Abstract
Purpose of Review
The explosive growth of the nanotechnology industry has necessitated the examination of engineered nanomaterials (ENMs) for their toxicity. The unique properties that make ENMs useful also makes them a health risk, and individuals with pre-existing diseases such as asthma are likely more susceptible. This review summarizes the current literature on the ability of ENMs to both exacerbate and directly cause asthma.
Recent Findings
Recent studies highlight the ability of metal nanoparticles (NPs) and carbon nanotubes (CNTs) to not only exacerbate pre-existing asthma in animal models, but also initiate allergic airway disease directly. CNTs alone are shown to cause airway mucus production, elevated serum IgE levels, and increased TH2 cytokine levels, all key indicators of asthma.
Summary
The ability of ENMs to modulate the immune response in asthma varies depending on their physicochemical properties and exposure timing. CNTs consistently exacerbate asthma, as do Ni and TiO2 NPs, whereas some NPs like Au attenuate asthma. Evidence is strong that ENMs can contribute to allergic airway disease; however, more work is required to determine their mechanisms, and more epidemiological studies are needed to validate results from animal models.
Keywords: Asthma, allergy, lung, nanoparticles, nanotubes, nanomaterials
Introduction
Asthma is a chronic inflammatory airway disease of increasing prevalence, which affects roughly 26 million people in the U.S. and 300 million worldwide [1, 2]. Asthma, in general, is characterized by airway hyperresponsiveness (AHR) as well as inflammation and mucous overproduction, which leads to airway obstruction [3]. Asthma is a highly heterogeneous disease and can result from exposure to a variety of allergens like house dust mites, pollen or mold, as well as other environmental toxicants such as cigarette smoke, diesel exhaust particles or ozone [4]. Multiple types of asthma have been identified clinically (i.e. allergic and nonallergic); however, the most common and well-studied is allergic asthma [4]. In the classic paradigm of allergic asthma, antigen presenting cells in the lung, mainly dendritic cells, take up and process inhaled allergens and induce activation of naïve T helper cells to TH2 type cells which go on to produce a variety of chemokines and cytokines to promote recruitment and activation of other immune cells [3–5]. TH2 cells produce interleukin (IL)-13 and IL-4 and activate B cells, which produce antigen-specific IgE, which subsequently binds to allergen and Fc receptors on mast cells in the lung, crosslinking them and causing degranulation and release of cytokines, leukotrienes and histamine, leading to inflammation [3]. In addition to these key players, multiple other cell types in the lung have critical roles in asthma. Innate immune cells like macrophages, eosinophils and neutrophils have multifaceted roles in promoting the inflammatory environment in airways, facilitating allergen processing and releasing cytokines [3]. Epithelial cells participate in the inflammatory signaling cascade as well, and fibroblasts residing beneath the epithelium contribute to collagen deposition and fibrosis in chronic asthma [3]. Moreover, smooth muscle cells around airways undergo hypertrophy and hyperplasia that contribute to increased AHR during episodes of bronchospasm [6].
Environmental factors capable of causing asthma exacerbations have been studied extensively and include viral infections and exposure to ambient air pollution [7]. Common components of air pollution like ozone, nitrogen dioxide, and particulate matter (PM) have been found to cause airway inflammation and exacerbate asthma [8]. Numerous epidemiological evidence suggests that both fine and ultrafine PM are associated with allergy and asthma, indicating that small particle size (≤2.5 μm and ≤0.1 μm, respectively) is an important factor when considering toxicology of PM [9]. Naturally then, with the advent of nanotechnology the pulmonary toxicology community has been interested in examining the effects that engineered nanomaterials (ENMs) may have on asthma.
ENMs can be generally defined as purposefully designed materials possessing at least one dimension less than or equal to 100 nm and unique physicochemical characteristics not present in their non-nanoscale counterparts of the same composition [10]. In recent years the number of different kinds of ENMs has grown exponentially, and ENMs are being used in a wide range of applications including electronics, engineering and medicine [11–13]. ENMs come in a diverse array of materials and shapes including metal nanoparticles (NPs) (TiO2, ZnO, Au, NiO), carbon NPs, silica NPs, and fullerenes like carbon nanotubes (CNTs). Metal nanoparticles may be spherical or irregular in shape, and CNTs may be either single- or multi-walled. There are also nanofibers, which are similar to CNTs, but are solid, and may be composed of carbon or metals. Examples of the physical characteristics of different kinds of ENMs are depicted in Figure 1 [14]. This diversity of ENMs means there are a variety of ways they could interact with biological systems to produce toxicity, and over the past 15 years there have been numerous studies into how ENMs may cause lung diseases like fibrosis and asthma [15]. The purpose of this review is to concisely summarize the current literature on the toxicology of ENMs in relation to asthma; studies on the uses of ENMs for asthma therapeutics will not be discussed. It will first focus on the way in which different types of ENMs exacerbate preexisting asthma, and follow by examining how ENMs may be able to initiate asthma directly, in the absence of allergens. These concepts are summarized and illustrated in Table 1 and Figure 2, respectively. There is a severe lack of human evidence for ENM toxicity, meaning this review will focus primarily on evidence from animal and cell models; however, later the few relevant human based studies will be discussed.
Figure 1.
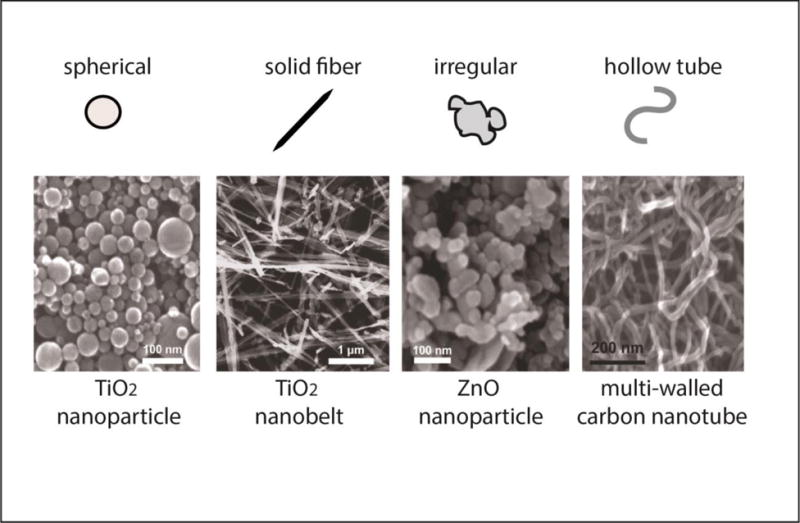
Examples of different types of engineered nanomaterials with varying shape. The scanning electron microscope images of the different types of engineered nanomaterials are from Xia et al., 2013 [14] and were reproduced from Environmental Health Perspectives (https://ehp.niehs.nih.gov/1306561/).
Table 1.
Summary of commonly studied asthma endpoints affected by different types of ENMs
| ENM | Asthma Models | AHR | BALF Cell Profile | Serum IgE | Cytokines | Direct Effects | References |
|---|---|---|---|---|---|---|---|
| TiO2 | OVA, TDI | ↑ | ↑↑neutrophils | ↑↑ | ↑IL-1, ↑IL-6, ↑IL-4 | ↑AHR, ↑mucus, ↑T cell proliferation | [16–21, 61–64] |
| Silica | OVA | ↑↑ | ↑↑eosinophils | ↑↑↑ | ↑↑IL-4, ↓IFN-γ, ↑IL-5, ↑IL-13, ↑IL-1 | – | [22–27] |
| Ag | OVA | ↓ | ↓total count | ↑ | ↓↓IL-4, ↓IL-13, ↓IL-5 | – | [28–31, 68] |
| Au | OVA, TDI | ↓ | ↓total counts, ↑eosinophils | No change | – | – | [21, 34–37] |
| Fe | OVA | – | ↓eosinophils | ↓ | ↓IL-4, ↓IFN-γ | – | [38, 39] |
| Zn | OVA | – | ↑eosinophils | No change | ↑IL-4, ↑IL-13, ↑IL-5 | ↑eosinophils, ↑IL-4, ↑IL-13 | [40] |
| Cu | OVA | ↑ | ↑total count | ↑ | – | ↑IL-6, ↑IL-8, ↑Muc5ac | [41, 65, 67] |
| Ni | T-bet–/– | – | ↑eosinophils | – | ↑CCL2 | ↑eosinophils, ↑eotaxin | [42, 66] |
| Ce | – | – | – | – | – | ↑IL-4, ↑IL-5 | [63] |
| C | OVA | ↑ | ↑macrophages | – | ↓IL-4, ↓IL-13 | – | [43–47] |
| Polymer | OVA | – | ↓total count, ↓eosinophils | ↓ | ↓IL-4, ↓IL-13 | – | [48–49] |
| CNT | OVA, HDM, TMA | ↑↑ | ↑↑eosinophils, ↑neutrophils | ↑↑↑ | ↑IL-4, ↑IL-13, ↑eotaxin, ↑IL-5 | ↑↑AHR, ↑IgE, ↑mucus, ↑IL-4, ↑IL-13 | [50–57, 69–74] |
Arrows indicate average relative enhancement/suppression.
Figure 2.
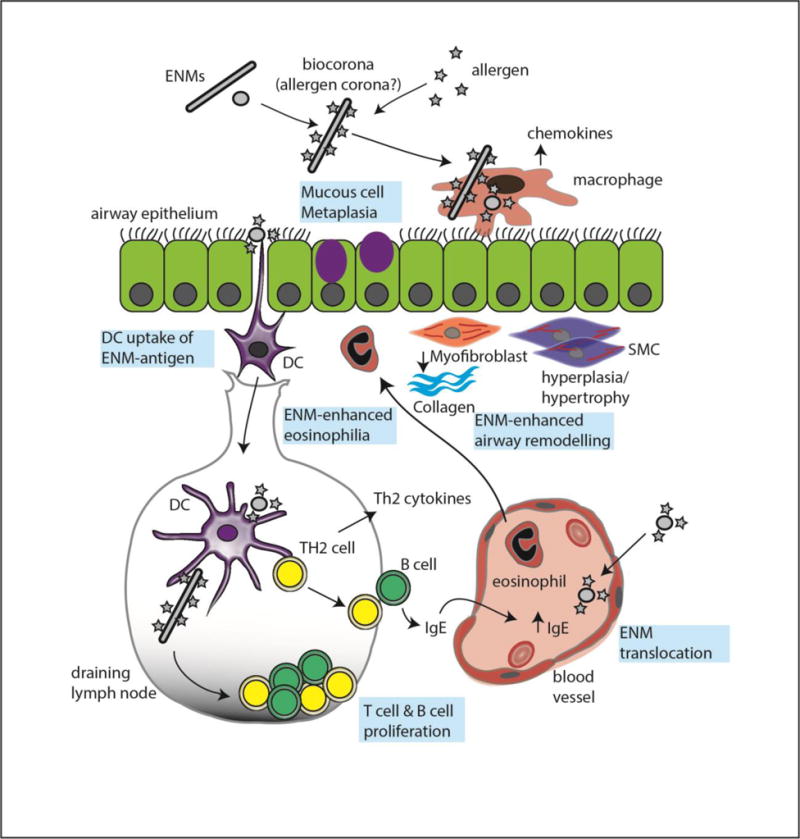
Illustration of interactions of engineered nanomaterials (ENMs) and allergens with the immune system. ENMs directly stimulate innate immune cells such as macrophages or epithelial cells to produce chemokines that stimulate recruitment of other inflammatory cells such as eosinophils. ENMs also interact with allergens to exacerbate innate immune responses. Dendritic cells transport ENMs to lymph nodes to program T cells as part of the acquired immune system. Phenotypic changes in cells and tissues are shown in blue boxes.
ENM-Induced Asthma Exacerbations
TiO2 Nanoparticles
A number of different asthma models have been utilized to study the effects TiO2 NPs on the immune response in asthma. Several studies utilize the common ovalbumin (OVA) mouse model, in which mice are sensitized and then challenged by OVA exposure to produce allergic airway disease[16–20]. One of the earliest TiO2/OVA studies by Rossi et al. compared nanosized and fine TiO2, and did not find significant differences in endpoints measured, and surprisingly exposure to either type of TiO2 decreased asthma endpoints like eosinophil numbers, airway mucous production and AHR [20]. This could be due to the timing of TiO2 NP exposure, because interestingly two other studies found that the order and timing of OVA and TiO2 NP exposure affected the observed immune modulation [17, 18]. Both of these studies found that TiO2/OVA exposed mice had increased AHR and eosinophilia when TiO2 NPs were given either between the OVA sensitization and challenge phases, or during sensitization, but not when TiO2 was given during the challenge phase or later, suggesting an adjuvant-like effect [17, 18]. Increases in the TH2 cytokines IL-4, IL-13 and IL-5 were seen, and Mishra et al. found elevated Socs3 expression, which is associated with airway inflammation, in TiO2 exposed mice which was NF-κB-dependent [18]. TiO2 NPs also increase levels of caspase-1 and activate the NLRP3 inflammasome to generate increased production of pro-inflammatory IL-1β, likely through reactive oxygen species (ROS) production [16]. Genetic susceptibility has also been shown to affect the immune response in the OVA model; Gustafsson et al. found differences in TiO2-induced inflammation in two susceptible rat strains, with both showing exacerbated IgE production and neutrophilia [19]. A study by Hussain et al. utilizing a toluene diisocyanate (TDI) mouse model of asthma similarly found that TiO2 NPs significantly increased lung inflammation; however, AHR was not increased [21].
Silica Nanoparticles
Several studies have used the OVA rodent model of asthma to examine the toxicity of silica NPs. In rats, SiO2 NPs administered with OVA were found to increase AHR and disrupt the TH2-TH1 balance by increasing IL-4 and decreasing IFN-γ levels in lung protein [22]. Conversely, eosinophil numbers in bronchoalveolar lavage fluid (BALF) were decreased by SiO2 exposure in this study [22]. The effect of silica NP size on asthma exacerbation was examined by another study, finding that the smaller 30 nm particles were the most bioactive, causing greatly enhanced IgE and IL-4 production when compared to the 70, 300 and 1000 nm particles they tested [23]. Polyethylene glycol-coated (PEGylated) silica NPs have been shown to enhance OVA-induced eosinophilia and neutrophilia, as well as BALF levels of numerous cytokines [24]. Most interesting in this study was the examination of tracheobronchial lymph node cell activation by assessing CD69+ cells by flow cytometry; in particular, alveolar macrophages and dendritic cells were found to have increased activation in silica NP/OVA treated mice compared to OVA alone [24]. Other studies have examined the differences between spherical, mesoporous (meaning they contain pores between 2 and 50 nm in diameter, giving them a high surface area) and PEGylated silica NPs in OVA mice [25, 26]. Of these, spherical silica NPs were generally the most inflammatory, causing enhanced eosinophilia compared with OVA alone, although there was no change in OVA-induced AHR [25]. In another study by the same group, mesoporous silica NPs were seen to be more inflammatory than spherical, however both types of silica NPs significantly increased IL-5, IL-13, IFN-γ and IL-1β over OVA alone [26]. Mesoporous silica NPs could be more inflammatory due to their increased surface area. It should be noted that this study used repeated co-exposure of NPs with OVA and found greater exacerbation than the previous study, which gave NPs only during the challenge phase, indicating again the importance of exposure timing. Silica NPs have been studied in vitro by using spleen-derived antigen presenting cells to present OVA peptides to T cells, followed by exposure to modified silica NPs [27]. This study found that silica NP exposure enhanced IL-2 and IFN-γ production by CD8+ T cells, indicating the ability of silica NPs to stimulate antigen-specific T cell responses [27].
Ag Nanoparticles
Ag NPs, which are well known for their antimicrobial properties, have been found to modulate inflammatory signaling in asthma models [28]. Ag NPs attenuate OVA induced allergic inflammation in mice, decreasing total BALF cell counts, IL-4 and IL-13 levels, and Muc5ac expression. [28]. This same study also found that Ag NPs decreased VEGF levels and had similar effects in vivo compared to the VEGF inhibitor SU5614, indicating this Ag NP inhibition of VEGF may be at least partly responsible for the attenuated inflammation [28]. Ag NP’s ability to attenuate allergic inflammation is supported by another study, which found Ag NPs decreased IL-13, IL-4, IL-5 and NF-κB levels, as well as AHR [29]. Conversely another study found that Ag NPs increased IgE and IL-13 levels in allergic mice, as well as ROS [30]. However, Ag NPs did not cause any increases in neutrophil or eosinophil BALF numbers, and lung histological changes were not striking [30]. Ag NPs effects on OVA-induced allergy were examined by proteomic analysis of BALF and plasma proteins by Su et al. who found a number of proteins induced in Ag NP exposed mice: apolipoprotein E, myosin light polypeptide 6, and several immunoglobulin components [31]. Even though some of these studies demonstrate suppression of allergic responses, suggesting that Ag NPs may not be deleterious in asthma, it is important to note that Ag NPs have been shown to increase non-allergy associated pro-inflammatory endpoints like neutrophilia and circulating levels of TNFα, independent of allergen challenge [32, 33].
Au Nanoparticles
Au NPs have been examined in several different ways in relation to asthma. In the study by Hussain et al. discussed above, Au NPs were also used in their TDI-induced asthma model and were found to be even more inflammatory than TiO2 NPs, showing increased AHR and total BALF cell counts [21]. In the OVA asthma model, Au NPs have been found to actually decrease OVA-induced allergy as seen by decreased inflammatory cell lung accumulation, decreased mucus production and lower cytokine levels [34]. Similarly, both PEGylated and citrated Au NPs have been shown to attenuate OVA-induced inflammation: both types of Au NPs decreased AHR, total BALF cell counts and eosinophil numbers [35]. This study also examined extrapulmonary uptake of Au NPs, finding that asthmatic mice had more NPs deposited in the spleen [35]. The effects of the protein corona (that is, the proteins that adsorb to the surface of NPs) on Au NP toxicity have been examined by conjugating Au NPs with coronas of common allergens, and Au NPs conjugated with the allergen Der p 1, which is a component of house dust mite, enhanced its protease activity and increased basophil activation in in vitro assays, suggesting that co-exposures of allergens with NPs could enhance inflammation in asthma through corona formation [36]. It has also been found that Au NPs are taken up by eosinophils on the airway surface in OVA-exposed mice [37]. More research will be needed to fully understand the impact of Au NPs on pre-existing asthma as the current studies show somewhat conflicting evidence, with the outcomes depending on the type of asthma model used.
Fe, Zn, Cu, and Ni Nanoparticles
Different sizes of iron oxide NPs have been tested in OVA asthma models and been found to inhibit allergic inflammation, with nanosized particles significantly decreasing eosinophil cell counts and OVA-specific IgE levels, and larger submicron iron oxide particles having no effect [38]. Hematite NPs have been observed to decrease total immune cell numbers in the lungs and lymph nodes of OVA sensitized mice, an effect which the authors speculate could be due to the acidic nature of the inflammatory environment causing Fe ion release from the nanoparticles, increasing ROS production [39]. Zinc NPs are well known for their toxicity, and ZnO NPs have been tested in the OVA asthma models and were found to increase BALF cell counts and serum IgE levels over OVA alone, an effect that was determined to be Zn ion-independent [40]. Copper oxide NPs have also been shown to exacerbate asthma: in an OVA model they exacerbate numerous endpoints including AHR, inflammatory cell counts, cytokines, IgE and ROS [41]. This study also found CuO NPs to increase phosphorylation of the MAPKs Erk, JNK and p38 [41]. Finally, Ni NPs have been found to exacerbate lung inflammation in a transgenic mouse model of asthma susceptibility [42]. This was determined by using mice lacking the T-bet transcription factor, which is involved with TH1 development, and mice lacking it consequently develop TH2-type allergic inflammation similar to asthma; this study found Ni NPs enhanced mucous production in T-bet knockout mice and increased BALF levels of the chemokine CCL2 [42]. Interestingly, through the use of an anti-CCL2 antibody it was determined that the Ni NP-induced mucous production was at least partly due to these increased CCL2 levels [42].
Carbon Nanoparticles
Carbon black NPs, which are produced through combustion processes, are often used as a control when testing more active types of NPs, however carbon black NPs themselves have been shown to have the potential to exacerbate asthma [43]. OVA-sensitized mice exposed to 14 nm carbon black NPs had increased numbers of dendritic cells, macrophages and B cells, as determined by cell surface markers; the larger 56 nm NPs used in this study did not elicit any change from OVA alone, indicating the importance of particle size [43]. These results are supported by another study, which found carbon black NPs given during OVA sensitization increased inflammatory cell numbers in the lungs as well as CD8+ T cells, CD4+ T cells and B cells in the lymph nodes [44]. These effects were likely not due to direct particle action on antigen presenting cells, as in vitro assays on dendritic cells only yielded dendritic cell activation with cell free-BALF from carbon black NP-exposed mice plus carbon black NPs [44]. Carbon black NPs also increases T cell activation in vitro; splenic leukocytes sensitized by OVA peptides with carbon black NPs had enhanced expression of TH2 associated genes, IL-13, IL-4 and IL-10 [45]. Graphene particles have also been studied in OVA models, yielding contrasting results to carbon black NPs [46, 47]. Graphene oxide was found to decrease markers of TH2 inflammation like IL-4, IL-13, IL-5 and eosinophils, while increasing airway remodeling and AHR; this increased remodeling may be due to production of chitinases by classically activated macrophages, as macrophages isolated from BALF had elevated acidic mammalian chitinase levels when treated with graphene oxide [46]. In vitro assays with sensitized mast cells and basophils indicate that C60 fullerenes inhibit allergic responses, an effect that is in part due to the inhibition of cellular ROS levels [47].
Polymer Nanoparticles
Limited work has been done on asthma and polymer NPs; polystyrene NPs have been examined by two studies [48, 49]. Glycine coated polystyrene NPs were tested in OVA-induced allergy in mice and were found to inhibit serum IgE, mucus production, and TH2 cytokines in the lung-draining lymph node [48]. The mechanisms of this inhibition were examined and polystyrene NPs decreased the numbers of migratory dendritic cells in the lymph nodes, as well as inhibited dendritic cell activation in the lung [48]. Extrapulmonary transport of polystyrene nanoparticles has been examined by using 64Cu-labeled NPs, and the OVA mouse model was used to determine how asthma affects transport [49]. Asthmatic mice had significantly less lung retention of NPs than control mice, and NPs were found in the liver, bladder and gastrointestinal tract; these results indicate that asthma may cause a predisposition for greater extrapulmonary toxicity of NPs [49].
Carbon Nanotubes
There is a plethora of literature on the effects of carbon nanotubes (CNTs) in mouse models of asthma. Among the first studies to show CNT exacerbation of asthma was a study by Ryman-Rasmussen et al. showing that multi-walled CNTs (MWCNTs) enhanced the development of airway fibrosis in OVA-induced asthma [50]. IL-13 was not increased by OVA/MWCNTs, however IL-5 and the profibrotic cytokine PDGF-AA were significantly enhanced by MWCNT, as well as airway collagen thickness [50]. Several OVA CNT studies share common results: OVA-induced TH2 inflammation is enhanced over OVA alone by CNT, as seen by increased eosinophilia, serum IgE and BALF IL-4 levels [51–53]. MWCNTs have also been shown to increase proliferation of OVA-specific T cells in vitro, indicating one possible mechanism of asthma exacerbation [51]. In vitro proliferation and activation of bone marrow-derived dendritic cells has also been observed, demonstrating that antigen presenting cells may be inappropriately activated resulting in more severe asthma [52]. Many of the studies looking at CNTs fail to measure AHR, however CNT-exacerbation of AHR was observed in two studies [53, 54]. Neutrophilia is enhanced by CNTs in a number of studies, though is less frequent than eosinophilia [50–53]. CNTs have been found to be more effective at exacerbating allergy than their similar nanosized cousins, carbon nanofibers, as seen by enhanced serum IgE levels; these differences are likely due to CNTs characteristic of being both longer and thinner than nanofibers [55]. Potential mechanisms of CNT-induced asthma exacerbation have been examined with one study looking at the role of COX-2 [56]. MWCNTs were found to be more effective at exacerbating asthma in COX-2 deficient mice when compared to wild-type: IL-13 in particular was greatly increased in the BALF of MWCNT/OVA-exposed COX-2−/− mice, as was serum IgE; interestingly, airway collagen thickness was actually decreased by MWCNTs in this study [56]. The role of STAT1 has also been investigated and similarly to COX-2 deficiency, STAT1−/− mice are more susceptible to asthma exacerbation by MWCNT: mucous cell metaplasia in airways is increased in knockouts, as are TGF-β1, osteopontin and IL-13, indicating a protective role of STAT1 in asthma [57].
The newer and more clinically relevant house dust mite allergen (HDM) model of asthma has also been used to investigate CNT exacerbation of asthma. As with the OVA model, markers of TH2 inflammation are increased in HDM/MWCNT exposed mice over HDM alone: serum IgE, BALF eosinophils, airway mucin, IL-13 and eotaxin [58, 59]. Airway fibrosis is also enhanced by co-exposure to HDM and MWCNTs as seen by lung collagen and higher BALF levels of the profibrotic cytokine TGF-β1 [58, 59]. Interestingly in one of these studies it was seen that MWCNTs induced inflammasome activation and subsequent IL-1β production by human THP-1 macrophages in vitro, which was suppressed by the TH2 cytokines IL-4 and IL-13, which are present in the allergic asthma environment [59]. CNTs have also been looked at in a chemically induced asthma model by using trimellitic anhydride (TMA) to induce allergy in rats [60]. MWCNTs were administered after sensitization and challenge with TMA and unlike all the above discussed studies, actually decreased TMA-induced serum IgE levels and BALF lymphocytes [60].
ENMs as a Direct Cause of Allergic Airway Disease
TiO2 Nanoparticles
TiO2 NPs can elicit several characteristics of allergic asthma directly, both in vivo and in vitro. In rats a single intratracheal dose of TiO2 NPs increased BALF eosinophils and neutrophils, the number of airway cells expressing Muc5ac, PAS-positive airway cells, and IL-13 expressing cells [61]. In vitro there is evidence for TiO2 NP-induced mucus secretion as well: human bronchial epithelial cells treated with TiO2 NPs show a dose dependent increase in mucus secretion, which was found to be dependent on Ca2+ influx into the cell [62]. Immune cell proliferation and activation is also affected by TiO2 NPs, which in vitro promote T cell proliferation directly, and TiO2 treated dendritic cells have an enhanced ability to stimulate CD4+ T cell proliferation [63]. Interestingly, TiO2 NPs have also been shown to modulate AHR in rats [64]. Rats exposed to TiO2 NPs by inhalation had increased expression of neurotrophins, which are closely involved with the responsiveness of airway sensory neurons [64]. AHR measurements in these animals mirrored these findings, showing a statistically significant increase with TiO2 NP exposure [64]. Notable however is that these effects were only seen in newborn and weanling rats, not adults, which could indicate that children are more susceptible to TiO2 NP-induced airway disease [64].
Zn, Ni, Cu, Ce, and Ag Nanoparticles
Zn, Ni and Cu NPs are able to recruit eosinophils to the lungs [40, 65, 66]. In epithelial cells in vitro both Zn and Cu NPs increased IL-8 secretion, and Cu NPs alone increased NF-κB activity; these effects were found to be ion-dependent [65]. This study, however, also showed Zn NP recruitment of eosinophils to the lung to be ion-independent [65]. Zn NPs also increase BALF levels of the TH2 cytokines IL-4, IL-13 and IL-5 [40]. In addition to eosinophil recruitment, Cu NPs also induce IL-6, IL-8 and Muc5ac production in bronchial epithelial cells in vitro, the latter of which is MAPK-dependent [67]. Ni NP-induced eosinophil recruitment to the lungs of rats was associated with increased eotaxin, likely released by alveolar macrophages [66]. Ce NPs have been shown to have contrasting effects to TiO2 NPs in that they act as an antioxidant and in vitro promote a TH2 type environment when dendritic cells are exposed, which go on to induce naïve T cells to produce IL-4 and IL-5 [63]. Ag NP have also been shown to promote allergic inflammation in mice when administered with LPS, which was dependent on CD4+ T cells and resulted in increased IL-17A, an effect not seen with Ag ions [68].
Carbon Nanotubes
With CNTs being one of the most heavily studied ENM in relation to asthma, there is a good deal of evidence that they can elicit allergic lung responses directly. Both single-walled CNTs (SWCNTs) and MWCNTs are able to cause AHR in mice [69, 70]. While a dose-dependent increase was seen in AHR with exposure to SWCNTs, BALF cell counts in these mice did not exhibit the eosinophilia usually associated with allergy, instead showing increased neutrophil numbers [69]. MWCNTs on the other hand exhibited an eosinophilic response in addition to neutrophilia and AHR [70]. The AHR and eosinophilia observed in this study was found to be dependent on both IL-13 and IL-33, but independent of T and B cells, indicating that MWCNTs are modulating the innate immune response to elicit allergy associated pathologies [70]. Intratracheal instillation of MWCNTs increases the proportion of B cells in the blood, while decreasing natural killer cells and T cells; serum IgE levels are also increased, the level of which rises over time post-exposure [71]. Additionally, IL-1, IL-6, IL-12 and especially IL-10 are significantly increased in the BALF of MWCNT-exposed mice [71]. The physicochemical properties of different types of CNTs have varying effects on the type of immune response they elicit, as examined by two studies [72, 73]. Rod-like MWCNTs, which are more rigid, are known to be more toxic than other more flexible MWCNTs, and are able to cause AHR, mucus secretion, eosinophilia and TH2 cytokine release (IL-4, IL-13) [72]. These finding are supported by another study, which compared rod-like and the more flexible tangled MWCNTs, finding that rod-like but not tangled MWCNTs induced IL-4, airway mucus production, and elevated serum IgE levels [73]. In vitro, MWCNTs are able to activate the NLRP3 inflammasome in human bronchial epithelial cells, indicating a potential to cause airway remodeling, a key feature of asthma [74].
Human Evidence for ENM-Induced Asthma
Though there is no direct human evidence of ENMs causing asthma, the findings of several studies suggest that ENMs may have the potential to cause or exacerbate asthma. A 2014 study used fractional exhaled nitric oxide (FENO) measurement as an endpoint to determine if NPs affect respiratory function [75]. FENO measurement has been recognized as an excellent diagnostic tool for airway inflammation, and FENO is elevated in patients with asthma, atopy and viral infection [75]. Workers from ENM-handling plants in Taiwan were recruited for the study, and associations were found between the risk level of NP exposure and FENO, with the strongest association being between TiO2 NPs and FENO [75]. It was also found that asthma and allergic rhinitis were associated with FENO [75]. This study indicates that ENMs, especially TiO2 NPs may have the potential to exacerbate asthma. More insight into the effect of NPs on allergic airway disease comes from a case report on a chemist who worked with Ni NPs [76]. Upon initiation of work with Ni NP on an open work bench, the patient experienced throat congestion, flushing of the face, and allergic skin reaction to metal jewelry [76]. The patient received bronchodilators, which improved forced expiratory volume, and the patient also tested positive to nickel in an allergy test [76]. The symptoms exhibited are associated with allergic rhinitis, and the spirometry testing suggests AHR, indicating that Ni NPs can cause allergy, or potentially allergic airway disease [76]. In 2015, a study was published which described CNTs, presumed to be from diesel exhaust, that were found in the airways of asthmatic children [77]. In the study, BALF was obtained from bronchoscopies of asthmatic children, and CNT-like structures were observed inside lung cells. This was intriguing since CNTs can be generated from high-temperature diesel exhaust engines in a laboratory setting [78]. However, no direct evidence was given in the study with asthmatic children that defined the source of the CNT-like structures in inflammatory cells and therefore this study lacked a definitive link between exposure and disease outcome. However, it is still worth mentioning because of its real-world relevance for environmental nanomaterial exposure. Future studies should carefully evaluate whether CNTs generated by diesel engines represent a human health risk.
Conclusion
It is clear from the existing studies in rodents that ENMs can modulate the immune response in asthma to either exacerbate or attenuate the disease. ENMs impact a variety of cellular and molecular targets in allergic airway disease including serum IgE, AHR, BALF cell counts, and pro-inflammatory cytokines. ENM modulation of the immune response in asthma is illustrated in Figure 2. The effects specific ENMs elicit depend on their, size, shape, composition, and coating. A summary of asthma outcomes that are affected by different types of ENMs can be found in Table 1. Because ENMs are so varied it will be necessary in the future to continue to assess their risk to individuals with lung diseases. Strides are being made towards a more mechanistic understanding of ENM-induced allergic lung disease, however much is still unknown. Additionally, there is a need for more epidemiological studies looking at workplace exposure to ENMs and their respiratory health effects in humans. Along these same lines, it would be advantageous for more studies to look at lower, occupationally relevant doses of ENMs and how exposure affects asthma induced by more relevant common allergens like HDM and cockroach allergens. There is also a need to assess early life exposure, as asthma is more frequent in children [1].
Acknowledgments
The authors would like to acknowledge the following funding sources: NIEHS Grant R01ES020897 (JCB), NIEHS Training Grant T32ES007046 (MDH), and NSF Grant 15-022 (JCB).
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Mark D. Ihrie and James C. Bonner declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Ozdoganoglu T, Songu M. The burden of allergic rhinitis and asthma. Ther Adv Respir Dis. 2012;6:11–23. doi: 10.1177/1753465811431975. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST. Allergy Allerg Dis. Wiley-Blackwell; Oxford, UK: Pathogenesis of Asthma; pp. 1608–1631. [Google Scholar]
- 4.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11:577–84. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402:B2–B4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 6.Royce SG, Cheng V, Samuel CS, Tang MLK. The regulation of fibrosis in airway remodeling in asthma. Mol Cell Endocrinol. 2012;351:167–175. doi: 10.1016/j.mce.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Wark PB, Gibson PG. Asthma exacerbations .3: Pathogenesis. Thorax. 2006;61:909–915. doi: 10.1136/thx.2005.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB, Bernstein JA. Health effects of air pollution. J Allergy Clin Immunol. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Baldacci S, Maio S, Cerrai S, Sarno G, Baïz N, Simoni M, Annesi-Maesano I, Viegi G. Allergy and asthma: Effects of the exposure to particulate matter and biological allergens. Respir Med. 2015;109:1089–1104. doi: 10.1016/j.rmed.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Auffan M, Rose J, Bottero J-Y, Lowry G, V, Jolivet J-P, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 11.Dreher KL. Health and environmental impact of nanotechnology: Toxicological assessment of manufactured nanoparticles. Toxicol Sci. 2004;77:3–5. doi: 10.1093/toxsci/kfh041. [DOI] [PubMed] [Google Scholar]
- 12.Elsaesser A, Howard CV. Toxicology of nanoparticles. Adv Drug Deliv Rev. 2012;64:129–137. doi: 10.1016/j.addr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Baughman RH, Zakhidov AA, de Heer WA. Carbon Nanotubes-the Route Toward Applications. Science (80-) 2002;297:787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 14.Xia T, Hamilton RF, Bonner JC, et al. Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ Health Perspect. 2013;121:683–90. doi: 10.1289/ehp.1306561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nel A, Xia T, Mädler L, Li N. Toxic Potential of Materials at the Nanolevel. Science (80-) 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 16.Kim B-G, Lee P-H, Lee S-H, Park M-K, Jang A-S. Effect of TiO 2 Nanoparticles on Inflammasome-Mediated Airway Inflammation and Responsiveness. Allergy Asthma Immunol Res. 2017;9:257. doi: 10.4168/aair.2017.9.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonasson S, Gustafsson Å, Koch B, Bucht A. Inhalation exposure of nano-scaled titanium dioxide (TiO 2) particles alters the inflammatory responses in asthmatic mice. Inhal Toxicol. 2013;25:179–191. doi: 10.3109/08958378.2013.770939. [DOI] [PubMed] [Google Scholar]
- 18.Mishra V, Baranwal V, Mishra RK, Sharma S, Paul B, Pandey AC. Titanium dioxide nanoparticles augment allergic airway inflammation and Socs3 expression via NF-κB pathway in murine model of asthma. Biomaterials. 2016;92:90–102. doi: 10.1016/j.biomaterials.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson Å, Jonasson S, Sandström T, Lorentzen JC, Bucht A. Genetic variation influences immune responses in sensitive rats following exposure to TiO2 nanoparticles. Toxicology. 2014;326:74–85. doi: 10.1016/j.tox.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Rossi EM, Pylkkänen L, Koivisto AJ, Nykäsenoja H, Wolff H, Savolainen K, Alenius H. Inhalation exposure to nanosized and fine TiO2 particles inhibits features of allergic asthma in a murine model. Part Fibre Toxicol. 2010;7:35. doi: 10.1186/1743-8977-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain S, Vanoirbeek JAJ, Luyts K, et al. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. Eur Respir J. 2011;37:299–309. doi: 10.1183/09031936.00168509. [DOI] [PubMed] [Google Scholar]
- 22.Han B, Guo J, Abrahaley T, et al. Adverse Effect of Nano-Silicon Dioxide on Lung Function of Rats with or without Ovalbumin Immunization. PLoS One. 2011;6:e17236. doi: 10.1371/journal.pone.0017236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida T, Yoshioka Y, Fujimura M, et al. Promotion of allergic immune responses by intranasally-administrated nanosilica particles in mice. Nanoscale Res Lett. 2011;6:195. doi: 10.1186/1556-276X-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandenberger C, Rowley NL, Jackson-Humbles DN, et al. Engineered silica nanoparticles act as adjuvants to enhance allergic airway disease in mice. Part Fibre Toxicol. 2013;10:26. doi: 10.1186/1743-8977-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HJ, Sohn J-H, Kim Y-J, et al. Acute exposure to silica nanoparticles aggravate airway inflammation: different effects according to surface characteristics. Exp Mol Med. 2015;47:e173. doi: 10.1038/emm.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han H, Park YH, Park HJ, Lee K, Um K, Park J-W, Lee J-H. Toxic and adjuvant effects of silica nanoparticles on ovalbumin-induced allergic airway inflammation in mice. Respir Res. 2016;17:60. doi: 10.1186/s12931-016-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Zhang Q, Kaplan BLF, Baker GL, Kaminski NE. Induced T cell cytokine production is enhanced by engineered nanoparticles. Nanotoxicology. 2014;8:11–23. doi: 10.3109/17435390.2013.848302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JW, Jang JW, Cha HR, Jung SY, Lee J, Jung SS, Kim JO, Kim S, Lee CS, Park H. Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation. Int J Nanomedicine. 2012;7:1329. doi: 10.2147/IJN.S27159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park HS, Kim KH, Jang S, et al. Attenuation of allergic airway inflammation and hyperresponsiveness in a murine model of asthma by silver nanoparticles. Int J Nanomedicine. 2010;5:505–15. doi: 10.2147/ijn.s11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng T-J, Chuang H-C, Hsiao T-C, Wu C-K, Chang H-H, Lee C-H, Chang C-C, Taiwan CardioPulmonary Research Group (T-CPR) Allergenicity and toxicology of inhaled silver nanoparticles in allergen-provocation mice models. Int J Nanomedicine. 2013;8:4495. doi: 10.2147/IJN.S52239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chuang H-C, Chien-Ling Su T-T, Tzu-Tao Chen C-C, et al. Comparative proteomics of inhaled silver nanoparticles in healthy and allergen provoked mice. Int J Nanomedicine. 2013;8:2783. doi: 10.2147/IJN.S46997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva RM, Anderson DS, Peake J, et al. Aerosolized Silver Nanoparticles in the Rat Lung and Pulmonary Responses over Time. Toxicol Pathol. 2016;44:673–86. doi: 10.1177/0192623316629804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becak DP, H N, Shannahan JH. Cardiac Ischemia Reperfusion Injury Following Instillation of 20 nm Citrate-capped Nanosilver. J Nanomed Nanotechnol. doi: 10.4172/2157-7439.S6-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barreto E, Serra MF, dos Santos RV, et al. Local Administration of Gold Nanoparticles Prevents Pivotal Pathological Changes in Murine Models of Atopic Asthma. J Biomed Nanotechnol. 2015;11:1038–1050. doi: 10.1166/jbn.2015.2024. [DOI] [PubMed] [Google Scholar]
- 35.Omlor AJ, Le DD, Schlicker J, et al. Local Effects on Airway Inflammation and Systemic Uptake of 5 nm PEGylated and Citrated Gold Nanoparticles in Asthmatic Mice. Small. 2017;13:1603070. doi: 10.1002/smll.201603070. [DOI] [PubMed] [Google Scholar]
- 36.Radauer-Preiml I, Andosch A, Hawranek T, Luetz-Meindl U, Wiederstein M, Horejs-Hoeck J, Himly M, Boyles M, Duschl A. Nanoparticle-allergen interactions mediate human allergic responses: protein corona characterization and cellular responses. Part Fibre Toxicol. 2015;13:3. doi: 10.1186/s12989-016-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geiser M, Wigge C, Conrad ML, Eigeldinger-Berthou S, Künzi L, Garn H, Renz H, Mall MA. Nanoparticle uptake by airway phagocytes after fungal spore challenge in murine allergic asthma and chronic bronchitis. BMC Pulm Med. 2014;14:116. doi: 10.1186/1471-2466-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ban M, Langonné I, Huguet N, Guichard Y, Goutet M. Iron oxide particles modulate the ovalbumin-induced Th2 immune response in mice. Toxicol Lett. 2013;216:31–39. doi: 10.1016/j.toxlet.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Gustafsson Å, Bergström U, Ågren L, Österlund L, Sandström T, Bucht A. Differential cellular responses in healthy mice and in mice with established airway inflammation when exposed to hematite nanoparticles. Toxicol Appl Pharmacol. 2015;288:1–11. doi: 10.1016/j.taap.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Huang K-L, Lee Y-H, Chen H-I, Liao H-S, Chiang B-L, Cheng T-J. Zinc oxide nanoparticles induce eosinophilic airway inflammation in mice. J Hazard Mater. 2015;297:304–312. doi: 10.1016/j.jhazmat.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Park J-W, Lee I-C, Shin N-R, Jeon C-M, Kwon O-K, Ko J-W, Kim J-C, Oh S-R, Shin I-S, Ahn K-S. Copper oxide nanoparticles aggravate airway inflammation and mucus production in asthmatic mice via MAPK signaling. Nanotoxicology. 2016;10:445–452. doi: 10.3109/17435390.2015.1078851. [DOI] [PubMed] [Google Scholar]
- 42.Glista-Baker EE, Taylor AJ, Sayers BC, Thompson EA, Bonner JC. Nickel Nanoparticles cause exaggerated lung and airway remodeling in mice lacking the T-box transcription factor, TBX21 (T-bet) Part Fibre Toxicol. 2014;11:7. doi: 10.1186/1743-8977-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koike E, Takano H, Inoue K-I, Yanagisawa R, Sakurai M, Aoyagi H, Shinohara R, Kobayashi T. Pulmonary Exposure to Carbon Black Nanoparticles Increases the Number of Antigen-Presenting Cells in Murine Lung. Int J Immunopathol Pharmacol. 2008;21:35–42. doi: 10.1177/039463200802100105. [DOI] [PubMed] [Google Scholar]
- 44.Kroker M, Sydlik U, Autengruber A, Cavelius C, Weighardt H, Kraegeloh A, Unfried K. Preventing carbon nanoparticle-induced lung inflammation reduces antigen-specific sensitization and subsequent allergic reactions in a mouse model. Part Fibre Toxicol. 2015;12:20. doi: 10.1186/s12989-015-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefebvre DE, Pearce B, Fine JH, Chomyshyn E, Ross N, Halappanavar S, Tayabali AF, Curran I, Bondy GS. In Vitro Enhancement of Mouse T Helper 2 Cell Sensitization to Ovalbumin Allergen by Carbon Black Nanoparticles. Toxicol Sci. 2014;138:322–332. doi: 10.1093/toxsci/kfu010. [DOI] [PubMed] [Google Scholar]
- 46.Shurin MR, Yanamala N, Kisin ER, et al. Graphene Oxide Attenuates Th2-Type Immune Responses, but Augments Airway Remodeling and Hyperresponsiveness in a Murine Model of Asthma. ACS Nano. 2014;8:5585–5599. doi: 10.1021/nn406454u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan JJ, Bateman HR, Stover A, Gomez G, Norton SK, Zhao W, Schwartz LB, Lenk R, Kepley CL. Fullerene nanomaterials inhibit the allergic response. J Immunol. 2007;179:665–72. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- 48.Hardy CL, LeMasurier JS, Belz GT, et al. Inert 50-nm Polystyrene Nanoparticles That Modify Pulmonary Dendritic Cell Function and Inhibit Allergic Airway Inflammation. J Immunol. 2012;188:1431–1441. doi: 10.4049/jimmunol.1100156. [DOI] [PubMed] [Google Scholar]
- 49.Enright HA, Bratt JM, Bluhm AP, Kenyon NJ, Louie AY. Tracking retention and transport of ultrafine polystyrene in an asthmatic mouse model using positron emission tomography. Exp Lung Res. 2013;39:304–313. doi: 10.3109/01902148.2013.819048. [DOI] [PubMed] [Google Scholar]
- 50*.Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled Multiwalled Carbon Nanotubes Potentiate Airway Fibrosis in Murine Allergic Asthma. Am J Respir Cell Mol Biol. 2009;40:349–358. doi: 10.1165/rcmb.2008-0276OC. One of the first papers examining the ability of CNTs to exacerbate asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue K, Koike E, Yanagisawa R, Hirano S, Nishikawa M, Takano H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol Appl Pharmacol. 2009;237:306–316. doi: 10.1016/j.taap.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Inoue K, Yanagisawa R, Koike E, Nishikawa M, Takano H. Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: Possible role of oxidative stress. Free Radic Biol Med. 2010;48:924–934. doi: 10.1016/j.freeradbiomed.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Mizutani N, Nabe T, Yoshino S. Exposure to Multiwalled Carbon Nanotubes and Allergen Promotes Early- and Late-Phase Increases in Airway Resistance in Mice. Biol Pharm Bull. 2012;35:2133–2140. doi: 10.1248/bpb.b12-00357. [DOI] [PubMed] [Google Scholar]
- 54.Li J, Li L, Chen H, et al. Application of vitamin E to antagonize SWCNTs-induced exacerbation of allergic asthma. Sci Rep. 2015;4:4275. doi: 10.1038/srep04275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nygaard UC, Samuelsen M, Marioara CD, Løvik M. Carbon Nanofibers Have IgE Adjuvant Capacity but Are Less Potent Than Nanotubes in Promoting Allergic Airway Responses. Biomed Res Int. 2013;2013:1–12. doi: 10.1155/2013/476010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sayers BC, Taylor AJ, Glista-Baker EE, et al. Role of Cyclooxygenase-2 in Exacerbation of Allergen-Induced Airway Remodeling by Multiwalled Carbon Nanotubes. Am J Respir Cell Mol Biol. 2013;49:525–535. doi: 10.1165/rcmb.2013-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson EA, Sayers BC, Glista-Baker EE, Shipkowski KA, Ihrie MD, Duke KS, Taylor AJ, Bonner JC. Role of Signal Transducer and Activator of Transcription 1 in Murine Allergen–Induced Airway Remodeling and Exacerbation by Carbon Nanotubes. Am J Respir Cell Mol Biol. 2015;53:625–636. doi: 10.1165/rcmb.2014-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ronzani C, Casset A, Pons F. Exposure to multi-walled carbon nanotubes results in aggravation of airway inflammation and remodeling and in increased production of epithelium-derived innate cytokines in a mouse model of asthma. Arch Toxicol. 2014;88:489–499. doi: 10.1007/s00204-013-1116-3. [DOI] [PubMed] [Google Scholar]
- 59.Shipkowski KA, Taylor AJ, Thompson EA, Glista-Baker EE, Sayers BC, Messenger ZJ, Bauer RN, Jaspers I, Bonner JC. An Allergic Lung Microenvironment Suppresses Carbon Nanotube-Induced Inflammasome Activation via STAT6-Dependent Inhibition of Caspase-1. PLoS One. 2015;10:e0128888. doi: 10.1371/journal.pone.0128888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staal YCM, van Triel JJ, Maarschalkerweerd TVP, Arts JHE, Duistermaat E, Muijser H, van de Sandt JJM, Kuper CF. Inhaled Multiwalled Carbon Nanotubes Modulate the Immune Response of Trimellitic Anhydride–induced Chemical Respiratory Allergy in Brown Norway Rats. Toxicol Pathol. 2014;42:1130–1142. doi: 10.1177/0192623313519874. [DOI] [PubMed] [Google Scholar]
- 61.Ahn M-H, Kang C-M, Park C-S, Park S-J, Rhim T, Yoon P-O, Chang HS, Kim S-H, Kyono H, Kim KC. Titanium dioxide particle – induced goblet cell hyperplasia : association with mast cells and IL-13. Respir Res. 2005;6:34. doi: 10.1186/1465-9921-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen EYT, Garnica M, Wang Y-C, Chen C-S, Chin W-C. Mucin Secretion Induced by Titanium Dioxide Nanoparticles. PLoS One. 2011;6:e16198. doi: 10.1371/journal.pone.0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schanen BC, Das S, Reilly CM, Warren WL, Self WT, Seal S, Drake DR. Immunomodulation and T Helper TH1/TH2 Response Polarization by CeO2 and TiO2 Nanoparticles. PLoS One. 2013;8:e62816. doi: 10.1371/journal.pone.0062816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scuri M, Chen BT, Castranova V, Reynolds JS, Johnson VJ, Samsell L, Walton C, Piedimonte G. Effects of Titanium Dioxide Nanoparticle Exposure on Neuroimmune Responses in Rat Airways. J Toxicol Environ Heal Part A. 2010;73:1353–1369. doi: 10.1080/15287394.2010.497436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho W-S, Duffin R, Poland CA, Duschl A, Oostingh GJ, MacNee W, Bradley M, Megson IL, Donaldson K. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo ; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology. 2012;6:22–35. doi: 10.3109/17435390.2011.552810. [DOI] [PubMed] [Google Scholar]
- 66.Lee S, Hwang S-H, Jeong J, et al. Nickel oxide nanoparticles can recruit eosinophils in the lungs of rats by the direct release of intracellular eotaxin. Part Fibre Toxicol. 2015;13:30. doi: 10.1186/s12989-016-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ko J-W, Park J-W, Shin N-R, et al. Copper oxide nanoparticle induces inflammatory response and mucus production via MAPK signaling in human bronchial epithelial cells. Environ Toxicol Pharmacol. 2016;43:21–26. doi: 10.1016/j.etap.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Hirai T, Yoshioka Y, Izumi N, et al. Metal nanoparticles in the presence of lipopolysaccharides trigger the onset of metal allergy in mice. Nat Nanotechnol. 2016;11:808–816. doi: 10.1038/nnano.2016.88. [DOI] [PubMed] [Google Scholar]
- 69.Hsieh W-Y, Chou C-C, Ho C-C, Yu S-L, Chen H-Y, Chou H-YE, Chen JJW, Chen H-W, Yang P-C. Single-Walled Carbon Nanotubes Induce Airway Hyperreactivity and Parenchymal Injury in Mice. Am J Respir Cell Mol Biol. 2012;46:257–267. doi: 10.1165/rcmb.2011-0010OC. [DOI] [PubMed] [Google Scholar]
- 70.Beamer CA, Girtsman TA, Seaver BP, Finsaas KJ, Migliaccio CT, Perry VK, Rottman JB, Smith DE, Holian A. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 2012;7:1070–1081. doi: 10.3109/17435390.2012.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park E-J, Cho W-S, Jeong J, Yi J, Choi K, Park K. Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation. Toxicology. 2009;259:113–121. doi: 10.1016/j.tox.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 72**.Rydman EM, Ilves M, Koivisto AJ, et al. Inhalation of rod-like carbon nanotubes causes unconventional allergic airway inflammation. Part Fibre Toxicol. 2014;11:48. doi: 10.1186/s12989-014-0048-2. An excellent paper that thoroughly examines direct allergic effects of rigid, rod-like CNTs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73*.Duke KS, Taylor-Just AJ, Ihrie MD, Shipkowski KA, Thompson EA, Dandley EC, Parsons GN, Bonner JC. STAT1-dependent and-independent pulmonary allergic and fibrogenic responses in mice after exposure to tangled versus rod-like multi-walled carbon nanotubes. Part Fibre Toxicol. 2017;14:26. doi: 10.1186/s12989-017-0207-3. A study that highlights the interactions of physicochemical features and genetic susceptibility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74*.Hussain S, Sangtian S, Anderson SM, Snyder RJ, Marshburn JD, Rice AB, Bonner JC, Garantziotis S. Inflammasome activation in airway epithelial cells after multi-walled carbon nanotube exposure mediates a profibrotic response in lung fibroblasts. Part Fibre Toxicol. 2014;11:28. doi: 10.1186/1743-8977-11-28. An important paper that shows the inflammasome mechanism in airway epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu W-T, Liao H-Y, Chung Y-T, Li W-F, Tsou T-C, Li L-A, Lin M-H, Ho J-J, Wu T-N, Liou S-H. Effect of Nanoparticles Exposure on Fractional Exhaled Nitric Oxide (FENO) in Workers Exposed to Nanomaterials. Int J Mol Sci. 2014;15:878–894. doi: 10.3390/ijms15010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Journeay WS, Goldman RH. Occupational handling of nickel nanoparticles: A case report. Am J Ind Med. 2014;57:1073–1076. doi: 10.1002/ajim.22344. [DOI] [PubMed] [Google Scholar]
- 77.Kolosnjaj-Tabi J, Just J, Hartman KB, Laoudi Y, Boudjemaa S, Alloyeau D, Szwarc H, Wilson LJ, Moussa F. Anthropogenic Carbon Nanotubes Found in the Airways of Parisian Children. EBioMedicine. 2015;2:1697–1704. doi: 10.1016/j.ebiom.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jung HS, Miller A, Park K, Kittelson DB. Carbon nanotubes among diesel exhaust particles: real samples or contaminants? J Air Waste Manage Assoc. 2013;63:1199–1204. doi: 10.1080/10962247.2013.812048. [DOI] [PubMed] [Google Scholar]


