M. Detection of diabetic nephropathy from advanced glycation endproducts (AGEs) differs in plasma and urine, and is dependent on the method of preparation. Amino Acids. 2014;46(2):311–319. doi: 10.1007/s00726-013-1533-x. [DOI] [PubMed] [Google Scholar]
- 50.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horie K, Miyata T, Maeda K, et al. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest. 1997;100(12):2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor betaindependent pathway. Am J Pathol. 2004;165(6):2033–2043. doi: 10.1016/s0002-9440(10)63254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolkow PP, Niewczas MA, Perkins B, et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19(4):789–797. doi: 10.1681/ASN.2007050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Titan SM, Vieira JM, Jr, Dominguez WV, et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications. 2012;26(6):546–553. doi: 10.1016/j.jdiacomp.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Verhave JC, Bouchard J, Goupil R, et al. Clinical value of inflammatory urinary biomarkers in overt diabetic nephropathy: a prospective study. Diabetes Res Clin Pract. 2013;101(3):333–340. doi: 10.1016/j.diabres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Tomita H, Sanford RB, Smithies O, Kakoki M. The kallikrein-kinin system in diabetic nephropathy. Kidney Int. 2012;81(8):733–744. doi: 10.1038/ki.2011.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merchant ML, Niewczas MA, Ficociello LH, et al. Plasma kininogen and kininogen fragments are biomarkers of progressive renal decline in type 1 diabetes. Kidney Int. 2013;83(6):1177–1184. doi: 10.1038/ki.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahluwalia A, Perretti M. Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1 beta in vivo in the mouse. J Immunol. 1996;156(1):269–274. [PubMed] [Google Scholar]
- 59.Bockmann S, Paegelow I. Kinins and kinin receptors: importance for the activation of leukocytes. J Leukoc Biol. 2000;68(5):587–592. [PubMed] [Google Scholar]
- 60.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3(9):2007–2018. [PubMed] [Google Scholar]
- 61.Hong SL. Effect of bradykinin and thrombin on prostacyclin synthesis in endothelial cells from calf and pig aorta and human umbilical cord vein. Thromb Res. 1980;18(6):787–795. doi: 10.1016/0049-3848(80)90201-7. [DOI] [PubMed] [Google Scholar]
- 62.Wheelock KM, Cai J, Looker HC, et al. Plasma bradykinin and early diabetic nephropathy lesions in type 1 diabetes mellitus. PloS One. 2017;12(7):e0180964. doi: 10.1371/journal.pone.0180964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tong PC, Lee KF, So WY, et al. White blood cell count is associated with macro- and microvascular complications in chinese patients with type 2 diabetes. Diabetes Care. 2004;27(1):216–222. doi: 10.2337/diacare.27.1.216. [DOI] [PubMed] [Google Scholar]
- 64.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(2):455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 65.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wheelock KM, Saulnier PJ, Tanamas SK, et al. White blood cell fractions correlate with lesions of diabetic kidney disease and predict loss of kidney function in Type 2 diabetes. Nephrol Dial Transplant. doi: 10.1093/ndt/gfx231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pertynska-Marczewska M, Kiriakidis S, Wait R, Beech J, Feldmann M, Paleolog EM. Advanced glycation end products upregulate angiogenic and pro-inflammatory cytokine production in human monocyte/macrophages. Cytokine. 2004;28(1):35–47. doi: 10.1016/j.cyto.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 68.Shurtz-Swirski R, Sela S, Herskovits AT, et al. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care. 2001;24(1):104–110. doi: 10.2337/diacare.24.1.104. [DOI] [PubMed] [Google Scholar]
- 69.Looker HC, Colombo M, Hess S, et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 2015;88(4):888–896. doi: 10.1038/ki.2015.199. [DOI] [PubMed] [Google Scholar]
- 70.Inker LA, Coresh J, Sang Y, et al. Filtration markers as predictors of ESRD and mortality: individual participant data meta-analysis. Clin J Am Soc Nephrol. 2017;12(1):69–78. doi: 10.2215/CJN.03660316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schardijn GH, Statius van Eps LW. Beta 2-microglobulin: its significance in the evaluation of renal function. Kidney Int. 1987;32(5):635–641. doi: 10.1038/ki.1987.255. [DOI] [PubMed] [Google Scholar]
- 72.Zhou LT, Lv LL, Pan MM, et al. Are urinary tubular injury markers useful in chronic kidney disease? A systematic review and meta analysis. PloS One. 2016;11(12):e0167334. doi: 10.1371/journal.pone.0167334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nauta FL, Boertien WE, Bakker SJ, et al. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care. 2011;34(4):975–981. doi: 10.2337/dc10-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uslu S, Efe B, Alatas O, et al. Serum cystatin C and urinary enzymes as screening markers of renal dysfunction in diabetic patients. J Nephrol. 2005;18(5):559–567. [PubMed] [Google Scholar]
- 75.Papale M, Di Paolo S, Magistroni R, et al. Urine proteome analysis may allow noninvasive differential diagnosis of diabetic nephropathy. Diabetes Care. 2010;33(11):2409–2415. doi: 10.2337/dc10-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rebholz CM, Inker LA, Chen Y, et al. Risk of ESRD and mortality associated with change in filtration markers. Am J Kidney Dis. doi: 10.1053/j.ajkd.2017.04.025. 10.1053j.ajkd.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mise K, Hoshino J, Ueno T, et al. Prognostic value of tubulointerstitial lesions, urinary N-acetyl-beta-d-glucosaminidase, and urinary beta2-microglobulin in patients with type 2 diabetes and biopsy-proven diabetic nephropathy. Clin J Am Soc Nephrol. 2016;11(4):593–601. doi: 10.2215/CJN.04980515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sabbisetti VS, Waikar SS, Antoine DJ, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25(10):2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coca SG, Nadkarni GN, Huang Y, et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol. doi: 10.1681/ASN.2017010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baker MA, Davis SJ, Liu P, et al. Tissue-specific microRNA expression patterns in four types of kidney disease. J Am Soc Nephrol. doi: 10.1681/ASN.2016121280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bijkerk R, Duijs JM, Khairoun M, et al. Circulating microRNAs associate with diabetic nephropathy and systemic microvascular damage and normalize after simultaneous pancreas-kidney transplantation. Am J Transplant. 2015;15(4):1081–1090. doi: 10.1111/ajt.13072. [DOI] [PubMed] [Google Scholar]
- 82.Bhatt K, Kato M, Natarajan R. Mini-review: emerging roles of microRNAs in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. 2016;310(2):F109–118. doi: 10.1152/ajprenal.00387.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ko YA, Mohtat D, Suzuki M, et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol. 2013;14(10):R108. doi: 10.1186/gb-2013-14-10-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siegfried Z, Eden S, Mendelsohn M, Feng X, Tsuberi BZ, Cedar H. DNA methylation represses transcription in vivo. Nat Genet. 1999;22(2):203–206. doi: 10.1038/9727. [DOI] [PubMed] [Google Scholar]
- 85.Yang XH, Cao RF, Yu Y, et al. A study on the correlation between MTHFR promoter methylation and diabetic nephropathy. Am J Transl Res. 2016;8(11):4960–4967. [PMC free article] [PubMed] [Google Scholar]
- 86.Aldemir O, Turgut F, Gokce C. The association between methylation levels of targeted genes and albuminuria in patients with early diabetic kidney disease. Ren Fail. 2017;39(1):597–601. doi: 10.1080/0886022X.2017.1358180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leung G, Kirpalani A, Szeto SG, et al. Could MRI be used to image kidney fibrosis? A review of recent advances and remaining barriers. Clin J Am Soc Nephrol. 2017;12(6):1019–1028. doi: 10.2215/CJN.07900716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li J, An C, Kang L, Mitch WE, Wang Y. Recent advances in magnetic resonance imaging assessment of renal fibrosis. Adv Chronic Kidney Dis. 2017;24(3):150–153. doi: 10.1053/j.ackd.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao J, Wang ZJ, Liu M, et al. Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol. 2014;69(11):1117–1122. doi: 10.1016/j.crad.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 90.Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22(8):1429–1434. doi: 10.1681/ASN.2010111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269(5232):1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 93.Lee CU, Glockner JF, Glaser KJ, et al. MR elastography in renal transplant patients and correlation with renal allograft biopsy: a feasibility study. Acad Radiol. 2012;19(7):834–841. doi: 10.1016/j.acra.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morrell GR, Zhang JL, Lee VS. Magnetic resonance imaging of the fibrotic kidney. J Am Soc Nephrol. doi: 10.1681/ASN.2016101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Looker HC, Nelson RG. Reading the tree leaves-how to enrich clinical trials of diabetic kidney disease. Kidney Int. 2017;92(1):23–25. doi: 10.1016/j.kint.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]