Significance
How terrestrial plants use N and respond to soil N loading is central to evaluating and predicting changing ecosystem structure and function with climate warming and N pollution. Here, evidence from NO3− in plant tissues has uncovered the uptake and assimilation of soil NO3− by Arctic tundra plants, which has long been assumed negligible. Soil NO3− contributed about one-third of the bulk N used by tundra plants of northern Alaska. Accordingly, the importance of soil NO3− for tundra plants should be considered in future studies on N and C cycling in Arctic ecosystems where C sequestration is strongly determined by N availability.
Keywords: Arctic tundra plants, nitrogen dynamics, plant nitrate, soil nitrate, stable isotopes
Abstract
Plant nitrogen (N) use is a key component of the N cycle in terrestrial ecosystems. The supply of N to plants affects community species composition and ecosystem processes such as photosynthesis and carbon (C) accumulation. However, the availabilities and relative importance of different N forms to plants are not well understood. While nitrate (NO3−) is a major N form used by plants worldwide, it is discounted as a N source for Arctic tundra plants because of extremely low NO3− concentrations in Arctic tundra soils, undetectable soil nitrification, and plant-tissue NO3− that is typically below detection limits. Here we reexamine NO3− use by tundra plants using a sensitive denitrifier method to analyze plant-tissue NO3−. Soil-derived NO3− was detected in tundra plant tissues, and tundra plants took up soil NO3− at comparable rates to plants from relatively NO3−-rich ecosystems in other biomes. Nitrate assimilation determined by 15N enrichments of leaf NO3− relative to soil NO3− accounted for 4 to 52% (as estimated by a Bayesian isotope-mixing model) of species-specific total leaf N of Alaskan tundra plants. Our finding that in situ soil NO3− availability for tundra plants is high has important implications for Arctic ecosystems, not only in determining species compositions, but also in determining the loss of N from soils via leaching and denitrification. Plant N uptake and soil N losses can strongly influence C uptake and accumulation in tundra soils. Accordingly, this evidence of NO3− availability in tundra soils is crucial for predicting C storage in tundra.
Nitrogen (N) is often the nutrient that most limits terrestrial plant growth, making plant N availability a key determinant of primary productivity in terrestrial ecosystems (1). Hence, improved knowledge of in situ plant N availability and consequent plant N use is crucial for better evaluating and predicting responses of vegetation to climate change and N loading (2, 3). However, the availability of N to terrestrial plants is difficult to evaluate using measurements of soil N because of strong plant–microbe and plant–plant competition for N and the resulting rapid turnover of soil N pools (4).
Arctic ecosystems are typically characterized by strong N limitation (1). Because of high carbon (C) stocks in permafrost soil and their sensitivity to environmental change, the Arctic C cycle has important implications for global C balance and C-climate feedbacks (5, 6). Although it remains difficult to budget N inputs in the Arctic, the Arctic biome is a potential sink for anthropogenic N pollutants (7). So far, long-term N addition experiments have revealed that elevated N inputs into Arctic tundra ecosystems change C accumulation and species diversity (5, 8, 9). Field observations and isotope labeling experiments provide evidence of how added N has altered the distribution, fate, biotic use, and losses of N in Arctic tundra ecosystems (10–15). These studies indicate that a better understanding of in situ N availability in Arctic ecosystems is important because C and N cycles are tightly coupled between the vegetation and soils, and elevated N loading can influence the Arctic’s C balance (5, 16).
Nitrate (NO3−) is a common and pivotal plant-available N form in addition to ammonium (NH4+) and some forms of dissolved organic N (DON) (1). Until the 1990s, researchers underestimated the availability of soil NO3− to microbes because microbial uptake of NO3− often results in very low NO3− standing stock and low or negative net NO3− production (nitrification) rates in soil, even when gross nitrification rates are high (17–19). However, it remains undetermined how important soil NO3− is for plants because of inadequate understanding of in situ plant NO3− use. In Arctic tundra, NO3− availability can be increased by direct release from thawing permafrost, melting snow, and increased nitrification resulting from elevated N loading and warming temperatures (7, 14, 20). Elevated NO3− availability to tundra plants can change interspecific N competition and N-use strategies of tundra plants (9, 13, 21), potentially resulting in the spread of NO3−-adapted species and altering the partitioning of above-ground vs. below-ground biomass (18, 22–24). These factors could alter CO2 fixation by vegetation and the quantity and quality of litter inputs to the soil, which would then change microbial breakdown of soil C and the emission and uptake of greenhouse gases (5, 8, 25–27). Accordingly, soil NO3− availability and plant NO3− use have important implications for both N and C cycles in Arctic tundra.
Despite its potential importance, NO3− availability and the contribution of different N forms to plant N use have been unclear in Arctic tundra (21, 28). Four decades of research show that tundra plants rely on soil NH4+ and DON (e.g., direct uptake of free amino acids) to meet growth requirements for N (12, 21, 28–31). In contrast, researchers generally have considered plant NO3− use to be negligible in the Arctic for several reasons. First, NO3− concentrations in soils are often low or undetectable, and soil net nitrification rates seldom show positive values (SI Appendix, Figs. S1 and S2), presumably because of low temperature, low soil NH4+ availability, and low soil pH, together with high microbial N demand (32, 33). Second, plant-tissue NO3−, a common marker of plant NO3− uptake, is rarely detected in tundra plants with conventional analytical methods (11, 12, 34).
We argue that the importance of NO3− to plants in such seemingly low-NO3− Arctic tundra ecosystems remains an open question for several reasons. First, although extractable soil NO3− concentrations are typically low in Arctic tundra soils, NO3− is sometimes present in measurable amounts and contributes nontrivial fractions of total extractable N (TEN) stocks similar to high-NO3− ecosystems (SI Appendix, Fig. S2B). Second, rates of in situ NO3− reductase activity (NRA), which is inducible and reflects the enzymatic NO3− reduction occurring in plants, are measurable in tundra plants and are not distinct from NRA rates measured in plants at lower latitudes (SI Appendix, Fig. S3). Accordingly, the abilities of Arctic tundra plants to assimilate NO3− are comparable to those of plants in relatively NO3−-rich ecosystems. Third, controlled experiments revealed that tundra plants took up NH4+ and NO3− at similar rates (9, 12, 29) or even took up NO3− at higher rates (33). Field 15N application (7, 13, 31) and modeling results (35) confirmed that tundra plants can assimilate NO3−, NH4+, and amino acids. All these observations illustrate that NO3− is an important soil N source in Arctic tundra and that tundra plants can use NO3−. However, the relative importance of soil NO3− for plants in Arctic tundra ecosystems is unknown because we lack measures of in situ plant NO3− use and how it compares to that of plants in other NO3−-poor or NO3−-rich ecosystems.
Results and Discussion
Using the highly sensitive denitrifier method (detailed in Materials and Methods), we analyzed concentrations and stable isotope compositions of NO3− in tissues of dominant plant species in Alaskan tundra ecosystems. We then compared our results with those for plants from relatively high-N or high-NO3− ecosystems in lower-latitude regions (Figs. 1 and 2). Such comparisons of Arctic sites to non-Arctic sites, using both traditional and new methods, are important for understanding soil N cycling (particularly soil NO3− availability) and for placing the N uptake abilities of tundra plants into a broader context.
Fig. 1.
Concentrations of NO3− in plant leaves (A) and roots (B) across different ecosystems. The box encompasses the 25th to 75th percentiles, and whiskers are the SD values. The line and square in each box mark the median and mean values of studied plants at each site, respectively. Unique letters above the boxes mark significant differences at the level of P < 0.05. Detailed site information, including site abbreviation definitions, and species-specific values are given in SI Appendix, Tables S1 and S2. dw, dry weight.
Fig. 2.
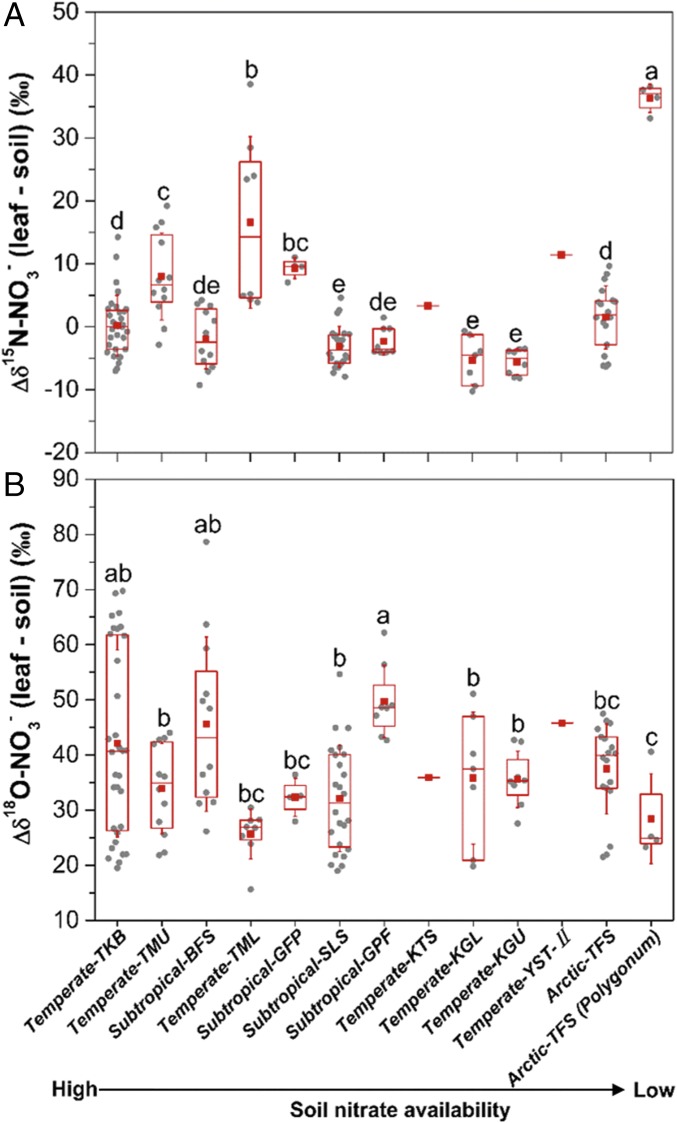
Differences (∆ values) in δ15N (A) and δ18O (B) between leaf NO3− and soil NO3− across different ecosystems. The box encompasses the 25th to 75th percentiles, whiskers are the SD values, and the red line and red square in each box mark the median and mean values, respectively. Unique letters above the boxes indicate significant differences at the level of P < 0.05. The ∆ values were calculated using replicate values of plant tissues minus mean values of soil in corresponding sites (SI Appendix, Fig. S8 and Table S1).
The Uptake of NO3− in Plants.
The existence of NO3− in plant tissues is evidence for NO3− uptake from the soil or atmosphere because NO3− production in non-N2 fixing plants is negligible under normal conditions (36–40). Although NO3− can be produced from the oxidation of nitric oxide (NO) both enzymatically and nonenzymatically in non-N2 fixing plants (37–40), the rates are very low in natural environments (41–44), especially compared with the pool sizes of NO3− detected in plants of this study. Besides, while NO3− production by nonsymbiotic hemoglobin is possible in anoxic conditions (38, 39) and with high ambient NO concentrations (40), neither anoxic conditions nor high ambient NO applies to the present study.
We detected unexpectedly high NO3− concentrations in leaves and roots of the tundra plant species studied (Fig. 1 and SI Appendix, Tables S1 and S2). First, of the 153 tundra plant samples analyzed, 143 had measurable NO3− concentrations (detailed in Materials and Methods). Some species (e.g., Polygonum bistorta) had higher foliar NO3− than low-latitude forest species, including those in high-NO3− environments (Fig. 1A and SI Appendix, Table S2). Second, ratios of leaf NO3− to soil NO3− and of root NO3− to soil NO3− were similar between tundra and lower-latitude ecosystems or even higher in tundra than in some lower-latitude ecosystems (SI Appendix, Fig. S4). These results provide evidence of high NO3− uptake of tundra plants despite much lower concentrations of NO3− in tundra soils. Thus, we conclude that tundra plants can take up NO3− as efficiently as plants from relatively NO3−-rich ecosystems in other biomes. In addition, NO3− additions to soils enhanced leaf NO3− concentrations in most tundra plants (SI Appendix, Figs. S5 and S6). This result is evidence that plant NO3− uptake is responsive to soil NO3− variations in Arctic tundra ecosystems. Such responses and patterns of NO3− uptake among studied species are useful for interpreting changes in functional traits and the structure of tundra plant communities in response to projected increases of soil NO3− with climate warming and elevated N deposition (1, 45).
The Sources of NO3− in Plants.
We used the ∆17O signatures of leaf NO3− (∆17Oleaf) to verify the mixing of atmospheric-derived NO3− [∆17Oatm > 0 per mille (‰) due to an enrichment in 17O during photochemical oxidization of nitrogen oxides (NOx) by O3] with soil-derived NO3− (∆17Osoil = 0‰ because of no 17O excess in atmospheric O2 and soil H2O molecules) (46–48). Leaf NO3− of P. bistorta showed no 17O isotope anomaly (∆17O values = 0.0‰; SI Appendix, Fig. S7), indicating that the NO3− detected in this species was purely soil derived. Clearly, soil NO3− is available to, and taken up by, tundra plants.
In contrast, positive ∆17Oleaf values in low-latitude forests (SI Appendix, Fig. S7) indicate the direct leaf absorption of atmospheric-derived NO3− (∆17O > 0‰) or possibly the root uptake of NO3− at the surface soil with positive ∆17O values (49). We used mean ∆17O values of precipitation NO3− measured in the Tama-Kyuryo Field Museum forest in temperate Japan (TML) (see SI Appendix, Table S1 for descriptions of the forest sites used in this study) (49); in Guiyang in subtropical China (this study); and in Jianfengling forests in Hainan, tropical China (49) as ∆17Oatm values in the studied temperate, subtropical, and tropical forests, respectively (SI Appendix, Fig. S7). We then estimated mixing ratios of atmospheric-derived NO3− (∆17Oleaf:∆17Oatm) for plants in lower-latitude ecosystems. The results showed that atmospheric-derived NO3− accounted for, on average, 35% (6 to 86%) of total leaf NO3− in measured samples from lower-latitude forests.
NO3− Assimilation in Plants.
Higher δ15N and δ18O values in plant-tissue NO3− relative to source NO3− could provide new evidence for in situ plant NO3− assimilation because NO3− reduction via NO3− reductase would cause 15N and 18O enrichments in the unassimilated NO3− (2, 50–52). Accordingly, we calculated differences (∆ values) between isotopic values of tissue NO3− (δ15N and δ18O) in each plant sample and mean values of soil NO3− in corresponding ecosystems (Fig. 2 and SI Appendix, Fig. S8).
In northern Alaska, δ15N values of soil NO3− were 1.0‰ at Toolik Field Station (TFS) (see SI Appendix, Table S1) (21, 53) and 0.5 ± 4.7‰ at Barrow (54). Atmospheric-derived NO3− in snowmelt had lower δ15N values of −4.8 ± 1.0‰ at Barrow (54) and much lower values of −8.6 ± 0.7‰ at a high Arctic site at Midtre Lovénbreen, Svalbard (55). Compared with δ15N values of soil- or atmospheric-derived NO3− (SI Appendix, Fig. S8A), the higher δ15N values of leaf NO3− in tundra of northern Alaska (positive Δδ15N values; Fig. 2A) are evidence for in situ NO3− assimilation in tundra plants (Fig. 2A). The δ18O values of NO3− produced in high-centered soil polygons averaged −4.4 ± 2.7‰ at Barrow (54). By comparison, distinctly higher δ18O values of leaf NO3− than those of soil NO3− (positive ∆δ18O values; Fig. 2B) also provide evidence for in situ NO3− assimilation in tundra plants.
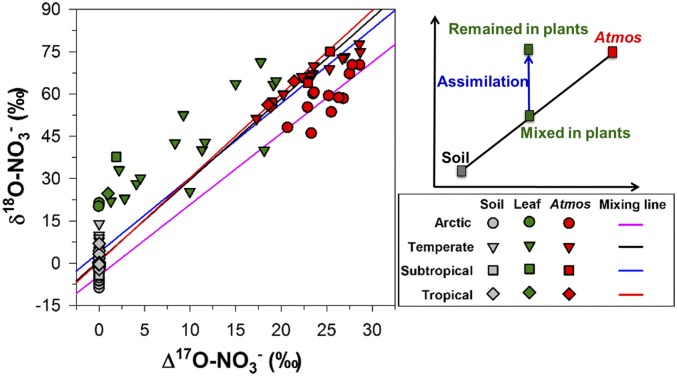
In non-Arctic sites, higher δ18O values of leaf NO3− than those of a soil- and atmospheric-derived NO3− mixture (distributed on the mixing line; Fig. 3) indicated assimilation of the mixed NO3− pool in the studied plants. However, higher 18O enrichments (SI Appendix, Fig. S8) might be due, in part, to contributions from high δ18O values of atmospheric-derived NO3− (57). Major uncertainties existed in fractional contributions of atmospheric-derived NO3− in leaf NO3− because of limited ∆17O data of leaf NO3− and lack of explicit ∆17O values of atmospheric NO3−. Precipitation NO3− might not fully represent all atmospheric NO3− contributions to plant leaves; in addition, it is even more difficult to determine reasonable δ15N and δ18O end-member values of atmospheric-derived NO3− in plant leaves. Despite these problems, NO3− isotopes in plant tissues did provide information on plant NO3− sources and uptake in disturbed ecosystems.
Fig. 3.
Δ17O vs. δ18O plots of NO3− in soil, leaves, and atmospheric (Atmos, as precipitation or snow) deposition across different ecosystems. The mixing lines of Arctic and tropical sites (y = 2.52x − 4.42 and y = 2.97x + 0.58, respectively) were based on isotopic values of soil NO3− (n = 18) (54) and snowpack NO3− (n = 12) (56) at Barrow, and of soil NO3− (n = 18) and precipitation NO3− (n = 3) at Jianfengling in tropical China (49), respectively. The mixing line of temperate sites (y = 2.64x + 3.82) was based on isotopic values of soil NO3− at Japanese temperate sites (n = 22) and precipitation at TML (n = 12) in this study. The mixing line of subtropical sites (y = 2.87x + 0.91) was based on isotopic values of soil NO3− (n = 29) at subtropical sites and precipitation NO3− at Guiyang, China (n = 3) in this study. The Δ17O of soil NO3− was assumed to be zero.
Contributions of Soil NO3− to Total N in Tundra Plants.
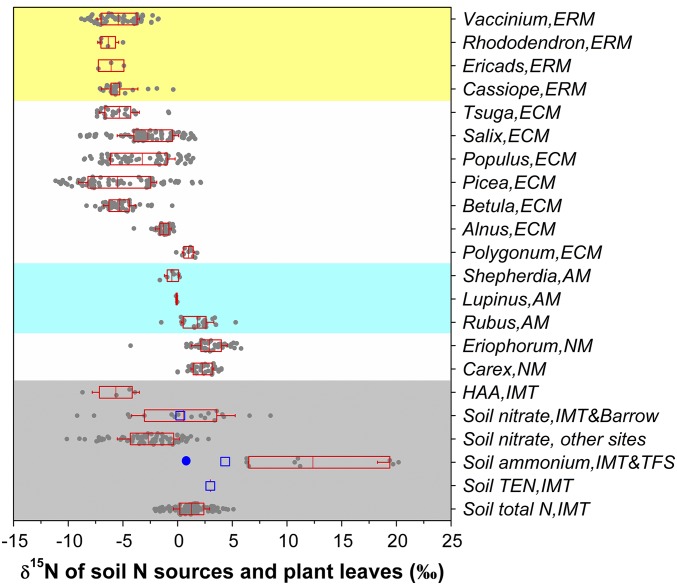
Compared with plants in relatively N-rich ecosystems, tundra plants showed a similar distribution of leaf total N concentrations but a much wider distribution of leaf total (bulk) δ15N values (SI Appendix, Fig. S9). The wider distribution of leaf total δ15N values arises because of the strong niche differentiation of N-use regimes among tundra plants (13, 58). However, δ15N values of total N in tundra plants (−11.2 to 5.8‰ in Alaska) are generally lower than those of soil NH4+ [around 12.3 ± 3.6‰ (this study); 4.4 ± 0.9‰ (53); and 1.4 ± 0.5‰ (21)], although some DON components are 15N depleted [around −5.7‰ for hydrolyzable amino acids (HAA) at Imnavait Creek (IMT) in northern Alaska; see SI Appendix, Table S1] (Fig. 4). This disparity between the δ15N signatures of plant total N vs. soil N sources exists even when isotopic fractionations for NH4+ and HAA assimilation by mycorrhizal plants are considered. Given plant NO3− uptake and assimilation as indicated by NO3− in plant tissues, soil NO3− should be considered when using δ15N methods to evaluate in situ contributions of soil N sources to total N of tundra plants.
Fig. 4.
δ15N values of leaf total N and soil N sources of tundra plants in Alaska. AM, arbuscular mycorrhiza; ECM, ectomycorrhiza; ERM, ericoid mycorrhiza; NM, nonmycorrhiza. The box encompasses the 25th to 75th percentiles, and whiskers are the SD values. The line in each box marks the mean value. Plant δ15N data were summarized from ref. 58 and those of SI Appendix, Fig. S9B. The empty squares show soil δ15N data reported at IMT (53) and the blue-filled circle shows data at TFS (21). Soil δ15N-NO3− values of other sites are summarized from available data of non-Arctic sites in this study; soil δ15N-NO3− values at Barrow are cited from ref. 54.
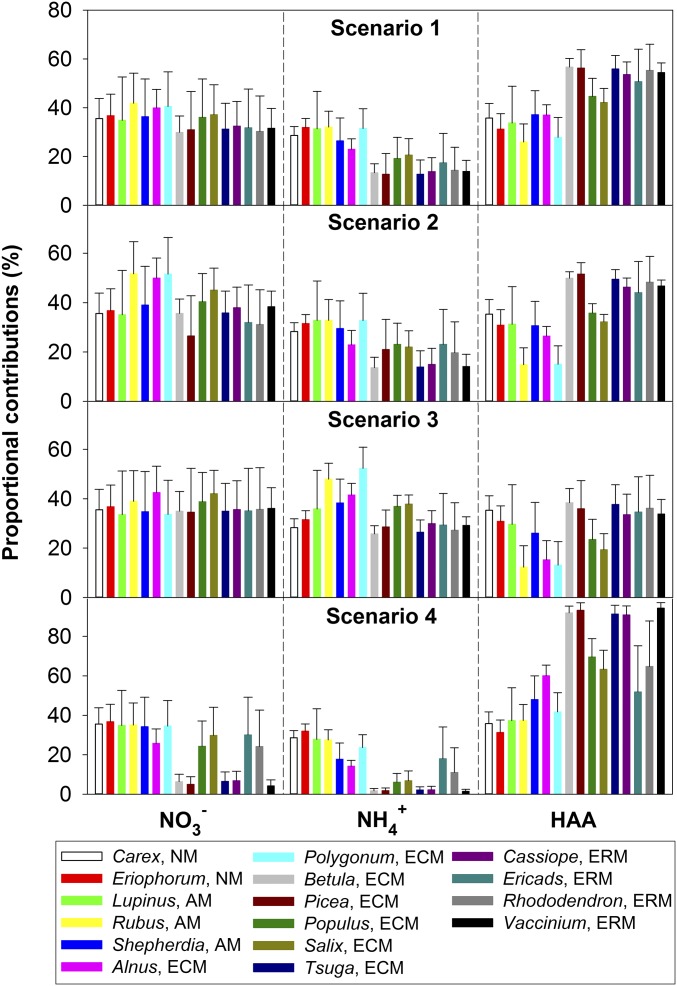
Proportional contributions (f, expressed as a percentage) of soil NO3− to total N in tundra plants were estimated using δ15N values of soil N (NO3−, NH4+, and HAA) and δ15N values of leaf total N in a Bayesian isotope-mixing model [Stable Isotope Analysis in R (SIAR) (cran.r-project.org/web/packages/siar/index.html) (59)] (Fig. 5). The SIAR model uses a Bayesian framework to establish a logical prior distribution (60) for estimating f values, and then determines the probability distribution for the f values of each source (soil NO3−, NH4+, and HAA, in this study) to the mixture (total N of plant leaves, in this study). We contend that this approach provides reliable estimations of fractional contributions of different N sources to plant total N because the mixing model considers isotope effects during plant N uptake (15ε values hereafter) and variability in both source δ15N values and plant δ15N values (61).
Fig. 5.
Proportional contributions (mean ± SD) of soil NO3−, NH4+, and HAA to leaf total N of tundra plants in Alaska. The 15ε values [0‰ for NM plants, −5.0‰ for AM plants, −6.9‰ for ECM plants, and −7.7‰ for ERM plants (21, 62)] were considered for NO3−, NH4+, and HAA (scenario 1); for NH4+ and HAA only (scenario 2); for HAA only (scenario 3); for none of NO3−, NH4+, and HAA (scenario 4).
In this study, the δ15N values (mean ± SD) of soil NO3− at Barrow [0.5 ± 4.7‰ (54)], soil NH4+ at IMT and TFS (11.5 ± 8.4‰, this study and ref. 21), and soil HAA at IMT [−5.7 ± 2.2‰; (53)] were used as source δ15N values. For nonmycorrhizal (NM) plants, leaf δ15N values were mainly controlled by the δ15N values and f values of source N (NO3−, NH4+, and HAA), assuming negligible isotope effects during the acquisition processes of source N from soil into NM plants (i.e., 15ε = 0‰). For mycorrhizal plants, the 15ε values during the acquisition processes of soil N sources were calculated as the net differences of leaf δ15N values between mycorrhizal and NM plants. The same 15ε value was assumed for plant species associated with the same type of mycorrhiza and for N forms absorbed through the same type of mycorrhiza. In Alaskan tundra, the 15ε values for plant species associated with arbuscular mycorrhizae (AM), ectomycorrhizae (ECM), and ericoid mycorrhizae (ERM) were estimated as net δ15N differences from NM plants—that is, −5.0‰, −6.9‰, and −7.7‰, respectively (21, 62), which differed from the 15ε values normalized for worldwide plants [−2.0‰, −3.2‰, and −5.9‰, respectively (63)]. Our 15ε values (0‰ for NM plants, −5.0‰ for AM plants, −6.9‰ for ECM plants, and −7.7‰ for ERM plants) were considered under four scenarios (scenario 1: for NO3−, NH4+, and HAA; scenario 2: for NH4+ and HAA only; scenario 3: for HAA only; and scenario 4: for none of NO3−, NH4+, and HAA) (Fig. 5). Estimates from natural 15N evidence were that NO3− assimilation accounted for 4 to 52% of species-specific leaf total N (around one-third, on average) of Alaskan tundra plants (Fig. 5), thereby demonstrating the importance of soil NO3− relative to soil NH4+ and HAA for N use by many tundra plants. These findings also enhance understanding of N competition among plant species and between plants and microbes in Arctic tundra ecosystems, and how that may affect changes in species community composition and productivity with climate change and N pollution.
Materials and Methods
Study Sites and Sampling.
To evaluate in situ NO3− uptake and assimilation in terrestrial plants in relation to NO3− availability, we selected 18 sites (see descriptions in SI Appendix, Table S1) across a distinct gradient of soil NO3− (SI Appendix, Fig. S2), including one tropical and four subtropical sites in southwestern China; nine temperate sites in central, southern, and western Japan; and four Arctic tundra sites in northern Alaska. Among them, Tsukuba Forest Experimental Watershed (TKB) and Tama-Kyuryo Field Museum upper slope (TMU) and lower slope (TML) (SI Appendix, Table S1) are characterized by high soil NO3− or N saturation (49, 64, 65), while the Arctic sites TFS, Sagavanirktok River Valley (SAG), and IMT (SI Appendix, Table S1) are characterized by unmeasurable nitrification rates and negligible soil NO3− and, thus, are assumed to be typically low-NO3− ecosystems (SI Appendix, Fig. S2). In total, 28 plant species in the above study sites were sampled for fine roots (roughly <5 mm in diameter and <20 cm in spatial distribution of soil depth) or mature sunlit leaves. The studied plants in each ecosystem include dominant indigenous species (SI Appendix, Table S1). The design of this study allows us to evaluate plant NO3− use at the species and ecosystem levels.
Soil N Analyses.
Soil N concentrations and net N transformation rates (mineralization and nitrification) were measured as indices of potentially available NO3− for both plants and soil microbes. Information on soil types and samplings, N variables, and corresponding methods used for each ecosystem are summarized in SI Appendix, Table S1. Concentrations of NO3− and NH4+ in soil solutions, extracts of fresh soils, and extracts of incubated soils (for net N mineralization and net nitrification rates) were determined colorimetrically. TEN was digested to NO3− using alkaline persulfate digestion and its concentration measured as NO3− on the autoanalyzer (specified in SI Appendix, Table S1). In-house standards (alanine, glycine, and histidine) dissolved in corresponding extracts were used for calibrating the concentrations of TEN and estimating the effect of the N blank from reagents (the same as that described in ref. 65). The soil extractable organic N was calculated as the difference between soil TEN and extractable inorganic N.
δ15N and δ18O ratios of soil NO3− were determined using the denitrifier (Pseudomonas aureofaciens) method (described in refs. 65 and 66) that converts NO3− to nitrous oxide (N2O) (67, 68). The calibration curve between measured isotope ratios of N2O and those of NO3− was prepared using US Geological Survey (USGS)-32, USGS-34, USGS-35, and International Atomic Energy Agency (IAEA) NO3 standards. Soil NH4+ in 100-mL extracts of IMT soil was separated onto glass filter papers (GF/D; Whatman) using the diffusion method (69), and then the NH4+ diffused on the filter papers was measured for δ15N values on an elemental analyzer coupled with an isotope ratio mass spectrometer (EA-IRMS) (70) at The Ecosystems Center, Marine Biological Laboratory (Woods Hole, MA). IAEA-N2 was run with the samples to check the accuracy of δ15N-NH4+ data. The analytical precision was better than 0.2‰ for δ15N-NO3−, 0.5‰ for δ18O-NO3−, and 0.5‰ for δ15N-NH4+. The respective natural abundances of 15N and 18O were reported as δ15N and δ18O values expressed in per mille units, as δ15N or δ18O = [(Rsample/Rstandard) – 1] × 1,000, where R = 15N/14N or 18O/16O and standards are atmospheric N2 and standard mean ocean water for N and O, respectively.
Plant N Analyses.
Leaf total N concentrations and total δ15N values of plant samples were analyzed using an EA-IRMS (detailed in SI Appendix, Table S1). The analytical precision for δ15N was better than 0.2‰. The leaf NRA assay, which has been used to evaluate the NO3−-reduction potential of tundra plants [expressed per either fresh or dry weight (58, 71)], was conducted for plants at pristine and control sites of IMT, SAG, TFS-MAT (moist acidic tundra), TFS-MNT (moist non-acidic tundra), and at fertilized plots of TFS-MAT (SI Appendix, Table S1 and Fig. S3 A and B). The method of leaf NRA determination was the same as that described in refs. 58, 72, and 73. The NRA data (only those uniformly reported in dry weight) of natural terrestrial plants in low-latitude ecosystems were compiled (SI Appendix, Fig. S3C) for comparing NRA levels between tundra and low-latitude ecosystems.
The concentrations and δ15N and δ18O of NO3− in plants were measured using the sensitive denitrifier method (67, 68) at the Tokyo University of Agriculture and Technology (TUAT; method details are described in refs. 74 and 75). In the present study, 1 of 7 root samples of Eriophorum vaginatum and 7 of 94 leaf samples of tundra plants showed measurable NO3− concentration as zero, including 5 of 15 Sphagnum samples, 1 of 8 Cassiope tetragona leaf samples, and 1 of 1 Juniperus communis leaf sample.
The ∆17O values of NO3− in plant leaves were determined by combining bacterial reduction [i.e., denitrifier method (67, 68)] and the thermal decomposition method (76). First, NO3− in plant extracts was converted to N2O using the denitrifier method (67, 68) at TUAT (method details are described in refs. 74 and 75). Next, the gold-tube conversion of bacteria-produced N2O into N2 and O2 was conducted, and Δ17O values (defined as Δ17O = [(1 + δ17O)/(1 + δ18O)β] − 1, where the constant β is 0.5247; see refs. 76 and 77) were measured on a Finnigan Delta Plus Advantage IRMS (Thermo Fischer Scientific) at the University of Washington (method details are described in ref. 78). A laboratory standard courtesy of Greg Michalski, Purdue University, West Lafayette, IN [NaNO3 with Δ17O = 19.9‰ (79)] and several standards that mimic the 5% and 10% of atmospheric NO3− (i.e., Δ17O = 1‰ and 2‰, respectively) were used to check the precision of low Δ17O samples. The average SDs for replicate analyses of an individual sample were ±0.2‰ for Δ17O.
Supplementary Material
Acknowledgments
We thank Laura Gough, Andrew J. Schauer, Muneoki Yoh, Nozomi Suzuki, Naohiro Yoshida, Yanbao Lei, Xiaodong Li, Erica Steve, Marshall Otter, Asami Nakanishi, Takahiro Hayashi, Ryo Kobayashi, Chieko Takahashi, Syuichiro Matsushima, Hiroyu Katoh, Azusa A. Hokari, Tomoko Makita, and colleagues and staff at TFS, TUAT, Center for Ecological Research, and the Institute of Geochemistry, Chinese Academy of Sciences for their assistance in the field and laboratory. We also thank Hideo Yamasaki for fruitful discussions on NO3− production by plants, and Erik Hobbie for helpful comments during the revision. This study was supported by the Kyoto University Foundation, the Sumitomo Foundation, Program for Next Generation World-Leading Researcher (Grant GS008) and Grant-in-Aid for Scientific Research (KAKENHI Grants 26252020, 26550004, 17H06297, and P09316) from the Japan Society for Promotion of Science, the National Natural Science Foundation of China (Grants 41730855, 41522301, and 41473081), the National Key Research and Development Program of China (Grants 2016YFA0600802 and 2017YFC0210101), and the 11th Recruitment Program of Global Experts (the Thousand Talents Plan) for Young Professionals granted by the central budget of China. Logistical support at Toolik Lake was provided by the US National Science Foundation Office of Polar Programs. Site selection, site maintenance, site descriptions, and field data were provided by the Arctic Long-Term Ecological Research program, funded by the US National Science Foundation Division of Environmental Biology (Grants 1026843, 1504006, and 1637459).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715382115/-/DCSupplemental.
References
- 1.Chapin FS, III, Matson PA, Vitousek PM. Principles of Terrestrial Ecosystem Ecology. 2nd Ed Springer; New York: 2011. [Google Scholar]
- 2.Bloom AJ, Burger M, Rubio Asensio JS, Cousins AB. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science. 2010;328:899–903. doi: 10.1126/science.1186440. [DOI] [PubMed] [Google Scholar]
- 3.Reich PB, Hobbie SE, Lee TD. Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nat Geosci. 2014;7:920–924. [Google Scholar]
- 4.Schimel JP, Bennett J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology. 2004;85:591–602. [Google Scholar]
- 5.Mack MC, Schuur EA, Bret-Harte MS, Shaver GR, Chapin FS., III Ecosystem carbon storage in Arctic tundra reduced by long-term nutrient fertilization. Nature. 2004;431:440–443. doi: 10.1038/nature02887. [DOI] [PubMed] [Google Scholar]
- 6.Schuur EA, et al. Climate change and the permafrost carbon feedback. Nature. 2015;520:171–179. doi: 10.1038/nature14338. [DOI] [PubMed] [Google Scholar]
- 7.Choudhary S, Blaud A, Osborn AM, Press MC, Phoenix GK. Nitrogen accumulation and partitioning in a High Arctic tundra ecosystem from extreme atmospheric N deposition events. Sci Total Environ. 2016;554-555:303–310. doi: 10.1016/j.scitotenv.2016.02.155. [DOI] [PubMed] [Google Scholar]
- 8.Shaver GR, et al. Species composition interacts with fertilizer to control long term change in tundra productivity. Ecology. 2001;82:3163–3181. [Google Scholar]
- 9.Hill PW, et al. Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nat Clim Chang. 2011;1:50–53. [Google Scholar]
- 10.Alexander V, Whalen SC, Klingensmith KM. Nitrogen cycling in arctic lakes and ponds. Hydrobiologia. 1989;172:165–172. [Google Scholar]
- 11.Chapin FS, III, Shaver GR, Kedrowski RA. Environmental controls over carbon, nitrogen and phosphorus fractions in Eriophorum vaginatum in Alaskan tussock tundra. J Ecol. 1986;74:167–195. [Google Scholar]
- 12.Chapin FS, III, Moilanen L, Kielland K. Preferential use of organic nitrogen for growth by a nonmycorrhizal arctic sedge. Nature. 1993;361:150–153. [Google Scholar]
- 13.McKane RB, et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature. 2002;415:68–71. doi: 10.1038/415068a. [DOI] [PubMed] [Google Scholar]
- 14.Yano Y, Shaver GR, Giblin AE, Rastetter EB, Nadelhoffer KJ. Nitrogen dynamics in a small arctic watershed: Retention and downhill movement of 15N. Ecol Monogr. 2010;80:331–351. [Google Scholar]
- 15.Yano Y, Shaver GR, Rastetter EB, Giblin AE, Laundre JA. Nitrogen dynamics in arctic tundra soils of varying age: Differential responses to fertilization and warming. Oecologia. 2013;173:1575–1586. doi: 10.1007/s00442-013-2733-5. [DOI] [PubMed] [Google Scholar]
- 16.Hobbie SE, Nadelhoffer KJ, Högberg P. A synthesis: The role of nutrients as constraints on carbon balances in boreal and arctic regions. Plant Soil. 2002;242:163–170. [Google Scholar]
- 17.Davidson EA, Hart SC, Firestone MK. Internal cycling of nitrate in soils of a mature coniferous forest. Ecology. 1992;73:1148–1156. [Google Scholar]
- 18.Atkin OK. Reassessing the nitrogen relations of arctic plants: A mini-review. Plant Cell Environ. 1996;19:695–704. [Google Scholar]
- 19.Stark JM, Hart SC. High rates of nitrification and nitrate turnover in undisturbed coniferous forests. Nature. 1997;385:61–64. [Google Scholar]
- 20.Finger RA, et al. Effects of permafrost thaw on nitrogen availability and plant-soil interactions in a boreal Alaskan lowland. J Ecol. 2016;104:1542–1554. [Google Scholar]
- 21.Hobbie JE, Hobbie EA. 15N in symbiotic fungi and plants estimates nitrogen and carbon flux rates in Arctic tundra. Ecology. 2006;87:816–822. doi: 10.1890/0012-9658(2006)87[816:nisfap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Hobbie SE, Chapin FS., III The response of tundra plant biomass, aboveground production, nitrogen, and CO2 flux to experimental warming. Ecology. 1998;79:1526–1544. [Google Scholar]
- 23.Scheurwater I, Koren M, Lambers H, Atkin OK. The contribution of roots and shoots to whole plant nitrate reduction in fast- and slow-growing grass species. J Exp Bot. 2002;53:1635–1642. doi: 10.1093/jxb/erf008. [DOI] [PubMed] [Google Scholar]
- 24.Iversen CM, et al. The unseen iceberg: Plant roots in arctic tundra. New Phytol. 2015;205:34–58. doi: 10.1111/nph.13003. [DOI] [PubMed] [Google Scholar]
- 25.Weintraub MN, Schimel JP. Interactions between carbon and nitrogen mineralization and soil organic matter chemistry in arctic tundra soils. Ecosystems. 2003;6:129–143. [Google Scholar]
- 26.Weintraub MN, Schimel JP. Nitrogen cycling and the spread of shrubs control changes in the carbon balance of Arctic tundra ecosystems. Bioscience. 2005;55:408–415. [Google Scholar]
- 27.Sistla SA, Asao S, Schimel JP. Detecting microbial N-limitation in tussock tundra soil: Implications for Arctic soil organic carbon cycling. Soil Biol Biochem. 2012;55:78–84. [Google Scholar]
- 28.Schimel JP, Chapin FS., III Tundra plant uptake of amino acid and NH4+ nitrogen in situ: Plants complete well for amino acid N. Ecology. 1996;77:2142–2147. [Google Scholar]
- 29.Koch GW, Bloom AJ, Chapin FS., III Ammonium and nitrate as nitrogen sources in two Eriophorum species. Oecologia. 1991;88:570–573. doi: 10.1007/BF00317721. [DOI] [PubMed] [Google Scholar]
- 30.Kielland K. Amino-acid absorption by arctic plants: Implications for plant nutrition and nitrogen cycling. Ecology. 1994;75:2373–2383. [Google Scholar]
- 31.Nordin AI, Schmidt K, Shaver GR. Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology. 2004;85:955–962. [Google Scholar]
- 32.Chapin FS, III, Kedrowski RA. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology. 1983;64:376–391. [Google Scholar]
- 33.Harms TK, Jones JB., Jr Thaw depth determines reaction and transport of inorganic nitrogen in valley bottom permafrost soils: Nitrogen cycling in permafrost soils. Glob Change Biol. 2012;18:2958–2968. doi: 10.1111/j.1365-2486.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Cleve K, Viereck LA. Forest Succession in Relation to Nutrient Cycling in the Boreal Forest of Alaska. Springer; New York: 1981. pp. 185–211. [Google Scholar]
- 35.Leadley PW, Reynolds JF, Chapin FS., III A model of nitrogen uptake by Eriophorum vaginatum roots in the field: Ecological implications. Ecol Monogr. 1997;67:1–22. [Google Scholar]
- 36.Hipkin CR, Simpson DJ, Wainwright SJ, Salem MA. Nitrification by plants that also fix nitrogen. Nature. 2004;430:98–101. doi: 10.1038/nature02635. [DOI] [PubMed] [Google Scholar]
- 37.Yamasaki H, et al. 2011. Nitric oxide synthase-like activities in plants. Nitrogen Metabolism in Plants in the Post-Genomic Era, Annual Plant Reviews, eds Foyer CH, Zhang H (Blackwell Publishing Ltd, West Sussex, UK), Vol 42, pp 103–125.
- 38.Mur LAJ, et al. Nitric oxide in plants: An assessment of the current state of knowledge. AoB Plants. 2013;5:pls052. doi: 10.1093/aobpla/pls052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limami AM, Diab H, Lothier J. Nitrogen metabolism in plants under low oxygen stress. Planta. 2014;239:531–541. doi: 10.1007/s00425-013-2015-9. [DOI] [PubMed] [Google Scholar]
- 40.Kuruthukulangarakoola GT, et al. Nitric oxide-fixation by non-symbiotic haemoglobin proteins in Arabidopsis thaliana under N-limited conditions. Plant Cell Environ. 2017;40:36–50. doi: 10.1111/pce.12773. [DOI] [PubMed] [Google Scholar]
- 41.Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot. 2002;53:103–110. [PubMed] [Google Scholar]
- 42.Perazzolli M, et al. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell. 2004;16:2785–2794. doi: 10.1105/tpc.104.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neill S, et al. Nitric oxide evolution and perception. J Exp Bot. 2008;59:25–35. doi: 10.1093/jxb/erm218. [DOI] [PubMed] [Google Scholar]
- 44.Romero-Puertas MC, Sandalio LM. Role of NO-dependent posttranslational modifications in switching metabolic pathways. Adv Bot Res. 2016;77:123–144. [Google Scholar]
- 45.Zaehle S. Terrestrial nitrogen-carbon cycle interactions at the global scale. Philos Trans R Soc Lond B Biol Sci. 2013;368:20130125. doi: 10.1098/rstb.2013.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michalski G, Scott Z, Kabiling M, Thiemens MH. First measurements and modeling of ∆17O in atmospheric nitrate. Geophys Res Lett. 2003;30:1870. [Google Scholar]
- 47.Michalski G, et al. Tracing atmospheric nitrate deposition in a complex semiarid ecosystem using Δ17O. Environ Sci Technol. 2004;38:2175–2181. doi: 10.1021/es034980+. [DOI] [PubMed] [Google Scholar]
- 48.Dejwakh NR, Meixner T, Michalski G, McIntosh J. Using ¹⁷O to investigate nitrate sources and sinks in a semi-arid groundwater system. Environ Sci Technol. 2012;46:745–751. doi: 10.1021/es203450z. [DOI] [PubMed] [Google Scholar]
- 49.Fang Y, et al. Microbial denitrification dominates nitrate losses from forest ecosystems. Proc Natl Acad Sci USA. 2015;112:1470–1474. doi: 10.1073/pnas.1416776112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu XY, Koba K, Makabe A, Liu CQ. Nitrate dynamics in natural plants: Insights based on the concentration and natural isotope abundances of tissue nitrate. Front Plant Sci. 2014;5:355. doi: 10.3389/fpls.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tcherkez G, Farquhar GD. Viewpoint: Isotopic fractionation by plant nitrate reductase, twenty years later. Funct Plant Biol. 2006;33:531–537. doi: 10.1071/FP05284. [DOI] [PubMed] [Google Scholar]
- 52.Carlisle E, Yarnes C, Toney MD, Bloom AJ. Nitrate reductase 15N discrimination in Arabidopsis thaliana, Zeamays, Aspergillus niger, Picheaangusta, and Escherichia coli. Front Plant Sci. 2014;3:195. doi: 10.3389/fpls.2014.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yano Y, Shaver GR, Giblin AE, Rastetter EB. Depleted 15N in hydrolysable-N of arctic soils and its implication for mycorrhizal fungi-plant interaction. Biogeochemistry. 2010;97:183–194. [Google Scholar]
- 54.Heikoop JM, et al. Isotopic identification of soil and permafrost nitrate sources in an arctic tundra ecosystem. J Geophys Res. 2015;120:1000–1017. [Google Scholar]
- 55.Wynn PM, Hodson AJ, Heaton THE, Chenery SR. Nitrate production beneath a high arctic glacier, Svalbard. Chem Geol. 2007;244:88–102. [Google Scholar]
- 56.Morin S, et al. An isotopic view on the connection between photolytic emissions of NOx from the Arctic snowpack and its oxidation by reactive halogens. J Geophys Res. 2012;117:D00R08. [Google Scholar]
- 57.Kendall C, Elliott EM, Wankel SD. Tracing anthropogenic inputs of nitrogen to ecosystems. In: Michener R, Lajtha K, editors. Stable Isotopes in Ecology and Environmental Science. 2nd Ed. Blackwell Publishing; Oxford, UK: 2007. pp. 375–449. [Google Scholar]
- 58.Nadelhoffer K, et al. 15N natural abundances and N use by tundra plants. Oecologia. 1996;107:386–394. doi: 10.1007/BF00328456. [DOI] [PubMed] [Google Scholar]
- 59.Parnell A, Jackson A. 2008 SIAR: Stable isotope analysis in R, Version 4.2. Available at cran.r-project.org/web/packages/siar/index.html. Accessed April 19, 2016.
- 60.Evans JSBT, Handley SJ, Perham N, Over DE, Thompson VA. Frequency versus probability formats in statistical word problems. Cognition. 2000;77:197–213. doi: 10.1016/s0010-0277(00)00098-6. [DOI] [PubMed] [Google Scholar]
- 61.Moore JW, Semmens BX. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol Lett. 2008;11:470–480. doi: 10.1111/j.1461-0248.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 62.Hobbie EA, Hobbie JE. Natural abundance of 15N in nitrogen-limited forests and tundra can estimate nitrogen cycling through mycorrhizal fungi: A review. Ecosystems. 2008;11:815–830. [Google Scholar]
- 63.Craine JM, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009;183:980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 64.Tabayashi Y, Koba K. Heterogeneous atmospheric nitrogen deposition effects upon the nitrate concentration of stream waters in a forested mountain area. Water Air Soil Pollut. 2011;216:105–115. [Google Scholar]
- 65.Takebayashi Y, Koba K, Sasaki Y, Fang Y, Yoh M. The natural abundance of 15N in plant and soil-available N indicates a shift of main plant N resources to NO3− from NH4+ along the N leaching gradient. Rapid Commun Mass Spectrom. 2010;24:1001–1008. doi: 10.1002/rcm.4469. [DOI] [PubMed] [Google Scholar]
- 66.Koba K, et al. 15N natural abundance of the N lost from an N-saturated subtropical forest in southern China. J Geophys Res. 2012;117:G02015. [Google Scholar]
- 67.Sigman DM, et al. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem. 2001;73:4145–4153. doi: 10.1021/ac010088e. [DOI] [PubMed] [Google Scholar]
- 68.Casciotti KL, Sigman DM, Hastings MG, Böhlke JK, Hilkert A. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem. 2002;74:4905–4912. doi: 10.1021/ac020113w. [DOI] [PubMed] [Google Scholar]
- 69.Koba K, Inagaki K, Sasaki Y, Takebayashi Y, Yoh M. Nitrogen isotopic analysis of dissolved inorganic and organic nitrogen in soil extracts. In: Ohkouchi N, Tayasu I, Koba K, editors. Earth, Life and Isotopes. Kyoto Univ Press; Kyoto: 2010. pp. 17–37. [Google Scholar]
- 70.Fry B, et al. Cryoflow: Cryofocusing nanomole amounts of CO2, N2, and SO2 from an elemental analyzer for stable isotopic analysis. Rapid Commun Mass Spectrom. 1996;10:953–958. [Google Scholar]
- 71.Atkin OK, Cummins WR. The effect of root temperature on the induction of nitrate reductase activites and nitrogen uptake rates in arctic plant species. Plant Soil. 1994;159:187–197. [Google Scholar]
- 72.Koba K, et al. Natural 15N abundance of plants and soil N in a temperate coniferous forest. Ecosystems. 2003;6:457–469. [Google Scholar]
- 73.Koyama L, Kielland K. Plant physiological responses to hydrologically mediated changes in nitrogen supply on a boreal forest floodplain: A mechanism explaining the discrepancy in nitrogen demand and supply. Plant Soil. 2011;342:129–139. [Google Scholar]
- 74.Liu XY, et al. Preliminary insights into δ15N and δ18O of nitrate in natural mosses: A new application of the denitrifier method. Environ Pollut. 2012;162:48–55. doi: 10.1016/j.envpol.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 75.Liu XY, et al. Dual N and O isotopes of nitrate in natural plants: First insights into individual variability and organ-specific pattern. Biogeochemistry. 2013;114:399–411. [Google Scholar]
- 76.Kaiser J, Hastings MG, Houlton BZ, Röckmann T, Sigman DM. Triple oxygen isotope analysis of nitrate using the denitrifier method and thermal decomposition of N2O. Anal Chem. 2007;79:599–607. doi: 10.1021/ac061022s. [DOI] [PubMed] [Google Scholar]
- 77.Miller MF. Isotopic fractionation and the quantification of 17O anomalies in the oxygen three-isotope system: An appraisal and geochemical significance. Geochim Cosmochim Acta. 2002;66:1881–1889. [Google Scholar]
- 78.Costa AW, et al. Analysis of atmospheric inputs of nitrate to a temperate forest ecosystem from Δ17O isotope ratio measurements. Geophys Res Lett. 2011;38:L15805–L15810. [Google Scholar]
- 79.Michalski G. Purification procedure for delta δ15N, δ18O, Δ17O analysis of nitrate. Int J Environ Anal Chem. 2010;90:586–590. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.