Abstract
Rationale: Expiratory central airway collapse is associated with respiratory morbidity independent of underlying lung disease. However, not all smokers develop expiratory central airway collapse, and the etiology of expiratory central airway collapse in adult smokers is unclear. Paraseptal emphysema in the paratracheal location, by untethering airway walls, may predispose smokers to developing expiratory central airway collapse.
Objectives: To evaluate whether paratracheal paraseptal emphysema is associated with expiratory central airway collapse.
Methods: We analyzed paired inspiratory and expiratory computed tomography scans from participants enrolled in a multicenter study (Genetic Epidemiology of Chronic Obstructive Pulmonary Disease) of smokers aged 45 to 80 years. Expiratory central airway collapse was defined as greater than or equal to 50% reduction in cross-sectional area of the trachea during expiration. In a nested case-control design, participants with and without expiratory central airway collapse were included in a 1:2 fashion, and inspiratory scans were further analyzed using the Fleischner Society criteria for presence of centrilobular emphysema, paraseptal emphysema, airway wall thickening, and paratracheal paraseptal emphysema (maximal diameter ≥ 0.5 cm).
Results: A total of 1,320 patients were included, 440 with and 880 without expiratory central airway collapse. Those with expiratory central airway collapse were older, had higher body mass index, and were less likely to be men or current smokers. Paratracheal paraseptal emphysema was more frequent in those with expiratory central airway collapse than control subjects (16.6 vs. 11.8%; P = 0.016), and after adjustment for age, race, sex, body mass index, smoking pack-years, and forced expiratory volume in 1 second, paratracheal paraseptal emphysema was independently associated with expiratory central airway collapse (adjusted odds ratio, 1.53; 95% confidence interval, 1.18–1.98; P = 0.001). Furthermore, increasing size of paratracheal paraseptal emphysema (maximal diameter of at least 1 cm and 1.5 cm) was associated with greater odds of expiratory central airway collapse (adjusted odds ratio, 1.63; 95% confidence interval, 1.18–2.25; P = 0.003 and 1.77; 95% confidence interval, 1.19–2.64; P = 0.005, respectively).
Conclusions: Paraseptal emphysema adjacent to the trachea is associated with expiratory central airway collapse. The identification of this risk factor on inspiratory scans should prompt further evaluation for expiratory central airway collapse.
Clinical trial registered with ClinicalTrials.gov (NCT 00608764).
Expiratory central airway collapse (ECAC) is an increasingly recognized smoking-related airway disease that is associated with substantial respiratory morbidity (1, 2). ECAC is characterized by excessive airway collapse during expiration due to either tracheomalacia or expiratory dynamic airway collapse resulting from weakened posterior membranous wall (1). Although traditionally diagnosed on bronchoscopy, ECAC defined as greater than or equal to 50% collapse of central airway lumen during expiration on computed tomographic (CT) imaging is frequently seen in cigarette smokers, especially those with chronic obstructive pulmonary disease (COPD) (2–4). ECAC diagnosed using CT imaging has been shown to be independently associated with worse respiratory quality of life as well as more frequent respiratory exacerbations (2).
The etiology of ECAC in adults is not clear, and proximal extension of inflammation from the distal small airways in smokers has been speculated to be a cause (5). However, not all smokers develop ECAC, and other factors likely play a role. In smokers with COPD, air trapping and the resulting higher positive pleural pressure during exhalation can result in collapse of weakened airway walls, but it is unclear why only some patients develop ECAC. Airway caliber depends not only on the strength of the airway walls but also on the interdependence with the surrounding parenchyma (6). The small airways remain patent during exhalation because of the opposing forces of positive airway pressure and the elastic recoil of alveoli adjacent to the airways. In emphysematous lungs, this elastic recoil is lost, and the untethered airways are more likely to collapse in the setting of high positive intrathoracic pressure (7, 8). It is our clinical observation that a number of smokers with ECAC have paraseptal emphysema adjacent to the trachea. We hypothesized that the presence of paratracheal paraseptal emphysema results in similar untethering of the larger airways and collapse of the airways. We tested our hypothesis by examining CT scans from a large cohort of smokers with and without COPD.
Methods
Study Population
We analyzed paired inspiratory–expiratory CT scans from the large multicenter COPDGene (Genetic Epidemiology of COPD) cohort study, the details of which have been previously published (9). The COPDGene study enrolled participants across 21 clinical centers and included 10,192 non-Hispanic white individuals and African Americans between the ages of 45 and 80 years, including current and former smokers with and without COPD. The cohort was enriched for COPD, such that approximately 45% of participants had COPD. COPD status was determined by post-bronchodilator spirometry in accordance with the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations (10).
Computed Tomographic Analysis
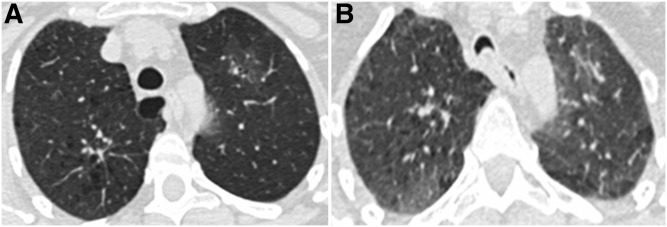
CT scans were acquired at maximal inspiration (total lung capacity) and at end-tidal expiration (functional residual capacity) in the same setting. At one center, expiratory CT scans were acquired at residual volume. CT assessment was performed in two stages. First, paired inspiratory and expiratory scans were analyzed by three readers (two chest radiologists and one pulmonologist) to determine the cross-sectional area of the central airways at three predetermined anatomic levels: the level of the aortic arch just below the origin of the left subclavian artery, the carina, and the bronchus intermedius just distal to the origin of the upper lobe bronchus, as previously described (2). A greater than or equal to 50% decrease in cross-sectional area of central airways from inspiration to expiration at any level was considered diagnostic of ECAC (Figure 1). We did not distinguish tracheomalacia from expiratory dynamic airway collapse on CT imaging and used the term ECAC to encompass both entities.
Figure 1.
Axial paired computed tomography images at (A) end-inspiration and (B) end-expiration showing expiratory central airway collapse at the level of the aortic arch in a representative subject. The cross-sectional area decreased 70% from end-inspiration to end-expiration.
For the current study, we selected 440 subjects with ECAC previously identified in this cohort (2) and 880 control subjects without ECAC. For each participant with ECAC, two control subjects were selected randomly from the remainder of the participants without ECAC, matched by GOLD stage. Inspiratory scans of these 1,320 participants were analyzed on lung windows by two readers (a trained medicine resident and a pulmonologist) blinded to the participants’ ECAC status. Visual characterization of emphysema and airway disease was performed according to the Fleischner Society criteria (11). Briefly, CT scans were categorized by the presence of emphysema and its subtypes as having centrilobular emphysema (CLE; none, trace, mild, moderate, confluent, and advanced destructive), paraseptal emphysema (PSE; none, mild, substantial), and by the presence of airway wall thickening (none, borderline, and definite) (Figure 2) (11). We further defined clinically substantial CLE as the presence of any of moderate, confluent, and advanced destructive CLE. For this analysis, we considered only substantial PSE and definite bronchial thickening as clinically significant. In addition, PSE located immediately adjacent to and abutting the central airways from the thoracic inlet to the carina was labeled paratracheal paraseptal emphysema (paratracheal PSE) when the maximal width of the PSE was greater than or equal to 0.5 cm.
Figure 2.
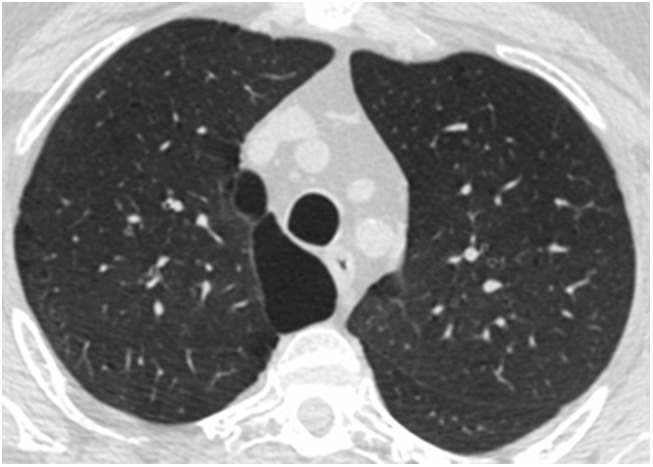
Axial inspiratory computed tomographic image at the level of the T4 vertebra of a representative study subject with substantial paratracheal paraseptal emphysema.
Statistical Analyses
Univariate comparisons between participants with and without ECAC were made using two-tailed independent t test for continuous variables and chi-square test for categorical variables. Univariate and multivariable associations were tested between emphysema subtypes and the presence of ECAC using conditional logistic regression models with GOLD stage as strata variable and adjustment for age, sex, race, body mass index (BMI), pack-years of smoking, current smoking status, and forced expiratory volume in 1 second (FEV1). We tested for interactions between sex and race with age categorized by the median on ECAC. We repeated these analyses for the relationship between paratracheal PSE and ECAC using varying thresholds for the definition of paratracheal PSE (≥0.5, ≥1.0, and ≥1.5 cm). We tested the relationship between paratracheal PSE and the probability of ECAC with adjustment for age, sex, race, BMI, pack-years of smoking, current smoking status, and FEV1. All results were determined to be statistically significant at a two-sided α of 0.05. Intra- and interobserver agreement were calculated using Cohen’s kappa analysis and intraclass correlation coefficients. All analyses were performed using Statistical Package for the Social Sciences (SPSS 24.0; SPSS Inc., Chicago, IL).
Results
Subject Characteristics
Overall, 1,320 participants were included in this study, 440 with ECAC and 880 without ECAC. Table 1 illustrates the baseline demographics and characteristics of participants. Of the 440 participants with ECAC, 211 (47.9%), 33 (7.5%), 93 (21.1%), 66 (15.0%), and 37 (8.4%) had GOLD stage 0 through 4, respectively, with an equal proportion of participants in each GOLD stage in the control group. Of these participants, 558 (42.3%) were men, 413 (17.7%) were African American, and 687 (52%) had COPD on the basis of GOLD criteria. The mean age of participants was 62.8 (standard deviation [SD], 9.0) years, with mean FEV1% predicted of 71.2 (26.7). Compared with those without airway collapse, those with airway collapse were older (mean ± SD, 65.0 ± 8.6 vs. 61.7 ± 8.9 yr), had higher BMI (31.2 ± 6.6 vs. 28.9 ± 6.4 kg/m2), and were less likely to be men (33.0 vs. 46.9%) or current smokers (28.0 vs. 38.2%). There was no difference in smoking burden on the basis of mean pack-years of smoking (Table 1).
Table 1.
Baseline characteristics of participants with and without expiratory central airway collapse
| Variable | Overall (N = 1,320) | Control Subjects (n = 880) | ECAC (n = 440) |
|---|---|---|---|
| Age, yr | 62.8 (9.0) | 61.7 (8.9) | 65.0 (8.6) |
| Male sex, % | 558 (42.3) | 413 (46.9) | 145 (33.0) |
| African American race, % | 233 (17.7) | 166 (18.9) | 67 (15.2) |
| Body mass index, kg/m2 | 29.7 (6.6) | 28.9 (6.4) | 31.2 (6.6) |
| Pack-years of smoking | 47.3 (27.8) | 47.0 (28.3) | 47.9 (26.7) |
| Current smoker, % | 459 (34.8) | 336 (38.2) | 123 (28.0%) |
| FEV1, L | 1.98 (0.87) | 2.05 (0.90) | 1.82 (0.79) |
| FEV1% predicted | 71.2 (26.7) | 71.7 (27.1) | 70.2 (25.8) |
| FVC, L | 3.0 (1.0) | 3.2 (1.0) | 2.8 (0.9) |
| FVC% predicted | 83.7 (19.3) | 84.2 (19.5) | 82.6 (18.8) |
| FEV1/FVC | 0.64 (0.17) | 0.64 (0.17) | 0.63 (0.17) |
Definition of abbreviations: ECAC = expiratory central airway collapse; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
All values expressed as mean (standard deviation) or as n (%) where indicated.
Imaging Results
Table E1 in the online supplement shows the distribution of emphysema subtypes in participants with and without ECAC. Kappa (standard error) values for detecting substantial CLE, substantial PSE, definite airway wall thickening, and paratracheal PSE were 0.73 ± 0.13, 0.55 ± 0.16, 0.86 ± 0.10, and 0.80 ± 0.10, respectively, and for interobserver variability were 0.79 ± 0.12, 0.93 ± 0.07, 0.26 ± 0.16, and 0.80 ± 0.11, respectively. The intraclass correlation coefficient for within and between observers for the size of paratracheal PSE was 0.88 (95% confidence interval [CI], 0.62–0.96) and 0.98 (95% CI, 0.93–0.99), respectively. Participants with ECAC were more likely to have mild PSE (28.0 vs. 19.8%; P = 0.001) and borderline bronchial wall thickening (48.2 vs. 34.7%; P < 0.0001), but there were no differences in CLE, substantial PSE, and definite bronchial thickening. Paraseptal emphysema in the paratracheal location was more frequent in those with ECAC than control subjects (16.6 vs. 11.8%; P = 0.016). On univariate analysis, older age (odds ratio [OR], 1.04 per year; 95% CI, 1.03–1.06; P < 0.001) and greater BMI (OR, 1.05 per kg/m2; 95% CI, 1.04–1.07; P < 0.0001) were associated with ECAC. Female sex was associated with ECAC (OR, 1.79; 95% CI, 1.41–2.27; P < 0.001), but there was no association between race and ECAC (OR for white race vs. African American, 1.30; 95% CI, 0.95–1.75; P = 0.10). Lower FEV1 was also associated with ECAC (OR per liter, 1.37; 95% CI, 1.20–1.59; P < 0.001). Emphysema subtypes, including CLE, substantial PSE, and definite airway wall thickening, were not associated with ECAC (OR, 1.15; 95% CI, 0.90–1.48; P = 0.26; 0.86; 95% CI, 0.62–1.20; P = 0.38; 1.17; 95% CI, 0.91–1.51; P = 0.21, respectively). However, paratracheal PSE was significantly associated with ECAC (OR, 1.48; 95% CI, 1.07–2.05; P = 0.02).
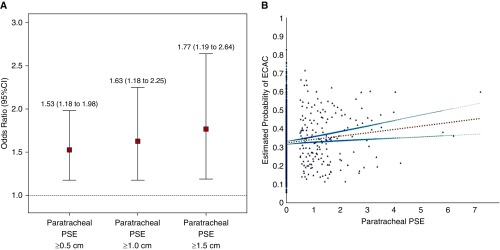
On multivariable analysis in separate models with adjustment for age, sex, race, BMI, pack-years of smoking, current smoking status, and FEV1, with GOLD stages as conditional blocks, there was no association between emphysema subtypes (CLE and PSE) and definite airway wall thickening with ECAC (Table 2). However, paratracheal PSE was associated with ECAC (adjusted OR, 1.53; 95% CI, 1.18–1.98; P = 0.001). We did not find any interactions between ECAC and sex, race, and age categorized by the median (63.4 yr) and hence did not perform any stratified analysis. To determine a dose–response effect, we further reclassified the presence of paratracheal PSE on the basis of different size criteria using the maximum width documented greater than or equal to 0.5 cm, greater than or equal to 1.0 cm, and greater than or equal to 1.5 cm. Figure 3A illustrates that with increasing maximal diameter of paratracheal PSE, there was a progressively stronger independent association between airway collapse and paratracheal PSE (adjusted OR, 1.63; 95% CI, 1.18–2.25; P = 0.003 and 1.77; 95% CI, 1.19–2.64; P = 0.005, for the 1-cm and 1.5-cm thresholds, respectively). When paratracheal PSE was treated as a continuous variable, a multivariable logistic model to predict the presence of ECAC by paratracheal PSE with adjustment for age, sex, race, BMI, pack-years of smoking, current smoking status, and FEV1 showed that a participant with 1 cm greater paratracheal PSE is 29% more likely to have ECAC (adjusted OR for probability of ECAC, 1.29; 95% CI, 1.07–1.56) (Figure 3B).
Table 2.
Multivariable associations between emphysema subtypes and airway disease with expiratory central airway collapse
| Variable | Crude OR (95% CI) | P Value | Adjusted OR* (95% CI) | P Value |
|---|---|---|---|---|
| Substantial centrilobular emphysema† | 1.15 (0.90–1.48) | 0.26 | 1.05 (0.82–1.35) | 0.70 |
| Substantial paraseptal emphysema | 0.86 (0.62–1.20) | 0.38 | 1.11 (0.83–1.48) | 0.49 |
| Definite bronchial wall thickening | 1.17 (0.91–1.51) | 0.21 | 1.11 (0.89–1.40) | 0.35 |
| Paratracheal paraseptal emphysema | 1.48 (1.07–2.05) | 0.02 | 1.53 (1.18–1.98) | 0.001 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Adjusted for age, sex, race, body mass index, pack-years smoking history, current smoking status, and forced expiratory volume in 1 second, with Global Initiative for Obstructive Lung Disease stage as strata condition.
Substantial centrilobular emphysema = presence of any of moderate, confluent, or advanced destructive centrilobular emphysema.
Figure 3.
(A) Adjusted odds ratios for the association between paratracheal paraseptal emphysema (paratracheal PSE) when defined as greater than or equal to 0.5 cm, greater than or equal to 1 cm, and greater than or equal to 1.5 cm in maximal diameter. All associations are adjusted for age, sex, race, body mass index, pack-years smoking history, current smoking status, and forced expiratory volume in 1 second, with Global Initiative for Obstructive Lung Disease stage as strata variable. (B) Relationship between paratracheal PSE as a continuous measure and the probability of expiratory central airway collapse (ECAC). The graph illustrates the positive linear slope (b = 0.018, P = 0.002) between paratracheal PSE and the predicted probability of ECAC obtained from the multivariable logistic model to predict the presence of ECAC by paratracheal PSE with adjustment for age, sex, race, body mass index, pack-years of smoking, current smoking status, and forced expiratory volume in 1 second. Each scattered dot represents the predicted probability of ECAC for a subject. The linear slope for the relationship between paratracheal PSE and ECAC is presented with 95% confidence interval (CI). Multivariable logistic model showed that a participant with 1-cm greater paratracheal PSE is 29% more likely to have ECAC.
Discussion
In a case-control study of smokers with and without ECAC, we found that paraseptal emphysema in the paratracheal location is independently associated with ECAC. The presence of ECAC was more likely with progressively greater size of the paratracheal paraseptal emphysema.
ECAC is an underrecognized entity associated with significant respiratory morbidity (2, 4). ECAC has been reported in adults secondary to several conditions, including endotracheal intubation; tracheostomy; lung transplantation and airway anastomosis; external airway compression from goiter, abscesses, cysts, malignancy; relapsing polychondritis; vascular rings; and genetic disorders such as Mounier-Kuhn syndrome (1, 5, 12). In the absence of these rare conditions, ECAC is most commonly associated with chronic bronchitis and emphysema (3). Because of the nonspecific respiratory symptoms of cough, dyspnea, and wheezing associated with central airway collapse, studies evaluating the prevalence and factors underlying its occurrence are mostly from biased populations of patients presenting with underlying lung disease (5, 13–17). A recent large cohort study of current and former smokers showed that approximately 5% of smokers have ECAC, and the prevalence in smokers without airflow obstruction is only slightly lower than the prevalence in those with chronic obstructive pulmonary disease (2). The presence of ECAC was associated with symptoms and respiratory morbidity, independent of underlying emphysema and lung function (2).
It is not clear why only a subset of smokers with chronic obstructive pulmonary disease develop ECAC. Previous studies have not shown any relationship between the extent of air trapping on computed tomography imaging and ECAC (2, 18). Chronic inflammation from cigarette smoking usually manifests as small airway inflammation, and it is speculated that proximal extension of this inflammation results in weakened walls in the central airways (5, 12). Subjects with ECAC were less likely to be active smokers; this finding is likely due to greater symptom burden resulting in higher quit rates, an association also seen in patients with chronic obstructive pulmonary disease, where patients with more severe disease are more likely to have quit smoking. Similarly to prior studies, we also found an association between greater body mass index and ECAC (2, 19). Obesity is associated with elevated pleural and intrathoracic pressure that may result in predisposed central airways to collapse during expiration (20). In the absence of ECAC or predisposition to ECAC, the equal pressure point where the intraluminal pressure drops below the intrapleural pressure usually occurs in the cartilaginous central airways. Weakened and narrowed central airways will predispose these airways to collapse at the choke point (21). We did note that lower FEV1 was independently associated with the presence of ECAC. Although we cannot prove directionality, it is plausible that ECAC results in lower lung function.
For the first time, we show that paratracheal emphysema abutting the central airways is associated with ECAC. This association with adjacent emphysema is a biologically plausible mechanism for central airway collapse, as evidenced by studies of airway–parenchyma interdependence in the smaller airways (6–8, 22). The small airways in chronic obstructive pulmonary disease remain patent during expiration in the face of opposing forces, including positive intrathoracic pressure and the elastic recoil of adjacent alveoli as well as the intrinsic bronchial tone. Multiple studies have documented the importance of peribronchiolar alveolar attachments in the maintenance of airway shape and patency (23, 24). When adjacent alveoli become emphysematous, there is a loss of the tethering action, and the small airways are more likely to collapse for a given intrathoracic pressure (7, 8, 22). This process likely occurs in the large airways as well. This may especially occur in the presence of weakened airway walls. The increased small airways resistance in chronic obstructive pulmonary disease is likely due to a combination of increased luminal narrowing due to thickened and stenotic airway walls (25) and airway destruction and dropout (25). However, other studies have also found that the average bronchiolar wall area is similar in emphysematous and nonemphysematous lungs, but with an association between airflow obstruction and the number of attachments between bronchioles and adjacent alveoli and the mean interalveolar attachment distance (7, 8, 23). Although we did not specifically quantify paratracheal centrilobular emphysema because of its diffuse nature, it is likely that paraseptal emphysema, by nature of the complete loss of alveolar attachments, exerts a greater impact on alveolar traction. We also found that a greater size of paraseptal emphysema adjacent to the trachea was associated with progressively greater risk for ECAC, supporting this concept.
Our study has a few limitations. We used paired inspiratory and expiratory images that were not obtained during dynamic exhalation. However, ECAC defined on static end-respiratory cycle scans detect more severe cases and are more specific, as tidal exhalation results in fewer pressure changes than dynamic forced exhalation (26–28). We included participants who were current or former smokers, with an oversampling of patients with chronic obstructive pulmonary disease, and our results may not be generalizable to other patients with chronic obstructive pulmonary disease. We combined computed tomographic diagnosis of tracheomalacia and expiratory dynamic airway collapse. Although the latter is more common in chronic obstructive pulmonary disease, the physiological consequences are similar. The difference in frequency of paratracheal paraseptal emphysema is not high between those with and without ECAC; however, an absolute prevalence difference of 5% and a 53% increased odds of paratracheal paraseptal emphysema being associated with the presence of ECAC makes this an important consideration in a subset of patients with unexplained symptoms. The study also has many strengths. We included participants from a large cohort study that is well characterized with extensive phenotyping using spirometry and computed tomographic measurements that are stringently quality controlled. The cohort included a substantial number of African Americans and included participants across the spectrum of disease severity.
Conclusions
Paraseptal emphysema in the paratracheal location is associated with expiratory central airway collapse. ECAC is associated with respiratory morbidity independent of underlying emphysema and may explain some cases of dyspnea that is out of proportion to lung function abnormalities. The identification of this risk factor on inspiratory scans should lower the threshold for evaluation for ECAC.
Supplementary Material
Footnotes
Supported by the COPDGene (Genetic Epidemiology of COPD) study National Institutes of Health grants R01 HL089897 and R01 HL089856, and National Institutes of Health grant K23HL133438 (S.P.B.). The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprising AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline.
Author Contributions: Study design: C.R.C. and S.P.B.; statistical analyses: Y.-i.K. and S.P.B.; data acquisition: C.R.C., H.N., N.L.J.T., C.G.W., D.A.L., and S.P.B.; data interpretation: C.R.C., H.N., N.L.J.T., C.G.W., D.A.L., S.B., J.M.W., M.T.D., A.A.D., G.R.W., M.G.F., and S.P.B.; manuscript writing: C.R.C. and S.P.B.; critical review of the manuscript for important intellectual content: C.R.C., H.N., N.L.J.T., C.G.W., Y.-i.K., D.A.L., S.B., J.M.W., M.T.D., A.A.D., G.R.W., M.G.F., and S.P.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the Genetic Epidemiology of COPD (COPDGene) Investigators
References
- 1.Murgu S, Colt H. Tracheobronchomalacia and excessive dynamic airway collapse. Clin Chest Med. 2013;34:527–555. doi: 10.1016/j.ccm.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt SP, Terry NL, Nath H, Zach JA, Tschirren J, Bolding MS, et al. Genetic Epidemiology of COPD (COPDGene) Investigators. Association between expiratory central airway collapse and respiratory outcomes among smokers. JAMA. 2016;315:498–505. doi: 10.1001/jama.2015.19431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandaswamy C, Balasubramanian V. Review of adult tracheomalacia and its relationship with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2009;15:113–119. doi: 10.1097/MCP.0b013e328321832d. [DOI] [PubMed] [Google Scholar]
- 4.Ochs RA, Petkovska I, Kim HJ, Abtin F, Brown M, Goldin J. Prevalence of tracheal collapse in an emphysema cohort as measured with end-expiration CT. Acad Radiol. 2009;16:46–53. doi: 10.1016/j.acra.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jokinen K, Palva T, Sutinen S, Nuutinen J. Acquired tracheobronchomalacia. Ann Clin Res. 1977;9:52–57. [PubMed] [Google Scholar]
- 6.Paré PD, Mitzner W. Airway-parenchymal interdependence. Compr Physiol. 2012;2:1921–1935. doi: 10.1002/cphy.c110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petty TL, Silvers GW, Stanford RE. Radial traction and small airways disease in excised human lungs. Am Rev Respir Dis. 1986;133:132–135. doi: 10.1164/arrd.1986.133.1.132. [DOI] [PubMed] [Google Scholar]
- 8.Lamb D, McLean A, Gillooly M, Warren PM, Gould GA, MacNee W. Relation between distal airspace size, bronchiolar attachments, and lung function. Thorax. 1993;48:1012–1017. doi: 10.1136/thx.48.10.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 11.Lynch DA, Austin JH, Hogg JC, Grenier PA, Kauczor HU, Bankier AA, et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology. 2015;277:192–205. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127:984–1005. doi: 10.1378/chest.127.3.984. [DOI] [PubMed] [Google Scholar]
- 13.Jokinen K, Palva T, Nuutinen J. Chronic bronchitis: a bronchologic evaluation. ORL J Otorhinolaryngol Relat Spec. 1976;38:178–186. doi: 10.1159/000275273. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa I, Boiselle PM, Raptopoulos V, Hatabu H. Tracheomalacia incidentally detected on CT pulmonary angiography of patients with suspected pulmonary embolism. AJR Am J Roentgenol. 2003;181:1505–1509. doi: 10.2214/ajr.181.6.1811505. [DOI] [PubMed] [Google Scholar]
- 15.Inoue M, Hasegawa I, Nakano K, Yamaguchi K, Kuribayashi S. Incidence of tracheobronchomalacia associated with pulmonary emphysema: detection with paired inspiratory-expiratory multidetector computed tomography using a low-dose technique. Jpn J Radiol. 2009;27:303–308. doi: 10.1007/s11604-009-0342-3. [DOI] [PubMed] [Google Scholar]
- 16.Sverzellati N, Rastelli A, Chetta A, Schembri V, Fasano L, Pacilli AM, et al. Airway malacia in chronic obstructive pulmonary disease: prevalence, morphology and relationship with emphysema, bronchiectasis and bronchial wall thickening. Eur Radiol. 2009;19:1669–1678. doi: 10.1007/s00330-009-1306-9. [DOI] [PubMed] [Google Scholar]
- 17.Ferretti GR, Jankowski A, Perrin MA, Chouri N, Arnol N, Aubaud L, et al. Multi-detector CT evaluation in patients suspected of tracheobronchomalacia: comparison of end-expiratory with dynamic expiratory volumetric acquisitions. Eur J Radiol. 2008;68:340–346. doi: 10.1016/j.ejrad.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Lee KS, Ashiku SK, Ernst A, Feller-Kopman D, DeCamp M, Majid A, et al. Comparison of expiratory CT airway abnormalities before and after tracheoplasty surgery for tracheobronchomalacia. J Thorac Imaging. 2008;23:121–126. doi: 10.1097/RTI.0b013e3181653c41. [DOI] [PubMed] [Google Scholar]
- 19.Boiselle PM, Litmanovich DE, Michaud G, Roberts DH, Loring SH, Womble HM, et al. Dynamic expiratory tracheal collapse in morbidly obese COPD patients. COPD. 2013;10:604–610. doi: 10.3109/15412555.2013.781149. [DOI] [PubMed] [Google Scholar]
- 20.Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. 1985;2010:212–218. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen OF, Butler JP. Expiratory flow limitation. Compr Physiol. 2011;1:1861–1882. doi: 10.1002/cphy.c100025. [DOI] [PubMed] [Google Scholar]
- 22.Saetta M, Ghezzo H, Kim WD, King M, Angus GE, Wang NS, et al. Loss of alveolar attachments in smokers. A morphometric correlate of lung function impairment. Am Rev Respir Dis. 1985;132:894–900. doi: 10.1164/arrd.1985.132.4.894. [DOI] [PubMed] [Google Scholar]
- 23.Anderson AE, Jr, Foraker AG. Relative dimensions of bronchioles and parenchymal spaces in lungs from normal subjects and emphysematous patients. Am J Med. 1962;32:218–226. doi: 10.1016/0002-9343(62)90291-7. [DOI] [PubMed] [Google Scholar]
- 24.Linhartová A, Anderson AE, Jr, Foraker AG. Radial traction and bronchiolar obstruction in pulmonary emphysema. Observed and theoretical aspects. Arch Pathol. 1971;92:384–391. [PubMed] [Google Scholar]
- 25.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell CR, Litmanovich D, Loring SH, Boiselle PM. Age and sex dependence of forced expiratory central airway collapse in healthy volunteers. Chest. 2012;142:168–174. doi: 10.1378/chest.11-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Donnell CR, Bankier AA, O’Donnell DH, Loring SH, Boiselle PM. Static end-expiratory and dynamic forced expiratory tracheal collapse in COPD. Clin Radiol. 2014;69:357–362. doi: 10.1016/j.crad.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boiselle PM, O’Donnell CR, Bankier AA, Ernst A, Millet ME, Potemkin A, et al. Tracheal collapsibility in healthy volunteers during forced expiration: assessment with multidetector CT. Radiology. 2009;252:255–262. doi: 10.1148/radiol.2521081958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.