Abstract
To clarify the origins of parent-child resemblance for drug abuse (DA), using national Swedish data, we fit path models to information on DA in parents and children from six informative family types: i) not-lived-with father, ii) not-lived-with mother, iii) step-father, iv) step-mother, v) triparental, and vi) adoptive. From these families, we estimated parent-offspring resemblance reflecting the effects of genes + rearing, genes only and rearing only. The estimates of parent-offspring correlations were statistically homogenous across family types. The weighted estimate of the father-offspring correlation for DA for genes + rearing, genes only and rearing only relationships were, respectively, +0.26, +0.19 and +0.06. Parallel figures for mother-offspring relationships were +0.19, +0.13 and +0.09. In both genes + rearing and genes only parent-offspring relationships, DA correlations were stronger for fathers than for mothers. Both genetic and environmental factors contribute substantially to parent-offspring resemblance for DA and appear to be additive.
Keywords: drug abuse, Sweden, genetics, parent-offspring, rearing, environment
INTRODUCTION
Because of the wide availability of twin samples (Hur and Craig, 2013), the genetic epidemiology of drug abuse (DA) has focused, in recent years, largely on clarifying the contributions of genetic and environmental factors to the well-demonstrated within-generation familial aggregation of DA (Bierut et al., 1998; Merikangas et al., 1998). While this is a critical endeavor, for the purposes of primary prevention, understanding the causes of the cross-generational transmission of DA (that is, parent to child transmission) is probably of greater import.
With this goal in mind, we have recently conducted a series of inter-related studies examining the sources of the association of drug abuse between parents and offspring. Each study used a different design to address aspects of cross-generational transmission. These have included a classical adoption design (Kendler et al., 2012), a study of “not-lived-with” and step-parents (Kendler et al., 2015c), and most recently an investigation of triparental families containing a rearing biological mother and a step-father (Kendler et al., 2015b), and a not-lived-with father (where not-lived-with parents were defined as biological parents who never resided with or near their offspring). The analyses of these studies all utilized an epidemiological framework and calculated odds or hazard ratios for DA in offspring as a function of the DA status of various classes of parents. Such statistical models have a number of advantages and are very commonly used as measures of association in general medical and psychiatric, and drug abuse epidemiology (Kraemer, 2006).
However, modern genetic epidemiological approaches to DA have sought to decompose resemblance among various relatives into its genetic and environmental components utilizing liability-threshold models initially developed by Pearson (Pearson, 1901) and later Falconer (Falconer, 1965). This model assumes that disease liability is the result of very large numbers of genetic and environmental risk factors each of small effect. As dictated by statistical theory and confirmed by simulation (Kendler and Kidd, 1986), this liability assumes a normal or “Gaussian” shape. It is further assumed that those above a threshold on this liability become affected.
This approach, operationalized using path or structural equation models, is quite different from the standard epidemiological methods used to calculate odds or hazard ratios. For example, there is no simple linear transformation from odds ratios (ORs) or hazard ratios (HRs) into correlations of liability which can in turn be decomposed into genetic and environmental pathways. In particular, for a given correlation of liability, the OR or HR will change as a function of the prevalence of the disorder becoming higher as the disorder becomes rarer.
Our goal in this paper is to further clarify the sources of parent-offspring transmission for DA. Therefore, we apply liability-threshold models as part of a path analysis to patterns of parent-offspring resemblance for DA from six unique family types from Swedish national data: i) not-lived-with fathers, ii) not-lived-with mothers, iii) step-fathers, iv) step-mothers, v) triparental, and vi) adoptive. We compare across samples estimates for the role of genetic and environmental factors in the cross-generational transmission of risk to DA from parents. To do this, we divide possible parenting figures into three categories: those who contribute, to a first approximation, i) both genes and rearing, ii) genes only and iii) rearing only. We then jointly estimate across samples parent-offspring resemblance for these three parental types. These analyses will also permit us to compare the heritability of DA estimated from biological-parent correlations with those obtained using twins from the same country and utilizing the same diagnostic definition (Kendler et al., 2013).
METHODS
We used linked data from multiple Swedish nationwide registries and healthcare data. Linking was achieved via the unique individual 10-digit personal ID number assigned at birth or immigration to all Swedish residents. In order to preserve confidentiality this ID number was replaced by a serial number.
The following sources were used to create our database: Total Population Register, containing annual data on family and geographical status; Multi-Generation Register, providing information on family relations; Swedish Hospital Discharge Register, containing all hospitalizations for all Swedish inhabitants from 1964–2010; Swedish Prescribed Drug Register, containing all prescriptions in Sweden picked up by patients from 2005 to 2010; Outpatient Care Register, containing information from all outpatient clinics from 2001 to 2010; Primary Health Care Register, containing outpatient diagnoses from 2001–2007 for 1 million patients from Stockholm and middle Sweden; Swedish Crime Register, containing national data on all convictions from 1973–2011; Swedish Suspicion Register, containing national data on all individuals strongly suspected of crime from 1998–2011; Swedish Mortality Register, containing all causes of death; and the Population and Housing Censuses that provided information on household and geographical status in 1960, 1965, 1970, 1975, 1980, and 1985. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (No. 2008/409).
DA was identified in the Swedish medical and mortality registries by ICD codes (ICD8: Drug dependence (304); ICD9: Drug psychoses (292) and Drug dependence (304), Nondependent abuse of drugs (305; excluding 305.0); ICD10: Mental and behavioral disorders due to psychoactive substance use (F10–F19), except those due to alcohol (F10) or tobacco (F17)); in the Suspicion Register by codes 3070, 5010, 5011, and 5012, which reflect crimes related to DA; and in the Crime Register by references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). DA was identified in individuals (excluding those suffering from cancer) in the Prescribed Drug Register who had retrieved (in average) more than four defined daily doses a day for 12 months from either of Hypnotics and Sedatives (Anatomical Therapeutic Chemical (ATC) Classification System N05C and N05BA), or Opioids (ATC: N02A).
Sample
The database was created by entering all individuals in the Swedish population born in Sweden between 1960 and 1990 (N= 3,257,987). From this database, we defined 6 kinds of families: 1) Triparental (TP) father families: individuals who, during ages 0–15, were residing ≥15 years in the same household as their mother, never residing in the same geographical area as their father, and residing at least 10 years in the same household as their step-father; 2) Not lived with (NLW) - father families: individuals who, during ages 0–15, were residing ≥15 years in the same household as their mother and never resided in the same household or geographical area as their father, furthermore the family could not be included in the TP family sample; 3) NLW – mother families: individuals who, during ages 0–15, were residing ≥15 years in the same household as their father and never resided in the same household or small area as their mother; 4) Step-father families: individuals who, during ages 0–15, were residing ≥15 years in the same household as their mother and had a step-father residing ≥10 years in the same household, furthermore the family could not be included in the TP family sample; 5) Step-mother families: individuals who, during ages 0–15, were residing ≥15 years in the same household as their father and had a step-mother residing ≥ 10 years in the same household; 6) Adoptive families: individuals who had been adopted prior to age of 10, with information available on both adoptive parents and at least 1 biological parent. Individuals adopted by biological relatives or by an adoptive parent living with a biological parent were excluded. We do not know exactly the time of placement in the adoptive family. But descriptions of adoptions during these years indicate that the vast majority of formal adoption were in the first few years of life (Bohman, 1970; Nordlöf, 2001). We also used for comparison results from a seventh family type, Intact families: defined as individuals who, during ages 0–15, were residing ≥ 15 years in the same household as their biological mother and biological father.
Household was defined as follows: From 1960 to 1985 (every 5th year) we used householdID from the Population and Housing Census. The householdID includes all individuals living in the same dwelling. For the years we did not have information, we approximated the householdID with the information from the year closest in time. From 1986 and onwards (every year) we used the FamilyID from the Total Population Register. The FamilyID is defined by individuals that are related or married, and who are registered at the same property (a person can only be part of one family). In addition, adults who are registered at the same property and have common children, but are not married, are registered in the same family. Geographical area was defined as Small Areas for Market Statistics (SAMS) that are small geographical units defined by Statistics Sweden, the Swedish government-owned statistics bureau. There are approximately 9,200 SAMS throughout Sweden, their average population of ~ 1,000. From 1960 to 1970, when we had no information on SAMS areas, we used parishes as a proxy. The parishes serve as districts for the Swedish census and elections, and have approximately the same number of inhabitants as SAMS. From 1960 to 1985 we only had information every 5th year and for that reason we approximated the geographical status with the information from the year closest in time.
Step-father or mother was defined as an individual 18 to 50 years older than the offspring who lived in the same household as the offspring and was not a first, second or third degree biological relative. This means that, from 1986 and onwards, for an offspring living with his/her mother (father), we only capture the step-father (mother) if he (she) is either married with the mother (father) of the offspring and/or has a common child together with the mother (father) of the offspring.
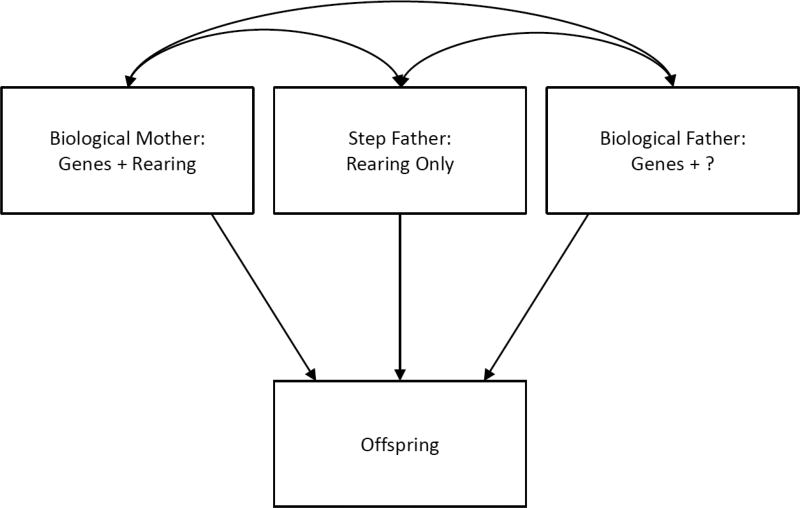
To each of the family types we fitted a path model, in which we modelled unidirectional paths (single-headed arrows) from each of the different parental figures in the families to the offspring and also the correlation between the parental figures in each family (double-headed arrow(s)). This model is illustrated in figure 1 for step-father families. Of note, we include the biological father for completeness so as to correctly model the spousal resemblance but do not include results from that relationship in our analyses because such fathers would have had variable rearing relationships with their offspring. The same would be true for biological mothers in step-mother families and the step-parents that were sometimes present in the not-lived-with father and mother families.
Figure 1.
A path model for our analysis of Paternal Not-Lived-With Families. These families, by definition, have a biological mother who reared the offspring and a biological father who neither lived with nor near the offspring during the offspring’s childhood and adolescence. The family may have contained a step-father but that was not a requirement. Therefore, we include information on the step-father, when present, in our model but do not utilize the information from that parent-offspring correlation in our further model fitting.
As a family could consist of several offspring from the same parents, each family was treated as a cluster in the analysis. We present the standardized path-coefficient for each of the unidirectional paths (equivalent to a tetrachoric correlation) and the estimated correlations between the different parental figures. Model fitting was done using Mplus version 7.2 (Muthen and Muthen, 2012) with the theta parameterization and the WLSMV as the fit function. In order to combine the results from the different samples, we used the Mantel-Haenszel meta-analysis method. We calculated the combined correlations and the p-values for the heterogeneity tests.
We compared the magnitude of the parent-offspring transmission from fathers and from mothers by examining the fit of a model where all the paths were equated to equality to one where they were permitted to be freely estimated. If this was significant, we then compared parent-offspring resemblance in fathers and mothers across the three classes of relationships: genes + rearing, genes only and rearing only.
Narrow sense heritability (which includes only additive genetic effects) can be estimated by doubling the parent-offspring correlation of liability when the parents were not involved in rearing their offspring (Falconer, 1989).
RESULTS
The sample size and prevalence rates for DA in parents and offspring from our six family types are seen in table 1 as well as comparison results from intact families. The largest sample sizes (aside from intact families) were seen with not-lived-with father, step-father and triparental families. Step-mother, not-lived-with mother and adoptive families were the rarest. In the offspring generation, rates of DA differed nearly five-fold across family types, being lowest in intact families, highest in not-lived with father, step-mother and not-lived-with mother families, and intermediate in step-father, triparental and adoptive families. Rates of drug abuse in the parental generation differ over 15-fold in mothers and 10-fold in fathers. The highest rates for DA in mothers were seen in step-mother and not-lived with mother families with much lower rates in the step-father, triparental and intact families. A broadly similar pattern was seen in fathers with highest rates in not-lived-with father and step-mother and step-father families. Rates for DA were consistently low in step- and adoptive-parents.
Table 1.
Sample Size and Prevalence Rates for Drug Abuse in Parents and Offspring from Seven Family Types
| Intact Families |
Triparental families |
Not-lived- with Father Families |
Not-lived- with Mother Families |
Step- Father Families |
Step- Mother Families |
Adoptive Families* |
|
|---|---|---|---|---|---|---|---|
| Sample Size of Offspring | 2,111,074 | 41,360 | 113,761 | 10,194 | 65,803 | 17,637 | 10,038 |
| Prevalence of Drug Abuse in Offspring (%) | 2.1 | 5.8 | 9.2 | 8.0 | 6.6 | 9.2 | 5.7 |
| Prevalence of Drug Abuse in Biological Mother (%) | 0.6 | 1.9 | 5.0 | 7.9 | 3.8 | 10.0 | 5.8 |
| Prevalence of Drug Abuse in Biological Father (%) | 0.6 | 5.4 | 7.5 | 5.3 | 5.4 | 7.1 | 3.8 |
| Prevalence of Drug Abuse in Step/Adoptive-Mother (%) | 1.1 | 0.9 | |||||
| Prevalence of Drug Abuse in Step/Adoptive-Father (%) | 1.3 | 1.4 | 0.5 |
number of adoptive fathers =8,512 and adoptive mothers=8,507.
Tetrachoric correlations (otherwise known as correlations of liability) for DA among the parents of the non-adoptive family types are seen in table 2. The correlations were widely variable and were generally higher between biological parents than between biological and step-parents. They were especially low and not significant when involving step-parents from not-lived-with and step-mother families. The highest correlations were seen between biological parents in the same not-lived-with and step-mother families. The correlations were especially high in biological parents from not-lived-with mother and step-mother and step-father families. In the adoptive families (table 3), parental correlations for DA were low and non-significant when involving an adoptive parent but quite high among biological parents.
Table 2.
Tetrachoric Correlations (and 95% CIs) for Drug Abuse among Parents in Six of our Family Types
| Nature of Sample/Design |
Correlation Biological Mother – Biological Father |
Correlation Biological Mother-Step- parent |
Correlation Biological Father-Step- parent |
|---|---|---|---|
| Intact Families | 0.32 (0.30; 0.34) | ||
| Triparental families | 0.18 (0.13; 0.24) | 0.36 (0.30; 0.43) | 0.21 (0.16; 0.27) |
| Not-lived-with Father families | 0.40 (0.38; 0.41) | 0.26 (0.17; 0.34) | 0.17 (0.08; 0.26) |
| Not-lived-with Mother families | 0.60 (0.56; 0.64) | 0.03 (−0.14; 0.19) | 0.01 (−0.18; 0.21) |
| Stepfather families | 0.51 (0.48; 0.54) | 0.24 (0.20; 0.29) | 0.22 (0.17; 0.27) |
| Stepmother families | 0.65 (0.62; 0.68) | 0.03 (−0.08; 0.13) | 0.01 (−0.12; 0.14) |
Table 3.
Tetrachoric Correlations (and 95% CIs) for Drug Abuse among Adoptive and Biological Parents in Adoptive Families
| Parents | Correlations |
|---|---|
| Adoptive Father – Adoptive Mother | 0.11 (−0.19; 0.41) |
| Adoptive Father – Biological Mother | 0.11 (−0.06; 0.29) |
| Adoptive Father – Biological Father | 0.14 (−0.04; 0.33) |
| Adoptive Mother– Biological Mother | 0.12 (−0.02; 0.25) |
| Adoptive mother – Biological Father | −0.02 (−0.20; 0.17) |
| Biological Mother – Biological Father | 0.48 (0.41; 0.54) |
The standardized path estimates for father-offspring and mother-offspring resemblance for DA across the six key family types as well as for intact families are seen in table 4. In both fathers and mothers, the degree of parent offspring resemblance was lower with parents providing both genes + rearing from intact families than from not-lived-with, step and triparental families. This supported our view that results from intact families should not be included in the weighted estimates as they appear to reflect different rearing environments and would have an overwhelming influence due to the very large sample size.
Table 4.
Path Estimates for Parent-Offspring Resemblance for Drug Abuse from Seven Different Family Types in a Swedish National Sample
| Family Sample | Sample Size |
Father | Mother | ||||
|---|---|---|---|---|---|---|---|
| Genes and Rearing |
Genes Only | Rearing Only | Genes and Rearing |
Genes Only | Rearing Only | ||
| Intact | 2,111,074 | 0.18 (0.16; 0.19) | 0.16 (0.14; 0.17) | ||||
| Not-Lived-With Father | 113,761 | 0.20 (0.18; 0.22) | 0.18 (0.15; 0.21) | ||||
| Not-Lived-With Mother | 10,194 | 0.24 (0.16; 0.33) | 0.17 (0.09; 0.26) | ||||
| Step-Father | 65,803 | 0.06 (0.01; 0.11) | 0.21 (0.17; 0.25) | ||||
| Step-Mother | 17,637 | 0.26 (0.20; 0.33) | 0.10 (0.01; 0.20) | ||||
| Triparental | 41,360 | 0.15 (0.11; 0.19) | 0.07 (0.01; 0.14) | 0.19 (0.13; 0.24) | |||
| Ado`ptive | 10,038 | 0.17 (0.06; 0.28) | 0.04 (−0.16; 0.24) | 0.05 (−0.05; 0.15) | 0.06 (−0.10; 0.21) | ||
| Weighted Estimate without Intact Families | 0.26 (0.20; 0.31) | 0.19 (0.17; 0.20) | 0.06 (0.02; 0.11) | 0.19 (0.17; 0.22) | 0.13 (0.07; 0.19) | 0.09 (0.01; 0.17) | |
| P-value for test of heterogeneity | 0.71 | 0.08 | 0.94 | 0.48 | 0.05 | 0.64 | |
Heterogeneity test for fathers versus mothers: “genes and rearing” p=0.04; “genes only” p=0.02;” rearing only” p=0.59
The results of the weighted estimates across these six special family types are provided in table 4 (second to last row). Six results were noteworthy. First, all of the weighted estimates for the parental effects were statistically significant. Second, heterogeneity tests across samples were non-significant for five of the parental types and marginally significant for mothers providing genes only. Such a pattern of findings is consistent with chance expectations. Third, for both fathers and mothers, the correlations were highest for parents providing genes and rearing, intermediate for those providing genes only and lowest for those providing rearing only. Fourth, for both fathers and mothers, the sum of the correlations for parents providing genes only and rearing only were close to those observed from parents providing both genes and rearing. Fifth, we could confidently reject a model that constrained to equality the parent-offspring resemblance in fathers and mothers (p=0.0003) demonstrating a statistical difference in the strength of the cross-generational transmission of DA from mothers and fathers. We then examined individually the three kinds of parent-offspring pairs and found that they were statistically significantly different for fathers and mothers providing genes + rearing (p=0.04) and genes only (p=0.02), but not for parents providing rearing only (p=0.59). Thus, unexpectedly, that for both parents providing genes + rearing and genes only, correlations were significantly higher for father-offspring than mother-offspring pairs.
Finally, narrow-sense heritability can be estimated by doubling the correlation between biological parents and offspring who have had minimal environmental contact (Falconer, 1989). From fathers and mothers providing “genes only”, we can estimate this narrow-sense heritability for DA (and 95% CIs) for DA to equal 38% (34–40%) and 26% (14–38%), respectively.
DISCUSSION
The goal of this report was to quantify, using liability-threshold path analytic models accounting for all inter-parental correlations, the sources of parent-offspring resemblance for DA utilizing 6 different informative family samples. All of these families were ascertained from a complete survey of the Swedish population. In particular, we were able to examine across multiple family types, parents who provided for their offspring both genes and rearing, genes only or rearing only.
The rates of DA differed substantially across the family types in both the offspring and especially the parents. Thus, these family types differ substantially in the level of risk for DA, likely from both genetic and environmental sources. Statistically, these effects can be easily accounted for by varying the threshold but it raises the empirical question of the underlying comparability of estimates across these family types. These widely varying base rates also mean that the comparison of results across families when using more traditional epidemiological statistical tools (e.g. logistic or Cox models) might be problematic.
We found widely varying parental correlations for DA across the family and parental types. Our evidence for spousal resemblance for DA in our intact families (+0.32) is somewhat higher than has been reported elsewhere for alcohol use disorders (Grant et al., 2007; Maes et al., 1998) but in the range of those reported previously for cannabis abuse/dependence (+0.33–0.40)(Hopfer et al., 2003).
An examination of the estimates of parent-offspring paths from mothers and fathers who provided both genes and rearing, genes only or rearing only suggests reasonable congruence across the six different family types. This was confirmed by formal heterogeneity testing. Congruent results coming from distinct family constellations should increase our confidence in the underlying validity of the results. Consistent with the prior analyses of these samples utilizing logistic and Cox models (Kendler et al., 2012; Kendler et al., 2015c), resemblance was lowest for the rearing only parent-child relationships and greater for the genes + rearing versus the genes only relationships. Surprisingly, a clear trend was seen for stronger father-offspring than mother-offspring relationships for both genes + rearing and genes only parental types. These results were statistically significant. These findings are inconsistent with substantial environmental risk for DA passing from mother to child via the intra-uterine or immediate post-partum environment which would be expected to produce stronger biological-mother offspring vs. biological-father offspring correlations.
Unexpectedly, parent-offspring correlations for parents providing both genes and rearing were appreciably higher in not-lived-with, step- and triparental families than in intact families. Since genetic factors are, perforce, the same across these relationships, these results provide further unexpected support for rearing effects. Our results suggest that the stable biological parental figure in disrupted families has a greater environmental influence on risk for DA in the offspring than is seen in intact families.
Results from our not-lived-with and adoptive families provided aggregate estimates of the parent-offspring correlation results solely from genetic effects. Doubling these figures provided estimates of narrow heritability for DA of between 26 and 38%. In our prior twin-sibling analysis of DA in Sweden, we estimated that the heritability was considerably higher ranging from 55 to 73% (Kendler et al., 2013). Lower heritability estimates from adoption versus twin studies has been found before in meta-analyses of two other externalizing traits: antisocial behavior (Rhee and Waldman, 2002) and alcoholism (Verhulst et al., 2014). This discrepancy could arise for several possible reasons two of which are particularly likely. First, non-additive genetic effects could explain this pattern because they contribute to heritability estimated from twin studies (e.g. “broad sense heritability) but not from parent-offspring relationships (narrow-sense heritability) (Falconer, 1989). Given the presence of shared environmental for DA in Sweden (Kendler et al., 2013), such non-additive effects would be likely undetectable in twin and sibling studies. Second, gene×age or gene×cohort interactions – which both predict that genetic effects within the same generation would be more highly correlated than between generations – would also result in higher estimates heritability from twin studies – where the subjects are exactly the same age -- than parent-offspring studies, where the subjects are a generation apart. This is particularly plausible for DA because of the changes over historical time in the availability of drugs of abuse and the social attitudes towards them. Furthermore, we have shown cohort effects for DA in Sweden over the last 35 years (Giordano et al., 2013).
We found evidence that rearing effects contribute to the cross-generational transmission of DA. Our results permit us to estimate that, when parents provide both genes and rearing, between 25 and 40% of the parent-offspring correlation for DA results from environmental processes. In our prior twin-sibling study of DA, we found evidence for robust shared environmental effects – accounting for 23% of the variance in liability – but only in males (Kendler et al., 2013). Our estimates of parent-offspring resemblance suggest that only a modest proportion of the shared environmental effects for DA are a result of parental behaviors. This is consistent with other evidence of important peer influences on DA (Brook et al., 1983; Kandel, 1985; Kendler et al., 2014; Kendler et al., 2015a).
Overall, our current results complement and extend our previous epidemiologically oriented analyses of adoptive, step-, not-lived with and triparental families in Sweden (Kendler et al., 2012; Kendler et al., 2015b; Kendler et al., 2015c) in demonstrating the complexity of the familial transmission of DA and the utility of a range of family constellations in elucidating such mechanisms. Importantly, our findings suggest that despite substantial differences in base rates for DA across different family constellations, they are providing us with the same broad estimates for the important of genetic versus rearing parental effects.
Limitations
These results should be interpreted in the context of four potentially important methodological limitations. First, our results are obtained in Sweden and may or may not extrapolate to other countries. Second, subjects with DA were detected from medical, legal and pharmacy records. This method does not require respondent cooperation or accurate recall. However, such registry data surely includes both false negative and false positive diagnoses. We cannot estimate precisely these biases as no major epidemiological study has reported rates of DA in Sweden. However, such a survey, done in neighboring Norway, which has similar rates of drug use and abuse (Hibell et al., 2007; Kraus et al., 2003), found lifetime prevalence rates of (American Psychiatric Association, 1987) DA and dependence of 3.4% (Kringlen et al., 2001), in the range of those we detected in our different family samples in Sweden. Furthermore, the validity of our ascertainment method is supported by the very high ORs (mean of 52.2 (Kendler et al., 2012)) for registration for DA across our different sources.
Third, we set 10 years as a minimum duration of cohabitation for step-parents and step-children because the sample size declined substantially with longer periods. Therefore, we could not match perfectly for duration of rearing between intact and step-families. Could we have underestimated the impact of parental rearing on transmission of DA? We explored this in a previous paper (Kendler et al., 2015c) and these results suggest that our approach could, at most, have modestly underestimated the impact of rearing effects on the cross-generational transmission of DA.
Finally, our design permitted us to examine genetic relationships between parents and children and their broadly defined cohabitation history but not other more subtle aspects of the parent-offspring relationship. For example, we do not know how often biological parents visited their “not-lived-with” offspring nor the emotional depth of the step-parent step-child relationship. Our hope is that our analyses accurately assess aggregate effects of genetic factors and rearing environment. Other research designs are needed to investigate more fine-grained parenting processes.
Conclusions
This paper applied the liability threshold model popular in psychiatric genetics, and especially in twin studies, to results from six unique family types in Swedish national data (not-lived-with fathers, not-lived-with mothers, step-fathers, step-mothers, triparental, and adoptive) to clarify the sources of parent-offspring transmission of DA. We found substantial consistency of results across these different family types. Both genetic and environmental factors contribute substantially to parent-offspring resemblance for DA and appear to be additive in their effect. Surprisingly, a clear trend was seen for stronger father-offspring than mother-offspring genetic effects. We can estimate from our results that, when parents provide both genes and rearing, between 25 and 40% of the parent-offspring resemblance for DA results from environmental processes with the remainder the result of genetic transmission.
Acknowledgments
GRANT SUPPORT & ACKNOWLEDGEMENTS: Michael Neale PhD provided helpful methodological advice. This study was funded by grant RO1 DA030005 from the National Institute of Drug Abuse, the Swedish Research Council 2008–3110, 2008–2638, the Swedish Research Council for Health, Working Life and Welfare (Reg.nr: 2013-1836), the ALF project grant, Lund, Sweden and the Swedish Council for Information on Alcohol and Other Drugs (CAN).
Footnotes
Conflicts of Interest and Source of Funding: None of the authors have conflicts to report.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Bohman M. Adopted children and their families: A follow-up study of adopted children, their background, environment and adjustment. Proprius, [Solna, Seelig] 1970 [Google Scholar]
- Brook JS, Whiteman M, Gordon AS. Stages of drug abuse in adolescence: personality, peer, and family correlates. Dev Psychol. 1983;19:269–277. [Google Scholar]
- Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet. 1965;29:51–76. [Google Scholar]
- Falconer DS. Introduction to Quantitative Genetics. Wiley; New York: 1989. [Google Scholar]
- Giordano GN, Ohlsson H, Kendler KS, Winkleby MA, Sundquist K, Sundquist J. Age, period and cohort trends in drug abuse hospitalizations within the total Swedish population (1975–2010) Drug Alcohol Depend. 2013;134:355–361. doi: 10.1016/j.drugalcdep.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JD, Heath AC, Bucholz KK, Madden PA, Agrawal A, Statham DJ, Martin NG. Spousal concordance for alcohol dependence: evidence for assortative mating or spousal interaction effects? Alcohol Clin Exp Res. 2007;31:717–728. doi: 10.1111/j.1530-0277.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- Hibell B, Guttormsson U, Ahlstrom S, Balakireva O, Bjarnason T, Kokkevi A, Kraus L. The 2007 ESPAD Report: Substance Use Among Students in 35 European Countries. The Swedish Council for Information on Alcohol and Other Drugs (CAN), Sweden 2007 [Google Scholar]
- Hopfer CJ, Stallings MC, Hewitt JK, Crowley TJ. Family transmission of marijuana use, abuse, and dependence. J Am Acad Child Adolesc Psychiatry. 2003;42:834–841. doi: 10.1097/01.CHI.0000046874.56865.85. [DOI] [PubMed] [Google Scholar]
- Hur YM, Craig JM. Twin registries worldwide: an important resource for scientific research. Twin Res Hum Genet. 2013;16:1–12. doi: 10.1017/thg.2012.147. [DOI] [PubMed] [Google Scholar]
- Kandel DB. On processes of peer influences in adolescent drug use: a developmental perspective. Adv Alcohol Subst Abuse. 1985;4:139–163. doi: 10.1300/J251v04n03_07. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kidd KK. Recurrence risks in an oligogenic threshold model: the effect of alterations in allele frequency. Ann Hum Genet. 1986;50(Pt 1):83–91. doi: 10.1111/j.1469-1809.1986.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Maes HH, Sundquist K, Ohlsson H, Sundquist J. Genetic and Family and Community Environmental Effects on Drug Abuse in Adolescence: A Swedish National Twin and Sibling Study. AJP. 2013;171:209–17. doi: 10.1176/appi.ajp.2013.12101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Mezuk B, Sundquist K, Sundquist J. Exposure to peer deviance during childhood and risk for drug abuse: a Swedish national co-relative control study. Psychol Med. 2015a;45:855–64. doi: 10.1017/S0033291714001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist J, Sundquist K. Triparental Families: A New Genetic-Epidemiological Design Applies to Drug Abuse, Alcohol Use Disorder and Criminal Behavior in a Swedish National Sample. AJP. 2015b;172:553–60. doi: 10.1176/appi.ajp.2014.14091127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K, Sundquist J. Peer Deviance, Parental Divorce, and Genetic Risk in the Prediction of Drug Abuse in a Nationwide Swedish Sample: Evidence of Environment-Environment and Gene-Environment Interaction. JAMA Psychiatry. 2014;71:439–45. doi: 10.1001/jamapsychiatry.2013.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K, Sundquist J. The Causes of parent-offspring transmission of drug abuse: a Swedish population-based study. Psychol Med. 2015c;45:87–95. doi: 10.1017/S0033291714001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Sundquist K, Ohlsson H, Palmer K, Maes H, Winkleby MA, Sundquist J. Genetic and familial environmental influences on the risk for drug abuse: a national Swedish adoption study. Arch Gen Psychiatry. 2012;69:690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC. Correlation coefficients in medical research: from product moment correlation to the odds ratio. Stat Methods Med Res. 2006;15:525–545. doi: 10.1177/0962280206070650. [DOI] [PubMed] [Google Scholar]
- Kraus L, Augustin R, Frischer M, Kummler P, Uhl A, Wiessing L. Estimating prevalence of problem drug use at national level in countries of the European Union and Norway. Addiction. 2003;98:471–485. doi: 10.1046/j.1360-0443.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- Kringlen E, Torgersen S, Cramer V. A Norwegian psychiatric epidemiological study. AJP. 2001;158:1091–1098. doi: 10.1176/appi.ajp.158.7.1091. [DOI] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Kendler KS, Hewitt JK, Silberg JL, Foley DL, Meyer JM, Rutter M, Simonoff E, Pickles A, Eaves LJ. Assortative mating for major psychiatric diagnoses in two population-based samples. Psychol Med. 1998;28:1389–1401. doi: 10.1017/s0033291798007326. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User's Guide: 1998–2012. Muthen & Muthen; Los Angeles, CA: 2012. [Google Scholar]
- Nordlöf B. FoU-rapport 2001. Socialtjänstförvaltningen, Forsknings-och utvecklingsenheten; Stockholm: 2001. Svenska Adoptioner i Stockholm 1918–1973 [Swedish Adoptions in Stockholm 1918–1973] p. 8. [Google Scholar]
- Pearson K. Mathematical contributions to the theory of evolution. VII. On the correlation of characters not quantitatively measurable. Proc Roy Soc. 1901;66:241–244. [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. PB. 2002;128:490–529. [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med AUG. 2014:1–12. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]