Significance
Circadian clocks throughout the body control mammalian physiology. Disruption of circadian rhythms associated with modern 24/7 societies results in adverse metabolic consequences, but the underlying mechanisms are unknown. Internal desynchrony between the phase of central and peripheral clocks is believed to be a major contributor to these adverse consequences. Here, in the first direct test of this assumption, we show that a ∼6-h phase misalignment between circadian clocks in brain and peripheral tissues is insufficient to induce obesity and glucose intolerance in mice. These results suggest that the adverse consequences of circadian disruption may arise by multiple mechanisms, rather than arising from internal desynchrony alone.
Keywords: clock genes, suprachiasmatic nucleus, peripheral oscillator, PER2::luciferase, circadian
Abstract
Circadian disruption as a result of shift work is associated with adverse metabolic consequences. Internal desynchrony between the phase of the suprachiasmatic nuclei (SCN) and peripheral clocks is widely believed to be a major factor contributing to these adverse consequences, but this hypothesis has never been tested directly. A GABAergic Cre driver combined with conditional casein kinase mutations (Vgat-Cre+ CK1δfl/fl εfl/+) was used to lengthen the endogenous circadian period in GABAergic neurons, including the SCN, but not in peripheral tissues, to create a Discordant mouse model. These mice had a long (27.4 h) behavioral period to which peripheral clocks entrained in vivo, albeit with an advanced phase (∼6 h). Thus, in the absence of environmental timing cues, these mice had internal desynchrony between the SCN and peripheral clocks. Surprisingly, internal desynchrony did not result in obesity in this model. Instead, Discordant mice had reduced body mass compared with Cre-negative controls on regular chow and even when challenged with a high-fat diet. Similarly, internal desynchrony failed to induce glucose intolerance or disrupt body temperature and energy expenditure rhythms. Subsequently, a lighting cycle of 2-h light/23.5-h dark was used to create a similar internal desynchrony state in both genotypes. Under these conditions, Discordant mice maintained their lower body mass relative to controls, suggesting that internal desynchrony did not cause the lowered body mass. Overall, our results indicate that internal desynchrony does not necessarily lead to metabolic derangements and suggest that additional mechanisms contribute to the adverse metabolic consequences observed in circadian disruption protocols.
Circadian clocks provide endogenous control of daily rhythms in physiology and behavior. At the cellular level, circadian rhythms are controlled by cell-autonomous transcription–translation feedback loops. In mammals these feedback loops consist of a positive limb (heterodimers of BMAL1, CLOCK, and NPAS2) that induces expression of a negative limb (PERIOD1-3, CRYPTOCHROME1-2), which inhibit the binding of positive-limb proteins to E-box elements in their promoters (1–4). The importance of these gene products in transcription–translation feedback loops is demonstrated by the loss of rhythmicity in mice lacking all members of any of these gene families (4–9). The speed of the clock depends on the degradation rate of negative-limb proteins. Phosphorylation of PERIOD proteins by CASEIN KINASE 1 delta and epsilon (CK1δ/ε) is a major determinant of circadian cycle length (10–14).
The suprachiasmatic nuclei (SCN) directly control behavioral rhythms. The SCN are entrained to environmental lighting cycles by retinal photoreceptors. Cell-autonomous circadian clocks are also present throughout the body, and are synchronized (entrained) by rhythmic timing signals (Zeitgebers) controlled by the main clock in the SCN (15–18). Circadian rhythms in peripheral organs are controlled by the interaction of local tissue clocks and SCN-generated rhythms in physiology and behavior (19–21).
The importance of circadian rhythms is illustrated by the adverse health outcomes observed in humans engaged in shift work (22–24), who often live in a state of circadian disruption (25, 26). Although the underlying mechanisms are largely unknown, disruption of phase alignment between clocks in the SCN and periphery (internal desynchrony) (27) has been hypothesized as a major factor underlying the adverse consequences of shift work (17, 24, 28). Because physiological rhythms throughout the body are under the control of both the SCN and local clocks, internal desynchrony is expected to result in disruption of these rhythmic processes (29). Studies of circadian desynchrony using human subjects under controlled laboratory conditions show that altering the phase relationship between the timing of behavior and endogenous rhythms can result in poor metabolic regulation (30, 31). Several rodent studies indicate that circadian disruption leads to obesity (32–37). Although these studies used different circadian disruption protocols, internal desynchrony was a common factor. Conversely, in models of environmental circadian disruption, obesity can be prevented by restricting food availability to the night, an intervention that maintains internal synchrony in nocturnal rodents (refs. 36 and 38–41, but see ref. 42). Together, these and other studies have led to the widespread belief that internal desynchrony plays a major role in producing the adverse health consequences of shift work and circadian disruption (17, 28, 29, 43). Remarkably, this hypothesis has never been tested directly.
Here we describe a mouse model that allows an unprecedented opportunity to test the hypothesis that internal desynchrony between the phase of clocks in the brain and in peripheral tissues results in adverse metabolic consequences. We generated a chimeric mouse line with a discordant circadian system in which the intrinsic free-running period of the SCN was slowed by deletion of three alleles of CK1 in GABAergic cells (Discordant mice; Vgat-Cre+ CK1δfl/fl εfl/+), while the intrinsic period of clocks in the rest of the body was unaffected. In vivo studies reveal that these mice have a long behavioral period to which peripheral clocks were entrained, albeit with an advanced phase relationship compared with the timing of activity. Metabolic parameters were assessed in these mice and the role of internal desynchrony in driving metabolic alterations was subsequently tested in mice whose internal desynchrony was ameliorated by exposure to a lighting cycle with 2 h light and 23.5 h darkness. Surprisingly, the internal desynchrony in our mouse model did not result in obesity or glucose intolerance. These results indicate that internal desynchrony does not necessarily lead to adverse metabolic consequences, and suggest that internal desynchrony alone may not be sufficient to produce the adverse metabolic phenotypes observed in humans and animals exposed to chronic circadian disruption.
Results
Discordant Mice Have a Slow but Robust Central Circadian Clock.
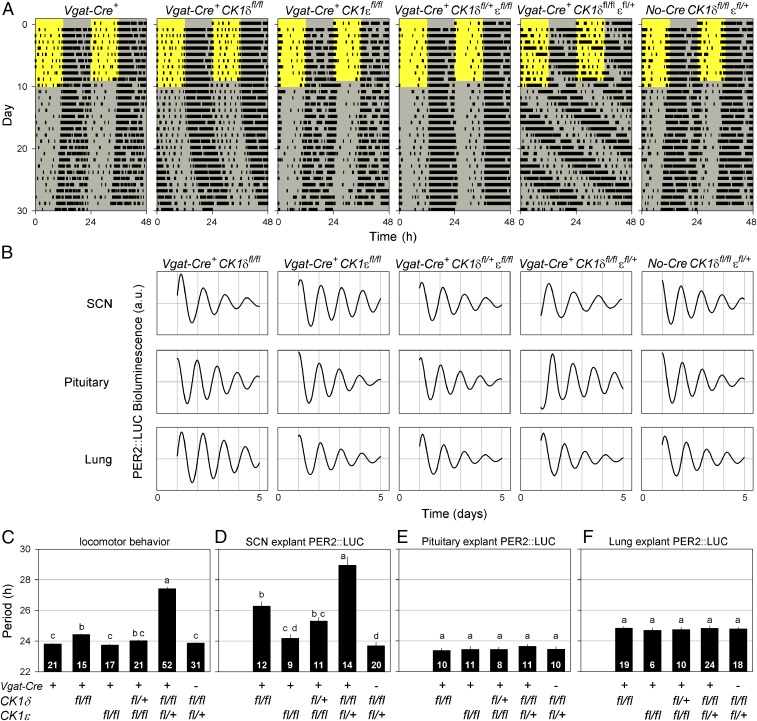
The GABAergic nature of SCN neurons (44) allowed the use of the Vgat-Cre+ driver to target conditional alleles of CK1δ and CK1ε (12) in the SCN and other GABAergic brain areas (45, 46), although not directly affecting circadian clocks in peripheral tissues. Deletion of different combinations of CK1δ/ε alleles in GABAergic neurons significantly lengthened the circadian period of both behavioral (F5,150 = 356.5, P < 0.0001; Fig. 1 A and C) and SCN-explant PER2::LUC bioluminescence rhythmicity (F4,63.67 = 36.14, P < 0.0001; Fig. 1 B and D), but did not significantly alter the PER2::LUC bioluminescence period of pituitary (F4,24.49 = 1.933, P = 0.1369; Fig. 1 B and E), lung (F4,71.92 = 0.1009, P = 0.9818; Fig. 1 B and F), or liver explants (F1,11.53 = 0.1334, P = 0.7216; Fig. S1E). The greatest lengthening of period was observed in the behavioral (27.4 h) and SCN-explant PER2::LUC (28.9 h) rhythms of mice with deletions of both alleles of CK1δ and one allele of CK1ε (Vgat-Cre+ CK1δfl/fl εfl/+). Combining the Vgat-Cre+ driver with these conditional CK1δ/ε alleles thus resulted in the generation of chimeric mice with a discordant circadian system in which the intrinsic circadian period of the SCN was substantially longer than that in peripheral tissues.
Fig. 1.
Disruption of CK1δ/ε alleles in GABAergic cells alters the period of behavioral and SCN rhythms but not peripheral clocks. (A) Representative, double-plotted wheel-running actograms of different Vgat-Cre+ CK1δ/ε genotypes illustrate the role of CK1δ/ε in setting the speed of the circadian clock. Mice were housed in a 12-h light/12-h dark lighting cycle for 10 d, followed by 20 d in constant darkness. The light and dark phases are indicated by the yellow and gray background, respectively. (B) Representative PER2::LUC bioluminescence traces of SCN, pituitary, and lung explants for different genotypes. High-amplitude oscillations in all genotypes show that disruption of CK1δ/ε alleles does not impair molecular circadian oscillations. (C–F) Period comparisons between different Vgat-Cre CK1δ/ε genotypes of locomotor behavior (C) and PER2::LUC bioluminescence rhythms of SCN (D), pituitary (E), and lung explants (F). Deletion of CK1δ/ε alleles in GABAergic cells results in alterations in locomotor and SCN period but does not affect the endogenous period of peripheral clocks. Bar graphs are mean ± SEM. The number of animals per genotype is indicated at the base of each bar. Genotypes identified with different letters are significantly different.
Most Discordant mice were unable to entrain their activity rhythm to a 12-h light/12-h dark lighting cycle (Fig. 1A). This is likely due to the 24-h lighting cycle falling outside the limits of entrainment due to the long endogenous period of these mice. To test this hypothesis, we first determined whether Discordant mice respond appropriately to light exposure. Phase shifts in response to 1-h light pulses at different times of the circadian cycle did not differ between the genotypes examined (Vgat-Cre+; Vgat-Cre+ CK1δfl/+ εfl/fl; Vgat-Cre+ CK1δfl/fl εfl/+; genotype × time interval: F22,373.2 = 1.183, P = 0.2592; Fig. S1B). Subsequently, the limits of entrainment were examined directly by exposing mice to environmental lighting cycles with different periods (T-cycles). Cre-negative controls (No-Cre CK1δfl/fl εfl/+) entrained to 24-h but not to 28-h T-cycles, whereas Discordant mice (Vgat-Cre+ CK1δfl/fl εfl/+) entrained to 28-h but not to 24-h T-cycles (Fig. S1 C and D). Together, these experiments showed that the SCN and behavioral circadian rhythms of Discordant mice had a long period, but that the light responsiveness of the central clock was intact.
Internal Desynchrony Between SCN and Peripheral Clocks in Discordant Mice.
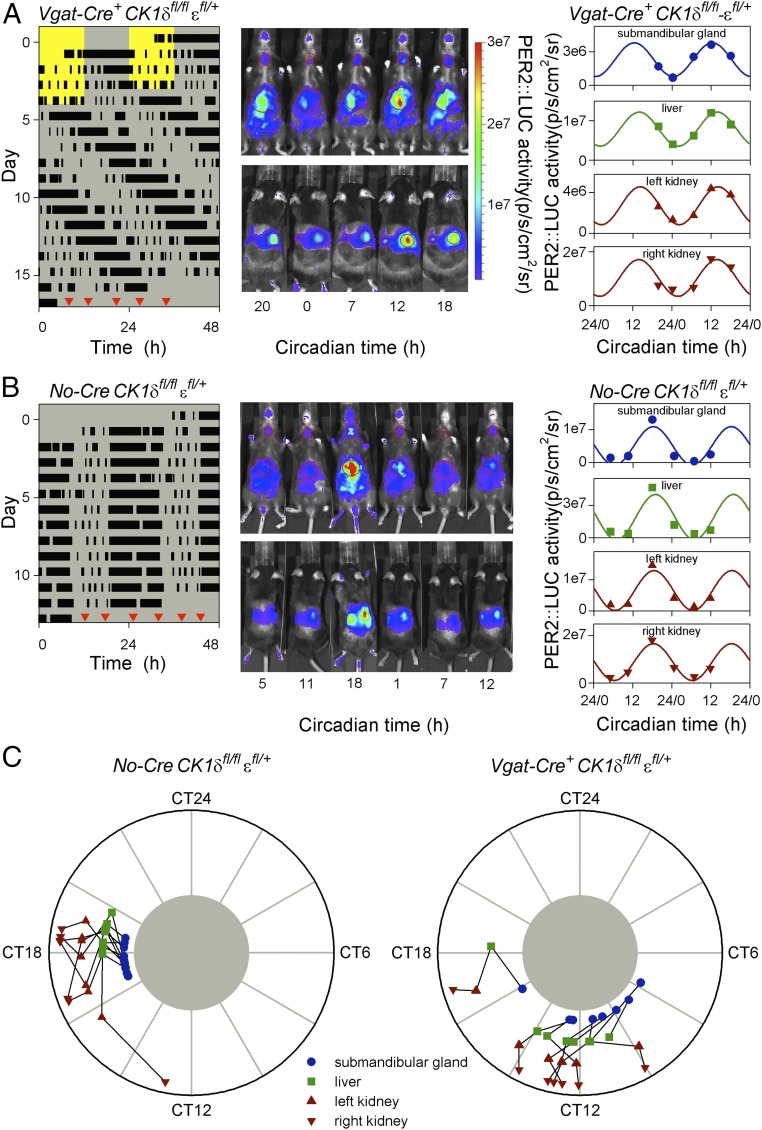
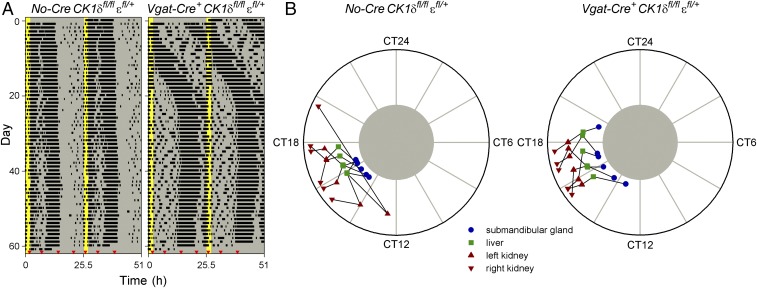
The internal phase relationship between SCN and peripheral clocks in vivo was assessed by measuring PER2::LUC bioluminescence in anesthetized mice at different times of day (47). After having been housed in constant darkness for 12–13 d to exclude interference by external Zeitgebers, PER2::LUC bioluminescence of the submandibular gland, liver, and kidneys was measured 5–6 times at ∼6-h intervals over ∼26–30 h (Fig. 2 A and B). Measurements of individual organs revealed reliable sinusoidal-shaped oscillations (Fig. 2 A and B and Fig. S2 B and C), with different peripheral organs in the same animal peaking at similar times (Fig. 2C). The phase of oscillations in all four organs was significantly clustered for both Discordant and Cre-negative control mice (Fig. 2C and Table S1), whereas the relative amplitude of these oscillations was not significantly different between genotypes (Fig. S2A). These observations showed that despite the difference in endogenous period between the SCN and periphery in Discordant mice, clocks in peripheral organs were entrained to a consistent phase in vivo. This consistent phase indicates that peripheral clocks were entrained to the long period dictated by the SCN, and to achieve this, peripheral clocks must be slowed by ∼3.5 h each circadian cycle.
Fig. 2.
Peripheral clocks of Discordant mice entrain to the long behavioral period with a shifted peak phase. (A and B) Representative examples of in vivo PER2::LUC bioluminescence imaging of a Discordant (Vgat-Cre+ CK1δfl/fl εfl/+ Per2::Luc/+; A) and Cre-negative control (No-Cre CK1δfl/fl εfl/+ Per2::Luc/+; B) mouse. The timing of running-wheel activity was measured for 12–13 d in constant darkness (actograms shown at Left) before mice were imaged 5–6 times at ∼6-h intervals from both the ventral and dorsal side (Center). Red triangles in each actogram indicate the order and timing of bioluminescence image collection, and images are shown from left to right in the order they were collected. Circadian time was determined by extrapolation of the onset of activity from the actogram. By definition, circadian time 12 corresponds to the onset of activity and a circadian cycle lasts 24 circadian hours. In vivo PER2::LUC bioluminescence was quantified for the submandibular gland, liver and each kidney (values from each measurement from these two animals are shown at Right). Cosinor curve fits with a period of 24 circadian hours are presented for each of the four organs. (C) Peak PER2::LUC bioluminescence phase plots of Cre-negative control (Left) and Discordant mice (Right) for submandibular gland, liver, and each kidney. PER2::LUC bioluminescence in peripheral organs peaked in the middle of the active phase (circadian time 18) for Cre-negative controls but was significantly phase advanced in Discordant mice (circadian time 12). The timing of peak PER2::LUC bioluminescence is depicted separately for each of the four organs, with black lines connecting different tissues of the same animal. CT, circadian time.
As expected, the long period of the SCN and behavioral rhythms resulted in a dramatic advance in phase angle of peripheral clocks relative to the behavioral rhythm (Fig. 2C and Table S1). Whereas PER2::LUC bioluminescence peaked in the middle of the active phase (circadian time 18) in Cre-negative controls, peripheral clocks in Discordant mice were phase advanced by ∼6 h, with PER2::LUC bioluminescence peaking around the onset of activity (circadian time 12). The in vivo measurement of peripheral clock phase thus revealed a state of internal desynchrony in Discordant mice, where the phase of peripheral clocks was severely misaligned with the timing of the SCN and behavior.
Internal Desynchrony Does Not Shift Body Temperature and Energy Expenditure Rhythms.
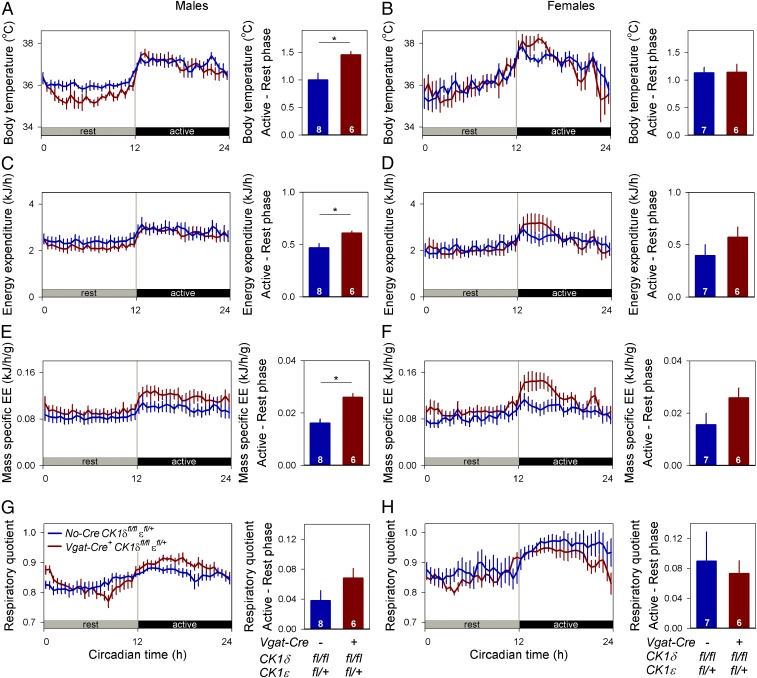
Based on the wealth of studies suggesting internal desynchrony influences metabolism, we expected metabolic consequences of internal desynchrony between the SCN and peripheral organs. One assessment of the consequences of internal desynchrony was made by comparing body temperature and energy expenditure. Measurements were collected throughout a single rest–activity cycle for Discordant and Cre-negative control mice housed in constant darkness with locomotor activity assessed by telemetry. Circadian rhythms in body temperature (Fig. 3 A and B) and energy expenditure (Fig. 3 C and D) were detected in both genotypes, with significant differences between the rest (circadian time 0–12) and active (circadian time 12–24) phase (Table S2). The phase of these rhythms was unaffected by genotype, with body temperature and energy expenditure rhythms being strictly aligned with the locomotor activity rhythm (Fig. 3 A–D). Minor but significant differences in amplitude were detected in the body temperature, energy expenditure, and mass-specific energy expenditure rhythms of males but not females (Table S2), with Cre-negative control males maintaining a higher body temperature throughout the resting phase (Fig. 3A and Fig. S3). Average metabolic state was assessed by comparing the mean body temperature, energy expenditure, mass-specific energy expenditure, and respiratory quotient over the full rest–activity cycle (Fig. S3). These comparisons did not reveal significant differences between genotypes, except for a small reduction in average body temperature in male Discordant mice (Table S2). In summary, body temperature and indirect calorimetry measurements did not reveal major changes in systemic metabolism between Discordant mice and their Cre-negative controls. The continued rhythmicity, with peak phases aligned with the behavioral active phase, suggested that rhythms in body temperature and energy expenditure were controlled by the SCN, and their phase was unaffected by the genetically induced internal desynchrony.
Fig. 3.
Phase of body temperature and energy expenditure rhythms are unaltered in Discordant mice. Body temperature (A and B), energy expenditure (C and D), mass-specific energy expenditure (E and F), and respiratory quotient (G and H) were measured throughout a full circadian rest–activity cycle in male (A, C, E, and G) and female (B, D, F, and H) mice housed in the absence of environmental rhythmicity (constant darkness). These metabolic circadian rhythms had similar timing relative to activity and rest in Discordant mice (Vgat-Cre+ CK1δfl/fl εfl/+, red) and in Cre-negative controls (No-Cre CK1δfl/fl εfl/+, blue). Small increases in the amplitude of the circadian rhythm in body temperature and energy expenditure were observed in male Discordant mice but not in females. Data are represented as mean ± SEM. Sample size per genotype is indicated at the base of each bar. Asterisks indicate significant differences between genotypes. EE, energy expenditure.
Discordant Mice Are Not Prone to Obesity and Glucose Intolerance.
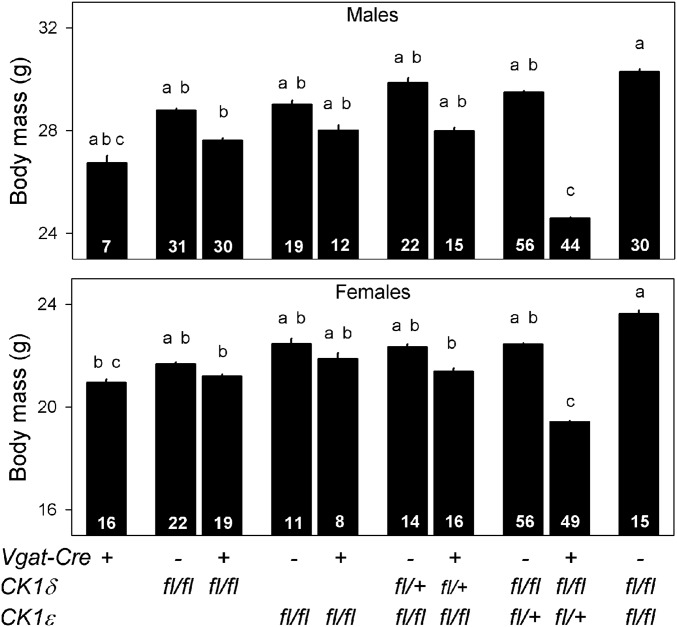
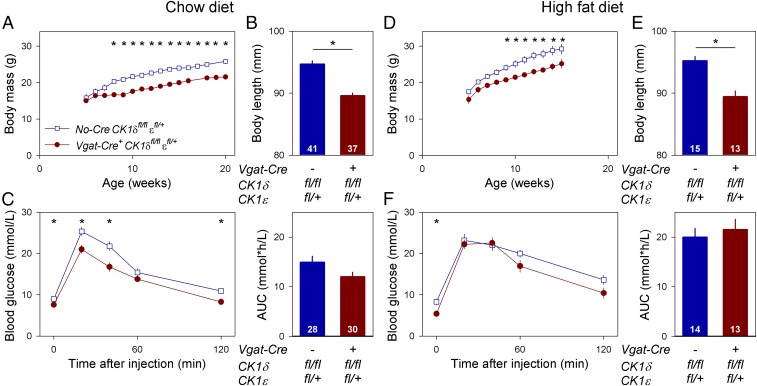
As noted previously, adverse metabolic outcomes were expected to result from internal desynchrony between clocks in the SCN and peripheral tissues. We next examined the body mass of different Vgat-Cre CK1δ/ε genotypes. As an initial assessment, all viable Vgat-Cre+ CK1δ/ε genotypes and their Cre-negative control lines were group housed in our colony room and exposed to a 12-h light/12-h dark lighting cycle and weighed repeatedly. A comparison of body mass at ∼125 d of age revealed significant differences between genotypes in both male (F9,254.3 = 12.75, P < 0.0001) and female mice (F9,214.2 = 11.01, P < 0.0001). Post hoc pairwise comparisons between each Vgat-Cre+ CK1δ/ε genotype and its Cre-negative control line showed that the only significant differences were observed in Discordant mice, with a 4.9 g and 3.0 g lower body mass in males and females, respectively (Fig. 4). These body mass differences were maintained in 18-mo-old mice (males: Discordant mice 31.6 ± 0.7 g, control mice 35.2 ± 1.0, t14 = 2.950 P = 0.0105; females: Discordant mice 24.9 ± 1.5 g, control mice 30.3 ± 1.1, t14 = 2.950 P = 0.0106). The surprising finding that body mass was dramatically reduced in Discordant mice suggested the possibility of metabolic disruptions in these mice.
Fig. 4.
Body mass comparison of different Vgat-Cre CK1δ/ε genotypes in male and female mice. Discordant mice (Vgat-Cre+ CK1δfl/fl εfl/+) had a significantly lower body mass compared with Cre-negative controls (No-Cre CK1δfl/fl εfl/+), whereas none of the other Vgat-Cre+ CK1δ/ε genotypes showed significant differences compared with its Cre-negative control group (Tukey HSD post hoc test). All mice were between 100 and 150 d old and group housed in a 12-h light/12-h dark lighting cycle. Bar graphs are mean ± SEM. Sample size per genotype is indicated at the base of each bar. Genotypes identified with different letters are significantly different.
One possible explanation for the lower body mass in Discordant mice is their lack of entrainment to the 12-h light/12-h dark lighting cycle (Fig. 1A) which would, over time, expose Discordant mice to light at all phases of the circadian cycle. To test the hypothesis that the lower body mass was a consequence of interactions with the lighting cycle, a follow-up experiment assessed differences in body mass, body length, and glucose tolerance in Discordant mice and Cre-negative littermate controls. To exclude the possibly confounding effects of disruptive environmental rhythmicity present in the previous experiment, mice were housed in constant darkness throughout life without access to a running wheel. Development in the absence of light exposure did not alter the free-running period of the locomotor activity rhythm as assessed by passive infrared motion detectors (Fig. S4A). Weekly weighing showed that Discordant mice had a significantly lower body mass starting at 8 wk of age (genotype × age: F15,367.1 = 6.137, P < 0.0001; Fig. 5A), and a significantly shorter body length as adults (F1,73.06 = 52.28, P < 0.0001; Fig. 5B). These differences could not be explained by changes in food intake (mass-specific food intake: genotype × age: F12,292.7 = 1.243, P = 0.2526; genotype: F1,24.42 = 1.699, P = 0.2046; Fig. S5 C and G) or the circadian phase of feeding (genotype × time interval: F11,132 = 0.6413, P = 0.7906; Fig. S4B). Blood glucose levels showed a significant circadian oscillation peaking during the rest phase (Discordant mice: F2,33 = 5.732, P = 0.0073; Cre-negative controls: F2,36 = 5.177, P = 0.0106), whereas no significant differences in rhythmicity were detected between genotypes (CircWave analysis: F3,70 = 2.120, P = 0.1055; Fig. S4C). Glucose tolerance tests performed in the middle of the resting phase revealed that Discordant mice were not glucose intolerant. If anything, glucose tolerance was slightly improved in Discordant mice relative to controls [area under the curve (AUC): F1,54.99 = 3.230, P = 0.0778] (Fig. 5C). Although the glucose dose administered was based on the animal’s weight, and this could confound comparison of groups that differ systematically in body mass, the small genotype difference in glucose tolerance was not due to the difference in administered glucose dose (Fig. S4D).
Fig. 5.
Internal desynchrony does not result in obesity and glucose intolerance. Discordant mice (Vgat-Cre+ CK1δfl/fl εfl/+, red) and Cre-negative littermates (No-Cre CK1δfl/fl εfl/+, blue) housed in constant darkness throughout their life were fed either regular chow (A–C) or high-fat diet (D–F). (A) Weekly body mass measurements from 5 to 20 wk of age revealed a significantly lower body mass in Discordant mice fed regular chow. (B) Body length (distance from nose to base of tail) was significantly shorter in Discordant mice fed regular chow. (C) Glucose tolerance tests performed in the middle of the rest phase on mice fed regular chow showed that glucose tolerance was not reduced in Discordant mice. The phase of activity was determined by passive infrared detectors. (D) Weekly body mass measurements from 5 to 16 wk of age revealed a significantly lower body mass in Discordant mice fed high-fat diet. (E) Body length (distance from nose to base of tail) was significantly shorter in Discordant mice fed high-fat diet. (F) Glucose tolerance tests performed in the middle of the rest phase on mice fed high-fat diet resulted in reduced glucose tolerance compared with chow-fed mice. The effect of the high-fat diet was not significantly worse in the Discordant genotype. Values represent the sex-corrected average of males and females. Data are represented as mean ± SEM. Sample size per genotype is indicated at the base of each bar. Asterisks indicate significant differences between genotypes at the indicated time point. AUC, area under curve.
To provide an additional metabolic challenge, a second cohort of mice was fed a high-fat diet starting at 5 wk of age. This resulted in significantly faster body mass increases in both Discordant (food type × age: F11,268.1 = 9.482, P < 0.0001) and Cre-negative control mice (food type × age: F11,288.9 = 4.742, P < 0.0001; Fig. S6 C and G), but the effect of the high-fat diet was not more pronounced in Discordant mice (food type × genotype × age: F11,557.2 = 0.9987, P = 0.4461). As with mice fed regular chow (Figs. 4 and 5A), Discordant mice had significantly lower body mass compared with Cre-negative controls when fed a high-fat diet (genotype × age: F11,271.2 = 2.311, P = 0.0101; Fig. 5D) and a significantly shorter body length (F1,20.52 = 69.65, P < 0.0001; Fig. 5E). Glucose tolerance tests showed that high-fat diet resulted in reduced glucose tolerance in both genotypes, but no significant differences in glucose tolerance were observed between genotypes (AUC: F1,20.66 = 0.1056, P = 0.7485; Fig. 5F). The metabolic phenotypes were similar in male and female mice (Figs. S5 and S6). In summary, these data show that Discordant mice were not more prone to develop obesity or become glucose intolerant compared with Cre-negative controls, even when challenged with a high-fat diet. Conversely, Discordant mice had a reduced body mass and, if anything, an improved glucose tolerance.
Reduced Size of Discordant Mice Is Not Caused by Internal Desynchrony.
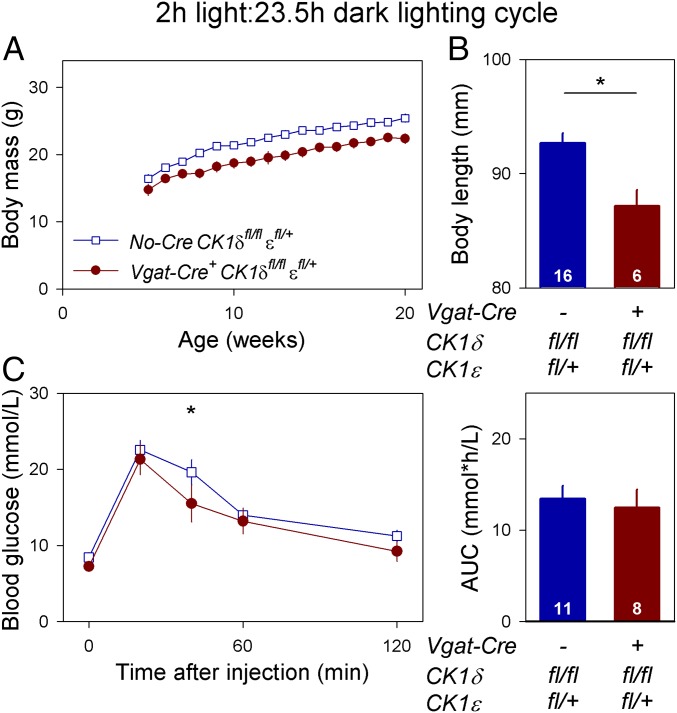
The reduced body mass and body length observed in Discordant mice could be caused by internal desynchrony or, alternatively, these phenotypes could be due to unrelated effects of the genotype. To distinguish between these possibilities, Discordant and Cre-negative control mice were both entrained to a T-cycle of 25.5 h with a short (2 h) light phase. This intermediate T-cycle entrained the behavioral circadian rhythm of both genotypes to a 25.5-h period with the active phase occurring during the dark phase, undisturbed by possible masking effects of light (Fig. 6A). Because both genotypes had genetically identical peripheral clocks and the SCN was entrained to the same 25.5-h period, Discordant and Cre-negative control mice were expected to maintain a similar internal phase relationship between SCN and peripheral clocks under these conditions. Indeed, peripheral PER2::LUC bioluminescence peaked in the first half of the active phase (circadian time 16) in both genotypes (Fig. 6B and Table S3). The similar state of internal desynchrony in Discordant and Cre-negative control mice did not significantly change the differences in body mass (lighting condition × genotype × age: F15,682.1 = 1.102, P = 0.3500; Fig. 7A), body length (lighting condition × genotype: F1,112.7 = 0.2376, P = 0.6269; Fig. 7B) and glucose tolerance (AUC: lighting condition × genotype: F1,70.43 = 0.2376, P = 0.6289; Fig. 7C) between the two genotypes. Overall, comparisons between Discordant and Cre-negative control mice, when both were entrained to a 25.5-h T-cycle, showed that the reduced body mass and body length of Discordant mice were not a consequence of internal desynchrony between SCN and peripheral clocks, but resulted from an unrelated pleiotropic effect.
Fig. 6.
Entrainment to a 2-h light/23.5-h dark lighting cycle induces a similar state of internal desynchrony in Discordant and Cre-negative control mice. (A) Representative, double-plotted wheel-running actograms of Cre-negative control (No-Cre CK1δfl/fl εfl/+ Per2::Luc/+) and Discordant (Vgat-Cre+ CK1δfl/fl εfl/+ Per2::Luc/+) mice housed in a 2-h light/23.5-h dark lighting cycle. This 25.5-h T-cycle entrained both genotypes, with the active phase uninterrupted by light. The light and dark phase are indicated by the yellow and gray background, respectively. The timing of in vivo PER2::LUC-bioluminescence imaging sessions is indicated by red triangles. (B) Peripheral organ in vivo PER2::LUC-bioluminescence peak-phase plots for Cre-negative control (Left) and Discordant (Right) mice. PER2::LUC bioluminescence in peripheral organs peaked at the same phase of the behavioral cycle in both Discordant and Cre-negative control mice. The timing of peak PER2::LUC bioluminescence is depicted separately for each of the four organs, with black lines connecting different tissues of the same animal. The timing of peak PER2::LUC bioluminescence is shown relative to the timing of behavior (as indicated by the circadian time scale) to ease the comparison of the internal desynchrony state between genotypes, even though the mice were exposed to a lighting cycle. CT, circadian time.
Fig. 7.
Rearing mice in a 2-h light/23.5-h dark lighting cycle does not reverse the reduced body mass and body length of Discordant mice. Discordant mice (Vgat-Cre+ CK1δfl/fl εfl/+, red) and Cre-negative littermates (No-Cre CK1δfl/fl εfl/+, blue) were housed in a 25.5-h T-cycle throughout their life. (A) Weekly body mass measurements between 5 and 20 wk of age show that Discordant mice maintained a lower body mass. (B) Body length (distance from nose to base of tail) was significantly shorter in Discordant mice. (C) Glucose tolerance tests performed in the middle of the rest phase revealed that glucose tolerance of Discordant mice was not worse than that of Cre-negative control mice. Values represent the sex-corrected average of males and females. Data are represented as mean ± SEM. Sample size per genotype is indicated at the base of each bar. Asterisks indicate significant differences between genotypes at the indicated time point. AUC, area under curve.
Discussion
Circadian disruption has been identified as a risk factor for the development of adverse metabolic outcomes (22–24, 30), but the mechanisms responsible for these adverse consequences remain unknown (43). The present study was designed to specifically test the hypothesis that internal desynchrony between circadian clocks in the brain and peripheral tissues results in adverse metabolic consequences. Discordant mice were generated using Cre-lox technology to lengthen the intrinsic period of the SCN while not altering peripheral clocks (Fig. 1). This resulted in internal desynchrony as shown by a dramatic ∼6-h phase advance of peripheral clock phase relative to the timing of behavior and systemic rhythmicity (Figs. 2 and 3). Unexpectedly, this type of internal desynchrony did not result in obesity or glucose intolerance, even when mice were tested in an environment without external timing cues (Figs. 4 and 5). The internal desynchrony between the phase of clocks in the brain and in peripheral organs produced in this model is thus insufficient to induce adverse metabolic consequences such as obesity and diabetes, although it remains possible that different types of internal desynchrony could induce adverse metabolic outcomes.
The absence of obesity and glucose intolerance in our Discordant mice contradicts the widely held belief that internal desynchrony between SCN and peripheral clocks is sufficient to result in adverse metabolic consequences (17, 28, 29, 43). This belief is based on experiments showing that discordance between the timing of activity and (peripheral) circadian phase is associated with adverse consequences on metabolic responses (30–33, 36, 37) and other health outcomes (48–52). Additionally, experiments restricting food intake to the night, thereby preventing internal desynchrony, ameliorated adverse metabolic outcomes (36, 38–40). These experiments suggested internal desynchrony as a causal factor in modulating the adverse consequences of circadian disruption, but, to our knowledge, the effects of internal desynchrony have previously only been tested in the presence of disruptive environmental timing signals. Our Discordant model appears to differ from other models of circadian desynchrony by being a relatively “clean” model of internal desynchrony; desynchrony is generated by intrinsic period differences between tissues, rather than through exposure to external timing cues. Other models are, to varying degrees, subject to external desynchrony (inappropriately timed light exposure and/or food intake). External desynchrony has been implicated as a potential contributing factor underlying the adverse consequences of circadian disruption (32, 39, 41, 51, 53, 54). One study appears to be an outlier from this literature by showing that daytime restricted access to a high-fat diet improved metabolic outcomes in mice, not only compared with unscheduled high-fat diet, but also compared with a group fed unscheduled low-fat diet consuming a similar number of calories (42). Food access was restricted to only 4 h per day in this study, however. The consequences of inappropriately timed food access may thus have been overcome by the prolonged daily fasting state (55). The present results, indicating an absence of adverse metabolic consequences in response to internal desynchrony between central and peripheral clocks, show that the adverse consequences of circadian disruption are likely the result of mechanisms other than internal desynchrony alone.
Another feature of the Discordant model that may contribute to the absence of adverse metabolic phenotypes is the relationship between central and peripheral intrinsic periods. In this model, peripheral tissues have a faster intrinsic period length than the SCN (∼24 h vs. ∼27.5 h, respectively). To maintain the stable phase relationship observed, the SCN must delay peripheral oscillators by several hours each cycle. Phase delays are more easily achieved than phase advances (56, 57), and the adverse consequences of repeated phase shifting are more pronounced with advances than with delays (50). Testing this directionality hypothesis will require development of new, chimeric animal models in which the SCN has a shorter period than the periphery, with the latter remaining genetically unperturbed.
A potential caveat in our assessment of the phase of peripheral tissues in anesthetized mice is that these animals had access to a running wheel for 12–13 d before imaging to precisely determine the timing of the locomotor activity rhythm. The wheel was not present on the day of measurement. It is possible that running wheel access increases the amplitude of internal Zeitgebers responsible for entrainment of peripheral oscillators, such as the body temperature rhythm. Thus, prior wheel access might influence the amplitude or phase of peripheral rhythms in the Discordant mice, such that the Discordant mice used for peripheral phase measurements may not have the same phase relationship between central and peripheral clocks as the Discordant animals used for metabolic analyses. This hypothesis could be tested using virally introduced reporters for long-term imaging of peripheral tissues (58) in freely moving Discordant mice, and would also be useful for assessing the long-term stability of peripheral tissue phase in this model.
Disruption of circadian rhythms by genetic mutations has been used to provide an increasingly detailed understanding of the importance of the circadian system in the regulation of metabolism. Initial reports documented obesity and altered blood glucose levels in mice with whole-body mutations of core clock genes Clock and Bmal1 (59, 60), while more recent studies have described adverse metabolic consequences following tissue-specific deletion of Bmal1 in the liver (61), pancreas (62–64), and adipocytes (65). Together, these studies illustrate the importance of core clock genes in maintaining proper metabolic health (66). Notably, however, these experimental paradigms cannot discern between the loss of transcription-factor activity and the loss of intrinsic rhythmicity resulting from clock gene deletion (67, 68). To circumvent these difficulties, the present study was performed using a chimeric mouse model in which peripheral organs remained effectively wild type.
Limiting mutations to GABAergic neurons allowed for a comprehensive assessment of the effects of different combinations of CK1δ/ε mutations on setting behavioral period (Fig. 1). In line with previous reports (11–13, 69, 70), deletion of both alleles of CK1ε did not result in substantial alteration of behavioral period. Mutation of both CK1δ alleles did increase the free-running period of our mice to 24.4 h, in line with previous cell- and tissue-culture experiments (12, 14, 69); it has not previously been possible to assess the impact of loss of CK1δ on behavioral rhythmicity because whole-body disruption of CK1δ is perinatal lethal (12, 71). Disruption of all four alleles of CK1δ/ε in GABAergic cells did not produce viable mice. Disruption of three of four CK1δ/ε alleles illustrated differences between these genes in setting circadian period. Although disruption of both CK1ε alleles and one allele of CK1δ in GABAergic neurons did not significantly change period, dramatic increases in circadian period (27.4 h) were evident following disruption of both alleles of CK1δ and one allele of CK1ε. The comparison between all of these genotypes clearly showed that CK1δ and CK1ε have overlapping roles in setting circadian period, with CK1δ exerting a relatively stronger influence compared with CK1ε (11–14).
Outside of its role in setting circadian period, CK1 has been shown to be involved in numerous noncircadian processes (72, 73). The reduced body mass and body length in Discordant mice (Figs. 4 and 5), unrelated to the state of internal desynchrony (Figs. 6 and 7), is likely a consequence of altered signaling in one of these nonclock pathways. Vgat-Cre is expressed in GABAergic neurons throughout the brain, including leptin-responsive cells in the arcuate nucleus that regulate food intake and energy homeostasis (45). Given the central role of leptin signaling in the regulation of body mass and metabolism, it is possible that alterations in arcuate nucleus leptin signaling could contribute to the lowered body mass in our Discordant mice. Similarly, it is possible that Discordant mice are protected from adverse metabolic consequences because of disruption of the casein kinase genes elsewhere in brain. Despite their lower body mass and body length (Fig. 5), however, Discordant mice were still able to become obese and glucose intolerant when fed a high-fat diet. This shows that the lower body mass of Discordant mice relative to Cre-negative controls was not due to a general inability to gain weight, confirming the suitability of Discordant mice as a model to study the metabolic consequences of internal desynchrony. Future studies with more localized disruption of the casein kinase genes could address sites and mechanisms by which CK1 disruption results in reduced body mass, and whether kinase gene disruption elsewhere in brain masks adverse consequences of internal desynchrony in the Discordant model. Generation of Cre-sensitive alleles of other genes involved in circadian period regulation could provide additional models of internal desynchrony to further test the extent to which our finding may be specific to mice with disruption of casein kinase genes.
Our characterization of metabolism focused on body mass, energy expenditure, and glucose clearance. Future work could extend our studies by assessment of additional physiological and molecular endpoints. Assessment of leptin signaling in the brain, insulin response, metabolic hormones and metabolites in serum, as well as clock and metabolism-related genes in peripheral tissues may help to further define the unexpected phenotypes of Discordant mice.
The discordant circadian system resulting from differences in endogenous period between tissues allowed us to study the role of the SCN in controlling peripheral rhythmicity. Previously it has been shown that SCN-controlled systemic rhythms can resynchronize peripheral clocks (74) and even drive a subset of rhythmic transcripts in an endogenously arrhythmic liver (19). The present results extend these findings and show that the SCN can impose its period on peripheral clocks, although the altered peripheral phase relationship illustrates the continued importance of endogenous peripheral clocks in setting an organ’s internal time (Fig. 2). Despite the large phase advance of peripheral clocks, feeding (Fig. S4B), body temperature, energy expenditure (Fig. 3) and blood glucose (Fig. S4C) rhythms, subject to both central and peripheral regulation, remained synchronized with the timing of locomotor activity. We anticipate that in the Discordant model, SCN rhythmicity, systemic physiology, food intake, and expression rhythms of peripheral genes driven by the timing of food intake remain relatively synchronized. Indeed, in genetic models of circadian rhythm disruption as well as in wild-type mice, the time of food availability plays a more important role in regulating peripheral gene expression rhythms than does the lighting cycle or the local clock (75–77). Overall, the present study suggests that although local clock gene expression is essential for an organ’s proper functioning (61–65), the internal time of peripheral tissues seems to be primarily dictated by timing signals emanating from the central clock. What role the local clock plays in setting the phase of peripheral gene expression rhythms remains to be determined and we believe that Discordant mice will provide a useful tool for discerning the competing roles of central and local clock control of peripheral gene expression.
Given the recent societal changes contributing to a higher frequency of circadian disruption (78), the incidence of associated adverse health outcomes is likely to increase. Unfortunately, the specific aspects of circadian disruption responsible for its adverse consequences are currently unknown. The hierarchical nature of the circadian system in mammals (20, 21) hinders the mechanistic interpretation of most circadian disruption studies because their experimental paradigms cause disruption at multiple levels (32–37). The present study was specifically designed to produce internal desynchrony between the SCN and peripheral clocks while not disrupting other aspects of circadian organization. It remains possible that other phase relationships between brain and peripheral clocks or internal desynchrony between other internal clocks (e.g., liver versus kidneys) is associated with adverse health outcomes (29). Conditional alleles of circadian-relevant genes allow site-specific alterations of the circadian system, generating paradigms to test the involvement of specific aspects of circadian disruption in causing its adverse consequences. These targeted approaches are essential to identify aspects of circadian disruption that are responsible for its adverse consequences. Mechanistic understanding of the proximate causes of these adverse consequences is essential in formulating rational countermeasures to help people cope with the physiological challenges posed by our contemporary 24/7 society.
Materials and Methods
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School or Williams College. All mice used in this study were generated by crossing previously described genotypes (12, 45, 79). Circadian locomotor activity rhythms were measured in individually housed mice in running wheel cages. PER2::LUC bioluminescence measurements in tissue explants were performed as previously described (80, 81). In vivo PER2::LUC bioluminescence measurement procedures were adapted from ref. 47 and involved ∼15-min isoflurane anesthesia of animals at 5- to 6-h intervals over 26–30 h and injection of luciferin (0.1 mL at 2.5 mg/mL, i.p.) followed by image capture. Mice used for body mass, size, and blood glucose measurements in constant darkness were housed in constant darkness throughout their life. More detailed descriptions of the methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Christopher Lambert, Jamie Black, and Mark Bingaman for technical assistance; Linh Vong and Brad Lowell for generously providing Vgat-Cre+ mice; and Joseph Takahashi for generously providing Per2::Luc/+ founder mice. This work was supported by NIH Grants R01 NS056125 and R21 ES024684 (to D.R.W.) and R15 HL120072 (to S.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712324115/-/DCSupplemental.
References
- 1.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gekakis N, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 3.Kume K, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 4.DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitaterna MH, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Horst GTJ, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 7.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng B, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 9.Bae K, et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 10.Lowrey PL, et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng QJ, et al. Setting clock speed in mammals: The CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etchegaray JP, et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol Cell Biol. 2009;29:3853–3866. doi: 10.1128/MCB.00338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walton KM, et al. Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J Pharmacol Exp Ther. 2009;330:430–439. doi: 10.1124/jpet.109.151415. [DOI] [PubMed] [Google Scholar]
- 14.Etchegaray JP, Yu EA, Indic P, Dallmann R, Weaver DR. Casein kinase 1 delta (CK1delta) regulates period length of the mouse suprachiasmatic circadian clock in vitro. PLoS One. 2010;5:e10303. doi: 10.1371/journal.pone.0010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 16.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 18.Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: Effects of transplanting the pacemaker. J Neurosci. 2006;26:6406–6412, and erratum (2006) 26. doi: 10.1523/JNEUROSCI.4676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 21.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans JA, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 2013;119:283–323. doi: 10.1016/B978-0-12-396971-2.00010-5. [DOI] [PubMed] [Google Scholar]
- 25.Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31:87–98. doi: 10.3109/07853899908998783. [DOI] [PubMed] [Google Scholar]
- 26.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–523. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- 27.Moore-Ede MC, Schmelzer WS, Kass DA, Herd JA. Internal organization of the circadian timing system in multicellular animals. Fed Proc. 1976;35:2333–2338. [PubMed] [Google Scholar]
- 28.Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: Findings from animal models. Best Pract Res Clin Endocrinol Metab. 2010;24:785–800. doi: 10.1016/j.beem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roenneberg T, Merrow M. The circadian clock and human health. Curr Biol. 2016;26:R432–R443. doi: 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris CJ, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA. 2015;112:E2225–E2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salgado-Delgado R, Angeles-Castellanos M, Buijs MR, Escobar C. Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience. 2008;154:922–931. doi: 10.1016/j.neuroscience.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 33.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23:372–381. doi: 10.1016/j.cub.2013.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coomans CP, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27:1721–1732. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 36.Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kettner NM, et al. Circadian dysfunction induces leptin resistance in mice. Cell Metab. 2015;22:448–459. doi: 10.1016/j.cmet.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–1029. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- 39.Hatori M, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barclay JL, et al. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS One. 2012;7:e37150. doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman H, et al. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 43.Turek FW. Staying off the dance floor: When no rhythm is better than bad rhythm. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1672–R1674. doi: 10.1152/ajpregu.00160.2008. [DOI] [PubMed] [Google Scholar]
- 44.Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver DR, et al. Functionally complete excision of conditional alleles in the mouse suprachiasmatic nucleus by Vgat-ires-Cre. J Biol Rhythms. doi: 10.1177/0748730418757006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tahara Y, et al. In vivo monitoring of peripheral circadian clocks in the mouse. Curr Biol. 2012;22:1029–1034. doi: 10.1016/j.cub.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Endo A, Watanabe T. Effects of non-24-hour days on reproductive efficacy and embryonic development in mice. Gamete Res. 1989;22:435–441. doi: 10.1002/mrd.1120220409. [DOI] [PubMed] [Google Scholar]
- 49.Filipski E, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 50.Davidson AJ, et al. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martino TA, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1675–R1683. doi: 10.1152/ajpregu.00829.2007. [DOI] [PubMed] [Google Scholar]
- 52.Preuss F, et al. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2034–R2040. doi: 10.1152/ajpregu.00118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.West AC, et al. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat Commun. 2017;8:417. doi: 10.1038/s41467-017-00462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- 55.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aschoff J, Hoffmann K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase-shifts of the zeitgeber. Chronobiologia. 1975;2:23–78. [PubMed] [Google Scholar]
- 57.Illnerová H, Vanĕcek J, Hoffmann K. Adjustment of the rat pineal N-acetyltransferase rhythm to eight-hour shifts of the light-dark cycle: Advance of the cycle disturbs the rhythm more than delay. Brain Res. 1987;417:167–171. doi: 10.1016/0006-8993(87)90194-6. [DOI] [PubMed] [Google Scholar]
- 58.Saini C, et al. Real-time recording of circadian liver gene expression in freely moving mice reveals the phase-setting behavior of hepatocyte clocks. Genes Dev. 2013;27:1526–1536. doi: 10.1101/gad.221374.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perelis M, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paschos GK, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18:1768–1777. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang G, et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med. 2016;8:324ra16. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu EA, Weaver DR. Disrupting the circadian clock: Gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 2011;3:479–493. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuchiya Y, et al. Effect of multiple clock gene ablations on the circadian period length and temperature compensation in mammalian cells. J Biol Rhythms. 2016;31:48–56. doi: 10.1177/0748730415613888. [DOI] [PubMed] [Google Scholar]
- 70.Smyllie NJ, Chesham JE, Hamnett R, Maywood ES, Hastings MH. Temporally chimeric mice reveal flexibility of circadian period-setting in the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 2016;113:3657–3662. doi: 10.1073/pnas.1511351113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Y, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 72.Knippschild U, et al. The casein kinase 1 family: Participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 73.Schittek B, Sinnberg T. Biological functions of casein kinase 1 isoforms and putative roles in tumorigenesis. Mol Cancer. 2014;13:231. doi: 10.1186/1476-4598-13-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo H, Brewer JM, Champhekar A, Harris RBS, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vollmers C, et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adamovich Y, et al. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19:319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atger F, et al. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc Natl Acad Sci USA. 2015;112:E6579–E6588. doi: 10.1073/pnas.1515308112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 2015;112:1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoo SH, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeBruyne JP, Weaver DR, Reppert SM. Peripheral circadian oscillators require CLOCK. Curr Biol. 2007;17:R538–R539. doi: 10.1016/j.cub.2007.05.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.