Abstract
In the present study, an attempt was made to eliminate apple chlorotic leaf spot virus, apple mosaic virus, apple stem grooving virus and apple stem pitting virus from apple cultivar ‘Oregon Spur-II’. Thermotherapy was carried out at 37–40 °C for 4 weeks followed by culturing of meristems of different sizes. During establishment of explants, highest survival percentage (62.35%) and proliferation (30.68%) was recorded during summer season. However, size of meristems and position of buds from where meristems were excised also influenced their survival. The meristems of size 0.6–0.7 mm were found to be the most appropriate for maximum establishment. Meristems excised from buds positioned on distil portions of actively growing shoots showed better results. MS medium supplemented with BA (1.0 mg/l), IBA (0.05 mg/l) and GA3 (0.1 mg/l) resulted in 56.62% establishment of explants, while maximum number of meristems proliferated with low BA (0.5 mg/l), IBA (0.08 mg/l) and same GA3 concentration. Two to fourfold multiplication was observed. Virus indexing of shoots raised from different sizes of meristems was carried out and found that 0.3–0.6 mm size was able to eliminate ACLSV, ApMV, ASGV and ASPV. However, some of 0.5–0.6 mm sized shoots were found infected with ACLSV. Larger meristems could not completely eliminate the viruses under study.
Electronic supplementary material
The online version of this article (10.1007/s13337-018-0437-5) contains supplementary material, which is available to authorized users.
Keywords: Meristem culture, ELISA, Apple, Thermotherapy, Viruses
Introduction
Apple is infected with more than 20 viruses [35]. In commercially cultivated apple trees, apple chlorotic leaf spot virus (ACLSV), apple stem grooving virus (ASGV) and apple stem pitting virus (ASPV) are the most widely occurred viruses and usually appear in mixed infection, which greatly decrease the growth and productivity of infected trees [7]. In order to prevent the productivity and quality losses of fruit trees due to viral diseases, selection and development of healthy planting material is very important. Popular apple variety ‘Oregon Spur-II’ is recommended for higher altitudes of Himachal pradesh. It forms spur heavily. Fruit is medium to large, conical in shape and blushed with dark red colour, firm, juicy and sweet in taste. However, this variety is infected with many viruses such as apple mosaic, apple chlorotic leaf spot, apple stem pitting, apple stem grooving and prunus necrotic ringspot virus.
Tissue culture, the method of growing isolated tissues and organs, enables the elimination of plant viruses and the propagation of virus free materials [23, 24, 33, 36]. Meristem culture is a widely used method for virus eradication from horticultural plants [9]. Because of the uneven distribution of viruses in plants, it is anticipated that meristem would be virus free [32]. Therefore, it would be useful to determine the size of meristem tip related to higher efficiency of virus elimination. Mostly the apical meristems are often virus free but some viruses are known to actually invade the meristematic region of the growing tips, in that case it has also been possible to obtain virus free plants by combining meristem culture with thermotherapy.
Quite scanty efforts have been made to grow shoot meristem of critical minimum size to eliminate viruses in apple. Bhardwaj et al. [1] reported that apple mosaic virus could be detected in meristems longer than 0.2 mm while taking meristems ranging in size from 0.1 to 1.0 mm. Hu et al. [15] concluded that fourteen apple survival grafting plants from five varieties were detected virus free after subjecting ten apple varieties to thermotherapy coupled with in vitro meristem culture and in vivo shoot tip grafting. A major advantage of working with such a small explant is the potential that this holds for excluding pathogenic organisms that may have been present in the donor plants from the in vitro culture. A second advantage is the genetic stability inherent in the technique, since plantlet production is from an already differentiated apical meristem and propagation from adventitious meristems is avoided.
In addition, phenolics interfere with the virus and render them undetectable through biological means or traditional serological techniques. ELISA, therefore, offers an easy method for their detection as a prerequisite for any budwood certification programme. Modern day ELISA is one of the most widely used serological test for the detection of plant viruses. The test departs from the classical serologicial procedure in which immuno-precipitin reactions are used. Immuno-specificity is recognized through the action of the associated enzyme label on a suitable substrate antigen–antibody complex. The basic principle of the ELISA lies in immobilizing the antigen on a solid surface and probing with specific immunoglobulins carrying an enzyme label.
The purpose of the present study was to develop an efficient procedure for in vitro virus eradication in important apple variety ‘Oregon Spur-II’, considering the evaluation of different sizes of meristems, their survival and proliferation on different media combinations and further indexing of the virus to determine whether their propagation by tissue culture would yield virus free shoots.
Materials and methods
Survey of plant material
Survey were conducted in apple orchards of Regional Horticultural Research Station, Mashobra and fields of Department of Fruit Science of Dr. Y. S. Parmar university of Horticulture and Forestry Nauni, Solan (H.P.) to ascertain and identify the apple cultivar ‘Oregon Spur-II’ plants infected with apple mosaic virus (ApMV), apple chlorotic leaf spot virus (ACLSV), apple stem grooving virus (ASGV), apple stem pitting virus (ASPV) and prunus necrotic ringspot virus (PNRSV). Infected plants expressing prominent symptoms of apple mosaic virus and apple chlorotic leaf spot virus were used for initial plant materials.
Serological virus indexing of mother plants
Leaf samples of ‘Oregon Spur-II’ growing in above mentioned fields marked on the basis of visual symptoms of apple mosaic virus and apple chlorotic leaf spot virus were collected in spring season and were tested for the presence of APMV, ACLSV, ASGV (ACD, Inc) and ASPV, PNRSV (BIOREBA, Switzerland), through Double Antibody Sandwich Enzyme Linked Immunosorbent Assay (DAS-ELISA) as described by Clarks and Adams [5] with modifications. The results were assessed visually and colorimetrically at 405 nm in a microtiter plate reader (Micro Scan M S 5605 A, Electronics corporation of India Limited).
Source of meristems as explants
The tree samples which tested positive for different viruses were considered as the source of meristem isolation. Infected shoots were collected from these trees in spring/summer and dormant seasons and exposed to 37–40 °C for 4 weeks under hot air treatment. The apical and lateral buds positioned on the distal and basal portions of the shoots were harvested from heat treated shoots, rinsed with 70% ethanol for 40 s and then treated with 2% sodium hypochlorite for 15 min. They were rinsed four times with sterilized distilled water and remained in it until the excision of meristems.
Excision of meristems
Meristems were excised from buds of which as many bud scale leaves as possible were removed without the aid of microscope. The remaining bud scales were removed aseptically followed by leaf primordia to expose the growing point with the help of a stereomicroscope (Olympus). Each leaf primordium was dissected out from shoot tip, using separate sterile, fine, pointed forceps and needles. The meristematic dome with 1–4 leaf primordia were carefully excised after measuring the size.
For excision of meristems from dormant cuttings, axillary bud was trimmed first on both the sides, then tip portion was removed and finally it was cut length wise on the upper side, taking care not to damage the leaf primordia. Then, under the stereomicroscope, leaf primordia were removed subsequently with separate sterile needles as done above.
Establishment and proliferation of meristems
Immediately after dissection, meristems (0.2–0.7 mm) were placed in 15 × 20 cm glass culture tubes containing 12 ml of Murashige and Skoog medium [28] medium with 30 g/l sucrose and various plant growth regulators i.e. benzyladenine (BA, 0.5–1.0 mg/l), gibberellic acid (GA3, 0.1 mg/l) and indole butyric acid (IBA) or naphthalene acetic acid (NAA, 0.01–0.l mg/l) or indole acetic acid (IAA, 0.12–0.15 mg/l), which was further supplemented with adsorbent polyvinyl pyrolidone (PVP, 0.5 g/l) or antioxidant ascorbic acid (100 mg/l). All the media were solidified with 7 g/l agar. Whenever agar was omitted from the medium, the meristem explants were placed on filter paper supports contained in culture tubes.
After 6 weeks, viable cultures which showed first leaves were transferred on to newly prepared MS medium of same composition in 250 ml flasks with 30 ml medium, for further growth of stems and axillary shoots. The number of shoots formed on different sized meristems were counted before transfer. Length and number of meristem derived shoots were further increased by subculturing them on MS medium supplemented with different growth regulators i.e. BA (0.5–1.0 mg/l), IBA (0.01–0.1 mg/l) and GA3 (0.1–0.5). Each surviving culture was labeled so that it could be traced back to the size of original shoot tip/meristem explant. At least 20 explants per treatment were inoculated with respect to size of meristem, position of buds, season of the year and different combinations of growth regulators. Each experiment was done twice. The data on explant contamination, their establishment on medium with different growth regulator combinations as well as effect of size of the explants on their establishment and proliferation were recorded after 6 weeks of culturing. The effect of season of year and position of buds on shoots were also studied and data was recorded.
For virus indexing of established shoots, leaf samples were taken from labeled multiple shoot clusters raised from different sizes of meristems and subjected to DAS ELISA. Rest of the shoots in these clusters were maintained on the same medium.
Shoot multiplication of virus tested shoots
After indexing, the shoots which were found free from tested viruses were cultured for further multiplication on MS medium supplemented with various BA, GA3 and IBA combinations. The shoots which revealed positive results were discarded. Rate of shoot multiplication and shoot length were recorded after 5 weeks in each treatment. The rate was determined by counting the number of new shootlets produced per shoot. All the cultures were maintained in a temperature controlled room at 25 ± 2 °C. Light consisted of white fluorescent tubes and an incandescent lamp. The intensity of light at the level of cultures was 4000 lx. Photoperiod was 16 h light and 8 h dark.
Statistical analysis
The data recorded for the different parameters were subjected to completely randomized design, CRD [13]. Percent data was subjected to arcsine transformstions prior to statistical analysis. The statistical analysis based on mean values per treatment was made using analysis of variance technique (ANOVA) for CRD.
Results and discussion
Preliminary survey of mother plants
After conducting the survey of apple plantation, it was found that typical viral symptoms such as chlorotic leaf spot, mosaic and puckering of leaves were exhibited by the diseased trees. Chlorotic leaf spots were recorded to be the predominant symptoms at the location surveyed. Symptoms specific of the disease on the leaves indicated the presence of the ApMV and ACLSV. Overall, two trees (marked S4 and S21) showed positive reactions for ACLSV, ASGV & ASPV and ApMV, ASGV & ASPV respectively. Heat treatment to shoots of ‘Oregon Spur-II’ was given to eliminate viruses from infected plants because many viruses are sensitive to elevated temperatures. Kassanis [18] and Kassanis and Posnette [19] reported that about half of the viruses affecting horticultural plants could be eliminated by heat treatment. In the present studies, various factors like season for bud excision, position of source of explants, heat treatment and size of excised shoot tips seem to have great influence on the efficiency of virus eradication which is in agreement with Tan et al. [34].
Effect of size of meristems on their establishment
It has been observed that maximum number of meristems (37–49%) of the size range 0.2–0.4 mm could not survive because of desiccation, while small number of explants were dessicated in size range of 0.5–0.7 mm (Table 1). Negligible amount of phenols exuded from 0.2 to 0.5 mm sized meristems while the browning intensity increased with larger explants. Phenolic exudates were seen to accumulate around larger meristems cultured on solid medium while phenols rinses in liquid medium and less toxic which proved effective for establishment of primary meristem cultures. Jones and Hatfield [17] also used liquid medium with explants supported on filter paper bridges.
Table 1.
Effect of size of meristems on their establishment
| Sr. no. | Size of meristem (mm) | Degree of phenolic exudation on medium | Meristems died due to desiccation (%) | Meristems died due to contamination (%) | Percent establishment | No. of explants turned to callus | No. of explants proliferated | No of shoots developed per explant |
|---|---|---|---|---|---|---|---|---|
| 1. | 0.2–0.3 | − | 48.91 (44.38) |
31.52 (34.14) |
20.65 (27.00) |
14 | – | – |
| 2. | 0.3–0.4 | − | 37.14 (37.55) |
37.14 (39.49) |
25.71 (30.44) |
11 | 3 | 1–2 |
| 3. | 0.4–0.5 | − | 25.64 (30.41) |
42.30 (40.57) |
32.05 (34.48) |
14 | 6 | 1 |
| 4. | 0.5–0.6 | + | 17.07 (24.38) |
47.56 (43.60) |
32.92 (35.01) |
16 | 6 | 1 |
| 5. | 0.6–0.7 | ++ | 12.50 (20.70) |
48.86 (44.35) |
36.36 (37.08) |
17 | 9 | 1–2 |
| S.E. | 1.48 | 2.76 | 1.61 | |||||
| C.D.(0.05) | 3.31 | 6.15 | 3.60 |
Values in parenthesis are arc sine transformed values
S.E. standard error, C.D. critical difference, very less phenols; + less phenols; ++ more phenols
During initial establishment stage in each season, both fungal and bacterial (at cut ends) contamination were observed. Contamination rate increased with the increase in size of meristems because maximum contamination (47–48.86%) was observed in 0.5–0.7 mm sized shoot tips while minimum (31.52%) in case of 0.2–0.3 mm. Rate of contamination with sizes 0.3–0.4 and 0.4–0.5 mm were at par with each other (Table 1). It has been suggested that the survival of meristem explants depends on their rate of microbial contamination and of explant browning, which pertain not only to the explants used for culture initiation but also to the physiological state of mother plants and to the season when explants are collected [8, 27].
In order to take advantage of virus free cells, the smallest possible meristem has to be taken but small explants donot regenerate into whole plants while large meristems do. These two factors have to be balanced to identify optimal explants size. In our study, it has been seen that after 5 weeks of culturing, 0.2–0.3 mm sized meristems were found to be relatively less prone to contamination but were comparatively difficult to establish. They initially resulted in 20.65% establishment, but after sometime, developed callus. Welander and Huntriester [40] also observed very less microbial contamination in 0.2–0.4 mm explants, which supports our results. Only the explants exceeding 0.3 mm in length formed shoots. Highest percentage of establishment (36.36%) was achieved when meristems of size 0.6–0.7 mm were cultured which also resulted in half of the explants proliferated to shoots, whereas others callused suggesting that size of the meristem is one of the most important factor governing its regeneration capacity. Our results are supported by Mangal et al. [25] who found that survival increased with the increase in size reaching 100% beyond 0.7 mm of meristem tip in case of carnation. The establishment frequency (32–33%) of meristems of size 0.4–0.5 and 0.5–0.6 mm were at par with each other. After establishment, more than half explants turned to callus while rest formed the shoots.
It was seen that the maximum survival percentage of 24.32 and 35.29 were observed in the buds collected both from basal and distal portions respectively of the actively growing shoots (Table 2). The results obtained here are similar with observations of various workers [16, 30, 40] who suggested that the meristems from growing shoot tips developed significantly better than the meristems obtained from dormant buds, the reason being that endogenous cytokinins regulate the growth of meristems from axillary buds [2]. On the other hand, in dormant season, 27.08 percent survival was found in meristems excised from the distal portion of shoots and showed better survival and growth, while 7.40 percent in case of basal buds, which is in agreement with the report of Golosin and Radojevic [12].
Table 2.
Effect of position of buds on shoots on meristem survival
| Type of shoots | Bud position on shoots | Survival percentage of meristems |
|---|---|---|
| Dormant shoots | Basal | 7.40 (15.73) |
| Distal | 27.08 (31.34) |
|
| Actively growing shoots | Basal | 24.32 (29.54) |
| Distal | 35.29 (36.44) |
|
| S.E. | 1.78 | |
| C.D.(0.05) | 4.10 |
Effect of growth regulators on establishment of meristem cultures
It is evident from Table 3 of Supplementary Material that growth regulators composition added in MS medium significantly affected the establishment of meristems. Out of twelve combinations attempted, maximum percent establishment i.e., 56.62% was found in combination 7 which contained 1.0 mg/l BA, 0.05 mg/l IBA and 0.1 mg/l GA3 followed by 37.50% in combination 4 containing 0.5 mg/l BAP, 0.08 mg/l IBA and 0.1 mg/l GA3. However, after first subculture, maximum number of meristems were proliferated in combination 4 with decreased BA and slight increase in IBA and considered the best. Similar growth regulators were used by previous workers [1, 42] in establishing the meristems of apple, however, concentrations of IBA and GA3 were different which may be dependent on different genotypes. Meristems cultured on MS medium supplemented with BAP and IAA showed very low frequency of establishment whereas three combinations of BA and NAA did not promote any growth of meristems while least survival (9.5%) was observed in 0.5 mg/l BAP, 0.05 mg/l NAA and 0.1 mg/l GA3. Here, it was seen that most of the meristems turned into callus and could not grow further. Callus formation may be due to the addition of NAA, although in low concentration.
It was further observed that when shoots with leaf rosettes were subcultured on fresh medium for their elongation and further growth, most of them turned brown and necrotic. Some remained green for a long time and showed very slow growth. As a result, very few meristems attained growth during initial stages and number of proliferated shoots brought to multiplication stage were decreased. After about eight weeks of culture, shoots regenerated from meristems reached the size of 0.4–0.7 cm.
In vitro shoot multiplication
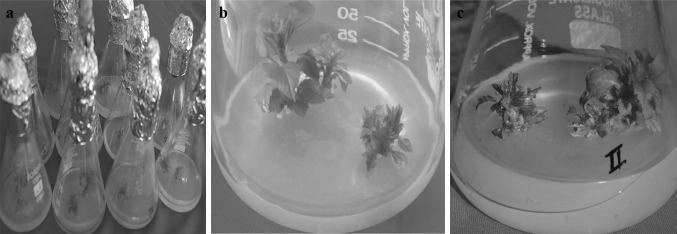
The proliferated explants resulted in multiple shoots in all the combinations tested (Table 4 of Supplementary Material; and Fig. 1a). Two to fourfold multiplication with 1.0–2.5 cm long shoots were observed on 0.5 mg/l BAP, 0.05 mg/l IBA and 0.1 mg/l GA3 supplemented MS medium (Fig. 1b). However, somewhat similar multiplication rate was found on combination 1 which contained 1.0 mg/l BAP, 0.01 mg/l IBA and 0.5 mg/l GA3 (Fig. 1c) but length of the shoots was shorter. The multiplication rates in combination 2, 3, 5 were at par with each other i.e., two to threefolds and approx. 0.5–2 cm long shoots. These results contrast to those reported by Ochatt and Caso [29] for meristems of M4 apple, because in ‘Oregon spur-II’, number of shoots per explant were increased when both BA and GA3 concentrations in multiplication medium decreased. Moreover, greatest size and internode enlargement made it possible to separate the shoots. A proliferation rate of 2.2 was observed in various apple rootstocks by Gabova [10]. The location of explants e.g., terminal versus lateral shoots can also affect the growth of the meristems and multiplication capability of the shootlets [26].
Fig. 1.
a Different sized meristem derived multiple shoots after indexing, b Shoots on MS multiplication medium with mg/l: BAP 0.5 + IBA 0.05 + GA3 0.1 and, c BAP 1.0 + IBA 0.01 and GA3 0.5
Virus indexing of in vitro established shoots
In our studies, virus indexing provided evidence that combination of thermotherapy alongwith meristem culture were efficient in elimination of viruses in ‘Oregon Spur-II’. These results support the findings of various workers [10, 14, 20] who were successful in eliminating viruses by meristem culture combined with thermotherapy. It was observed that shoots developed from 0.3 to 0.5 mm meristems were able to retrieve ACLSV, as the values are slightly more than negative control. However, one shoot from 0.5 to 0.6 mm size was found positive and other negative. All the shoots derived from 0.6 to 0.7 mm size meristems were still infected with ACLSV as indicated by colour reactions and OD values (Table 3). ApMV was found to be absent from shoots raised from meristems of the size range 0.3–0.6 mm while present in some of the shoots raised from 0.6 to 0.7 mm sized meristems as shown by their colour reaction and O.D. values.
Table 3.
Virus indexing of meristem derived shoots showing OD values
| Name of the virus | Size of meristems (mm) | |||
|---|---|---|---|---|
| 0.3–0.4 | 0.4–0.5 | 0.5–0.6 | 0.6–0.7 | |
| 1. ACLSV | 0.099 (−ve) | 0.113 (−ve) | 0.168 (−ve) | 0.201 (+ve) |
| 0.195 (+ve) | 0.231 (+ve) | |||
| 0.248 (+ve) | ||||
| 2. ApMV | 0.076 (−ve) | 0.124 (−ve) | 0.159 (−ve) | 0.193 (−ve) |
| 0.139 (−ve) | 0.213 (+ve) | |||
| 3. ASGV | 0.087 (−ve) | 0.098 (−ve) | 0.139 (−ve) | 0.174 (−ve) |
| 0.092 (−ve) | 0.116 (−ve) | 0.148 (−ve) | 0.181 (−ve) | |
| 0.125 (−ve) | 0.153 (−ve) | 0.210 (+ve) | ||
| 0.219 (+ve) | ||||
| 0.224 (+ve) | ||||
| 4. ASPV | 0.081 (−ve) | 0.102 (−ve) | 0.132 (−ve) | 0.166 (−ve) |
| 0.063 (−ve) | 0.113 (−ve) | 0.135 (−ve) | 0.170 (−ve) | |
| 0.117 (−ve) | 0.141 (−ve) | 0.178 (−ve) | ||
| 0.193 (+ve) | ||||
| 0.207 (+ve) | ||||
| *+ve control | **+ve control | ***+ve control | ****+ve control | |
| 0.312 | 0.302 | 0.293 | 0.311 | |
| *−ve control | **−ve control | ***−ve control | ****−ve control | |
| 0.097 | 0.103 | 0.104 | 0.093 | |
−ve shows absence of virus, +ve shows presence of virus
*ACLSV, **ApMV, ***ASGV and ****ASPV
In case of ASGV, all the shoots derived from meristem size range 0.3–0.6 mm were observed to show negative values which are either less than negative control or slightly more than it, hence were considered not to be carrying ASGV infection. However, some of shoots from 0.6 to 0.7 mm sized meristems were found negative and others positive for ASGV. Yamaga and Munakata [41] found it impossible to eradicate ASGV from 0.2 to 0.25 mm size shoot tips in infected apple tissuesby shoot tip culture alone but by combining heat therapy, bigger sized shoot tips at 1 mm size recommended. Our findings that ASGV and ApMV could be eliminated from 0.6 mm long shoot tips due to added heat treatment, supports the above report. In a previous report, all cultures regenerated from 1 mm long tips kept at 37 °C treated for 35 days were free of these viruses [34]. ASGV is more tolerant to high temperatures than ACLSV and the elimination of the virus could take a longer heat treatment period than that for ACLSV eradication [3, 21]. On the contrary, by using same heat treatment, it was possible to remove ASGV from 0.6 to 0.7 mm long shoot tips in ‘Oregon Spur-II’ while ACLSV was present in this size even smaller than that. It may be eliminated by using longer duration of heat treatment as shown in studies conducted previously [14] during the elimination of PNRSV and ACLSV while Welander and Huntrieser [40] recommended a combination of heat treatment for 2 weeks at 37 °C with shoot tip culture of apple and produced shoot tips free from heat tolerant viruses. Similarly for ASPV, it is clear from Table 3 that 0.3–0.6 mm sized meristems were observed to eliminate this virus. It has been observed that 0.3–0.4 mm sized meristems showed values less than negative control but as size increased, values also increased but less than double value of negative control. Only few meristems of size 0.6–0.7 mm were found infected with ASPV, while others were not. When we traced back the maintained cultures it has been found that shoots developed from shoot tips excised from axillary buds of distal portions of branches were found free of ApMV, ASGV and ASPV except ACLSV. Calculation of efficiency of heat treatment combined with meristem culture showed that all the shoots developed from 0.3 to 0.5 mm long tips were able to eradicate four viruses. Similar results were found with 0.5–0.6 mm long tips except for ACLSV. However, size range 0.6–0.7 was found less efficient (Table 4). Thermotherapy for 35 days significantly decreased the titer of viruses in cultures raised from tips of main and axillary shoots in apple [37]. These differences may be due to plant physiology and culture growth conditions which exert further influences on virus accumulation as shown by Knapp et al. [22]. They further reported that virus distribution was less localized in actively growing plants, whereas plants in the stationary phase accumulated higher virus levels which is in agreement with the present findings. Both ASGV and ACLSV showed high concentrations in the tip of the pear shoots and lower concentrations in the middle stem. However, the heat treatment was less effective to reduce virus titers in the bottom shoots [39]; whereas different distribution patterns of these viruses in apple plants were observed by Knapp et al. [22] who showed that ASGV and ACLSV distribute throughout the epidermis, cortex and vascular bundles tissues of apple stem, at higher levels in the basal stem and in lower levels towards the meristem tip of apple shoot. The different distribution patterns of both viruses in pear and apple shoots could be related to the host–virus interactions [11, 20, 21, 37].
Table 4.
Efficiency of thermotherapy combined with meristem culture on generation of virus free apple shoots of ‘Oregon Spur-II’
| eat treatment | Size of meristems (mm) | Efficiency of generating virus free plants | |||
|---|---|---|---|---|---|
| ACLSV | ApMV | ASGV | ASPV | ||
| 37–40 °C for 4 weeks. | 0.3–0.4 | 1/1 | 1/1 | 2/2 | 2/2 |
| 0.4–0.5 | 1/1 | 2/2 | 3/3 | 3/3 | |
| 0.5–0.6 | 1/2 | 1/1 | 3/3 | 3/3 | |
| 0.6–0.7 | 0/3 | 1/2 | 2/5 | 3/5 | |
A little is known about the mechanism how thermotherapy enhances virus eradication from shoot tips. Some early studies indicated that high temperature could augment the virus free areas by inhibiting virus replication or movement and improving the growth speed of treated plants [6, 18], which resulted in the subsequent virus eradication from meristem tips. Recently, it was found that viral RNA silencing could be significantly enhanced at the high temperatures [4, 31]. Similarly, RNA silencing was enhanced and viral RNA was degraded in shoot tips of raspberry during thermotherapy [38].
In conclusion, the present results indicated that DAS ELISA is a useful technique for virus indexing which facilitate the production of virus free propagation materials. High virus elimination efficiency in apple cultivar ‘Oregon Spur-II’ was achieved by a combination of thermotherapy and in vitro culture of up to 0.6 mm meristem tips which resulted in complete elimination of four viruses (ACLSV, ApMV, ASGV, ASPV). Further studies are underway to achieve efficient in vitro rooting and acclimatization of virus tested shoots, from which healthy plants could be produced.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Financial assistance provided by the ICAR—central assistance scheme, office of the Dean of college of Horticulture, Dr. YSP UHF Nauni, Solan, (H.P.), India is highly acknowledged.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13337-018-0437-5) contains supplementary material, which is available to authorized users.
References
- 1.Bhardwaj SV, Rai SJ, Thakur PD, Handa A. Meristem tip culture and heat therapy for production of apple mosaic virus free plants in India. Acta Hort. 1998;472:135–140. doi: 10.17660/ActaHortic.1998.472.13. [DOI] [Google Scholar]
- 2.Borkowska B. Variation in activity of cytokinin like compounds in apple buds during release during dormancy. Biochem Physiol Pflanzen. 1976;170:153–157. doi: 10.1016/S0015-3796(17)30194-4. [DOI] [Google Scholar]
- 3.Campell AI. Heat sensitivity of some apple viruses. Tagungsber. Dal DDR, Berlin; 1968. 97: 311–316.
- 4.Chellappan P, Vanitharani R, Ogbe F, Fauquet CM. Effect oftemperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 2005;138:1828–1841. doi: 10.1104/pp.105.066563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark AD, Adams AS. Characteristics of the microplate method of ELISA for detection of plant viruses. J Gen Virol. 1977;34:475–483. doi: 10.1099/0022-1317-34-3-475. [DOI] [PubMed] [Google Scholar]
- 6.Cooper VC, Walkey DGA. Thermal inactivation of cherry leafroll virus in tissue cultures of Nicotiana rustica raised from seeds and meristem tips. Ann Appl Biol. 1978;88:273–278. doi: 10.1111/j.1744-7348.1978.tb00706.x. [DOI] [Google Scholar]
- 7.Desvignes JC, Boye R. Different diseases caused by chlorotic leaf spot virus on the fruit trees. Acta Hort. 1989;235:31–38. doi: 10.17660/ActaHortic.1989.235.3. [DOI] [Google Scholar]
- 8.Dobranszki J, Magyar TK, Jambor BE, Lazanyi J, Buban T, Szalai J. Influence of aromatic cytokinins on shoot multiplication and their effects on rooting of apple cv. ‘Husveti Rozmaring’. Int J Hortic Sci. 2000;6:84–87. [Google Scholar]
- 9.Faccioli VC, Marani F. Virus elimination by meristem tipculture and tip micrografting. In: Hadidi A, Khetarpal RK, Koganezawa H, editors. Plant virus disease control. St. Paul: APS Press; 1998. pp. 346–380. [Google Scholar]
- 10.Gabova RN. Virus free pome fruits through meristem tip culture. Acta Hort. 1988;235:69–75. [Google Scholar]
- 11.Gella R, Errea P. Application of in vitro therapy for ilarvirus elimination in three prunus species. J Phytopathol. 1998;146:445–449. doi: 10.1111/j.1439-0434.1998.tb04779.x. [DOI] [Google Scholar]
- 12.Golosin B, Radojevic L. Micropropagation of apple rootstocks. Acta Hort. 1987;212:589–591. [Google Scholar]
- 13.Gomez KA, Gomez AA. Statistical procedures for agricultural research. New York: Wiley; 1984. [Google Scholar]
- 14.Hanke V, Fischer C, Wolfram B. Production of virus free plant material for fruit breeding by in vitro methods. Gartenba. 1988;35(6):176–178. [Google Scholar]
- 15.Hu GJ, Dong YF, Zhang ZP, Fan XD, Ren F, Li ZN. Efficacy of virus elimination from apple by thermotherapy coupled with in vivo shoot tip grafting and in vitro meristem culture. J Phytopathol. 2017;165:701–706. doi: 10.1111/jph.12610. [DOI] [Google Scholar]
- 16.Huang SC, Millikan DF. In vitro micrografting of apple shoot tips. Hort Sci. 1980;15:740–741. [Google Scholar]
- 17.Jones OP, Hatfield SGS. Root initiation in apple shoots cultured in vitro with auxins and phenolic compounds. J Hortic Sci. 1976;51:495–499. doi: 10.1080/00221589.1976.11514718. [DOI] [Google Scholar]
- 18.Kassanis B. Effects of changing temperature on plant virus diseases. Adv Virus Res. 1957;4:221–241. doi: 10.1016/S0065-3527(08)60600-4. [DOI] [PubMed] [Google Scholar]
- 19.Kassanis B, Posnette AF. Thermotherapy of virus infected plants. Recent Adv Bot. 1961;1:557–563. [Google Scholar]
- 20.Knapp E, da Camara MachadoA, Puhringer Q, Wang H, Hanzer V, Weiss H, Katinger H, da Camara Laimer, Machado M. Localization of fruit tree viruses by immuno-tissue printing in infected shoots of Malus sp. and Prunus sp. J Virol Methods. 1995;55:157–173. doi: 10.1016/0166-0934(95)00033-Q. [DOI] [PubMed] [Google Scholar]
- 21.Knapp E, Hanzer V, Weiss H, Da Camara MachadoA, Weiss B, Wang Q, Katinger H, da Câmara Laimer, Machado M. New aspects of Virus elimination in fruit trees. Acta Hort. 1995;386:409–418. doi: 10.17660/ActaHortic.1995.386.56. [DOI] [Google Scholar]
- 22.Knapp E, Hanzer V, Weiss H, Da Camara MachadoA, Wang Q, Weiss B, Katinger H, Laimer da Camara Machado M. Distribution of apple chlorotic leafspot virus in apple shoots cultivated in vitro. Acta Hortic. 1995;386:187–194. doi: 10.17660/ActaHortic.1995.386.24. [DOI] [Google Scholar]
- 23.Knapp E, Hanzer V, Mendonca D, da Camara MachadoA, Katinger H, da Camara Laimer, Machado M. Improved virus detection in rosaceous fruit trees in vitro. Plant Cell Tissue Organ Cult. 1998;52:3–6. doi: 10.1023/A:1005987505435. [DOI] [Google Scholar]
- 24.Maliogka VI, Skiada FG, Eleftheriou EP, Katis NI. Elimination of a new ampelovirus (GLRaV-Pr) and grapevine rupestris stem pitting associated virus (GRSPaV) from two vitis vinifera cultivars combining in vitro thermotherapy with shoot tip culture. Sci Hortic. 2009;123:280–282. doi: 10.1016/j.scienta.2009.08.016. [DOI] [Google Scholar]
- 25.Mangal M, Bhardwaj SV, Sharma DR. In vitro plantlet regeneration from meristem tip culture in carnation cv. Scania. Crop Improv. 2001;28(1):14–18. [Google Scholar]
- 26.Mert C, Soylu A. Shoot location and collection time effects on meristem tip culture of some apple rootstocks. Pak J Bot. 2010;42(1):549–557. [Google Scholar]
- 27.Modgil M, Sharma DR, Bhardwaj SV. Micropropagation of apple cv. ‘Tydeman’s Early Worcestor’. Sci Hortic. 1999;81:179–188. doi: 10.1016/S0304-4238(98)00259-3. [DOI] [Google Scholar]
- 28.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant. 1962;15:473–479. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 29.Ochatt SJ, Caso OH. In vitro meristem culture of M4 apple in optimal nutrient medium. Plant Cell Tissue Organ Cult. 1982;2(1):39–41. doi: 10.1007/BF00033551. [DOI] [Google Scholar]
- 30.Ozturk O. Micropropagation of some apple clonal rootstocks. M.Sc. Thesis. Bursa: Uludag University, Institute of Science; 2004. p. 42.
- 31.Qu F, Ye XH, Hou GC, Sato S, Clemente TE, Morris TJ. RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana benthamiana. J Virol. 2005;79:15209–15217. doi: 10.1128/JVI.79.24.15209-15217.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quak F. Therapy of individual plants. In: De Bokx JA, van der Want JPH, editors. Viruses of potatoes and seed-potato production. Wageningen: Pudoc; 1987. pp. 151–161. [Google Scholar]
- 33.Sahar AY, Al-Dhaher MMA, Shalaby AA. Elimination of grapevine fanleaf virus (GFLV) and grapevine leaf roll- associated virus-1(GLRaV-1) from infected grapevine plants using meristem tip culture. Int J Virol. 2009;5:89–99. doi: 10.3923/ijv.2009.89.99. [DOI] [Google Scholar]
- 34.Tan R, Wang L, Hong N, Wang G. Enhanced efficiency of virus eradication following thermotherapy of shoot tip cultures of pear. Plant Cell Tiss Organ Cult. 2010;101:229–235. doi: 10.1007/s11240-010-9681-0. [DOI] [Google Scholar]
- 35.Wang J. Apple shoot tip and embryo culture. In: Chen Z, Evans DA, Sharp WR, Ammirato PV, editors. Handbook of plant cell culture. Perennial crop. New York, USA:Macmillan; 1990.
- 36.Wang QC, Valkonen JPT. Elimination of two viruses which interact synergistically from sweetpotato by shoot tip culture and cryotherapy. J Virol Methods. 2008;154:135–145. doi: 10.1016/j.jviromet.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Wang G, Hong N, Tang R, Deng X, Zhang H. Effect of thermotherapy on elimination of apple stem grooving virus and apple chlorotic leaf spot virus for in vitro cultured pear shoot tips. Hort Sci. 2006;41(3):729–732. [Google Scholar]
- 38.Wang QC, Cuellar WJ, Rajam Aki M, Hirata Y, Valkonen JPT. Combined thermotherapy and cryotherapy for efficient virus eradication: relation of virus distribution, subcellular changes, cell survival and viral RNA degradation in shoot tips. Mol Plant Pathol. 2008;9:237–250. doi: 10.1111/j.1364-3703.2007.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LP, Hong N, Wang GP, Xu WX, Michelutti R, Wang AM. Distribution of apple stem grooving virus and apple chlorotic leaf spot virus in infected in vitro pear shoots. Crop Prot. 2010;29(12):1447–1451. doi: 10.1016/j.cropro.2010.08.003. [DOI] [Google Scholar]
- 40.Welander M, Huntrieser I. Rooting ability of shoots raised in vitro from apple rootstock A2. Physiol Plantarum. 1991;53:301–306. doi: 10.1111/j.1399-3054.1981.tb04504.x. [DOI] [Google Scholar]
- 41.Yamaga H, Munakata T. Production of virus free apple planting stock by meristem culture. Biol Control Plant Dis. 1991;126:96–102. [Google Scholar]
- 42.Yepes LM, Aldwinckle HS. Micropropagation of thirteen Malus cultivars and rootstocks and effect of antibiotics on proliferation. Plant Growth Regul. 1994;15(1):55–67. doi: 10.1007/BF00024677. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.