Abstract
Purpose
To investigate glaucomatous damage in KPro patients via structural analysis of the optic nerve head and digital planimetric quantification of Goldmann visual fields (GVF), a novel method of monitoring perimetric changes in KPro patients.
Methods
Records of patients undergoing KPro implantation from 2007 to 2015 at a single institution were reviewed. Parameters related to glaucoma status and KPro outcomes were analyzed.
Results
22 eyes from 21 patients met inclusion criteria, with mean follow up of 49.4 months (range 15–90). Mean results for the following parameters pre-KPro and at last follow-up were (pre-Kpro; at last follow-up): best-corrected visual acuity (BCVA) [2.07; 0.70 logMAR], number of glaucoma medications [1.14; 1.05], intraocular pressure (IOP) [18.4; 18.4 mmHg], vertical cup-to-disc ratio (C/D) [0.48; 0.50], and horizontal C/D [0.52; 0.52]. IOP-lowering procedures were performed pre-KPro (5/22), concurrently with KPro (10/22), post-KPro (6/22), or never (6/22). Increase in C/D ≥ 0.1 and loss of V4e isopter area > 30% occurred in 22.7% and 12.5%, respectively. Development of post-KPro glaucoma, progression of pre-existing or post-KPro glaucoma, and no glaucoma development as evidenced by objective assessment of structural and functional parameters were seen in 2/22 (9.1%), 7/22 (31.8%), and 6/22 (27.3%) eyes, respectively.
Conclusion
Clinicians should strive to vigilantly monitor for glaucoma despite the inherent difficulties in tonometry, optic nerve visualization and imaging, and visual field testing in KPro patients. Meticulous glaucoma surveillance with structural and functional testing combined with earlier IOP-lowering surgical intervention may result in decreased rates of glaucomatous vision loss in KPro patients.
Keywords: Boston Keratoprosthesis, keratoprosthesis, corneal transplant, glaucoma, secondary glaucoma
Introduction
Implantation of the Boston Keratoprosthesis Type I (KPro) is an established surgical treatment for a variety of corneal pathologies including recurrent corneal graft failure, infectious keratitis, chemical or thermal injury, and inflammatory keratitis.1 Contemporary advancements in KPro device implantation and its post-operative management have increased rates of success and long-term device retention.1 However, glaucomatous optic neuropathy associated with otherwise successful keratoprosthesis implantation may negatively impact visual outcomes. Glaucoma is the second most common complication of KPro implantation after retroprosthetic membrane formation and is a leading cause of permanent visual loss.1
Patients requiring KPro implantation are at particularly high risk of glaucoma for multiple reasons; the underlying ocular conditions necessitating KPro implantation may independently predispose to glaucoma (e.g. aniridia, thermal injury, chemical injury, or multiple prior ocular surgeries) and the corneal pathology often precludes adequate glaucoma surveillance (e.g. inability to adequately visualize optic nerve head and inability to reliably obtain intraocular pressure measurements).2 Furthermore, intraoperative factors (e.g. mechanical distortion of the iridocorneal angle at the time of KPro implantation) and post-operative factors (e.g. corticosteroid use, inflammation, or mechanical compromise of the iridocorneal angle) may also increase the risk of glaucoma.2–8
Previous retrospective studies, have revealed a high incidence of pre-existing glaucoma in KPro patients (40.2–89.3%); however, there is limited information regarding the development of de novo glaucoma and glaucoma progression after KPro implantation.2,5,7,9–21 In this study, the authors investigate glaucomatous optic neuropathy in KPro patients via structural analysis of the optic nerve head as well as digital planimetric quantification of Goldmann visual fields (GVF), a novel method of monitoring perimetric changes in KPro patients.
Materials and Methods
Study Design
Electronic medical records were reviewed from consecutive patients that underwent Boston Keratoprosthesis Type I implantation performed by one of two surgeons (M.S.C and J.D.C) at the University of Illinois at Chicago from 2007 to 2015. The study protocol was approved by the Institutional Review Board of the University of Illinois at Chicago and adhered to the tenets of the Declaration of Helsinki.
Participants
Inclusion criteria limited patients to those with ≥ 1 year of post-implantation follow-up and ≥ 2 separate good quality optic nerve head photographs separated by ≥ 6 months. Additionally, most included patients also had at least 2 visual fields separated by ≥ 6 months using a Goldmann perimeter (Haag-Streit International, Koeniz, Switzerland).
Patient records were analyzed for patient age, sex, ethnicity, ocular history including presence or absence of glaucoma, baseline pre-operative intraocular pressure (IOP), pre-operative best corrected visual acuity (BCVA), pre-operative number of glaucoma medications, and post-operative IOP, BCVA, and number of glaucoma medications within 1 month of the following time points – 1, 3, 6, 12, 18, 24, 30, 36, 48, 60, and 72 months or at last follow-up. Serial optic disc photographs were analyzed and the vertical and horizontal cup-to-disc ratios (C/D) were graded by an independent ophthalmologist.
Intraocular pressure measurement
Intraocular pressures were measured using scleral pneumotonometry (Mentor 30 Classic pneumatonometer; Mentor Inc, Norwell, Massachusetts, USA) and digital palpation techniques. When IOP was measured by both scleral pneumatonometry (performed 2mm posterior to the inferotemporal limbus) and digital palpation in a single clinical visit, preference was given to the IOP value obtained by scleral pneumatonometry unless there was documented concern regarding the reliability of the pneumatonometry measurement such as in excessive eyelid squeezing. The technique and utility of scleral pneumatonometry has been described previously.22,23 When IOP measurements by digital palpation were recorded as a range of values (e.g. 10–15 mmHg), the mean of the two values was used for statistical analysis. The proportion of patients with IOPs > 20 mmHg and > 25 mmHg on at least two separate clinical examinations was then determined.
Optic nerve head imaging and analysis
All optic nerve head photographs were performed by one of two experienced ophthalmic photographers. These photographs were subsequently examined by an independent glaucomatologist who was masked to all additional clinical data. Using stereopair images in the Axis Image Management software (Sonomed Escalon, New Hyde Park, NY) the examiner delineated the optic disc and cup margins and recorded the vertical and horizontal cup-to-disc ratios. Measurements were made using the software’s cup-to-disc measurement tool when available or calculated from millimeter rule measurements made on magnified images. Poor quality photographs in which the optic disc or cup margins could not reliably be delineated by the examiner were excluded.
Visual field testing and analysis
Goldmann visual fields were performed by experienced ophthalmic technicians. Goldmann visual fields were scanned as digital images (Figure 1) and analyzed in Adobe Photoshop CC 2015.1.1 (Adobe Systems Inc, San Jose, CA). Prior to analysis, the measurement scale for all visual fields was digitally calibrated such that the pixel distance from the visual field center to the 10 degrees radius was equivalent to 1.1 cm (Image: Analysis: Set measurement scale: Custom: Pixel length usually set at 94 pixels). This length was determined by using the ruler tool to measure the pixel distance from the visual field center to the 10 degrees radius: logical length = 1.1: logical units = cm). Under high digital magnification (400–700%), the magnetic lasso tool (Feather = 0 px, Width = 10 px, Contrast = 10%, Frequency = 85) was used to carefully delineate the outer edges of the technician-drawn lines for each isopter. If present, scotomas were also similarly delineated using the magnetic lasso tool, though with the “subtract from selection” option selected. The final total area was then calculated (Image: Analysis: Record measurements). Visual field deterioration was defined as a loss of more than 30% of V4e isopter area. The V4e isopter was delineated on all patients and was therefore used for progression analysis; additionally, it was thought to be the most reproducible and least extinguishable isopter given its high luminescence and large target size.
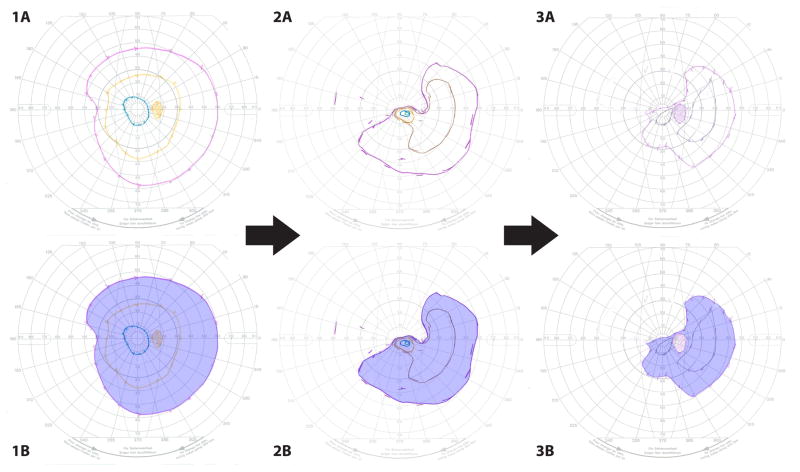
Figure 1.
Goldmann visual fields (GVF) in a patient who demonstrated progressive visual field constriction. The baseline Goldmann visual fields are depicted in components 1A–3A. Components 1B–3B highlight the V4e isopter; the areas bound by the V4e isopter were measured via digital planimetric quantification as described in the methods section (V4e isopter areas: GVF 1A/B = 99.15 cm2, GVF 2A/B = 56.23 cm2, and GVF 3A/B = 38.24 cm2)
Glaucoma analysis
Patients were considered to demonstrate progression of pre-existing glaucomatous optic neuropathy or development of de novo glaucoma after KPro implantation when an increase of ≥ 0.1 in the cup-to-disc ratio was observed, or when there was a decrease in the V4e isopter area of greater than 30% in the GVF.
Statistical analysis
All subjects underwent visual acuity testing using a Snellen chart. The best-corrected visual acuity was converted to the logarithm of the minimal angle of resolution (logMAR) for statistical analysis. When Snellen visual acuity could not be obtained, semi-quantitative assessments of visual acuity were made (e.g. count fingers, hand motions, light perception, or no light perception). For the purpose of statistical analysis, the following logMAR values were assigned: count fingers – logMAR 2.0, hand motions – logMAR 2.3, light perception – logMAR 2.6, no light perception – logMAR 3.0.24,25
Results
A total of 115 eyes of 109 patients underwent Boston Keratoprosthesis type 1 implantation at the University of Illinois at Chicago from 2007 to 2015. A total of 22 eyes from 21 patients met inclusion criteria for final analysis. The demographic and clinical information of included patients are shown in Table 1. Primary KPro implantation, defined as KPro implantation performed without a history of prior corneal transplantation was performed in 7/22 eyes. Secondary KPro implantation, defined as KPro implantation performed in eyes with a history of prior corneal transplantation with subsequent immunologic rejection, occurred in 15/22 eyes. Post-KPro complications are listed in Table 2 and outcomes are summarized in Table 3.
TABLE 1.
PATIENT CHARACTERISTICS
| Females | 11/22 (50.0%) |
|
| |
| Mean Age at time of KPro | 50.0 ± 15.1 (10–81 yrs) |
|
| |
| Race | |
| African American | 12/22 (54.5%) |
| Caucasian | 8/22 (36.4%) |
| Hispanic | 2/22 (9.1%) |
|
| |
| Indications for KPro | |
| Non-Inflammatory Dystrophies/Degenerations | 8/22 (36.4%) |
| Corneal dystrophy (1 CHED, 1 lattice, 2 gelatinous droplike) | 4/22 (18.2%) |
| Pseudophakic bullous keratopathy | 1/22 (4.5%) |
| Keratoconus | 1/22 (4.5%) |
| Iridocorneal endothelial syndrome (Chandler syndrome) | 1/22 (4.5%) |
| Limbal stem cell deficiency | 1/22 (4.5%) |
| Burns | 5/22 (22.7%) |
| Chemical burn | 4/22 (18.2%) |
| Thermal burn | 1/22 (4.5%) |
| Autoimmune | 4/22 (18.2%) |
| Uveitis | 2/22 (9.1%) |
| Stevens-Johnson syndrome | 2/22 (9.1%) |
| Infectious | 3/22 (15/6%) |
| Herpetic keratitis | 3/22 (15.6%) |
| Aniridia | 2/22 (9.1%) |
|
| |
| Primary KPro | 7/22 (31.8%) |
| Secondary Kpro (following prior immunologic transplant rejection) | 15/22 (68.2%) |
|
| |
| # of Patients who had IOP-Lowering Procedures | |
| None | 6/22 (27.3%) |
| At Any Time | 16/22 (72.7%) |
| Pre-KPro | 5/22 (22.7%) |
| Concurrently | 10/22 (45.5%) |
| Post-KPro | 6/22 (27.3%) |
TABLE 2.
POST-OPERATIVE COMPLICATIONS
| None | 6/22 (27.3%) |
| Cystoid macular edema | 6/22 (27.3%) – 4/6 required intravitreal injections |
| Retroprosthetic membrane | 5/22 (22.7%) |
| Epiretinal membrane | 4/22 (18.2%) |
| Rhegmatogenous retinal detachment | 4/22 (18.2%) |
| Endophthalmitis | 2/22 (9.1%) |
| Corneal extrusion/melt | 2/22 (9.1%) |
| Iris obstruction of visual axis requiring argon laser iridoplasty | 2/22 (9.1%) |
| Required ocular surface rehabilitation (e.g. tarsorrphaphy) | 2/22 (9.1%) |
| Floppy eyelid syndrome requiring repair | 1/22 (4.5%) |
| Hypotony and choroidal effusion | 1/22 (4.5%) |
| Myopic choroidal neovascular membrane with macular scar | 1/22 (4.5%) |
| Posterior capsule opacificiation requiring yag capsulotomy | 1/22 (4.5%) |
| Episcleritis | 1/22 (4.5%) |
| Macular hole | 1/22 (4.5%) |
| Infectious keratitis | 1/22 (4.5%) |
TABLE 3.
OUTCOMES
| Clinical Data | Mean Clinical Follow Up Time | 49.4 ± 20.0 (15 – 90) months |
| Mean Interval Between Baseline and Final Disc Photos | 28.7 ± 14.7 (6 – 73) months | |
| Mean Interval Between Baseline and Final Goldmann Visual Fields | 31.0 ± 19.0 (6 – 72) months | |
|
| ||
| BCVA At Last Follow-Up | ||
| ≥ 20/200 | 19/22 (86.4%) | |
| ≥ 20/100 | 17/22 (77.3%) | |
| ≥ 20/80 | 15/22 (68.2%) | |
| ≥ 20/40 | 8/22 (36.4%) | |
|
| ||
| Mean BCVA | ||
| Pre-KPro | 2.07 ± 0.33 logMAR | |
| At Last Follow-Up | 0.70 ± 0.78 logMAR | |
|
| ||
| Mean # Of Glaucoma Meds | ||
| Pre-KPro | 1.14 ± 1.7 | |
| At Last Follow-Up | 1.05 ± 1.1 | |
|
| ||
| Mean IOP | ||
| Pre-KPro | 18.4 ± 6.7 mmHg | |
| At Last Follow-Up | 18.4 ± 5.6 mmHg | |
|
| ||
| Elevated IOP | ||
| >25 mmHg at 2 Separate Visits | 2/22 (9.1%) | |
| >20 mmHg at 2 Separate Visits | 11/22 (50%) | |
|
| ||
| Structural Data | Mean Initial C/D | |
| Vertical | 0.48 ± 0.13 | |
| Horizontal | 0.52 ± 0.11 | |
|
| ||
| Mean Final C/D | ||
| Vertical | 0.50 ± 0.13 | |
| Horizontal | 0.52 ± 0.12 | |
|
| ||
| Vertical or Horizontal C/D Increase | ||
| ≥ 0.1 | 5/22 (22.7%) | |
| ≥ 0.2 | 1/22 (4.5%) | |
|
| ||
| Functional Data | Loss Of V4e Isopter Area | |
| > 30% | 2/16 (12.5%) | |
|
| ||
| Overall Glaucoma Rates | Both C/D Increase + Loss of Isopter Area | 0/16 (0.0%) (0 cases/person-year) |
|
| ||
| No Glaucoma | 6/22 (27.3%) (0.078 cases/person-year) | |
| Post-KPro Glaucoma | 2/22 (9.1%) (0.026 cases/person-year) | |
| Pre-KPro Glaucoma | 14/22 (63.6%) (0.18 cases/person-year) | |
| Progression of Pre-KPro Glaucoma | 5/14 (35.7%) (0.11 cases/person-year) | |
| Progression of Pre or Post-KPro Glaucoma: | ||
| By ≥ 0.1 Increase In C/D | 5/22 (22.7%) (0.065 cases/person-year) | |
| By > 30% Loss of Isopter Area | 2/22 (9.1%) (0.026 cases/person-year) | |
| By Either Method | 7/22 (31.8%) (0.091 cases/person-year) | |
| In Primary Kpro | 2/7 (28.6%) (0.073 cases/person-year) | |
| In Secondary KPro | 5/15 (33.3%) (0.101 cases/person-year) | |
Ninety-three eyes were excluded from analysis for various reasons, often multifactorial. Nineteen eyes (20.4% of excluded eyes) were lost to follow-up, 8 eyes (8.6%) had pale nerves with end-stage cupping prior to KPro implantation, and 4 eyes (4.3%) belonged to patients who became deceased during the study period. Two eyes (2.2%) received glaucoma care by outside providers. Many excluded eyes had media opacities precluding adequate fundus photography at the time of the study, including 34 eyes (37%) with primary and recurrent retroprosthetic membranes, 6 (6.5%) with prior vitrectomy surgery and silicone oil fill (including cases of egress into the anterior chamber), 3 (3.2%) with significant posterior capsule opacification, 2 (2.2%) with persistent epithelial disease and corneal haze, and 2 (2.2%) with chronic vitritis. Of eyes with clear media, 17 (18.3%) had nystagmus that prevented adequate fundus photography. Seventeen eyes (18.3%) also underwent KPro explantation (12 from corneal melts and 5 from endophthalmitis). Six eyes (6.5%) became no light perception (NLP) vision during the study; these eyes had hand motion or light perception vision pre-operatively, and reasons for vision loss included acanthamoeba infection with inconsistent follow-up, choroidal hemorrhage during KPro surgery, recurrent retinal detachments, endophthalmitis, and worsening of pre-existing end-stage glaucoma. Finally, one eye was exenterated for basal cell carcinoma.
In total, 11 eyes in this series (50%) experienced elevated intraocular pressures above 20 mmHg on two separate visits after Kpro implantation. A vertical or horizontal C/D increase of ≥ 0.1 or ≥ 0.2 was seen in 5 (22.7%) and 1 (4.5%) eyes, respectively. Visual field deterioration (loss of >30% of V4e isopter area) occurred in 2 out of 16 eyes (12.5%) with Goldmann visual fields at least 6 months apart (Figure 1). Development of post-KPro glaucoma, progression of pre-existing or post-KPro glaucoma, and no glaucoma development as evidenced by objective assessment of structural and clinical parameters were seen in 2/22 (9.1%), 7/22 (31.8%), and 6/22 (27.3%) eyes, respectively (Table 3).
IOP-lowering surgical treatments in this series included Baerveldt-350 glaucoma drainage implants (Abbott Medical Optics, Inc, Santa Ana, CA), Ahmed-FP7 valve glaucoma drainage implants (New World Medical, Inc, Rancho Cucamonga, CA), Molteno drainage implants (Molteno Ophthalmic Ltd, Dunedin, New Zealand), trabeculectomy, and transscleral cyclophotocoagulations. All glaucoma drainage devices implanted concurrently or post-KPro implantation were inserted through the pars plana, while glaucoma drainage devices implanted prior to KPro implantation were revised and repositioned through the pars plana at the time of KPro implantation. Of the 12 eyes that had IOP-lowering surgeries prior to or concurrent with KPro implantation, 5/12 showed evidence of progression and 2/12 underwent additional IOP-lowering surgery. Of the remaining 10 eyes that did not have prior or concurrent IOP-lowering surgeries, 4/10 ultimately underwent IOP-lowering surgeries.
Discussion
With increased rates and longer durations of KPro retention, it has become evident that careful surveillance and treatment of glaucoma is essential to ensure the best possible visual outcomes in KPro patients.1 The pathophysiology of glaucoma progression and development in KPro patients is complex and multifactorial.2–8 Several conditions that lead patients to eventually require a keratoprosthesis are also independently associated with the development of glaucoma as evidenced by the rates of glaucoma in the following conditions: chemical burns (prevalence 22–55%), aniridia (incidence 6–75%, with prevalence as high as 91%), and penetrating keratoplasty (incidence 9–31% in the early post-operative period, 18–35% in the late post-operative period).2 Furthermore, glaucoma is particularly challenging to diagnose, monitor, and treat in KPro patients. Preoperatively, glaucoma surveillance (e.g. optic nerve evaluation, intraocular pressure monitoring, visual field testing, and optical coherence tomography testing) is often limited by corneal pathology and other comorbid conditions, thereby potentially permitting glaucomatous damage to proceed without being detected, monitored, or intervened upon. Likewise, postoperatively, glaucoma surveillance may be hindered by the keratoprosthesis itself and possibly further limited by secondary complications such as a retroprosthetic membrane. The most notably compromised glaucoma surveillance modality is intraocular pressure monitoring, but the quality of visual field testing, optical coherence tomography testing, and optic disc evaluation may also be affected. Intra-operatively, it is posited that there may be mechanical distortion of the iridocorneal angle at the time of KPro implantation which may further contribute to glaucoma risk.6 Numerous post-operative factors may also play an important role, including mechanical compromise and crowding of the iridocorneal angle by the KPro backplate,2,4–6,8 obstruction of the trabecular meshwork (by inflammatory debris, erythrocytes, or vitreous debris),2,5,7 retained viscoelastic material, intraocular inflammation,2,5,7 corticosteroid use,4,5,8 possibly decreased effectiveness of topical IOP-lowering medications (given the reduced corneal surface area),4 and possibly reduced compliance with topical IOP-lowering medications (given the requirement to comply with multiple long-term topical medications). Importantly, a prospective study involving serial anterior segment optical coherence tomography has suggested high rates of progressive iridocorneal angle shallowing, peripheral anterior synechiae formation, and secondary angle closure following KPro implantation (7/11 eyes, 63.6%).6
The impact of glaucoma in KPro patients has been described previously. Several retrospective studies have revealed a high incidence of preoperatively diagnosed glaucoma in Kpro patients, ranging from 33.3–89.3% (Table 4 – Supplemental Digital Content). While not as well studied, high rates of progression of preoperatively diagnosed glaucoma (ranging from 18.5–72.2%) and postoperatively newly diagnosed glaucoma (0.0–75.0%) have also been reported in other retrospective studies (Table 4 – Supplemental Digital Content). As a result, this study endeavored to better investigate the incidence of pre-existing glaucoma, de novo glaucoma, and progression of glaucoma in KPro recipients. While many previous papers (Table 4 – Supplemental Digital Content) have used elevated IOP or subjective assessments of glaucomatous damage by fundoscopy or review of visual fields by examining physicians, ours is among the few to utilize objective measurements of structural changes (analysis of cup-to-disc ratio measurements in disc photographs graded by an independent glaucomatologist) and functional changes (utilizing a novel method for the quantification of Goldmann visual fields in KPro patients) as the primary basis for determining whether and how glaucoma has affected KPro patients.
The incidence of pre-existing glaucoma in our patient population (14/22, 63.6%) is comparable to prior studies (mean 63.1%, 33.3–89.3%, Table 4 – Supplemental Digital Content). The incidence of progression of pre-existing glaucoma in this study (5/14, 35.7%) is also comparable to prior studies (mean 39.4%, Table 4 – Supplemental Digital Content), though the incidence of post-KPro glaucoma is substantially lower in this study (2/22, 9.1%) compared to prior studies (mean 45.6%). This represents amongst the lowest reported post-KPro glaucoma rate, second only to a prior case series from our institution (Table 4 – Supplemental Digital Content).13 We hypothesize that this may be at least somewhat attributable to a high rate of procedural or surgical IOP-lowering interventions in our study occurring concurrently with KPro implantation (45.5% versus a mean of 21.7% among prior studies, Table 4 – Supplemental Digital Content) or post-KPro implantation (27.3% versus a mean of 12.9% among prior studies, Table 4 – Supplemental Digital Content).
It is also important to note that in this case series, 19/22 (86.4%) eyes retained visual acuity ≥ 20/200 at last follow-up and no eyes had loss of visual acuity related to glaucoma progression. Additionally, the mean intraocular pressure pre-KPro and at last follow-up were identical (18.4 vs 18.4 mmHg), and the mean cup-to-disc ratios pre-KPro and at last follow-up were comparable (vertical, 0.48 vs 0.50; horizontal, 0.52 vs 0.52). A number of patients were excluded from the study for reasons such as severe media opacities precluding adequate fundus photography, thereby potentially biasing the final results towards better visual acuity and glaucoma management parameters. Nonetheless, we hypothesize that when a stringent methodology used for glaucoma assessment such as in this study is possible, it may allow for early detection of glaucomatous changes and minimization of glaucoma-associated visual acuity loss.
Given that glaucoma surveillance in KPro patients is particularly challenging, the investigation and implementation of novel surveillance methods in these patents is essential. The methods employed in our study, including the use of digital planimetric analysis of Goldmann visual fields, may serve well as the foundations for larger future studies investigating glaucoma in KPro patients. Options for functional visual field assessment include static automated perimetry or kinetic perimetry (GVF). Both methods may be significantly affected by refractive and ocular media changes as well as glare after keratoprosthesis.26 In this series, Goldmann visual fields were selected for various reasons. GVFs were administered by experienced technicians, providing accuracy and repeatability. Furthermore, the ability to utilize standardized stimulus sizes and fixation targets with static automated perimetry is limited in KPro patients with central visual acuity <20/200 or nystagmus. Additionally, assessment of the visual field beyond the central 24 degrees was preferred as little is known about patterns of field loss in KPro patients. The use of V4e may be less sensitive to detecting smaller glaucomatous changes as compared to dimmer or smaller isopters. However, we limited our analysis to the V4e isopter for two major reasons: (1) a study by Sayegh et al utilized the Goldmann V4e isopter to study the optical functional properties of KPro devices, providing reasonable baseline parameters with which to compare progressive field loss;26 (2) all GVFs performed included the V4e isopter, allowing for consistent comparison between patients. Although the V4e isopter was chosen for analysis in this study, the technique of digital planimetric quantification can be readily applied to any isopter. We believe that the same method of planimetric analysis of Goldmann visual fields may also be extended to other non-KPro glaucoma patients as well, along with other non-glaucomatous conditions such as inherited retinal degenerations.27
There are certain limitations to this study. It is a retrospective study and the number of eyes included for final analysis is small relative to the overall number of KPro procedures performed at our institution. As mentioned earlier, there are several factors that likely account for this. Firstly, the inclusion criteria requiring two separate, high-quality, gradable, disc photographs separated in time are more difficult to meet in KPro patients with potentially poor anterior segment transparency than the average glaucoma patient. Secondly, the smaller sample size may at least partially be attributable to the changing views and priorities of examining physicians regarding glaucoma surveillance throughout the KPro era – in earlier years, baseline and follow-up disc photographs may not have been thought of as being a necessary part of routine care for KPro patients, whereas, nowadays at our institution, a shift has occurred to image nearly all patients with sufficiently transparent media. Despite the strict exclusion criteria, a long follow-up was achieved in this study (49.6 months), allowing for assessment and detection of short-term to long-term glaucomatous structural and functional changes in the included patients. Compared to other studies listed in table 4 (Supplemental Digital Content), only 1 other study had a longer mean follow-up duration.
This study adds to the medical literature on glaucoma in KPro patients in two distinct ways. Firstly, it bases an assessment of glaucoma development or progression on objective deterioration in structural and functional parameters. Secondly, it introduces the use of digital planimetric quantification of Goldmann visual fields for the purpose of analyzing visual field changes in KPro patients. These methods may serve useful to aide in the important and challenging task of glaucoma surveillance.
Based on the findings of this study, we conclude that meticulous glaucoma surveillance with structural and functional testing combined with earlier IOP-lowering surgical intervention may result in decreased rates of glaucomatous vision loss in KPro patients. However, we acknowledge that successfully obtaining optic nerve imaging and visual fields in a number of patients with implanted KPro can be challenging, and further studies focusing on developing better ways to assess intraocular pressure, glaucoma detection and progression are needed.
Supplementary Material
Summary of previous studies assessing glaucoma in Boston keratoprosthesis patients. All studies were retrospective except for those by Lekhanont et al (2014) and Oliveira et al (2014) which were both prospective. There was considerable heterogeneity in the definitions of glaucoma, glaucoma progression, and elevated intraocular pressure. Some studies considered glaucoma or glaucoma progression to simply be an elevated intraocular pressure, while other studies required both characteristic structural (e.g. optic nerve head changes) and functional (e.g. visual field loss) glaucomatous changes. A few studies did not clearly state the definitions of glaucoma or elevated intraocular pressure.
Acknowledgments
Funding: This work is supported by an unrestricted departmental grant from Research to Prevent Blindness and a National Institute of Health Core Grant (EY001792).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose relevant to this study.
Contributor Information
Mohsin H. Ali, University of Illinois at Chicago, Illinois Eye and Ear Infirmary, Department of Ophthalmology and Visual Sciences, 1855 West Taylor Street, M/C 648, Chicago, IL 60612.
Mark S. Dikopf, University of Illinois at Chicago, Illinois Eye and Ear Infirmary, Department of Ophthalmology and Visual Sciences, 1855 West Taylor Street, M/C 648, Chicago, IL 60612.
Anthony G. Finder, University of Illinois at Chicago, Illinois Eye and Ear Infirmary, Department of Ophthalmology and Visual Sciences, 1855 West Taylor Street, M/C 648, Chicago, IL 60612.
Ahmad A. Aref, University of Illinois at Chicago, Illinois Eye and Ear Infirmary, Department of Ophthalmology and Visual Sciences, 1855 West Taylor Street, M/C 648, Chicago, IL 60612.
Thasarat Vajaranant, University of Illinois at Chicago, Illinois Eye and Ear Infirmary, Department of Ophthalmology and Visual Sciences, 1855 West Taylor Street, M/C 648, Chicago, IL 60612.
Jose de la Cruz, University of Illinois at Chicago, Illinois Eye and Ear Infirmary, Department of Ophthalmology and Visual Sciences, 1855 West Taylor Street, M/C 648, Chicago, IL 60612.
Maria Soledad Cortina, University of Illinois at Chicago, Illinois Eye and Ear Infirmary, Department of Ophthalmology and Visual Sciences, 1855 West Taylor Street, M/C 648, Chicago, IL 60612.
References
- 1.Lee WB, Shtein RM, Kaufman SC, et al. Boston Keratoprosthesis: Outcomes and Complications: A Report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:1504–11. doi: 10.1016/j.ophtha.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Kamyar R, Weizer JS, de Paula FH, et al. Glaucoma associated with Boston type I keratoprosthesis. Cornea. 2012;31:134–9. doi: 10.1097/ICO.0b013e31820f7a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li JY, Greiner MA, Brandt JD, et al. Long-term complications associated with glaucoma drainage devices and Boston keratoprosthesis. Am J Ophthalmol. 2011;152:209–18. doi: 10.1016/j.ajo.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Banitt M. Evaluation and management of glaucoma after keratoprosthesis. Curr Opin Ophthalmol. 2011;22:133–6. doi: 10.1097/ICU.0b013e328343723d. [DOI] [PubMed] [Google Scholar]
- 5.Talajic JC, Agoumi Y, Gagné S, et al. Prevalence, progression, and impact of glaucoma on vision after Boston type 1 keratoprosthesis surgery. Am J Ophthalmol. 2012;153:267–274. e1. doi: 10.1016/j.ajo.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Kang JJ, Allemann N, Cruz J, de la, et al. Serial Analysis of Anterior Chamber Depth and Angle Status Using Anterior Segment Optical Coherence Tomography After Boston Keratoprosthesis. Cornea. 2013;32:1369–74. doi: 10.1097/ICO.0b013e3182a0cff5. [DOI] [PubMed] [Google Scholar]
- 7.Crnej A, Paschalis EI, Salvador-Culla B, et al. Glaucoma progression and role of glaucoma surgery in patients with Boston keratoprosthesis. Cornea. 2014;33:349–54. doi: 10.1097/ICO.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mahmood AM, Al-Swailem SA, Edward DP. Glaucoma and corneal transplant procedures. J Ophthalmol. 2012;2012:576394. doi: 10.1155/2012/576394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldave AJ, Sangwan VS, Basu S, et al. International results with the Boston type I keratoprosthesis. Ophthalmology. 2012;119:1530–8. doi: 10.1016/j.ophtha.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Chang H-YP, Luo ZK, Chodosh J, et al. Primary implantation of type I Boston keratoprosthesis in nonautoimmune corneal diseases. Cornea. 2015;34:264–70. doi: 10.1097/ICO.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 11.Chew HF, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28:989–96. doi: 10.1097/ICO.0b013e3181a186dc. [DOI] [PubMed] [Google Scholar]
- 12.Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, Davis. Ophthalmology. 2011;118:1543–50. doi: 10.1016/j.ophtha.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Kang JJ, de la Cruz J, Cortina MS. Visual outcomes of Boston keratoprosthesis implantation as the primary penetrating corneal procedure. Cornea. 2012;31:1436–40. doi: 10.1097/ICO.0b013e31823f7765. [DOI] [PubMed] [Google Scholar]
- 14.Lekhanont K, Thaweesit P, Muntham D, et al. Medium-term outcomes of boston type 1 keratoprosthesis implantation in Bangkok, Thailand. Cornea. 2014;33:1312–9. doi: 10.1097/ICO.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 15.Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998;105:751–7. doi: 10.1016/S0161-6420(98)94034-9. [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira LA, Pedreira Magalhães F, Hirai FE, et al. Experience with Boston keratoprosthesis type 1 in the developing world. Can J Ophthalmol. 2014;49:351–7. doi: 10.1016/j.jcjo.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Patel AP, Wu EI, Ritterband DC, et al. Boston type 1 keratoprosthesis: the New York Eye and Ear experience. Eye (Lond) 2012;26:418–25. doi: 10.1038/eye.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert M-C, Harissi-Dagher M. Boston type 1 keratoprosthesis: the CHUM experience. Can J Ophthalmol. 2011;46:164–8. doi: 10.3129/i10-103. [DOI] [PubMed] [Google Scholar]
- 19.Srikumaran D, Munoz B, Aldave AJ, et al. Long-term outcomes of boston type 1 keratoprosthesis implantation: a retrospective multicenter cohort. Ophthalmology. 2014;121:2159–64. doi: 10.1016/j.ophtha.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Aravena C, Bozkurt TK, Yu F, et al. Long-Term Outcomes of the Boston Type I Keratoprosthesis in the Management of Corneal Limbal Stem Cell Deficiency. Cornea. 2016;35:1156–64. doi: 10.1097/ICO.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 21.Goins KM, Kitzmann AS, Greiner MA, et al. Boston Type 1 Keratoprosthesis: Visual Outcomes, Device Retention, and Complications. Cornea. 2016;35:1165–74. doi: 10.1097/ICO.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 22.Kapamajian MA, de la Cruz J, Hallak JA, et al. Correlation between corneal and scleral pneumatonometry: an alternative method for intraocular pressure measurement. Am J Ophthalmol. 2013;156:902–906. e1. doi: 10.1016/j.ajo.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 23.Kuo DS, Ou Y, Jeng BH, et al. Correlation of Serial Scleral and Corneal Pneumatonometry. Ophthalmology. 2015;122:1771–6. doi: 10.1016/j.ophtha.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange C, Feltgen N, Junker B, et al. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT) Graefes Arch Clin Exp Ophthalmol. 2009;247:137–42. doi: 10.1007/s00417-008-0926-0. [DOI] [PubMed] [Google Scholar]
- 25.Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236–40. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 26.Sayegh RR, Avena Diaz L, Vargas-Martín F, et al. Optical functional properties of the Boston Keratoprosthesis. Invest Ophthalmol Vis Sci. 2010;51:857–63. doi: 10.1167/iovs.09-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zahid S, Peeler C, Khan N, et al. Digital quantification of Goldmann visual fields (GVFs) as a means for genotype-phenotype comparisons and detection of progression in retinal degenerations. Adv Exp Med Biol. 2014;801:131–7. doi: 10.1007/978-1-4614-3209-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of previous studies assessing glaucoma in Boston keratoprosthesis patients. All studies were retrospective except for those by Lekhanont et al (2014) and Oliveira et al (2014) which were both prospective. There was considerable heterogeneity in the definitions of glaucoma, glaucoma progression, and elevated intraocular pressure. Some studies considered glaucoma or glaucoma progression to simply be an elevated intraocular pressure, while other studies required both characteristic structural (e.g. optic nerve head changes) and functional (e.g. visual field loss) glaucomatous changes. A few studies did not clearly state the definitions of glaucoma or elevated intraocular pressure.