Abstract
Longstanding theory predicts that competitive interactions set species' range limits in relatively aseasonal, species-rich regions, while temperature limits distributions in more seasonal, species-poor areas. More recent theory holds that species evolve narrow physiological tolerances in aseasonal regions, with temperature being an important determining factor in such zones. We tested how abiotic (temperature) and biotic (competition) factors set range limits and structure bird communities along strong, opposing, temperature-seasonality and species-richness gradients in the Himalayas, in two regions separated by 1500 km. By examining the degree to which seasonal elevational migration conserves year-round thermal niches across species, we show that species in the relatively aseasonal and speciose east are more constrained by temperature compared with species in the highly seasonal west. We further show that seasonality has a profound effect on the strength of competition between congeneric species. Competition appears to be stronger in winter, a period of resource scarcity in the Himalayas, in both the east and the west, with similarly sized eastern species more likely to segregate in thermal niche space in winter. Our results indicate that rather than acting in isolation, abiotic and biotic factors mediate each other to structure ecological communities.
Keywords: body mass, elevational distributions, range limits, seasonality, species richness
1. Background
The role of abiotic versus biotic factors in setting species range limits is contentious, despite over a century of investigation. Under the assumption that pronounced temperature seasonality is physiologically stressful, longstanding theory suggests that temperature sets range limits in highly seasonal and species-poor regions, while interspecific competition constrains ranges in aseasonal, species-rich regions (the ‘species-interactions/abiotic-stress hypothesis’; hypotheses A1 and B1 in table 1) [1–4]. A more recent, contrasting theory—the ‘climatic variability hypothesis'—posits that species evolve narrow temperature tolerances in aseasonal regions and are more temperature-limited than species in climatically variable zones (table 1: A2) [5,6]. This hypothesis, however, ignores the role of competition in limiting species distributions. While much work has focused separately on temperature or competition in limiting species distributions [7,8], how these factors might interact to structure the ranges of multiple species—and therefore entire communities—remains unknown.
Table 1.
Hypotheses pertaining to the strength and nature of abiotic versus biotic range limitation, their expected ecological outcomes, the tests performed in this study and predicted region of limitation.
| ID | name (the ‘…’ ) | hypothesis (‘…states that…’ ) | ecological outcome (‘…resulting in…’ ) | test of outcome (‘…evidenced by…’ ) | predicted region of limitation (‘…in…’ ) |
|---|---|---|---|---|---|
| abiotic predictions | |||||
| A1 | species-interactions/abiotic stress hypothesis | increased temperature fluctuations impose thermoregulatory stress | thermal niche tracking | greater thermal niche overlap | seasonal environments (western Himalayas) |
| A2 | climatic variability hypothesis | decreased seasonality limits adaptation to variable temperatures | thermal niche tracking | greater thermal niche overlap | aseasonal environments (eastern Himalayas) |
| A3 | thermoregulatory capacity hypothesis | smaller species, given poorer thermoregulatory capacity, are more strongly limited by abiotic factors than larger species | thermal niche tracking | negative correlation between body size and thermal niche overlap | all environments; stronger in the region where abiotic factors more important |
| biotic predictions | |||||
| B1 | species-interactions/abiotic stress hypothesis | increased species richness enhances competitive interactions | competitive exclusion | lower congeneric niche overlap | aseasonal environments (eastern Himalayas) |
| B2 | ecological similarity hypothesis | competition increases with greater ecologically similarity between species | competitive exclusion | positive correlation between congeneric body size differences and congeneric niche overlap | all environments; stronger in the region where biotic factors more important |
| abiotic–biotic prediction | |||||
| AB | resource availability hypothesis | competition increases in resource-scarce periods, and relaxes in resource-abundant periods | competitive exclusion | lower congeneric niche overlap in resource-limiting season | all environments; stronger in the region with greater seasonality (western Himalayas) |
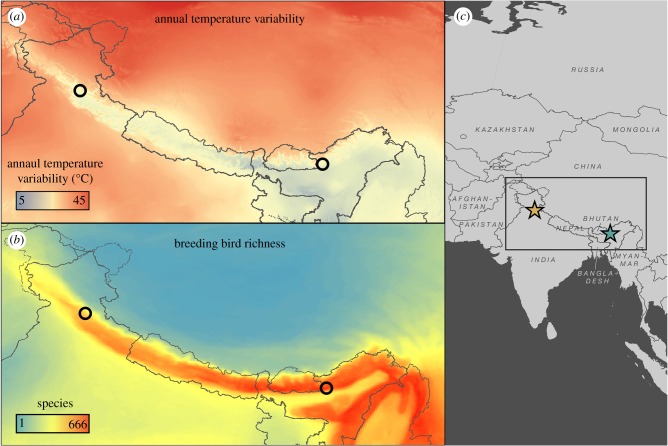
One of the fundamental constraints to understanding how temperature and competition interact to structure ecological communities is the lack of suitable systems that simultaneously span large temperature and species richness gradients, while also retaining a suite of species with similar biogeographic histories and adaptive strategies. Spanning over 2000 km, the Himalayas exhibit strong opposing abiotic (temperature seasonality) and biotic (species richness) gradients along an east–west axis (figure 1). The western Himalayas are twice as variable in annual temperature as the east, but have half the number of breeding bird species (figure 1) [9], patterns qualitatively similar to tropical–temperate species richness and temperature seasonality gradients globally [10]. In addition, a majority of bird species in the Himalayas breed at higher elevations in summer and migrate over short distances to winter at lower elevations, thereby potentially occupying similar thermal niches in both summer and winter. The starkly different abiotic and biotic environments within the Himalayas thus allow for a robust test of how temperature and competition act and interact to structure elevational range limits for a highly diverse bird community.
Figure 1.
Opposing abiotic and biotic gradients in the Himalayas. Annual temperature variability (a) decreases moving eastward across the Himalayas while breeding bird richness (b) increases. Black circles in (a) and (b) indicate western and eastern Himalayan survey regions, with their position within India shown by brown and green stars in (c).
Temperature is an important determinant of species abundances and distributions [11–17]. Indeed, some species have moved to track temperatures through recent climate change [18], while other species migrate hundreds to thousands of kilometres each year to occupy similar temperatures in summer and winter [19]. However, under what conditions temperature or competition assume primacy in enforcing range limits is far from clear. Empirical support for the species-interactions/abiotic-stress hypothesis is equivocal. While some studies report patterns that are consistent with temperature constraining ranges in highly seasonal environments and competition limiting distributions in climatically benign regions [20,21], other studies have produced results more consistent with the predictions of the climatic variability hypothesis [7,22].
(a). Testing abiotic predictions
In regions where species are more sensitive to temperature, we expect that elevational migration by birds should result in more strongly conserved thermal niches by maximizing overlap between summer and winter thermal distributions. Greater thermal niche tracking through elevational migration in the highly seasonal western Himalayas would be consistent with predictions of the species-interactions/abiotic-stress hypothesis (table 1: A1), while greater thermal niche tracking in the relatively aseasonal eastern Himalayas would be consistent with predictions of the climatic variability hypothesis (table 1: A2). Further, we expect that smaller species should have greater seasonal thermal niche overlap than larger species because larger species are better thermoregulators, and thus better adapted to greater seasonal temperature fluctuations [23]; this relationship should be stronger in the region where temperature more strongly structures ranges (table 1: A3). Additionally, within the subset of bird species common to both the eastern and western Himalayas, we expect that populations will more closely track temperature across seasons in the region where the abiotic environment sets stronger range limits (table 1: A3).
(b). Testing biotic predictions
In addition to temperature, interspecific competition—for instance, for portions of geographical space that are thermally optimal—can also structure species ranges, and interspecific interactions leading to competitive exclusion also appear to limit species ranges at various scales [8,17,24,25]. We expect greater segregation in thermal niche space between potentially competing congeneric species in the region where competition is a stronger determinant of range limits, which the species-interactions/abiotic-stress hypothesis predicts would be the more speciose eastern Himalayas (table 1: B1). We also expect that segregation in thermal niche space should increase as a function of body size similarity between congeners, because similarly sized species are likely to have similar thermoregulatory capacities and thermal niches. We further expect that this relationship should be stronger in the region where biotic interactions more strongly structure ranges (table 1: B2).
(c). Testing abiotic–biotic predictions
Finally, evidence indicates that temperature can mediate the strength of competition and limit species ranges in plants and animals [26,27]. We expect that seasonal resource fluctuations should alter competition, and therefore coexistence or segregation in thermal niche space. Specifically, we expect greater congeneric segregation in thermal space in winter (when resources are scarce) than in summer (when resources are abundant; table 1: AB) [28]. Because the degree of seasonality is heightened in the west, we expect these patterns to be stronger for species in the western Himalayas (table 1: AB).
2. Material and methods
(a). Study regions and elevational transects
We exhaustively surveyed birds along five near-continuous elevational transects within old growth forest spanning a longitudinal gradient in temperature variability and bird species richness in the Himalayas (figure 1). Sampling was limited to old growth forest—the original, unaltered habitat type at all elevations in our study areas—to avoid potential biases arising from sampling highly modified habitats such as agriculture and plantation. Three transects were located in the western Himalayas in Great Himalayan National Park, Himachal Pradesh (‘west’; 31.70° N, 77.50° E) spanning 2000–3750 m in elevation. Two transects were located in the eastern Himalayas in Eaglenest Wildlife Sanctuary, Arunachal Pradesh (‘east’; 27.10° N, 92.40° E) spanning 1000–3000 m in elevation. The west is characterized by cool temperate vegetation and contains roughly 150 breeding bird species whereas the east is characterized by warm subtropical and moist temperate forests with over 350 breeding bird species [9,29]. These two regions were chosen to maximize differences of both temperature variability and species richness along the abiotic and biotic gradients (figure 1) [9], while retaining species with similar evolutionary histories that are broadly situated within the same biogeographic context. The elevational range surveyed reflects the largest gradient possible within protected old growth forest in each region. For purposes of illustration only (maps, figure 1), temperature variability was obtained from WorldClim [30] and breeding species richness from Jenkins et al. [31]. Bird taxonomy and nomenclature presented in the manuscript follow Gill & Donsker [32], but we also used an alternative taxonomy from the Handbook of the Birds of the World (v. 2.0, December 2017, available at http://datazone.birdlife.org/species/taxonomy) to ensure our results were robust to the choice of taxonomic classification.
(b). Bird and temperature surveys
Given the extreme topography of the landscape, we surveyed birds using a modified line transect technique. Transects in both regions were situated along existing trail networks, with some portions along trails of our own construction. We surveyed birds between 05.00 and 10.30 h and 17.00–19.00 h during the breeding season (summer) in late April–late June in 2013 and 2014 in the west and April–May 2015 in the east, and during the winter months of October–December 2012 in the west and October–December 2015 in the east; these periods do not coincide with periods of ongoing seasonal migration. Each elevational transect was surveyed three times per field season during summer and six times during the field season in winter in the west, and six times per field season in the east, amounting to six surveys in summer and winter in both the west and east. While surveying, we identified all birds by sight and sound and recorded the elevation, time, distance to observer and count for all birds while walking at a slow, constant pace. We had two simultaneous observers for all surveys to maximize detectability, resulting in one combined count in each region.
We deployed temperature data loggers spaced approximately every 350 vertical metres along all elevational transects to simultaneously record temperature every 5 min. We used HOBO Microstation data loggers (Onset H21-002, Natick, Massachusetts, USA) fitted with temperature/relative humidity sensors (Onset S-THB-M002, accuracy ± 0.21°C) in the west and iButton data loggers (Thermochron DS1922 L, Maxim Integrated, San Jose, California, USA, accuracy ± 1°C) in the east.
(c). Defining and comparing seasonal thermal niches
We follow the definition of the niche as a set of environmental conditions that restricts a species to a geographical range through ‘physiological and psychological respects' [13,33]. While there are many definitions and concepts surrounding the niche [14], we focus strictly on temperature to define a species’ thermal niche. For each species, we define a seasonal thermal niche as the two-dimensional space composed of all pairs of minimum and maximum daily temperatures observed for a given bird species in a region and within a season. We calculated the minimum and maximum daily temperatures for each bird observation by linearly interpolating the temperature readings from the closest data loggers situated above and below the observation during the daily period over which we surveyed for birds.
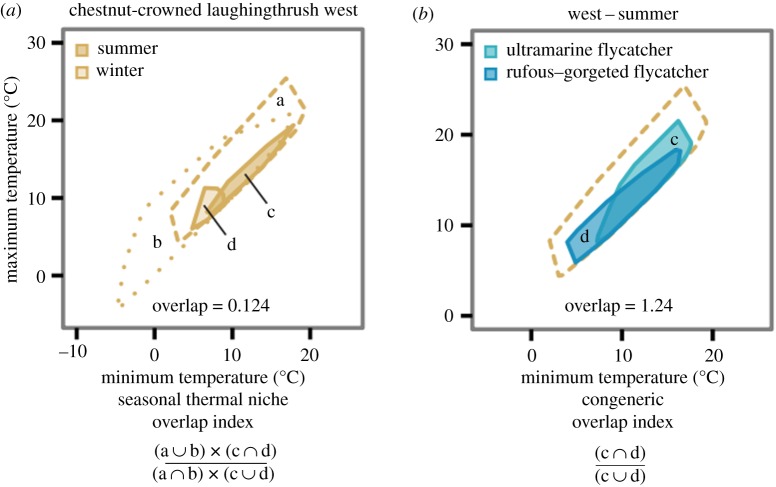
We limited analyses to species that were observed during summer and winter in a given region and met a minimum sample size requirement of ≥5 observations in each season. Based on these criteria, 120 species were included for further analyses (electronic supplementary material, table S1). For each species within a region, we calculated a 95% minimum convex polygon in environmental space based on the distribution of minimum and maximum temperatures to define a seasonal thermal niche using the package rgeos in the program R ([34,35]; equation and example in figure 2a). For each species in each region, we calculated the overlap between summer and winter thermal niche spaces occupied by the species (c ∩ d in figure 2a) relative to the thermal niche space occupied by the species across seasons (c ∪ d in figure 2a). While the use of minimum convex polygons prohibited us from analysing finer-scale seasonal use of niche space based on kernel density functions, they enabled us to account for differences in regional temperature availability (sensu [36]), which can bias comparisons of environmental niches. Consequently, we scaled the resulting value by the overlap between total available summer and winter thermal niche spaces within a region (a ∩ b in figure 2a) relative to the overlap of overall thermal niche space across seasons within a region (a ∪ b in figure 2a). This was done to make seasonal overlap values comparable across regions. This calculation scales the overlap metric higher when the available environmental space provides ample potential segregation of seasonal niches (i.e. null expectation = less overlap), and scales the overlap metric lower when the available environmental space favours seasonal overlap (i.e. null expectation = more overlap; figure 2a; electronic supplementary material, table S1). Therefore, our calculation of seasonal thermal niche overlap explicitly accounts for and corrects regional differences in thermal regimes, allowing for unbiased comparisons across regions.
Figure 2.
Calculating seasonal thermal niche overlap and congeneric overlap while accounting for regional differences in temperature seasonality. (a) Calculation of thermal niche overlap for an example species, the chestnut-crowned laughingthrush (Trochalopteron erythrocephalum), while accounting for regional temperature space. Here, a and b represent the overall thermal spaces in the western Himalayas during summer and winter, respectively, while c and d represent the respective 95% minimum convex polygons of summer and winter thermal niche spaces of the example species. Seasonal thermal niche overlap is a measure of the degree of thermal niche conservatism across seasons, taking into account the regional availability of thermal space, both thermal space common to both seasons (a ∩ b) and overall region-specific thermal space (a ∪ b). Greater seasonal thermal niche overlap thus corresponds to greater temperature tracking across seasons, which reflects greater sensitivity to temperature seasonality. (b) Calculation of congeneric thermal niche overlap in one season for two example species. Here, c and d represent the 95% minimum convex polygons of summer thermal niche spaces for two congeneric species, ultramarine flycatcher (Ficedula superciliaris) and rufous-gorgeted flycatcher (Ficedula strophiata), in the west. Yellow dashes signify the overall thermal space in the summer in the west. Congeneric thermal niche overlap is a measure of the degree of congeneric co-occurrence. Lower congeneric thermal niche overlap thus corresponds to greater congeneric segregation in temperature space, which reflects stronger competition through competitive exclusion.
(d). Body mass and thermal sensitivity
We obtained bird body masses from Dunning [37]. To relate seasonal thermal niche overlap to body size, we used the quasi-Poisson family of generalized linear models (GLMs) because seasonal thermal niche overlap (a) followed a Poisson-like variance structure (variance increasing with the mean), (b) was non-integer and (c) was over-dispersed (variance greater than the mean). Because survey transects did not cover the full elevational range of all species across both seasons (owing largely to seasonal elevational migrations), we weighted the regression to give more weight to species that were more completely sampled across the elevational range surveyed [38]. We calculated the weight as the total number of observations across seasons scaled by the proportion of each species's published elevational range (separately in the west [39] and east [40]) captured in our surveys.
To ensure that modelled relationships between each covariate and seasonal thermal niche overlap were not affected by the non-independence of species arising from their joint evolutionary histories, we computed and interpreted the Pagel's λ [41] for seasonal temperature overlap in the R packages ape [42] and geiger [43], using a comprehensive phylogeny of Himalayan birds [29]. We found no evidence that phylogeny influenced seasonal thermal niche overlap (pχ2 = 0.99, d.f. = 1; for test to distinguish phylogenetic signal from Brownian motion), and consequently present results from the weighted quasi-Poisson model.
(e). Evaluating congeneric co-occurrence
To evaluate the role of biotic interactions as determinants of seasonal thermal niches, we calculated the thermal niche space overlap for congeneric species pairs within each season for each region (figure 2b; electronic supplementary material, table S2). We focused our analyses on congeneric species because they are typically very similar ecologically and thought to compete extensively in many cases, especially along elevational gradients [2,25,44,45]. Unlike for the calculation of species- and region-specific seasonal thermal niche overlap, which accounts for differences in seasonal thermal availability across regions (figure 2a), total available thermal space in a given season is identical for all species within a region. Therefore, the calculation of within-region, within-season thermal niche overlap between pairs of species does not require accounting for differences in thermal availability across regions, and is calculated more simply as the overlap of two congeners' thermal niche spaces within a season (c ∩ d in figure 2b) relative to the total thermal niche space occupied by both species within a season (c ∪ d in figure 2b). This was done to make congeneric overlap values within a season comparable across species pairs. Consequently, congeneric species pairs with greater overlap in thermal space indicate greater co-occurrence, while pairs with lower overlap reflect segregation in thermal niche space, consistent with competitive exclusion in geographical space. Overall, we compared thermal overlap values between 201 pairs of congeneric species from 41 genera (electronic supplementary material, table S2). At the genus level, Pagel's λ was not significantly different from random (pχ2 = 0.72, d.f. = 1), indicating no phylogenetic dependence of congeneric thermal niche overlap.
We modelled seasonal congeneric overlap using a weighted quasi-Poisson model as above, weighting the regression by the minimum number of observations of a species within a given congener pair scaled by the proportion of the published range within the Himalayas captured by our data within a season. We parametrized the weighted model with thermal niche overlap between congeneric pairs as the response variable, and included region, season and the relative difference in body masses between the two competitors (as a proxy for ecological similarity) as predictors, along with all pairwise interactions. Relative difference in body sizes between species was calculated as the absolute difference in body masses divided by the body mass of the larger species in the pair. This index is therefore bound between zero and a trivial maximum value approaching one. A value of zero indicates that species in a congener pair are identically sized, while higher values indicate greater differences in body size.
In the analyses evaluating congeneric co-occurrence, several species occurred multiple times because of comparisons with a large number of congeners (electronic supplementary material, table S2), potentially leading to non-independence. We therefore conducted an additional analysis to test whether multiple occurrences of these species in congener pairs led to any bias in our results. To do this, we used the glmmPQL function in the R package MASS [46] to run a weighted quasi-Poisson generalized linear mixed-effects model (GLMM) with the same formulation as the GLM (see above), but with crossed random effects, these being the focal species and the congeneric species being compared [47]. We then compared the predicted (fitted) values from the GLM and the GLMM to evaluate the potential for bias in the GLM results.
3. Results
We recorded and analysed 12 846 detections of 120 species (electronic supplementary material, table S1) from 12 repeated samples of each of five transects across the western and eastern Himalayas in summer and winter that met our criteria for inclusion. These included 201 pairings of congeneric species from 41 genera (electronic supplementary material, table S2). Ten species were common to both the eastern and western Himalayas. A total of 87 species from the western Himalayas and 105 species from the eastern Himalayas were excluded from analysis (electronic supplementary material, table S3). Roughly 74% and 68% of exclusions were due to species being observed in only one season for the western and eastern Himalayas, respectively. The remainder of exclusions were due to low (less than 5) sample sizes during summer, winter or both seasons within a region.
(a). Abiotic predictions: seasonal thermal niche overlap
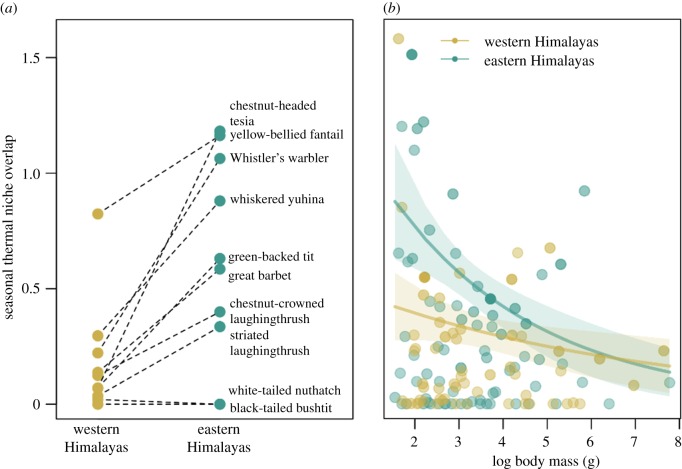
Using all included species, overlap between summer and winter thermal niches was not different between the relatively aseasonal eastern and highly seasonal western Himalayas (βregion = –3.20, 95% CI = [–12.27, 2.18]; McFadden's pseudo-R2 = 0.07). However, for the 10 species we recorded in both the east and west, eastern populations tracked their seasonal thermal niches more strongly than their western counterparts, accounting for differences in seasonality between the two regions (paired t-test; t9 = –4.00; difference in seasonal thermal niche overlap = 0.44 higher in the east [0.19, 0.70]; figure 3a). Further, and as expected, body mass was inversely related to seasonal thermal niche overlap in both regions, but this relationship was twice as strong in the relatively aseasonal east than in the highly seasonal west (βeast = –0.30 [–0.46, –0.14]; βwest = –0.15 [–0.31, –0.01]; McFadden's pseudo-R2 = 0.20; figure 3b).
Figure 3.
Relationships between species traits and seasonal thermal niche overlap of bird species in the western and eastern Himalayas (n = 120 bird species). (a) Species common to both east and west showed greater seasonal thermal niche overlap in the east. (b) Body mass was significantly negatively associated with seasonal thermal niche overlap across regions, and this pattern was twice as strong in the eastern Himalayas. Bold lines and shaded regions reflect predicted relationships from weighted quasi-Poisson regressions with 95% confidence intervals, respectively. The transparency of each point indicates its relative weight in the regression—bolder points contribute more towards model fit (see Material and methods).
(b). Biotic predictions: thermal niche overlap between congeners
Across seasons, congeneric thermal niches were more segregated in the highly speciose east than in the relatively species-poor west (βregion = 0.66 [0.44, 0.93]; McFadden's pseudo-R2 = 0.17). Unexpectedly, with summer and winter congeneric niche overlap data pooled, thermal niche overlap was unrelated to body size similarity between congeneric species pairs in either the east or the west (βeast = –1.37 [–2.87, 0.09]; βwest = 0.69 [–0.54, 1.89]; McFadden's pseudo-R2 = 0.00), indicating that, in general, species do not segregate in thermal niche space as a result of similarity in body size. These results did not change with re-analysis using the alternative taxonomy of the Handbook of the Birds of the World (electronic supplementary material, figure SM1, and table S4).
(c). Abiotic–biotic interactions: seasonality and thermal niche overlap between congeners
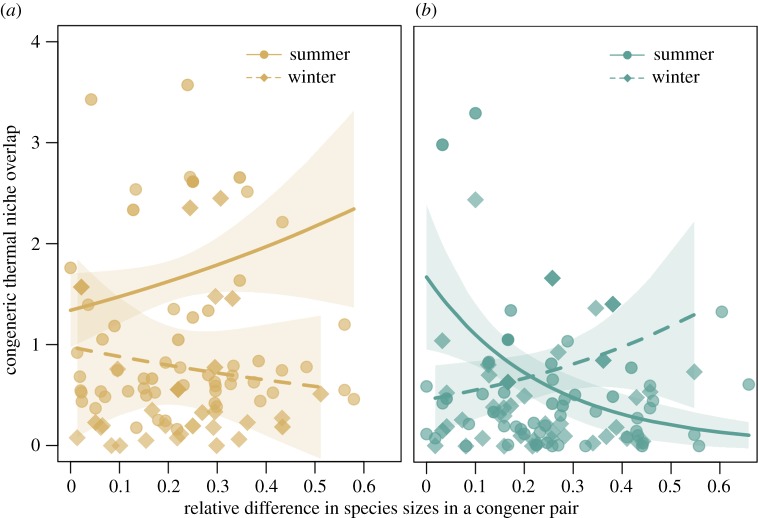
Congeners in the relatively species-poor west segregated in thermal space more in winter than in summer (β = –0.75 [–1.37, –0.23]; McFadden's pseudo-R2 global model = 0.31), but the degree of congeneric segregation was unrelated to body size similarity between congeners (βsummer = 0.97[–0.44, 2.37]; βwinter = –1.02 [–3.85, 1.81]; figure 4a). Results using the alternative taxonomy were similar (electronic supplementary material, figure SM1). By contrast, we found important interactions between body size similarity and seasonality in the highly speciose (but less seasonal) east. Similarly sized congeners were more likely to segregate in thermal space in the winter in the east (βwinter = 1.91 [0.17, 3.67]; figure 4b). This relationship reversed in summer, with similarly sized species in the east more likely to share thermal niche space (βsummer = –4.17 [–6.51, –2.00]; figure 4b). These relationships were preserved with the alternative taxonomy, albeit more weakly (electronic supplementary material, figure SM1). Finally, there was no significant difference between the results from the GLM and GLMM, indicating that multiple occurrences of the same species in the analysis did not bias our results (Pearson's R between fitted values of GLM and GLMM = 0.98).
Figure 4.
Relationship between thermal niche overlap and relative size differences between congeneric species pairs of Himalayan birds (n = 201 pairs). (a) In the west, thermal niche overlap between congeners was not related to differences in body size in either summer or in winter. (b) In the east, similarly sized congeners segregated in thermal space in winter, but occupied similar thermal space in summer. Bold lines and shaded regions reflect predicted relationships from weighted quasi-Poisson regressions with 95% confidence intervals, respectively. The transparency of each point indicates its relative weight in the regression—bolder points contribute more towards model fit (see Material and methods).
4. Discussion
We examined how temperature and competition interact to structure species ranges for an entire community of birds in regions that differ greatly in their abiotic and biotic environments, making this one of the first studies to examine the roles of both abiotic and biotic factors as determinants of species ranges across large environmental gradients within a unified framework [4]. Our results suggest that: (i) species in the relatively aseasonal eastern Himalayas are more sensitive to temperature than species in the highly seasonal western Himalayas; (ii) competition is greater in the species-rich eastern Himalayas compared with the species-poor western Himalayas; and (iii) winter enhances competition in both the western and eastern Himalayas, but in different ways.
(a). Abiotic determinants of community structure
Temperature appears to be an important factor structuring eastern Himalayan bird communities (figure 3), more so than for western Himalayan birds. For species common to both the eastern and western Himalayas, eastern populations more closely tracked their thermal niches across seasons, suggesting they are more sensitive to changing temperatures than populations in the west (figure 3a, table 1: A2). Further, body size was much more strongly negatively correlated with seasonal temperature tracking in the relatively aseasonal eastern Himalayas compared with the more seasonal west (figure 3b, table 1: A3). In other words, for a given body size, species tracked temperature twice as strongly in the east than in the west (figure 3b), also indicating greater thermoregulatory constraints on eastern Himalayan species (table 1: A2–3). These results are consistent with the climatic variability hypothesis, which postulates that species—or even populations of a species—evolve narrow physiological tolerances in aseasonal environments, and are, therefore, more constrained by the abiotic environment than species in more seasonal, temperate zones [5,6,22]. Thus, we report patterns that support the climatic variability hypothesis (table 1: A2) and contradict the abiotic predictions of the species-interactions/abiotic-stress hypothesis (table 1: A1).
Notably, a large proportion of the western Himalayan avifauna is the result of colonization by tropical clades of southeast Asian origin from the eastern Himalayas [48]. Post-glacial colonization of the western Himalayas from the east is likely to have filtered out species unable to tolerate the marked seasonal fluctuations in the west [49], thus selecting for a western avifauna less structured by thermal sensitivity. This is consistent with recent evidence suggesting that phylogenetic conservatism and the retention of ancestral thermal traits in birds and mammals is more pronounced in the tropics [50]. Seasonality as an abiotic filter can give rise to communities of climate generalists in highly seasonal regions such as the western Himalayas, resulting in patterns (figure 3) that support the climatic variability hypothesis [5] (table 1: A3) and contradict the species-interactions/abiotic-stress hypothesis (table 1: A1).
(b). Biotic determinants of community structure
The greater segregation of potentially competing species pairs in the eastern Himalayas is consistent with the biotic predictions of the species-interactions/abiotic-stress hypothesis (table 1: B1) [1,4], which suggests that competitive interactions can set range limits in species-rich, relatively aseasonal, regions. Our results thus indicate that both temperature specialization and competition are likely to be important in structuring bird communities in the relatively aseasonal, species-rich eastern Himalayas (table 1: A2, B1).
(c). Abiotic–biotic interactions
We find that seasonality appears to have an important effect on the co-occurrence of species in thermal space in both the eastern and western Himalayas (table 1: AB). Winter conditions appear to enhance competition in birds across the Himalayas, but in different, context-specific ways that depend on the underlying abiotic and biotic environment. In the species-poor west, congeneric co-occurrence is greater in summer than in winter, indicating heightened competition in winter (figure 4a). In the species-rich east, while we found no difference in congeneric co-occurrence across seasons, congeneric co-occurrence increased with body size differences in winter; in the summer, similarly sized congeners were more likely to co-occur in thermal niche space (figure 4b). These results also point to a relaxation of competition in summer and heightened competition in winter along a thermal dimension (figure 4). Given the size-linked thermal sensitivity of eastern Himalayan birds (figure 3b, table 1: A3), similarly sized congeners may be constrained by the abiotic environment to occupy coinciding thermal niches in summer.
Seasonality throughout the Himalayas causes drastic fluctuations in resource abundance, with arthropod densities in the peak of winter falling to half those of early winter densities [28]. Summer spikes in arthropod abundance allow for greater congeneric coexistence [50], including in the Himalayas [51]. Greater resource availability in summer is therefore a likely mechanism by which competition between similarly sized congeners is relaxed, allowing congeners to coexist in the portion of temperature space optimal for their size. This summertime relaxation of competition could be more pronounced in the Eastern Himalayas because summer prey densities in the east are twice as high as densities in the west [51].
Our results are consistent with studies showing seasonality can influence the strength of interspecific interactions for a range of taxa [50,52,53]; our results also show that, for the Himalayan bird community, temperature seasonality can promote alternating periods of coexistence and competitive exclusion along elevational and thermal dimensions. Thus, seasonality results in different summer and winter distributional patterns in the western and eastern Himalayas that—depending on region and season—both support and contradict the species-interactions/abiotic-stress hypothesis (table 1: A1, B1). For instance, segregation in thermal space between congeners in the speciose east in winter, and congener coexistence in the depauperate west in summer, are consistent with the idea that competition is more important in limiting ranges in species-rich, aseasonal regions (table 1: B1) [1,4]. However, patterns of overlap in thermal niche space between congeners in summer in the species-rich east (coexistence) and winter in the less speciose west (segregation) contradict predictions made by Darwin [1]. These seasonally alternating patterns further reinforce the limitations of a single explanatory framework (either the climate variability or the species-interactions/abiotic-stress hypotheses) in adequately explaining complex interactions between abiotic and biotic environments in structuring species ranges.
(d). Caveats and considerations
While our study considers both the abiotic and biotic factors in a unified framework, we characterize the influence of both these factors within the context of thermal niche space. Doing so potentially underestimates the importance of other abiotic factors such as precipitation, which are important components of ecological niches [14] and known to influence species ranges [18]. Techniques that incorporate multiple dimensions of niche space and make unbiased estimates of niche overlap are advancing [36], yet further work is needed to implement such techniques at fine spatial scales that are appropriate for simultaneously assessing competitive dynamics. Second, we also note that species do not solely compete for optimal thermal space, but also for food resources, nesting sites and perches to attract mates [54]. Additional metrics of competition based on morphological characteristics (such as beak size or tarsus length) or direct measurements of resource competition through behavioural experiments [8] could help elucidate how the abiotic environment might influence these other important determinants of community structure. Third, many Himalayan bird species are also capable of using agricultural lands, particularly during winter [55], and may experience different (probably warmer) thermal niches in these landscapes compared with forest. Because our surveys were restricted to forests, it is possible we underestimated the size of some species' winter thermal niches (though this underestimation would presumably be constant across regions). Further research exploring whether bird species use agricultural lands to track seasonal temperature changes or to exploit other seasonal resource fluctuations would further elucidate the nature of biotic and abiotic limitations of species ranges. Finally, exploring the potential for competition between ecologically similar—but phylogenetically distinct—species across a range of abiotic conditions could bolster our understanding of the ways in which the abiotic environment, species ecology and evolutionary history interact to influence community structure.
5. Conclusion
Taken together, our results suggest that in ecological time, pronounced abiotic sensitivity regulates range limits to a greater degree in the seasonally more benign eastern Himalayas (figure 3), and that winter resource scarcity promotes competitive segregation in both highly seasonal, species-poor environments as well as less seasonal, species-rich environments (figure 4). As ultimate mechanisms over evolutionary time, stronger breeding competition in the more speciose east could have resulted in greater congener segregation along an elevational (or thermal) dimension, subsequently influencing adaptation to the abiotic environment and leading to pronounced breeding thermal specialization in eastern Himalayan bird species [29]. These results, while consistent with recent research linking temperature variability and elevational range size [22], underscore how a fluctuating abiotic environment can mediate both the strength and the direction of biotic interactions to structure entire communities. Importantly, while our results suggest that eastern Himalayan bird species may be more sensitive to the abiotic environment than western Himalayan species, previous research disentangling multiple drivers of bird elevational range limits indicated that most species’ breeding ranges in the western Himalayas are also set by temperature [17]. Perhaps seasonal increases in resource availability during the breeding season reduce interspecific competition, making temperature assume greater importance in structuring breeding species distributions.
The opposing abiotic and biotic gradients in the Himalayas are directly analogous to latitudinal gradients in temperature variability and species richness globally, with higher species richness in the tropics and greater seasonality at higher latitudes. Our results therefore may help to explain the factors setting range limits and structuring ecological communities at broader spatial scales, especially for endotherms such as birds. Based on our findings, we expect that thermal specialization and competition are both likely to play important roles in structuring communities in the tropics, while heightened competition during resource-scarce periods is a likely determinant of communities at higher latitudes. In the case of Himalayan birds, we find evidence consistent with both mechanisms at play. We emphasize that rather than abiotic or biotic factors operating independently and consistently to differentially influence species ranges and structure ecological communities in temperate and tropical regions [56–58], these factors probably interact at various spatial and temporal scales to magnify or diminish each other, resulting in heretofore unexpected patterns.
Supplementary Material
Acknowledgements
We thank A. Sood, P. Sood, K. Ramesh, I. Glow, N. Tsering and P. Sharma for logistical support. We are grateful to R. Kalyanaraman, P. Negi, L. Chand, B. Thakur, D. Thakur, H. Negi, D. Subba, S. Rai, B. Tamang, M. Rai, D. K. Pradhan and B. Rajjak for help in the field. We thank R. K. Colwell for detailed, helpful comments on the manuscript, and J. Daskin for enhancing its clarity. We also thank the editor and two anonymous reviewers for constructive comments that improved the manuscript.
Ethics
We thank the Forest Departments of the states of Himachal Pradesh and Arunachal Pradesh for providing permissions for this study (Himachal Pradesh permit no. 534; Arunachal Pradesh permit no. 3682-83).
Data accessibility
Available as electronic supplementary material.
Authors' contributions
U.S. and P.R.E. designed the study, collected the data, performed the analyses and wrote the manuscript. M.W.T. and D.S.W. co-designed the study, and contributed to the statistical analyses and writing.
Competing interests
We have no competing interests.
Funding
Funding for this study came from the High Meadows Foundation, Princeton University, the University of Connecticut, and the Princeton Environmental Institute Walbridge Fund.
References
- 1.Darwin C. 1859. On the origin of species by means of natural selection. London, UK: Murray. [Google Scholar]
- 2.MacArthur RH. 1972. Geographical ecology: patterns in the distribution of species. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Brown J. 1995. Macroecology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 4.Louthan AM, Doak DF, Angert AL. 2015. Where and when do species interactions set range limits? Trends Ecol. Evol. 30, 780–792. ( 10.1016/j.tree.2015.09.011) [DOI] [PubMed] [Google Scholar]
- 5.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 6.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 7.Khaliq I, Hof C, Prinzinger R, Böhning-Gaese K, Pfenninger M. 2014. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. R Soc. B 281, 20141097 ( 10.1098/rspb.2014.1097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowski JE, Robinson SK, Levey DJ. 2012. Squeezed at the top: interspecific aggression may constrain elevational ranges in tropical birds. Ecology 91, 1877–1884. ( 10.1890/09-2063.1) [DOI] [PubMed] [Google Scholar]
- 9.Price TD, Mohan D, Tietze DT, Hooper DM, Orme CDL, Rasmussen PC. 2011. Determinants of northerly range limits along the Himalayan bird diversity gradient. Am. Nat. 178, S97–S108. ( 10.1086/661926) [DOI] [PubMed] [Google Scholar]
- 10.Gaston KJ. 2000. Global patterns in biodiversity. Nature 405, 220–227. ( 10.1038/35012228) [DOI] [PubMed] [Google Scholar]
- 11.Root T. 1988. Energy constraints on avian distributions and abundances. Ecology 69, 330–339. ( 10.2307/1940431) [DOI] [Google Scholar]
- 12.Perry AL, Low PJ, Ellis JR, Reynolds JD. 2005. Climate change and distributional shifts in marine fishes. Science 308, 1912–1915. ( 10.1126/science.1111322) [DOI] [PubMed] [Google Scholar]
- 13.Soberon J. 2007. Grinellian and Eltonian niches and geographic distribution of species. Ecol. Lett. 10, 1115–1123. ( 10.1111/j.1461-0248.2007.01107.x) [DOI] [PubMed] [Google Scholar]
- 14.Soberon J, Nakamura M. 2009. Niches and distributional areas: concepts, methods, and assumptions. Proc. Natl. Acad. Sci. USA 106, 19 644–19 650. ( 10.1073/pnas.0901637106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiens JJ. 2011. The niche, biogeography and species interactions. Phil. Trans. R. Soc. B 366, 2331–2335. ( 10.1098/rstb.2011.0059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boucher-Lalonde V, Kerr JT, Currie DJ. 2014. Does climate limit species richness by limiting individual species ranges? Proc. R. Soc. B 281, 20132695 ( 10.1098/rspb.2013.2695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsen PR, Tingley MW, Kalyanaraman R, Ramesh K, Wilcove DS. 2017. The role of competition, ecotones and temperature in the elevational distribution of Himalayan birds. Ecology 98, 337–348. ( 10.1002/ecy.1669) [DOI] [PubMed] [Google Scholar]
- 18.Tingley MW, Monahan WB, Beissinger SR, Moritz C. 2009. Birds track their Grinellian niche through a century of climate change. Proc. Natl Acad. Sci. USA 106, 19 637–19 643. ( 10.1073/pnas.0901562106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez C, Tenerio EA, Montoya P, Cadena CD. 2016. Niche-tracking migrants and niche switching residents: evolution of climatic niches in New World warblers (Parulidae). Proc. R. Soc. B 283, 20152458 ( 10.1098/rspb.2015.2458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross SD, Price TD. 2000. Determinants of the northern and southern range limits of a warbler. J. Biogeogr. 27, 869–878. ( 10.1046/j.1365-2699.2000.00440.x) [DOI] [Google Scholar]
- 21.Ettinger AK, Ford KR, HilleRisLambers J. 2011. Climate determines upper, but not lower, altitudinal range limits of Pacific Northwest conifers. Ecology 92, 1323–1331. ( 10.1890/10-1639.1) [DOI] [PubMed] [Google Scholar]
- 22.Chan WP, Chen IC, Colwell RK, Liu WC, Huang CY, Shen SF. 2016. Seasonal and daily climate variation have opposite effects in species elevational range size. Science 351, 1437–1439. ( 10.1126/science.aab4119) [DOI] [PubMed] [Google Scholar]
- 23.Porter WP, Kearney M. 2009. Size, shape and the thermal niche of endotherms. Proc. Natl Acad. Sci USA 106, 19 666–19 672. ( 10.1073/pnas.0907321106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connell JH. 1961. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42, 710–723. ( 10.2307/1933500) [DOI] [Google Scholar]
- 25.Terborgh J. 1971. Distribution on environmental gradients: theory and a preliminary interpretation of distributional patterns in the avifauna of the Cordillera Vilcabamba, Peru. Ecology 52, 23–40. ( 10.2307/1934735) [DOI] [Google Scholar]
- 26.Taniguchi Y, Nakano S. 2000. Condition-specific competition: implications for the altitudinal distribution of stream fishes. Ecology 81, 2027–2039. ( 10.1890/0012-9658(2000)081%5B2027:CSCIFT%5D2.0.CO;2) [DOI] [Google Scholar]
- 27.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 28.Ghosh M, Singh P, Mohan D. 2011. Seasonal variation in foraging ecology of three species of overwintering leaf warblers (genus Phylloscopus) in the Himalayan foothills. J. Ornith. 152, 869–877. ( 10.1007/s10336-011-0670-9) [DOI] [Google Scholar]
- 29.Price TD, et al. 2014. Niche filling slows the diversification of Himalayan songbirds. Nature 509, 222–225. ( 10.1038/nature13272) [DOI] [PubMed] [Google Scholar]
- 30.Hijmans RJ, Cameron PR, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climat. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 31.Jenkins CN, Pimm SL, Joppa LN. 2013. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 110, E2602–E2610. ( 10.1073/pnas.1302251110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill F, Donsker D.. 2016. IOC World Bird list (v 6.3). ( 10.14344/IOC.ML.6.3). [DOI]
- 33.Grinnell J. 1917. The niche-relationships of the California thrasher. Auk 34, 427–433. ( 10.2307/4072271) [DOI] [Google Scholar]
- 34.Bivand R, Rundel C, Pebesma E, Hufthammer KO, Stuetz R. 2016. rgeos: interface to geometry engine—open source. See https://cran.r-project.org/web/packages/rgeos/index.html .
- 35.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 36.Broennimann O, et al. 2012. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeog. 21, 481–497. ( 10.1111/j.1466-8238.2011.00698.x) [DOI] [Google Scholar]
- 37.Dunning JB. 2008. CRC handbook of avian body masses. Baco Raton, FL: CRC Press. [Google Scholar]
- 38.Korn EL, Graubard BI. 1995. Examples of differing weighted and unweighted estimates from a sample survey. Am. Stat. 49, 291–295. [Google Scholar]
- 39.Grimmett R, Inskipp C, Inskipp T. 1991. Birds of India, Pakistan, Nepal, Bhutan, Sri Lanka, and the Maldives. Princeton, NJ: Princeton University Press. [Google Scholar]
- 40.Spierenburg P. 2005. Birds in Bhutan: status and distribution. Bedford, UK: Oriental Bird Club. [Google Scholar]
- 41.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884. ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 42.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 43.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 44.Terborgh J, Weske JS. 1975. The role of competition in the distribution of Andean birds. Ecology 56, 562–576. ( 10.2307/1935491) [DOI] [Google Scholar]
- 45.Noon BR. 1981. The distribution of an avian guild along a temperate elevational gradient: the importance and expression of competition. Ecol. Monog. 51, 105–124. ( 10.2307/2937309) [DOI] [Google Scholar]
- 46.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 47.Tobias JA, Cornwallis CK, Derryberry EP, Claramunt S, Brumfield RT, Seddon N. 2014. Species coexistence and the dynamics of phenotypic evolution in adaptive radiation. Nature 506, 359 ( 10.1038/nature12874) [DOI] [PubMed] [Google Scholar]
- 48.Packert M, Martens J, Sun YH, Severinghaus LL, Nazarenko AA, Ting J, Topfer T, Tietze DT. 2012. Horizontal and elevational phylogeographic patterns of Himalayan and Southeast Asian forest passerines (Aves: Passeriformes). J. Biogeog. 39, 556–573. ( 10.1111/j.1365-2699.2011.02606.x) [DOI] [Google Scholar]
- 49.Srinivasan U, Tamma K, Ramakrishnan U. 2014. Past climate and species ecology drive nested species richness patterns along an east-west axis in the Himalayas. Glob. Ecol. Biogeog. 23, 52–60. ( 10.1111/geb.12082) [DOI] [Google Scholar]
- 50.DuBowy P. 1988. Waterfowl communities and seasonal environments: temporal variability in interspecific competition. Ecology 69, 1439–1453. ( 10.2307/1941641) [DOI] [Google Scholar]
- 51.Ghosh-Harihar M, Price TD. 2014. A test for community saturation along the Himalayan bird diversity gradient, based on within-species geographic variation. J. Anim. Ecol. 83, 628–638. ( 10.1111/1365-2656.12157) [DOI] [PubMed] [Google Scholar]
- 52.Hu SS, Tessier AJ. 1995. Seasonal succession and the strength of intra- and interspecific competition in a Daphnia assemblage. Ecology 76, 2278–2294. ( 10.2307/1941702) [DOI] [Google Scholar]
- 53.Bertness MD, Ewanchuk PJ. 2002. Latitudinal and climate-driven variation in the strength and nature of biological interactions in New England Salt Marshes. Oecologia 132, 392–401. ( 10.1007/s00442-002-0972-y) [DOI] [PubMed] [Google Scholar]
- 54.Dhont AA. 2012. Interspecific competition in birds. Oxford, UK: Oxford University Press. [Google Scholar]
- 55.Elsen PR, Kalyanaraman R, Ramesh K, Wilcove DS. 2017. The importance of agricultural lands for Himalayan birds in winter. Conserv. Biol. 31, 416–426. ( 10.1111/cobi.12812) [DOI] [PubMed] [Google Scholar]
- 56.Jiguet F, Gado AS, Julliard R, Neaston SE, Couvet D. 2007. Climate envelope, life-history traits and the resilience of birds facing global change. Glob. Change Biol. 13, 1672–1684. ( 10.1111/j.1365-2486.2007.01386.x) [DOI] [Google Scholar]
- 57.Cahill AE, et al. 2012. How does climate change cause extinction? Proc. R. Soc. B 383, 20121890 ( 10.1098/rspb.2012.1890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cahill AE, et al. 2014. Causes of warm-edge range limits: systematic review, proximate factors and implications for climate change. J. Biogeog. 41, 429–442. ( 10.1111/jbi.12231) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available as electronic supplementary material.