Abstract
Purinergic P2X4 receptors (P2X4Rs) belong to the P2X superfamily of ionotropic receptors that are gated by adenosine-5’-triphosphate (ATP). Accumulating evidence indicates that P2X4Rs play an important role in regulation of ethanol intake. At the molecular level, ethanol’s inhibitory effects on P2X4Rs are antagonized by ivermectin (IVM), in part; via action on P2X4Rs. Behaviorally, male mice deficient in p2rx4 gene [P2X4R knockout (KO)] have been shown to exhibit a transient increase in ethanol intake over a period of 4 days as demonstrated by social and binge drinking paradigms. Furthermore, IVM reduced ethanol consumption in male and female rodents, whereas, male P2X4R KO mice were less sensitive to anti-alcohol effects of IVM compared to wildtype (WT), further supporting a role for P2X4Rs as targets of IVM’s action. The current investigation extends testing the hypothesis that P2X4Rs play a role in regulation of ethanol intake. First, we tested the response of P2X4R KO mice to ethanol for a period of 5 weeks. Second, to gain insights into the changes in ethanol intake, we employed a lentivirus-shRNA (LV-shRNA) methodology to selectively knockdown P2X4R expression in the nucleus accumbens (NAc) core in male C57BL/6J mice. In agreement with our previous study, male P2X4R KO mice exhibited higher ethanol intake than WT mice. Additionally, reduced expression of P2X4Rs in NAc core significantly increased ethanol intake and preference. Collectively, the findings support the hypothesis that P2X4Rs play a role in regulation of ethanol intake and that P2X4Rs represent a novel drug target for treatment of alcohol use disorder.
Keywords: P2X4 receptors, alcohol use disorder, ethanol, nucleus accumbens, shRNA
INTRODUCTION
P2X receptors (P2XRs) are becoming a focus of investigation in neuroscience and ethanol studies (Litten et al. 2012, Burnstock 2008, Asatryan et al. 2011, Gum et al. 2012, Franklin et al. 2014, Xu et al. 2016). P2XRs are fast acting cation-permeable ion channels that are gated by synaptically released extracellular adenosine 5’-triphosphate (ATP) (Chizh & Illes 2001, Khakh 2001, North 2002). In the CNS, ATP directly mediates fast excitatory synaptic transmission by acting on P2XRs located on postsynaptic membranes. In addition, ATP can produce neuromodulator responses by promoting neurotransmitter release of other major ionotropic targets (e.g., GABA and glutamate), known to play important roles in ethanol drinking and other behaviors by acting on P2XRs located on pre- and postsynaptic membranes (Khakh 2001, Jo & Schlichter 1999, Hugel & Schlichter 2002, Baxter et al. 2011, Xu et al. 2016).
P2X4Rs are the most abundantly expressed P2XR subtype in the CNS ranging from neurons to microglia (Buell et al. 1996, Soto et al. 1996) and are the most sensitive P2XR subtype to ethanol. In vitro studies report that ethanol concentrations starting at approximately 5 mM modulate ATP-activated currents in neurons (Li et al. 1994, Li et al. 1998, Li et al. 1993, Weight et al. 1999, Xiao et al. 2008) and recombinant models (Xiong et al. 2000, Xiong et al. 2001, Davies et al. 2002, Davies et al. 2005, Asatryan et al. 2008, Asatryan et al. 2010). This concentration of ethanol is well below the 17 mM (i.e., 0.08%) blood ethanol concentration (BEC) that is considered “under the influence” in the U.S. In addition, P2X4Rs are located in brain regions identified as neural substrates of ethanol [e.g., hippocampus, cerebellum, ventral tegmental area (VTA), nucleus accumbens (NAc), hypothalamic nuclei including paraventricular nucleus (PVN) nd arcuate nucleus (Arc)] (McCool 2011, Pankratov et al. 2009, Sim et al. 2006, Gonzales et al. 2004, Xu et al. 2016).
Recent studies implicate P2X4Rs in the regulation of multiple CNS functions including neuropathic pain (Tsuda et al. 2000, Ulmann et al. 2008), neuroendocrine functions (Zemkova et al. 2010) and hippocampal plasticity (Baxter et al. 2011, Lorca et al. 2011, Sim et al. 2006). In addition, P2X4Rs have been recently shown to modulate the function of other major ionotropic targets, such as γ-amino butyric acid receptors (GABAARs) (Jo et al. 2011) and glutamate N-Methyl-D-aspartate receptors (NMDA) (Baxter et al. 2011) receptors. Many of these physiological and behavioral functions linked to P2XRs are known to be affected by ethanol.
Building evidence links P2X4Rs to ethanol consumption including investigations using microarray techniques, which found an inverse relationship between p2rx4 gene expression and innate rodent intake and preference for ethanol (Kimpel et al. 2007, Tabakoff et al. 2009). In agreement with this hypothesis, we recently demonstrated that male P2X4 knockout (KO) mice (i.e., p2rx4 deleted) consumed significantly more ethanol than wildtype (WT) controls (Wyatt et al. 2014). The present paper extends the investigation of the role of P2X4Rs in ethanol intake and addresses two unresolved questions from the recent Wyatt et al paper. First, we significantly increased the length of time of the ethanol investigation to gain insights regarding the transient nature of the increased drinking reported by Wyatt and colleagues (Wyatt et al. 2014). This was accomplished by testing male P2X4KO mice and WT littermates for changes in ethanol intake and preference for 5 weeks using a 24 hr access two-bottle choice paradigm. Second, in that the increase in ethanol intake previously measured in male P2X4KO mice could partially reflect compensatory developmental changes, we also utilized a lentiviral-mediated shRNA knockdown strategy (LV-shRNA) to knockdown P2X4R expression in the NAc core and measured changes in ethanol intake and preference.
MATERIALS AND METHODS
Animals
We used experimentally naïve 2–3 month old male WT and P2X4R KO mice from our breeding colony at the University of Southern California. The generation of P2X4R KO mice and the breeding scheme has been described previously (Sim et al. 2006, Wyatt et al. 2013). For the LV-shRNA experiments, 2–3 month old male C57BL/6 mice were obtained from Jackson laboratories (Bar Harbor, ME). Mice were group housed (i.e. 5 per cage) in the vivarium maintained at 22 °C and a 12 hr/12 hr light: dark cycle with free access to food and water. All procedures are carried out in compliance with the guidelines of National Institute of Health and approved by the Institutional Animal Care and Use Committee of University of Southern California.
Drugs
The ethanol solution was prepared as a 10% v/v ethanol solution in tap water from 190 proof USP grade ethanol solution (Koptec, King of Prussia, PA).
Short hairpin RNA (shRNA) constructs and LV production
cDNA encoding two shRNA sequences targeting different regions of P2X4 mRNA (S1 and S2) were subcloned into the Clontech biscistronic pLVX-shRNA2 vector where the shRNA expression was driven by human U6 promoter, located just upstream of the MCS (Mountain View, CA). The vector also expressed ZsGreen1 reporter, a human codon optimized variant of the coral reef green fluorescent protein (GFP), under CMV promoter control. The shRNA sequences were 5’-CCACAAATACTCAGGGTTG-3’ and 5’-CTCAGATGGGCTTCAGATA-3’. We have observed that the simultaneous use of both the sequences resulted in higher extent inhibition of P2X4R expression. LV was produced by mixing both shRNA constructs with psPAX2 and pMD2.G packaging vectors obtained from Addgene (Cambridge, MA) and transfected HEK 293T cells. Virus-containing supernatant was collected, concentrated and resultant viral titers were determined via the ELISA method. Concentrator and titration kits were obtained from Clontech Laboratories (Mountain View, CA).
Stereotaxic surgery and microinjection procedure
Mice were anesthetized with a ketamine/xylazine cocktail and placed in a mouse stereotaxic frame (David Kopf Instruments, Tujunga, CA). A small incision was made to the skin exposing the skull. Bregma and lambda were measured to ensure an even plane and a small area of dura removed in the area for microinjection. A ten-microliter syringe (Hamilton, Reno, NV) was used to deliver 1μL of LV (4.1 × 107 to 5.3 × 109 IU/mL) to each NAc (bregma coordinates: anteriorposterior 1.2 mm; mediolateral 1.0 mm; dorsoventral 4.5 mm) at a rate of 0.1 μL/min. After infusion, the syringe was left in place for a further 5 min. Mice were allowed to recover from surgery in cages placed on heating pads for 2 days, after which they were transported to the vivarium. Mice were subsequently single housed in the vivarium with ad libitum access to food and water during their resting period for 1 week prior to start of the ethanol drinking experiments.
Anatomical verification of LV microinfusion into the NAc core by fluorescence microscopy
Following the stereotaxic surgery and microinjection of LV, transcardial perfusion was performed on mice using 0.9% NaCl followed by 4% phosphate buffered paraformaldehyde. Brains were post fixed in 4% phosphate buffered paraformaldehyde overnight followed by storage in 20% sucrose for 48 hr and frozen in 4-Methylbutane on dry ice. Striatal sections were cut coronally at 25 μm thickness in a cryostat and later stored in a cryoprotective solution containing 30% sucrose in PBS at 4 °C until further use. The striatal sections were then examined for GFP immunofluorescence under a fluorescent microscope (Olympus BX61 microscope, Shinjuku, Tokyo, Japan).
Verification of LV-shRNA mediated knockdown of P2X4Rs in vitro and in vivo using Western immunoblotting
BV-2 transduction
Knockdown of P2X4Rs by LV-shRNA strategy in vitro was verified through transduction of mouse microglial BV-2 cells, which have a high endogenous P2X4R expression. BV-2 cells were cultured in 6-well plates in DMEM/ F12 medium supplemented with penicillin/streptomycin and fetal bovine serum until they reached approximately 80% confluence. Cells were transduced with 106 infectious units/ml of shRNA-based LV. Confirmation of virus expression was visualized by GFP fluorescence after 48 hr.
Microinjection into NAc core
P2X4R knockdown in mouse nucleus accumbens core by LV-shRNA methodology was verified at 14 days post infusion of LV-shRNA. 2 and 3 mice were stereotactically injected with the LV alone and LV-shRNA-p2rx4 respectively. A separate cohort of mice that have never undergone surgery (will be described as naïve mice) was used as a positive control for this study. After surgery, mice were allowed to rest for period of 1 week. At the end of the recovery period, the mice remained in their cages for period of 14 and 42 days during which they had ad libitum access to food and water. We chose 14 day time-point since the mice are exposed to ethanol 2 weeks post surgery and 42 day time-point since the final week during which they receive ethanol is 6 weeks post surgery. At their respective time points, mice were euthanized using CO2 asphyxiation and striatum was dissected out as per landmarks described in the mouse brain atlas (Franklin & Paxinos 2007).
BV-cell lysate or striatal tissue homogenate preparation
Cells or striatal tissues were treated with lysis buffer containing 50mM tris-HCl pH (7.4), 150 mM NaCl, 0.5% sodium deoxycholate, 1% Triton-X-100, 0.1% SDS, 1% proteinase inhibitor cocktail (Millipore, Temecula,CA). BV-2 cell lysates were spun at 13,000 rpm for 10 min at 4 °C and the protein-containing supernatant was collected. Protein content for BV-2 cells and ventral striatum was measured by using BCA protein assay (Thermo Scientific, Rockford, IL).
Immunoblotting procedure
Striatal homogenates or cell lysates ( respectively of 50 μg and 10 μg per lane) ran on 10% SDS PAGE gels and transferred onto polyvinylidine fluoride membranes using a semi-dry transfer method (Trans turbo blot, BioRad, Hercules, CA). Non-specific binding was blocked using 5% non fat dry milk (BioRad, Hercules, CA) followed by incubation with rabbit anti-P2X4 receptor antibody (Alomone Labs, Jerusalem, Israel) overnight at 4 °C. Membranes were then incubated with goat anti-rabbit secondary antibody for 1 hr at room temperature. In between incubation steps for antibodies and blocking, membranes were washed 3 times, 5 min each time, with TBS containing 0.05% Tween-20. After secondary antibody incubation, membranes were incubated with ECL substrate, (BioRad, Hercules, CA) and bands were visualized using chemilumenescent method (ChemiDoc system, BioRad, Hercules, CA).
24 hr access two-bottle choice paradigm
The 24 hr access two bottle choice paradigm is a model that mimics social drinking and is used to investigate differences in ethanol intake in genetically modified animals or upon pharmacological treatment (Yoneyama et al. 2008, Middaugh & Kelley 1999, Belknap et al. 1993). We used the procedure previously described (Wyatt et al. 2014, Yardley et al. 2012, Asatryan et al. 2014). Briefly, singly housed WT/P2X4R KO or naïve/LV alone/LV-shRNA-p2rx4 infused mice had 24 hr access to 2 inverted graduated tubes (25 mL) with metal sippers positioned on stainless steel cage tops. Food was evenly distributed on the cage tops to avoid association with either of the tubes. The mice had access to tubes containing water only for the first week post acclimation. In the second week, one of the tubes contained water and the other with 10% ethanol solution (10E). 10E and water intake was recorded by measuring the lower meniscus. It was ensured that positions of the tubes were switched every alternate day to avoid side preferences. Mice were given fresh solution of 10E and water once a week. Body weights were measured and used to calculate the g/kg/24hr ethanol intake. Percent ethanol preference was determined by multiplying the ratio of volume of 10E intake (mL) over total fluid intake (10E + water) by 100.
For the LV-shRNA-p2rx4 drinking experiment, mice underwent a baseline drinking session post acclimation during which their voluntary consumption of ethanol, water, total fluid intake and ethanol preference was measured. Upon stable baseline drinking, mice were randomly assigned to one of the three treatment options: 1) LV-shRNA-p2rx4, LV alone or naïve mice (i.e. mice that have never undergone the surgery). ANOVA was used to ensure that the ethanol intake, preference, water or total fluid intake did not significantly differ between the three treatment groups.
STATISTICAL ANALYSES
Repeated measures two way ANOVA (genotype x week) was used to investigate differences in 10E intake, 10E preference, water and total fluid intake between WT and P2X4R KO mice, followed by Bonferroni post hoc test for multiple comparisons. For the LV-shRNA drinking studies, separate two way ANOVAs followed by Bonferroni post hoc comparisons were conducted between LV alone and LV-shRNA-p2rx4 groups to analyze the effect of LV-shRNA on drinking behavior as well as between naïve mice and LV alone group to determine whether injection of LV alone has any impact on drinking behavior. One way ANOVA with Tukey’s post hoc test was also used to compare the efficiency of LV-shRNA-p2rx4 transfusion and transfection on P2X4R knockdown in mouse striatum and BV-2 cells. Significance was set at P<0.05. All data was analyzed using Graph Pad software (Prism, San Diego, CA).
RESULTS
P2X4R KO mice exhibited increased voluntary ethanol consumption in the 24 hour access drinking paradigm
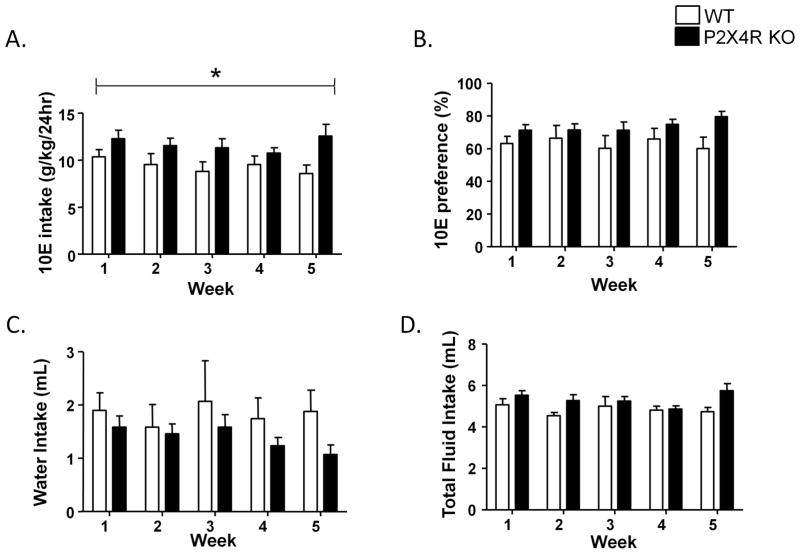
We tested the effects of global knockout of p2rx4 gene on ethanol intake using a 24 hr two bottle choice paradigm (10E versus water). As illustrated in Fig 1A, there was a significant effect of genotype [F(1,16)=4.88, p<0.05], but not week or genotype x week interaction for 10E intake. There was no significant effect of genotype, week or genotype x week interaction for 10E preference or water intake between WT and P2X4R KO mice (Fig 1B & C). There was a non-significant trend towards effect of genotype [F(1,16)=3.28, p=0.0891] but not week or genotype x week interaction for total fluid intake. (Fig 1D). Considering that there were changes in 10E intake, but not preference, we evaluated the effect of genotype and week on body weight. There was a significant effect of genotype [F(1,16)=4.68, p<0.05] since the P2X4R KO mice weighed significantly more than their WT counterparts. There was also significant effect of week [F(4,64)=59.08, p<0.001] on body weight. There was no significant interaction between the two factors on body weight between WT and P2X4R KO mice.
Figure 1.
P2X4R KO mice exhibited significantly higher 10E intake compared to WTcontrols (A) and tended to have higher total fluid intake (D) without any significant changes in 10E preference (B) or water intake (C). For each week, the 10E intake, preference and water intake was measured as an average of 5 days. Values represent mean ± SEM for a duration of 5 days each week for 8 WT and 10 P2X4R KO mice. * P <0.05 versus WT mice, two way-ANOVA.
Transfection of BV-2 cells or transfusion in mouse striatum with LV-shRNA-p2rx4 reduced P2X4R expression
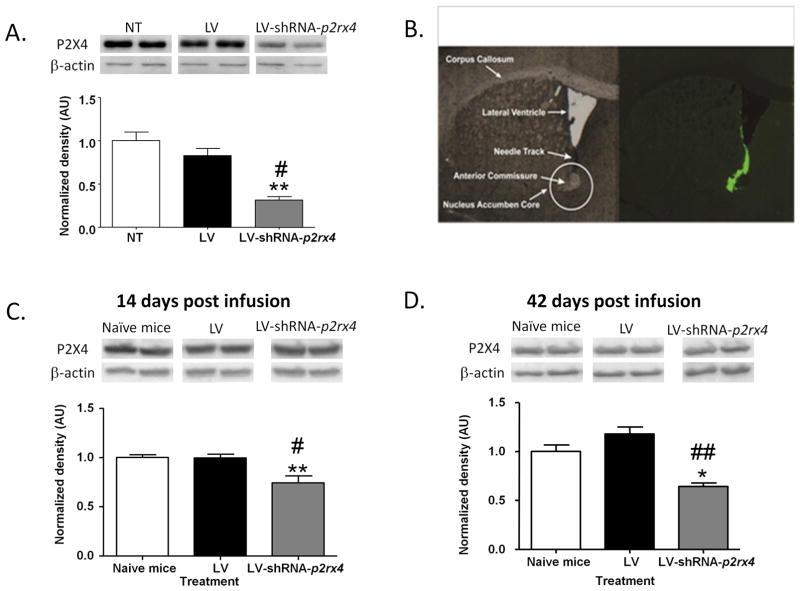
We first investigated the efficiency of knockdown of P2X4Rs using a LV-shRNA strategy in BV-2 cells. As depicted in Fig 2A, LV-shRNA treatment significantly reduced P2X4R expression [F(2,4)=27.88, p<0.01] in BV-2 cells. Tukey’s post hoc test confirmed that LV-shRNA treatment significantly reduced P2X4R expression as compared to untreated cells (q=9.875, p<0.01) and cells treated with LV alone (q=7.377, p<0.05).
Figure 2.
Microglial BV-2 cells transinfected with LV-shRNA-p2rx4 reduced P2X4R expression by 68% and 62% as compared to non-treated cells (NT) and LV alone treated cells respectively (A). Microinfusion of LV into the NAc core was verified by detecting ZsGreen1 immunofluorescence (B). Stereotaxic injection of LV-shRNA-p2rx4 in NAc core significantly reduced P2X4R expression as compared to naïve mice and LV alone infused mice respectively after 14 (C) and 42 days (D). Values represent mean ± SEM for 3–8 mice per treatment group for (C) and (D), **P<0.01 v/s non-treated cells,# P<0.05 v/s LV alone treated cells for (A), *P<0.05, **P<0.01v/s naïve controls , #P<0.05, ##P<0.01 v/s LV alone group for (C) and (D), Tukey’s post hoc test.
We next tested the efficiency of LV-shRNA-p2rx4 infusion on P2X4R knockdown in the mouse striatum at 14 and 42 days post infusion. As illustrated in Fig 2C, LV-shRNA significantly reduced P2X4R expression in the striatum at 14 days post infusion [F(2,12)=9.266, p<0.01].Tukey’s post hoc test confirmed significant reduction in P2X4R expression in mice that received LV-shRNA-p2rx4 treatment as compared to both naive mice (q=5.565,p<0.01) and mice that only received the LV infusion after a period of 14 days (q=4.889,p<0.05). In Fig 2D, LV-shRNA treatment significantly reduced P2X4R expression at 42 days post infusion [F( 2,14)=10.37,p<0.05] with Tukey’s post hoc text confirming significant reduction in P2X4R expression in mice injected with LV-shRNA-p2rx4 in comparison to naïve controls (q=4.300, p<0.05) and mice that received LV infusion alone (q=6.435,p<0.01)..
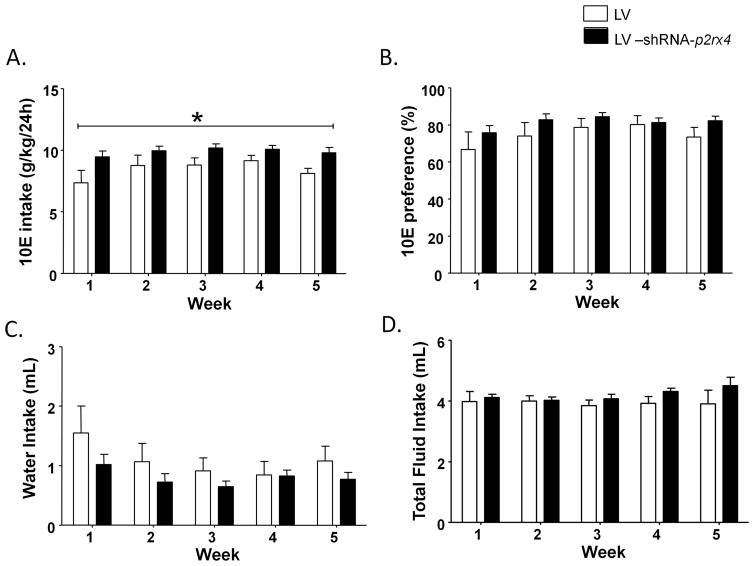
Infusion of LV alone did not have any significant effect on ethanol intake or preference in comparison to the naïve mice
There was non-significant trend towards effect of week [F(4,76)=2.35, p=0.0618] without any significant effect of treatment or week x treatment interaction for 10E intake in mice infused with LV alone relative to naïve mice (Fig 3A). Similarly, in the context of 10E preference, there was a non-significant trend towards effect of week [F(4,76)=2.03, p=0.0982] without any significant effect of treatment or week x treatment interaction (Fig 3B). There was a significant effect of week [F(4,76)=2.59, p<0.05] but not treatment and the week x treatment interaction trended towards significance [F(4,76)=2.19, p=0.0782] for water intake between the two groups (Fig 3C). There was a non-significant trend towards effect of week [F(4,76)=2.21, p=0.0753] and treatment [F(1,19)=3.86, p=0.0642] on total fluid intake between the two groups of mice. However, there was a significant week x treatment interaction [F(4,76)=2.75, p<0.05] with Bonferroni post hoc test indicating reduced total fluid intake in mice receiving LV infusion relative to naïve mice at week 5 (t=3.329, p<0.01) (Fig 3D). Finally, there was a non-significant trend towards effect of week on body weight [F(4,76)=2.49, p=0.0503] without any significant effect of treatment or week x treatment interaction between LV alone infused mice and naïve mice.
Figure 3.
The LV-shRNA-p2rx4 group exhibited significantly higher 10E intake as compared to mice infused with LV alone (A).. No significant changes in 10E preference (B), water intake (C) or total fluid intake (D) between the groups . Values represent mean ± SEM for a duration of 5 days for each week for 10 mice infused with LV alone and 14 mice infused with LV-shRNA-p2rx4. *P<0.05, versus LV alone infused mice,two way ANOVA.
The LV-shRNA-p2rx4 infused mice exhibited a higher ethanol intake as compared to mice that only received LV infusion
There was a significant effect of week [F(4,80)=3.87, p<0.01] and treatment [F(1,20)=6.08, p<0.05] on 10E intake in the LV-shRNA-p2rx4 group as compared to the group that received LV infusion , without any significant week x treatment interaction. (Fig 3A). There was a significant effect of week [F(4,80)=3.96, p<0.01] but not treatment or week x treatment interaction for 10E preference between the two groups (Fig 3B). Similarly, there was a significant effect of week [F(4,80)=5.57, p<0.001] but not treatment or week x treatment interaction for water intake (Fig 3C). There was no significant effect of week, treatment or week x treatment interaction for total fluid intake (Fig 3D). There was a significant effect of week [F(4,80)=16.60, p<0.001],but not treatment on body weight between two groups. However, there was a significant week x treatment interaction [F(4,80)=3.39, p<0.05] for body weight since LV-shRNA-p2rx4 infused mice gained more weight across the 5 weeks period. .
DISCUSSION
The current study investigated the role of P2X4Rs in regulation of ethanol drinking behavior. Overall, the findings support the hypothesis that P2X4Rs play an important role in the regulation of ethanol intake by demonstrating that reduced P2X4R expression results in changes in ethanol drinking behavior. Using a global knockout strategy, we demonstrated that P2X4R KO mice exhibited significantly increased ethanol intake. The increased body weights of P2X4R KO mice may partially contribute to their increased ethanol intake as suggested by lack of significant change in ethanol preference. On the other hand, there was no significant change in water intake suggesting that p2rx4 deficiency affects mechanism of ethanol drinking, without perturbing the physiolgy of drinking per se. These results are in good agreement with our previous study wherein we showed that male P2X4R KO mice exhibited higher ethanol intake over a period of 4 days without changes in ethanol preference or water intake as compared to their WT littermates (Wyatt et al. 2014).
Taking into consideration the complex compensatory changes that occur in a knockout mouse model, the constitutive deficiency of P2X4Rs does not necessarily represent the full pharmacological blockade of the receptor. At present, we do not have any selective P2X4R antagonists that can be used to provide a direct link between P2X4R antagonism and increased ethanol consumption. As a complementary strategy, we employed a LV-shRNA methodology to address this issue. In the present investigation, we targeted the NAc core as the site for the LV-shRNA injection since this is a critical site of the dopamine (DA) mesolimbic circuitry for various drugs of abuse including ethanol to induce their reinforcing and rewarding effects (Bassareo et al. 2017, Cador et al. 1991, Corbit et al. 2016, Di Chiara 2002). Moreover, P2X4Rs are expressed in the striatum (Amadio et al. 2007) and endogenous ATP (possibly via activation of P2XRs) has been implicated in modulation of DA neurotransmission in various regions of the mesolimbic circuitry including the VTA and NAc (Xiao et al. 2008, Krugel et al. 2001). In agreement with our previous and current findings from the male P2X4R KO study, we found that mice with reduced P2X4R expression ( via LV-sh-RNA-p2rx4 infusion) exhibited greater ethanol consumption relative to naïve mice and mice infused with LV alone. There were no significant changes in ethanol intake upon infusion of LV alone in relation to naïve mice indicating that the increased ethanol intake in mice with reduced P2X4R expression is due to shRNA mediated knockdown of P2X4Rs and not infusion of the virus alone. The coherence in findings from both the P2X4R KO and LV-shRNA-p2rx4 studies indicates that functional deletion of p2rx4 gene increases ethanol intake. Nevertheless, considering there have been compensatory changes in receptors such as NMDARs, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) as well as DA receptors in P2X4R KO mice (Wyatt et al. 2013, Khoja et al. 2016), it is possible that alterations in these receptor systems may occur upon infusion of LV-shRNA in the NAc core. Hence, future studies involving measuring expression levels of glutamatergic and dopaminergic receptors in LV-shRNA-p2rx4 infused mice would be warranted to delieanate the interaction of P2X4Rs with other neurotransmitter systems in regulation of ethanol intake.
Additionally, shRNA mediated knockdown of P2X4Rs significantly increased ethanol preference relative to naïve mice but not mice infused with LV alone. The increased ethanol preference in LV-shRNA-p2rx4 infused mice may account for the increased ethanol intake relative to naïve mice since there were no significant differences in body weights between these group. Unlike in the LV-shRNA methodology where there is knockdown of a particular gene at the adult stage, the P2X4R KO mice may exhibit neurodevelopmental adaptations to compensate for constitutive deficiency of p2rx4 gene, and such adaptations could nullify the effect of p2rx4 knockout on ethanol preference. Moreover, the p2rx4 knockdown is in the NAc core which is a key brain region for expression of ethanol reinforcement and has an important role in acquisition and maintenance of ethanol seeking behavior (Gonzales et al. 2004). Thus, potential neurodevelopmental changes in this brain region of P2X4R KO mice could significantly interfere with motivational behavior towards seeking ethanol. The somewhat differing data with respect to ethanol preference from P2X4R KO and LV-shRNA-p2rx4 infused mice suggests the need for additional investigations using operant chamber technique to monitor self-administration or conditioned place preference in P2X4R KO mice or LV-shRNA-p2rx4 infused mice to better understand the role of P2X4Rs in ethanol seeking behavior.
The findings from LV-shRNA-p2rx4 and P2X4R KO experiments are in agreement with mutiple studies that have reported an inverse correlation between P2X4R expression and ethanol consumption. For example, Kimpel and colleagues compared gene expression in brain areas associated with reward in inbred alcohol preferring (iP) v/s non-preferring (iNP) rat lines and found that functional p2rx4 expression was significantly reduced in iP rats (Kimpel et al. 2007). Similarly, Tabakoff and colleagues found lower levels (i.e., inverse relationship) of whole brain expression of p2rx4 mRNA in inbred rats that display a high ethanol-drinking phenotype compared to those with a lower ethanol-drinking phenotye (Tabakoff et al. 2009). On the other hand, McBride and colleagues reported that p2rx4 gene expression was significantly increased in high alcohol drinking female (HAD2) rats, relative to their low-alcohol drinking (LAD2) counterparts (McBride et al. 2012). In addition, increased expression of P2X4Rs was detected in the periaqueductal gray (PAG), a region associated with fear and anxiety (Behbehani 1995), in adolescent male P rats (McClintick et al. 2016). To further validate the correlation of increased P2X4R expression with increased ethanol intake, LV-shRNA mediated knockdown of P2X4R expression in the posterior VTA was recently shown to significantly decrease ethanol intake in female HAD2 rats (Franklin et al. 2015). Although there are differences regarding the direction of change in drinking behavior in these reports , a common theme emerging from these investigations is that manipulation of p2rx4 expression is associated with significant changes in ethanol consumption.
Multiple investigations from our laboratory as well as others have supported the hypothesis that inhibition of P2X4R activity increases ethanol drinking behavior and potentiation of P2X4Rs reduces ethanol intake as well as the propensity to seek ethanol Based on recent work, we suggested that ethanol acts as an open channel blocker of P2X4Rs (Ostrovskaya et al. 2011, Popova et al. 2010) and that positive modulation of P2X4Rs by ivermectin (IVM) antagonized ethanol induced inhibition of P2X4Rs (Asatryan et al. 2010). Positive modulation of P2X4Rs by IVM can reduce ethanol drinking behavior as illustrated in previous reports showing that IVM administration in C57BL/6J mice reduced ethanol intake and preference using variety of paradigms that mimic social drinking and motivation to seek ethanol (Yardley et al. 2012, Asatryan et al. 2014, Wyatt et al. 2014).The anti-alcohol effects of IVM can be, in part, linked to P2X4R activity in that the degree of reduction of ethanol intake was decreased in male P2X4R KO mice (Wyatt et al. 2014). Similar findngs were obtained from Franklin and colleagues, where IVM significantly reduced ethanol intake in male and female HAD-1 and HAD-2 rats. Furthermore, intracerebroventricular (ICV) administration of IVM significantly reduced ethanol intake in female HAD2 rats (Franklin et al. 2015). Although previous findings from our laboratory have suggested IVM as a positive modulator of P2X4Rs, IVM has been reported to be a positive modulator of ligand gated ion channels(LGICs) belonging to Cys-loop superfamily such as GABAARs (Dawson et al. 2000), glycine receptors (GlyRs) (Shan et al. 2001) and nicotininc acetylcholine receptors (nAchRs) (Krause et al. 1998). Thus, the role of other ionotropic receptors in the behavioral effects of IVM cannot be disregarded. The physiological significance of ethanol induced inhibition of P2X4Rs within the mesolimbic circuitry is unknown and currently under investigation in our laboratory. However, based on previous reports, it is thought that ethanol induced inhibition of P2XRs on the GABA releasing terminals in the VTA is linked to disinhibition of VTA DA neurons as suggested previously (Xiao et al. 2008). Therefore, the study by Xiao and colleagues provides indirect evidence for P2X4Rs’s role in modulation of firing of DA neurons in VTA. One possible mechanism for P2X4Rs in modulation of firing of DA neurons is via its localization in the Arc region of the hypothalamus. P2X4Rs have been reported to be involved in regulating presynaptic GABA release onto proopiomelanocortin (POMC) neurons in the Arc region (Xu et al. 2016) and GABAergic activity in this region has been associated with an inhibitory influence on the VTA DA neurons (Tabakoff et al. 2009). However, additional electrophysiological studies would be needed to test this hypothesis before definitive conclusions can be drawn. Overall, we do not have sufficient data to conclusively state that there is a direct link between P2X4R potentiation/inhibition and firing of DA neurons in the VTA. This is an area of work that is ongoing. Moreover, elucidating the GABAergic tone or firing of VTA DA neurons in P2X4R KO mice or mice that received LV-shRNA-p2rx4 infusion would also be important to help provide novel insights into the mechanism leading to increased ethanol intake in these mice.
While the aforementioned studies highlight the importance of neuronal P2X4Rs in regulation of ethanol intake, the role of microglial P2X4Rs in mediating ethanol induced responses cannot be excluded. P2X4Rs have been implicated in regulation of mciroglial function and have been associated with pathophysiology of several neurodegenerative and neuroimmune disorders (Burnstock 2008, Potucek et al. 2006, Tsuda et al. 2013). Ethanol has been shown to upregulate P2X4R mRNA and protein expression in microglial cells, suggesting involvement of P2X4Rs in microglial responses (Gofman et al. 2014). For instance, P2X4Rs have been reported to play a role in mediating ethanol-induced macrophage and microglial phagocytosis as well as microglial migration (Gofman et al. 2014). In addition, P2X4Rs have also been reported to regulate alcohol induced effects on signaling molecules such as phosphotidylinositol-3-kinase (PI3K-Akt) , extracellular regulated kinase 1/2 (ERK 1/2) and transcription factors such as cyclic-AMP regulated phosphoprotein (CREB) (Gofman et al. 2016), all of which are linked to multiple physiological functions such as proliferation, differentiation, neurodevelopment and inflammation (Kim & Choi 2010). Interestingly, we observed significant increase in CREB phosphorylation in ventral striatum of P2X4R KO mice (Khoja et al. 2016), indicating a role for CREB activation in P2X4Rs’ effects on ethanol drinking behavior. The in vitro findings reported above suggest that alcohol can regulate mitogen activated protein kinase (MAPK) signaling pathways in microglial cells in brain sites linked to reward circuitry. Thus, infusion of LV-shRNA-p2rx4 could possibly lead to perturbation of ethanol-dependent MAPK signaling pathways in microgilal cells in the NAc core, as a result of which the LV-shRNA-p2rx4 infused mice consume significantly more ethanol than those injected with LV alone. Hence, the contribution of P2X4Rs to ethanol intake in each cell type needs to be thoroughly examined in future investigations.
In conclusion, the results from our previous work using P2X4R KO mouse model coupled with current LV-shRNA studies which corroborate findings from others, supports r the hypothesis that P2X4Rs play an important role in regulation of ethanol intake. Moreover, the results support the discovery and development of P2X4R allosteric modulators as novel therapeutic agents for treatment of AUD.
HIGHLIGHTS.
Genetic deficiency of p2rx4 increases ethanol intake.
Reduced P2X4 expression in nucleus accumbens increases ethanol intake.
P2X4 allosteric modulators represent novel drug therapy for alcohol addiction.
Acknowledgments
We would like to thank Dustin Lieu, Jamie Thuy, Neel Patel for their technical assistance. This work was conducted in partial fulfillment for PhD degree in Molecular Pharmacology and Toxicology (S.K.) as well as in Clinical Pharmacy and Experimental Therapeutics (N.H.). This work was supported by National Institute on Alochol Abue and Alcoholism (NIAAA) Grant R01 AA022448 (D.L.D) and USC School of Pharmacy. Authors have no conflict of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amadio S, Montilli C, Picconi B, Calabresi P, Volonte C. Mapping P2X and P2Y receptor proteins in striatum and substantia nigra: An immunohistological study. Purinergic signalling. 2007;3:389–398. doi: 10.1007/s11302-007-9069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Nam HW, Lee MR, Thakkar MM, Dar MS, Davies DL, Choi DS. Implication of the purinergic system in alcohol use disorders. Alcoholism, clinical and experimental research. 2011;35:584–594. doi: 10.1111/j.1530-0277.2010.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Perkins DI, Trudell JR, Alkana RL, Davies DL. Ivermectin antagonizes ethanol inhibition in P2X4 receptors. Journal of Pharmacology And Experimental Therapeutics. 2010;334:720–728. doi: 10.1124/jpet.110.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Woodward JJ, King BF, Alkana RL, Davies DL. Roles of ectodomain and transmembrane regions in ethanol and agonist action in purinergic P2X2 and P2X3 receptors. Neuropharmacology. 2008;55:835–843. doi: 10.1016/j.neuropharm.2008.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Yardley MM, Khoja S, Trudell JR, Hyunh N, Louie SG, Petasis NA, Alkana RL, Davies DL. Avermectins differentially affect ethanol intake and receptor function: implications for developing new therapeutics for alcohol use disorders. The International Journal of Neuropsychopharmacology. 2014;17:907–916. doi: 10.1017/S1461145713001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Cucca F, Frau R, Di Chiara G. Changes in Dopamine Transmission in the Nucleus Accumbens Shell and Core during Ethanol and Sucrose Self-Administration. Frontiers in Behavioral Neuroscience. 2017;11:71. doi: 10.3389/fnbeh.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AW, Choi SJ, Sim JA, North RA. Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. European Journal Neuroscience. 2011;34:213–220. doi: 10.1111/j.1460-9568.2011.07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Progress in Neurobiology. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Suprenant A. An antagonist insensitive P2X receptor expressed in epithelia and brain. The EMBO journal. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nature Reviews Drug Discovery. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- Cador M, Taylor JR, Robbins TW. Potentiation of the effects of reward-related stimuli by dopaminergic-dependent mechanisms in the nucleus accumbens. Psychopharmacology. 1991;104:377–385. doi: 10.1007/BF02246039. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Illes P. P2X receptors and Nociception. Pharmacol Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- Corbit LH, Fischbach SC, Janak PH. Nucleus accumbens core and shell are differentially involved in general and outcome-specific forms of Pavlovian-instrumental transfer with alcohol and sucrose rewards. European Journal of Neuroscience. 2016;43:1229–1236. doi: 10.1111/ejn.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology. 2005;49:243–253. doi: 10.1016/j.neuropharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, Alkana RL. Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcoholism, clinical and experimental research. 2002;26:773–778. [PubMed] [Google Scholar]
- Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, Schaeffer JM, Meinke PT, McKernan RM. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid A receptor. Journal of Pharmacology And Experimental Therapeutics. 2000;295:1051–1060. [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural brain research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Franklin BJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; 2007. [Google Scholar]
- Franklin KM, Asatryan L, Jakowec MW, Trudell JR, Bell RL, Davies DL. P2X4 receptors (P2X4Rs) represent a novel target for the development of drugs to prevent and/or treat alcohol use disorders. Frontiers in Neuroscience. 2014;8:176. doi: 10.3389/fnins.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, Bell RL, McBride WJ. Involvement of Purinergic P2X4 Receptors in Alcohol Intake of High-Alcohol-Drinking (HAD) Rats. Alcoholism, clinical and experimental research. 2015;39:2022–2031. doi: 10.1111/acer.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofman L, Cenna JM, Potula R. P2X4 receptor regulates alcohol-induced responses in microglia. Journal of Neuroimmune Pharmacology. 2014;9:668–678. doi: 10.1007/s11481-014-9559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofman L, Fernandes NC, Potula R. Relative Role of Akt, ERK and CREB in Alcohol-Induced Microglia P2X4R Receptor Expression. Alcohol and Alcoholism. 2016;51:647–654. doi: 10.1093/alcalc/agw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gum RJ, Wakefield B, Jarvis MF. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic signalling. 2012;8:41–56. doi: 10.1007/s11302-011-9272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neuroscience. 2002;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Donier E, Martinez A, Garret M, Toulme E, Boue-Grabot E. Cross-talk between P2X4 and gamma-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. The Journal of Biological Chemistry. 2011;286:19993–20004. doi: 10.1074/jbc.M111.231324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nature Neuroscience. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nature Reviews, Neuroscience. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- Khoja S, Shah V, Garcia D, Asatryan L, Jakowec MW, Davies DL. Role of purinergic P2X4 receptors in regulating striatal dopamine homeostasis and dependent behaviors. Journal of Neurochemistry. 2016;139:134–148. doi: 10.1111/jnc.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochimica et biophysica acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Kimpel MW, Strother WN, McClintick JN, Carr LG, Liang T, Edenberg HJ, McBride WJ. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol. 2007;41:95–132. doi: 10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: A positive allosteric effector of the alpha 7 meuronal nicotinic acetylcholine receptor. Molecular pharmacology. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Krugel U, Kittner H, Illes P. Mechanisms of adenosine 5'-triphosphate-induced dopamine release in the rat nucleus accumbens in vivo. Synapse. 2001;39:222–232. doi: 10.1002/1098-2396(20010301)39:3<222::AID-SYN1003>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Ethanol inhibits a neuronal ATP-gated ion channel. Mol Pharmacol. 1993;44:871–875. [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Alcohol action on a neuronal membrane receptor: Evidence for a direct interaction with the receptor protein. Proceedings in Natural Academy of Sciences USA. 1994;91:8200–8204. doi: 10.1073/pnas.91.17.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. British Journal of Pharmacology. 1998;123:1–3. doi: 10.1038/sj.bjp.0701599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, et al. Medications development to treat alcohol dependence: a vision for the next decade. Addiction biology. 2012;17:513–527. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca RA, Rozas C, Loyola S, Moreira-Ramos S, Zeise ML, Kirkwood A, Huidobro-Toro JP, Morales B. Zinc enhances long-term potentiation through P2X receptor modulation in the hippocampal CA1 region. European Journal of Neuroscience. 2011;33:1175–1185. doi: 10.1111/j.1460-9568.2010.07589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, Edenberg HJ, Lumeng L, Bell RL. Gene expression in the ventral tegmental area of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Pharmacology, biochemistry, and behavior. 2012;102:275–285. doi: 10.1016/j.pbb.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, McBride WJ, Bell RL, Ding ZM, Liu Y, Xuei X, Edenberg HJ. Gene Expression Changes in Glutamate and GABA-A Receptors, Neuropeptides, Ion Channels, and Cholesterol Synthesis in the Periaqueductal Gray Following Binge-Like Alcohol Drinking by Adolescent Alcohol-Preferring (P) Rats. Alcoholism, clinical and experimental research. 2016;40:955–968. doi: 10.1111/acer.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:185–194. doi: 10.1016/s0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiological Reviews. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ostrovskaya O, Asatryan L, Wyatt L, Popova M, Li K, Peoples RW, Alkana RL, Davies DL. Ethanol is a fast channel inhibitor of P2X4 receptors. The Journal of pharmacology and experimental therapeutics. 2011;337:171–179. doi: 10.1124/jpet.110.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. P2X receptors and synaptic plasticity. Neuroscience. 2009;158:137–148. doi: 10.1016/j.neuroscience.2008.03.076. [DOI] [PubMed] [Google Scholar]
- Popova M, Asatryan L, Ostrovskaya O, Wyatt LR, Li K, Alkana RL, Davies DL. A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. Journal of Neurochemistry. 2010;112:307–317. doi: 10.1111/j.1471-4159.2009.06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potucek YD, Crain JM, Watters JJ. Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochemistry International. 2006;49:204–214. doi: 10.1016/j.neuint.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. Journal of Biological Chemistry. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. Journal of Neuroscience. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor clonned from rat brain. Proc Natl Acad Sci USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, et al. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biology. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Koizumi S, Kita A, Shigemoto Y, Ueno S, Inoue K. Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons. The Journal of Neuroscience. 2000;20:RC90. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. P2X4 receptors and neuropathic pain. Front Cell Neurosci. 2013;7:191. doi: 10.3389/fncel.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. Journal of Neuroscience. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight FF, Li C, Peoples RW. Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors) Neurochemistry International. 1999;35:143–152. doi: 10.1016/s0197-0186(99)00056-x. [DOI] [PubMed] [Google Scholar]
- Wyatt LR, Finn DA, Khoja S, Yardley MM, Asatryan L, Alkana RL, Davies DL. Contribution of P2X4 Receptors to Ethanol Intake in Male C57BL/6 Mice. Neurochemical Research. 2014;39:1127–1139. doi: 10.1007/s11064-014-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt LR, Godar SC, Khoja S, Jakowec MW, Alkana RL, Bortolato M, Davies DL. Sociocommunicative and sensorimotor impairments in male P2X4-deficient mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1993–2002. doi: 10.1038/npp.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhou C, Li K, Davies DL, Ye JH. Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action. J Pharmacol Exp Ther. 2008;327:196–205. doi: 10.1124/jpet.108.139766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Li C, Weight FF. Inhibition by ethanol of rat P2X 4 receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;130:1394–1398. doi: 10.1038/sj.bjp.0703439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong KM, Li C, Weight FF. Differential modulation by short chain and long chain n -alcohols of rat P2X 4 receptors expressed in Xenopus oocytes. Alcoholism, clinical and experimental research. 2001;25:7A. [Google Scholar]
- Xu J, Bernstein AM, Wong A, et al. P2X4 Receptor Reporter Mice: Sparse Brain Expression and Feeding-Related Presynaptic Facilitation in the Arcuate Nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:8902–8920. doi: 10.1523/JNEUROSCI.1496-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Wyatt L, Khoja S, et al. Ivermectin reduces alcohol intake and preference in mice. Neuropharmacology. 2012;63:190–201. doi: 10.1016/j.neuropharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. American Journal Physiology Endocrinology and Metabolism. 2010;298:E644–651. doi: 10.1152/ajpendo.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]