Abstract
The retinoic acid receptor-related orphan receptors α and γ (RORα and RORγ), are key regulators of helper T (Th)17 cell differentiation, which is involved in the innate immune system and autoimmune disorders. In this study, we investigated the effects of isoflavones on RORα/γ activity and the gene expression of interleukin (IL)-17, which mediates the function of Th17 cells. In doxycycline-inducible CHO stable cell lines, we found that four isoflavones, biochanin A (BA), genistein, formononetin, and daidzein, enhanced RORα- or RORγ-mediated transcriptional activity in a dose-dependent manner. In an activation assay of the Il17a promoter using Jurkat cells, these compounds enhanced the RORα- or RORγ-mediated activation of the Il17a promoter at concentrations of 1 × 10−6 M to 1 × 10−5 M. In mammalian two-hybrid assays, the four isoflavones enhanced the interaction between the RORα- or RORγ-ligand binding domain and the co-activator LXXLL peptide in a dose-dependent manner. In addition, these isoflavones potently enhanced Il17a mRNA expression in mouse T lymphoma EL4 cells treated with phorbol myristate acetate and ionomycin, but showed slight enhancement of Il17a gene expression in RORα/γ-knockdown EL4 cells. Immunoprecipitation and immunoblotting assays also revealed that BA enhanced the interaction between RORγt and SRC-1, which is a co-activator for nuclear receptors. Taken together, these results suggest that the isoflavones have the ability to enhance IL-17 gene expression by stabilizing the interactions between RORα/γ and co-activators. This also provides the first evidence that dietary chemicals can enhance IL-17 gene expression in immune cells.
Keywords: Interleukin 17, Isoflavone, Luciferase assay, Retinoic acid receptor-related orphan receptor, Th17
1. Introduction
The retinoic acid receptor-related orphan receptors, RORα-γ (NR1F1-3), are members of the nuclear receptor superfamily and function as ligand-dependent transcription factors that are involved in the regulation of a wide range of physiological processes, including immune response, lipid/glucose homeostasis, and circadian rhythm (Jetten, 2009; Jetten et al., 2013; Takeda et al., 2014a,b). They are considered to be orphan receptors because their endogenous ligands have not yet been agreed upon definitively, and they have been identified with distinct tissue distributions and biological activities (Jetten, 2009). In the thymus, RORα and two isoforms, γ1 and γ2 (also referred to RORγt), have been identified (He et al., 1998), with RORγt differing from the RORγ1 isoform in that it lacks the amino terminus of RORγ1. Recent studies have shown that the RORα and RORγ regulate the transcription of interleukin 17 (IL-17) and other pro-inflammatory cytokines in T helper 17 (Th17) cells (Ivanov et al., 2006; Yang et al., 2008). As Th17 cells play important roles in host defense against bacterial and fungal infections, as well as in a wide variety of autoimmune diseases, including psoriasis and rheumatoid arthritis (Harrington et al., 2005; Park et al., 2005; Weaver et al., 2007; Stockinger and Veldhoen, 2007), the identification of ligands that regulate the RORs has been the focus of significant interest due to their potential for clinical use (Huh and Littman, 2012; Solt and Burris, 2012).
Unlike most members of the nuclear receptor superfamily, RORs bind as monomers to ROR response element (RORE), consisting of an AGGTCA element preceded by an AT-rich sequence, in the promoter regulatory region of the target genes. Subsequent transcriptional regulation by RORs is mediated through constitutively recruiting co-repressors and co-activators, including NCOR1, RIP140, NCOA1 and PGC-1α (Jetten, 2009). Recent studies have shown that a synthetic chemical SR1001, digoxin derivatives, and ursolic acid act as RORα and/or RORγ inverse agonists to inhibit basal transcription mediated by ROR itself, and ameliorate the severity of experimental autoimmune encephalomyelitis (EAE), which is dependent on Th17 cell function (Huh et al., 2011; Solt et al., 2011; Xu et al., 2011). In our previous paper, we reported that some azole-type fungicides, such as imibenconazole, inhibited Il17a mRNA expression via RORα/γ in mouse lymphoma EL4 cells, and that some environmental chemicals can also act as modulators of IL-17 gene expression in immune cells (Kojima et al., 2012). Thus, extensive study has been undertaken on inhibitory small molecules, including several ROR inverse agonists, and some of them may have potential applications to drug-therapy for autoimmune diseases in the future. On the other hand, there have been few reports on chemicals that enhance ROR activity, although some hydroxycholesterols, cholesterol sulfate and a synthetic chemical SR1078 have been reported to act as ROR agonists (Kallen et al., 2002; Wang et al., 2010). The identification of ROR agonistic compounds would aid in the development of therapeutic means for fighting certain bacterial or fungal infections and cancers through the augmentation of ROR and Th17 cell activity (Huh and Littman, 2012; Solt and Burris, 2012).
Isoflavones are naturally occurring plant chemicals, and their plant-based dietary intake may play a beneficial role in the treatment/prevention of obesity, cancer, osteoporosis, and cardiovascular disease (Setchell and Cassidy, 1999). Two of the major isoflavones found in humans are genistein (GE) and daidzein (DA), which are metabolized from their plant precursors, biochanin A (BA) and formononetin (FN), respectively. These isoflavones share a common diphenolic structure that resembles that of the potent synthetic estrogens diethylstilbestrol and hexestrol (Fig. 1). Therefore, the effects of isoflavones on human health have been the focus of much attention due to their estrogenic activity via estrogen receptors (Takeuchi et al., 2009). To date, there have been no reports on the effects of isoflavones on Th17 cell function, although the effects of GE on immunity have been extensively studied (Yellayi et al., 2002).
Fig. 1.
Chemical structures of the isoflavones used in this study.
In this study, we investigated the potential RORα and RORγ activities of isoflavones using Chinese hamster ovary (CHO)-K1 and Jurkat T cell-based reporter gene assays. As a result, we found that isoflavones, such as BA and FN, enhanced the constitutive activation of RORα and RORγ, and also enhanced the interactions between RORs and the co-activator NCOA1. In addition, these compounds were found to enhance Il17a gene expression in EL-4 cells in a ROR-dependent manner. Thus, we here provide the first evidence that dietary isoflavones might increase IL-17 gene transcriptional activity through their actions as RORα and RORγ agonists.
2. Materials and methods
2.1. Chemicals and antibodies (Ab)
Formononetin (FN, >99% pure), biochanin A (BA, >99% pure), daidzein (DA, >99% pure) and genistein (GE, >98% pure) were purchased from Sigma–Aldrich (St. Louis, MO, USA). A synthetic ROR inverse agonist, T0901317 and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma–Aldrich. Ionomycin was purchased from LKT Laboratories (St. Paul, MN, USA). Dimethylsulfoxide was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan), and used as a vehicle. All compounds tested were dissolved in DMSO at a concentration of 1 ×10−2 M. Anti-FLAG and anti-SRC-1 Abs were obtained from Sigma–Aldrich and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively.
2.2. Cell line and cell culture materials
CHO-K1 cells, human lymphoma T Jurkat cells, mouse lymphoma EL4 cells, and human cervix carcinoma HeLa cells were obtained from the American Type Culture Collection. Fetal bovine serum (FBS) and charcoal-dextran treated FBS (CD-FBS) were obtained from Hyclone (Logan, UT, USA). Dulbecco’s modified Eagle’s medium (DMEM), DMEM plus Ham’s F-12 nutrient mixture (DMEM/F-12) and RPMI-1640 medium were obtained from GIBCO-BRL (Invitrogen Rockville, MD, USA). Glutamine and penicillin–streptomycin (antibiotics) solutions were obtained from Dainippon Pharmaceutical Co., Ltd. (Osaka, Japan), and 0.25% trypsin/0.02% ethylenediamine tetra-acetic acid (EDTA) disodium salt solution was obtained from Life Technologies (Paisley, UK). CHO-K1 cells were maintained in DMEM/F-12 containing 5% FBS and antibiotics. EL4 and Jurkat cells were maintained in RPMI-1640 medium containing 10% FBS and antibiotics. HeLa cells were maintained in DMEM containing 10% FBS and antibiotics.
2.3. ROR-reporter gene assays using CHO Tet-on cells
Doxycycline-inducible ROR stable cell lines were generated by transfecting a pTRE2 expression vector (Clontech, Mountain View, CA, USA) containing RORα or RORγ, into CHO Tet-on cells (Clontech) followed by transfection with the pGL4.27 luciferase (LUC) reporter vector (Promega, Madison, WI, USA) driven by 5xRORE. Single clones of pGL4.27-5xRORE- and pTRE2-ROR-expressing cells were selected from a medium containing hygromycin (Invitrogen, Grand Island, NY, USA) and puromycin (Sigma–Aldrich), respectively. CHO Tet-on cell lines were cultured in F12 medium supplemented with 10% FBS, and suitable for use in the Tet-on system (Clontech). To induce ROR expression, cells were treated for 20 h with 1 μM doxycycline in the presence or absence of a dilution series of the isoflavones. All the assays were performed in triplicate and repeated independently at least twice. RORE-mediated activation of the LUC reporter was measured with a Luciferase Assay Substrate Kit (Promega). cAMP-based cell viability was evaluated by CellTiter-Glo Luminescent Cell Viability Assay (Promega).
2.4. Activation assay of the Il17a promoter in Jurkat cells
Jurkat cells were co-transfected with a pCMV-β-Gal plasmid (Clontech), pCMV10-3xFlag-RORα or pCMV10-3xFlag-RORγ plasmid, and a pGL4.14 reporter plasmid (Promega) under the control of human Il17a-3kb-CNS promoter (Zhang et al., 2012), using Lipofectamine 2000 (Invitrogen), and then treated with the vehicle, or 0.1, 1, 10 μM of the isoflavones. After 24 h, the firefly luciferase and β-galactocidase activities were measured using a Luciferase Assay Substrate Kit (Promega) and a Luminescent β-galactosidase Detection Kit II (Clontech), respectively. The firefly luciferase activity was normalized against β-galactocidase activity. All transfections were performed in triplicate and repeated at least twice.
2.5. Mammalian two-hybrid assay
CHO-K1cellswereco-transfectedwithapGL4.27-(UAS)5reporter plasmid, containing 5 copies of the Gal4 upstream activating sequence (UAS) in the reporter vector pGL4.27 (Promega), pCMV-β-Galplasmid, pM-EBIP96(LXXLL)plasmid, andVP16-RORα(LBD)or VP16-RORγ(LBD) plasmid, containing the ligand-binding domain (LBD) of ROR (Kurebayashi et al., 2004; Takeda and Jetten, 2013), using Lipofectamine 2000 (Invitrogen), and then treated with the vehicle, or 0.1, 1, 10 μM of the isoflavones. After 24 h, the firefly luciferase and β-galactocidase activities were measured as described above and their ratios calculated. All transfections were performed in triplicate and repeated at least twice.
2.6. Il17a mRNA expression in EL4 cells or RORα/γ-knockdown EL4 cells
We plated host EL4 cells or RORα/γ-knockdown EL4 cells (Kojima et al., 2012) in 12-well microtiter plates (Nalge, Nunc, Denmark) at a density of 2 × 6 106 cells/ml in RPMI-1640 medium containing 10% FBS. Next day, the cells were treated with the vehicle or both PMA (5 ng/ml) and ionomycin (1 μM) for the induction of Il17a. At the same time, the cells were treated with the vehicle, or 0.1,1 or 10 μM of the isoflavones, and incubated at 37 °C for 6 h. The cells were harvested and homogenized in 1 ml of TRIzol Reagent (Molecular Research Center, Cincinnati, OH, USA), followed by total RNA isolation. The complementary DNA (cDNA) was synthesized using 1μg of total RNA by Revertra Ace reverse transcriptase (TOYOBO, Osaka, Japan). The cDNAs were then stored at −20 °C until analysis.
2.7. Real-time quantitative PCR for Il17a and β-actin
We measured Il17a and β-actin mRNA levels using TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). cDNA prepared from the total RNA of the EL4 cells was subjected to real-time quantitative PCR on a 7900HT Fast Real Time PCR system (Applied Biosystems). The following probes were designed by Applied Biosystems: for Il17a, 5′-FAM d(CTTCATCTGTGTCTCTGATGCTGTT) NFQ-3′; and for β-actin, 5′-FAM d(ACTGAGCTGCGTTTTACACCCTTTC) NFQ-3′. The amount of Il17a mRNA was normalized against that of β-actin mRNA.
2.8. Interaction assay between RORγt and NCoA-1 using immunoprecipitation and immunoblotting
The expression plasmids FLAG-RORγt and FLAG-RORγtΔAF2 were constructed according to our previously described method (Matsuda et al., 2001). HeLa cells were plated on 6-cm dishes at 1 × 106 cells/dish, and transfected with FLAG-RORγt or FLAG-RORγtΔAF2 using jetPEI (Polyplus Transfection, Strasbourg, France), according to the manufacturer’s instructions. At 36 h after transfection, the cells were left untreated or were treated with BA (1 μM) for an additional 15 min. Immunoprecipitation and western blotting assays were performed as described previously (Matsuda et al., 2001). The immunoprecipitates from the cell lysates and the cell lysates were resolved on G6Pase and FGF21 and transferred to polyvinylidene difluoride transfer membrane (PerkinElmer, Boston, MA, USA). The membranes were then immunoblotted with anti-FLAG or anti-SRC-1 Ab. Immunoreactive proteins were visualized using Immobilon™ Western Chemiluminescent HRP Substrate (Millipore).
2.9. Data analysis
We used a one-way analysis of variance (ANOVA) followed by Bonferroni correction to evaluate the differences in transcriptional levels between the control group and each of the chemical groups in the reporter gene assays and quantitative RT-PCR assays. Statistical significance was set at p < 0.05. Data were presented as means ± SD of three triplicate experiments.
3. Results
3.1. Effects of isoflavones on transcriptional activity in doxycycline-inducible Tet-on CHO cell line for RORα and RORγ
We examined the effects of isoflavones on RORα- and RORγ-mediated transcriptional activity using CHO Tet-on cells. This system consists of the CHO cells stably integrated with a doxycycline-inducible RORα or RORγ expression vector and a RORE-responsive-LUC reporter (Slominski et al., 2014). The system allows a sensitive detection of RORE-mediated LUC activation by the inducible expression of ROR on addition of doxycycline. Fig. 2A and B shows the transcriptional activity of the four isoflavones in CHO Tet-on cells expressing RORα and RORγ, respectively. In the RORα assay, the isoflavones functioned as agonists of RORα-mediated activation in a dose-dependent manner from 1 × 10−8 to 1 × 10−5 M (Fig. 2A). The order of the relative potency of RORα agonistic activity was BA, FN > GE > DZ. In the RORγ assay, these compounds acted as RORγ agonists in a dose-dependent manner from 1 × 10−8 to 1 × 10−5 M (Fig. 2B). The order of the relative potency of RORγ agonistic activity was FN > BA > GE, DZ.
Fig. 2.
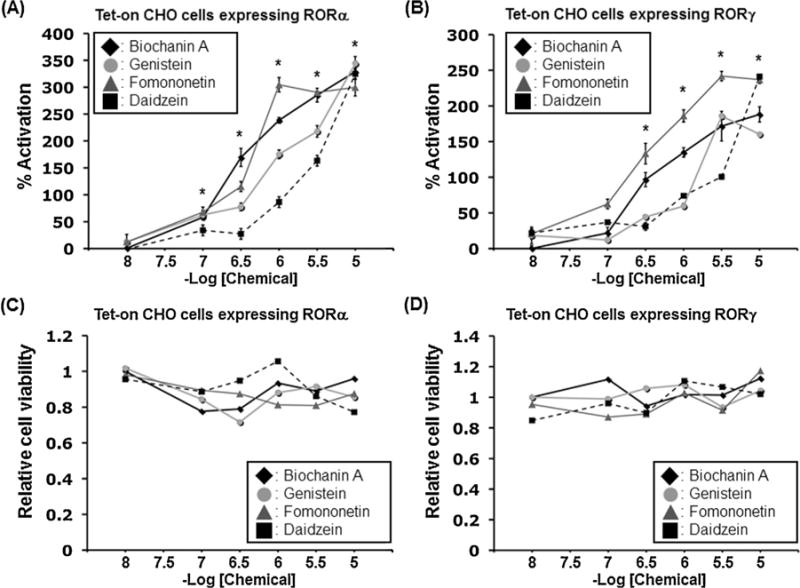
Effects of isoflavones on ROR-dependent transcriptional activation using CHO Tet-on cells. Reporter gene assay was performed using doxycycline-inducible Tet-on CHO cell line for RORα (A) and RORγ (B) under treatment for 20 h with 1 μM doxycycline in the presence or absence of a dilution series of isoflavones, such as biochanin A, genistein, formononetin, and daidzein. Relative LUC activity driven by 5xRORE was determined as described in Section 2. Reporter activation was plotted as a percentage of the control value [presence of vehicle]. Values represent the means ± SEM (n = 3) and are presented as percentage induction, with 100% activity defined as the constitutive activity induced by the vehicle control (DMSO). Significant differences from the vehicle control are indicated by asterisks (*P < 0.05 in all 4 isoflavones; 1-way ANOVA). Cytotoxicity of the isoflavones was evaluated by the total amount of ATP in each cells using CellTiter-Glo Luminescent Cell Viability Assay in RORα-expressing cells (C) and RORγ-expressing cells (D).
Based on cytotoxicity testing using CellTiter-Glo none of the compounds tested showed any cytotoxic effects at concentrations from 1 × 10−8 to 1 × 10−5 M (Fig. 2C and D).
3.2. Effects of isoflavones on the activation of the Il17a promoter in Jurkat T cells
RORα and RORγ have been reported to directly regulate the transcription of the IL-17 gene (Jetten, 2009; Yang et al., 2008). To examine the effect of isoflavones on the activation of the Il17a promoter, we cotransfected Jurkat cells with the RORα or RORγ expression plasmid and pGL4.14 reporter plasmid under the control of the Il17a promoter. As shown in Fig. 3A, we found that all four isoflavones enhanced the activation of the Il17a promoter via RORα in a dose-dependent manner, with the activation by BA and GE, in particular, observed at a concentration of 1 × 10−6 M. In the cells transfected with RORγ, these isoflavones significantly enhanced the activation of the Il17a promoter at a concentration of 1 × 10−5 M, with the activation by BA and FN again observed at the lower concentration of 1 × 10−6 M (Fig. 3B).
Fig. 3.
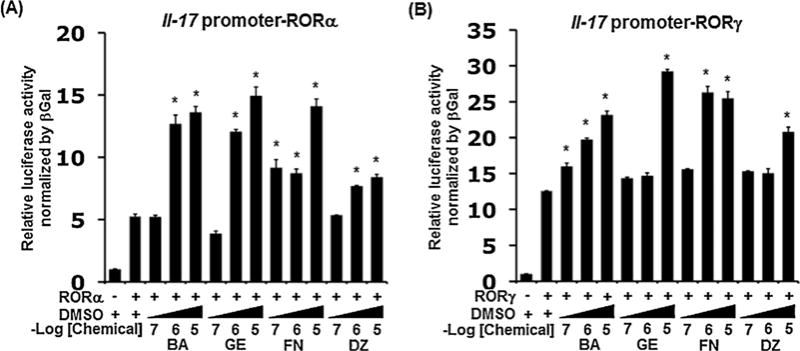
Isoflavone-induced enhancement of RORα- and RORγ-mediated Il17a promoter activation in Jurkat cells. Cells were co-transfected with the pCMV-β-Gal and pCMV10-3xFlag-RORα (A) or pCMV10-3xFlag-RORγ (B) and pGL4.14 reporter plasmid under the control of the Il17a promoter and treated with increasing concentrations of the isoflavones at 0.1, 1, and 10 μM. After 24 h, relative LUC activity was determined as described in Materials and Methods. The firefly luciferase activity was normalized against β-galactosidase activity. BA, GE, FN, and DZ represent each isoflavone, biochanin A, genistein, formononetin, and daidzein, respectively. Values represent the means ± SEM (n = 3). Significant differences from the vehicle control (DMSO) plus RORα or RORγ are indicated by asterisks (*P < 0.05; 1-way ANOVA).
3.3. Effects of isoflavones on the interaction of ROR-LBD with the LXXLL-peptide EBIP96 assessed using a mammalian two-hybrid assay
Transcriptional activation by RORs is mediated through their interaction with co-activators, which interact with the activation domain (AD) of ROR through LXXLL-like motifs (Jetten, 2009). Therefore, we examined the effects of isoflavones on the interaction of the LBD of RORα or RORγ with the LXXLL-peptide EBIP96 using a mammalian two-hybrid system (Kurebayashi et al., 2004; Takeda and Jetten, 2013). In the RORα-LBD assay, the isoflavones enhanced the interaction between the LBD of RORα and the LXXLL-peptide in a dose-dependent manner (Fig. 4A). The order of the relative potency was FN > BA > GE, DZ. In the RORγ-LBD assay, the isoflavones also enhanced the interaction between the LBD of RORγ and the LXXLL-peptide in a dose-dependent manner (Fig. 4B). The order of the relative potency was FN > BA, GE, DZ.
Fig. 4.
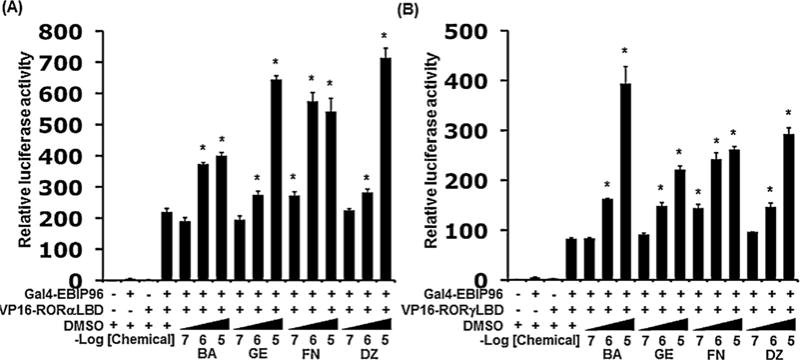
Effects of isoflavones on interactions between ROR-LBD and the co-activator LXXLL peptide in mammalian two-hybrid assays. The analysis was performed by co-transfecting CHO-K1 cells with a pGL4.27-(UAS)5 reporter plasmid, pCMV-β-Gal pM-EBIP96 peptide, and either VP16-RORα(LBD) (A) or VP16-RORγ(LBD) (B). Cells were treated in the presence of the vehicle (DMSO), or increasing concentrations of the four isoflavones as indicated. After 24 h, relative LUC activity was determined as described in Section 2. The firefly luciferase activity was normalized against β-galactosidase activity. BA, GE, FN, and DZ represent each isoflavone, biochanin A, genistein, formononetin, and daidzein, respectively. Values represent the means ± SEM (n = 3) and are presented as the mean n-fold induction over the vehicle control. Significant differences from the vehicle control (DMSO) are indicated by asterisks (*P < 0.05; 1-way ANOVA).
3.4. Effects of isoflavones on the in vitro expression of Il17a mRNA in mouse T lymphoma EL4 cells
Our previous study showed that several azole-fungicides, showing RORα/γ inverse agonistic activity in CHO-reporter gene assays, inhibited Il17a gene expression in PMA/ionomycin-stimulated EL4 cells (Kojima et al., 2012). In this study, we examined whether isoflavones affect Il17a gene expression level in EL4 cells. Fig. 5 shows the effects of isoflavones and T0901317 on Il17a gene expression in PMA/ionomycin-stimulated EL4 cells. Real-time PCR analysis revealed that four isoflavones significantly enhanced Il17a mRNA expression at concentrations from 1 ×10−7 to 1 × 10−5 M in PMA/ionomycin-stimulated EL4 cells. Of the four isoflavones, GE (1 × 10−5 M) induced the most potent increase of Il17a mRNA expression, and BA (1 × 10−5 M) followed it. In addition, treatment with FN and DZ (each 1 × 10−6 M) significantly enhanced Il17a mRNAs in comparison with vehicle treatment. In contrast, ROR inverse agonist T0901317 (1 × 10−5 M) potently suppressed Il17a mRNA expression in PMA/ionomycin-stimulated EL4 cells, as described in our previous paper (Kojima et al., 2012).
Fig. 5.
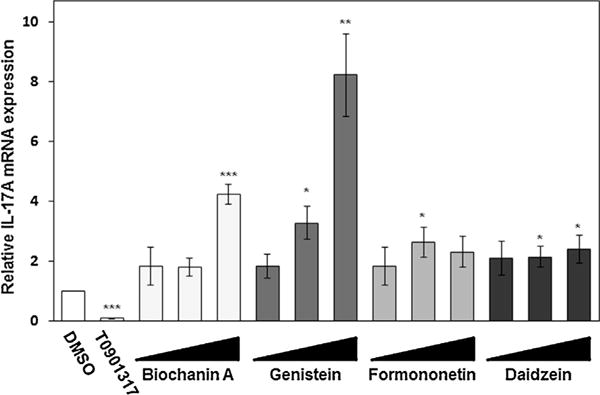
Enhancement of Il17a mRNA expression by isoflavones in EL4 cells treated with PMA and ionomycin. EL4 cells were treated with PMA (5 ng/ml) and ionomycin (1 μM) for Il17a gene induction. At the same time, the cells were treated with the vehicle, or 0.1, 1 or 10 μM of the isoflavones, and incubated at 37 °C for 6 h. Il17a mRNA expression in the cells was amplified by real-time RT-PCR and normalized against the expression of the β-actin housekeeping gene. BA, GE, FN, and DZ represent each isoflavone, biochanin A, genistein, formononetin, and daidzein, respectively. Values are expressed relative to the vehicle control with PMA/ionomycin stimulation (taken as 1) and represent the mean ± SD of three experiments. Significant differences from the vehicle control with PMA/ionomycine stimulation are indicated by asterisks (*P < 0.05 and **P < 0.01; 1-way ANOVA).
3.5. Effects of biochanin A on Il17a mRNA expression in the RORα/γ knockdown EL4 cells
We previously reported the establishment of EL4/shRorac and EL4/shNC cells as endogenous RORα and RORγ double-knockdown EL4 and control EL4 cell lines, respectively (Kojima et al., 2012). RORα and RORγ mRNA expression in EL4/shRorac cells respectively decreased at about 50 and 30% of those in EL4/shNC cells when the cells were treated with PMA/ionomycin (Kojima et al., 2012). In this study, we examined whether the enhancement of Il17a gene expression by isoflavones was RORα/γ-dependent using PMA/ionomycin-stimulated EL4/shRorac and EL4/shNC cells. Fig. 6 show the respective effects of BA and T0901317 (each 1 × 10−5 M) on Il17a mRNA levels in EL4/shRorac and EL4/shNC cells treated with PMA/ionomycin. Il17a mRNA expression in the EL4/shRorac cells was found to be markedly decreased in comparison with that in EL4/shNC cells. Although BA induced a potent increase in Il17a mRNA expression in EL4/shNC cells, this compound showed slight enhancement of Il17a mRNA expression in EL4/shRorac cells. In contrast, T0901317 showed inhibitory effects on Il17a mRNA expression in both cell lines as described by our previous paper (Kojima et al., 2012)
Fig. 6.
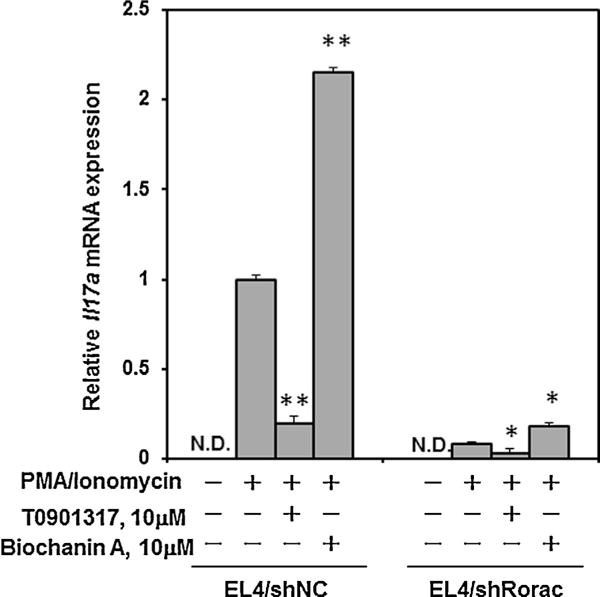
Comparison of enhancement of Il17a mRNA expression by biochanin A using EL4/shRorc cells and EL4/shNC cells. Il17a mRNA expression was measured in EL4/shRorac cells and EL4/shNC cells in the presence or absence of biochanin A (1 × 10−5 M) with PMA/ionomycin stimulation, and normalized against the expression of the β-actin housekeeping gene. Values are expressed relative to the vehicle control with PMA/ionomycin stimulation in EL4/shNC cells (taken as 1) and represent the mean ± SD of three experiments. Significant differences from the vehicle control with PMA/ionomycine stimulation are indicated by asterisks (*P < 0.05 and **P < 0.01; 1-way ANOVA). ND, not detected (<0.01).
3.6. Effects of biochanin A on the protein interaction between RORγt and co-activator SRC-1
The steroid receptor co-activator-1 (SRC-1) is one of co-activators able to physically interact with RORγ and enhance RORγ-mediated transcriptional activation (Kurebayashi et al., 2004). We investigated the effect of BA on the protein-protein interaction between FLAG-RORγt or FLAG-RORγtΔAF2 and endogenous SRC-1 using immunoprecipitation and immunoblotting. As shown in Fig. 7, the presence of BA (1 × 10−6 M) enhanced the recruitment of SRC-1 by RORγt. On the other hand, deletion of the carboxyl terminal amino acids (477–495) of RORγt containing the activation function 2 (AF2) (RORγtΔAF2) resulted in the total loss of BA-induced SRC-1 recruitment by RORγt.
Fig. 7.
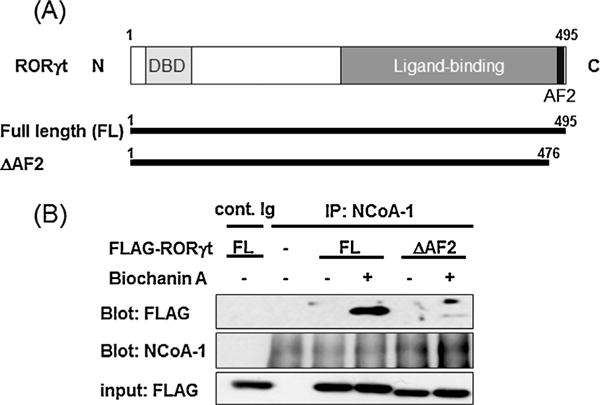
Effects of biochanin A on protein interactions between RORγt and NCoA-1 (SRC-1). (A) The schematic diagram shows the amino acids structures of RORγt and RORγtΔAF2, including DNA binding domain (DBD) and ligand binding domain (LBD). (B) HeLa cells in a 6-cm dish were transfected with expression plasmid FLAG-RORγt or FLAG-RORγtΔAF2. At 36 h after transfection, the cells were left untreated or were treated with BA (1 × 10−6 M) for 15 min. The cells were lysed, immunoprecipitated with control Immunogloblin (Ig) or anti-NCoA-1 (anti-SRC-1) Ab, and immunoblotted with anti-FLAG or anti-SRC-1 Ab. An aliquot of each total-cell lysate was analysed by immunoblotting with anti-FLAG Ab.
4. Discussion
RORs are orphan nuclear receptors, for which the endogenous ligands remain unknown, that are constitutively active and retain the ability to interact with a range of co-activator NR box peptides even in the absence of agonistic ligands (Jetten et al., 2001). Therefore, it may be relatively difficult to identify compounds that further increase ROR activity beyond a plateau of regulation by endogenous ligand (Huh and Littman, 2012). Indeed, most of the small molecules identified as ROR modulators are RORα and/or RORγ inverse agonists, and reports on newly identified ROR agonists are limited. To our knowledge, there has been only one report, by Wang et al. (2010), on the identification of ROR agonists among exogenous agents. They reported that the synthetic chemical SR1078 acts as a RORα/γ dual agonist, and stimulates the expression of two ROR target genes, G6Pase and FGF21, in hepatocarcinoma cells (Wang et al., 2010). More recently, this chemical was reported to be useful as an anti-cancer therapeutic agent through a novel mechanism for the stabilization of p53 protein expression by RORα (Wang et al., 2012), and as an insulin-inducing agent through the activation of insulin transcription by RORα (Kuang et al., 2014). Thus, the identification of RORα/γ agonist would be of great value with regard to not only the augmentation of the innate immune system via Th17 cell activity, but also other beneficial physiological processes.
In this study, we demonstrated for the first time that dietary isoflavones act as agonists of RORα- and RORγ-mediated transactivation. We used several cell-based assays to demonstrate that isoflavones act as ROR agonists. The CHO Tet-on assay system is useful for the evaluation of ROR agonists and inverse agonists among a large range of chemicals using doxycycline-inducible RORα or RORγ. In these assays, four isoflavones were found to enhance RORE activation in a ROR-dependent manner (Fig. 2A and B). FN and BA, in particular, both of which possess a methoxy group at 4′ position, showed higher agonistic activity to RORα and RORγ than did GE and DZ, which possess a hydroxyl group at the same position (Fig. 1), suggesting that isoflavones with a hydrophobic rather than a hydrophilic structure at that position can preferentially enhance RORE activation via RORα/γ. In addition, using Jurkat cells, we found that isoflavones enhance Il17a promoter activation as well as RORE activation (Fig. 3A and B). These findings suggest that isoflavones might act as ROR agonists that can up-regulate IL-17 gene expression in immune cells.
Transcriptional activation by nuclear receptors is mediated through interaction with nuclear co-factors that are part of a larger co-activator complex (McKenna and O’Mally, 2002). This implies that RORs contain an activation function (AF-2) located at the C terminus of its LBD, and agonist binding induces the AF-2 helix to adopt a conformation that encourages interactions with the LXXLL motifs of co-activator proteins such as SRCs (Fujita-Sato et al., 2011; Jin et al., 2010; Kurebayashi et al., 2004). On the basis of the results from two-hybrid assay, we showed that the LBDs of RORα and RORγ can interact with the LXXLL motifs of EBIP96 even in the absence of any exogenous ligands (Fig. 4A and B). Interestingly, four isoflavones, including BA, were found to further enhance these high basal interactions between the LBD of RORα or RORγ and EBIP96 in a dose-dependent manner. In addition, based on the results of an immunoprecipitation assay, we demonstrated that BA remarkably enhanced the protein binding of RORγt with SRC-1, but failed to do so for RORγt lacking AF2 (Fig. 7). These results suggest that isoflavones enhance RORα- and RORγ-mediated transcriptional activity through the stabilization of the interactions between the AF2 of ROR and its co-activators, such as EBIP96 and SRC1, in immune cells.
In our previous study, we used PMA/ionomycin-stimulated EL4 cells to evaluate the influence of azole-type fungicides on Il17a gene expression, and also established RORα/γ knockdown EL4 (EL4/shRorac) cells using shRNA technology (Kojima et al., 2012). Similarly, in this study, we examined whether isoflavones affect Il17a gene expression in EL4 or EL4/shRorac cells. As a result, we found that four isoflavones enhanced Il17a mRNA expression in PMA/ionomycin-stimulated EL4 cells (Fig. 5). In addition, BA was found to potently enhance Il17a expression in EL4/shNC cells, but slightly affect in EL4/shRorac cells (Fig. 6). This may imply that the remained RORα/γ are involved in the slight enhancement of Il17a mRNA expression by BA. Taken together, these results suggest that isoflavones, including GE and BA, can positively modulate IL-17 production in a ROR-dependent manner. However, the isoflavone dose-dependency observed in Il17a expression assays using EL4 cells (Fig. 5) differs from the dose-dependency observed in reporter gene assays using CHO-K1 or Jurkat cells (Figs. 2–4). This may indicate a difference in response between endogenous RORs in EL4 cells and over-expressed RORs in CHO-K1 or Jurkat cells. In addition, it has been reported that the enhancement of Il17a gene expression might involve not only ROR-mediated activation, but also other additional mechanisms, such as the activation of aryl hydrocarbon receptor (AhR) and signal transducer and activator of transcription 3 (STAT3) (Rutz et al., 2013). Interestingly, GE was reported to be able to activate mouse AhR and STAT3 (Zhang et al., 2003; Chau et al., 2007). These findings appear to support our data showing that GE induced the most potent increase of Il17a mRNA expression in EL4 cells. Fig. 5 also shows that the effects of FN and DZ on Il17a mRNA expression in EL4 cells were not dose-dependent. Although we cannot clearly explain this, their effects may indicate unknown inhibitory effects on Il17a mRNA expression by high doses of FN and DZ, and may involve specific structural requirements such as the lack of a hydroxyl group at position 5 in isoflavones (Fig. 1).
Isoflavones are mainly found in legumes, especially in plants from the family Fabaceae such as soy and red clover (Coward et al., 1993), although they are also found in trace amounts in several fruits and plant seeds such as sesame and sunflowers (Liggins et al., 2000). According to many epidemiological studies, the daily intake of isoflavones among Southeast Asians ranges between 15 and 47 mg, while the Western population consumes only 0.15 to 1.7 mg of isoflavones per day (Medjakovic et al., 2010). Although the physiological concentrations reached during exposure to these isoflavones might be lower than the concentrations at which ROR activity is affected, an epidemiological study has reported that the mean concentration of GE in serum samples from Japanese men was 492.7 nM, compared with 33.2 nM in those from men in the United Kingdom (Morton et al., 2002). Many studies have provided evidence regarding multiple health and toxic effects of isoflavones (Setchell and Cassidy, 1999). Thus, isoflavones are speculated to be of great values with regard to the augmentation of the innate immune system through the enhancement of Th17 cell activity as well as possessing health benefits when used as supplements for the amelioration of symptoms associated with menopause.
The present study also provides clues to the structural design of potent and selective ROR ligands with potential applications to the treatment of metabolic and immune disorders. The development of ROR agonists represents a promising therapeutic strategy in the treatment of immunosuppressive diseases (Huh and Littman, 2012; Solt and Burris, 2012). RORα/γ agonists, such as isoflavones, enhance the beneficial functions of Th17 cells in fighting pathogens, and might be useful as lead compounds for the development of drugs for the treatment of immune dysfunction diseases involving the loss of Th17 cells. On the other hand, as the enhancement of RORα/γ activity might adversely affect Th17 cell function in autoimmune diseases, the effect on Th17 cells associated with the intake of isoflavones needs to be assessed in in vivo studies using autoimmune animal models, such as EAE. Further studies, including in vivo tests using mice, are needed to fully elucidate the effect of isoflavones on IL-17 production in the immune system.
5. Conclusion
Our findings indicate that dietary chemicals, such as isoflavones, may act as RORα/γ dual agonists to regulate Th17 cell function, leading to potential effects on the immune system in organisms. As the physiological roles of RORs are shown to more and more important, ROR agonists, such as isoflavones, might become valuable agents for the regulation of various diseases involving RORs.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research (C) (21590675 and 24590773) from the Japan Society for the Promotion of Science (JSPS). and by the Intramural Research of the NIH, NIEHS (AMJ; Z01-ES-100485)
Abbreviations
- AF
activation function
- ANOVA
analysis of variance
- BA
biochanin A
- CD-FBS
charcoal-dextran treated FBS
- CHO
Chinese hamster ovary
- CNS
conserved noncoding sequence
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- DZ
daidzein
- EAE
experimental autoimmune encephalomyelitis
- EDTA
ethylenediamine tetra-acetic acid
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- FN
formononetin
- G6Pase
glucose-6-phosphatase
- Gal
galactocidase
- GE
genistein
- HRP
horse-radish peroxidase
- IL-17
interleukin 17
- LBD
ligand-binding domain
- LUC
luciferase
- NCOA1
nuclear receptor co-activator-1
- NCOR1
nuclear receptor co-repressor-1
- NR
nuclear receptor
- PCR
polymerase chain reaction
- PMA
phorbol 12-myristate 13-acetate
- RIP140
receptor interacting protein 140
- ROR
retinoic acid receptor-related orphan receptor
- RORE
ROR response element
- PGC-1α
peroxisome proliferator-activated receptor γ co-activator-1α
- RT-PCR
reverse transcriptase-PCR
- shRNA
short hairpin RNA
- SD
standard deviation
- SDS
sodium dodecyl sulfate
- SDS-PAGE
SDS-polyacrylamide-gel electrophoresis
- SEM
standard error of mean
- SRC-1
steroid receptor co-activator-1
- Th17
T-helper 17
- TRE
tetracycline responsive element
- UAS
upstream activating sequence
Footnotes
Conflicts of interest
None.
Transparency document
The Transparency document associated with this article can be found in the online version.
References
- Chau MN, Touny EI, Jagadeesh LH, Banerjee S. Physiologically achievable concentrations of genistein enhance telomerase activity in prostate cancer cells via the activation of STAT3. Carcinogenesis. 2007;28:2282–2290. doi: 10.1093/carcin/bgm148. [DOI] [PubMed] [Google Scholar]
- Coward L, Barnes NC, Setchell KDR, Barnes S. Genistein and daidzein, and their β-glycoside conjugates: anti-tumor isoflavones in soybean foods from American and Asian diets. J Agric Food Chem. 1993;41:1961–1967. [Google Scholar]
- Fujita-Sato S, Ito S, Isobe T, Ohyama T, Wakabayashi K, Morishita K, Ando O, Isono F. Structural basis of digoxin that antagonizes RORγt receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J Biol Chem. 2011;286:31409–31417. doi: 10.1074/jbc.M111.254003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- He YW, Deftos ML, Ojala EW, Bevan MJ. RORγt, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 1998;9:797–806. doi: 10.1016/s1074-7613(00)80645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Littman DR. Small molecule inhibitors of RORγt: targeting Th17 cells and other applications. Eur J Immunol. 2012;42:2232–2237. doi: 10.1002/eji.201242740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RRV, Chow J, Manel N, Ciofani M, Kim SV, Cuesta A, Santori FR, Lafaille JJ, Xu HE, Gin DY, Rastinejad F, Littman DR. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Mckenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jetten AM. Retinoid-related orphan receptors (RORs): critical role in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- Jetten AM, Kang HS, Takeda Y. Retinoic acid-related orphan receptors α and γ: key regulators of lipid/glucose metabolism, inflammation, and insulin sensitivity. Front Endocrinol. 2013;4:1. doi: 10.3389/fendo.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Martynowski D, Zheng S, Wada T, Xie W, Li Y. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORγ. Mol Endocrinol. 2010;24:923–929. doi: 10.1210/me.2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B. X-ray structure of the hRORα LBD at 1.63 Å: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORα. Structure. 2002;10:1697–1707. doi: 10.1016/s0969-2126(02)00912-7. [DOI] [PubMed] [Google Scholar]
- Kojima H, Muromoto R, Takahashi M, Takeuchi S, Takeda Y, Jetten AM, Matsuda T. Inhibitory effects of azole-type fungicides on interleukin-17 gene expression via retinoic acid receptor-related orphan receptors α and γ. Toxicol Appl Pharmacol. 2012;259:338–345. doi: 10.1016/j.taap.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J, Hou X, Zhang J, Chen Y, Su Z. Identification of insulin as a novel retinoic acid receptor-related orphan receptor α target gene. FEBS Lett. 2014;588:1071–1079. doi: 10.1016/j.febslet.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Kurebayashi S, Nakajima T, Kim SC, Chang CY, McDonnell DP, Renaud JP, Jetten AM. Selective LXXLL peptides antagonize transcriptional activation by the retinoid-related orphan receptor RORγ. Biochem Biophys Res Commun. 2004;315:919–927. doi: 10.1016/j.bbrc.2004.01.131. [DOI] [PubMed] [Google Scholar]
- Liggins J, Blunk LJ, Runswick S, Atkinson C, Coward WA, Bingham SA. Daidzein and genistein content of fruits and nuts. J Nutr Biochem. 2000;11:326–331. doi: 10.1016/s0955-2863(00)00085-1. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Yamamoto T, Muraguchi A, Saatcioglu F. Cross-talk between transforming growth factor-beta and estrogen receptor signaling through Smad3. J Biol Chem. 2001;276:42908–42914. doi: 10.1074/jbc.M105316200. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O’Mally BW. Minireview: nuclear receptor coactivators—an update. Endocrinology. 2002;143:2461–2465. doi: 10.1210/endo.143.7.8892. [DOI] [PubMed] [Google Scholar]
- Medjakovic S, Mueller M, Jungbauer A. Potential health-modulating effects of isoflavones and metabolites via activation of PPAR and AhR. Nutrients. 2010;2:241–279. doi: 10.3390/nu2030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132:3168–3171. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood I, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz S, Eidenschenk C, Ouyang W. IL-22: not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, Jetten AM. RORα and RORγ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Burris TP. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol Metab. 2012;23:619–627. doi: 10.1016/j.tem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidović D, Schürer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Jetten AM. Prospero-related homeobox 1 (Prox 1) functions as a novel modulator of retinoic acid-related orphan receptors α- and γ- mediated transactivation. Nucleic Acids Res. 2013;41:6992–7008. doi: 10.1093/nar/gkt447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Kang HS, Freudenberg J, DeGraff LM, Jothi R, Jetten AM. Retinoic acid-related orphan receptor γ (RORγ): a novel participant in the diurnal regulation of hepatic gluconeogenesis and insulin sensitivity. PLoS Genet. 2014a;10:e1004331. doi: 10.1371/journal.pgen.1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Kang HS, Lih FB, Jiang H, Blaner WS, Jetten AM. Retinoid acid-related orphan receptor γ, RORγ, participates in diurnal transcriptional regulation of lipid metabolic genes. Nucleic Acids Res. 2014b;42:10448–10459. doi: 10.1093/nar/gku766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Takahashi T, Sawada Y, Iida M, Matsuda T, Kojima H. Comparative study on the nuclear hormone receptor activity of various phytochemicals and their metabolites by reporter gene assays using Chinese hamster ovary cells. Biol Pharm Bull. 2009;32:195–202. doi: 10.1248/bpb.32.195. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORγt protein. J Biol Chem. 2011;286:22707–22710. doi: 10.1074/jbc.C111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kumar N, Nuhant P, Cameron MD, Istrate MA, Roush WR, Griffin PR, Burris TP. Identification of SR1078, a synthetic agonist for the orphan nuclear receptor RORα and RORγ. ACS Chem Biol. 2010;5:1029–1034. doi: 10.1021/cb100223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Solt LA, Kojetin DJ, Burris TP. Regulation of p53 stability and apoptosis by a ROR agonist. PLoS one. 2012;7:e34921. doi: 10.1371/journal.pone.0034921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu B, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns K, Watowich SS, Tian Q, Jetten AM, Dong C. TH17 lineage differential is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellayi S, Naaz A, Szewczykowski MA, Sato T, Woods JA, Chang J, Segre M, Allred CD, Helferich WG, Cooke PS. The phytoestrogen genistein induces thymic and immune changes: a human health concern. Proc Natl Acad Sci U S A. 2002;99:7616–7621. doi: 10.1073/pnas.102650199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang J, Fang L, Zhou L, Wang S, Xiang Z, Li Y, Wisely B, Zhang G, An G, Wang Y, Leung S, Zhong Z. Increasing human Th17 differentiation through activation of orphan nuclear receptor retinoid acid-related orphan receptor γ (RORγ) by a class of aryl amide compounds. Mol Pharmacol. 2012;82:583–590. doi: 10.1124/mol.112.078667. [DOI] [PubMed] [Google Scholar]


