Abstract
Stimulation with antibodies to CD3 and CD28 coimmobilized on beads can be used to significantly expand T cells ex vivo. With CD4 T cells from HIV-infected patients, this expansion usually is accompanied by complete suppression of viral replication, presumed to be caused by down-regulation of the viral coreceptor CCR5 and up-regulation of CCR5 ligands. Here we show that this suppression occurs in total CD4 T cells acutely infected with R5 HIV, but not in purified CD62L− memory CD4 T cells. The lack of complete suppression in these memory cells, typically comprising 10–40% of total CD4 T cells, occurs despite high levels of CCR5 ligand secretion and down-regulation of CCR5. Significantly, adding back naïve or CD62L+ memory CD4 T cells inhibits the viral replication in the CD62L− cells, with the naïve cells capable of completely repressing the virus. Although this inhibition was previously thought to be specific to bead-bound anti-CD3/CD28 stimulation, we show that the same suppression is obtained with sufficiently strong anti-CD3/B7.1 stimulation. Our results show that inhibitory mechanisms, expressed predominantly by strongly stimulated naïve CD4 T cells and mediated independently of CCR5-binding chemokines, play a role in the inhibition of R5 HIV replication in CD4 T cells upon CD28 costimulation.
Keywords: CD62L, CCR5, B7, CD3, proliferation
Resting CD4 T cells can be infected with HIV, but productive HIV infection requires T cell activation (1). Compared with stimulation via the T cell receptor–CD3 complex (2), the interaction of CD28 on the surface of CD4+ T cells with its ligands CD80 (B7.1) and CD86 (B7.2) or with anti-CD28 antibodies (Abs) increases HIV replication in peripheral blood mononuclear cells (PBMC) (3, 4). Consistent with these findings, immune activation in vivo increases HIV replication (5).
In contrast to these observations, Levine et al. (6) reported that stimulation of CD4 T cells of HIV-infected patients with anti-CD3 and anti-CD28 coimmobilized on Sepharose beads “clears” HIV from infected cultures, allowing ex vivo expansion of autologous CD4 T cells in the absence of antiretroviral drugs. The same authors later reported that this mode of stimulation decreases expression of the main HIV coreceptor CCR5 (7) and induces high levels of the HIV-inhibitory CCR5 ligands RANTES, macrophage inflammatory protein (MIP)-1α, and MIP-1β (hereafter, CCR5 ligands) (8), that block access to CCR5, resulting in inhibition of CCR5-dependent (R5), but not of CXCR4-dependent (X4), HIV replication.
Therefore, anti-CD3/CD28 stimulation can result in decreased or enhanced HIV replication, apparently depending on the mode of stimulation [i.e., soluble Abs vs. Abs immobilized on beads (3, 6)]. The differential effects of these modes of stimulation on CCR5 expression and CCR5 ligand secretion do not appear to resolve the controversy. Creson et al. (9) reported that continuous passage of CD4 T cells on plastic culture dishes coated with anti-CD3/CD28 Abs failed to inhibit R5 HIV infection, despite decreased expression of CCR5 and secretion of CCR5 ligands at levels comparable to those achieved by stimulation with anti-CD3/CD28 mAb-conjugated beads.
Our findings indicate that the resolution of this paradox lies in the strength of T cell stimulation and in the heterogeneity of CD4 T cells. CD4 T cells can be divided into naïve T cells that express both CD45RA and CD62L and memory T cells (10, 11). Memory cells can be further divided into a number of subsets, including M1 (CD45RA−CD62L−) and M2 (CD45RA−CD62L+). We and others (12–15) have shown that naïve T cells support productive X4 HIV infection much less efficiently than memory T cells after CD3/CD28 stimulation.
In this article, we show that, as for X4 viruses, CD28-costimulated naïve T cells do not support productive R5 HIV infection. We demonstrate that, in contrast to the suppression of R5 HIV replication in total CD4 stimulated by anti-CD3/CD28 antibody-conjugated beads (6), R5 HIV can replicate at high levels in M1 cells stimulated with anti-CD3/CD28 beads, despite down-regulation of CCR5 and secretion of high levels of CCR5 ligands. Furthermore, addition of purified naïve CD4 T cells to M1 cells reproduces the inhibition of HIV replication observed in total CD4 T cell cultures. Finally, inhibition of R5 HIV replication in CD4 T cells, but not in purified M1 cells, can be obtained with the more physiologically relevant anti-CD3/B7.1 stimulation, by increasing the strength of the stimulus, under conditions that induce minimal levels of CCR5 ligands.
Taken together, these results suggest that the strength of stimulation is critical for inhibition of R5 HIV replication and that mechanisms independent of CCR5 ligands, expressed by naïve CD4 T cells, are involved in this inhibition. Thus, we have identified a mechanism for the inhibition of R5 viruses that is a cross-regulatory phenomenon between subsets of CD4 T cells.
Materials and Methods
Cell Culture Conditions.
Cells were cultured at 37°C, 5% CO2 in RPMI 1640 (GIBCO/BRL) supplemented with 2 mM l-glutamine, 100 units/ml penicillin, 50 nM streptomycin (GIBCO/BRL), 20 mM Hepes (Sigma), 10% FBS (Atlanta Biologicals, Norcross, GA), and human recombinant IL-2 (50 units/ml, from Maurice Gately, Hoffmann-La Roche, obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health) (cRPMI).
Antibodies (Abs).
Biotin, FITC, phycoerythrin (PE), Cy5PE, and allophycocyanin (APC)-conjugated Abs, and purified Abs to CD4, CD8, CD45RA, CD62L, and RANTES were from PharMingen; Cy5.5 and Cy7 were from Amersham Pharmacia; PE and APC were from ProZyme (San Leandro, CA), and Cascade blue was from Molecular Probes. Purified Abs were conjugated to the indicated fluorochromes in our laboratory. Neutralizing and control Abs to MIP-1α and MIP-1β were from R&D Systems.
Cell Isolation.
Human PBMC were isolated by Ficol-Paque (Amersham Pharmacia). After overnight culture at 2 × 106/ml in cRPMI without IL-2, nonadherent PBMC (2 × 108) were stained with biotinylated anti-CD14, anti-CD19, and anti-CD8 followed by avidin-coated magnetic beads and purified over a MACS column (Miltenyi Biotech, Auburn, CA). CD4-enriched T cells (≥80% purity) were stained with FITC anti-CD62L, PE anti-CD45RA, and Cy5PE anti-CD4 at room temperature, washed twice, and sorted by FACS (FACStarPlus, Becton Dickinson), as described (15).
Flow Cytometry Analysis.
For analysis of CCR5 expression, cells were stained with FITC or allophycocyanin anti-CCR5 for 30 min at 37°C. Other Abs were then added for 15 min on ice, and cells were washed three times and fixed with 0.5% paraformaldeyde. Data were compensated and analyzed by using flowjo (Tree Star, San Carlos, CA).
Acute HIV Infection.
HIV-1BaL [obtained from S. Gartner, M. Popovic, and R. Gallo (16), AIDS Reference Reagent Program] was prepared by infection of IL-2-stimulated PBMC (17). Virus-containing supernatants were harvested 6 days later and stored at −80°C. The TCID50 was determined in IL-2-stimulated PBMC. Sorted cells were cultured overnight in cRPMI without IL-2, then infected by a 2 h incubation with HIV-1BaL (1,500 TCID50/1 × 106 cells), washed three times, resuspended in cRPMI, and stimulated as indicated. Every 3 days, cells were split and fed with fresh cRPMI supplemented with stimuli. Half of the cells were used to assay cell proliferation; cell-free supernatants were stored at −80°C for determination of HIV-reverse transcriptase (RT) activity and chemokine concentrations.
Cell Stimulation.
HIV-infected cells were plated in 96-well plates at 0.5 × 106/ml in cRPMI with appropriate stimuli. OKT3 (kindly provided by M. Feldmann, Kennedy Institute, London) was used at 50 ng/ml. P815/B7.1 cells (kind gift of L. Lanier, DNAX) were fixed in 0.5% paraformaldeyde, washed extensively with RPMI, and added at the indicated ratio. Beads coated with anti-CD3 (OKT3) and anti-CD28 (9.3) (kind gift of C. June, Univ. of Pennsylvania, Philadelphia) were used at a ratio of 3:1 to cells, unless otherwise indicated.
Quantitative Viral and Cellular Assays.
HIV replication was monitored as Mg-dependent RT activity in supernatants (17). Cell number was determined with a standard MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. MIP-1α, MIP-1β, and RANTES concentrations were assayed by ELISA (R & D Systems).
Results
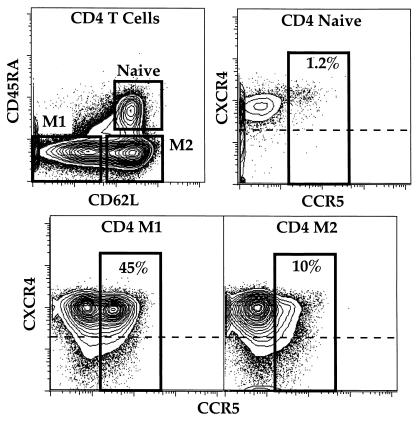
We previously reported that the X4 HIV-1LAI strain does not replicate in naïve CD45RA+CD62L+ CD4 T cells stimulated by cross-linking CD3 and CD28 with anti-CD3/B7.1. In contrast, anti-CD3/B7.1 stimulation induced high levels of HIV-1LAI replication in CD4 memory T cells (15). Memory CD45RA− CD4 T cells can be subdivided in CD62L− (M1) and CD62L+ (M2) cells. We did not find any significant difference in the ability of HIV-1LAI to productively infect M1 and M2 cells. The inhibition of naïve T cells occurs postentry, consistently with the uniformly high expression of the HIV coreceptor CXCR4 on all CD4 subsets (Fig. 1).
Figure 1.
Flow cytometric analysis of CD4 T cell subpopulations. Fresh total PBMC isolated from healthy uninfected individuals were stained with antibodies to CCR5 (FITC), CXCR4 (PE), CD3 (Cy5PE), CD4 (Cy7PE), CD8 (Cy5.5APC), CD45RA (Cascade blue), and CD62L (Cy5.5PE) as described in Materials and Methods. Naive, M1 and M2 CD4 T cells are identified on the basis of expression of CD45RA and CD62L (Upper Left). The other plots show the expression of CXCR4 and CCR5 on gated subsets of CD4 T cells. Numbers indicate the percentage of each subset that expresses CCR5. Cells above the dashed line express CXCR4; as expected, a majority of all subsets are CXCR4+. The antibody for CCR5 (2D7) binds competitively with CCR5 ligands; however, there are no chemokines in these unstimulated cultures so no blocking can occur.
The R5 HIV coreceptor CCR5 is expressed mainly by memory cells (18). In agreement with previous results (19, 20), we found that CCR5 is preferentially expressed by M1 (CD62L−) cells (Fig. 1). Thus, we were interested to determine the ability of R5 HIV to replicate not only in naïve T cells, but in individual memory subsets as well.
Total CD4 T cells and the naïve M1 and M2 subsets of CD4 T cells were sorted by flow cytometry on the basis of forward and side-scattered light and the expression of CD4, CD45RA, and CD62L (Fig. 1), with a purity always >98% at reanalysis. After overnight culture, cells were infected with the R5 HIV-1BaL, washed extensively, and stimulated with anti-CD3/B7.1 by using previously defined conditions of 0.2 P815/B7 per T cell (15).
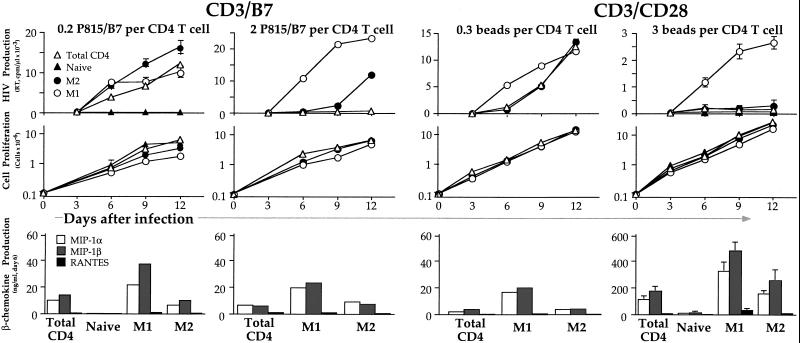
High levels of RT activity could be detected in total CD4 T cell cultures (Fig. 2). As previously shown for the X4 virus HIV-1LAI (15), naïve CD4 T cells did not replicate the R5 HIV-1BaL upon CD3/B7.1 stimulation. Both the M1 and M2 cell subsets efficiently replicated HIV, indicating that the low expression of CCR5 on M2 is more than sufficient for infection to occur at levels comparable to those found in M1, which express far greater amounts of CCR5.
Figure 2.
R5 HIV replication is suppressed in total CD4 but not in purified M1 memory cells upon sufficient CD3/CD28 costimulation, and suppression does not correlate with CCR5 ligand secretion. CD4 T cell subsets were sorted as described in Fig. 1, infected with HIV-1BaL and stimulated with anti-CD3/B7 (50 ng/ml anti-CD3 plus either 0.2 or 2 fixed P815/B7 cells/T cell) or with anti-CD3/CD28 beads (0.3 or 3 beads/T cell). Supernatants and cells were harvested every 3 days for analysis of viral replication by RT activity (Top) and cell proliferation by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay (Middle); data are the average ± SD of triplicate cultures and are representative of 3–10 experiments. For some experiments, MIP-1α, MIP-1β, and RANTES levels were measured 6 days after stimulation and infection (Bottom).
Levine et al. (6) demonstrated a differential regulation of R5 HIV replication depending on the mode of CD28 costimulation, with inhibition seen when stimulation was provided by bead-immobilized anti-CD3 and anti-CD28. We repeated our experiments using these beads at a 3:1 bead/cell ratio (6). We found minimal levels of HIV replication in total CD4 T cells (Fig. 2), consistent with the suppression observed in CD4 T cells from HIV-infected patients (6).
For purified subsets, HIV replication was undetectable in naïve cells and was very low in M2 cells. Surprisingly, however, high levels of RT activity could be measured in M1 cells (Fig. 2). In 10 independent experiments, after 6 days of stimulation with anti-CD3/CD28 beads, the M1 subset produced 6- to 40-fold more HIV than total CD4 T cell cultures (data not shown). Moreover, spreading of HIV at day 12 was observed only in M1 cell cultures, and not in total CD4. This occurred despite the fact that M1 cells typically comprise 10–40% of the total CD4 T cells.
We next investigated whether the strength of stimulation is important to suppress HIV replication in total CD4 T cells. We stimulated cells with anti-CD3/CD28 beads at the lower ratio of 0.3 beads/cell. All subsets exhibited exponential growth, only marginally slower than seen with 3 beads/cell. Importantly, although, no suppression of virus replication was observed in total CD4 T cells (Fig. 2): by day 12 there was no difference in HIV replication among total CD4, M2 or M1 cells, and total CD4 T cells replicated HIV to levels comparable with those achieved by CD3/B7.1 stimulation. It is interesting to note that previous reports indicated that repeated addition of the anti-CD3/CD28 beads was necessary to achieve inhibition of R5 replication (4); we confirmed this result in a separate experiment (not shown). Because the CD4 cells are dividing rapidly, the bead/cell ratio quickly diminishes from 3:1 to much smaller (noninhibitory) levels (Fig. 2). Thus, complete viral inhibition requires maintenance of a high stimulatory signal, and therefore replenishment of beads to match the cellular proliferation.
We then asked whether the same suppression of HIV replication observed in total CD4 cells with a high concentration of anti-CD3/CD28 beads might be achieved by increasing the strength of CD3/B7.1 stimulation. P815/B7.1 cells express human B7.1 and also express Fc receptors that bind soluble anti-CD3 Abs (thereby cross-linking both CD3 and CD28 or CTLA-4 on target cells). In the presence of optimal amounts of anti-CD3, increasing the ratio of P815/B7.1/CD4 T cells should result in increasing the strength of CD3/CD28 stimulation by providing a greater degree of cross-linking. Indeed, by increasing the P815/B7.1 ratio 10-fold, inhibition of virus replication was observed in total CD4 T cells (Fig. 2), to a level of inhibition comparable to that achieved with a high concentration of beads. Again, despite suppression of HIV replication in total CD4 T cells, the virus replicated at very high levels in M1.
The ability of M1 cells to replicate HIV, as compared with total CD4 T cells, upon stimulation with a high concentration of anti-CD3/CD28 beads or anti-CD3/B7.1 might be explained by a higher growth rate of the cells. Although some variability was observed among different donors, with all of the stimuli the levels of cell proliferation were greater in M2 and total CD4 T cells compared with M1 cells (1- to 2.2-fold and 1.3- to 2.5-fold, respectively, upon stimulation with anti-CD3/CD28 beads, n = 10). Therefore, the degree of HIV replication in purified subsets does not correlate with the degree of cell proliferation.
Stimulation of CD4 T cells with anti-CD3/CD28 beads induces high levels of CCR5 ligands (8) that block access to CCR5, likely inhibiting the spread of HIV among naturally infected CD4 T cell cultures (6). We measured MIP-1α, MIP-1β, and RANTES in the supernatants of acutely infected CD4 T cell subsets 6 days after stimulation in the different conditions (Fig. 2). Preliminary experiments had shown that this is the peak of induction. High levels of CCR5 ligands could be detected in total CD4 T cells stimulated with a ratio of 3:1 anti-CD3/CD28 beads/cells, and much lower levels upon stimulation with a ratio of 0.3:1, roughly correlating with HIV replication.
In M1 cells stimulated with anti-CD3/CD28 beads at the bead/cell ratio of 3:1, HIV replicates much less efficiently than in M1 cells stimulated with anti-CD3/B7.1, indicating that production of CCR5 ligands and CCR5 down-regulation induced by the beads play a significant role in the regulation of HIV replication. However, in purified M1 cells stimulated with anti-CD3/CD28 beads (3:1 ratio) HIV replicates at much higher levels than in total CD4 T cells, despite greater CCR5 ligand production. Furthermore, inhibition of viral replication in total CD4 T cells was observed with the strongest anti-CD3/B7.1 stimulation, despite modest production of CCR5 ligands. Thus, the ability of purified M1 cells to replicate HIV after CD3/CD28 bead stimulation is not because of an inability to produce CCR5 ligands. Importantly, we cannot explain suppression of HIV replication in bulk CD4 cultures solely by CCR5 regulation or CCR5 ligand production.
Previous reports showed that CCR5 ligand secretion does not account for the entire CD28 antiviral effect: for example, high levels of CCR5 ligands can be induced by CD2 or CD5 costimulation in the absence of suppression of HIV infection (8). It was proposed that the CD28 costimulation induced suppression of HIV replication is mediated by induction of high levels of CCR5 ligands in addition to down-regulation of CCR5 (7). We asked whether an inability of M1 to down-regulate CCR5 upon stimulation with anti-CD3/CD28 beads might account for the high levels of HIV replication detected in the purified subset as compared with total CD4 T cell cultures. After stimulation, only trace levels of CCR5 were detectable in any subsets (data not shown). Compared with CCR5 expression at day 0 (Fig. 1), CCR5 was therefore efficiently down-regulated in M1 cells. The high level of virus replication in M1 despite down-regulation of CCR5 is not surprising, because in the presence of optimal amounts of CD4, trace amount of CCR5 are sufficient for infection and virus replication to occur (21–23), as seen for CD3/B7.1-stimulated M2 cells (Fig. 2). Thus, the high level of HIV replication observed in purified M1 cells after anti-CD3/CD28 beads stimulation is not caused by a failure to down-regulate CCR5.
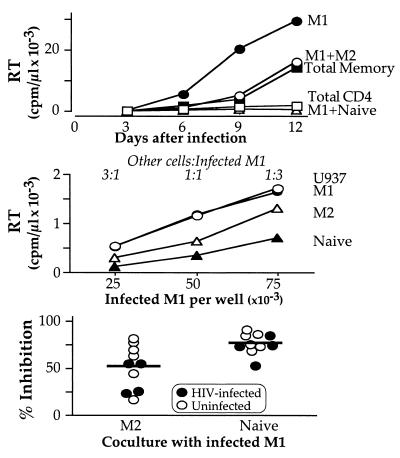
It seems paradoxical that HIV efficiently replicates in purified M1 cells, but does not replicate in these cells when present in bulk CD4 cultures (Fig. 2). We tested whether this suppression of HIV replication might be caused by inhibitory mechanisms expressed by M2 or naïve cells. Purified M2 or naïve cells were cocultured with purified M1 cells and RT activity over 12 days after infection was measured (Fig. 3). In this experiment, all cells were infected with HIV. Consistent with previous experiments, when total CD4 were infected and stimulated with anti-CD3/CD28 beads, very low levels of HIV replication were observed; however, HIV could replicate in purified M1 at very high levels. But when M1 were remixed with M2 at a 1:1 cell/cell ratio, some inhibition was observed; over time, the virus could once again spread in these cultures. Importantly, when naïve CD4 T cells were added to M1 cells, the complete inhibition of HIV replication observed for total CD4 T cells was reproduced.
Figure 3.
Coculture of infected M1 with M2 or naïve inhibits HIV replication. Cells were sorted and infected as described in Fig. 1 and stimulated with anti-CD3/CD28 beads. (Top) Both M2 and naïve cells inhibit HIV replication when added to M1 cells; only naïve T cells reproduce the suppression observed in total CD4 T cell cultures. (Middle) RT activity in culture supernatants 6 days after infection and stimulation with anti-CD3/CD28 beads correlates with the initial number of infected M1 cells in all coculture conditions. M1 cells were plated at the indicated concentrations and stimulated with anti-CD3/CD28 beads (3 beads/cell); uninfected U937, M1, M2, or naïve cells were premixed with anti-CD3/CD28 beads and added to M1 cells at the final concentration of 5 × 105 cells/ml (1 × 105 cells/well), resulting in different cell/cell ratios (1:3, 1:1, and 3:1). Both naïve and M2 cells inhibited viral replication in M1 cells; addition of uninfected M1 or U937 cells had no effect. A mild dose-dependent effect of the naïve and M2 cells was observed (not shown). (Bottom) HIV-infected (filled symbols) or uninfected (empty symbols) M2 or naïve cells were premixed with antiCD3/CD28 beads and added to infected and infected M1 cells at a 1:1 ratio; data are shown for 10 independent experiments. The inhibitor effect of naïve T cells was consistently greater than that of M2 cells and was independent of whether or not they were infected.
In this experiment the levels of RT activity are higher than the levels reported in Fig. 2 (5-fold higher in M1 cells at day 6; subsequently, the virus spreads more efficiently). We infected cells from more than 10 different donors throughout our experiments and observed significant variation in the absolute levels of RT activity in purified M1 stimulated with anti-CD3/CD28 beads (range 600–30,000 cpm/μl). Variability in HIV replication in CD4 T cells from different donors has been reported and might be caused by differential expression of CCR5 or other reasons (24). The experiment reported here is one of two in which we observed the highest RT levels in purified M1 cells. Despite this high level of viral replication in purified M1 cells, inhibition by addition of naïve cells was still complete.
To quantitate the ability of M2 or naïve cells to suppress HIV replication in acutely infected M1 cells and to minimize effects of differential cell growth, we chose to look at RT activity at an early time point (day 6). HIV-infected stimulated M1 were cocultured with stimulated M2 or naïve cells at different ratios. U937 cells also were used as a filler in control cultures of infected M1, allowing us to compare cultures with the same starting cell concentration. All cultures were equivalently split at day 3, to compare cultures with the same number of infected M1 cells. Using a flow cytometry-based assay for cell division, we found that the proliferation of M1 cells was similar whether cocultured with naive, M2, or other M1 cells (see additional text and Fig. 6, which are published as supporting information on the PNAS web site, www.pnas.org).
In the cocultures, there was a linear relationship between RT activity measured at day 6 and the starting number of infected M1 cells in culture, and the presence of uninfected M1 cells or U937 did not affect the degree of HIV replication (Fig. 3). However, naïve, and to a lesser extent M2 cells, inhibited HIV replication. At a 1:1 ratio, M2 demonstrated 45% suppression and naïve 70% suppression of M1 HIV replication (Fig. 3). Note that the naïve suppression becomes complete by day 12, whereas the M2 suppression is ameliorated.
We determined whether previous exposure to HIV might affect the ability of naive and M2 cells to inhibit HIV replication in M1 cells. In a series of 10 different experiments, infected or uninfected M2 or naïve cells, or M1 as a control, were cocultured with infected M1 cells at a 1:1 ratio. As shown in Fig. 3, the naïve and M2 ability to inhibit HIV replication is unaffected by previous exposure of these cells to the virus.
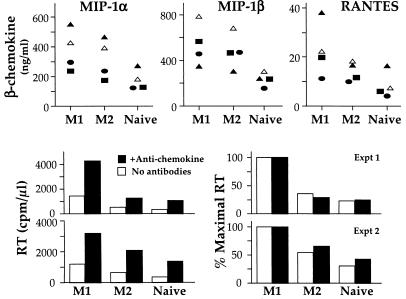
We next addressed whether coculture of M1 with M2 or naïve cells could induce higher levels of CCR5 ligands that might be responsible for inhibition of HIV replication. We compared the levels of MIP-1α, MIP-1β, and RANTES in cocultures of M1 with M2 or naïve cells with the levels of CCR5 ligands in cocultures of M1 with M1 cells (Fig. 4). CCR5 ligands concentrations in cocultures were as predicted based on the ability of the subsets to secrete CCR5 ligands (Fig. 2) multiplied by the proportions of each subset. The highest levels of CCR5 ligands were produced by cocultures of infected M1 with uninfected M1 cells, in which no viral inhibition was observed; slightly lower levels in cocultures of M1 with M2 cells, and much lower in cocultures of M1 with naïve cells, in which the greatest inhibition of HIV replication was observed. The lowest secretion of chemokines in cocultures of M1 with naïve as compared with cocultures of M1 with M1 or M2 cells is consistent with the inability of naïve cells to secrete chemokines (Fig. 2). Therefore, addition of M2 or naïve to M1 cells did not modify the pattern of CCR5 ligand production, and the levels of the ligands in cocultures cannot explain the total inhibition of HIV replication observed for CD4 cultures.
Figure 4.
M2- or naïve-mediated suppression of M1 HIV replication is independent of CCR5 ligands. Cells were sorted, infected, and stimulated as described in Figs. 1 and 2. (Top) Infected M1 cells were cocultured with either infected (filled symbols) or uninfected (empty symbols) M1, M2, or naïve cells as described in Fig. 3. MIP-1α, MIP-1β, and RANTES levels were measured 6 days after infection and stimulation. (Middle and Bottom) Infected M1 cells were cocultured with uninfected M2 or naïve cells, in the absence or presence of a mixture of neutralizing levels of anti-CCR5 ligand Abs (anti-MIP-1α, anti-MIP-1β, anti-RANTES; 50 μg/ml). RT activity was measured in culture supernatants 6 days after infection and stimulation in two independent experiments; data are the average of duplicate cultures for each. (Left) The absolute RT activity. (Right) Relative activity normalized to the amount observed in the comparable M1 culture. Although CCR5 ligands inhibit HIV replication by ≈50%, their action does not account for the total inhibition of viral replication observed in CD4 T cells nor by the activity expressed by naïve cells.
To conclusively rule out the contribution of CCR5 ligands to naïve- and M2-mediated suppression of HIV replication, infected M1 were cocultured with uninfected M2 or naïve cells, or M1 as a control, in the presence of a mixture of anti-CCR5 ligand Abs. Indeed, higher levels of RT activity could be measured in all coculture conditions (Fig. 4 Lower Left), indicating that CCR5 ligands do inhibit viral replication (to some extent) in this system and that the neutralizing Abs blocked chemokine effects. Nonetheless, the presence of anti-β-chemokine Abs did not change the pattern of inhibition exerted by naïve and M2 cells upon R5 HIV replication in M1 cells (Fig. 4 Lower Right).
Discussion
Previous studies (12–14, 25) revealed that naïve CD4 T cells are intrinsically resistant to X4 HIV replication when stimulated with anti-CD3/CD28. This mode of stimulation induces significant cellular proliferation in the absence of HIV replication, even though the cells are infected with the virus. Memory T cells, on the other hand, were productively infected by X4 HIV after CD3/CD28 stimulation. Here we extend those observations to show that the same intrinsic resistance of naïve T cells to HIV is true for R5 viruses. However, we also show that naïve T cells are capable of inhibiting R5 virus replication when cocultured with memory cells.
HIV replication is linked to lymphocyte activation and proliferation; it was commonly accepted that stronger mitogenic stimulation of infected T cells would result in greater HIV production. Therefore, it was originally puzzling that CD28 costimulation, one of the most powerful stimulants of cell activation and proliferation, could result in inhibition of HIV replication (6). However, these studies showed that inhibition occurs only with R5 viruses and with a particular mode of CD3/CD28 stimulation (i.e., Abs coimmobilized on beads)—a condition that induces very high levels of CCR5 ligands and down-regulation of the viral coreceptor CCR5 (7, 8). Nonetheless, Creson et al. (9) reported that down-regulation of CCR5 and secretion of high amounts of CCR5 ligands are not sufficient to inhibit R5 HIV replication.
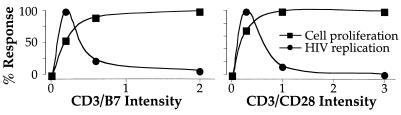
The data we present here show that CD28 costimulation, mediated either by the CD3/CD28 beads or by the more physiological cross-linking by anti-CD3 and B7.1, can enhance or suppress R5 HIV replication in acutely infected CD4 T cells. The enhancement or suppression depends on the strength of the stimulus. Specifically, the use of high concentrations of CD3/CD28 cross-linking agents results in maximal cellular replication as well as a minimal viral replication. Lower concentrations of cross-linking agents result in only slightly decreased cellular proliferation but substantial viral production (Fig. 5). Indeed, the relative inhibition or enhancement of HIV replication compared with cellular replication is identical whether CD28 costimulation is effected by mAbs binding solely to CD28 or by B7.1 that binds both CD28 and CTLA-4.
Figure 5.
Total CD4 T cells were sorted and infected as described in Figs. 1 and 2. Stimulation intensity was varied by increasing the ratio of P815/B7 cells to CD4 cells (Left) or anti-CD3/CD28 beads to CD4 cells (Right). Cell number and viral replication are expressed as percent of maximum. These data show that the strength of the stimulus differentially impacts cellular and viral replication. At slightly suboptimal stimulations (where cellular replication rate is about 90% of maximal), viral replication is substantial. As the stimulation strength is increased, viral replication is suppressed. Thus, the stronger stimulation is required to evince the suppressive activity expressed by naïve, and to a lesser extent, M2 CD4 T cells.
Previous studies showed that stimulation with anti-CD3 and natural ligands for CD28 (B7.1 and B7.2) enhance both X4 and R5 HIV replication (3, 4). Both B7.1 and B7.2 also bind to the CD28 homologue CTLA-4. Although the role of CTLA-4 in T cell activation is controversial, it appears to limit the extent of immune activation and proliferation, counteracting CD28-mediated signaling. It was proposed that the effect of CD3/B7 stimulation on HIV replication might be different compared with stimulation with anti-CD3/CD28 beads caused by binding of B7 to CTLA-4 (26). Indeed, stimulation with beads coated with anti-CD3/CD28/CTLA-4 does not inhibit HIV replication, induces high levels of CCR5 and inhibits CCR5 ligand secretion, as opposed to stimulation with beads coated only with anti-CD3/CD28, which inhibits CCR5 expression and induces high levels of CCR5 ligand secretion (26, 27).
Our data confirm that stimulation with anti-CD3/B7 induces very low levels of CCR5 ligands compared with stimulation with anti-CD3/CD28 beads (Fig. 2), but further show that, by increasing the strength of CD3/B7 stimulation, it is possible to suppress HIV replication, even with very low levels of CCR5 ligands. We propose that the previously reported differential effect of costimulation using anti-CD28 vs. B7 on HIV replication is therefore caused not by the engagement of CTLA-4, but by quantitatively different signal strength (Fig. 5).
This hypothesis has precedent in the report that the degree of CD28 cross-linking can activate distinct signaling pathways (28). Furthermore, the strength of T cell signaling has been shown to affect the balance of T helper (Th)1/Th2 responses (29). Most recently, Riley et al. (27) showed that a high strength of CD28 costimulation (mediated by immobilized anti-CD28) is necessary for inhibition of HIV replication, suggesting that full activation suppresses HIV replication. We further show that the same suppression of HIV replication can be achieved by using a high strength of B7 stimulation.
Previous studies using soluble or plate-bound anti-CD3/CD28 did not show inhibition of HIV replication (3, 9). In this case, we believe that the strength of the CD3/CD28-mediated stimulation was insufficient to generate the inhibitory signals expressed by naïve cells. Note that these conditions may be sufficient to down-regulate CCR5 expression (9)—but it is clear that exceedingly low levels of CCR5 are sufficient for R5 viral infection [for example, R5 HIV replication was efficient in M2 cells (Fig. 2) despite the low levels of CCR5, or on M1 cells sorted for undetectable CCR5 expression (data not shown)].
Perhaps the strongest evidence that CCR5 ligand secretion (or CCR5 down-regulation) is not crucial to the CD3/CD28-mediated inhibition of R5 HIV comes from our study of viral replication in purified CD4 subsets. Here we found that the cells that expressed the highest levels of CCR5 ligands (M1 or CD45RA−CD62L− CD4 T cells) showed the highest viral replication. Furthermore, simply adding back purified naïve CD4 T cells (which secrete very low levels of CCR5 ligands) was sufficient to suppress viral replication in the M1 cells. It is particularly interesting that naïve T cells express this inhibitory mechanism, because naïve T cells are not effector cells in other ways (e.g., expression of effector cytokines, chemokines, or other effector functions).
The inhibition of R5 viral replication in cocultures of CD4 T cells containing the permissive M1 cells and the inhibitory naïve T cells could not be explained by an inhibition of the replication of M1 cells themselves, to a change in CCR5 ligand secretion, and did not depend on exposure of naïve T cells to HIV. Finally, the inhibitory effect of naïve T cells on viral replication in M1 cells is specific to R5 virus, as total CD4 infected with the X4 HIV-1IIIB at the same TCID50 as with HIV-1BaL could replicate virus at very high levels independently of the strength of the costimulation.
We propose that this mode of inhibition (expressed by naïve CD4 T cells) is principally responsible for inhibiting viral replication in bulk CD4 cultures—and that while CCR5 ligands and CCR5 down-regulation clearly inhibit virus, their activity is insufficient to explain the total inhibition of replication in the bulk cultures. At present, we do not understand the basis for this inhibitory mechanism. It depends on the presence of strongly stimulated naïve CD4 T cells; strongly stimulated M2 cells (CD45RA−CD62L+) also exhibit some level of this inhibition (although for a limited amount of time). The inhibition is not evident when naïve T cells are suboptimally stimulated—even when the cell division rate is 90% of maximum.
It is remarkable that naïve T cells express this inhibitory mechanism. Naïve T cells generally do not express effector functions; this ability of a product of naïve T cells to regulate viral replication in memory cells demonstrates a cross-regulatory mechanism expressed by naïve T cells.
Supplementary Material
Acknowledgments
We are grateful to Dr. Thomas Merigan for making the Stanford Center for AIDS Research available for these studies. We thank Dr. David Parks and the Stanford Shared FACS Facility for flow cytometry support, Iwan Tjioe for expert technical help, Dr. Guido Poli for critical reading of the manuscript, and Drs. Pietro Ghezzi, Nicole Baumgarth, and members of the Herzenberg laboratory for advice and help. M. Mengozzi is a fellow of Istituto Superiore di Sanita', Rome. This work was supported by National Institutes of Health Grants CA-42509–14 and CA-81543–02.
Abbreviations
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- RT
reverse transcriptase
- MIP
macrophage inflammatory protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Korin Y D, Zack J A. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran P A, Diegel M L, Sias J C, Ledbetter J A, Zarling J M. AIDS Res Hum Retroviruses. 1993;9:455–464. doi: 10.1089/aid.1993.9.455. [DOI] [PubMed] [Google Scholar]
- 3.Smithgall M D, Wong J G, Linsley P S, Haffar O K. AIDS Res Hum Retroviruses. 1995;11:885–892. doi: 10.1089/aid.1995.11.885. [DOI] [PubMed] [Google Scholar]
- 4.Barker E, Bossart K N, Levy J A. J Immunol. 1998;161:6223–6227. [PubMed] [Google Scholar]
- 5.Stanley S, Ostrowski M A, Justement J S, Gantt K, Hedayati S, Mannix M, Roche K, Schwartzentruber D J, Fox C H, Fauci A S. N Engl J Med. 1996;334:1222–1230. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- 6.Levine B L, Mosca J D, Riley J L, Carroll R G, Vahey M T, Jagodzinski L L, Wagner K F, Mayers D L, Burke D S, Weislow O S, et al. Science. 1996;272:1939–1943. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- 7.Carroll R G, Riley J L, Levine B L, Feng Y, Kaushal S, Ritchey D W, Bernstein W, Weislow O S, Brown C R, Berger E A, et al. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 8.Riley J L, Carroll R G, Levine B L, Bernstein W, St. Louis D C, Weislow O S, June C H. J Immunol. 1997;158:5545–5553. [PubMed] [Google Scholar]
- 9.Creson J R, Lin A A, Li Q, Broad D F, Roberts M R, Anderson S J. J Virol. 1999;73:9337–9347. doi: 10.1128/jvi.73.11.9337-9347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay C R. Immunol Today. 1991;12:189–192. doi: 10.1016/0167-5699(91)90051-T. [DOI] [PubMed] [Google Scholar]
- 11.Picker L J, Treer J R, Ferguson-Darnell B, Collins P A, Buck D, Terstappen L W. J Immunol. 1993;150:1105–1121. [PubMed] [Google Scholar]
- 12.Cayota A, Vuillier F, Scott-Algara D, Dighiero G. Lancet. 1990;336:941. doi: 10.1016/0140-6736(90)92311-5. [DOI] [PubMed] [Google Scholar]
- 13.Woods T C, Roberts B D, Butera S T, Folks T M. Blood. 1997;89:1635–1641. [PubMed] [Google Scholar]
- 14.Spina C A, Prince H E, Richman D D. J Clin Invest. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roederer M, Raju P A, Mitra D K, Herzenberg L A. J Clin Invest. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 17.Kinter A L, Poli G, Fox L, Hardy E, Fauci A S. J Immunol. 1995;154:2448–2459. [PubMed] [Google Scholar]
- 18.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou W, Foussat A, Houhou S, Durand-Gasselin I, Dulioust A, Bouchet L, Galanaud P, Levy Y, Emilie D. AIDS. 1999;13:455–463. doi: 10.1097/00002030-199903110-00003. [DOI] [PubMed] [Google Scholar]
- 20.Lee B, Sharron M, Montaner L J, Weissman D, Doms R W. Proc Natl Acad Sci USA. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedroza-Martins L, Gurney K B, Torbett B E, Uittenbogaart C H. J Virol. 1998;72:9441–9452. doi: 10.1128/jvi.72.12.9441-9452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicenzi E, Bordignon P P, Biswas P, Brambilla A, Bovolenta C, Cota M, Sinigaglia F, Poli G. J Virol. 1999;73:7515–7523. doi: 10.1128/jvi.73.9.7515-7523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roederer M, Dubs J G, Anderson M T, Raju P A, Herzenberg L A. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll R G, Riley J L, Levine B L, Blair P J, St Louis D C, June C H. Semin Immunol. 1998;10:195–202. doi: 10.1006/smim.1998.0131. [DOI] [PubMed] [Google Scholar]
- 27.Riley J L, Schlienger K, Blair P J, Carreno B, Craighead N, Kim D, Carroll R G, June C H. J Exp Med. 2000;191:1987–1997. doi: 10.1084/jem.191.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledbetter J A, Imboden J B, Schieven G L, Grosmaire L S, Rabinovitch P S, Lindsten T, Thompson C B, June C H. Blood. 1990;75:1531–1539. [PubMed] [Google Scholar]
- 29.Lenschow D J, Walunas T L, Bluestone J A. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.