Abstract
Coordination of vascular smooth muscle cell tone in resistance arteries plays an essential role in the regulation of peripheral resistance and overall blood pressure. Recent observations in animals have provided evidence for a coupling between adrenoceptors and Panx1 (pannexin-1) channels in the regulation of sympathetic nervous control of peripheral vascular resistance and blood pressure; however, evidence for a functional coupling in humans is lacking. We determined Panx1 expression and effects of treatment with the pharmacological Panx1 channel inhibitor probenecid on the vasoconstrictor response to α1- and α2-adrenergic receptor stimulation in the human forearm and leg vasculature of young healthy male subjects (23±3 years). By use of immunolabeling and confocal microscopy, Panx1 channels were found to be expressed in vascular smooth muscle cells of arterioles in human leg skeletal muscle. Probenecid treatment increased (P<0.05) leg vascular conductance at baseline by ≈15% and attenuated (P<0.05) the leg vasoconstrictor response to arterial infusion of tyramine (α1- and α2-adrenergic receptor stimulation) by ≈15%, whereas the response to the α1-agonist phenylephrine was unchanged. Inhibition of α1-adrenoceptors prevented the probenecid-induced increase in baseline leg vascular conductance, but did not alter the effect of probenecid on the vascular response to tyramine. No differences with probenecid treatment were detected in the forearm. These observations provide the first line of evidence in humans for a functional role of Panx1 channels in setting resting tone via α1-adrenoceptors and in the constrictive effect of noradrenaline via α2-adrenoceptors, thereby contributing to the regulation of peripheral vascular resistance and blood pressure in humans.
Keywords: forearm, hypertension, norepinephrine, sympathetic nervous system, tyramine
Coordination of vascular smooth muscle cell (VSMC) tone in resistance arteries plays an essential role in the regulation of peripheral resistance and blood flow and thus overall blood pressure.1 VSMC tone is mainly determined by myogenic tone and the activity of the sympathetic nervous system2 where norepinephrine released from sympathetic nerve endings acts on α1- and α2-adrenergic receptors on VSMCs leading to vasoconstriction. Recent studies from several research groups conducted in vitro and in genetically modified animals have provided evidence that stimulation of α-adrenoceptors leads to opening of the Panx1 (pannexin-1) channel and efflux of ATP from VSMCs.3–7 ATP released through Panx1 induces vasoconstriction in an autocrine manner via purinergic receptors, thereby regulating the effect of norepinehrine.5,6 This vasoconstrictor effect of ATP acting directly on VSMCs is in contrast to the endothelium-dependent vasodilator effect of intravascular ATP mediated via formation of NO and prostanoids and activation of inwardly rectifying potassium channels.8,9 The divergent vasoactive property of ATP exemplifies the dual role of purinergic signaling in the cardiovascular system.10 Importantly, evidence for a functional role of the Panx1 channel in human vasculature is lacking, and its presence and role in skeletal muscle tissue resistance vessels in general have not been established.
Here, we examined the effect of treatment with the pharmacological Panx1 channel inhibitor probenecid on the vasoconstrictor response to α1- and α2-adrenergic receptor stimulation in the human forearm and leg vasculature. We hypothesized that Panx1 channels would be expressed in VSMCs of skeletal muscle arterioles and that probenecid would attenuate α1-adrenergic receptor–mediated vasoconstriction. The effect of probenecid on the adrenergic receptor–mediated vasoconstriction was expected to be lower in the arm because of the lower vascular hydrostatic pressure and lower adrenergic responsiveness in arm than leg.11,12
Methods
A total of 15 healthy recreationally active males, aged 23±3 years, participated in the study (Table S1 in the online-only Data Supplement). The subjects underwent screening by means of a medical examination, 12-lead ECG, and blood sampling from an antecubital vein. On the day of screening, subjects also performed an incremental bicycle ergometer exercise test in which pulmonary (L/min) was determined (Oxycon Pro, Intramedic, Denmark; VO2max Table S1). Exclusion criteria were history or symptoms of cardiovascular disease, renal dysfunction, insulin resistance, diabetes mellitus, or hypercholesterolemia. All subjects were nonsmokers and were not taking prescription medicine. The study was approved by the Ethics Committee of the Capital Region of Copenhagen (H-15014050) and conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all subjects before enrollment into the study.
Experimental Protocol 1: Role of Panx1 in Total α-Adrenergic and α1-Adrenergic Mediated Vasoconstriction in the Upper and Lower Extremities
The study was conducted as a double-blinded, placebo-controlled, balanced crossover study. Subjects (n=10) reported to the laboratory on 2 different days separated by a washout period of 14 to 21 days (Figure S1). Subjects refrained from caffeine, alcohol, and exercise for 24 hours before each experimental day, and the subjects were instructed to eat the same breakfast. An oral dose of either probenecid (3000 mg; Meda, Denmark) or calcium as placebo (800 mg; Orkla Health, Denmark) was given at ≈08:00 AM, and the first arterial infusion was started 4 hours later as probenecid has been shown to reach maximal plasma concentration after this period of time.13 On the basis of the literature, 3000 mg probenecid should result in plasma concentrations of ≈700 μmol/L,13 which has been shown to effectively inhibit 5,6,14 Intake of the present dose of Panx1 channels (IC50=150 μmol/L).calcium tablets did not change the vascular response to various stimuli (tyramine infusion, passive and active limb movement) as evidenced from a series of pilot experiments. Under aseptic conditions and local anesthesia, catheters (20 gauge; Arrow, Reading, PA) were placed in the femoral artery and vein of the experimental leg 2 cm below the inguinal ligament (lidocaine, 20 mg/mL; Astra Zeneca, Denmark). A catheter was also placed in the brachial artery (20 gauge, arterial cannula; BD) and cephalic vein (20 gauge, venflon; BD) of the experimental arm. A 3-port connector was placed in series so that infusion and blood pressure measurements could be performed simultaneously. Subjects were positioned in the supine position with the experimental arm extended laterally at heart level where they received continuous brachial artery infusion of tyramine (Sigma-Aldrich, St. Louis, MO) for 3 minutes at each dose (0.3 and 1.0 μmol min−1 L forearm volume−1), and measurements (blood flow, blood pressure and blood samples) were performed during the last minute at each dose. After 30 minutes of rest, subjects received continuous femoral arterial infusion of tyramine for 3 minutes at each dose (0.3 and 1.0 μmol min−1 L leg volume−1) in the supine position. Tyramine evokes norepinephrine release from neuronal vesicles and consequent release of norepinephrine out of nerve terminals, thus resulting in stimulation of α1- and α2-adrenergic receptors, without having any intrinsic vasoconstrictor properties.15 After 30 minutes of rest, subjects then received continuous brachial artery infusion of phenylephrine (selective α1-adrenergic receptor agonist; SAD, Denmark) for 3 minutes at each dose (0.75, 1.5, and 3.0 μg min−1 L forearm volume−1) followed by additional 30 minutes of rest and femoral arterial infusion of phenylephrine (0.75, 1.5, and 3.0 μg min−1 L leg volume−1). The order of the infusions was kept similar during both experimental days. This approach was used to minimize any potential influence of diurnal variations in vascular function as the protocol ensured that infusions were performed at the same time of day during each visit. To avoid residual systemic effects of the prior compound infusion, blood pressure and blood flow were measured frequently to ensure that these variables had returned to baseline level before initiation of the next infusion.
A biopsy was obtained from the middle portion of m. vastus lateralis of the experimental leg using the percutaneous needle biopsy technique with suction 15 minutes before initiating femoral arterial tyramine infusion. Muscle biopsies were quickly embedded in mounting medium (TissueTek OCT, Sakura), frozen in precooled isopentane and stored at −80° until further analysis.
Experimental Protocol 2: Role of Panx1 in α2-adrenergic Mediated Vasoconstriction in the Lower Extremity
Similar to protocol 1, a double-blinded, balanced crossover placebo approach was used. Subjects (n=5) reported to the laboratory on 2 different days separated by a washout period of ≈14 days (Figure S2). Subjects refrained from caffeine, alcohol, and exercise for 24 hours before each experimental day, and the subjects were instructed to eat the same breakfast. An oral dose of either probenecid (3000 mg; Meda) or placebo (800 mg calcium; Orkla Health) was given at ≈08:00 AM. Under aseptic conditions, catheters (20 guage; Arrow, Reading) were placed in the femoral artery and vein of the experimental leg 2 cm below the inguinal ligament under local anesthesia (lidocaine, 20 mg/mL; Astra Zeneca). An oral dose of terazosin (selective α1-adrenergic receptor antagonist, 0.05 mg kg−1; Amdipharm Limited, Ireland) was then given 2 hours after intake of placebo/probenecid. This dosage of terazosin provides ≈80% α1 blockade.16 After additional 2 hours, subjects were placed in the supine position where they received continuous femoral arterial infusion of tyramine (Sigma-Aldrich) for 3 minutes at each dose (0. 15, 0.3, and 1.0 μmol min−1 L leg volume−1). After 30 minutes of rest, continuous femoral arterial infusion of phenylephrine (0.75, 1.5, and 3.0 μg min−1 L leg volume−1; SAD) was performed.
Measurements and Calculations
Arterial blood flow (femoral arterial [leg] blood flow and brachial arterial blood flow) was measured with ultrasound Doppler (Vivid E9; GE Healthcare) equipped with a linear probe operating at an imaging frequency of 4.0/8.0 MHz and Doppler frequency of 4.3 MHz. The site of measurements in the common femoral artery was distal to the inguinal ligament but above the bifurcation into the superficial and deep femoral branches to avoid turbulence from the bifurcation. For measurements in the brachial artery, the probe was placed approximately half way up the upper arm. All recordings were obtained at the lowest possible insonation angle and always <60°. Sample volume maximized according to the width of the vessel and kept clear of the vessel walls. Doppler traces and B-mode images were recorded continuously, and Doppler traces were averaged over ≈30 s. Arterial diameter measures (in triplicate) were assessed during systole from arterial B-mode images with the vessel parallel to the transducer after each Doppler recording. Intra-arterial and intravenous pressure and heart rate were monitored with transducers (Pressure Monitoring Set; Edwards Lifesciences, Irvine, CA) positioned at the level of the tip of the catheters. Leg and forearm volumes were estimated by anthropometry and vascular conductance (leg vascular conductance [LVC] and forearm vascular conductance) as arterial blood flow/(mean arterial [femoral/brachial] blood pressure–mean venous [femoral/brachial] blood pressure). Norepinephrine was analyzed by enzyme immunoassay (LDN, Nordhorn, Germany), hemoglobin by color absorption photometry (Sysmex XN, Sysmex, Denmark), CRP by turbidimetry (Cobas 8000, c702 module; F. Hoffmann-La Roche Ltd, Rotkreuz, Switzerland), glycated hemoglobin by absorption photometry (Tosoh G7 and G8, Tosoh, Japan), total cholesterol, low-density lipoprotein, and high-density lipoprotein were analyzed by enzymatic absorption photometry (Cobas 8000, c702 module; F. Hoffmann-La Roche Ltd, Rotkreuz, Switzerland).
Fluorescence Immunolabeling
Sections (10 μm) from m. vastus lateralis muscle biopsies were fixed with ice-cold acetone for 10 minutes, air-dried for 10 minutes, and blocked for 30 minutes with 5% fish skin gel, 0.5% BSA, and 0.25% Triton X-100 in PBS. Colocalization of Panx1 with VSMCs was visualized using an affinity-purified custom-made rabbit anti-human Panx1 polyclonal antibody (1:500, PANX1 CT-412, 0.5 μg/mL; Genemed Synthesis, San Francisco, CA) and mouse anti–α-smooth muscle actin (1:250; Sigma-Aldrich) antibody diluted in blocking solution at 4°C overnight. Secondary antibodies were Alexa Fluor 594 (1:400, goat anti-rabbit IgG, A-11012; Thermo Fisher, Waltham, MA) and Alexa Fluor 568 (1:400, goat anti-mouse, A-11019; Thermo Fisher) incubated at room temperature for 1 hour. Finally, sections were mounted with Prolong Gold Antifade with DAPI (4′,6-diamidino-2-phenylindole; P36931; Thermo Fisher) and stored at 4°C until image acquisition. Negative controls were performed by staining without primary antibodies. Confocal images were collected on a Zeiss LSM 880 Axio Observer microscope through a DIC M27 Plan-Appchromat 63/1.40 Oil objective (Carl Zeiss, Oberkochen, Germany).
Statistical Analysis
Differences in baseline hemodynamics were detected using paired Student t tests. A linear mixed-model approach was used to investigate differences within and between treatments. Fixed factors were intervention (placebo, probenecid), infusion dose, and extremity (leg, forearm). Subjects were specified as a repeated factor and identifier of random variation. Homogeneity of covariance and normal distribution were confirmed through residual and Q–Q plots. Pairwise differences were identified using Tukey honestly significant difference post hoc procedure. The number of subjects was selected on the basis of detecting a 15% difference in tyramine-induced vasoconstriction with intake of probenecid. Statistical analyses were performed with R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) through the interface R studio (R Foundation for Statistical Computing). Data are reported as means±SE unless otherwise stated. P <0.05 was considered statistically significant.
Results
Panx1 Is Expressed in VSMCs of Skeletal Muscle Arterioles
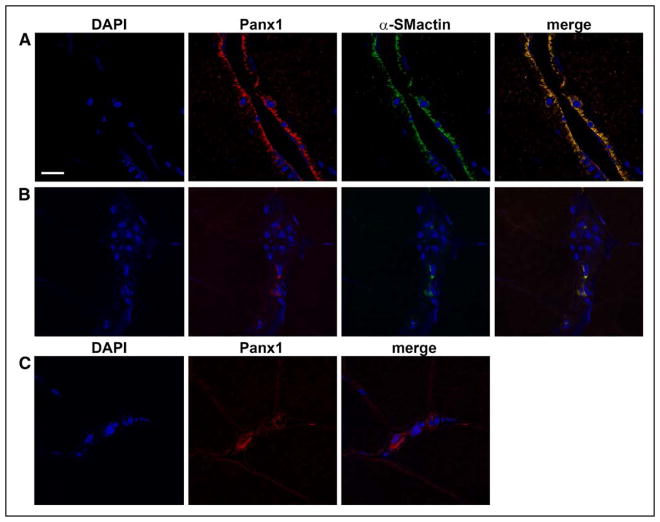
By use of immunohistochemistry and confocal microscopy, colocalization of Panx1 channels with VSMCs was confirmed in arterioles in muscle samples obtained from m. vastus lateralis of human subjects (Figure 1).
Figure 1.
Panx1 (pannexin-1) is expressed in vascular smooth muscle cells in human skeletal muscle. A, Representative immunofluorescence micrographs showing colocalization of Panx1 (red) and α-smooth muscle cell actin (α-SMactin; green) in sections of arterioles isolated from human vastus lateralis muscle. Nuclei are stained with DAPI (4′,6-diamidino-2-phenylindole; blue). B, Negative controls were performed by staining with secondary antibody only. C, Positive controls were performed by staining with primary and secondary antibody for Panx1 only.
Role of Panx1 in α1-Adrenergic Mediated Vasoconstriction in the Upper and Lower Extremities
Baseline
At baseline, leg blood flow and LVC were ≈15% higher (P<0.05) with probenecid compared with placebo (Table 1). When normalized to tissue volume, leg blood flow and LVC were ≈55% lower (P<0.05) compared with brachial arterial blood flow and forearm vascular conductance with both placebo and probenecid. Mean blood pressure was higher (P<0.05) in the brachial artery compared with femoral artery. Although probenecid did not affect femoral arterial blood pressure or brachial arterial blood pressure, it significantly increased heart rate (P<0.05).
Table 1.
Baseline Hemodynamics—Protocol 1
| Variable | Leg | Forearm | ||
|---|---|---|---|---|
| PLA | PROB | PLA | PROB | |
| Blood flow, mL min−1 | 377±48 | 433±39* | 103±13† | 126±15† |
| Blood flow, mL min−1 L−1 | 36±4 | 42±3* | 81±12† | 96±10† |
| Mean arterial blood pressure, mm Hg | 84±2 | 82±1 | 82±2† | 80±0† |
| Vascular conductance, mL min−1 mm Hg−1 | 4.80±0.67 | 5.54±0.53* | 1.36±0.17† | 1.70±0.24† |
| Vascular conductance, mL min−1 L−1 mm Hg−1 | 0.46±0.05 | 0.53±0.04* | 1.07±0.17† | 1.28±0.14† |
| Heart rate, bpm | 58±2 | 61±2* | 56±2 | 60±2* |
| Venous norepinephrine, nmol L−1 | 2.2±0.4 | 1.7±0.3 | 1.8±0.3 | 1.6±0.4 |
Values are means±SE. n=10. PLA indicates placebo; and PROB, probenecid.
Significantly different from PLA (P<0.05).
Significantly different from leg within same condition (P<0.05).
Leg and Forearm Vasoconstrictor Responses to Tyramine
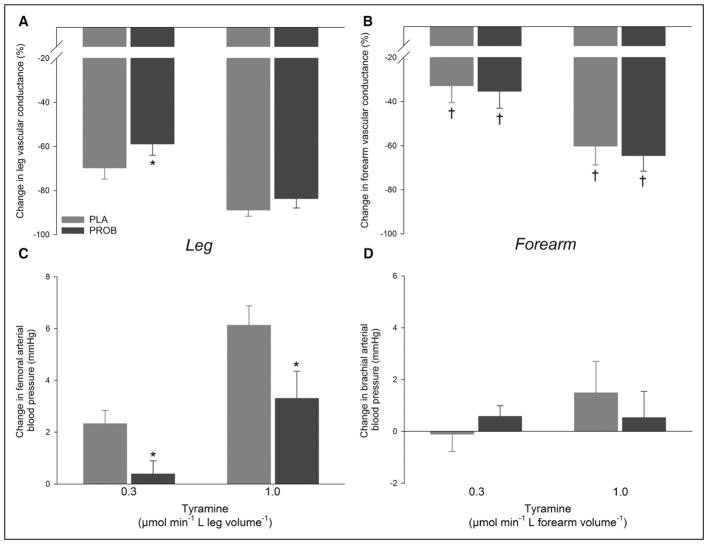
In the leg, the vasoconstrictor response to the lowest dose of tyramine (0.3 μmol min−1 L leg volume−1) was ≈15% lower (P<0.05) with probenecid compared with placebo, whereas probenecid failed to attenuate vasoconstriction at the higher dose of tyramine (Figure 2; Table 2). The tyramine-induced increase in femoral arterial blood pressure was blunted (P<0.05) with probenecid compared with placebo during both infusion rates. In the forearm, no differences were detected between placebo and probenecid in the vasoconstrictor response to tyramine.
Figure 2.
Leg and forearm vasoconstrictor responses to tyramine. Percentage changes in leg and forearm vascular conductance (A, B) and blood pressure (C, D) with arterial tyramine infusion without placebo (PLA) and with probenecid (PROB). n=10. *Significantly different from PLA (P<0.05), †significantly different from leg within same condition (P<0.05).
Table 2.
Changes in Hemodynamics With Arterial Infusion of Tyramine and Phenylephrine—Protocol 1
| PLA | PROB | PLA | PROB | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leg | ||||||||||
| Variable | TYR 0.3 | TYR 1.0 | TYR 0.3 | TYR 1.0 | PE 0.75 | PE 1.5 | PE 3.0 | PE 0.75 | PE 1.5 | PE 3.0 |
| Blood flow, % | −69.1±5.2 | −88.0±3.1 | −58.7±5.1* | −83.3±4.2 | −52.5±7.2 | −54.3±5.1 | −59.7±3.9 | −54.3±3.9 | −58.2±4.1 | −61.6±3.8 |
| Mean arterial blood pressure, mm Hg | 2±1 | 6±1 | 0±1* | 3±1* | 1±1 | 2±1 | 5±1 | 1±1 | 2±1 | 3±0 |
| Vascular conductance, % | −69.7±5.1 | −88.8±2.9 | −58.8±5.2* | −83.6±4.3 | −53.1±7.2 | −55.3±4.9 | −61.4±4.0 | −54.6±4.0 | −59.1±4.0 | −62.7±3.7 |
| Heart rate, bpm | 0±1 | 1±2 | −1±1 | 1±1 | −1±1 | 0±1 | 0±1 | 0±1 | 0±1 | −1±1 |
| Venous norepinephrine, nmol L−1 | 0.8±0.8 | 6.4±1.6 | 0.6±0.5 | 9.8±1.8 | … | … | … | … | … | … |
| Forearm | ||||||||||
| Variable | TYR 0.3 | TYR 1.0 | TYR 0.3 | TYR 1.0 | PE 0.75 | PE 1.5 | PE 3.0 | PE 0.75 | PE 1.5 | PE 3.0 |
| Blood flow, % | −32.8±7.4† | −60.0±8.0† | −34.4±8.0† | −64.2±6.9† | −42.5±4.9 | −55.9±5.9 | −71.5±4.7 | −36.3±6.2 | −50.3±6.6 | −68.2±5.1 |
| Mean arterial blood pressure, mm Hg | 0±1 | 1±1† | 1±0 | 1±1 | 1±0 | 1±1 | 1±1† | 0±1 | 1±1 | 1±1 |
| Vascular conductance, % | −32.8±7.7† | −60.2±8.5† | −35.3±7.8† | −64.5±7.1† | −43.8±4.9 | −56.7±5.9 | −72.0±4.7 | −37.1±6.4 | −51.3±6.5 | −68.9±5.2 |
| Heart rate, bpm | 0±1 | 2±1 | 1±1 | 2±2 | 0±1 | 0±1 | −1±1 | 0±1 | 0±1 | −1±1 |
| Venous norepinephrine, nmol L−1 | 0.4±0.3 | 6.5±3.0 | 1.4±0.4 | 4.5±1.1 | … | … | … | … | … | … |
Values are means±SE. n=10. PLA indicates placebo; and PROB, probenecid.
Significantly different from PLA (P<0.05).
Significantly different from leg within same condition (P<0.05).
The vasoconstrictor response to both infusion rates of tyramine was higher (P<0.05) in the leg compared with forearm with placebo and probenecid (Table 2).
Leg and Forearm Vasoconstrictor Responses to Phenylephrine
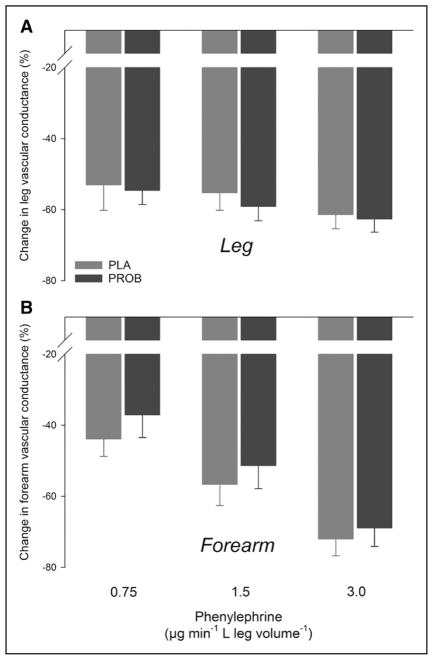
In the leg and forearm, no differences were detected between placebo and probenecid in the vasoconstrictor response to phenylephrine (Figure 3; Table 2). Similarly, no differences in the vasoconstrictor response to phenylephrine were detected between the leg and forearm.
Figure 3.
Leg and forearm vasoconstrictor responses to phenylephrine. Percentage changes in leg (A) and forearm (B) vascular conductance with arterial phenylephrine infusion without placebo (PLA) and with probenecid (PROB). n=10.
Role of Panx1 in α2-Adrenergic Mediated Vasoconstriction in the Lower Extremity
Baseline
leg blood flow, femoral arterial blood pressure, LVC, and heart rate were similar with placebo and probenecid (Table 3).
Table 3.
Baseline Hemodynamics—Protocol 2
| Variable | Leg | |
|---|---|---|
| TER+PLA | TER+PROB | |
| Blood flow, mL min−1 | 558±111 | 556±132 |
| Blood flow, mL min−1 L−1 | 46±7 | 46±9 |
| Mean arterial blood pressure, mm Hg | 75±1 | 75±1 |
| Vascular conductance, mL min−1 mm Hg−1 | 7.6±1.5 | 7.6±1.8 |
| Vascular conductance, mL min−1 L−1 mm Hg−1 | 0.63±0.09 | 0.63±0.12 |
| Heart rate, bpm | 56±1 | 55±1 |
| Venous norepinephrine, nmol L−1 | 3.8±0.9 | 4.8±1.2 |
TER+PLA indicates terazosin+placebo; and TER+PROB, terazosin+probenecid.
Leg Vasoconstrictor Responses to Tyramine and Phenylephrine
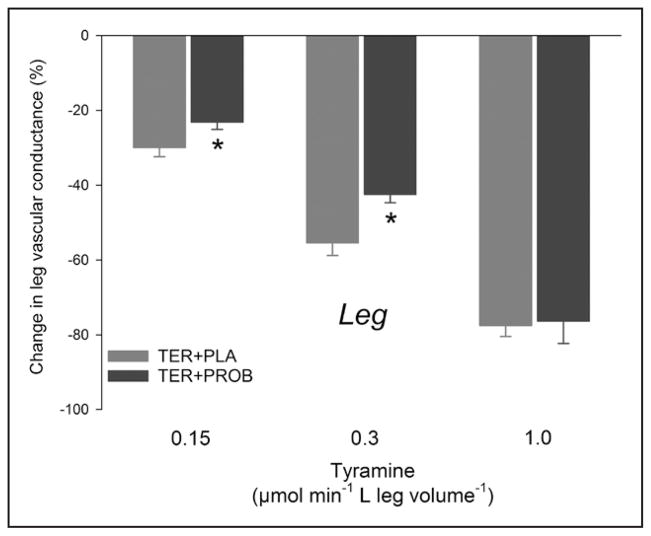
The vasoconstrictor response to the 2 lowest doses of tyramine (0.15 and 0.3 μmol min−1 L leg volume−1) was ≈24% lower (P<0.05) with terazosin+probenecid compared with terazosin+placebo (Figure 4; Table 4). During α1-adrenoceptor blockade, phenylephrine increased rather than decreased LVC, and although this effect was numerically lower after probenecid treatment, no differences were detected between terazosin+placebo and terazosin+probenecid in the vascular response to phenylephrine.
Figure 4.
Leg α2-adrenergic mediated vasoconstrictor responses to tyramine. Percentage changes in leg vascular conductance with arterial tyramine infusion with terazosin+placebo (TER+PLA) and with terazosin+probenecid (TER+PROB). n=5. *Significantly different from PLA (P<0.05).
Table 4.
Changes in Hemodynamics With Arterial Infusion of Tyramine and Phenylephrine—Protocol 2
| Leg | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TER+PLA | TER+PROB | TER+PLA | TER+PROB | |||||||||
| Variable | TYR 0.15 | TYR 0.3 | TYR 1.0 | TYR 0.15 | TYR 0.3 | TYR 1.0 | PE 0.75 | PE 1.5 | PE 3.0 | PE 0.75 | PE 1.5 | PE 3.0 |
| Blood flow, % | −29.1±2.3 | −55.2±3.1 | −76.1±2.9 | −22.3±2.7* | −41.5±3.0* | −74.3±7.1 | 7.9±6.4 | 19.4±9.1 | 21.4±9.4 | 6.1±5.8 | 28.6±9.4 | 46.5±18.0 |
| Mean arterial blood pressure, mm Hg | 1±0 | 0±1 | 5±1 | 1±1 | 1±1 | 5±1 | 0±1 | −1±1 | 2±1 | 0±1 | 0±1 | 2±1 |
| Vascular conductance, % | −30.0±2.4 | −55.5±3.3 | −77.5±2.9 | −23.2±1.9* | −42.5±2.2* | −76.4±5.9 | 8.1±7.2 | 21.0±10.7 | 18.5±10.4 | 6.4±6.2 | 27.8±11.3 | 42.7±16.4 |
| Heart rate, bpm | 1±0 | 1±0 | 5±1 | 1±1 | 0±1 | 2±2 | 1±1 | 0±1 | 3±1 | 0±1 | 1±1 | 2±1 |
| Venous norepinephrine, nmol L−1 | −1.4±1.6 | 0.8±2.0 | 5.0±2.5 | 0.4±1.9 | 0.8±1.5 | 8.0±2.8 | … | … | … | … | … | … |
Values are means±SE. n=5. TER+PLA indicates terazosin+placebo; and TER+PROB, terazosin+probenecid.
Significantly different from PLA (P<0.05).
Discussion
The primary novel findings were (1) Panx1 channels were expressed in VSMCs of arterioles in human leg skeletal muscle, (2) probenecid treatment increased baseline LVC and attenuated the leg vasoconstrictor response to arterial infusion of tyramine, whereas the response to the α1-agonist phenylephrine was unchanged, and (3) inhibition of α1-adrenoceptors prevented the probenecid-induced increase in baseline LVC but did not alter the effect of probenecid on the vascular response to tyramine infusion. Taken collectively, these observations provide the first line of evidence in humans for a functional role of Panx1 channels in setting resting tone via α1-adrenoceptors and in the constrictive effect of noradrenaline via α2-adrenoceptors, thereby contributing to the regulation of peripheral vascular resistance and blood pressure in humans.
First Evidence for a Functional Coupling Between Adrenoceptors and Panx1 Channels in Humans
Tyramine induces vascular constriction by inducing a release of norepinephrine and stimulation of α1- and α2-adrenoceptors on VSMCs. Hence, the present finding that the Panx1 channel inhibitor probenecid attenuated the leg vasoconstrictor response to tyramine provides the first evidence of a coupling between these receptors and Panx1 in the sympathetic control of vascular tone in humans. Although this coupling is in general agreement with observations made in vitro and in animal models, it has been clearly demonstrated that this complementary vasoconstrictor mechanism is coupled to α1-adrenoceptor activation in these models.5–7 In the present study, we did not find evidence for this coupling as probenecid did not affect the vasoconstrictor response to the α1-agonist phenylephrine. Furthermore, inhibition of α1-adrenoceptors did not alter the attenuating effect of probenecid on the vasoconstrictor response to tyramine infusion. This discrepancy may be a consequence of differences in the investigated vessels as previous studies have used large thoracodorsal arteries5,6 and mesenteric arterioles,7 whereas the present findings are likely to primarily reflect arterioles of skeletal muscle. In addition, species differences, degree of baseline stimulation of Panx1 channels, degree of α1-adrenoceptor stimulation caused by phenylephrine, or the targeting of different receptor populations by mode of agonist administration may explain the discrepancy with previous findings.5–7 Along these lines, as probenecid increased LVC at baseline and because this effect was absent when blocking α1-adrenoceptors, it could be argued that the Panx1-mediated α1-response was already fully stimulated. If so, further stimulation of α1-receptors not coupled to Panx1 would be insensitive to probenecid treatment. On the other hand, outcome may also depend on the mode of agonist delivery as the adrenoceptor subtype composition at the neuroeffector junction is likely different from that outside.17,18 Because tyramine releases norepinephrine (as well as ATP and neuropeptide Y) at the neuroeffector junction, it may well target a different population of receptors compared with phenylephrine delivered through the blood stream. As a consequence, exogenous phenylephrine may predominately activate receptors that do not couple to Panx1 and therefore the response would be insensitive to probenecid.
Smaller resistance vessels play a major role in the regulation of vascular resistance and the distribution of blood flow within skeletal muscle.19 Data from various animal models suggest that α1-adrenoceptors are primarily located upstream in larger arterioles and conduit arteries, whereas α2-adrenoceptors are primarily located in the smaller resistance vessels.20,21 In the current study, we were able to show that Panx1 channels are present in smaller arterioles in human skeletal muscle supporting the proposition of a coupling between Panx1 channels and α2-adrenoceptors in leg skeletal muscle vasculature for the regulation of peripheral vascular resistance in this tissue. Such an interaction between Panx1 channels and α-adrenoceptors would allow for a point of amplification and integration through which other signaling pathways could effectively modify the contractile response. With regard to the latter, skeletal muscle can increase blood flow during contractile activity substantially despite large increases in sympathetic outflow,22 a phenomenon known as functional sympatholysis.23 Hence, Panx1 channels may provide 1 point of intercept for vasodilator substances in contracting skeletal muscle.24 As the mechanisms underlying the sympatholytic properties of intravascular ATP remain undisclosed,25 it is tempting to speculate that ATP acting on luminal endothelial purinergic receptors may ultimately lead to reduced Panx1 activity and α-adrenergic mediated vasoconstriction. One such mode of action could be hyperpolarization of endothelial and adjacent smooth muscle cells, thus leading to altered smooth muscle Panx1 conformation and activity.26
At rest, Panx1 channel inhibition increased LVC and heart rate, effects that were not detected during α1-adrenoceptor antagonism. These findings are in accordance with experiments performed in vitro and in gene modified animals demonstrating a coupling between Panx1 channels and α1-adrenoceptors4–6 and suggest a functional role of Panx1 channels in setting resting tone via α1-adrenoceptors in humans. The increase in heart rate probably represents a baroreflex-mediated effect to maintain MAP at a reduced total peripheral resistance, but direct cardiac effects cannot be excluded.27
Probenecid only reduced the vasoconstriction to the low and intermediate doses of tyramine, whereas the response to the highest dose was unaffected. This finding may relate either to differential effects of α-adrenergic receptors inside and outside the neuroeffector junctions or to nonlinearity in the mechanism underlying signal transduction. As noted above, the population of α1-adrenoceptor subtypes is different in the neuroeffector junction, which means that lower levels of tyramine may selectively activate the receptors in this region. In contrast, neurotransmitters could spill over to extrajunctional regions at higher concentrations and signal via receptors that act independent of Panx1. In addition, and not mutually exclusive, the underlying signaling may be nonlinear and thus allow high levels of stimulation to overcome partial inhibition of any step in the chain. For example, if α-adrenoceptor stimulation releases more ATP than needed for maximal vasoconstriction, then a reduction in the maximal release capacity (by Panx1 inhibition) right shifts the tyramine-to-vasoconstriction dose–response curve rather than reducing the maximum response.
Because of its characterization as a selective α1-agonist, phenylephrine has commonly been used to study the pharmacology and physiology of α1-mediated vasoconstrictor effects in both animal models and humans. Interestingly, femoral arterial infusion of phenylephrine was in the present study found to induce a vasodilator response during concomitant inhibition of α1-adrenoceptors. This vasodilator effect of phenylephrine may have been mediated via β2-receptors28,29 as phenylephrine-induced vasodilation during nonselective α-adrenoceptor inhibition was shown to be abrogated by the nonselective β-blocker propranolol in the human forearm.30 This potential β-mediated vasodilation induced by phenylephrine deserves further investigation.
Differences in Vascular Responsiveness in the Upper and Lower Extremities
Unlike quadrupeds, the legs of humans are subjected to marked changes in hydrostatic pressure relative to the arms.11 Thus, it is not surprising that the legs display greater arterial wall thickness,31 enhanced adrenergic responsiveness,12 and a reduced vascular responses to vasodilator substances.32 In accordance, vascular conductance and blood flow per volume of tissue were found to be higher in the forearm during baseline conditions, and the vasoconstrictor response to tyramine was lower in the forearm compared with the leg. In contrast, no difference between the extremities was detected in the vascular response to phenylephrine, indicating a similar responsiveness to α1-adrenoceptor but a less pronounced role of α2-adrenoceptors in the arm than the leg. This evidence for a more pronounced overall role of α2-adrenoceptors in the leg compared with the arm is in line with the previous findings of overall greater adrenergic responsiveness in the lower extremities.12 The greater importance of vasoconstriction for the regulation of vascular resistance, combined with the greater role of the α2-adrenoreceptors in the lower than the upper extremities, could explain the apparent coupling between this receptor subtype and Panx1 channels specifically in the lower limbs of humans.
Experimental Considerations
In the present study, we chose to inhibit Panx1 channels with probenecid as this compound has been successfully used in vitro and in vivo in animal models to target Panx1-mediated signaling processes5,6,14 and is approved for use in humans (gout remedy).33 Probenecid also lists the mildest and most acceptable side effects compared with mefloquine which is the other approved pharmaceutical known to inhibit Panx1 channels. In addition to its well-known inhibitory effect on Panx1 channels, probenecid has been shown to have inhibitory effects on other proteins such as anion transporters.34 Specifically, probenecid also inhibits the MRP4 and MRP5 (multidrug resistance proteins 4 and 5),35 which have been implicated in the export of cAMP from arterial smooth muscle cells.36 If cAMP secretion plays a role in the response to tyramine, the effect of blocking it is difficult to predict. On one hand, reduced cAMP secretion could increase intracellular cAMP37 and cause vasodilation, but on the other hand, reduced cAMP secretion would also reduce the formation of the vasodilator adenosine (from extracellular cAMP breakdown).38 As our study does not address the possible role of cAMP, we are unable to evaluate its possible involvement at present. More specific inhibitors of VSMC Panx1 should be developed for human use to fully elucidate the physiological function of this channel.
Perspectives
In the resistance vessels, sympathetic nervous activity plays an essential role in the control of blood pressure by regulating peripheral vascular resistance.1 In a recent study, knockout mice deficient in VSMC Panx1 channels were shown to display hypotension, and in wild-type mice, intraperitoneal infusion of a Panx1 channel inhibitor induced an acute reduction in blood pressure.5 In the present study, we provide evidence for a similar importance of Panx1 channels for regulation of vascular tone and thereby blood pressure in humans. It should be noted, that the reduction in blood pressure at rest with probenecid treatment did not reach statistical significance; however, given the initially low mean arterial blood pressure in the young healthy subjects, this lack of effect may reflect a baroreceptor-mediated compensation to preserve blood pressure and sufficient perfusion of vital organs. Such preservation of blood pressure is supported by the higher heart rate with probenecid treatment as this chronotropic response is indicative of a higher cardiac output, which would serve to increase blood pressure. Collectively, our results suggest that Panx1 channels may be a target for therapeutic interventions aimed at lowering blood pressure in disease states characterized by increased blood pressure or in conditions where muscle function is compromised by lack of functional sympatholysis.39 Targeting Panx1 channels with interfering peptides that recognize a part of intracellular amino acid sequence may be 1 approach.5 Future research should also aim at investigating the effect of Panx1 channel inhibition in individuals with essential hypertension.
Supplementary Material
Novelty and Significance.
What Is New?
This is the first study to explore the potential functional coupling between adrenoceptors and Panx1 (pannexin-1) channels in the regulation of sympathetic nervous control of peripheral vascular resistance and blood pressure in humans.
What Is Relevant?
Panx1 channels may be a target for therapeutic interventions aimed at lowering blood pressure in disease states characterized by increased blood pressure.
Summary
The present observations provide the first line of evidence in humans for a functional role of Panx1 channels in setting resting tone via α1-adrenoceptors and in the constrictive effect of norepinephrine via an interaction with α2-adrenoceptors, thereby contributing to the regulation of peripheral vascular resistance and blood pressure in humans.
Acknowledgments
Sources of Funding
The study was supported by The Danish Ministry of Culture: Sports Research and The Danish Council for Independent Research, Medical Sciences (No. 6110-00632).
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.117.10251/-/DC1.
Disclosures
None.
References
- 1.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- 2.Guimarães S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- 3.Chiu YH, Jin X, Medina CB, Leonhardt SA, Kiessling V, Bennett BC, Shu S, Tamm LK, Yeager M, Ravichandran KS, Bayliss DA. A quantized mechanism for activation of pannexin channels. Nat Commun. 2017;8:14324. doi: 10.1038/ncomms14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kauffenstein G, Tamareille S, Prunier F, et al. Central role of P2Y6 UDP receptor in arteriolar myogenic tone. Arterioscler Thromb Vasc Biol. 2016;36:1598–1606. doi: 10.1161/ATVBAHA.116.307739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billaud M, Chiu YH, Lohman AW, et al. A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells. Sci Signal. 2015;8:ra17. doi: 10.1126/scisignal.2005824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE. Pannexin1 regulates α1-adrenergic receptor- mediated vasoconstriction. Circ Res. 2011;109:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus JA, Wright CE. Novel α1-adrenoceptor antagonism by the fluroquinolone antibiotic trovafloxacin. Eur J Pharmacol. 2016;791:179–184. doi: 10.1016/j.ejphar.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 8.Mortensen SP, González-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1140–R1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- 9.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. ATP-mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans. J Physiol. 2012;590:5349–5359. doi: 10.1113/jphysiol.2012.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic signaling in the cardiovascular system. Circ Res. 2017;120:207–228. doi: 10.1161/CIRCRESAHA.116.309726. [DOI] [PubMed] [Google Scholar]
- 11.Rowell LB. Human Cardiovascular Control. New York, NY: Oxford University Press; 1993. [Google Scholar]
- 12.Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol (1985) 2002;92:2105–2113. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- 13.Selen A, Amidon GL, Welling PG. Pharmacokinetics of probenecid following oral doses to human volunteers. J Pharm Sci. 1982;71:1238–1242. doi: 10.1002/jps.2600711114. [DOI] [PubMed] [Google Scholar]
- 14.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandão F, Rodrigues-Pereira E, Guilherme Monteiro J, Osswald W. Characteristics of tyramine induced release of noradrenaline: mode of action of tyramine and metabolic fate of the transmitter. Naunyn Schmiedebergs Arch Pharmacol. 1980;311:9–15. doi: 10.1007/BF00500297. [DOI] [PubMed] [Google Scholar]
- 16.Jones H, Lewis NC, Green DJ, Ainslie PN, Lucas SJ, Tzeng YC, Grant EJ, Atkinson G. α1-Adrenoreceptor activity does not explain lower morning endothelial-dependent, flow-mediated dilation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1437–R1442. doi: 10.1152/ajpregu.00042.2011. [DOI] [PubMed] [Google Scholar]
- 17.Townsend SA, Jung AS, Hoe YS, Lefkowitz RY, Khan SA, Lemmon CA, Harrison RW, Lee K, Barouch LA, Cotecchia S, Shoukas AA, Nyhan D, Hare JM, Berkowitz DE. Critical role for the alpha-1B adrenergic receptor at the sympathetic neuroeffector junction. Hypertension. 2004;44:776–782. doi: 10.1161/01.HYP.0000145405.01113.0e. [DOI] [PubMed] [Google Scholar]
- 18.Yang XP, Chiba S. Existence of different alpha(1)-adrenoceptor subtypes in junctional and extrajunctional neurovascular regions in canine splenic arteries. Br J Pharmacol. 2001;132:1852–1858. doi: 10.1038/sj.bjp.0704020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol. 2004;561(pt 2):535–545. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGillivray-Anderson KM, Faber JE. Effect of acidosis on contraction of microvascular smooth muscle by alpha 1- and alpha 2-adrenoceptors. Implications for neural and metabolic regulation. Circ Res. 1990;66:1643–1657. doi: 10.1161/01.res.66.6.1643. [DOI] [PubMed] [Google Scholar]
- 21.Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- 22.Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. Compr Physiol. 2015;6:1–32. doi: 10.1002/cphy.c140080. [DOI] [PubMed] [Google Scholar]
- 23.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen MS. Sympathetic vasoconstriction takes an unexpected pannexin detour. Sci Signal. 2015;8:fs4. doi: 10.1126/scisignal.aaa7312. [DOI] [PubMed] [Google Scholar]
- 25.Hearon CM, Jr, Richards JC, Racine ML, Luckasen GJ, Larson DG, Joyner MJ, Dinenno FA. Sympatholytic effect of intravascular ATP is independent of nitric oxide, prostaglandins, Na(+)/K(+) -ATPase and KIR channels in humans. J Physiol. 2017;595:5175–5190. doi: 10.1113/JP274532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dora KA. Endothelial-smooth muscle cell interactions in the regulation of vascular tone in skeletal muscle. Microcirculation. 2016;23:626–630. doi: 10.1111/micc.12322. [DOI] [PubMed] [Google Scholar]
- 27.Rubinstein J, Lasko VM, Koch SE, Singh VP, Carreira V, Robbins N, Patel AR, Jiang M, Bidwell P, Kranias EG, Jones WK, Lorenz JN. Novel role of transient receptor potential vanilloid 2 in the regulation of cardiac performance. Am J Physiol Heart Circ Physiol. 2014;306:H574–H584. doi: 10.1152/ajpheart.00854.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the L-arginine/nitric oxide pathway on vasodilation caused by beta-adrenergic agonists in human forearm. Circulation. 1997;95:2293–2297. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- 29.Eklund B, Kaijser L. Effect of regional alpha- and beta-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. J Physiol. 1976;262:39–50. doi: 10.1113/jphysiol.1976.sp011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torp KD, Tschakovsky ME, Halliwill JR, Minson CT, Joyner MJ. beta-Receptor agonist activity of phenylephrine in the human forearm. J Appl Physiol (1985) 2001;90:1855–1859. doi: 10.1152/jappl.2001.90.5.1855. [DOI] [PubMed] [Google Scholar]
- 31.Astrand H, Sandgren T, Ahlgren AR, Länne T. Noninvasive ultrasound measurements of aortic intima-media thickness: implications for in vivo study of aortic wall stress. J Vasc Surg. 2003;37:1270–1276. doi: 10.1016/s0741-5214(02)75344-5. [DOI] [PubMed] [Google Scholar]
- 32.Proctor DN, Newcomer SC. Is there a difference in vascular reactivity of the arms and legs? Med Sci Sports Exerc. 2006;38:1819–1828. doi: 10.1249/01.mss.0000230340.79247.52. [DOI] [PubMed] [Google Scholar]
- 33.Good ME, Begandt D, DeLalio LJ, Keller AS, Billaud M, Isakson BE. Emerging concepts regarding pannexin 1 in the vasculature. Biochem Soc Trans. 2015;43:495–501. doi: 10.1042/BST20150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahl G, Qiu F, Wang J. The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology. 2013;75:583–593. doi: 10.1016/j.neuropharm.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63:1094–1103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- 36.Cheng D, Ren J, Jackson EK. Multidrug resistance protein 4 mediates cAMP efflux from rat preglomerular vascular smooth muscle cells. Clin Exp Pharmacol Physiol. 2010;37:205–207. doi: 10.1111/j.1440-1681.2009.05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin ZP, Zhu YL, Johnson DR, Rice KP, Nottoli T, Hains BC, McGrath J, Waxman SG, Sartorelli AC. Disruption of cAMP and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signaling and nociceptive response. Mol Pharmacol. 2008;73:243–251. doi: 10.1124/mol.107.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol. 2004;66:571–599. doi: 10.1146/annurev.physiol.66.032102.111604. [DOI] [PubMed] [Google Scholar]
- 39.Nyberg M, Hellsten Y. Reduced blood flow to contracting skeletal muscle in ageing humans: is it all an effect of sand through the hourglass? J Physiol. 2016;594:2297–2305. doi: 10.1113/JP270594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.