Significance
Stress cannot be avoided in the present competitive world, and chronic stress is associated with detrimental effects on physical health, including the progression of inflammatory bowel disease (IBD). However, the mechanisms behind it are less clearly understood. This study showed that chronic stress disturbed gut microbiota, thereby triggering immune system response and facilitating dextran sulfate sodium-induced colitis. Results also showed stress-deficient expression of mucin-2 and lysozyme, which may contribute to the disorder of gut microbiota. This study adds to our understanding of interactions between microbiota and host and provides the basis for future clinical studies of microbiota manipulation and transplantation and the development of new therapeutic strategies for depression or IBD.
Keywords: chronic stress, DSS-induced colitis, immune reaction, gut microbiota, mucin-2
Abstract
Chronic stress is known to promote inflammatory bowel disease (IBD), but the underlying mechanism remains largely unresolved. Here, we found chronic stress to sensitize mice to dextran sulfate sodium (DSS)-induced colitis; to increase the infiltration of B cells, neutrophils, and proinflammatory ly6Chi macrophages in colonic lamina propria; and to present with decreased thymus and mesenteric lymph node (MLN) coefficients. Circulating total white blood cells were significantly increased after stress, and the proportion of MLN-associated immune cells were largely changed. Results showed a marked activation of IL-6/STAT3 signaling by stress. The detrimental action of stress was not terminated in IL-6−/− mice. Interestingly, the composition of gut microbiota was dramatically changed after stress, with expansion of inflammation-promoting bacteria. Furthermore, results showed stress-induced deficient expression of mucin-2 and lysozyme, which may contribute to the disorder of gut microbiota. Of note is that, in the case of cohousing, the stress-induced immune reaction and decreased body weight were abrogated, and transferred gut microbiota from stressed mice to control mice was sufficient to facilitate DSS-induced colitis. The important role of gut microbiota was further reinforced by broad-spectrum antibiotic treatment. Taken together, our results reveal that chronic stress disturbs gut microbiota, triggering immune system response and facilitating DSS-induced colitis.
Inflammatory bowel diseases (IBDs), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic disabling gastrointestinal (GI) disorders, impacting the quality of patients’ lives and creating a great challenge to health care systems. IBD has a high rate of prevalence in the Western world and in East Asia, and the disease has drawn wide attention (1). Factors behind the increase in IBD are thought to be city living and consumption of convenience food. However, the stressful modern lifestyle also contributes much to IBD. Stress, a feeling of being overwhelmed, worried, or run down, cannot be avoided in the present competitive world. Even though stress is important for people’s rapid reaction to threats, chronic stress is associated with detrimental effects on physical health and adversely affects the immune, neuroendocrine, and central nervous systems, promoting the progression of diseases such as cancer and IBD (2, 3). Many studies have found an association between IBD and psychiatric diagnoses, and IBD was even classified as a psychosomatic disorder (4), but the mechanisms behind it are less clearly understood.
The GI tract is an extremely complex ecosystem generated by the alliance of GI epithelium, mucus layer, immune cells, and resident microbiota (5). Under physiological conditions, the GI immune system develops tolerances and balances the appropriate responses to threatening pathogens and commensal bacteria. Although the etiology remains unclear, IBD is thought to be caused by a hyperactive immune system attacking the GI tract, causing inflammation and ulceration (6). The degree of immune activation and the level of proinflammatory cytokines are linked to the severity of IBD. Unfortunately, the positive effect of immune-suppressor or antiinflammatory treatment is modest, and IBD remains notoriously difficult to treat. Recently, instead of assuming that the immune system is inherently faulty as a result of genetics or environment, gut microbiota is being thought to be the factor that provokes the immune system, and it is accepted that disturbance of the microbiota–host relationship is associated with IBD (7, 8). The gut is colonized by ∼1 × 1014 cfu of bacteria, almost 10 times the amount of host cells (9). Even though the composition of the microbiota remains relatively constant throughout adult life, it could be influenced by many factors that then influence the host’s health status. Diet with or without fiber (10), whole grain (11), or emulsifiers (12) can alter gut microbiome composition, which essentially contribute to the state of the host’s inflammation and metabolic syndrome. Regardless of diet, loss of the immune-related sensors type I IFN (13), TRAF6 (14), and AIM2 (15) appear to exert their effects on colitis or colitis-related cancer affecting the gut microbiome. The crucial role of the gut microbiome in IBD has been powerfully confirmed by the beneficial effect of fecal microbiota therapy (FMT) (16, 17). Others also reported that microbiota transfer could endow the recipient with the donor’s response to therapy (18) or even the longevity (19). Growing evidence has also linked depression or stress to microbial dysbiosis (20, 21). However, a causal relationship between microbial dysbiosis and detrimental effects of stress has never been elucidated, and little is known about the interactions among microbiota, GI epithelium, and the immune system after stress in IBD. A more detailed understanding of the interactions should certainly provide insight into the disease state and will direct future clinical research in this area.
Herein, we observed that chronic stress induced low-grade inflammation and accelerated progression of DSS-induced colitis. The effect was associated with an increased immune response as well as increased proinflammatory cytokines. By cohousing or antibiotic treatment, we found that the perturbed microbial flora was the real mechanism behind the IBD-promoting effect of chronic stress.
Results
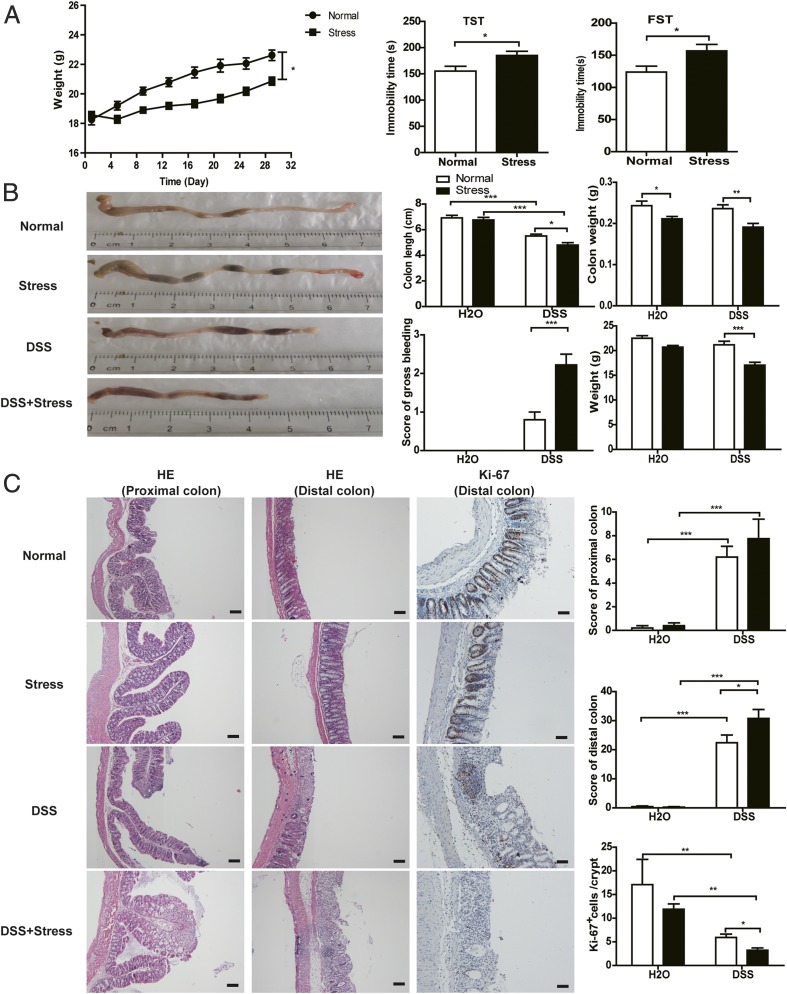
Chronic Stress Facilitated the Development of Colitis.
It is increasingly understood that chronic stress needs to be tightly controlled because, even though acute stress is beneficial, prolonged stress has deleterious effects. To assess the possibility that chronic stress can promote colitis, we used an established model of colitis induced by DSS. Before the administration of DSS, mice were subjected to a well-established chronic stress paradigm (22) for 1 mo. After 1 mo of continuous stress, the weight of mice was significantly decreased compared with the normal group, and the immobility times in a tail suspension test (TST) and forced swim test (FST) were significantly increased, indicating depression-like behavior (Fig. 1A). In normal treated conditions, DSS induced colitis in mice, with shortened colon length, decreased body weight, and obvious gross bleeding (Fig. 1B). In stress-treated conditions, even though chronic stress had no appreciable influence itself, it dramatically enhanced the severity of DSS-induced colitis, with further shortened colon length, decreased body and colon weights, and more obvious gross bleeding. In DSS-treated groups, chronic stress also caused severe pathologic processes throughout the proximal and distal colon, with extensive epithelial damage and inflammatory infiltrates, as well as decreased numbers of Ki-67–positive cells, compared with the nonstressed group (Fig. 1C). Collectively, these results reveal that chronic stress promotes the development of DSS-induced colitis.
Fig. 1.
Chronic stress accelerated DSS-induced colitis. (A) Body weight of mice in normal and stress groups before DSS and immobility time of mice after 1 mo stress in TST and FST using an EthoVision XT 11.5 system (n = 10). (B) Representative colon pictures and colon length, colon weight, bleeding score, and body weight (n = 9–10). (C) Representative H&E staining, Ki-67 staining, pathological score, and mean positive Ki-67 staining cells counted in 20 crypts (n = 4–5; *P < 0.05, **P < 0.01, and ***P < 0.001). (Scale bars: H&E stain, 100 μm; Ki-67 stain, 50 μm.)
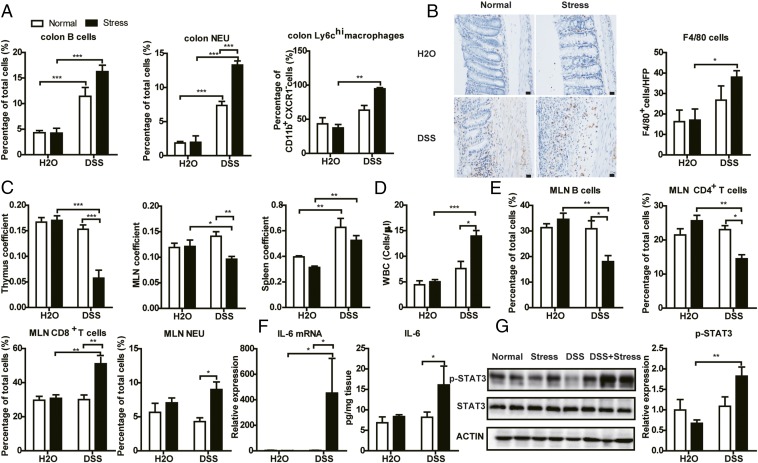
Chronic Stress Disturbed Immune System and Activated the IL-6/STAT3 Signaling Pathway in DSS-Induced Mice.
Infiltration of immune cells and elevated levels of proinflammatory markers are hallmarks of the IBD, an immune-related disorder. We first investigated the influence of chronic stress on the colonic immune system. Flow cytometric analysis revealed increased infiltration of B cells and neutrophils (NEUs) in colonic lamina propria in mice treated with DSS, and NEUs were further increased in the stress-treated group. It is known that ly6Chi macrophages in the inflamed colon give rise to proinflammatory effector cells. We also detected a significantly accumulation of ly6Chi macrophages induced by DSS in stress-treated conditions but not in normal conditions (Fig. 2A and Fig. S1). Immunohistochemistry also showed an increase of infiltrated macrophages in the colon of mice in the DSS+stress group (Fig. 2B). CD4+ and CD8+ T cells showed no obvious differences in all groups (Fig. S3A). Next, we studied the impact of chronic stress on systemic immunity. As shown in Fig. 2C, even though stress and DSS itself had no influence on thymus or mesenteric lymph node (MLN) coefficient, DSS+stress significantly decreased the thymus and MLN coefficient. There was obvious splenomegaly in the DSS and DSS+stress groups. We then used an automated hematology analyzer (ADVIA2120i; Siemens) to determine the number and influx of circulating blood cells from the peripheral blood. DSS induced an increase in circulating total white blood cells (WBCs) and the proportion of large unstained cells (LUCs) in only the stress conditions (Fig. 2D and Fig. S3B). However, there were no differences between stressed and nonstressed groups in all other cell types (Fig. S3B). We further studied the prevalence of immune cells in spleen and MLN by using flow cytometric analysis. Even though no differences in splenic immune cells were seen, the proportions of MLN-associated B cells and CD4+ T cells were significantly decreased in the DSS+stress group, and the proportion of MLN-associated CD8+ T cells and NEU cells was significantly increased in the DSS+stress group (Fig. 2E and Figs. S2 and S3C). These results indicated that stress alone induced subclinical inflammation and caused an extreme hyperactive mucosal immune response after DSS.
Fig. 2.
Chronic stress disturbed the immune system. (A) Flow cytometric analysis of B cells (CD45+, CD19+), NEUs (CD45+, Ly6G+), and ly6chi macrophages in colonic lamina propria (n = 4–5). (B) Representative F4/80 staining images and mean positive cells counted in five high-power fields (HFPs; n = 4–5). (Scale bar: 20 μm.) (C) Thymus coefficient, MLN coefficient, and spleen coefficient (n = 9–10). Thymus/MLN/spleen coefficient is calculated as thymus/MLN/spleen weight/body weight × 100. (D) WBC count in peripheral blood (n = 4–5). (E) Flow cytometric analysis of B (CD45+, CD19+), CD4+ T (CD45+, CD4+), CD8+ T (CD45+, CD8+), and NEU (CD45+, Ly6G+) cells in MLN (n = 4–5). (F) RT-PCR and ELISA results of IL-6 in colon (n = 5). (G) Western blot results of p-STAT3 in colon (n = 5; *P < 0.05, **P < 0.01, and ***P < 0.001).
As for inflammatory cytokines, we observed an increase of IL-6 mRNA in the DSS+stress group (Fig. 2F), but not of TNF-α, iNOS, CCL2, or IFN-γ mRNA (Fig. S4A). Furthermore, the antiinflammatory IL-10 mRNA level was dramatically decreased as a result of stress or DSS treatment (Fig. S4A). By using ELISA analysis, we detected a significant increase of IL-6 but not TNF-α or NO in the DSS+stress group compared with the DSS group (Fig. 2F and Fig. S4B). IL-6 binds to soluble or membrane-bound IL-6 receptor polypeptides, triggering activation of STAT3 (23). In line with increased IL-6 in the colon, we found increased phosphorylation of only STAT3, but not ERK, JNK, P38, or P65 induced by DSS in stress conditions, indicating activation of the STAT3 pathway (Fig. 2G and Fig. S4C). Collectively, these results suggest the proinflammatory signature of stress on DSS treatment, especially the activation of the IL-6/STAT3 pathway.
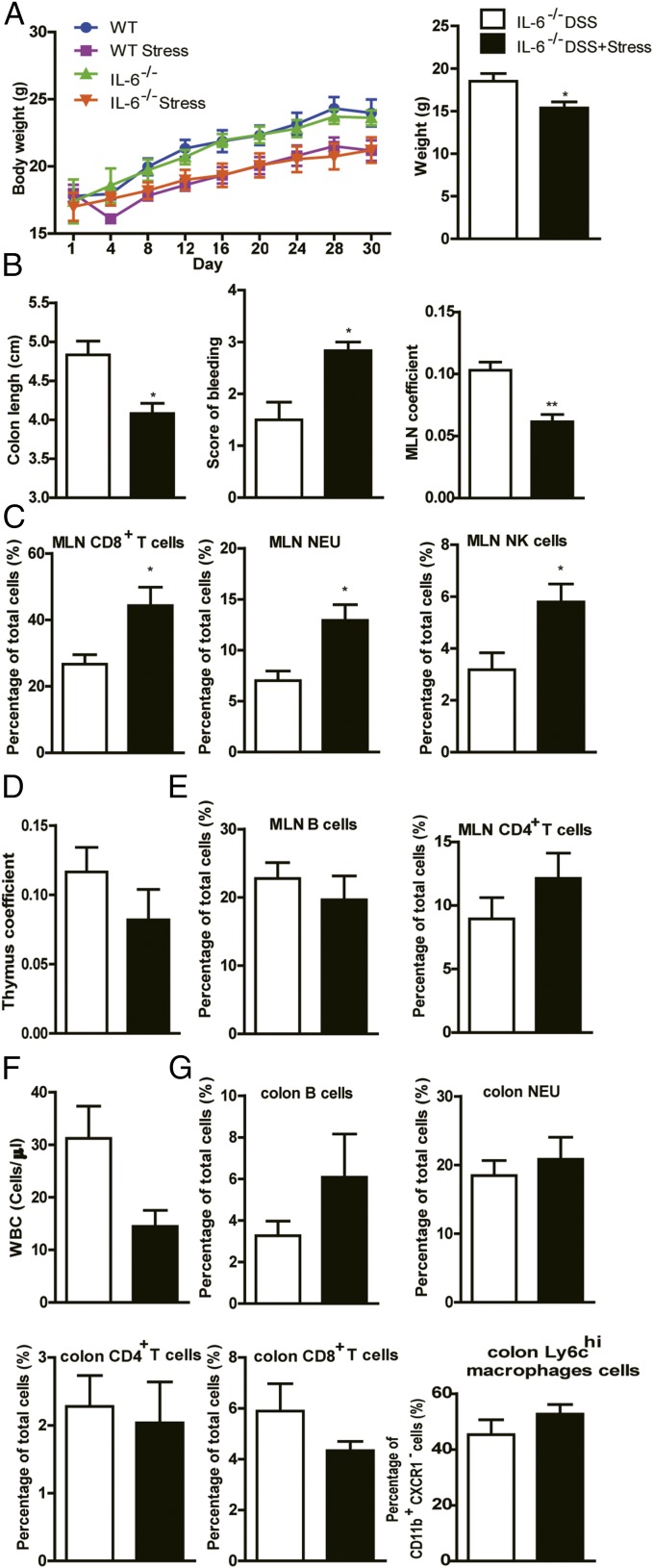
Genetic Suppression of IL-6 Failed to Terminate the Effect of Chronic Stress on Colitis.
IL-6 is a pleiotropic cytokine with central roles in immune and inflammatory reactions. A growing body of evidence suggests a correlation between circulating or local IL-6 levels and the clinical activity of IBD, even suggesting the IL-6/STAT3 pathway as a therapeutic target for IBD (24–26). Because we found a dramatic activation of IL-6/STAT3 signaling in the DSS+stress group (Fig. 2 F and G), we were interested in elucidating if the activation of IL-6 is the reason why stress promoted colitis. To explore this causal relationship, we induced stress in IL-6−/− mice. Surprisingly, suppression of IL-6 did not terminate the effect of chronic stress, as no differences were observed between the weights of IL-6−/− mice and WT mice, no matter whether the mice were exposed to stress (Fig. 3A). In agreement with these data, stressed IL-6−/− mice also tended to lose more body weight compared with nonstressed mice at the end of DSS treatment (Fig. 3A). In IL-6−/− mice, chronic stress also enhanced the severity of colitis induced by DSS, with shortened colon length, increased bleeding score, decreased MLN coefficient, and increased proportion of MLN-associated CD8+ T cells, NEU cells, and natural killer (NK) cells (Fig. 3 B and C), indicating that IL-6 suppression failed to abrogate the effect of chronic stress. For the thymus coefficient, the percentages of B cells and CD4+ T cells in MLN; WBC in the peripheral blood; and B cells, NEUs, CD4+ T cells, CD8+ T cells, and ly6Chi macrophages in colon, there are no differences between DSS and DSS+stress groups in IL-6−/− mice (Fig. 3 D–G), but these were insufficient to block the effect of stress. Taken together, these results suggest that the increase of IL-6 in DSS+stress group only accompanies the facilitated colitis, but is not the reason.
Fig. 3.
Genetic deletion of IL-6 failed to terminate the effect of chronic stress.(A) Body weight before DSS and body weight at the time of euthanasia (n = 5). (B) Colon length, bleeding score, and MLN coefficient (n = 5). (C) Flow cytometric analysis of CD8+ T, NEU, and NK cells in MLN (n = 5). (D) Thymus coefficient (n = 5). (E) Flow cytometric analysis of B and CD4+ T cells in MLN (n = 5). (F) WBC counts in the peripheral blood (n = 5). (G) Flow cytometric analysis of B, NEU, CD4+ T, and CD8+ T cells and ly6chi macrophages in colon (n = 5; *P < 0.05 and **P < 0.01).
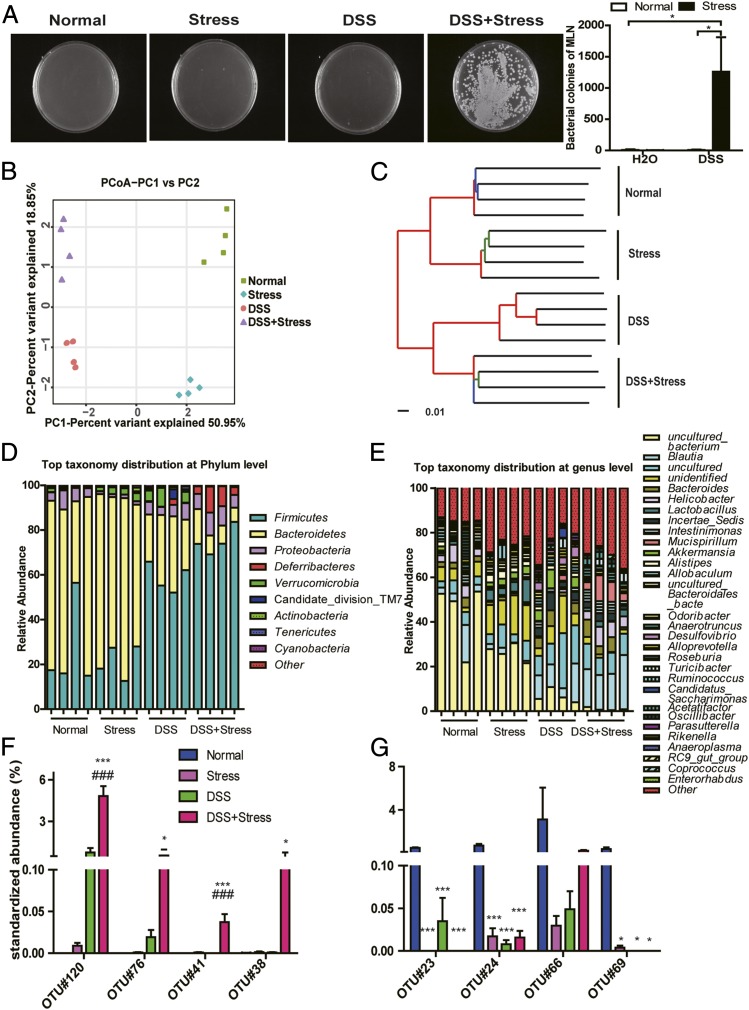
Chronic Stress Changed Composition of Gut Microbiota and Degraded the Colonic Mucus Barrier.
As increased inflammation is not the reason behind the effect of chronic stress, we attempted to find the underlying mechanism. Gut microbiota has emerged as a principal factor in the development and function of the immune system, and research has inextricably linked the gut microbiome to IBD and depression (27–29). However, how chronic stress influences the composition of gut microbiota in normal and DSS-treated mice, and the causality of microbial ecology and the colitis-promoting effect, are poorly understood. We first found a dramatic increase of cultured bacteria in the MLN in the DSS+stress group (Fig. 4A). To further assess the composition of gut microbiota, we performed 16S rRNA gene sequence analysis of the gut microbiota. Principal coordinates analysis (PCoA) revealed a markedly distinct colon microbial landscape in mice in the normal, stress, DSS, and DSS+stress groups (Fig. 4B). Unweighted pair group method with arithmetic mean (UPGMA) also confirmed the distinct microbial communities among the four groups (Fig. 4C). At the phylum and genus levels, the composition of microbiota all dramatically changed in stress- or DSS-treated mice (Fig. 4 D and E). Relative to normal mice, stressed mice harbored increased levels of inflammation-promoting operational taxonomic units (OTUs) related to Helicobacter, Peptostreptococcaceae, Streptococcus, and Enterococcus faecalis, but decreased levels of Rikenella, Roseburia, and Lachnospiraceae. In DSS treated mice, these inflammation-promoting OTUs also bloomed, whereas DSS+stress mice showed a dramatic further increase, especially of Helicobacter and Streptococcus (Fig. 4 F and G).
Fig. 4.
Chronic stress changed composition of gut microbiota and degraded the colonic mucus barrier. (A) Representative images and numbers of bacterial colonies in MLN (n = 4; *P < 0.05). (B) PCoA (n = 4). (C) UPGMA method (n = 4). (D) Composition of microbiota at phylum level (n = 4). (E) Composition of microbiota at genus level (n = 4). (F) Bacterial species from colon content that had the greatest increase in abundance between different groups and their relative abundance. OTU#120 is related to Helicobacter, OTU#76 to Peptostreptococcaceae, OTU#41 to Streptococcus, and OTU#38 to E. faecalis (n = 4). (G) Bacterial species from colon content that had the greatest decrease in abundance between different groups and their relative abundance (*P < 0.05 and ***P < 0.001 vs. normal group; ###P < 0.001 vs. DSS group). OTU#23 is related to Rikenella, OTU#24 to Rikenella, OTU#66 to Roseburia, and OTU#69 to Lachnospiraceae bacterium 6-1 (n = 4).
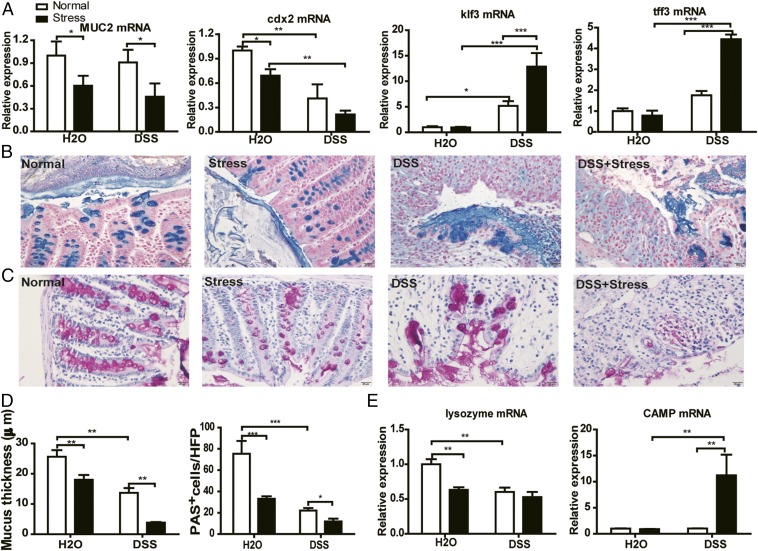
Gut microflora in the intestinal lumen are in closely contact with mucin and antimicrobial peptides (Amps) and are profoundly regulated by them. The mucus layer and Amps act together as the first line of defense against commensal microbes and invading pathogens (30). We then rationalized that the integrity of this critical barrier could be compromised by chronic stress. Quantitative PCR results showed a significant decrease in mRNA of Mucin-2 (MUC2), the building block of colonic mucus, and Cdx2, a positive regulator of MUC2 expression, in all stress-treated groups, suggesting a weakened ability for mucus production in stressed mice (Fig. 5A). However, we found a clear increase in mRNA of Klf3 and Tff3, factors involved in barrier function, in the DSS group, and a further increase in the DSS+stress group (Fig. 5A). These increases could be compensatory responses of the host to offset the mucosal damage induced by DSS and even stress. To further ascertain the effect of stress on mucus, we performed Alcian blue and periodic acid–Schiff (PAS) staining in each mouse. In agreement with the changes in transcripts, results revealed a thinner mucus thickness and a decreased goblet cell number in stressed mice compared with nonstressed mice (Fig. 5 B–D). We also examined the production of Amps, and results showed a significant decrease of lysozyme mRNA expression in all stress- and DSS-treated groups. For cathelicidin Amp (CAMP), however, only the DSS+stress group showed greatly increased expression (Fig. 5E).
Fig. 5.
Chronic stress degraded the colonic mucus barrier. (A) RT-PCR results of MUC2, Cdx2, Klf3, and Tff3 (n = 5). (B) Representative images of Alcian blue-stained inner mucus layer. (Scale bar: 20 μm.) (C) Representative PAS-stained goblet cell pictures. (Scale bar: 20 μm.) (D) Quantification of inner mucus layer thickness and mean PAS+ goblet cells in the colon (n = 4). (E) RT-PCR results of lysozyme and CAMP (n = 5; *P < 0.05, **P < 0.01, and ***P < 0.001).
All these results indicate that mice exposed to chronic stress experience deficiency of mucus layer and lysozymes and changed composition of gut microbiota despite no overt signs of disease, but develop robust colitis after DSS treatment.
Gut Microbiota Is Responsible for the Susceptibility of DSS Colitis to Chronic Stress.
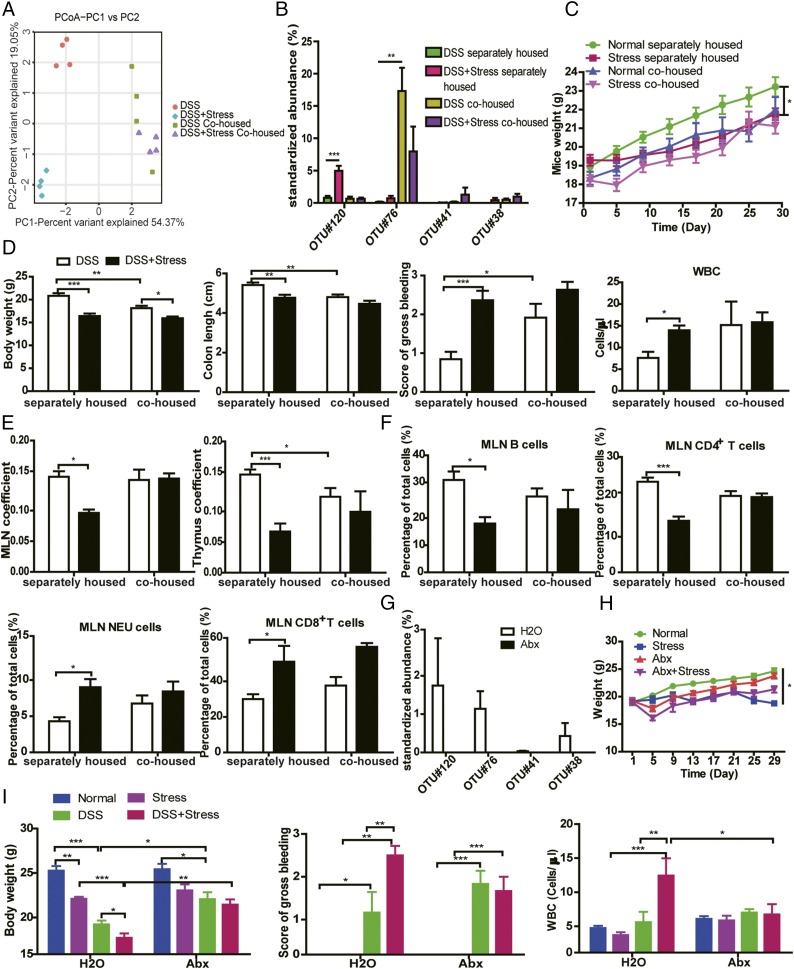
To probe if the changed gut microbiota triggered the deleterious effect of stress, we took advantage of the transmissible nature of the gut microbiota (15) to determine whether susceptibility to DSS could be enhanced in normal mice by cohousing them with stressed mice. PCoA revealed an equilibrated colon microbial landscape in cohoused mice (Fig. 6A). The abundance of some inflammation-promoting OTUs related to Streptococcus and E. faecalis were increased in the DSS group after cohousing (Fig. 6B). The composition of microbiota at the phyla and genus levels after cohousing are shown in Fig. S5. Interestingly, even though the weight of mice in the separately housed stress group was significantly lower than in the normal separately housed group, no appreciable differences were seen in mouse weights between normal cohoused and stressed cohoused groups (Fig. 6C). We also observed significant decrease in colon length and body weight and significant increase of bleeding score (Fig. 6D) in DSS cohoused mice compared with DSS separately housed mice, indicating that the transferred gut microbiota from stressed mice to nonstressed mice by cohousing contributes to more severe colitis. Consistent with these findings, the effect of stress on the immune system was compromised by cohousing, as differences in thymus coefficient, MLN coefficient, WBC count, and proportion of MLN-associated immune cells (Fig. 6 D–F) disappeared between cohoused groups.
Fig. 6.
Gut microbiota is responsible for the susceptibility of DSS colitis to chronic stress. (A) PCoA of each mouse (n = 4). (B) Bacterial species from colon content that had the greatest increase in abundance between different groups and their relative abundance. OTU#120 is related to Helicobacter, OTU#76 to Peptostreptococcaceae, OTU#41 to Streptococcus, and OTU#38 to E. faecalis (n = 4). (C) Body weight before DSS. (D) Body weight at the time of euthanasia, colon length, bleeding score, and WBC count in peripheral blood. (E) MLN and thymus coefficient. (F) Flow cytometric analysis of B cells, CD4+ T cells, NEUs, and CD8+ T cells in MLN (n = 5–6). (G) Bacterial species from colon content in the DSS+stress group in H2O and antibiotic-treated conditions (n = 4). (H) Body weight before DSS (n = 6). (I) Body weight at the time of euthanasia, bleeding score, and WBC count in the peripheral blood (n = 6; *P < 0.05, **P < 0.01, and ***P < 0.001).
To further confirm the effect of gut microbiota, we treated mice with antibiotic agents according to a previous study (31). After antibiotic treatment, inflammation-promoting OTUs related to Helicobacter, Peptostreptococcaceae, Streptococcus, and E. faecalis were all not detectable (Fig. 6G). Results showed that antibiotic treatment blocked the decrease in body weight induced by stress (Fig. 6 H and I) and partially inhibited the decrease in body weight induced by DSS (Fig. 6I). Remarkably, stress could no longer sensitize mice to DSS colitis in the antibiotic-treated condition and could no longer increase the circulating WBC count (Fig. 6I).
Together, these findings reveal the interesting concept that stress-induced microbiota dysbiosis is responsible for susceptibility to DSS-induced colitis.
Discussion
This study shows that chronic stress changed the microbiota and increased the susceptibility of mice to DSS-induced colitis. Our observation that ablation of the inflammatory cytokine IL-6 did not terminate stress sensitization to DSS colitis suggests that the hyperinflammatory response is not the real culprit. In contrast, the disappearance of differences between stressed and nonstressed groups when the gut microbial landscape was equilibrated by cohousing or microbiota was abolished by antibiotic treatment, unequivocally showing that gut microbiota are responsible for the deleterious effects of stress.
Colitis is an immune-related disease, and chronic stress is well known to affect the immune system. Mucosal inflammation was triggered in DSS-induced colitis, with outnumbered infiltration of B cells, NEUs, and macrophages in colonic lamina propria, and significant destruction of the crypt architecture. Colitis was greatly accelerated in chronic stress-treated groups. Despite the inflamed colon, systemic immunity also participated in colitis. The relationships between the lymphatic system and immune response/inflammation are undeniable. Studies have reported that lymphangitis, compromised lymph drainage and lymphatic pumping, bacterial infiltration, and lymph node infection are likely players in inflammatory disorders and IBD (32–34). We found a decreased MLN coefficient and increased bacterial infiltration in MLN of mice in the DSS+stress group, reflecting more severe colitis in this group. We also discovered compromised thymus in the DSS+stress group, as the thymus coefficient was significantly reduced. All these indicated a colon submucosal hyperinflammation and systemic immune inhibition induced by stress, which contribute to the development of colitis.
Multiple signals are involved in the coordination of the immune system, serving to activate and attenuate its responses to attack (35). IL-6 is released upon stimulation of inflammatory cells to activate signaling pathways such as STAT3, and is up-regulated in colitis. Neutralization of IL-6 with antibody showed beneficial effects on colitis (25, 36), even though reports have also found genetic deletion of IL-6–promoted colitis (37). We found an increase of IL-6 in the DSS+stress group, and deletion of IL-6 failed to mitigate the effect of stress, suggesting that the hyperinflammatory responses may contribute to the detrimental effect of stress but not to the underlying mechanism behind stress. Despite the driving inflammatory mechanisms, we also found a remarkable decrease of IL-10 mRNA not only in DSS-treated groups but also in the stress only-treated group. This decrease in IL-10 would also contribute to the effect of stress, as IL-10 deficiency manifests in severe intestinal inflammation (38, 39), and deletion of IL-10 in animals is one of the oldest colitis models used (40).
Immune system can be triggered by gut microbiota (41), and the microbiome of patients with IBD is altered, with cause-and-effect relationships with disease. Numerous studies have shown that colitis can be profoundly affected by transmissible microbial communities that arise from diet changes or host genetic defects (12–15). Here, we found a distinct colon microbial landscape in each group, indicating a dramatically changed composition of microbiota after stress or DSS treatment. Notably, inflammation-promoting OTUs related to Helicobacter, Peptostreptococcaceae, Streptococcus, and E. faecalis were increased in the stress-treated group. These findings fit well with evidence that Helicobacter is linked to IBD (42), as are Peptostreptococcaceae (43), Streptococcus (44), and E. faecalis (45). Lachnospiraceae, which has shown protection against colitis (46), were significantly decreased after stress.
MUC2, the major component of the mucus in the colon, and Amps, the endogenous antibiotics with antimicrobial activities, are expressed in the intestinal lining in close contact with the gut microflora, keeping the majority of gut bacteria away from epithelial cells and also regulating the intestinal microbial habitat (47, 48). Here we observed a decrease in MUC2 mRNA expression and goblet cell numbers and a thinner mucus layer in stress-treated groups. Mucolytic bacteria like Akkermansia were increased after stress or DSS treatment, which may also contribute to the thinner mucus layer. However, the relative abundance of Akkermansia in the DSS+stress group showed no increase, and reasons for this might be the lack of mucin, as Akkermansia grow only on mucin O-glycans as a sole polysaccharide source (10). The underlying mechanisms still need further study. The decrease in mucus layer induced by chronic stress might facilitate the development of colitis. It has been reported that MUC2-deficient 129SV mice spontaneously developed colitis (49) even though it is not always the case in C57BL/6J mice (50–52). However, in C57BL/6J mice, MUC2 deficiency was associated with low levels of subclinical chronic inflammation (53), and MUC2 protected against colitis (54). Amps, secreted by goblet cells, intestinal epithelial cells, Paneth cells, or immune cells, are also important players in IBD (47). It was reported that colonic MUC2 mucin regulates the expression and antimicrobial activity of β-defensin (55), and that MUC2 mucin contributes to the synthesis of CAMP (56). We found significantly decreased expression of lysozyme in stress- and DSS-treated groups, but an increased expression of CAMP in the DSS+stress group. A previous study showed that lysozyme might increase mucin gene expression and promote colonic barrier integrity (57). It was also reported that the expression of Amps is increased in colitis, which represents a self-defense response (47). Reduced expression of MUC2 and lysozyme might contribute to shape the microbial composition of the stress condition. However, the modulation of gut microbiota is remarkably complex and poorly understood, and how chronic stress modulates gut microbiota still needs further study.
Of note is that, in the case of cohousing, when the gut microbial habitat is shared between stress and nonstress groups, the increased severity of colitis induced by stress was abrogated, as the severity of DSS colitis was indistinguishable between stressed and nonstressed mice. Microbiota transfer endowed the normal mice with the stress-treated mice’s response to DSS, suggesting that the detrimental effect of stress is attributed to the disturbed gut microbiome. Furthermore, the immune system was influenced by microbiota, as differences in thymus coefficient, lymph node coefficient, and infiltrated immune cells disappeared between the cohoused groups. The important role of gut microbiota was further reinforced by antibiotic treatment, as the increased susceptibility to colitis of stressed mice was also abolished after antibiotic treatment. It should be noted that antibiotic treatment only partially blocked the decreased body weight induced by DSS without affecting the colon length or bleeding score. This does not contradict the findings of previous studies because, even though some colitis models show improvement under germfree conditions or antibiotic treatment, the reports in a DSS model conflict with reports of improvement or promotion (58, 59). However, the reasons for these discrepancies are still unknown. Collectively, the combined effect of cohousing and antibiotic treatment on stress provides strong evidence pointing to the disturbed gut microbiome as the real mechanism behind the effect of stress.
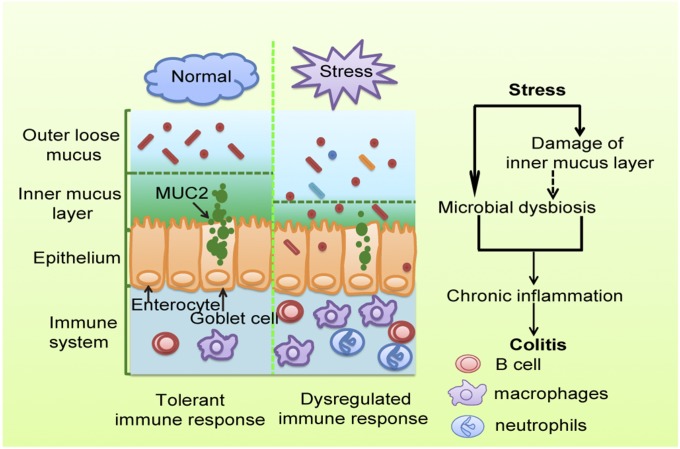
Taken together, our results reveal that chronic stress disturbs gut microbiota, triggering an immune system response, and then facilitates DSS-induced colitis (Fig. 7). This study adds to our understanding of interactions between microbiota and host and provides the basis for future clinical studies for microbiota manipulation and transplantation, and for development of new therapeutic strategies for depression or IBD.
Fig. 7.
A proposed model illustrating influences of chronic stress on colitis. Chronic stress disturbs gut microbiota and impairs the mucus layer, which then triggers the immune system and facilitates colitis.
Materials and Methods
Mice.
Male C57BL/6 mice were used for all studies. IL-6−/− mice on C57BL/6 background were purchased from the Model Animal Research Center of Nanjing University, and age-matched C57BL/6 mice were used as WT control. Mice were housed in a room with a 12-h/12-h light/dark cycle and habituated in the room for 3 d before experiments. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (60), with the approval of Center for New Drug Safety Evaluation and Research, China Pharmaceutical University.
Chronic Restraint Stress.
Mice were randomly assigned to home cage control conditions or chronic restraint stress. The restraint-stress procedure was performed based on previous studies (2, 22). In brief, mice were restrained in well-ventilated conical-bottom centrifuge tubes (50 mL; Corning) for 3 h daily during the procedure, not allowing forward and backward movement. For cohousing experiments, equal numbers of stressed mice and nonstressed mice were housed in the same cage during the procedures.
Behavior Tests.
Mice behavior was tested by TST and FST by using an EthoVision XT 11.5 system. For TST, mice were individually suspended by the distal portion of their tails with adhesive tape for a period of 6 min (30 cm from the floor) in a visually isolated area. For FST, each mouse was placed in a cylinder container (diameter, 28 cm; height, 33 cm) containing 20 cm of water at 25 ± 1 °C. Water was replaced between every trial. Following swim sessions, mice were removed from the cylinder, dried with a towel, and returned to their home cages. Activity monitor software was used to record and quantify the immobility time in both tests.
Colitis Induction with DSS.
Mice were given 2.5% DSS (36–50 kDa; MP Biomedicals) in drinking water for 7 d to induce colitis and killed on day 8. The length of whole colon was measured, and the weight was recorded after opening longitudinally and flushing with PBS solution. The colon was carefully examined, and the bleeding score was recorded. Half of the colon was used for flow cytometry analysis, and half of the colon was divided into three sections (proximal, middle, and distal). The proximal and distal colon sections were fixed flat on filter paper in 4% phosphate-buffered formaldehyde for histological analyses. The middle colon was then divided into three sections and was snap-frozen for subsequent molecular analyses.
Histology and Immunohistochemistry.
Proximal and distal colon tissues were fixed in 4% phosphate-buffered formaldehyde solution for 24 h and embedded in paraffin. Sections of 5 μm were stained with H&E. Inflammation and tissue damage of each colon was scored based on the degree of epithelial damage and inflammatory infiltrate in the mucosa, submucosa, and muscularis/serosa as previously described (61). Each of the four scores was multiplied by 1 if the change was focal, 2 if it was patchy, and 3 if it was diffuse. The four individual scores per colon were added, resulting in a total scoring range of 0–36 per mouse. For immunohistochemistry, distal colon tissues were cut to 5-μm sections. After dewaxing and rehydration, the sections were soaked in sodium citrate buffer for heat-induced epitope retrieval, and incubated with 10% goat serum for 1 h to block the nonspecific binding sites. Then, sections were incubated with anti–Ki-67 antibody (1:200; Abcam) and anti-F4/80 antibody (1:100; Abcam) overnight at 4 °C, followed by incubation with HRP secondary antibodies for 1 h. The sections were developed by using a diaminobenzidine substrate kit (TIANGEN) and counterstained with hematoxylin. Images were obtained with an Olympus BX41 microscope.
Cell Isolation and Flow Cytometry.
Isolation of colonic lamina propria cells was performed following a previously established method (62). In brief, luminal content, extraintestinal fat tissue, and blood vessels were removed, and colons were then cut into 0.5-cm pieces. Colon pieces were first incubated with HBSS (without Ca2+ and Mg2+) containing 5% FBS, 2 mM EDTA, and 1 mM DTT to remove epithelial cells and mucus, and then digested in PBS solution containing 5% FBS, 1 mg/mL collagenase VIII (Sigma), and 0.1 mg/mL DNase I (Roche). Digested cell suspension was then washed with PBS solution and filtered with a 45-μm cell strainer. Antibodies used for colonic lamina propria staining included CD45 (FITC), CD19 (PECY5), CD11b (PE), Ly6G (PECY7), CD4 (APC), CD8 (PECY7), F4/80 (FITC), CX3CR1 (BV510), and Ly6C (APC). MLN homogenate and spleen homogenate after lysis of red blood cells were also washed with PBS solution and filtered through a 45-μm cell strainer to obtain cell suspension. Antibodies used for spleen and MLN staining included CD45 (FITC), CD19 (PECY5), CD4 (APC), CD8 (PECY7), CD11b (PE), Ly6G (PECY7), and CD49 (APC). Cells were analyzed with MACSQuant Analyzer 10 (Miltenyi Biotec). Flow cytometry analysis was done with FlowJo software.
Real-Time PCR Analysis.
Total RNA from colon tissue was extracted by using TRIzol (Invitrogen) and reverse transcribed into cDNA by using a cDNA synthesis kit (Takara). Quantitative PCR was done with a Step One Plus Real-Time PCR system (Applied Biosystems) with gene-specific primers. Expression data were normalized to β-actin mRNA expression.
Inflammatory Mediator Measurement.
Colon tissues were weighed and homogenized by using a tissue mixer (PRO Scientific) with three volumes of PBS solution. The tissue samples were then centrifuged at 15,000 × g for 10 min. Tissue supernatants were collected for the assay. TNF-α and IL-6 concentrations were measured by ELISA (Dakewe Biotech). NO concentration was determined by Griess reagent (Beyotime Biotechnology).
Western Blotting.
Colon tissues were homogenized with lysis buffer and centrifuged. Protein samples were boiled for 5 min, electrophoresed in 10% SDS polyacrylamide gel, and transferred onto PVDF membranes (Millipore). The blots were blocked with 5% skim milk in Tris-buffered saline solution–Tween 0.1% for 1 h at room temperature and probed with primary antibodies at the appropriate dilutions overnight at 4 °C. The blots were washed and incubated for 1 h at room temperature with the HRP-conjugated secondary antibody, then developed with enhanced chemiluminescence (Millipore). The densitometry of protein bands was quantified by using ImageJ software (National Institutes of Health).
Immunostaining of Mucins and Goblet Cells.
Mucus immunostaining was performed according to a previous study (12). Briefly, mice colon containing fecal material was fixed in methanol-Carnoy’s solution (60% methanol, 30% chloroform, 10% glacial acetic acid). Tissues were embedded in paraffin in a vertical orientation and cut into 5-μm sections. Tissue sections were dewaxed, hydrated, and stained with Alcian blue/Nuclear Fast Red. Thickness of inner mucus was measured in 10 different areas of one section and at least 10 sections of each mouse. For immunostaining of goblet cells, tissue sections were dewaxed, hydrated, and stained with PAS/hematoxylin. PAS+ goblet cells were counted in five different areas of the section and at least 10 sections of each mouse. Measurement and observation were performed with an Olympus BX41 microscope.
Bacterial Incubation of MLNs.
MLNs were aseptically removed, weighed, and homogenized in PBS solution. The homogenates (100 μL) were plated onto Luria–Bertani agar and incubated at 37 °C under aerobic conditions for 24 h, and then the colonies were counted.
Compositional Analysis of the Gut Microbiota by Pyrosequencing and Data Analysis.
Colon content homogenates in PBS solution were immediately frozen (−80 °C) and stored until further processing. Next-generation sequencing library preparations and Illumina MiSeq sequencing were conducted at GENEWIZ. In brief, 30–50 ng DNA was used to generate amplicons by using a MetaVx Library Preparation kit (GENEWIZ). The QIIME data analysis package was used for 16S rRNA data analysis. Sequences were grouped into OTUs using the clustering program VSEARCH (1.9.6) against the Silva 119 database preclustered at 97% sequence identity. β-Diversity was calculated by using weighted and unweighted UniFrac, and PCoA was performed. A UPGMA tree from the β-diversity distance matrix was built.
Antibiotic Treatment.
For antibiotic treatment, broad-spectrum antibiotic agents including ampicillin (1 g/L), neomycin (1 g/L), and metronidazole (0.5 g/L) were administered in drinking water during the time of intervention (31).
Statistical Analysis.
Data are presented as mean ± SEM. Statistical significance was determined by Student’s t test between two groups and two-way ANOVA in groups of more than two. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Intramural Research Program Grant Z01-ES101684 (to L.B.); National Science Foundation of China Grants 81603132, 81673468, 91529304, 81473230, 81273547, 81403020, 91129731, 81502407, and 81673559; Natural Science Foundation of Jiangsu Province Grants BK20160753 and BK20140666; China Postdoctoral Science Foundation Grant 2016M591965; the 111 Project of B18056; and State Key Laboratory of Natural Medicines Innovation Research and Incubation Foundation Grant SKLNMZZCX201609.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720696115/-/DCSupplemental.
References
- 1.Chi KR. Epidemiology: Rising in the East. Nature. 2016;540:S100–S102. doi: 10.1038/540S100a. [DOI] [PubMed] [Google Scholar]
- 2.Le CP, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. doi: 10.1038/ncomms10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mawdsley JE, Rampton DS. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel GL. Psychological factors in ulcerative colitis in man and gibbon. Gastroenterology. 1969;57:362–365. [PubMed] [Google Scholar]
- 5.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3:e982426. doi: 10.4161/21688370.2014.982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YZ, Li YY. Inflammatory bowel disease: Pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilott NE, et al. Defining the microbial transcriptional response to colitis through integrated host and microbiome profiling. ISME J. 2016;10:2389–2404. doi: 10.1038/ismej.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 10.Desai MS, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez I, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7:269–280. doi: 10.1038/ismej.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chassaing B, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tschurtschenthaler M, et al. Type I interferon signalling in the intestinal epithelium affects Paneth cells, microbial ecology and epithelial regeneration. Gut. 2014;63:1921–1931. doi: 10.1136/gutjnl-2013-305863. [DOI] [PubMed] [Google Scholar]
- 14.Vlantis K, et al. TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice. Gut. 2016;65:935–943. doi: 10.1136/gutjnl-2014-308323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man SM, et al. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell. 2015;162:45–58. doi: 10.1016/j.cell.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vrieze J. Medical research. The promise of poop. Science. 2013;341:954–957. doi: 10.1126/science.341.6149.954. [DOI] [PubMed] [Google Scholar]
- 17.Pigneur B, Sokol H. Fecal microbiota transplantation in inflammatory bowel disease: The quest for the holy grail. Mucosal Immunol. 2016;9:1360–1365. doi: 10.1038/mi.2016.67. [DOI] [PubMed] [Google Scholar]
- 18.Vétizou M, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Nam HG, Valenzano DR. The short-lived African turquoise killifish: An emerging experimental model for ageing. Dis Model Mech. 2016;9:115–129. doi: 10.1242/dmm.023226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Mahony SM, Clarke G, Dinan TG, Cryan JF. Early-life adversity and brain development: Is the microbiome a missing piece of the puzzle? Neuroscience. 2017;342:37–54. doi: 10.1016/j.neuroscience.2015.09.068. [DOI] [PubMed] [Google Scholar]
- 21.Moloney RD, et al. Stress and the microbiota-gut-brain axis in visceral pain: Relevance to irritable bowel syndrome. CNS Neurosci Ther. 2016;22:102–117. doi: 10.1111/cns.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thaker PH, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 23.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsuyama K, et al. Therapeutic strategies for targeting the IL-6/STAT3 cytokine signaling pathway in inflammatory bowel disease. Anticancer Res. 2007;27:3749–3756. [PubMed] [Google Scholar]
- 25.Ito H, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989–996. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Ito H. Treatment of Crohn’s disease with anti-IL-6 receptor antibody. J Gastroenterol. 2005;40:32–34. doi: 10.1007/BF02990576. [DOI] [PubMed] [Google Scholar]
- 27.Chu H, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meisel M, et al. Interleukin-15 promotes intestinal dysbiosis with butyrate deficiency associated with increased susceptibility to colitis. ISME J. 2017;11:15–30. doi: 10.1038/ismej.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The central nervous system and the gut microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ijssennagger N, et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci USA. 2015;112:10038–10043. doi: 10.1073/pnas.1507645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Kruiningen HJ, Colombel JF. The forgotten role of lymphangitis in Crohn’s disease. Gut. 2008;57:1–4. doi: 10.1136/gut.2007.123166. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, et al. The pro-inflammatory cytokine TNF-α inhibits lymphatic pumping via activation of the NF-κB-iNOS signaling pathway. Microcirculation. 2017;24:e12364. doi: 10.1111/micc.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von der Weid PY, Rehal S, Ferraz JG. Role of the lymphatic system in the pathogenesis of Crohn’s disease. Curr Opin Gastroenterol. 2011;27:335–341. doi: 10.1097/MOG.0b013e3283476e8f. [DOI] [PubMed] [Google Scholar]
- 35.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Sommer J, et al. Interleukin-6, but not the interleukin-6 receptor plays a role in recovery from dextran sodium sulfate-induced colitis. Int J Mol Med. 2014;34:651–660. doi: 10.3892/ijmm.2014.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dann SM, et al. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol. 2008;180:6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Begue B, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol. 2011;106:1544–1555. doi: 10.1038/ajg.2011.112. [DOI] [PubMed] [Google Scholar]
- 39.Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–519. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 41.Cekanaviciute E, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114:10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Q, et al. Enterohepatic Helicobacter species as a potential causative factor in inflammatory bowel disease: A meta-analysis. Medicine (Baltimore) 2015;94:e1773. doi: 10.1097/MD.0000000000001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsoi H, et al. Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology. 2017;152:1419–1433.e5. doi: 10.1053/j.gastro.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Al-Jashamy K, Murad A, Zeehaida M, Rohaini M, Hasnan J. Prevalence of colorectal cancer associated with Streptococcus bovis among inflammatory bowel and chronic gastrointestinal tract disease patients. Asian Pac J Cancer Prev. 2010;11:1765–1768. [PubMed] [Google Scholar]
- 45.Zhou Y, et al. Increased Enterococcus faecalis infection is associated with clinically active Crohn disease. Medicine (Baltimore) 2016;95:e5019. doi: 10.1097/MD.0000000000005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surana NK, Kasper DL. Moving beyond microbiome-wide associations to causal microbe identification. Nature. 2017;552:244–247. doi: 10.1038/nature25019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho S, Pothoulakis C, Koon HW. Antimicrobial peptides and colitis. Curr Pharm Des. 2013;19:40–47. doi: 10.2174/13816128130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salzman NH, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann P, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–119. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wenzel UA, et al. Spontaneous colitis in Muc2-deficient mice reflects clinical and cellular features of active ulcerative colitis. PLoS One. 2014;9:e100217. doi: 10.1371/journal.pone.0100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasnain SZ, et al. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology. 2013;144:357–368.e9. doi: 10.1053/j.gastro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 53.Yang K, et al. Interaction of Muc2 and Apc on Wnt signaling and in intestinal tumorigenesis: Potential role of chronic inflammation. Cancer Res. 2008;68:7313–7322. doi: 10.1158/0008-5472.CAN-08-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergstrom KS, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cobo ER, Kissoon-Singh V, Moreau F, Chadee K. Colonic MUC2 mucin regulates the expression and antimicrobial activity of β-defensin 2. Mucosal Immunol. 2015;8:1360–1372. doi: 10.1038/mi.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cobo ER, Kissoon-Singh V, Moreau F, Holani R, Chadee K. MUC2 mucin and butyrate contribute to the synthesis of the antimicrobial peptide cathelicidin in response to Entamoeba histolytica- and dextran sodium sulfate-induced colitis. Infect Immun. 2017;85:e00905-16. doi: 10.1128/IAI.00905-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee M, et al. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Agric Food Chem. 2009;57:2233–2240. doi: 10.1021/jf803133b. [DOI] [PubMed] [Google Scholar]
- 58.Hernández-Chirlaque C, et al. Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. J Crohn’s Colitis. 2016;10:1324–1335. doi: 10.1093/ecco-jcc/jjw096. [DOI] [PubMed] [Google Scholar]
- 59.Kitajima S, Morimoto M, Sagara E, Shimizu C, Ikeda Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim. 2001;50:387–395. doi: 10.1538/expanim.50.387. [DOI] [PubMed] [Google Scholar]
- 60.National Research Council 2011. Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed.
- 61.Lamkin DM, et al. Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav Immun. 2012;26:635–641. doi: 10.1016/j.bbi.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zigmond E, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.