Key Points
Question
Is uveal melanoma associated with myotonic dystrophy?
Findings
In this case series, 6 patients were found to have myotonic dystrophy–associated uveal melanoma, including a case of myotonic dystrophy–associated iris melanoma and myotonic dystrophy–associated uveal melanoma in relatives. With variable follow-up of 6, 6, 41, 42, and 87 months (5 patients), findings included melanoma regression (4 of 5 tumors), melanoma recurrence (1 of 5 tumors), and no metastatic disease (5 of 5 patients); 1 patient declined treatment or follow-up.
Meaning
These findings suggest an association of myotonic dystrophy and uveal melanoma; patients with myotonic dystrophy may benefit from periodic ophthalmic examination.
Abstract
Importance
Patients with myotonic dystrophy (MD) have an increased risk of malignancy including uveal melanoma. This case series further explores the association between these 2 diseases.
Objective
To describe a cohort of patients with uveal melanoma associated with MD, including a case of iris melanoma, and MD-associated uveal melanoma in relatives.
Design, Setting, and Participants
Retrospective case series at 3 tertiary referral centers (Wills Eye Hospital, Philadelphia, Pennsylvania; Mayo Clinic, Rochester, Minnesota; and Moorfields Eye Hospital, London, England), between January 1, 2000, and August 31, 2017. The study included 6 patients with MD and uveal melanoma.
Main Outcomes and Measures
Melanoma response to treatment and development of metastatic disease.
Results
There were 6 patients, 4 men and 2 women, with MD and uveal melanoma. The mean patient age at melanoma diagnosis was 47 years (median, 43 years; range, 30-67 years), and the tumor involved the choroid in 5 patients (83%) and iris in 1 patient (17%). The diagnosis of MD was known since young adulthood in 2 patients (33%) and was discovered in adulthood in 4 patients (67%). The main clinical features of MD included muscle weakness (n = 5; 83%), myotonia (n = 4; 67%), polychromatic cataract (n = 4; 67%), complications with general anesthesia (n = 4; 67%), myalgia (n = 3; 50%), cardiac arrhythmia (n = 2; 33%), and frontal baldness (n = 2; 33%). Genetic testing revealed MD type 1 (4 of 4 tested patients), and 2 patients demonstrated positive family history of MD with classic clinical features and preferred no testing. Melanoma treatment included plaque radiotherapy (n = 4; 67%), photodynamic therapy (n = 1; 17%), and declined treatment (n = 1; 17%). At follow-up of 6, 6, 41, 42, and 87 months (5 patients), findings included melanoma regression (4 of 5 tumors), melanoma recurrence (1 of 5 tumors), and no metastatic disease (5 of 5 patients).
Conclusions and Relevance
Six adult patients with MD demonstrated uveal melanoma involving the choroid or iris, emphasizing the association between these 2 diseases. Further research seems warranted to explore the pathogenesis of uveal melanoma in MD. These findings support the consideration of ophthalmic examination for uveal melanoma in patients with MD.
This case series describes a relatively large cohort of patients with uveal melanoma associated with myotonic dystrophy, including a case of iris melanoma, and myotonic dystrophy–associated uveal melanoma in relatives.
Introduction
Myotonic dystrophy (MD) is an autosomal dominant muscular dystrophy characterized by progressive muscle weakness, myotonia, cardiac conduction deficits, and early onset cataracts.1 It is one of the most common genetic muscle disorders affecting patients of European descent (prevalence, approximately 1 in 8000) and there are 2 genetic subtypes.1 Myotonic dystrophy type 1 (MD1; Steinert disease) is caused by a CTG trinucleotide repeated expansion in the DMPK gene on chromosome 19 (OMIM 160900).1 Myotonic dystrophy type 2 (MD2; Ricker syndrome) is caused by a CCTG tetranucleotide repeated expansion in the CNBP (ZNF9) gene on chromosome 3 (OMIM 602668).1
Several studies have confirmed that patients with MD have an increased risk of malignancy including choroidal melanoma, non-Hodgkin lymphoma, and cancers of the brain, colon, endometrium, ovary, testicle, and thyroid.2,3,4,5,6 In patients with MD, malignancies account for 10% of all deaths, and the absolute risk of cancer mortality increases from 2% at age 50 years to 6% at age 70 years.6
To our knowledge, there is only 1 prior report describing 3 patients with uveal melanoma associated with MD1.7 We describe 6 additional cases of MD-associated uveal melanoma.
Methods
This study is in compliance with the Health Insurance Portability and Accountability Act and received institutional review board approval from Wills Eye Hospital. Records from 3 ocular oncology practices (Wills Eye Hospital, Philadelphia, Pennsylvania; Mayo Clinic, Rochester, Minnesota; and Moorfields Eye Hospital, London, England) were retrospectively reviewed for concurrent MD and uveal melanoma. All patients agreed to participate in research per each institution’s unique protocol. While consent for research was obtained, specific consent for this study was waived because some patients were deceased or lost to follow-up at the time of retrospective review.
Results
Six patients with uveal melanoma were found to have underlying MD (Table). Two cases were included in a prior large series,3 but none of the included cases have been previously reported in detail.
Table. Uveal Melanoma in 6 Patients With Myotonic Dystrophya.
| Patient No./Race/Sex/Age Range | Genetic Mutation | Myotonic Dystrophy Features | Presenting Visual Acuity | Uveal Melanoma Features | Follow-up Visual Acuity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systemic MD Features | Family History | Melanoma Location | Largest Basal Diameter, mm | Tumor Thickness, mm | Subretinal Fluid | Orange Pigment | Treatment | Follow-up After Melanoma Diagnosis, mo | Metastatic Uveal Melanoma | |||||
| MD | Cancer | |||||||||||||
| 1/W/F/30s | DMPK | Muscle weakness, muscle pain, general anesthesia complication, and PC IOL | Positive | Positive | 20/70 | Choroid | 6.0 | 2.5 | Negative | Positive | I-125, TTT | 6 | No | CF |
| 2/W/M/40s | DMPK | Myotonia, cardiac arrhythmia, and CT cataract | Negative | Negative | 20/30 | Choroid | 7.0 | 1.8 | Negative | Positive | I-125, TTT | 87 | No | 20/400 |
| 3/W/M/50s | DMPK | Myotonia, muscle weakness, muscle pain, frontal baldness, cardiac arrhythmia, general anesthesia complication, and CT cataract | Positive | Positive | 20/30 | Choroid | 8.5 | 4.6 | Positive | Positive | Ru-106 | 41 | No | 20/100 |
| 4/W/F/60s | DMPK | Myotonia, muscle weakness, muscle pain, frontal baldness, and general anesthesia complication, PC IOLb | Positive | Positive | 20/50 | Choroid | 6.0 | 1.5 | Negative | Positive | PDT | 6 | No | 20/30 |
| 5/W/M/40s | Not tested | Muscle weakness, general anesthesia complication, and CT cataract | Positive | Positive | 20/40 | Iris | 8 clock hours | 2.0 | NA | NA | I-125 | 42 | No | HM |
| 6/ME/M/50s | Not tested | Myotonia and muscle weakness | Positive | Positive | 20/20 | Choroid | 14.0 | 3.7 | Negative | Positive | Advised I-125 | 0 | NA | NA |
Abbreviations: CF, count fingers; CT cataract, polychromatic (Christmas tree) cataract; F, female; HM, hand motion; I-125, iodine 125 plaque radiotherapy; M, male; ME, Middle Eastern; NA, not applicable; PC IOL, posterior chamber intraocular lens; PDT, photodynamic therapy; Ru-106, ruthenium 106 plaque radiotherapy; TTT, transpupillary thermotherapy; W, white.
Patients were free of metastatic disease at the latest reported follow-up time. Metastatic screening protocols included abdominal imaging every 6 to 12 months with or without liver function tests and chest imaging. Specific imaging modalities varied by institution.
Presence of CT cataract prior to cataract extraction could not be ascertained.
Case 1
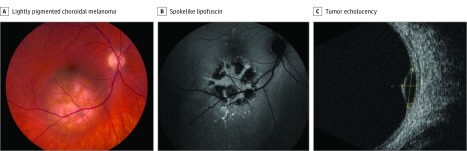
A young white woman with MD1 diagnosed when she was a teenager (100 trinucleotide repeats in DMPK) had an intraocular mass. Review of systems was remarkable for foot drop and marked oxygen desaturation following general anesthesia. Family history was positive for cutaneous melanoma, Merkel cell carcinoma, and MD. Ophthalmic examination showed pseudophakia in both eyes and a macular pigmented choroidal melanoma in the right eye with overlying orange pigment (Figure 1A), measuring 6 mm in largest basal diameter. Fundus autofluorescence revealed spokelike lipofuscin overlying the lesion (Figure 1B). B-scan ultrasonography depicted an echolucent choroidal mass, measuring 2.5 mm in thickness (Figure 1C). The melanoma was treated with iodine 125 plaque radiotherapy with transpupillary thermotherapy consolidation. On 6-month follow-up, the tumor demonstrated regression and there was no evidence of metastases.
Figure 1. Case 1.
A young woman with myotonic dystrophy had a lightly pigmented choroidal melanoma in the right eye, inferior macular region, with overlying orange pigment (A), confirmed on autofluorescence as spokelike lipofuscin (orange pigment) (B), and tumor echolucency on B-scan ultrasonography (C).
Case 2
A middle-aged white man with no history of MD displayed aged facial features, pasty white skin tone, and transient atrial fibrillation. Slitlamp biomicroscopy revealed polychromatic (Christmas tree) cataract in both eyes. Funduscopy of the right eye revealed a pigmented choroidal melanoma temporal to the macula (eFigure, A in the Supplement) with overlying lipofuscin (eFigure, B in the Supplement), measuring 7 mm in largest basal diameter and 1.8 mm in thickness with acoustic hollowness on ultrasonography (eFigure, C in the Supplement). Owing to clinical suspicion, genetic testing was performed and revealed MD1 (644 trinucleotide repeats in DMPK). The patient was treated with iodine 125 plaque radiotherapy and transpupillary thermotherapy. At 87 months follow-up, tumor regression was documented, with no evidence of metastases.
Case 3
A middle-aged white man with adult-onset MD1 was referred for evaluation of a pigmented choroidal mass in the right eye. There was no personal or family history of cancer. Examination revealed Christmas tree cataract in both eyes. Funduscopy of the right eye showed a pigmented choroidal melanoma with overlying orange pigment and subretinal fluid. Treatment with ruthenium-106 plaque radiotherapy resulted in tumor regression, with no evidence of metastases at 41 months follow-up. The patient subsequently died following myocardial infarction.
Case 4
A middle-aged white woman (a relative of case 3) with adult-onset MD1 had a choroidal mass in the left eye. She had no personal history of cancer, but her relative had choroidal melanoma. Examination revealed pseudophakia in both eyes and a pigmented choroidal melanoma in the left eye with orange pigment and thickness 1.5 mm. The patient was treated with 3 sessions of double duration photodynamic therapy but had lesion growth. Cardiac status precluded general anesthesia for plaque placement; therefore, stereotactic radiotherapy was advised. She had no metastatic disease 6 months after diagnosis.
Case 5
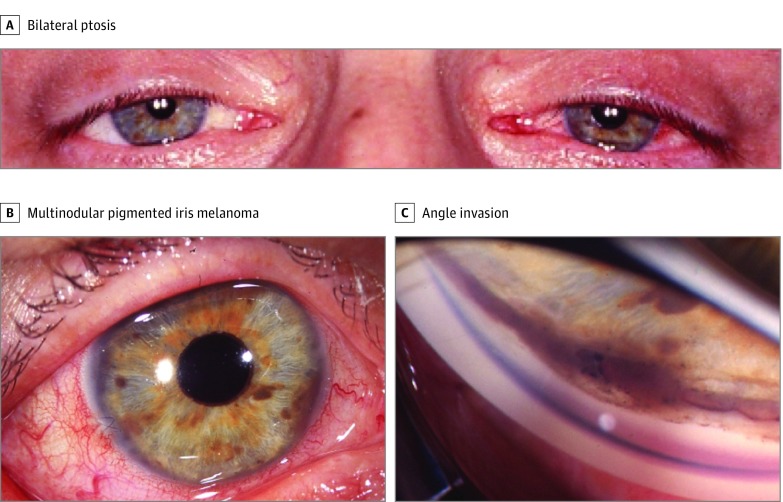
A middle-aged white man with history of MD diagnosed in young adulthood and Christmas tree cataract in both eyes removed prior to referral was seen for evaluation of an iris lesion in the right eye. Family history was positive for MD and breast cancer. Examination disclosed belpharoptosis (Figure 2A) and pseudophakia in both eyes. Anterior segment examination of the right eye demonstrated a pigmented, multinodular, diffuse, ring-shaped iris melanoma (Figure 2B) with invasion into the trabecular meshwork (Figure 2C), measuring 3.0 mm in thickness. The patient was treated with epicorneal iodine 125 plaque radiotherapy, and the tumor regressed with no metastatic disease at 42 months’ follow-up.
Figure 2. Case 5.
A middle-aged man with myotonic dystrophy (MD), bilateral ptosis, and weak compensatory brow elevation, consistent with MD-associated facial weakness (A), had a ring-shaped, multinodular pigmented iris melanoma (B), with 8 clock hours of angle invasion on gonioscopy (C).
Case 6
A middle-aged Middle Eastern man with adult-onset MD was referred for evaluation of a pigmented choroidal lesion in the right eye. Family history was significant for MD, lymphoma, and lung cancer and a personal history of basal cell carcinoma. Examination showed nuclear sclerotic cataract without polychromasia in both eyes. Funduscopy in the right eye revealed a choroidal melanoma with overlying orange pigment, measuring 14 mm in basal diameter and 3.7 mm in thickness. The patient was offered iodine 125 plaque radiotherapy but declined treatment or further follow-up (alive at 2 months by telephone).
Discussion
To our knowledge, this is the largest series of MD-associated uveal melanoma to date. We include the first reported case of MD-associated iris melanoma and MD-associated uveal melanoma in relatives. There were 6 patients (4 of 6 [67%] were men) diagnosed with MD in young adulthood (n = 2; 33%) or older (n = 4; 67%), each with features of MD, including muscle weakness (n = 5; 83%), myotonia (n = 4; 67%), polychromatic cataract (n = 4; 67%), complications with general anesthesia (n = 4; 67%), myalgia (n = 3; 50%), cardiac arrhythmia (n = 2; 33%), and frontal baldness (n = 2; 33%). Four patients had genetically confirmed MD1. Two patients declined genetic testing but had positive family history of MD and classic clinical features.
In each case, uveal melanoma was diagnosed in adulthood (mean age, 47 years; median, 43 years; range, 30-67 years) and was treated with plaque radiotherapy (4 of 6; 67%), PDT (1 of 6; 17%), or declined treatment (1 of 6; 17%). After mean follow-up of 36 months for 5 treated patients (median, 41 months; range, 6-87 months), all plaque-treated tumors (4 of 4) demonstrated regression without recurrence, and the PDT-treated tumor (1 of 1) was unresponsive to 3 sessions. No patient developed metastatic disease.
While MD reportedly incurs a 27.5-fold increased risk of choroidal melanoma,3 the mechanism behind this risk remains uncertain. A hypothesis suggests that DMPK may function as a tumor suppressor in the Wnt/β-catenin signaling pathway, imparting an increased risk of cancer to patients with MD1 who have loss of heterozygosity of DMPK.8,9 Increased β-catenin expression has been demonstrated in aggressive class 2 uveal melanomas,10 making this is a plausible culprit for the link between uveal melanoma and MD1.
Limitations
Limitations of our study include lack of genetic testing in 2 of 6 patients, preventing us from definitively stating that uveal melanoma has been exclusively associated with MD1. Additionally, while neither our series nor prior reports have associated metastatic uveal melanoma with MD,7 longer follow-up is necessary to better evaluate the risk of tumor spread in these patients.
Conclusions
Given the association between uveal melanoma and MD, consideration should be given to ophthalmic examinations with careful attention to any iris, ciliary body, or choroidal lesions. Future studies may further delineate the pathogenesis of uveal melanoma in patients with MD and define best cancer screening practices.
eFigure. Case 2.
References
- 1.Thornton CA. Myotonic dystrophy. Neurol Clin. 2014;32(3):705-719, viii. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott D, Johnson NE, Cannon-Albright LA. A population-based survey of risk for cancer in individuals diagnosed with myotonic dystrophy. Muscle Nerve. 2016;54(4):783-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Win AK, Perattur PG, Pulido JS, Pulido CM, Lindor NM. Increased cancer risks in myotonic dystrophy. Mayo Clin Proc. 2012;87(2):130-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Torrón R, García-Puga M, Emparanza JI, et al. Cancer risk in DM1 is sex-related and linked to miRNA-200/141 downregulation. Neurology. 2016;87(12):1250-1257. [DOI] [PubMed] [Google Scholar]
- 5.Gadalla SM, Lund M, Pfeiffer RM, et al. Cancer risk among patients with myotonic muscular dystrophy. JAMA. 2011;306(22):2480-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadalla SM, Pfeiffer RM, Kristinsson SY, et al. Quantifying cancer absolute risk and cancer mortality in the presence of competing events after a myotonic dystrophy diagnosis. PLoS One. 2013;8(11):e79851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velazquez-Martin JP, Pavlin CJ, Simpson ER. Association between uveal melanoma and myotonic dystrophy: a series of 3 cases. JAMA Ophthalmol. 2013;131(2):246-249. [DOI] [PubMed] [Google Scholar]
- 8.Mueller CM, Hilbert JE, Martens W, Thornton CA, Moxley RT III, Greene MH. Hypothesis: neoplasms in myotonic dystrophy. Cancer Causes Control. 2009;20(10):2009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9(5):534-546. [DOI] [PubMed] [Google Scholar]
- 10.Onken MD, Ehlers JP, Worley LA, Makita J, Yokota Y, Harbour JW. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res. 2006;66(9):4602-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Case 2.