Abstract
Stressful life events (SLEs) are exceedingly common and have been associated with a range of psychological disorders, perhaps through dysregulation in the hypothalamic-pituitary-adrenal (HPA) axis. The use of certain emotion regulation strategies in response to stress, such as expressive suppression and cognitive reappraisal, has additionally been linked to heightened HPA axis reactivity to acute stress. However, it is unclear how emotion regulation may interact with SLEs to affect HPA axis reactivity, particularly concerning relationship stressors (RSs). Using cross-sectional data from 117 men and 85 women aged 18–55 years old (M = 39.9 ± 10.7), we investigated whether trait use of suppression or reappraisal interacted with recent negatively perceived SLEs and relationship stressors to impact HPA axis response to an acute stressor. Separate linear mixed models revealed that trait suppression interacted with SLEs and RSs to predict cortisol response to stress, while reappraisal did not. Findings indicate higher trait expressive suppression may influence the cortisol response to acute stress after exposure to more recent stressful events, particularly when those stressful events include relationship stress.
Keywords: acute stress, suppression, cortisol, relationship stress, emotion regulation, HPA axis
1. Introduction
Stressful life events (SLEs) are exceedingly common and have been associated with a range of mental health disorders, including increased risk of depression, posttraumatic stress disorder (PTSD), and anxiety disorders (Cameron et al., 2010; Francis et al., 2012; Kessler, 1997; Moitra et al., 2011). These disorders are also linked to dysregulated hypothalamic-pituitary-adrenal (HPA) axis reactivity to stress (Jacobson, 2014; Palazidou, 2012; Sherin & Nemeroff, 2011), suggesting that dysregulated HPA axis functioning may be a pathway by which SLEs give rise to psychopathology (Holsboer, 2001; Pariante & Lightman, 2008). Nonetheless, few studies have examined moderators of the relationship between SLEs and HPA axis reactivity, and nothing to date has investigated the impact of relationship stressors on HPA axis reactivity to acute stress.
1.1 Stressful life events and neuroendocrine dysregulation
Exposure to traumatic SLEs is linked with dysregulated HPA axis stress reactivity (Carpenter et al., 2007; Heim et al., 2002; Jacobson, 2014; Palazidou, 2012; Pariante & Lightman, 2008; Sherin & Nemeroff, 2011). While it is challenging to determine whether dysregulated HPA axis reactivity or psychological disorders come first, exaggerated HPA axis reactivity to stress can occur both prior to and during depressive episodes (Ehlert et al., 2001; Holsboer, 2001; Pariante & Lightman, 2008), suggesting changes in HPA axis functioning may precipitate the development of psychopathology. As such, dysregulated HPA axis functioning, including reactivity to stress, following recent SLE(s) may be a neurobiological risk factor for the development of mental health disorders. While research exists concerning the effects of traumatic SLEs on the HPA axis, no known research has explored the possible impact of stressful interpersonal events, specifically, those that involve relationships, on HPA axis reactivity to acute stress.
Social relationships can facilitate better quality and quantity of social support, as well as reduced perceived stress, enhanced immune function, and improved mental and physical health (Cohen, 2004; Fagundes et al., 2011; Kiecolt-Glaser et al., 1997). Notably, SLEs that include threat to social relationships may deeply impact psychological wellbeing. Relationship stress and loss are associated with increased anxiety, lower levels of life satisfaction and higher rates of mental illness (Aseltine & Kessler, 1993; Knopfli et al., 2016; Simon & Barrett, 2010; Rhoades et al., 2011). Furthermore, stressful events that involve relationship stressors (RSs) may involve not only the stressor itself, but also the possible stress of a sudden loss of social support and sense of belonging (Cohen & Wills, 1985; Williamson & Schulz, 1992). The importance of relationships for wellbeing (Fagundes et al., 2011) and a threat to such relationships may be one reason for the disparate responses to interpersonal and noninterpersonal traumas.
Interpersonal events (e.g., physical attack, sexual assault) typically lead to greater psychological distress and higher rates of PTSD than events caused by accident or nature (e.g., automobile accident, natural disaster; Greene et al., 2000). Further, interpersonal traumas that are perpetrated by someone that is close to and trusted by the victim are more distressing than those caused by strangers (Freyd, 1996; Goldsmith, Freyd, & DePrince, 2011). Together, the importance of good relationships to health, the negative impacts of relationship loss, and the additive effects of relationships on traumatic responses suggests that when considering SLEs, stressors that include relationships may be perceived as especially stressful and could have a particularly potent effect on HPA axis stress reactivity; however, no research to date has specifically examined this association.
While SLEs and RSs are common, not everyone who experiences them develops dysregulated HPA axis functioning or poorer health. In fact, the majority of individuals exposed to stressful or traumatic events exhibit resilience (Bonanno et al., 2011). Continued healthy functioning for most people suggests other individual factors, such as emotion regulation, may influence vulnerability to the negative psychological and physiological effects of SLEs and RSs.
1.2 Emotion regulation strategies
Emotion regulation strategies are ways individuals exhibit control over their emotions, when they have them, and how they are expressed (Gross, 1998). Two common emotion regulation strategies are expressive suppression and cognitive reappraisal. Expressive suppression involves withholding emotional expression despite internal arousal (Gross, 1998; Gross & John, 2003). It is related to less social sharing, heightened sympathetic nervous system activation (Gross & Levenson, 1993, 1997), less positive emotion, and greater negative emotion (Gross & John, 2003), higher rumination and depressive symptoms (Gross & John, 2003; John & Gross, 2004) and its frequent use is generally considered to be maladaptive (John & Gross, 2004).
In comparison, cognitive reappraisal involves re-interpreting an emotion-eliciting event or situation to alter its emotional impact (Gross & John, 2003). Reappraisal is typically regarded as an adaptive emotion regulation strategy; increased reappraisal use is associated with healthier patterns of affect, social functioning, and well-being than suppression (John & Gross, 2004). Increased reappraisal ability is also related to fewer depressive symptoms (Troy et al., 2010) and is protective against the negative impact of increased stress on body mass index and type II diabetes (Sagui & Levens, 2016). Additionally, reappraisal is associated with an increased ability to recover psychologically from negative emotionally arousing situations both on a day-to-day basis as well as in response to experimentally-induced negative stimuli (Augustine & Hemenoover, 2009; Gross & John, 2003; Meyer et al., 2012).
1.3 Emotion regulation and cortisol reactivity
Although suppression and reappraisal are different emotion regulation strategies, habitual use of both is linked with greater HPA axis reactivity to acute stress (Lam et al., 2009). Further, when participants were instructed to use reappraisal during an acute stressor, they exhibited a greater cortisol response than their counterparts who were given no instruction (Denson et al., 2014). As an elevated HPA axis response is typically viewed as detrimental to health (e.g., Kirschbaum et al, 1995; Morris & Rao, 2014; Puig-Perez et al., 2016), these findings raise the question of how an adaptive emotion regulation strategy, such as reappraisal, and a maladaptive strategy, such as suppression, could both give rise to elevated HPA axis stress reactivity.
One potential explanation may be in the short- versus long-term effects of reappraisal. Reappraisal, an approach-oriented strategy, requires individuals to exert cognitive effort to engage with and process negative emotion, identify ways in which negative content may be framed more positively, and then cognitively reinterpret the situation (Sheppes et al., 2011). Consequently, reappraisal in the short-term may increase negative affect and stress as the individual engages more cognitive resources to process and reframe the negative stimuli. When exposed to more stressors, however, habitual use of an adaptive strategy such as reappraisal may increase ability, ease of reappraisal, and meaning finding, possibly resulting in greater psychological and physiological habituation to stress and lower reactivity in the future. Although reappraisal is effortful in the short-term (Shafir et al., 2015), it is associated with long-term reduction of negative affect and adaptive outcomes following traumatic events and stressors (Denson et al., 2014; Moore et al., 2008; Troy et al., 2010). Hence, reappraisal may exacerbate HPA axis activity in the short-term in response to stress for those with less stressor experience, but result in a well-regulated system in the long-term for those with higher reappraisal, leading to a habituated stress response.
Conversely, expressive suppression is cognitively and physiologically taxing because it involves the behavioral inhibition of ongoing emotion expression during emotional arousal, but it does not change the subjective experience, which increases negative affect and risk for cardiovascular disease when used habitually (Gross, 1998; Gross & John, 2003; Hu et al., 2014; Mauss & Gross, 2004). As suppression contributes to greater negative affect and is physiologically demanding due to the increased cognitive and behavioral effort required to suppress expression, habitual suppression may additively interact with the experience of recent stressful events to uniquely heighten HPA axis reactivity. Despite the psychological effects of reappraisal and expressive suppression in response to stressors as well as their association with HPA axis reactivity, no research thus far has investigated the possible interaction between these SLEs and emotion regulation strategies on HPA axis reactivity to stress.
1.4 Current study
The aim of this study was to determine whether reappraisal or suppression modulates HPA axis reactivity to acute stress within the context of recent SLEs and, in particular, RSs. We hypothesized that consistent with prior research, individuals higher in reappraisal and suppression would have exaggerated cortisol reactivity to an acute stressor. We also predicted that suppression would interact with SLEs and RSs exposure such that as an individual experiences more SLEs, those who habitually suppress would have exaggerated HPA axis reactivity to stress as their SLEs and RSs increase. Furthermore, we expected higher reappraisal would buffer the effects of SLEs and RSs exposure on HPA axis stress reactivity as long-term or well-practiced reappraisal skills would dampen HPA axis stress reactivity among those with greater reappraisal compared to lower levels of reappraisal.
2. Methods
Secondary data were obtained from the Pittsburgh Cold Study 3 (PCS 3) within the Common Cold Project. The data were collected by the Laboratory for the Study of Stress, Immunity, and Disease at Carnegie Mellon University under the directorship of Sheldon Cohen, PhD; and were accessed via the Common Cold Project website (www.commoncoldproject.com; grant number NCCIH AT006694). The PCS 3 was originally a viral challenge study conducted between 2007 and 2011; including an acute stressor laboratory session before and after the viral challenge. Data used in these analyses were from the baseline (i.e., pre-viral challenge) period of the study. This study received approval by the Carnegie Mellon University and University of Pittsburgh Internal Review Boards and all participants gave informed consent via the telephone during screening and in writing before study procedures began.
2.1 Participants
Participants included in analyses were 117 men and 85 women aged 18–55 years old (M = 29.9, SD = 10.7), who experienced negative SLEs ranging from 0 to 9 in the past 12 months (M = 2.5, SD = 2.2). In regards to relationship stressors (RSs), participants endorsed a range of 0–5 negatively perceived RSs with an average of 0.96 (SD = 1.10). A full summary of sample characteristics is shown in Table 1. All volunteers were from the Pittsburgh, Pennsylvania, metropolitan area who responded to advertisements and were judged to be in good health via a medical history screener and a medical examination by a study physician. Further exclusion criteria included: regular use of psychoactive medications, antidepressants, sleeping pills, or tranquilizers; use of steroids or immunosuppressants in the past three months; a history of any psychiatric disorder treated within the past 12 months; acute illness in the past 30 days, a history of chronic illness (e.g., diabetes, asthma, cardiovascular disease); females currently pregnant or lactating. Female participants were also tested for pregnancy, and participants with positive results were excluded from the study.
Table 1.
Descriptive characteristics of sample population including zero order correlations
| Variable | M ± SD/n (%) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age (years) | 29.9 ± 10.7 | -- | |||||||||||
| 2. Sex (female) | 85 (42%) | .04 | -- | ||||||||||
| 3. HM use (yes) 20 (10%) | 20 (10%) | −.16 * | .43** | -- | |||||||||
| 4. Race (white) | 138 (68%) | −.15* | −.09 | .09 | -- | ||||||||
| 5. Smoker (yes)a | 93 (46%) | .06 | −.12 | −.14 | .01 | -- | |||||||
| 6. BMI (kg/m2) | 27.2 ± 6.4 | .34** | .13 | −.10 | −.22** | −.06 | -- | ||||||
| 7. Educationb | 5.3 ± 1.7 | −.07 | .10 | .21** | .32** | −.21* | −.14* | -- | |||||
| 8. Baseline cortisolc | 6.28 ± 3.92 | −.14 | −.24** | −.03 | .05 | .10 | −.04 | −.07 | -- | ||||
| 9. SLEs | 2.5 ± 2.2 | −.07 | .15* | .02 | −.01 | −.05 | .02 | .17* | −.05 | -- | |||
| 10. RSs | 1.0 ± 1.1 | −.06 | .05 | −.05 | −.05 | .05 | .03 | .11 | .01 | .77** | -- | ||
| 11. Non-RSs | 1.5 ± 1.5 | −.05 | .18* | .07 | 02 | −.11 | .00 | .16* | −.08 | .89** | .40** | -- | |
| 12. Reappraisal | 28.8 ± 6.7 | .07 | −.01 | −.07 | 00 | .11 | .01 | .00 | .04 | −.03 | .05 | −.08 | -- |
| 13. Suppression | 13.5 ± 4.9 | .07 | −.25** | .00 | −.07 | −.04 | −.04 | −.09 | .06 | −.02 | −.01 | −.02 | -.07 |
Note. N = 202;
p < .05,
p < .01,
HM = Hormonal Medication; BMI = Body Mass Index; kg/m2 = kilograms per meters squared; SLEs = Stressful Life Events in the past 12 months; RSs = Relationships Stressors in the past 12 months.
Participant smoked a cigarette at least once in the last two weeks.
Education at level 5 is equivalent to an associate’s degree or 2 years of college.
Salivary cortisol units = nanomoles per liter (nmol/L).
2.2 Procedure
After undergoing screening and providing informed consent, participants completed a series of psychosocial questionnaires within 3 weeks prior to the laboratory visit. To ensure the most accurate assessment of salivary cortisol, participants were asked to refrain from drinking alcohol for 48 hours, from taking any nonprescription medications for 24 hours, from eating and drinking (except water) for two hours, and from smoking for one hour prior to their laboratory session. To control for diurnal variation in cortisol, the laboratory session in which the participants underwent the social-evaluative stress task occurred in the afternoon thru early evening. Specifically, all participants’ baseline cortisol samples were taken between 3:00pm and 7:30pm following a 20-minute baseline (acclimation) period.
Acute Stress Provocation
Participants underwent a modified version of the Trier Social Stress Test (TSST; Kirschbaum et al., 1993), a well-validated, reliable method for provoking HPA axis response (Dickerson & Kemeny, 2004). In the modified version, participants were instructed to prepare a speech to defend themselves against either an alleged traffic violation or shoplifting incidence. Participants were told that the speech would be evaluated. They were given 5 minutes to prepare, and then were videotaped delivering their speech for another 5 minutes. At the end of the 5-minute speech, an evaluator entered the room and instructed the participants to serially subtract 13 from 1,022 as quickly and accurately as possible for an additional 5 minutes. If a participant made an error, they were told to restart from the beginning. The total time for the TSST was 15 minutes. Saliva samples were taken immediately prior to the TSST, directly following completion, and then at 10-minute intervals for 50 minutes, totaling 7 samples. In the parent study, two acute stress lab sessions occurred pre- and post-viral challenge; thus, two topics (e.g., traffic violation and shoplifting) for the public speaking were used and counterbalanced across visits. The project examines the cortisol response from the pre-viral challenge stress lab visit.
2.3 Measures
Stressful Life Events
The Life Events List (LEL; Cohen et al., 1991) is a 24-item questionnaire used to assess major stressful life events occurring within the past 12 months. Participants indicated whether they had personally experienced each of the possible life events within the past year or if someone they were close to had experienced the event. Notably, if a life event such as death or worsening relationship was endorsed, participants were asked to specify who the event happened with/to, allowing for greater information collection and multiple exposures to one of the 24 possible stressful life events. For the purposes of this study, life events that happened personally to the participant and which were either normatively considered as negative (e.g., deaths, crime) or perceived as negative (e.g., job change, moving) were summed for analyses with possible scores ranging from 0 to 39. Examples include “Lost or changed job,” “Suffered a significant business/investment loss,” and “House was broken into and/or burgled.” In addition, a relationship stressor (RS) variable was created by summing endorsement of a subset of the SLEs related to interpersonal stressors, including events that were normatively negative or perceived as negative: death of a loved one, ending of romantic relationship, ending of significant friendship, worsening of a significant relationship, and separation or divorce. Possible RS values ranged from 0 to 15. Non-RSs was calculated by subtracting RS from SLEs, resulting a possible range of 0–24. For all variables, greater values indicate greater exposure to stressful life events.
Emotion Regulation Strategies
The Emotion Regulation Questionnaire (ERQ) is a 10-item self-report scale used to assess the habitual use of two strategies for managing emotions: cognitive reappraisal and expressive suppression (Gross & John, 2003). The suppression subscale consists of 4 items such as “I keep my emotions to myself” and “I control my emotions by not expressing them”. The reappraisal subscale consists of 6 items, including “When I want to feel less negative emotion (such as sadness or anger), I change what I’m thinking about” and “When I am faced with a stressful situation, I make myself think about it in a way that helps me stay calm.” Respondents indicate how strongly they agree with statements on a 7-point Likert scale (1 = strongly disagree, 7 = strongly agree). The ERQ reliably captures both suppression (Cronbach’s α = .76) and reappraisal (Cronbach’s α = .83) across a variety of populations (Gross & John, 2003; Moore et al., 2008; Troy et al., 2013). Higher values indicate greater likelihood in using suppression or reappraisal.
2.4 Salivary Cortisol
All saliva samples were collected using the Salivette® (Sarstedt, Rommelsdorft, Germany). Saliva samples were placed in an ultra-low freezer and shipped to Dr. Clemens Kirschbaum (Dresden, Germany) for cortisol assessment. Cortisol levels were determined by time-resolved fluorescence immunoassay with a cortisol-biotin conjugate as a tracer (Dressendofer et al, 1992). Intra- and inter-assay variabilities were each less than 12%.
2.5 Analytic Plan
Participants missing their baseline cortisol value or with 3 or less cortisol values were removed from all analyses (N=11), resulting in a final sample size of 202 participants that are summarized in the participant description above and in Table 1. Acute cortisol stress reactivity was analyzed via two outcomes, 1) as area under the curve with respect to increase (AUCi) and 2) cortisol response trajectories over time. All cortisol models adjusted for continuous potential confounders of age, BMI, education level and time of day the stressor task started, as well as categorical variables of smoking status (1 = current vs. 0 = nonuser), race (1 = all minority vs. 0 = white), sex (1 = female vs. 0 = male), public speaking topic (0 = traffic violation vs. 1 = shoplifting), and hormonal medication use (1= user vs. 0 = nonuser). Using PROCESS model 1 (Hayes, 2013), we examined the moderating role of emotion regulation (reappraisal and suppression, independently) on the relationship between negative stressful life events to self (SLEs) and cortisol AUCi. Any significant finding with SLEs was repeated using the relationship stressors (RSs) variable while controlling for non-relationship SLEs (non-RSs) to examine the role of relationship stress in the overall results. Due to missing cortisol values, AUCi analyses only included 178 participants.
Because several cortisol values were measured within each subject, linear mixed models were also used to examine the effects of SLEs and emotion regulation while taking into account the correlation within subjects over time (Bennett et al., 2013; Diggle, Heagerty, Liang, & Zeger, 2002). A diagonal variance-covariance structure was fitted to estimate error variance. The models were fit using Linear Mixed Model in SPSS with a REPEATED statement (IBM SPSS 23, Armonk, NY, USA). Each linear mixed model also included random effects for slope, baseline cortisol levels, and start time of the stress session as well as continuous fixed effects of time post-stressor, SLEs, emotion regulation (reappraisal and suppression, independently) and all 2- and 3-way interactions among time, SLEs, and emotion regulation strategies. Any significant finding with SLEs was repeated using relationship stressors (RSs) variable while controlling for non-relationship SLEs to examine the role of relationship stress in the overall results. To interpret significant interactions, predictors were mean split to summarize the cortisol values over time (Schielzeth, 2010). Linear mixed models can handle missing cortisol values, thus, these analyses included 202 participants summarized in Table 1. Following a previously reported data analysis plan (Stone et al., 2001), cortisol data were log10 transformed for all participants’ raw values. It is important to note that all analyses remained significant regardless of predicting raw or transformed cortisol data. A two-sided significance level of α=0.05 was used for all tests.
3. Results
3.1 Cortisol Area Under the Curve with respect to increase (AUCi) Analyses
3.1.1 Examination of Interaction between SLEs and Emotion Regulation Strategies
Suppression moderated the relationship between stressful life events and AUCi response to the acute stressor (β = .083; ΔR2 = .022, p<.05). Specifically, among those who reported higher suppression, the greater the number of stressful life events, the larger cortisol’s AUCi (β= .690, t (165) = 2.20, p<.05), while there was no relationship between SLEs and AUCi for those with lower suppression (β= −.123, t (165) = −.43, n.s.). Being female and shoplifting topic were significant covariates related to a smaller AUCi. See Table 2 for the summary of the moderated regressions. Reappraisal had no main effect as well as no interaction with SLEs to predict AUCi levels.
Table 2.
Summary of moderated linear regression for negative stressful life events (left) and relationship stressors (right) interactions with suppression predicting cortisol area under the curve with respect to increase (AUCi) in response to an acute lab stressor
| Stressor Type | ||||||||
|---|---|---|---|---|---|---|---|---|
| SLEs | RSs | |||||||
|
| ||||||||
| Variable | β | S.E. | R2 | ΔR2 | β | S.E. | R2 | ΔR2 |
| Constant | 6.388 | 7.598 | 7.771 | 7.570 | ||||
| Smoker (yes) | −1.964 | 1.007 | −2.166* | 1.020 | ||||
| Race (white) | −1.069 | 1.164 | −.957 | 1.160 | ||||
| Speech Topica | 2.240* | .988 | 2.049* | .980 | ||||
| Sex (Female) | −2.519* | 1.185 | −2.327 | 1.180 | ||||
| HM use (yes) | 1.063 | 1.909 | 1.000 | 1.897 | ||||
| Age | .005 | .056 | .005 | .055 | ||||
| Education | .396 | .331 | .403 | .331 | ||||
| BMI | .072 | .083 | .062 | .083 | ||||
| Stressor Start Time | −.635 | .440 | −.701 | .438 | ||||
| Non-RSs | n/a | n/a | −.048 | .367 | ||||
| Stressor type (ST) | .302 | .225 | .777 | .491 | ||||
| Suppression (Sup) | −.182 | .106 | −.196 | .106 | ||||
| ST x Sup | .083* | .041 | .118* | .022* | .201* | .083 | .134* | .031* |
Note. N = 178; due to missing cortisol values.
p < .05.
As part of parent study, participants experienced two acute stress lab sessions; speech topics were counterbalanced across visits and included shoplifting or traffic violation.
SLEs = Stressful Life Events experienced in the last 12 months; RSs = Social Relationship Stressful Events in the last 12 months; β = standardized beta weight; S.E. = standard error; HM = Hormonal medication; BMI = Body Mass Index. The interaction term is the product of the mean center ST and Sup variables.
3.1.2 Examination of Interaction between RSs and Emotion Regulation Strategies
Mirroring the SLE analyses, suppression interacted with relationship stressors (RSs) to predict cortisol AUCi, while controlling for non-RSs (β = .201; ΔR2 = .031, p<.05). Again, as number of RSs increased, the AUCi increased for those who reported higher levels of suppression (β= 1.717, t (165) = 2.73, p<.01), yet, there was no significant relationship observed among those with lower suppression (β= −.251, t (165) = −.39, n.s.). Due a potential concern with reduced variability in RSs, the analyses were also conducted with RSs as dichotomous (0= no RSs vs 1= 1+ RSs). Similar results occurred whether RSs was included as a continuous or dichotomous variable; reported statistics summarize the results from analyses with RSs as a continuous variable.
3.2 Cortisol Linear Mixed Model Analyses
3.2.1 Response to TSST
Overall, there was a significant effect of time; salivary cortisol significantly increased immediately after the stressor and significantly decreased during recovery (p<.001). As expected, sex and age were significant covariates of the cortisol response. However, smoking status, race, education level, hormonal medication use, and BMI were not significant predictors and their exclusion did not modify the outcome of model. A significant main effect for the public speaking topic (i.e., traffic violation or shoplifting) was observed and remained a covariate throughout all analyses. Number of SLEs did not significantly affect the cortisol stress response, and neither suppression nor reappraisal had a significant main effect on the cortisol stress response.
3.2.2 Examination of Interaction between SLEs and Emotion Regulation Strategies
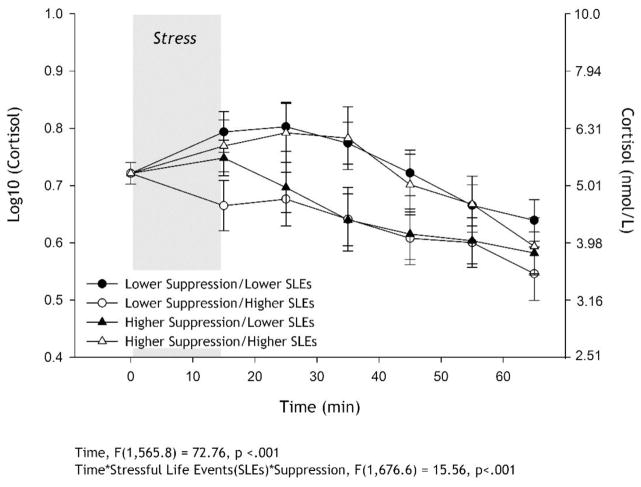
In addition to the significant time effect, the 3-way interaction of time X SLEs X suppression was significant (p<.001; summary statistics can be found in Table 3). As displayed in Figure 1, for individuals with lower suppression, those with fewer SLEs had what appears to be a “typical” cortisol reactivity to the lab stressor with a rise and fall, while greater exposure to SLEs was related to lower overall cortisol production due to what appears to be a non- or minimal response to the lab stressor. Among those who reported a higher tendency to use suppression, the immediate response to acute stress appears to be similar, but their recovery trajectories are different. Specifically, those with greater SLEs have larger cortisol response overall with their recovery mirroring the lower suppression/lower SLE trajectory, while those who experience lower SLEs appear to recover very quickly from the acute stressor. All 2-way interactions were not statistically significant. No 2-way or 3-way interactions including reappraisal reached statistical significance.
Table 3.
Summary of random and fixed effects for linear mixed models examining the moderating role of suppression on the association between negative stressful life events (left) and relationship stressors (right) and cortisol reactivity to an acute lab stressor
| Stressor Type | ||||
|---|---|---|---|---|
| SLEs | RSs | |||
|
| ||||
| Variables | Estimate | S.E. | Estimate | S.E. |
| Random Effects | ||||
| Baseline Cortisol | .414 | .588 | .406 | .575 |
| Start Time | .000 | .001 | .001 | .001 |
| Fixed Effects | ||||
| Intercept | .577*** | .102 | .605*** | .101 |
| Smoker (yes) | −.023 | .014 | −.025 | .014 |
| Race (white) | .001 | .016 | .004 | .016 |
| Speech Topica | −.009 | .014 | −.011 | .013 |
| Sex (Female) | −.116*** | .017 | −.114*** | .017 |
| HM use (yes) | −.003 | .025 | .004 | .025 |
| Age | −.003*** | .001 | −.003*** | .001 |
| Education | .006 | .004 | .007 | .004 |
| BMI | .001 | .001 | .001 | .001 |
| Non-RSs | n/a | n/a | −.005 | .005 |
| Time | −.031*** | .004 | −.031*** | .004 |
| Stressor type (ST) | −.005 | .006 | −.011 | .012 |
| Suppression (Sup) | −.003 | .003 | −.003 | .003 |
| Time x ST | .002 | .002 | .006 | .003 |
| Time x Sup | −.001 | .001 | −.001 | .001 |
| Time x ST x Sup | .001*** | .000 | .002*** | .000 |
Note. N = 202; due to missing cortisol values.
p < .001.
As part of parent study, participants experienced two acute stress lab sessions; speech topics were counterbalanced across visits and included shoplifting or traffic violation.
SLEs = Stressful Life Events experienced in the last 12 months; RSs = Social Relationship Stressful Events in the last 12 months; S.E. = standard error; HM = Hormonal medication; BMI = Body Mass Index. The interaction term is the product of the mean center ST and Sup variables.
Figure 1.
Salivary cortisol (mean ±SEM) response to acute lab stressor (log transformed on the left axis and raw on the right axis). Linear mixed model controlled for age, sex, race, smoking status, speech topic, hormone medication use, education, and body mass index (BMI) as fixed effects and baseline cortisol and time of day for baseline sample as the random effects. Number of recent stressful life events (SLEs), trait emotion suppression, time and all 2- and 3-way interactions were fixed effect predictors. To approximate the significant time * SLEs * suppression interaction, the sample was mean split on SLEs and suppression for interpretation and display purposes; however, SLEs and suppression were entered as continuous predictors in the mixed linear model, not as categorical. For individuals with lower suppression, greater exposure to SLEs (○) was related to lower overall cortisol production due to what appears to be a minimal response to the lab stressor, while those with fewer SLEs (●) had what appears to be a “typical” cortisol reactivity to the lab stressor with a rise and fall, resulting in comparatively more cortisol production. Among those who reported higher tendency to use suppression, the immediate response to acute stress appears to be similar, but their recovery trajectories are different. Specifically, those with greater SLEs (△) have greater cortisol response overall with their recovery mirroring the lower suppression/lower SLE trajectory, while those who experience lower SLEs (▲) appear to recover very quickly from the acute stressor.
3.2.3 Examination of the interaction between relationship stressors (RSs) and suppression
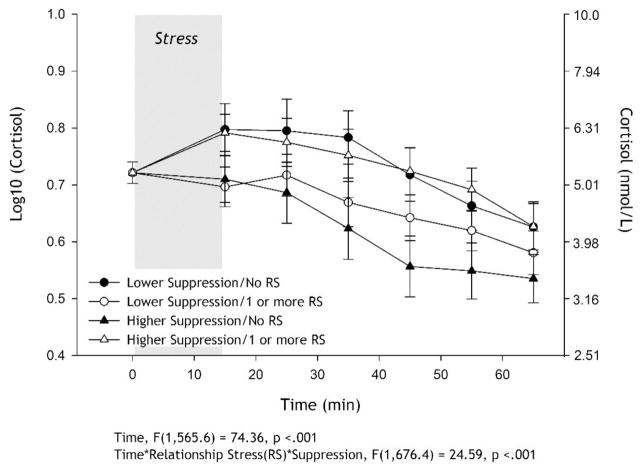
In addition to the significant time effect, there was a significant time X RSs X suppression effect (p<.001; summary statistics can be found in Table 3). In contrast to the lower suppression findings for SLEs, the cortisol curves for those with lower suppression levels appear to be similar regardless of exposure to RSs, mirroring the AUCi analysis. Among those who endorsed higher suppression, lower RSs were related to a minimal cortisol response followed by quick recovery, while individuals with greater RSs appear to display the typical cortisol reactivity with a significant rise and recovery to baseline within 50 minutes of the stressor ending, following a comparable trajectory to individuals with lower levels of suppression (see Figure 2). None of the 2-way interactions were statistically significant. RSs results remained significant regardless if analysis controlled for non-relationship stressful life events. Mirroring AUCi analyses, these analyses were also conducted with RSs as dichotomous (0= no RSs vs 1= 1+ RSs); the 3-way interaction was significant. All analyses summarized in the paper are based on the continuous RSs analyses.
Figure 2.
Salivary cortisol (mean ±SEM) response to acute lab stressor (log transformed on the left axis and raw on the right axis). Linear mixed model controlled for age, sex, race, smoking status, speech topic, hormone medication use, education, body mass index (BMI), non-relationship stressors as fixed effects and baseline cortisol and time of day for baseline sample as the random effects. Number of recent relationship stressors (RSs), trait emotion suppression, time and all 2- and 3-way interactions were fixed effect predictors. To approximate the significant time * RSs * suppression interaction, the sample was mean split on suppression and RSs as a dichotomous variable (0 = no RSs; 1 = 1 or more RSs) for interpretation and display purposes; however, RS and suppression were entered as continuous predictors in the mixed linear model, not as categorical. Among those who reported greater suppression, no RSs (▲) was related to a minimal cortisol response followed by quick recovery, while individuals with RSs (△) appear to display the typical cortisol reactivity with a significant rise and recovery to baseline within 50 minutes of the stressor ending. For those who reported lower suppression, the experience of RSs does not significantly affect their cortisol response over time.
Post-hoc analyses to further examine the relationship among SLEs, RSs, suppression and acute cortisol reactivity
We ran additional analyses using non-RSs as the primary predictor and suppression as the moderator, while controlling for RSs. For AUCi analyses, the interaction between non-RSs and suppression was not significant (β = .0733; ΔR2 = .007, n.s). In the linear mixed model, non-RSs interacted with suppression to significantly predict cortisol reactivity while controlling for RSs (β= .000493, t (671.5) = 2.160, p = .031).
4. Discussion
The purpose of this study was to determine whether individual differences in reappraisal or suppression moderated the relationship between recent SLEs and the HPA axis response to an acute stressor. Further, the analyses were re-conducted to examine the role of RSs. While there was no main effect of tendency to reappraise or suppress on the acute cortisol stress response in this healthy adult sample, suppression uniquely interacted with both SLEs and RSs to predict individual differences in the HPA axis acute stress response (i.e., AUCi and cortisol slope over time). Our first analyses revealed that among people with higher suppression tendencies, those who endorsed more SLEs had a larger AUCi due a larger cortisol rise and slower recovery, while those with fewer SLEs had a smaller AUCi due to a smaller cortisol response and quicker recovery. While controlling for non-relationship SLEs (non-RSs), our second analysis focusing particularly on RSs mirrored the SLEs by suppression interaction findings for those who have a greater tendency to utilize suppression.
As emotion regulation strategies are expected to be used in response to SLEs, the assessment of HPA axis stress reactivity following these events can reveal whether the strategies may buffer or exacerbate reactivity of stress systems in the long term. The present pattern of findings suggests that the interaction between recent negative stressful experiences and the tendency to suppress emotion expression has the potential to modulate HPA axis reactivity to stress in the long term, possibly due to suppression preventing the psychological and physiological adaptation to the SLEs encountered. Habitual suppression may be detrimental when the individual has experienced fewer SLEs supporting other research that suggests suppression as generally being damaging to psychological and physical health (Gross & John, 2003; Hu et al., 2014; John & Gross, 2004; Mauss & Gross, 2004) and that suppression may mediate the relationship between SLEs and psychological disorders (Compare et al., 2014).
The significant interactive effect when examining only relationship stress (i.e., the worsening or loss of a close relationship) and suppression while controlling for non-RSs suggests interpersonal stressors may explain the more global SLEs and suppression findings. Our follow-up exploratory analyses, examining whether non-RSs interacted with suppression while controlling for RSs, suggest that RSs do not fully explain the interaction of SLEs and suppression on acute cortisol reactivity. However, when reviewing the summary statistics, RSs in conjunction with suppression explain more of the variance in cortisol reactivity than non-RSs do, supporting a large literature that focuses on the role of close relationships on physical health. Close relationships, as well as the social support they bring, are imperative to psychological and physical health (Robles et al., 2014; Soulsby & Bennett, 2015), while poor relationship quality, relationship dissolution, and the death of a loved one are associated with poorer psychological and physical health outcomes (Kiecolt-Glaser et al., 1987; Knopfli et al., 2016; Rote, 2016; Stroebe et al., 2007). Thus, the importance of social relationships for health and well-being may mean that the effects of suppression on health are amplified when stressful events include the loss or ending of a relationship. This hypothesis was supported by our data.
In addition to negative relationships events being more stressful for the individual than non-RSs (e.g., losing a job, home burglary, etc.), the impact of suppression on health may be greater in these circumstances because an effort to conceal emotions can affect not only psychological and physical well-being, but also social functioning (Gross & John, 2003). For example, an effort to conceal emotions during a relationship conflict or ending, either with the partner or others on whom the individual may normally depend for support, may impact social communication and reduce empathic caring as well as social support. This situation can compound the stress incurred beyond the primary relationship stressor and may shift an acute stressful life event into a chronic stressful experience. Unfortunately, these relational processes were unable to be addressed directly due to the secondary nature of the data.
In addition, we note that in Figure 1, higher levels of suppression may not universally lead to a dysregulated cortisol response. For individuals with more SLEs, higher suppression was associated with a greater cortisol response compared to those with lower levels of suppression, mirroring Lam and colleagues’ (2009) previous report. However, habitual suppressors with less exposure to SLEs recovered from the lab stressor more quickly as indexed by an earlier decrease in cortisol levels compared to their lower suppression counterparts. Non-habituation to stress among those with lower suppression may explain the effect. Alternatively, the TSST is a task in which suppression is contextually appropriate (i.e. suppression of anxiety is expected in the context of public speaking); thus, higher habitual suppression may be beneficial when SLEs are lower, as the individual is practiced in not conveying their emotions. Overall, more research is needed to determine the reproducibility of the effects found here and delineate the cause of differential acute stress responses with fewer or greater recent major SLEs.
While suppression seems to have varying effects on HPA axis response to acute stress contingent upon SLE occurrence, the interaction between SLEs and reappraisal did not significantly explain differences in the HPA axis response. Thus, our hypotheses were partially supported by the findings. Although reappraisal is typically considered an adaptive emotion regulation strategy (John & Gross, 2004), it is possible that under novel acute stress conditions, the effects of reappraising require more physiological arousal to support psychological transition that may blur or lead to unpredictable HPA axis reactivity. We also cannot be sure that the tendency to reappraise stressful events translated to actual use in response to the recent SLEs or the acute lab stressor.
Furthermore, as mentioned above, the TSST may provide a context that allows individuals to use habitual suppression more readily than reappraisal. The TSST relies on an individual’s concern with a social evaluation (Dickerson & Kemeny, 2004); thus, to hide or minimize their inner emotions from the experiment evaluator/video recording, individuals who tend to utilize suppression may have used it readily during the TSST to reduce the appearance of anxiety while “publically” speaking and serial subtracting, leading to a quicker cortisol recovery. However, attempting to utilize reappraisal when experiencing a social evaluative stressor may not be as natural or easily implemented given the context of the stressor, limiting our ability to observe reappraisal effects. The adaptiveness of emotion regulation appears to rely on the context in which the strategy is used (Lovallo, 2011; Sagui & Levens, 2016). Therefore, our findings have the capacity to contribute to a larger literature on context variable emotion regulation strategies, stressors and SLEs. To further elucidate the ways in which contextually appropriate emotion regulation strategies influence cortisol acute stress reactivity, future research should assess what emotion regulation strategies were used in response to particular events and their impact.
Surprisingly, neither reappraisal nor suppression had main effects on HPA axis reactivity. The lack of significant results was also true when examining only relationship stress. These main effect findings contradict prior research by Lam and colleagues (2009) which found that trait reappraisal and suppression were associated with an exaggerated cortisol response. Given that only one prior study has examined the association between trait use of these emotion regulation strategies and HPA axis reactivity, thus far, more research is necessary before we can make conclusions as to the effect of trait emotion regulation on HPA axis reactivity. Additionally, the current study utilized an altered TSST protocol in which the speech portion of the task was performed in front of a video camera as opposed to an in-person group of confederates, which may reduce the social pressure of the TSST potentially resulting in a reduced HPA axis response (Hawn et al., 2015). Future studies should include confederates during this portion of the stress provocation to ensure that that the TSST has the desired effect and potentially elicit stronger HPA axis responses.
Further, the characteristics of our sample are different from Lam and colleagues (2009). On average, the present sample is 10 years older and contains 30% more males compared with the prior study. Age and gender may modulate emotion processing, including perception and regulation (Gross & John, 2003; Nolen-Hoeksema & Aldao, 2011). For example, younger adults have reported using suppression at a greater frequency compared to older adults (Schirda et al., 2016). Moreover, women, who are evolutionarily adapted and societally expected to be more expressive (Nolen-Hoeksema & Aldao, 2011), may be particularly impacted by the suppression of emotions, especially when stress reactivity is elicited using a social evaluation (Benenson et al., 2013). Hence, our more gender-balanced sample combined with age differences may explain the different set of findings. Unfortunately, we were unable to analyze the effects of sex or age using a 4-way interaction because the original data collection does not provide sufficient power; this lack of power is a limitation of utilizing secondary data in the current study. Additional studies must be designed and powdered appropriately to examine how age and sex may directly interact with emotional regulation and SLEs (i.e., 4-way interaction) to modulate HPA axis stress reactivity and provide consensus.
The present investigation was strengthened by including only psychologically and physically healthy adults, demonstrating that this effect can be seen even without a clinical diagnosis of a psychological disorder such as major depression or posttraumatic stress disorder. Additionally, most research examining life events focuses on traumatic events and ignores the possible influence of stressful events that are not typically considered traumatic (e.g., relationship breakup, divorce) on physiological functioning; our findings demonstrate that even events that are commonly experienced and thus considered normative across the lifespan may still affect stress reactivity and physiological functioning.
Although this study was well controlled, some limitations should be considered. Because this study investigated healthy adults with no recent history of psychopathologies, findings may not generalize to clinical populations (e.g., major depression, posttraumatic stress disorder, etc.). Future research should explore the effects of SLEs and emotion regulation strategies on stress reactivity in clinical populations or populations with more extreme stress (e.g., refugees), as well as longitudinally to determine if their effects on HPA axis reactivity do indeed contribute to the development of psychological disorders.
Additionally, participants in this study would have presumably used the emotion regulation strategy reported to cope with SLEs and the acute lab stressor. However, as previously stated we cannot know that these strategies were utilized during the lab stressor. It is possible that the use of different strategies in the lab may have altered cortisol reactivity. Future research should explore whether the same or different strategies were used in response to the recent SLEs, as well as whether participants used an emotion regulation strategy during the acute stressor. Lastly, severity of psychological distress following SLEs was not captured. Because more severe stressors may require more utilization of emotion regulation strategies and may result in either greater habituation or sensitization to acute stress, this severity should be assessed and controlled for in future studies.
This research provides new insight into how the use of a suppressive emotion regulation strategy interacts with stressful life events, particularly regarding relationship stress, to predict HPA axis reactivity. To test the impact of suppression on stress systems further, future research could use a repeated-stress paradigm (i.e., two consecutive days) to experimentally examine whether suppressing emotions in response to a stressor leads to nonhabituation of HPA axis reactivity, as well as whether these effects extend to other physiological responses to stress (i.e., cardiovascular and inflammatory responses). Reappraisal is a proactive regulatory strategy, so while effects of reappraisal were not found in the present study, it is possible that reappraisal could have an effect when the stressor was repeated and/or had a more chronic nature. It would also be worth investigating whether the short-term HPA axis stress reactivity changes demonstrated here lead to psychological disorders, such as depression, and long-term effects (i.e., past 12-months) on the HPA axis and the immune system. If using a suppressive emotion regulation strategy does indeed serve as a mediator, future intervention studies should examine whether reducing suppression during interpersonally stressful situations (e.g., relationship loss) could result in normalizing cortisol response to future stress and a decrease in the incidence of psychological disorders and physiological dysregulation.
Overall, the current study expands upon prior knowledge of how SLEs and emotion regulation affect HPA axis stress reactivity. While previous research has reported greater cortisol reactivity for individuals habitually using reappraisal and suppressive emotion regulation strategies (Lam et al., 2009), this study is the first to examine how habitual use of these emotion regulation strategies may change HPA axis reactivity following recent exposure to stressful life events, when the strategies would presumably be used more frequently. The findings suggest the use of suppression following SLEs may lead to changes in HPA axis reactivity, while reappraisal does not appear to have significant interactive effects. Our results are the first to highlight the importance of considering recent SLEs—no previous research has investigated the effect of emotion regulation strategies and SLEs on HPA axis response to acute stress. Furthermore, the relationship was replicated when examining only relationship stressors, suggesting that social/relational stress and trait suppression may be particularly potent when examining acute stress to a social evaluative stressor. Finally, the current study reveals that events not inherently viewed as traumatic (e.g., relationship stressors) may still modify physiological functioning and should be considered when assessing the effects of life events on stress reactivity.
Highlights.
Examined the interactive effect of emotion regulation and stressful events on cortisol reactivity
Suppression moderated the relation between recent stressful events and cortisol reactivity
Reappraisal had no main or interactive effects
Relationship stressors as predictor mirrors all stressful life events analyses
Acknowledgments
The data used for this article were collected by the Laboratory for the Study of Stress, Immunity, and Disease at Carnegie Mellon University under the directorship of Sheldon Cohen, PhD and were accessed via the Common Cold Project (CCP) website (www.commoncoldproject.com). CCP data are made publically available through a grant from the National Institute of Allergy and Infectious Diseases Center for Complementary and Integrative Health (R01 AT006694). The conduct of the studies was supported by a grant from the National Institute of Allergy and Infectious Disease (R01 AI066367). Secondary funding was provided by the National Institutes of Health (UL1 RR024153 and UL1 TR000005). The authors additionally thank the study staff and participants. No direct support was received from any aforementioned grant for these analyses or manuscript preparation. In addition, LGR received the Everett Graduate Student Fellowship that supported her time to conduct analyses and draft the paper.
Footnotes
Conflicts of Interest: Authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aseltine RH, Kessler RC. Marital disruption and depression in a community sample. J Health Soc Behav. 1993;34:237–251. [PubMed] [Google Scholar]
- Augustine AA, Hemenover SH. On the relative effectiveness of affect regulation strategies: a meta-analysis. Cognition Emotion. 2009;23:1181–1220. [Google Scholar]
- Benenson JF, Markovits H, Hultgren B, Nguyen T, Bullock G, Wrangham R. Social exclusion: More important to human females than males. PLoS ONE. 2013;8:e55851. doi: 10.1371/journal.pone.0055851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JM, Glaser R, Andridge RR, Peng J, Malarkey WB, Kiecolt-Glaser JK. Long lasting effects of smoking: Breast cancer survivors’ inflammatory responses to acute stress differ by smoking history. Psychoneuroendocrinology. 2013;38:179–187. doi: 10.1016/j.psyneuen.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA, Westphal M, Mancini AD. Resilience to loss and potential trauma. Annu Rev Clin Psycho. 2011;7:511–535. doi: 10.1146/annurev-clinpsy-032210-104526. [DOI] [PubMed] [Google Scholar]
- Cameron A, Palm K, Follette V. Reaction to stressful life events: what predicts symptom severity? J Anxiety Disord. 2010;24:645–649. doi: 10.1016/j.janxdis.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Mello AF, Anderson GM, Price LH, Wilkinson CW, Mello MF, Wier LM. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiat. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Social support and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. Am Psychol. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. New Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Compare A, Zarbo C, Shonin E, Van Gordon W, Marconi C. Emotional regulation and depression: A potential mediator between heart and mind. Cardiovasc. Psychiatry Neurol. 2014;2014 doi: 10.1155/2014/324374. Article ID 324374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson TF, Creswell JD, Terides MD, Blundell K. Cognitive reappraisal increases neuroendocrine reactivity to acute social stress and physical pain. Psychoneuroendocrino. 2014;49:69–78. doi: 10.1016/j.psyneuen.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. Clarendon Press; Oxford: 2002. [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Bennett JM, Derry HM, Kiecolt-Glaser JK. Relationships and inflammation across the lifespan: Social developmental pathways to disease. Soc Personal Psychol Compass. 2011;5:891–903. doi: 10.1111/j.1751-9004.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis JL, Moitra E, Dyck I, Keller MB. The impact of stressful life events on relapse of generalized anxiety disorder. Depress Anxiety. 2012;29:386–391. doi: 10.1002/da.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64(6):970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Hawn SE, Paul L, Thomas S, Miller S, Amstadter AB. Stress reactivity to an electronic version of the Trier Social Stress Test: a pilot study. Front Psychol. 2015;6:724. doi: 10.3389/fpsyg.2015.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; New York: 2013. [Google Scholar]
- Heim C, Newport J, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: A multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disorders. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- Hu T, Zhang D, Wang J, Mistry R, Ran G, Wang X. Relation between Emotion Regulation and Mental Health: A Meta-analysis Review. Psychol Reports: Measures Statistics. 2014;114:341–362. doi: 10.2466/03.20.PR0.114k22w4. [DOI] [PubMed] [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. J Personal. 2014;72:1301–1334. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmith M, Kim C, Malarkey WB. Marital conflict in older adults: Endocrinological and immunological correlates. Psychosom Med. 1997;59:339–349. doi: 10.1097/00006842-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, … Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Knopfli B, Morselli D, Perrig-Chiello P. Trajectories of psychological adaptation to marital breakup after a long-term marriage. Gerontology. 2016;62:541–552. doi: 10.1159/000445056. [DOI] [PubMed] [Google Scholar]
- Jacobson L. Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Compr Physiol. 2014;4:715–738. doi: 10.1002/cphy.c130036. [DOI] [PubMed] [Google Scholar]
- Laboratory for the Study of Stress, Immunity, and Disease. Common Cold Project Data. 2016 http://www.commoncoldproject.com.
- Lam S, Dickerson SS, Zoccola PM, Zaldivar F. Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrino. 2009;34:1355–1362. doi: 10.1016/j.psyneuen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Do low levels of stress reactivity signal poor states of health? Biol Psychol. 2011;86:121–128. doi: 10.1016/j.biopsycho.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Gross JJ. Emotion suppression and cardiovascular disease: Is hiding your feelings bad for your heart? In: Temoshok LR, Vingerhoets A, Nyklicek I, editors. The expression of emotion and health. Brunner-Routledge; London: 2004. pp. 62–81. [Google Scholar]
- Mazurka R, Wynne-Edwards KE, Harkness KL. Stressful life events prior to depression onset and the cortisol response to stress in youth with first onset versus recurrent depression. J Abnorm Child Psychol. 2016;44:1173–1184. doi: 10.1007/s10802-015-0103-y. [DOI] [PubMed] [Google Scholar]
- Meyer T, Smeets T, Giesbrecht T, Merckelbach H. The efficiency of reappraisal and expressive suppression on everyday affective experiences. Psychiat Res. 2012;200:964–969. doi: 10.1016/j.psychres.2012.05.034. [DOI] [PubMed] [Google Scholar]
- Moitra E, Dyck I, Beard C, Bjornsson AS, Sibrava NJ, Weisberg RB, Keller MB. Impact of stressful life events on the course of panic disorder in adults. J Affect Disorders. 2011;134:373–376. doi: 10.1016/j.jad.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Zoellner LA, Mollenholt N. Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behav Res Ther. 2008;46:993–1000. doi: 10.1016/j.brat.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Rao U. Cortisol response to psychosocial stress during a depressive episode and remission. Stress. 2014;17:51–58. doi: 10.3109/10253890.2013.857398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Aldao A. Gender and age differences in emotion regulation strategies and their relationship to depressive symptoms. Pers Indiv Differ. 2011;51:704–708. [Google Scholar]
- Palazidou E. The neurobiology of depression. Brit Med Bull. 2012:1–19. doi: 10.1093/bmb/lds004. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Puig-Perez S, Villada C, Pulopulos MM, Hidalgo V, Salvador A. How are neuroticism and depression related to the psychophysiological stress response to acute stress in healthy older people? Physiol behav. 2016;156:128–136. doi: 10.1016/j.physbeh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Rhoades GK, Kamp Dush CM, Atkins DC, Stanley SC, Markman HJ. Breaking up is hard to do: The impact of unmarried relationship dissolution on mental health and life satisfaction. J Fam Psychol. 2011;25:366–374. doi: 10.1037/a0023627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: A meta-analytic review. Psychol Bull. 2014;140:140–187. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rote S. Marital disruption and allostatic load in late life. J Aging Health. 2017;29:688–707. doi: 10.1177/0898264316641084. [DOI] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol. 2010;1:103–113. [Google Scholar]
- Schirda B, Valentine TR, Aldao A, Prakash RS. Age-related differences in emotion regulation strategies: Examining the role of contextual factors. Dev Psychol. 2016;5:1370– 1380. doi: 10.1037/dev0000194. [DOI] [PubMed] [Google Scholar]
- Shafir R, Schwartz N, Blechert J, Sheppes G. Emotional intensity influences pre-implementation and implementation of distraction and reappraisal. Soc Cogn Affect Neur. 2015;10:1329–1337. doi: 10.1093/scan/nsv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G, Scheibe S, Suri G, Gross JJ. Emotion regulation choice. Psychol Sci. 2011;22:1391–1396. doi: 10.1177/0956797611418350. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. 2011;13:263–278. doi: 10.31887/DCNS.2011.13.2/jsherin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RW, Barrett AE. Nonmarital romantic relationships and mental health in early adulthood: Does the association differ for women and men? J Health Soc Behav. 2010;51:168–182. doi: 10.1177/0022146510372343. [DOI] [PubMed] [Google Scholar]
- Soulsby LK, Bennett KM. Marriage and psychological wellbeing: The role of social support. Psychology. 2015;6:1349–1359. [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, Grossman S. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrino. 2001;26:295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370:1960–1973. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- Troy AS, Wilhelm FH, Shallcross AJ, Mauss IB. Seeing the silver Lining: Cognitive reappraisal ability moderates the relationship between stress and depressive symptoms. Emotion. 2010;10:783–795. doi: 10.1037/a0020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy AS, Shallcross AJ, Mauss IB. A person-by-situation approach to emotion regulation: cognitive reappraisal can either help or hurt, depending on the context. Psychol Sci. 2013;24:2505–2514. doi: 10.1177/0956797613496434. [DOI] [PubMed] [Google Scholar]
- Williamson GM, Schulz R. Physical illness and symptoms of depression among elderly outpatients. Psychol Aging. 1992;7:343. doi: 10.1037//0882-7974.7.3.343. [DOI] [PubMed] [Google Scholar]