Abstract
Overcoming immune tolerance of the growth factors associated with tumor growth should be a useful approach to cancer therapy by active immunity. We used vascular endothelial growth factor (VEGF) as a model antigen to explore the feasibility of the immunogene tumor therapy with a vaccine based on a single xenogeneic homologous gene, targeting the growth factors associated with angiogenesis. To test this concept, we constructed a plasmid DNA encoding Xenopus homologous VEGF (XVEGF-p) and control vectors. We found that immunogene tumor therapy with a vaccine based on XVEGF was effective at both protective and therapeutic antitumor immunity in several tumor models in mice. VEGF-specific autoantibodies in sera of mice immunized with XVEGF-p could be found in Western blotting analysis and ELISA assay. The purified immunoglobulins were effective at the inhibition of VEGF-mediated endothelial cell proliferation in vitro, and at antitumor activity and the inhibition of angiogenesis by adoptive transfer in vivo. The elevation of VEGF in the sera of the tumor-bearing mice could be abrogated with XVEGF-p immunization. The antitumor activity and production of VEGF-specific autoantibodies, significantly elevated IgG1 and IgG2b, could be abrogated by the depletion of CD4+ T lymphocytes. The observations may provide a vaccine strategy for cancer therapy through the induction of autoimmunity against the growth factors associated with tumor growth in a cross reaction with single xenogeneic homologous gene and may be of importance in the further exploration of the applications of other xenogeneic homologous genes identified in human and other animal genome sequence projects in cancer therapy.
Active specific immunotherapies with cancer vaccines based on tumor antigens represent very promising approaches to cancer therapy (1, 2). However, to date, with the few exceptions of melanoma tumor antigens, there is still limited information on the identity and density of antigenic peptides and cytotoxic T lymphocyte epitopes presented by human solid tumors (1, 2). Efforts are therefore continuing to develop a new strategy for cancer vaccines.
The generation of new blood vessels, or angiogenesis, is regulated by some growth factors, including vascular endothelial growth factor (VEGF) and fibroblast growth factors (3–12). The proliferation of tumor cells themselves is also regulated by some growth factors, including transforming growth factor α, amphiregulin, and IL-6 under certain circumstances (13–15). It is conceivable that overcoming immune tolerance of these growth factors associated with angiogenesis or cancer cell growths may be a useful approach to cancer therapy by active immunity. However, an immune response to growth factors is presumably difficult to elicit with a vaccine based on autologous or syngeneic growth factors because of the immune tolerance acquired during the development of the immune system.
Many genes were highly conserved during the evolutionary process, which was characterized by varying degrees of gene similarity among different species (16). Many counterparts of the genes of human and mouse can be identified from the genome sequence of the fruit fly Drosophila melanogaster and of other animals such as Xenopus laevis (16). For example, a comparison analysis made in the present study by searching the Swissprot database at the National Center for Biotechnology Information indicates that the Xenopus homologue of VEGF (accession no. AAB63680.1) (4) is 75% and 73% identical in mouse VEGF 164 (accession no. AAB22253.1) (3) and human VEGF 165 (accession no. BAA78418.1) (5), respectively, at the amino acid level.
As a strategy for cancer therapy, antiangiogenic therapy attempts to stop new vessels from forming around a tumor and to break up the existing network of abnormal capillaries that feeds the cancerous mass (6, 10, 17–22). VEGF has been known to be a potent vasculogenic and angiogenic factor (7–12). It has been reported that the abrogation of VEGF-induced angiogenesis, including the passive immunization of a neutralizing antibody against VEGF, can suppress tumor growth in vivo (12, 21), suggesting that VEGF plays an important role in angiogenesis in tumor growth. Thus, VEGF may be used as a ideal model molecule to explore the feasibility of immunogene tumor therapy with a vaccine based on a single xenogeneic gene by overcoming the immune tolerance of growth factors associated with tumor growth in a crossreaction between xenogeneic homologous and self-molecules. To test this concept, we constructed a plasmid DNA encoding Xenopus VEGF (XVEGF-p). At the same time, the plasmid DNA encoding the corresponding mouse VEGF (MVEGF-p) and empty vector (e-p) were also constructed and used as controls. The vaccines were tested for the ability to induce antitumor immunity in several tumor models in mice.
Materials and Methods
Vaccine Preparation and Its Immunization.
A cDNA clone encoding Xenopus homologous VEGF and that encoding the corresponding mouse VEGF 164 were isolated by PCR with the use of a X. laevis cDNA library and a mouse skeletal muscle cDNA library (CLONTECH), respectively. The amplified products were inserted into PCR 2.1 plasmid (Invitrogen) and then subcloned into pSecTag 2A (Invitrogen), which contains a cytomegalovirus promoter. VEGF of Xenopus and mouse inserted into pSecTag 2A was named XVEGF-p and MVEGF-p, respectively. As a control, pure plasmid was used as an empty vector (c-p). The full-length sequence of Xenopus and mouse VEGF was confirmed by dideoxy sequence to be identical to those reported (3–5). Plasmids for DNA vaccination were purified by using two rounds of passage over Endo-free columns (Qiagen, Chatsworth, CA), as reported (23). The expression of plasmid DNA was confirmed in the transfected cells by reverse transcription–PCR and with the use of anti-VEGF antibodies in Western blotting analysis and ELISA. Meth A fibrosarcoma, MA782/5S mammary cancer, and H22 hepatoma models were established in BALB/c mice. Mice were immunized with different doses (5–150 μg per mouse) of DNA vaccine in normal saline by intramuscular injection once a week for 4 weeks. Additional control animals were injected with normal saline. All studies involving mice were approved by the institute's animal care and use committee.
Western Blot Analysis.
Western blot analysis was performed as described (24). Briefly, recombinant VEGF proteins and other proteins were separated by SDS/PAGE. Gels were electroblotted with Sartoblot onto a poly(vinylidene difluoride) membrane. The membrane blots were blocked at 4°C in 5% nonfat dry milk, washed, and probed with mouse sera at 1:500. Blots were then washed and incubated with a biotinylated secondary antibody (biotinylated horse anti-mouse IgG or IgM), followed by transfer to Vectastain ABC (Vector Laboratories). Recombinant mouse VEGF, human VEGF, and basic fibroblast growth factor (bFGF) were obtained from Sigma-Aldrich. Anti-placenta growth factor (PIGF) and bFGF antibodies were obtained from Santa Cruz Biotechnology. Recombinant Xenopus VEGF; mouse VEGF 120, 164, and 188 isoforms; VEGF-B and C; bFGF; and PlGF were expressed, refolded, and purified from Escherichia coli, as detailed (25).
Purification of Ig, Its Inhibition of Cell Proliferation in Vitro, and Its Adoptive Transfer in Vivo.
Immunoglobulins were purified from the pooled sera derived from the mice at day 7 after the fourth immunization or from control mice by affinity chromatography (CM Affi-gel Blue Gel Kit; Bio-Rad). The inhibition of VEGF-mediated endothelial cell proliferation was described (26).
To assess the efficacy of Ig in antitumor in vivo, the purified Ig (10–300 mg/kg) was adoptively intravenously transferred 1 day before mice were challenged with 1 × 105 to 1 × 107 tumor cells and then treated twice per week for 3 weeks.
Ig Subclass Response to VEGF Immunization in ELISA.
The Ig subclass was determined by ELISA as described (27). Briefly, VEGF was plated and blocked. Experimental mouse sera were serially diluted and added to wells. Plates were incubated for 2 h at 37°C, washed, and then incubated with serially diluted alkaline phosphatase-conjugated anti-mouse IgG subclass or anti-IgM or anti-IgA. Enzyme activity was measured with an ELISA reader (Bio-Rad).
Enzyme-Linked Immunospot Assay.
The enzyme-linked immunospot assay for the enumeration of specific antibody-secreting cells has been described (28). Briefly, poly(vinylidene difluoride)-bottomed 96-well Amicon multititer plates (Millipore) were coated with 30 μg/ml of VEGF. Mononuclear cells prepared from spleen were incubated on the plates at 37°C for 4 h. IgG bound to the membrane was revealed as spots with alkaline phosphatase-conjugated anti-mouse IgG antibodies.
In Vivo Depletion of Immune Cell Subsets.
Immune cell subsets were depleted as described (29, 30). Mice were injected i.p. with 500 μg of either the anti-CD4 (clone GK 1.5, rat IgG), anti-CD8 (clone 2.43, rat IgG), anti-natural killer (NK) (clone PK136) mAb, or isotype controls 1 day before the immunization, and then twice per week for 3 weeks. Tumor cells (1 × 106 to 1 × 107) were challenged after the fourth immunization. These hybridomas were obtained from the American Type Culture Collection. The depletion of CD4+, CD8+, and NK cells was consistently greater than 98%, as determined by flow cytometry (Coulter Elite ESP) (31).
Micropocket Assay.
The micropocket assay has been described (32). Briefly, five mice in each group were immunized by vaccines continuously for 4 weeks or treated by adoptive transfer of purified immunoglobulins as described above before VEGF-containing pellets were implanted into the cornea. Control groups included treatment with MVEGF-p, c-p, and saline alone or with immunoglobulins from the mice treated with MVEGF-p, c-p, and saline alone.
Immunohistochemistry.
Frozen sections were fixed in acetone, incubated, and stained with an antibody reactive to CD31 as described (33). The sections were then stained with labeled streptavidin biotin reagents (Dako LSAB kit, peroxidase; Dako). Vessel density was determined by counting the number of microvessels per high-power field in the sections as described (33).
Statistical Analyses.
For comparison of individual time points, ANOVA and an unpaired Student's t test were used (34). Survival curves were constructed according to the Kaplan–Meier method (35). Statistical significance was determined by the log-rank test (36).
Results
Induction of Protective Antitumor Immunity.
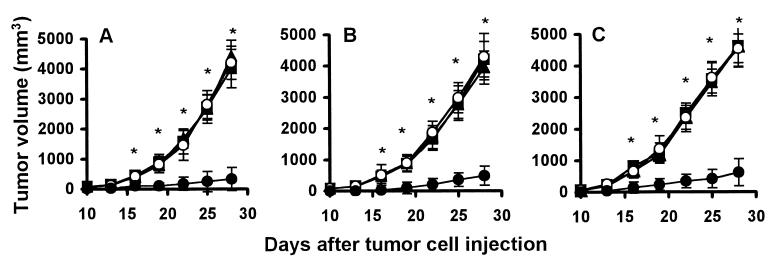
Mice were immunized i.m. once weekly continuously for 4 weeks with different doses (5–150 μg per mouse) of XVEGF-p, MVEGF-p, c-p, or saline alone, and then challenged with 1 × 104–107 Meth A fibrosarcoma cells, MA 782/5S mammary cancer cells, and H22 hepatoma cells at day 7 after the fourth immunization. As shown in Fig. 1, tumor grew progressively in all nonimmunized mice (saline alone) or in MVEGF-p or c-p-immunized mice, but there was significant protection from tumor growth in XVEGF-immunized mice. The protective effect was dose dependent. The dose (100 μg per mouse) used in Fig. 1 is an optimal one selected for immunization in several preliminary experiments. Treatment with a 150-μg dose did not show greater effect than that with 100 μg. Treatment with a 5- to 15-μg dose shows little effect.
Figure 1.
Induction of protective antitumor immunity. Mice (10 mice in each group) were immunized i.m. with 100 μg of XVEGF-p (●), MVEGF-p (○), or c-p (■) or were nonimmunized (saline alone) (▴) once a week for 4 weeks. Mice were then challenged with 1 × 106 Meth A cells (A) or H22 hepatoma (B) or MA 782/5S mammary cancer cells (C) s.c. 1 week after the fourth immunization. The results are expressed as mean ± SEM. Asterisks (*) indicate a significant difference in tumor volume (P < 0.05) between XVEGF-p-treated and control groups.
Induction of Therapeutic Antitumor Immunity.
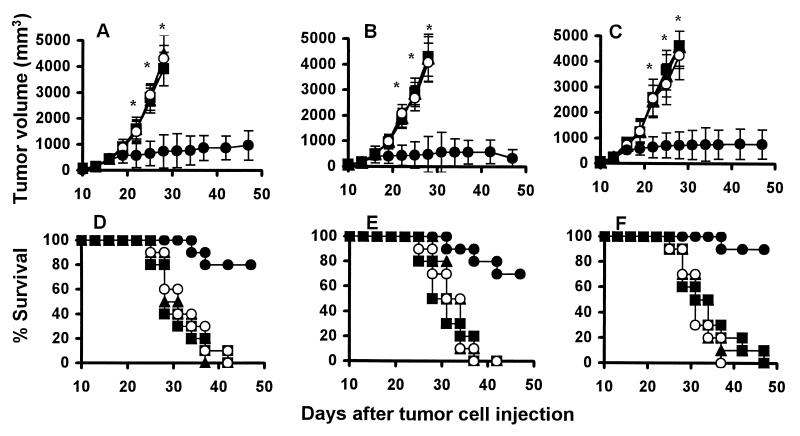
The therapeutic efficacy of XVEGF-p was next tested in the established tumors. The mice were treated starting at day 7 after the injection of Meth A fibrosarcoma cells, MA 782/5S mammary cancer cells, or H22 hepatoma cells, when the tumor was palpable. Treatment with XVEGF-p once weekly resulted in significant antitumor activity (Fig. 2). The survival of the tumor-bearing mice treated with XVEGF-p was also significantly greater than that of the controls (P < 0.0005).
Figure 2.
Induction of the therapeutic antitumor immunity. Mice (10 mice in each group) treated with i.m. injection of 100 μg of XVEGF-p (●), MVEGF-p (○), c-p (■), or saline alone (▴) once weekly for 4 weeks starting at day 7 after 1 ×106 live Meth A cells (A and D), H22 hepatoma cells (B and E), or MA 782/5S mammary cancer cells (C and F) were introduced s.c. into mice. The results are expressed as mean ± SEM. Asterisks (*) indicate a significant difference in tumor volume (P < 0.05) between XVEGF-p-treated and control groups. A significant increase in survival in XVEGF-p-treated mice, compared with the control groups (P < 0.0005, by log rank test), was found with all three tumor models. The XVEGF-p-treated mice have been followed for more than 3 months. The survival rate of the mice was 50%, 60%, and 60% at day 90 for Meth A fibrosarcoma, H22 hepatoma, and MA 782/5S mammary cancer, respectively.
The mice immunized with these vaccines have been investigated in particular for potential long-term toxicity for more than 1 year. No adverse consequences were indicated in gross measures such as weight loss, ruffling of fur, life span, behavior, and feeding. Furthermore, no pathologic changes in liver, lung, kidney, spleens, brain, heart, pancreas, intestines, or bone marrow were found by microscopic examination. In addition, hepatotoxicity was assessed once monthly for 14 months by the measurement of plasma alanine aminotransferase, aspartate aminotransferase, and direct bilirubin. At the same time, renal damage was assessed by measuring plasma urea nitrogen and creatinine. No abnormal changes were indicated by these biochemical analyses (data not shown).
Characterization of Autoantibodies Against VEGF and Their Antitumor Efficacy.
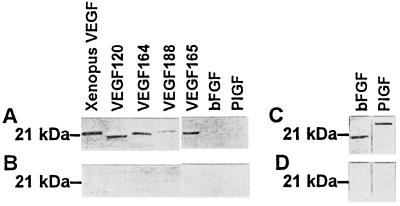
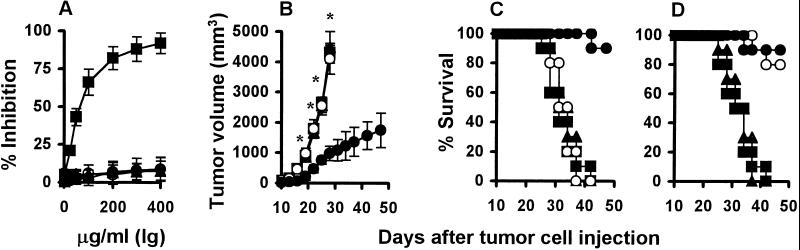
In an attempt to explore the possible mechanism by which antitumor activity was induced with XVEGF-p, we identified VEGF-specific autoantibodies in the immunized mice. Sera from mice (eight of eight mice) immunized with XVEGF-p recognized not only recombinant Xenopus VEGF, but also mouse and human VEGF in Western blotting analysis (Fig. 3A). However, the sera did not crossreact with VEGF-B and VEGF-C and other growth factors such as bFGF and PlGF. The sera isolated from controls show negative staining for VEGF and other growth factors (Fig. 3B). Treatment with purified immunoglobulins isolated from XVEGF-p-immunized mice resulted in the apparent inhibition of VEGF-mediated endothelial cell proliferation (Fig. 4A), but it had no effect on bFGF-mediated proliferation. In addition, the immunoglobulins had no direct inhibitory effect on the proliferation of tumor cells (Meth A fibrosarcoma cells, MA 782/5S mammary cancer cells, or H22 hepatoma cells) or endothelial cells (mouse SVEC4–10 cells and human umbilical vein endothelial primary cells) (data not shown). Furthermore, the abrogation of the elevation of VEGF in the sera of tumor-bearing mice was also confirmed with XVEGF-p immunization. For example, a median of 63 pg/ml or 67 pg/ml for VEGF values was present, respectively, in the sera of hepatoma-bearing mice that were not immunized or that were immunized with MVEGF-p in an ELISA assay, respectively, but the level for most mice immunized with XVEGF-p was undetectable, with a median of 2 pg/ml (P < 0.01), similar to that of normal mice. Furthermore, the number of anti-VEGF antibody-producing B cells was 76 ± 28 per 105 mononuclear cells in the spleen of mice immunized with XVEGF-p, but was undetectable in mice immunized with MVEGF-p or c-p or in nonimmunized mice (10 mice). In addition, adoptive transfer of sera or purified Ig isolated from XVEGF-p-immunized mice provided effective protection against tumor growth (Fig. 4 B and C). Adsorption of sera or Ig with recombinant VEGF before adoptive transfer could abrogate its antitumor activity (Fig. 4D).
Figure 3.
The identification of VEGF-specific antoantibody in sera in Western blot analysis. Recombinant Xenopus VEGF, VEGF 120, 164, 188, and 165 as well as other growth factors (bFGF and PlGF) were stained with sera isolated from mice immunized with XVEGF-p and other control sera. The positive bands for VEGF can be recognized with the sera isolated from mice immunized with XVEGF-p (A), but negative staining from mice immunized with MVEGF-p (B) or other control groups. (C) Positive staining for bFGF and PlGF was found with the antibodies against bFGF and PlGF, respectively, as positive controls for A, but (D) negative staining was found with control IgG.
Figure 4.
Inhibition of VEGF-mediated endothelial cell proliferation in vitro and the antitumor effect by the adoptive transfer of Igs in vivo. (A) Human umbilical vein endothelial cells were incubated with mouse or human VEGF (300 ng/ml) in the presence of various concentrations of immunoglobulins. Treatment with immunoglobulins from mice immunized with XVEGF-p (■) resulted in the apparent inhibition of endothelial cell proliferation, compared with mice immunized with MVEGF-p (○) or c-p (●) or with nonimmunized mice (▴). But it had no effect on bFGF-mediated endothelial cell proliferation (data not shown). (B) Adoptive transfer of immunoglobulins in vivo. The protective antitumor effect against Meth A cells was tested with purified immunoglobulins (25 mg/kg) from mice immunized with XVEGF-p (●), MVEGF-p (○), or c-p (■) or from nonimmunized mice (▴). Results are expressed as means ± SEM. Asterisks (*) indicate a significant difference in tumor volume (P < 0.05) between immunoglobulins from XVEGF-immunized mice and all other control groups. (C) The survival of the mice in B. Treatment of the mice with immunoglobulins from XVEGF-p-immunized mice (●) resulted in a significant increase in survival, compared with the other controls (P < 0.0005, by log rank test). (D) Protective antitumor effect against Meth A fibrosarcoma was tested with immunoglobulins from mice immunized with XVEGF-p and control vaccines, and immunoglobulins before adoptive transfer were adsorbed with VEGF or bFGF by the immunoadsorption method as detailed (51). The adsorption of the immunoglobulins with VEGF (■) could abrogate the antitumor activity of immunoglobulins from XVEGF-p-immunized mice (●) (P < 0.0005, by log rank test), but bFGF (○) had no effect (P > 0.05). The control groups include immunoglobulins from the mice treated with saline alone (▴) and from MVEGF-p- or c-p-immunized mice (not shown).
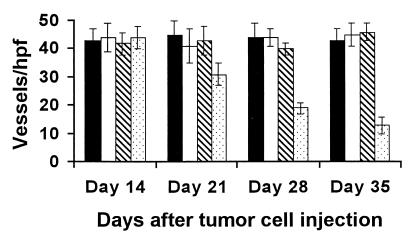
Angiogenesis was apparently suppressed within the tumors of mice treated by adoptive transfer of Ig isolated from XVEGF-p-immunized mice or with XVEGF-p. Sequential analysis of the microvessel density was performed. The microvessel density gradually decreased as a result of the prolongation of the treatment (Fig. 5). Moreover, the inhibition of angiogenesis in the mice treated by adoptive transfer of Ig from XVEGF-p-immunized mice or with XVEGF-p was confirmed by micropocket assay. Blood vessel length, clock hours (the proportion of the circumference that is vascularized if the eye is viewed as a clock), and area of neovascularization in micropocket assay were inhibited by 62 ± 8, 69 ± 7, and 82 ± 9%, respectively, in the mice treated with XVEGF-p, compared with those in nonimmunized mice (saline alone). Similar results could also be found compared with the other control groups.
Figure 5.
Sequential analysis of inhibition of angiogenesis within tumors. Mice treated were killed on days 14, 21, 28, and 35 after tumor cell injection. Vessel density was determined by counting the number of microvessels per high-power field in the sections stained with an antibody reactive to CD31, as described in Materials and Methods. Treatment with MVEGF-p (solid bar), e-p (open bar), saline alone (striped bar), XVEGF-p (dotted bar). The data are expressed as means ± SEM.
Role of CD4+ T Cells in Xenopus VEGF-Induced Antitumor Activity.
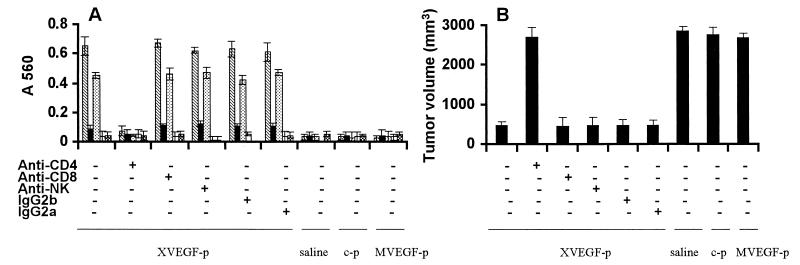
The VEGF-specific Ig subclass response to XVEGF-p was also determined by ELISA and found to be elevated significantly in IgG1 and Ig2b in sera obtained from mice at day 7 after the fourth immunization, compared with the controls (Fig. 6A). The mice depleted of CD4+ T lymphocytes did not develop detectable antibodies against VEGF (Fig. 6A) and were not protected from tumor challenge in XVEGF-p-immunized mice (Fig. 6B). In contrast, treatment with anti-CD8 or anti-NK mAb or control IgG had no effect (Fig. 6). These data suggest that the induction of the autoantibody against VEGF, which is probably responsible for XVEGF-p-induced antitumor activity, may be involved in CD4+ T lymphocytes.
Figure 6.
Abrogation of Ig subclass response and antitumor activity by the depletion of immune cell subsets. (A) Sera obtained from mice immunized with XVEGF-p were tested against mouse VEGF or Xenopus VEGF by ELISA. Immunization with XVEGF-p showed an apparent elevation of IgG1 (striped bar) and IgG2b (dotted bar), which can crossreact with mouse VEGF, and a slight increase in Ig2a (solid bar) without an increase in IgM (open bar) or IgA (hatched bar). Treatment with anti-CD4 can abrogate the elevation of IgG1 and IgG2b. In contrast, treatment with anti-CD8, anti-NK, or isotype controls (IgG2a and IgG2b) has no effect. Immunization with MVEGF-p, c-p, or saline alone showed no effect on the Ig subclass response to VEGF. The data are expressed as means ± SD. (B) Abrogation of antitumor activity by the depletion of immune cell subsets. Mice were immunized and then challenged with H22 hepatoma cells as described in Fig. 1. Depletion of immune cell subsets was described in Materials and Methods. Depletion of CD4+ T lymphocytes showed complete abrogation of the antitumor activity of the XVEGF-p vaccine. The results are expressed as means ± SEM. Data represent day 25 after tumor cell injection. Similar results can be found at other time points.
Discussion
Several observations have been made in the present study concerning the vaccine based on Xenopus VEGF as a model antigen, antitumor immunity, and angiogenesis. The vaccine based on the Xenopus homologue of VEGF as a model antigen could induce both protective and therapeutic antitumor immunity. The autoimmune response against VEGF may be provoked in a crossreaction by the immunization of Xenopus VEGF, and the autoantibody targeting of VEGF is probably responsible for the antitumor activity. These suggestions are supported by our findings in the present study. VEGF-specific autoantibodies were identified by Western blotting analysis and ELISA assay. VEGF-mediated endothelial cell proliferation was inhibited in vitro by immunoglobulins from XVEGF-p-immunized mice. The elevation of VEGF in the sera of tumor-bearing mice was abrogated with XVEGF immunization. The antitumor activity and the inhibition of angiogenesis were acquired by the adoptive transfer of purified immunoglobulins. IgG1 and IgG2b were substantially increased in response to XVEGF-p. There were antitumor activity and production of VEGF-specific autoantibodies that could be abrogated by the depletion of CD4+ T lymphocytes. Angiogenesis was apparently inhibited in tumor, and corneal angiogenesis was inhibited. In addition, the antitumor activity was also confirmed with the vaccine based on recombinant Xenopus VEGF protein and with the DNA vaccine based on quail VEGF (data not shown). Based on our findings mentioned above, we may rule out the possibility that the antitumor activity with Xenopus VEGF may result from the nonspecifically augmented immune response against tumor growth in host mice. Because our findings demonstrated that no increase in the NK activity of spleen cells or in the level of cytokines such as IFN-α, IFN-β, IFN-γ, tumor necrosis factor α, or β-chemokines in sera was found in the immunized mice (data not shown), we can also exclude the possibility that that the antitumor activity may result from a nonspecifically augmented immune response.
In the present study, we found that mice depleted of CD4+ T lymphocytes by the injection of anti-CD4 mAb and vaccinated with Xenopus VEGF were not protected from tumor challenge. At the same time, mice depleted of CD4+ T lymphocytes did not develop detectable autoantibodies against VEGF. In contrast, treatment with anti-CD8 or anti-NK mAb or control IgG failed to abrogate the antitumor activity. These findings suggest that the induction of the autoantibody response to VEGF, which is responsible for Xenopus VEGF-induced antitumor activity, may involve CD4+ T lymphocytes. It is known that CD4+ T lymphocytes can steer and amplify immune responses through the secretion of cytokines and expression of surface molecules (37, 38). It has been reported that antitumor immunity could be induced by DNA immunization against human gp75/tyrosinase-related protein-1 or tyrosinase-related protein-2 (the slaty locus protein) and has depended on CD4+ T lymphocytes in melanoma models (39–41). For the antibody-dependent immunity, CD4+ T lymphocytes can be required at the immunization phase as well as at the effector phase (42). Furthermore, CD4+ T lymphocytes have been reported to be required for the induction of antitumor immunity by vaccination with a recombinant vaccinia virus encoding self tyrosinase-related protein 1 in a mouse melanoma model (42, 43). In addition, it has been reported that CD4+ T lymphocytes play a prominent role in classic mouse models of autoimmunity, such as experimental allergic encephalitis, systemic lupus erythematosus, and autoimmune gastritis (44–47). These findings may help explain the requirement for CD4+ T lymphocytes in the induction of autoimmune response against mouse VEGF in a crossreaction.
Direct i.m. injection of naked plasmid DNA can induce a strong, long-lived immune response to the antigen encoded by the gene vaccine (48, 49). Gene transfer into muscle is simple, inexpensive, and safe (48–50). Cancer gene therapy using plasmid DNA is undergoing evaluation in clinical trials (50). In the present study, DNA vaccine based on Xenopus VEGF as an antigen could induce not only protective but also therapeutic antitumor activity in several tumor models in mice without adverse effects, as mentioned above. In addition, it has been known that the Xenopus homologue of VEGF is 75% and 73% identical in mouse VEGF 164 and human VEGF 165, respectively, at the amino acid level. Furthermore, the antibodies induced with XVEGF-p recognized not only mouse VEGF but also human VEGF. The findings mentioned above suggest that a XVEGF-p vaccine may have potential application to the treatment of cancer patients.
Taken together, our findings may provide a vaccine strategy for cancer therapy through the induction of an autoimmune response against self molecules for tumor growth in a crossreaction by immunization with a single xenogeneic homologous gene. This vaccine strategy may be used to target other growth factors or their receptors associated with tumor growth. This suggestion is also supported by our unpublished data that a vaccine based on VEGF receptor isolated from quail can induce an antitumor effect through autoimmunity against tumor endothelium in mouse, and that a vaccine based on EGF receptors from the fruit fly Drosophila melanogaster or avian EGF receptors can induce autoimmunity against EGF receptor-positive tumors in mouse models. Many counterparts of human genes can be identified from the genome sequence of D. melanogaster and of other animals such as X. laevis (4, 16). Thus, overcoming immune tolerance of self molecules involving angiogenesis or tumor cell proliferation with xenogeneic counterparts may be of importance to the further exploration of the applications of xenogeneic homologous genes identified in human and other animal genome sequence projects in cancer therapy.
Acknowledgments
This research was supported by the National 973 Project, the National Outstanding Young Scientist Foundation of China, the National Natural Sciences Foundation of China, the University Key Teacher Foundation, and the Chinese Medical Board Foundation.
Abbreviations
- VEGF
vascular endothelial growth factor
- XVEGF-p
plasmid DNA encoding homologous Xenopus VEGF
- MVEGF-p
plasmid DNA encoding homologous mouse VEGF
- c-p
e-p, empty vector
- bFGF
basic fibroblast growth factor
- NK cells
natural killer cells
- PlGF
placenta growth factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Boon T, Coulie P G, Van den Eynde B. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg S A. Immunol Today. 1997;18:175–178. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 3.Leung D W, Cachianes G, Kuang W J, Goeddel D V, Ferrara N. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 4.Cleaver O, Tonissen K F, Saha M S, Krieg P A. Dev Dyn. 1997;210:66–77. doi: 10.1002/(SICI)1097-0177(199709)210:1<66::AID-AJA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Breier D, Albrecht U, Sterrer S, Risau W. Development (Cambridge, UK) 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 7.Yancopoulos G D, Davis S, Gale N W, Rudge J S, Wiegand S J, Holash J. Nature (London) 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P, Jain R K. Nature (London) 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 9.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Alitalo K. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 11.Zetter B R. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 12.Bicknell R. In: Tumor Angiogenesis. Bicknell R, Lewis C E, Ferrara N, editors. London: Oxford Univ. Press; 1997. pp. 19–28. [Google Scholar]
- 13.De Luca A. Oncogene. 2000;19:5863–5871. doi: 10.1038/sj.onc.1203979. [DOI] [PubMed] [Google Scholar]
- 14.O-Charoenrat P, Rhys-Evans P, Eccles S. Int J Cancer. 2000;88:759–765. doi: 10.1002/1097-0215(20001201)88:5<759::aid-ijc12>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Treon S P, Anderson K C. Curr Opin Hematol. 1998;5:42–48. [PubMed] [Google Scholar]
- 16.Kornberg T B, Krasnow M A. Science. 2000;287:2218–2220. doi: 10.1126/science.287.5461.2218. [DOI] [PubMed] [Google Scholar]
- 17.Kerbel R S. Nature (London) 1997;390:335–336. doi: 10.1038/36978. [DOI] [PubMed] [Google Scholar]
- 18.Fidler I J, Ellis L M. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 19.Arap W, Pasqualini R, Ruoslahti E. Science. 1998;279:377–379. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 20.Fan T P, Jaggar R, Bicknell R. Trends Pharmacol Sci. 1995;16:57–66. doi: 10.1016/s0165-6147(00)88979-8. [DOI] [PubMed] [Google Scholar]
- 21.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Folkman J. J Natl Cancer Inst. 1996;88:1091–1092. doi: 10.1093/jnci/88.16.1091. [DOI] [PubMed] [Google Scholar]
- 23.Krieg A M, Wu T, Weeratna R, Efler S M, Homan L L, Yang L, Yi A-K, Short D, Davis H L. Proc Natl Acad Sci USA. 1998;95:12631–12636. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei Y-Q, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A. Cancer Res. 1994;54:4952–4956. [PubMed] [Google Scholar]
- 25.Keyt B A, Berleau L T, Nguyen H V, Chen H, Heinsohn H, Vandlen R, Ferrara N. J Biol Chem. 1996;271:7788–7795. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- 26.Stacker S A, Vitali A, Caesar C, Domagala T, Groenen L C, Nice E, Achen M G, Wilks A F. J Biol Chem. 1999;274:34884–34890. doi: 10.1074/jbc.274.49.34884. [DOI] [PubMed] [Google Scholar]
- 27.Pulendran B, Smith J L, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski C R. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedgwick J D, Holt P G A. J Immunol Methods. 1983;57:301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y-Q, Wang Q-R, Zhao X, Yang L, Tian L, Lu Y, Kang B, Lu C-J, Huang M-J, Lou Y-Y, et al. Nat Med. 2000;6:1160–1166. doi: 10.1038/80506. [DOI] [PubMed] [Google Scholar]
- 30.Horton H M, Anderson D, Hernandez P, Barnhart K M, Norman J A, Parker S E. Proc Natl Acad Sci USA. 1999;96:1553–1558. doi: 10.1073/pnas.96.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Y-Q, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A. Cancer Res. 1996;56:1104–1110. [PubMed] [Google Scholar]
- 32.Volpert O V, Lawler J, Bouck N P. Proc Natl Acad Sci USA. 1998;95:6343–6348. doi: 10.1073/pnas.95.11.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blezinger P, Wang J, Gondo M, Quezada A, Mehrens D, French M, Singhal A, Sullivan S, Rolland A, Ralston R, Wang M. Nat Biotechnol. 1999;17:343–348. doi: 10.1038/7895. [DOI] [PubMed] [Google Scholar]
- 34.Sauter B V, Martinet O, Zhang W J, Mandeli J, Woo S L C. Proc Natl Acad Sci USA. 2000;97:4802–4807. doi: 10.1073/pnas.090065597. . (First Published April 11, 2000; 10.1073/pnas.090065597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan E L, Meier P. J Am Stat Assoc. 1958;53:475–484. [Google Scholar]
- 36.Peto R, Peto J. J R Stat Soc. 1972;135:185–193. [Google Scholar]
- 37.Schwartz R H. Curr Opin Immunol. 1997;9:351–357. doi: 10.1016/s0952-7915(97)80081-7. [DOI] [PubMed] [Google Scholar]
- 38.Romagnani S. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 39.Weber L W, Bowne W B, Wolchok J D, Srinivasan R, Qin J, Moroi Y, Clynes R, Song P, Lewis J J, Houghton A N. J Clin Invest. 1998;102:1258–1264. doi: 10.1172/JCI4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowne W B, Srinivasan R, Wolchok J D, Hawkins W G, Blachere N E, Dyall R, Lewis J J, Houghton A N. J Exp Med. 1999;190:1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houghton A N, Gold J S, Blachere N E. Curr Opin Immunol. 2001;13:134–140. doi: 10.1016/s0952-7915(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 42.Pardoll D M. Proc Natl Acad Sci USA. 1999;96:5340–5342. doi: 10.1073/pnas.96.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Overwijk W W, Lee D S, Surman D R, Irvine K R, Touloukian C T, Chan C C, Carroll M W, Moss B, Rosenberg S A, Restifo N P. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosmann T R, Sad S. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 45.Ohashi P S. Curr Opin Immunol. 1996;8:808–814. doi: 10.1016/s0952-7915(96)80009-4. [DOI] [PubMed] [Google Scholar]
- 46.Kumar V, Stellrecht K, Sercarz E. J Exp Med. 1996;184:1609–1617. doi: 10.1084/jem.184.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Silva H D, Van Driel I R, La Gruta N, Toh B H, Gleeson P A. Immunology. 1998;93:405–408. doi: 10.1046/j.1365-2567.1998.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corr M, Lee D J, Carson D A, Tighe H. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinaldi M, Ria F, Parrella P, Signori E, Serra A, Ciafre S A, Vespignani I, Lazzari M, Farace M G, Saglio G, et al. Cancer Res. 2001;61:1555–1562. [PubMed] [Google Scholar]
- 50.Roth J A, Cristiano R J. J Natl Cancer Inst. 1997;89:21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- 51.Amagai M, Nishikawa T, Nousari H C, Anhalt G J, Hashimoto T. J Clin Invest. 1998;102:775–782. doi: 10.1172/JCI3647. [DOI] [PMC free article] [PubMed] [Google Scholar]