Abstract
Hepatitis C virus (HCV) is a common opportunistic pathogen especially among Human immunodeficiency virus (HIV) infected patients. Due to incongruous studies, the pathological effect of HCV on HIV induced disease are still not fully understood. While some studies have showed no effect of HCV on HIV infection, others reported a defined role of HCV in aggravating the rates of AIDS-related illnesses and mortality. The explanation of such variances may be due to the host immune response, viral genotypes, sub-type and quasi-species distribution. The factors that complicate the management of HIV/HCV patients are: (1) reduced HCV antibody production, (2) drug interactions, (3) liver disease and (4) different epidemiologic characteristics. However, it is abundantly clear that the morbidity and mortality caused by HCV have increased since the introduction of highly active antiretroviral therapy (HAART) against HIV. In this review, the consequence of HIV/HCV co-infection on host immune response, viral replication, disease progression, mortality and morbidity, viral load, persistence and current treatment options have been discussed. Based on the clinical studies, it is necessary to evaluate the effect of HCV therapy on HIV progression and to provide a fully active HCV treatment for patients receiving HIV treatment. In conclusion, it is recommended to provide fully active HAART therapy in combination with a known HCV therapy.
Keywords: Hepatitis C virus, HIV/HCV co-infection, HIV progression, Direct acting antivirals, Interferons
Introduction
The hepatitis C virus (HCV) belongs to family Flaviviridae and its infection is well known as a common infection, especially among human immunodeficiency virus (HIV) infected patients. Recent data showed that more than 35 million individuals worldwide are infected with HIV, while more than 150 million people are suffering from HCV infection [15].Due to their overlapping mode of transmission, the occurrence of HIV/HCV co-infection has increased dramatically. It has been estimated that around 5–7 million people are globally co-infected with HIV/HCV [21]. In the United States alone, around 25% HIV patients are co-infected with HCV. On the basis of genetic variations between HCV isolates, HCV is further classified into seven genotypes or clades (1–7) and 67 sub-types [44]. Genotype-4 (GT-4) is the most prevalent in the Saudi Arabia [32]. Genotype 3 is the most responsive while Genotypes 1 and 4 are known as less responsive. Sub-types of a particular HCV genotype are further divided into quasi-species based on their genetic diversity. The genomic composition of sub-types of a genotype varies between 20 and 25%. Subtypes 1a and 1b are the most widespread throughout the world and cause sixty percent of all cases. Several lines of evidences have supported the notion that viral genotypes are imperative in the outcome and required the duration of interferon (IFN) based therapy. The duration of the therapy is also variable, for example, standard IFN therapy for genotypes 1 and 4 is 48 weeks and for genotypes 2 and 3 are ~ 24 weeks. Sustained Virological Responses (SVRs) is, ~ 90% for genotypes 2 and 3, ~ 80% of genotype 6–70% of genotype 1, and 50% for genotype 4. Thus, with HCV-GT 1 and GT4 and the IFN resistance is a major issue. Infection with one genotype does not confer immunity against other GTs, and concurrent infections with two viral strains have been reported. In most of the cases, it has been observed that one viral strain removes the other from in a short time. This pronouncement opens the window of opportunity for hepatologist to replace non-responsive strains with easier to treat strains by mutual exclusion. The rate of liver-related complications among HIV/HCV co-infected patients has increased at an alarming rate in the western world. This is a leading cause of hospitalization and death in these countries among those patients associated with HIV coinfection. Latest survey has shown that 9% death in HIV patients happens due to this complication. The large number of hospitalization and death indicates the prevalence of viral hepatitis among HIV patients, despite the use of successful antiretroviral therapy [36]. The rate of co-infection with HCV has been reported to be up to 25% in HIV patients [36]. However, this rate is not always constant across the different populations globally. Compared to HCV mono-infected patients, A, co-infected patients have an increased risk of developing liver diseases like hepatocellular carcinoma, in absence of successful HCV treatment [23]. Interestingly, the level of HCV RNA increases due to absence of antiretroviral therapy and it has been observed that there is no linkage between HCV viremia and liver disease progression in co-infected individuals [40]. The end stage hepatic events and injury caused by drug use are the most important determinants for liver complications in HIV/HCV coinfected patients. Recently, the used antiretroviral drugs have lesser side effect and found to be safer in co-infected patients as compared to mono-infected individuals [39, 48]. It has been observed that, the chance of liver injury is less, if the eradication of HCV infection by antiviral drugs has been achieved before starting antiretroviral therapy. Direct acting antivirals (DAA) are the future hope for treatment of HCV infection in co-infected HIV/HCV patients due to their less side effect with better efficiency [45]. In those persons who are intravenous drug users or who received contaminated blood products, there is an increased risk of HIV/HCV co-infection. The efficient parenteral HCV transmission is behind the elevated rate of HIV/HCV co-infection. Recently, the intravenous drug abuse in Western Europe has been on the decline, since the eighties where it reached its peak, but it has been rapidly on the rise in Eastern Europe and in East Asian countries. Blood transfusion is a high-risk factor for HIV/HCV co-infection. Screening blood for both HCV and HIV co-infection has resulted in the reduction of transmission by blood transfusion in the most of the developed countries, but this is still not the case in many of the underdeveloped countries and as such, still is a main source of contamination. During the last decade, there has been a rising incidence towards the rate of HCV infection among homosexuals HIV patients. So, homosexuals are observed to be at higher risk of getting HCV infection as compared to mono-infected individuals [41]. Detection of acute hepatitis C is very essential for better treatment and management of HIV patients in co-infected individuals [50]. Additionally, threat among co-infected patients, there has recently been evidences which increases the occurrence of HCV re-infection among some HIV patients particularly among drug abusers [30] and homosexual men having multiple sex partners [24]. For this reason, people education, awareness and prevention strategy are a key aspect in HIV/HCV disease management.
Effect on immune response
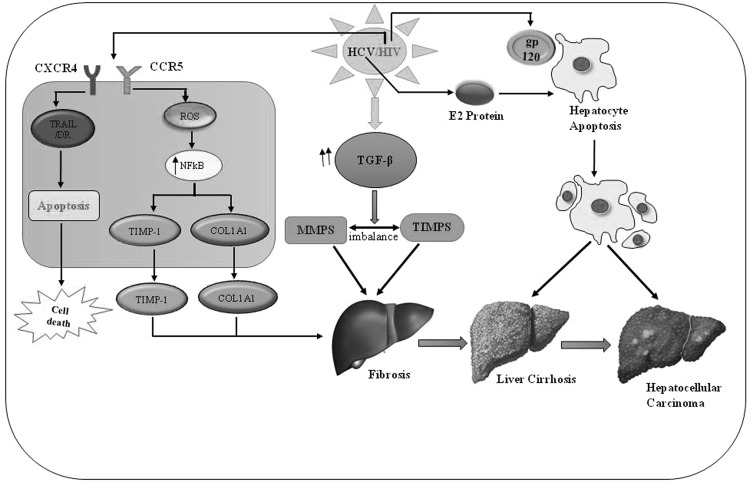
The consequence of immune response in HIV/HCV co-infection with respect to immune-regulatory mechanism is not well understood. The immune regulatory mechanism and their interaction with other regulatory molecules of the immune system are yet to be explored in HIV/HCV co-infected patients. The co-infection of HIV/HCV is persistently found to be associated with both extrinsic and intrinsic immune regulatory pathways. Example of extrinsic regulatory pathway include Fox P3+ CD4+ regulatory T cells, while intrinsic inhibitory pathway includes programmed death-1 (PD-1) and CTLA-4 (cytotoxic T lymphocytes antigen-4), with parentally reversible suppression of antiviral effectors T cells. There is an evidence of induction of multiple T cell inhibitory pathways in HIV/HCV co-infected patients. The T cell inhibitory pathway like FoxP3+ Tregs, PD-1 and CTLA-4 are inversely associated with overall CD4 T cells [7]. Both viruses, HIV and HCV are known to adopt the same route of infection. It is reported that the intensity of HCV infection is variable when patients are found to be HIV positive and the response of the immune system to the HCV infection exhibits various functional impairments and contributes to the liver damage and development of other diseases. The inflammatory response in the liver increases to a higher rate because of HCV infection. In HIV infected patient, it has been observed that the route of HCV infection and liver-related disease like liver cirrhosis and/or liver carcinoma are frequently occurs. This leads to higher death rates in HIV/HCV co-infected patients. It is also reported that, the response of T cells become weak in HIV/HCV co-infected patient and intact HCV specific immune response was more as compared to long-term HIV non-progressors. The IFN-α level in HIV/HCV co-infected patient was observed to be higher. In HIV/HCV co-infected patients, a rapid increase of TGF-β level, matrix metalloproteinases (MMPs) and tissue inhibitors was observed and finally resulted in development of fibrosis. It has been well known that viral envelop protein (gp 120) triggers the apoptosis of hepatocytes in vitro and causes liver damage. The hepatocyte apoptosis induces the inflammatory cytokine IL-8 production in hepatocyte-derived cell and stimulation gets higher by the viral encoded envelope proteins like HCV E2 and HIV gp120 (Fig. 1). The reduction of HCV RNA has been achieved upon using 10 kDa interferon-gamma-inducible protein in the first phase and finally a better response was observed against the chronic hepatitis by using this therapy [2]. Other than HIV envelop protein, it has been well known that HIV-1 Nef is transferred from expressing T cells to hepatocytic cells through conduits and enhances HCV replication. The extracellular Nef protein in HIV-1 infected individuals can pass out into hepatocytes and increase the size and shape of the lipid droplets inside the liver cells thus augmenting HCV replication and HCV mediated ROS production leading to HCC [34].
Fig. 1.
Schematic depicting the overall effect of HIV/HCV co-infection on progression of liver disease. TIMP1 (tissue inhibitors of metalloproteinase 1), COL1A1 (Collagen, type I, alpha 1), MMPs (matrix metalloproteinase), TRAIL (TNF-related apoptosis-inducing ligand), TGF-β (transforming growth factor beta), NFkB (nuclear factor kappa-B), ROS (reactive oxygen species), CCR5 (C–C chemokine receptor type 5)
Like a retrovirus, total three structural proteins known as Gag, Pol and Env and six accessory proteins Tat, Rev, Nef, Vpr, Vpu and Vif are encoded by HIV genome all these genes play a significant role in virus replication and pathogenesis. In vivo studies have shown that Nef is indispensable for HIV-1 pathogenesis. AIDS like disease can be developed by expressing the nef in mice and lower viral load and attenuated diseases in humanized mice, non-human primates and humans linked with nef deletion or defect [29]. Several crucial functions like protein trafficking, down-regulation of cell surface receptors, alteration of intracellular signaling, and enhancement of HIV-1 infectivity can be conferred by the localization of Nef on the plasma membrane. HIV-1 Nef plays a significant role in HIV/AIDS pathogenesis. Based on recent studies it has been observed that Nef can be transferred to neighboring cells and affects the biological function of these cells. Additionally, exosomes known as ChE+/CD81low/TSG101low and AChE-/CD81 high/TSG101 high play an important role in intercellular Nef transfer [29]. The HIV infection activates the HCV quasi-species compartmentalization both inside as well as outside the liver cells and particularly in monocytes/macrophages and lymphocytes.HIV infects the activated and proliferating CD4 T cells and after chronic HCV infection the HCV specific CD4 T cells continue to be activated and proliferate for a longer period. An elevated level of immune activation is associated with HIV infection resulting in an elevated level of transportation of activated CD8 T cells into liver cells by causing inflammation to liver. Due to HIV infection, dramatic changes occur in the TH1 to TH2 level resulting into alteration of immune response and cytokine profile after HCV infection [3].
Effect on virus replication
The enhanced replication of HCV is well known due to HIV infection in HIV/HCV co-infected patients [28]. HIV infection targets mainly to mono-nuclear leukocytes containing CD4, chemokine receptors CCR5 and CXCR4 cells (Fig. 1). Based on the published reports, it is well known that, the HCV replicates not only in hepatocytes, hepatocytic cell lines, and hematopoietic cells, but also in peripheral blood mononuclear cells (PBMC). It is also known that during natural infection, HCV binds to Dendritic cells receptors and replicates efficiently and these Myeloid Dendritic Cells serve as a reservoir for HCV replication. Due to the lack of reliable and efficient standardized methods to study the HCV replication with or without HIV infection has hampered the advances in this area and development of an effective disease management strategy. For this reason, there is an urgent need to elucidate the underlying mechanism behind the HIV/HCV interactions in order to design and develop effective antiviral therapies [35].
Effect on disease progression
HIV accelerates the progression of HCV infection by suppressing the immune system of immuno-compromised individuals. It has been reported that a drastic depletion of CD4 T cells occurs as a consequence of HIV infection resulting into the loss of immune response. It is worth noting that, during HIV/HCV co-infection, the rate of fibrosis and risk of liver cirrhosis increases, especially when the CD4 T cell counts becomes low with higher HCV viral load. It is strongly supported with published reports that the prevalence of liver-related diseases and death is higher in HIV/HCV co-infected patients as compared to mono-infected individuals [9]. Based on recent reports, after the introduction of highly active antiretroviral therapy (HAART), the life expectancy of HIV drug users (DUs) has been improved. However, this was not the case of HIV/HCV co-infected drug users who remained at a higher death risk from hepatitis/liver-associated diseases [43]. The death rate among HIV patient was lower after the introduction of combination of HAART. However, this was not the case among HIV/HCV co -infected patients, where the rate has increased [46]. HCV infection has become a major cause of death among HIV patients due to liver related disease. Recent data supports that cellular immunity gets hampered due to high liver inflammation and fibrosis in HIV/HCV co-infected patients. The prediction of liver fibrosis in HIV/HCV co-infected patients can be determined by using Aspartate aminotransferase (AST) and liver stiffness [16]. Recently, based on a meta-analysis results, it has been reported that the death rate among HIV/HCV co-infected patient was approximately 35% higher than those infected with HIV alone. But the mortality rate in AIDS patients is continuously increasing and is more than that of HIV-positive patient without being diagnosed with AIDS [33]. HCV infection is reported to reduce the CD4+ T cell response to HAART, resulting in a decrease in CD4+ T-cell counts. Additionally, the use of alcohol and drugs increases the high-risk of mortality rates in HIV/HCV co-infected patients. It is well known that chronic HCV infection alone can cause 50% mortality among AIDS patients globally [5].
Very recently a cross-sectional study was conducted by using Golden Gate assay in 219 patients to analyze the association between 2′5′oligoadenylate synthetase 1,2 and 3 (OAS1–3) and myxovirus resistance proteins 1 (Mx1) polymorphisms and severity of liver disease in human immunodeficiency virus (HIV)/hepatitis C virus (HCV)coinfected patients and it was observed that Mx1 and OAS1–2 polymorphisms were associated with the severity of liver disease in HIV/HCV-coinfected patients and results, suggested a significant role in the progression of hepatic fibrosis [14].
The co-infection of HCV in HIV patients accelerates the liver disease progression as compared to HCV mono-infection. Recent study suggests that the pool of natural killer cells significantly altered in HIV/HCV co-infection and might be also involved in rapid liver disease progression in co-infected patients [20]. Based on previous in vitro studies the hepatocyte function and HCV replication can be severely affected by soluble HIV proteins. It has also been reported that HIV can productively infect hepatocytes. In a recent study, the impact of HIV infection of hepatocytes on HCV expression was investigated and it was concluded that, the induction of HCV RNA synthesis and protein production in vitro can be modulated by HIV infection. Recently, in vivo study results revealed that the level of HCV RNA was elevated in HIV/HCV infected individuals as compared to those with HCV mono-infection. Based on the findings, the suppression of HIV could be a novel strategy in liver disease control [22].Very little information is available about the genomic basis of HIV/HCV co-infection and its regulation by microRNA (miRNA). Recently, the role of specific miRNAs in co-infection has been demonstrated which were correlated with important gene targets particularly playing a crucial role and functional relationships to many pathways in cancer, immune-function, and metabolism. A HCV-specific miR-122 in co-infection (FC450, p¼4.02E−06) was found to play a significant role in up regulation and alteration of biological functions [17].
Hemophiliacs with HIV/HCV co-infection
The HIV/HCV co-infection is a serious issue in hemophiliacs treated with clotting factors. It is estimated that 60–95% of the hemophiliacs who received clotting factor were infected with HIV. The progression of the HCV disease among co-infected hemophiliacs was observed to be rapid. Eyster and colleagues reported as higher incidence (8.8%) of liver failure among hemophiliacs infected with HCV than those HCV patients without hemophilia. In a cohort study of HCV infected hemophiliacs (40% co-infected with HIV), hepatic decompensation observed in 11 patients out of 181, whereas 10 of these 11 patients were co-infected with HIV. The hepatic decompensation rate was higher (21 times) in HIV co-infected patients than in HCV patients alone. Recently, in an interesting study conducted on hemophilic patients, it was observed that the HCV resides for longer time in Peripheral blood mononuclear cells (PMBC). The presence of HCV in PMBC can be attributed to therapeutic failure. So, care must be taken during treatment of hemophilic patients in case of co-infection. Thus, HIV alters the pattern of HCV progression, resulting in an increased mortality and morbidity rate among those individuals with co-infection [35].
Morbidity and mortality caused by HCV
The overall objective of HCV therapy is to permanently eliminate the risk of eventual development of hepatocellular carcinoma and liver failure during HIV/HCV co-infection, which poses a serious challenge for scientists and researchers. Among chronic hepatitis, co-infection with HIV accelerates the progression of disease and decreases the Spell out (SVR) rate to interferon based therapy. Newly introduced DAA therapy give hope to an increase in SVR. But in this case virological breakthrough or relapse may happen due to selection of preexisting resistance associated variants (RAV) [18]. Jabara et al. [18], conducted a study and based on the findings he concluded that genetic diversity of HCV is reduced in a well-controlled HIV co-infection indicating impaired cellular immunity but RAV frequency must not adversely affect the outcome of DAA therapy [18]. Pegylated interferon alfa-2a (PEG-IFN) plus ribavirin strategy has already been approved by FDA for treatment of HIV/HCV co-infected individuals. It has been observed that the binding of interferon takes place with specific cell-surface receptors in virus-infected cells resulting in rapid gene activation by protein–protein interaction. Inhibiting viral replication or translation, and viral assembly with release is mediated by the antiviral effects of interferon/Ribavirin. The mechanism for the antiviral effects is not well known but it is presumed that the interference happens through many guanosine-dependent processes. These include inhibiting the enzyme inosine monophosphate dehydrogenase and eventual interference with the replication of the viral nucleic acid. Studies have shown a marked difference in HIV/HCV co-infection groups with treatment of PEG-IFN vs. IFN and PEG-IFN in combination with ribavirin. Treatment with PEG-IFN and ribavirin was associated with the significantly higher rate of sustained virological response in comparison to treatment with IFN and ribavirin [6]. Additionally, in a study conducted and significant pancreatitis was observed in only one patient who was receiving didanosine. In this study, the same number of patients dropped due to laboratory abnormalities. Overall, most of the withdrawals were from the interferon plus ribavirin treatment and the least were from the PEG-IFN plus ribavirin treatment. In pegylated interferon groups, higher rate of neutropenia was observed resulted into significant differences [47].
Treatment options of HIV/HCV co-infection
Currently, multiple treatment options for HIV/HCV co-infections are available, but there is an urgent need for continuous education and awareness program about consequences of severe and acute HCV infection among HIV patients [31]. Antiviral treatment is equally important in preventing viral transmission of HIV/HCV co-infection [49]. The available ART treatment has been recommended for HIV/HCV co-infection as it delays the progression of liver cirrhosis despite of CD4+ T cell counts [1, 12]. But, the co-infected individuals receiving simultaneous treatment for both HIV and HCV, have a high pill burden besides an increased danger of unwanted drug–drug interactions and liver injury induced by drugs, especially in cases of advanced stages of liver cirrhosis. The treatment of co-infection by ART therapy should be utilized in a well-designed manner by reducing the drug–drug interactions with defined and standardized HCV therapy. Raltegravir and dolutegravir which are known as HIV integrase inhibitors have less side effects and drug–drug interactions. The use of antiviral agents against HCV protease can be minimized by using HIV protease inhibitors and non- nucleosides reverse transcriptase inhibitors. DAA are found to be a promising and another new option for treatment of HIV/HCV co-infected individual leading to the availability of interferon-free regimen with high SVR, even in cirrhosis patients having previous treatment failure. Currently, several new DAA are being use and many others are waiting for FDA approval [11]. DAA can provide shorter treatment options in case of patients having increased SVR rate (Table 1). A recent study showed that, after treatment with telaprevir, PEG-IFN and ribavirin therapy for 12 weeks against acute HCV genotype 1 infection, the SVR rate was found to be lower (84%) than in HIV infected patients [13]. It has been reported that the DAA plays a significant role, not only in treatment of HCV mono-infection but also in HIV/HCV co-infection by delaying the progression of fibrosis and hepatic decomposition. It is observed that, the use of first- generation HCV NS3/4A protease inhibitors like telaprevir or boceprevir with PEG-IFN and ribavirin resulted in a better response in HIV/HCV co-infected patients. Additionally, in the second phase of study conducted on HIV/HCV co-infected patients as it was observed that after 48 weeks of treatment, the SVR rate was found to be 63–74% respectively. In two spell out ANRS study with patients who did not responded well to interferon therapy, the ribavirin therapy has shown better efficiency. The SVR rate for telaprevir in combination with PEG-IFN and ribavirin was observed to be 83% after 48 week of treatment while boceprevir PEG-IFN and ribavirin treatment, the SVR was observed to be 56% [8]. Based on multiple study, it was observed that the protease inhibitors produces many adverse side effects including rashes and anemia because they were suggested to be taken three times per day resulted into negative The use of second generation HCV protease inhibitors showed better tolerability as compared to others. Simeprevir is a second-generation protease inhibitor. In a study conducted on HIV/HCV co-infected individuals by applying triple dose up to 12 weeks and the SVR was observed to be 57–74% as compared to null responders [10].
Table 1.
Properties of DAA in HCV genotypes
| S. no. | DAA | HCV genotype | Dose | Duration | Resistance barrier | References |
|---|---|---|---|---|---|---|
| 1. | Telaprevir | 1 | *** | • | L | [9] |
| 2. | Boceprevir | 1 | **** | •• | L | [1, 12] |
| 3. | Faldaprevir | 1,2,4 | * | • | L | [11] |
| 4. | Simeprevir | 1,2,4 | * | • | L | [11] |
| 5. | Sofosbuvir | 1-6 | * | •• | H | [8, 13] |
| 6. | Daclatasvir | 1-6 | * | •• | L | [10] |
| 7. | ABT-450 | 1 | * | • | L | [38] |
Dose: * 1 pill bid; duration: • 3 months; L, low; H, high; ABT-450 is an acylsulfonamide inhibitor of the NS3-4A serine protease
Faldaprevir is another protease inhibitor. A triple therapy with 1 day per dose was given on individuals with genotype 1 co-infection till 4 weeks and SVR rate was observed to be 74% after treatment. [38]. FDA has approved another DAA drug in December 2013, known as Sofosbuvir, which works as a direct acting NS5B nucleotide polymerase inhibitor. This has many novel characters like, potent antiviral activity against all HCV genotypes, high SVR rate, higher barrier to resistance and better tolerability as compared to other interferon-based therapies [19, 25]. Additionally, this avoids significant drug interaction with ART because; it does not rely on cytochrome P450 for metabolism. Overall synergetic approaches of applying two or more than two DAA found to have greater capacity to cure episodes of HCV mono-infections. This combination therapy was quite successful in LONESTAR study which was used in combinations of Sofosbuvir and NS5A inhibitor ledipasvir with or without ribavirin till 12 weeks and resulted into SVR rate of 95% in patients with genotype 1 infection [26]. More research need to be conducted to assess the contributions of multiple factors such as IL28B genotype in predicament of virological responses to all DAA [37].
Concluding remarks
In HIV/HCV co-infection, the replication of HCV increases resulting into liver disease development and contribute significant mortality in HCV infected patients globally. Since the start of the epidemic, approximately 70 million individuals got the infection with HIV with about 35 million deaths. Until the end of the year 2016, approximately, 36.7 million individuals were detected with HIV infection [15]. Although significant advances have been made in HIV/HCV treatments and development of multiple options for management of liver disease in HIV infected individuals but still its remain challenging due to many factors including limited resources, fragmented health care, and elevated level of injection drug use, alcohol use in the HIV-infected patient. The proportion of HIV individuals and HCV co-infected patients has been observed to be significantly similar due to similar transmission route of infection [4]. Bio-markers of HCV contact are present in a high percentage in HIV-infected individuals. HCV co-infection does appear to influence the rate of HIV progression but may be a surrogate for factors associated with HIV seroconversion. Based on analysis of data from several studies it is now well established that, the individuals co-infected with HIV and HCV have a higher risk of death than mono-infected patients. Therefore, it is necessary to evaluate the effect of HCV therapy on HIV progression and provide highly active treatment for patients receiving HIV/HCV treatment. The co-infection of HIV/HCV accelerates the faster disease progression to liver fibrosis and cirrhosis with a higher mortality rate as compared to HCV mono-infected individuals. Globally, the liver associated mortality has become a serious cause of death in HIV-positive individuals under combined anti-retroviral therapy (cART) [14, 20, 29, 42]. The immune-pathogenesis of liver disease in HIV/HCV coinfected patients is a multi-factorial process. Based on multiple published reports, the course of HCV infection worsens by HIV infection and increased the liver cirrhosis and hepatocellular carcinoma as well as elevated immunological defects and risk of comorbidities [27].
Acknowledgements
Authors would like to gratefully acknowledge the facility provided by King Fahd Medical Research Center (KFMRC), King Abdulaziz University, Jeddah, Saudi Arabia.
References
- 1.AIDSinfo. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. 2016. http://www.aidsinfonihgov/guidelines.
- 2.Askarieh G, Alsiö A, Pugnale P, Negro F, Ferrari C, Neumann AU, et al. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010;51(5):1523–1530. doi: 10.1002/hep.23509. [DOI] [PubMed] [Google Scholar]
- 3.Blackard JT, Yang Y, Bordoni P, Sherman KE, Chung RT. Team ACTGS. Hepatitis C virus (HCV) diversity in HIV-HCV-coinfected subjects initiating highly active antiretroviral therapy. J Infect Dis. 2004;189(8):1472–1481. doi: 10.1086/382959. [DOI] [PubMed] [Google Scholar]
- 4.Boesecke C, Rockstroh JK. Acute hepatitis C in patients with HIV. Semin Liver Dis. 2012;32(2):130–137. doi: 10.1055/s-0032-1316468. [DOI] [PubMed] [Google Scholar]
- 5.Branch AD, Van Natta ML, Vachon M-L, Dieterich DT, Meinert CL, Jabs DA, et al. Mortality in hepatitis C virus-infected patients with a diagnosis of AIDS in the era of combination antiretroviral therapy. Clin Infect Dis. 2012;55(1):137–144. doi: 10.1093/cid/cis404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chary A, Winters MA, Kottilil S, Murphy AA, Polis MA, Holodniy M. Impact of interferon-ribavirin treatment on hepatitis C virus (HCV) protease quasispecies diversity in HIV- and HCV-coinfected patients. J Infect Dis. 2010;202(6):889–893. doi: 10.1086/655784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho H, Kikuchi M, Li Y, Nakamoto N, Amorosa VK, Valiga ME, et al. Induction of multiple immune regulatory pathways with differential impact in HCV/HIV coinfection. Front Immunol. 2014 doi: 10.3389/fimmu.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotte L, Braun J, Sogni P, Vincent C, Valantin M-A, Vittecoq D, Michelet C, Batisse D, Morlat P, Gervais A, Pawlotsky J-M, Aboulker J-P, Molina J-M. High end-of-treatment (EOT) response rate with telaprevir-PegIFN-RBV in treatment-experienced HIV coinfected patients with HCV genotype 1: ANRS HC26 TelapreVIH study. In: 64th Annual Meeting of the American Association for the Study of Liver Diseases. 2013.
- 9.De Lédinghen V, Barreiro P, Foucher J, Labarga P, Castéra L, Vispo ME, et al. Liver fibrosis on account of chronic hepatitis C is more severe in HIV-positive than HIV-negative patients despite antiretroviral therapy. J Viral Hepat. 2008;15(6):427–433. doi: 10.1111/j.1365-2893.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 10.Dieterich D, Rockstroh J, Orkin C, et al. Simeprevir (TMC435) plus peginterferon/ribavirin in patients co-infected with HCV genotype-1 and HIV-1: primary analysis of the C212 study. In: 14th European AIDs conference. 2013.
- 11.Dorward J, Garrett N, Scott D, Buckland M, Orkin C, Baily G. Successful treatment of acute hepatitis C virus in HIV positive patients using the European AIDS Treatment Network guidelines for treatment duration. J Clin Virol. 2011;52(4):367–369. doi: 10.1016/j.jcv.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 12.EACS. European AIDS Clinical Society. Guidelines, Version 7.0. [online]. 2013. http://www.eacsocietyorg/Portals/0/Guidelines_Online.
- 13.Fierer DS, Dieterich DT, Mullen MP, Branch AD, Uriel AJ, Carriero DC, et al. Telaprevir in the treatment of acute hepatitis C virus infection in HIV-infected men. Clin Infect Dis. 2014;58(6):873–879. doi: 10.1093/cid/cit799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-Álvarez M, Berenguer J, Jiménez-Sousa MA, Pineda-Tenor D, Aldámiz-Echevarria T, Tejerina F, et al. Mx1, OAS1 and OAS2 polymorphisms are associated with the severity of liver disease in HIV/HCV-coinfected patients: a cross-sectional study. Sci Rep. 2017;7:41516. doi: 10.1038/srep41516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Global Health Observatory (GHO) data. http://www.who.int/gho/hiv/en/. Last accessed on 25.12.2017.
- 16.Gonzalez FA, Van den Eynde E, Perez-Hoyos S, Navarro J, Curran A, Burgos J, et al. Liver stiffness and aspartate aminotransferase levels predict the risk for liver fibrosis progression in hepatitis C virus/HIV-coinfected patients. HIV Med. 2015;16(4):211–218. doi: 10.1111/hiv.12197. [DOI] [PubMed] [Google Scholar]
- 17.Gupta P, Liu B, Wu JQ, Soriano V, Vispo E, Carroll AP, et al. Genome-wide mRNA and miRNA analysis of peripheral blood mononuclear cells (PBMC) reveals different miRNAs regulating HIV/HCV co-infection. Virology. 2014;450–451:336–349. doi: 10.1016/j.virol.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Jabara CB, Hu F, Mollan KR, Williford SE, Menezes P, Yang Y, et al. Hepatitis C Virus (HCV) NS3 sequence diversity and antiviral resistance-associated variant frequency in HCV/HIV coinfection. Antimicrob Agents Chemother. 2014;58(10):6079–6092. doi: 10.1128/AAC.03466-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 20.Kaczmarek DJ, Kokordelis P, Krämer B, Glässner A, Wolter F, Goeser F, et al. Alterations of the NK cell pool in HIV/HCV co-infection. PLoS ONE. 2017;12(4):e0174465. doi: 10.1371/journal.pone.0174465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim AY, Chung RT. Coinfection with HIV-1 and HCV—a one-two punch. Gastroenterology. 2009;137(3):795–814. doi: 10.1053/j.gastro.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong L, Welge JA, Powell EA, Blackard JT. HIV infection of hepatocytes results in a modest increase in hepatitis C virus expression in vitro. PLoS ONE. 2014;9(2):e83728. doi: 10.1371/journal.pone.0083728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacombe K, Rockstroh J. HIV and viral hepatitis coinfections: advances and challenges. Gut. 2012;61(Suppl 1):i47–i58. doi: 10.1136/gutjnl-2012-302062. [DOI] [PubMed] [Google Scholar]
- 24.Lambers FAE, Prins M, Thomas X, Molenkamp R, Kwa D, Brinkman K, et al. Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. AIDS. 2011;25(17):F21–F27. doi: 10.1097/QAD.0b013e32834bac44. [DOI] [PubMed] [Google Scholar]
- 25.Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13(5):401–408. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- 26.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383(9916):515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 27.Liberto MC, Zicca E, Pavia G, Quirino A, Marascio N, Torti C, et al. Virological mechanisms in the coinfection between HIV and HCV. Mediators Inflamm. 2015;2015:320532. doi: 10.1155/2015/320532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim K-A, et al. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134(3):803–811. doi: 10.1053/j.gastro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Luo X, Fan Y, Park I-W, He JJ. Exosomes are unlikely involved in intercellular Nef transfer. PLoS ONE. 2015;10(4):e0124436. doi: 10.1371/journal.pone.0124436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marco A, Esteban JI, Solé C, da Silva A, Ortiz J, Roget M, et al. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59(1):45–51. doi: 10.1016/j.jhep.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Martin TCS, Martin NK, Hickman M, Vickerman P, Page EE, Everett R, et al. Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS. 2013;27(16):2551–2557. doi: 10.1097/QAD.0b013e32836381cc. [DOI] [PubMed] [Google Scholar]
- 32.Nakano T, Lau GMG, Lau GML, Sugiyama M, Mizokami M. An updated analysis of hepatitis C virus genotypes and subtypes based on the complete coding region. Liver Int. 2012;32(2):339–345. doi: 10.1111/j.1478-3231.2011.02684.x. [DOI] [PubMed] [Google Scholar]
- 33.Omland LH, Jepsen P, Weis N, Christensen PB, Laursen AL, Nielsen H, et al. Mortality in HIV-infected injection drug users with active vs cleared hepatitis C virus-infection: a population-based cohort study. J Viral Hepat. 2010;17(4):261–268. doi: 10.1111/j.1365-2893.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 34.Park I-W, Fan Y, Luo X, Ryou M-G, Liu J, Green L, et al. HIV-1 Nef is transferred from expressing T cells to hepatocytic cells through conduits and enhances HCV replication. PLoS ONE. 2014;9(6):e99545. doi: 10.1371/journal.pone.0099545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parodi C, Belmonte L, Baré P, de Bracco MME, Ruibal-Ares B. Impact of human immune deficiency virus infection on hepatitis C virus infection and replication. Curr HIV Res. 2007;5(1):55–67. doi: 10.2174/157016207779316341. [DOI] [PubMed] [Google Scholar]
- 36.Puoti M, Moioli MC, Travi G, Rossotti R. The burden of liver disease in human immunodeficiency virus-infected patients. Semin Liver Dis. 2012;32(2):103–113. doi: 10.1055/s-0032-1316473. [DOI] [PubMed] [Google Scholar]
- 37.Rivero-Juarez A, Lopez-Cortes LF, Camacho A, Caruz A, Torres-Cornejo A, Martinez-Dueñas L, et al. The IL28B effect on hepatitis C virus kinetics among HIV patients after the first weeks of pegylated-interferon/ribavirin treatment varies according to hepatitis C virus-1 subtype. AIDS. 2013;27(12):1941–1947. doi: 10.1097/QAD.0b013e328360ea1e. [DOI] [PubMed] [Google Scholar]
- 38.Rockstroh NM, Soriano V, Arastéh K, Guardiola J, Bhagani S, Mallolas J, Tural C, et al. Start verso4 phase 3 trial of faldaprevir plus pegylated interferon α-2a and ribavirin in patients with HIV and HCV genotype-1 co-infection. 64th annual meeting of the American Association for the Study of Liver Diseases. 2013.
- 39.Rockstroh J, Teppler H, Zhao J, Sklar P, Harvey C, Strohmaier K, et al. Safety and efficacy of raltegravir in patients with HIV-1 and hepatitis B and/or C virus coinfection. HIV Med. 2012;13(2):127–131. doi: 10.1111/j.1468-1293.2011.00933.x. [DOI] [PubMed] [Google Scholar]
- 40.Rockstroh JK, Peters L, Grint D, Soriano V, Reiss P, Monforte A, et al. Does hepatitis C viremia or genotype predict the risk of mortality in individuals co-infected with HIV? J Hepatol. 2013;59(2):213–220. doi: 10.1016/j.jhep.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Sánchez C, Plaza Z, Vispo E, de Mendoza C, Barreiro P, Fernández-Montero JV, et al. Scaling up epidemics of acute hepatitis C and syphilis in HIV-infected men who have sex with men in Spain. Liver Int. 2013;33(9):1357–1362. doi: 10.1111/liv.12212. [DOI] [PubMed] [Google Scholar]
- 42.Sherman KE, Rockstroh J, Thomas D. Human immunodeficiency virus and liver disease: an update. Hepatology. 2015;62(6):1871–1882. doi: 10.1002/hep.28150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smit C, van den Berg C, Geskus R, Berkhout B, Coutinho R, Prins M. Risk of hepatitis-related mortality increased among hepatitis C virus/HIV-coinfected drug users compared with drug users infected only with hepatitis C virus: a 20-year prospective study. J Acquir Immune Defic Syndr. 2008;47(2):221–225. doi: 10.1097/QAI.0b013e31815d2f59. [DOI] [PubMed] [Google Scholar]
- 44.Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59(1):318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soriano V, Sherman KE, Rockstroh J, Dieterich D, Back D, Sulkowski M, et al. Challenges and opportunities for hepatitis C drug development in HIV-hepatitis C virus-co-infected patients. AIDS. 2011;25(18):2197–2208. doi: 10.1097/QAD.0b013e32834bbb90. [DOI] [PubMed] [Google Scholar]
- 46.Thein H-H, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22(15):1979–1991. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 47.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-García J, Lazzarin A, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351(5):438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 48.Vispo E, Fernández-Montero JV, Labarga P, Barreiro P, Soriano V. Low risk of liver toxicity using the most recently approved antiretroviral agents but still increased in HIV-hepatitis C virus coinfected patients. AIDS. 2013;27(7):1187–1188. doi: 10.1097/QAD.0b013e32835cb815. [DOI] [PubMed] [Google Scholar]
- 49.Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013;369(18):1715–1725. doi: 10.1056/NEJMsa1214720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webster DP, Wojcikiewicz T, Keller M, Castelnovo D, Mistry H, Gilleece Y, et al. Spontaneous clearance and treatment of acute hepatitis C infection in HIV-positive men with 48 weeks of interferon-alpha and ribavirin. Int J STD AIDS. 2013;24(3):179–183. doi: 10.1177/0956462412472317. [DOI] [PubMed] [Google Scholar]