Abstract
Black living kidney donors are at higher risk of developing kidney disease than white donors. We examined the effect of the APOL1 high-risk genotype on postdonation renal function in black living kidney donors and evaluated whether this genotype alters the association between donation and donor outcome. We grouped 136 black living kidney donors as APOL1 high-risk (two risk alleles; n=19; 14%) or low-risk (one or zero risk alleles; n=117; 86%) genotype. Predonation characteristics were similar between groups, except for lower mean±SD baseline eGFR (CKD-EPI equation) in donors with the APOL1 high-risk genotype (98±17 versus 108±20 ml/min per 1.73 m2; P=0.04). At a median of 12 years after donation, donors with the APOL1 high-risk genotype had lower eGFR (57±18 versus 67±15 ml/min per 1.73 m2; P=0.02) and faster decline in eGFR after adjusting for predonation eGFR (1.19; 95% confidence interval, 0 to 2.3 versus 0.4; 95% confidence interval, 0.1 to 0.7 ml/min per 1.73 m2 per year, P=0.02). Two donors developed ESRD; both carried the APOL1 high-risk genotype. In a subgroup of 115 donors matched to 115 nondonors by APOL1 genotype, we did not find a difference between groups in the rate of eGFR decline (P=0.39) or any statistical interaction by APOL1 status (P=0.92). In conclusion, APOL1 high-risk genotype in black living kidney donors associated with greater decline in postdonation kidney function. Trajectory of renal function was similar between donors and nondonors. The association between APOL1 high-risk genotype and poor renal outcomes in kidney donors requires validation in a larger study.
Keywords: kidney donation, renal function, human genetics
Kidney transplantation, particularly from a living donor, is the preferred treatment for patients with ESRD.1 Although live kidney donation is promoted worldwide, it is imperative to continue to clarify the long-term risks of unilateral nephrectomy in donors of all races.2 Recent studies have shown an increased risk of hypertension and ESRD in black donors compared with matched black nondonors.3,4 The risk of ESRD is also higher among donors biologically related to the recipients.4,5 In the United States, 88% of black donors are biologically related to their recipients.6 These findings suggest that contributing factors to the poorer renal outcomes observed in a small proportion of donors include interactions between the effect of donation and genetic predisposition.
Genetic variants in APOL1 have been associated with increased susceptibility to nondiabetic CKD in blacks.7,8 The APOL1 high-risk genotype is composed of two renal risk alleles, and it is associated with a greater risk of FSGS compared with the presence of one or zero renal risk alleles.7 Forty percent of blacks on dialysis carry two APOL1 renal risk alleles compared with approximately 12% in the general population.9 It is possible that the increased risk of kidney disease observed in black live kidney donors might be explained, at least in part, by APOL1 renal risk alleles that are shared between the donor and their related recipient. To date, there have been two patient reports of black live kidney donors developing FSGS 3–7 years after donation; both of them were found to carry two APOL1 renal risk alleles.10,11 A recent study reported that young blacks with good baseline health (suitable potential donors who did not donate) carrying two APOL1 renal risk variants were at highest risk of developing CKD in the future.12
For these reasons, we decided to expand and extend our prior study of black donor outcomes,3 and we performed APOL1 genotyping. We first evaluated whether the presence of the APOL1 high-risk genotype in black live kidney donors associates with pre- and postdonation renal function. We next evaluated whether the presence of the APOL1 high-risk genotype altered the association between donation and outcomes by studying a subpopulation of black donors who were successfully matched to suitable black nondonor controls with similar indicators of baseline health, family history of ESRD in a first degree relative, and APOL1 genotype.
Results
Comparing Renal Function between donors grouped by APOL1 Risk Status
A total of 136 donors were studied at a median (25th, 75th percentile) of 12 (9, 12.5) years after donation. Their mean age was 37±9 years old, 87 donors (64%) were women, and 106 donors (78%) were first degree relatives of the recipient. The frequencies of carrying zero, one, and two APOL1 renal risk alleles were 67 (49%), 50 (37%), and 19 (14%), respectively. The predonation characteristics of high- and low-risk genotype donors were similar except for predonation eGFR, which was lower in donors with high-risk genotype (98±17 versus 108±20 ml/min per 1.73 m2; P=0.03) (Table 1).
Table 1.
Predonation characteristics of live kidney donors on the basis of APOL1 renal risk genotype
| Predonation Characteristics | High-Risk Genotype, n=19 | Low-Risk Genotype, n=117 | P Value |
|---|---|---|---|
| Age, yr | 37±9 | 37±9 | 0.84 |
| Women, % | 61 | 63 | 0.85 |
| Weight, kg | 85±16 | 82±18 | 0.49 |
| Body mass index, kg/m2 | 30±5 | 29±6 | 0.45 |
| Systolic BP, mm Hg | 121±7 | 120±10 | 0.56 |
| Diastolic BP, mm Hg | 72±8 | 73±8 | 0.61 |
| Serum creatinine, mg/dl | 0.98±0.18 | 0.89±0.18 | 0.06 |
| eGFR, ml/min per 1.73 m2 | 98±17 | 108±20 | 0.03 |
| Fasting blood sugars, mg/dl | 82±12 | 81±12 | 0.62 |
| Education, % | 0.09 | ||
| None to 8th grade | 11 | 1 | |
| 9th–11th Grade | 6 | 15 | |
| High school | 40 | 34 | |
| Some college | 33 | 38 | |
| Bachelors | 11 | 6 | |
| Postgraduate | 0 | 6 | |
| Individual income, % | 0.97 | ||
| <$12,000 | 11 | 13 | |
| $12,000–$25,000 | 28 | 28 | |
| >$25,000 | 61 | 60 | |
| Employed, full or part time, % | 83 | 80 | 0.79 |
| Family history of hypertension, yes, % | 79 | 71 | 0.48 |
| Family history of ESRD, yes, % | 89 | 76 | 0.20 |
| Relationship to the recipient, % | 0.27 | ||
| Parent | 11 | 9 | |
| Sibling | 37 | 38 | |
| Child | 42 | 28 | |
| Spouse | 5 | 9 | |
| Other | 5 | 16 |
High-risk genotype is defined as carrying two APOL1 renal risk alleles, and low-risk genotype is defined as carrying one or zero APOL1 renal risk alleles. Data are presented as mean±SD. To convert serum creatinine from milligrams per deciliter to micromoles per liter, multiply by 88.4. Family history of hypertension and ESRD was defined as first degree relative with these conditions.
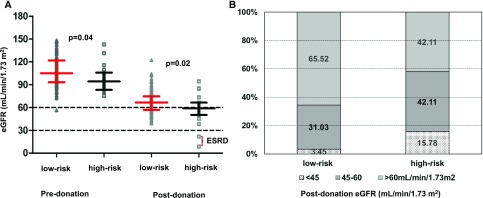
At last follow-up, there was no difference between the two groups of donors (APOL1 high-risk versus low-risk genotype) with respect to time from donation, weight, absolute values of systolic and diastolic BP, and the prevalence of hypertension (Table 2). The donors with high-risk genotype continued to have lower eGFR (57±18 versus 67±15 ml/min per 1.73 m2; P=0.02) (Figure 1A), and a greater proportion had eGFR<60 ml/min per 1.73 m2 (58% versus 34.5%; P=0.03) (Figure 1B). Results remained unchanged when postdonation eGFR was calculated via the Modification of Diet in Renal Disease study equation (Table 2).13 Although donors with APOL1 high-risk genotype had a higher urine albumin-to-creatinine ratio (uACR; 8.39 [2.6–23.0] versus 4.99 [2.5–11.6] mg/g; P=0.01), the proportion of donors developing microalbuminuria was not different between the two groups (P=0.86).
Table 2.
Postdonation outcomes between live kidney donors on the basis of APOL1 renal risk genotype
| Postdonation Outcomes | High-Risk Genotype, n=19 | Low-Risk Genotype, n=117 | P Value |
|---|---|---|---|
| Time since donation, yr | 11.3 [9.1–12.5] | 11.6 [9.1–13.6] | 0.75 |
| Postdonation weight, kg | 89±18 | 88±18 | 0.72 |
| Change in weight since donation, kg | +4.8±17 | +5.5±17 | 0.87 |
| Systolic BP, mm Hg | 128±12 | 130±19 | 0.70 |
| Change in systolic BP since donation, mm Hg | +7±12 | +10±19 | 0.48 |
| Diastolic BP, mm Hg | 82±10 | 83±27 | 0.81 |
| Change in diastolic BP since donation, mm Hg | +10±12 | +7±12 | 0.89 |
| Hypertension, % | 44 | 49 | 0.74 |
| Treated for hypertension with medication, % | 75 | 67 | 0.29 |
| Serum creatinine, mg/dl | 1.71±1.2 | 1.26±0.3 | 0.003 |
| CKD-EPI eGFR, ml/min per 1.732 m2 | 57±20 | 67±15 | 0.02 |
| CKD-EPI eGFR <60 ml/min per 1.732 m2, % | 67 | 36 | 0.01 |
| MDRD eGFR, ml/min per 1.732 m2 | 58±20 | 68±14 | 0.01 |
| MDRD eGFR <60 ml/min per 1.732 m2, % | 53 | 32 | <0.01 |
| ESRD, % | 11 | 0 | 0.02 |
| uACR, mg/g | 8.39 [2.6–23.0] | 4.99 [2.5–11.6] | 0.01 |
| Microalbuminuria, % | 16 | 10 | 0.86 |
High-risk genotype is defined as carrying two APOL1 renal risk alleles, and low-risk genotype is defined as carrying one or zero APOL1 renal risk alleles. Data are presented as mean±SD if normally distributed or median [interquartile range] in not normally distributed. To convert serum creatinine from milligrams per deciliter to micromoles per liter, multiply by 88. ESRD was defined by receipt of dialysis or kidney transplant. Microalbuminuria was defined as uACR>30 mg/g or >3 mg/mmol. MDRD, Modification of Diet in Renal Disease.
Figure 1.
Lower pre- and post-donation eGFR in donors with high-risk APOL1 genotype than low-risk APOL1 genotype. Part A shows that the predonation and postdonation eGFRs were lower in donors carrying two APOL1 renal risk alleles (or high-risk genotype) than those carrying zero or one APOL1 renal risk allele (or low-risk genotype). Part B shows that a greater fraction of donors with two APOL1 renal risk alleles (high-risk genotype) had postdonation eGFR<60 ml/min per 1.73 m2. eGFR was calculated via the CKD-EPI formula.
Two donors developed ESRD due to proteinuric kidney disease (not biopsied) at 10 and 18 years postdonation. Neither of these two donors had developed diabetes after donation, but both had hypertension. Both carried the APOL1 high-risk genotype and were related to the recipient. Both donors have undergone kidney transplantation. Per the United Network of Organ Sharing (UNOS), of our study cohort of 249 donors, five donors had died, and two donors reached ESRD and underwent a kidney transplant. None of the donors died from renal causes. Both of these donors with ESRD who underwent a kidney transplant participated in our study.
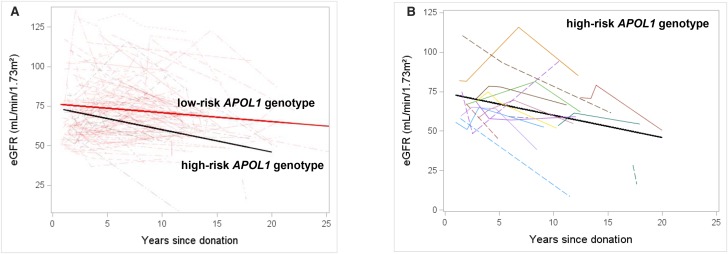
Figure 2A shows the rate of decline in postdonation eGFR in donors with APOL1 high-risk and low-risk genotypes. After adjusting for baseline predonation eGFR, donors with the high-risk APOL1 genotype showed a faster rate of decline in eGFR than donors with the low-risk genotype (1.1; 95% confidence interval [95% CI], 0 to 2.3 versus 0.4; 95% CI, 0.1 to 0.7 ml/min per 1.73 m2 per year; P=0.02). Figure 2B shows changes in postdonation eGFR for each donor carrying the high-risk APOL1 genotype.
Figure 2.
Rate of decline in postdonation eGFR is greater among donors with high-risk versus low-risk APOL1 genotype. Serum creatinine values spanning the period from 12 months after donation (to allow for renal compensation) to last follow-up were abstracted on all participating donors. eGFR was calculated at each of the time points via the CKD-EPI formula using serum creatinine and age of the donor at these time points. A shows that the donors carrying two APOL1 renal risk alleles (or high-risk genotype) had faster decline in postdonation eGFR than those carrying zero or one APOL1 renal risk allele (or low-risk genotype). B shows the postdonation eGFR trajectory on 19 APOL1 high-risk genotype donors.
Comparing Renal Function in Donors and Nondonors Matched for APOL1 Risk Status and Other Characteristics
Of the 136 donors who were in our primary analysis, 115 donors were successfully matched to 115 nondonor controls from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. The baseline characteristics between the two groups were similar, except for health insurance, education, and income (Supplemental Table 1). The donors were assessed at a median (25th, 75th percentile) of 11.6 (9.1, 12.8) years after cohort entry (which in the case of donors, was the date of nephrectomy); corresponding values in nondonors were 9.9 (9.3, 14.6) years.
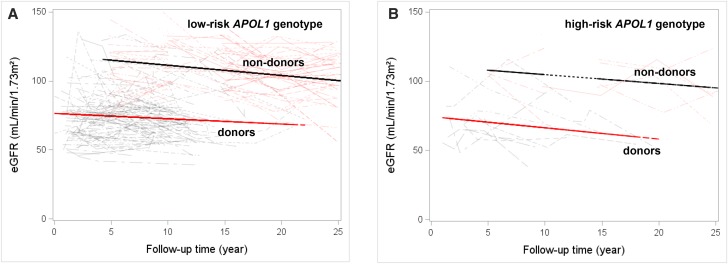
As anticipated, at follow-up, eGFR was lower in donors than nondonors (68±18 versus 109±21 ml/min per 1.73 m2; P<0.01) due to unilateral nephrectomy. However, the uACR was similar in donors and nondonors (4.8 [2.3–13.1] versus 4.9 [3.6–7.6] mg/g; P=0.10). One donor developed ESRD, and none developed ESRD in the nondonor cohort. Figure 3 shows that the rate of decline in eGFR was similar in donors and nondonors grouped by APOL1 renal risk genotype. After adjusting for socioeconomic differences, there was no difference in the rate of change in eGFR in the two groups (donors versus nondonors: 0.5; 95% CI, 0.1 to 0.8 versus 0.6; 95% CI, 0.3 to 0.9 ml/min per 1.73 m2 per year; P=0.39). There was no statistical interaction by APOL1 status (P=0.92; i.e., the presence of high-risk APOL1 [versus absence] did not modify the above associations between donation [versus nondonation] and eGFR outcomes).
Figure 3.
Similar rate of decline in eGFR between donors and matched nondonors grouped by APOL1 genotype. eGFR was calculated for donors and nondonors via the CKD-EPI formula from serum creatinine values spanning the period from 12 months postdonation (to allow for renal compensation in the donors) and at the subsequent examination year, which is at every 5 years (for nondonors), to the last follow-up. There was no difference in change in eGFR between donors and nondonors stratified by APOL1 renal risk status. A depicts comparison of eGFR between donors and nondonors with zero or one APOL1 renal risk allele (low-risk genotype), and B depicts comparison of eGFR between donors and nondonors with two APOL1 renal risk alleles (high-risk genotype).
Both systolic and diastolic BP readings were higher in donors compared with nondonors at follow-up (128±16 versus 119±13 mm Hg; P<0.001 and 83±27 versus 76±10 mm Hg; P=0.02, respectively). The prevalence of hypertension was higher in the donors compared with the nondonors: 47 of 115 (41%) versus 20 of 115 (17%; P<0.001). There was no difference in weight between the two groups (88±17 versus 87±18 kg; P=0.79).
Discussion
The APOL1 high-risk genotype in black live kidney donors is associated with lower pre- and postdonation eGFR as well as a faster decline in postdonation eGFR compared with in donors with the APOL1 low-risk genotype. However, we did not observe an exaggerated decline in kidney function that could be attributable to donation in the presence of the APOL1 high-risk genotype when donors were compared with similar genotype nondonor subjects who would have otherwise qualified for kidney donation.
This is the first study examining the effect of the APOL1 high-risk genotype in black live kidney donors on postdonation renal function. In this cohort, 80% of the donors were biologically related to the recipients, and 78% had first degree relatives with ESRD; both rates are similar to those reported nationally for blacks.6 The prevalence of the APOL1 high-risk genotype among black live kidney donor was 14%, similar to that reported in the general population but lower than the one reported for first degree relatives of blacks with nondiabetic ESRD, which is approximately 23%.8,14,15 Despite the high fraction of biologically related donors, the prevalence of two APOL1 renal risk alleles was not higher than in the general population, perhaps due to vigorous screening for hypertension and kidney disease before donation.
Persons with the APOL1 high-risk genotype are consistently reported to have a lower eGFR than those with the low-risk genotype.16 In this study, we also observed that predonation eGFR was lower among donors with the APOL1 high-risk genotype, and at follow-up, they continued to have lower eGFR, faster decline in eGFR, and a higher uACR than the low-risk group. There was a greater proportion of donors with eGFR<60 ml/min per 1.73 m2 in the high-risk genotype group. Two donors developed ESRD, and both participated in our study and carried the APOL1 high-risk genotype. Because of our inability to genotype the entire eligible study cohort and small sample size, we were unable to accurately determine ESRD rates among donors with the APOL1 high-risk genotype. Although the observed 11% rate in this sample was substantial, it could potentially be overestimated, and therefore, it should be interpreted with caution. A similar trajectory of renal function at follow-up between a subset of donors and healthy nondonors matched to APOL1 status suggests that donation did not accelerate loss of renal function in individuals carrying two APOL1 renal risk alleles.
Among donors carrying the APOL1 low-risk genotype, the postdonation eGFR, the rate of decline in eGFR, and the proportion developing microalbuminuria were similar to those reported among white donors by Ibrahim et al.17 (68±14 versus 64±11 ml/min per 1.73 m2, 0.4±0.2 versus 0.6±3.8 ml/min per 1.73 m2 per year, and 10% versus 9%, respectively). It is possible that the absence of the APOL1 high-risk genotype might mitigate the risk of poor renal outcomes traditionally observed in black donors.
There was no difference in the prevalence of hypertension between donors on the basis of the APOL1 genotype. Of note, one third to one quarter of kidney donors were unaware of being hypertensive, and therefore, they were not on treatment. The donors were more likely to develop hypertension than nondonors, even after matching for family history of ESRD and APOL1 genotype. The APOL1 high-risk genotype is not associated with postdonation development of hypertension among black live kidney donors.
Strengths of our study include long duration of follow-up of live kidney donors and nondonors. Study data were obtained in person for 96% of the donors and all of the nondonors. The careful selection and matching of nondonor controls for familial and genetic factors help to elucidate the risks directly associated with kidney donation. APOL1 genotyping of the donors and nondonors was performed by the same laboratory at the National Institutes of Health (NIH). Serum creatinine at last follow-up among the donors and for study visits in nondonors was measured via enzymatic assay.
Limitations of our study are the relatively small sample size and 55% recruitment of eligible donors. Modest ascertainment rates have been the Achilles heel for all prospective donor studies in the United States (37%–65%).17,18 The predonation characteristics and family history of ESRD did not differ between the donors who did and did not participate (Supplemental Table 2), ruling out selection bias. Furthermore, some controls were used more than once; however, they were selected from different examination years, and therefore, they had unique baseline examination and follow-up time. Although the most recent follow-up eGFR among donors was computed using serum creatinine measured via enzymatic assay, the interval eGFR (from donation to last follow-up) was computed using serum creatinine concentrations from two medical centers, and therefore, the assays were not uniform. Lastly, the nondonors differed from donors on socioeconomic variables, which could potentially have affected our outcomes.
Our study shows that the APOL1 high-risk genotype is associated with lower pre- and postdonation eGFR and a faster decline in renal function in living kidney donors compared with the low-risk genotype. The observed ESRD rate of 11% in our small study is substantial, but it may be inaccurate due to our inability to genotype the entire eligible study cohort. Compared with in healthy nondonors, live kidney donation per se does not amplify the risk associated with APOL1-related kidney disease. Given the small size of our study, we cannot make any definitive recommendations about genotyping all potential donors. However, in light of our findings and recent reports of the APOL1 high-risk genotype in the deceased donor being associated with inferior recipient allograft survival,19,20 additional studies are urgently needed to address the effect of the APOL1 genotype in live kidney donors on both donor and recipient outcomes. The NIH-initiated prospective study encompassing all deceased and live black kidney donors in the United States (i.e., the APOL1 Long-Term Kidney Transplantation Outcomes Network) is designed to address some of these issues.21
Concise Methods
Study Design and Oversight
We conducted an observational cohort study of donors who donated a kidney between 1993 and 2010 at two transplant centers in Detroit, Michigan (Supplemental Material). All donors provided written informed consent. The study protocols were approved by the institutional review boards at both recruitment sites and the genetic testing site (i.e., the NIH). The study adhered to the Declaration of Istanbul.
Study Subjects
Live Kidney Donors
Of the 244 donors eligible to participate in the study, 136 (56%) agreed (Supplemental Figure 1). At follow-up, height, weight, and BP were measured, and blood and urine samples were collected. Five donors unable to come for a study visit mailed a sample of their saliva for genotyping, and relevant study information was abstracted from their medical records. All serum creatinine values spanning the period from 12 months after donation (to allow for renal compensation to occur) to last follow-up were abstracted, and eGFR was calculated via the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation22 using serum creatinine value and age at the time of follow-up. Donors were queried about ever receiving chronic dialysis or being transplanted, and when this occurred, follow-up time was truncated at the time of ESRD onset. Their last serum creatinine value before starting dialysis was used to compute eGFR. The UNOS was also contacted by each participating center to determine if any of their donors who had donated during the study period (i.e., eligible for participation in the study) had initiated dialysis, were waitlisted or received a kidney transplant, or died.
Nondonor Control Subjects
A comparable group of blacks was selected from the CARDIA Study cohort.23 After reviewing baseline records of 2637 participants, 958 (36%) of the CARDIA Study participants fulfilled the restriction criteria to be considered suitable for live kidney donation. The second step consisted of finding nondonors who matched donors with regard to baseline age, sex, systolic BP, family history of ESRD in a first degree relative, APOL1 genotype, and duration of follow-up. At the end, 115 nondonors were successfully matched to 115 donors (Supplemental Figure 2).
Serum creatinine values from subsequent CARDIA Study examination years (after enrollment examination years) were used to calculate eGFR at various time points in follow-up via the CKD-EPI equation. Information on need for chronic dialysis or transplant among the CARDIA Study participants was also ascertained from their most recent follow-up interviews. Family history of ESRD in a first degree relative was also collected at the last visit.24
Groups by APOL1 Status
The donors with zero or one APOL1 renal risk allele were grouped together as low-risk genotype, and those with two risk alleles are referred to as high-risk genotype. The nondonors were also grouped similarly on the basis of the number of APOL1 renal risk alleles.
Outcomes
The primary outcome for all study subjects was average annual change in eGFR from 1 year after cohort entry. We also examined the following secondary outcomes: (1) urinary albumin excretion estimated via spot uACR and microalbuminuria defined by a random uACR >30 mg/g (SI units: >3.0 mg/mmol) and (2) hypertension defined by a systolic BP ≥140 mm Hg, a diastolic BP ≥90 mm Hg, or the use of antihypertensive medications.
These outcomes were compared between donors with and without the APOL1 high-risk genotype and also between donor and matched nondonor controls.
Statistical Analyses
Data are presented as mean (SD) for normally distributed data and median (interquartile range) for nonparametric data. We assessed differences in baseline characteristics between groups using independent sample t tests, Mann–Whitney U tests, and Fisher exact tests before matching and paired t tests, Wilcox signed rank tests, McNemar tests, or Mantel–Haenszel tests as appropriate after matching. We used the “greedy matching” macro25 to match nondonors to donor at a 1:1 ratio by the following characteristics: age (5-year interval), sex, duration of follow-up (5-year interval), family history of ESRD in a first degree relative, systolic BP (15-mm Hg interval), and APOL1 genotype (low versus high risk). The number of available eligible nondonors determined the donor sample size. The paired t tests or Wilcox signed rank tests were used, as appropriate, to compare BP, eGFR, and uACR at follow-up. The mixed effect model with repeated measure was used to compare the rate of decline in renal function. Mixed effect models were used to derive the eGFR decline rate, which has flexibility in treating follow-up time as a continuous variable. In the longitudinal analysis, we included baseline eGFR in the model, assuming that the baseline eGFR is independent of donation (intervention).26 We tested for interaction in the matched donor nondonor outcomes to see if the effects were modified by the presence or absence of APOL1 risk. All tests of statistical significance were two-tailed tests, and we interpreted α<0.05 as statistically significant. We used SAS version 9.4 (SAS Institute, Cary, NC) to perform the analyses.
Disclosures
A.X.G. received partnership funding from Astellas for a research grant on live kidney donation funded by the Canadian Institutes of Health Research.
Supplementary Material
Acknowledgments
Dr. Rohini Parshar (Henry Ford Transplant Institute, Detroit, MI) helped in retrieving follow-up information on some of the donors. This manuscript was prepared using Coronary Artery Risk Development in Young Adults (CARDIA) Study research materials obtained from the National Heart, Lung, and Blood Institute.
C.A.W. and J.B.K. acknowledge funding support from the Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. This project has been funded, in part, with federal funds from Center for Cancer Research, National Cancer Institute, National Institutes of Health contract HHSN26120080001E. A.X.G. is supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a Clinician Investigator Award from the Canadian Institutes of Health Research.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, and the mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Evaluation of Potential Living Kidney Donors in the APOL1 Era,” on pages 1079–1081.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017060658/-/DCSupplemental.
References
- 1.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S: High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med 333: 333–336, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Allen MB, Reese PP: Transforming living kidney donation with a comprehensive strategy. PLoS Med 13: e1001948, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doshi MD, Goggins MO, Li L, Garg AX: Medical outcomes in African American live kidney donors: A matched cohort study. Am J Transplant 13: 111–118, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, et al. : Risk of end-stage renal disease following live kidney donation. JAMA 311: 579–586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mjøen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Øyen O, et al. : Long-term risks for kidney donors. Kidney Int 86: 162–167, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Lentine KL, Schnitzler MA, Garg AX, Xiao H, Axelrod D, Tuttle-Newhall JE, et al. : Race, relationship and renal diagnoses after kiving kidney donation. Transplantation 99: 1723–1729, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, et al. : MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 40: 1175–1184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. : Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma L, Langefeld CD, Comeau ME, Bonomo JA, Rocco MV, Burkart JM, et al. : APOL1 renal-risk genotypes associate with longer hemodialysis survival in prevalent nondiabetic African American patients with end-stage renal disease. Kidney Int 90: 389–395, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwang NA, Shetty A, Sustento-Reodica N, Gordon EJ, Leventhal J, Gallon L, et al. : APOL1-associated end-stage renal disease in a living kidney transplant donor. Am J Transplant 16: 3568–3572, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Kofman T, Audard V, Narjoz C, Gribouval O, Matignon M, Leibler C, et al. : APOL1 polymorphisms and development of CKD in an identical twin donor and recipient pair. Am J Kidney Dis 63: 816–819, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Locke JE, Sawinski D, Reed RD, Shelton B, MacLennan PA, Kumar V, et al. : Apolipoprotein L1 and chronic kidney disease risk in young potential living kidney donors [published online ahead of print February 9, 2017]. Ann Surg 10.1097/SLA.0000000000002174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, et al. : The genetic structure and history of Africans and African Americans. Science 324: 1035–1044, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman BI: APOL1 and nephropathy progression in populations of African ancestry. Semin Nephrol 33: 425–432, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, et al. : Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol 27: 2842–2850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. : Long-term consequences of kidney donation. N Engl J Med 360: 459–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogueira JM, Weir MR, Jacobs S, Haririan A, Breault D, Klassen D, et al. : A study of renal outcomes in African American living kidney donors. Transplantation 88: 1371–1376, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. : The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant 11: 1025–1030, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman BI, Julian BA, Pastan SO, Israni AK, Schladt D, Gautreaux MD, et al. : Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant 15: 1615–1622, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.APOL1 Long-Term Kidney Transplantation Outcomes Network (APOLLO) Clinical Centers: (Collaborative U01), Bethesda, MD, National Institutes of Health, 2017 [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, et al. : CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 41: 1105–1116, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Exam Components-All Years CARDIA, 2010. Available at: http://www.cardia.dopm.uab.edu/exam-materials2/exam-components.
- 25.Available at: http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros#general.
- 26.Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV: Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat Med 28: 2509–2530, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.