Abstract
Perinatal encephalopathy remains a major cause of disability, such as cerebral palsy. Therapeutic hypothermia is now well established to partially reduce risk of disability in late preterm/term infants. However, new and complementary therapeutic targets are needed to further improve outcomes. There is increasing evidence that glia play a key role in neural damage after hypoxia-ischemia and infection/inflammation. In this review, we discuss the role of astrocytic gap junction (connexin) hemichannels in the spread of neural injury after hypoxia-ischemia and/or infection/inflammation. Potential mechanisms of hemichannel mediated injury likely involve impaired intracellular calcium handling, loss of blood-brain barrier integrity and release of adenosine triphosphate (ATP) resulting in over-activation of purinergic receptors. We propose the hypothesis that inflammation-induced opening of connexin hemichannels is a key regulating event that initiates a vicious cycle of excessive ATP release, which in turn propagates activation of purinergic receptors on microglia and astrocytes. This suggests that developing new neuroprotective strategies for preterm infants will benefit from a detailed understanding of glial and connexin hemichannel responses.
Keywords: hypoxia-ischemia, connexin hemichannels, spreading injury, connexin 43, astrocytes, hypoxic-ischemic encephalopathy
Introduction
Perinatal brain injury is a significant public health issue. It is associated with high mortality, serious morbidity and very high costs. Most of this cost is related to long-term neurodevelopmental disabilities, such as cerebral palsy (CP). For example, in 2003 the lifetime economic cost of CP was over US$ 11.5 billion (Honeycutt et al., 2004). This cost is largely attributed to the combined loss of productive members of society and the direct burden of care on the individual, family and social institutions (Honeycutt et al., 2004). A large cohort of preterm infants born from 1997 to 2011 in France suggests that premature birth continues to be associated with high risk of neurodevelopmental impairment (Pierrat et al., 2017). Encouragingly, survival without moderate to severe neuromotor or sensory disabilities increased from 45.5% to 62.3% at 25–26 weeks' gestation, but remained unchanged at 32–34 weeks' gestation, although fewer children survived with CP (P = 0.01). Similarly, although the Australian CP Register found no change in the overall risk of CP from 1993 to 2006, there was a trend for reduced risk of CP after extremely preterm birth (before 28 weeks of gestation) (Smithers-Sheedy et al., 2016). By contrast, in a population based study of 8 year-old children who were born preterm in the state of Victoria, Australia, rates of major neurosensory disability were similar for cohorts born in 1991–1992, 1997, and 2005 (Cheong et al., 2017). Of concern, academic performance was actually worse in 2005 than in previous cohorts, after controlling for other factors.
Triggers and Outcomes of Perinatal Brain Injury
The causes of perinatal brain injury are undoubtedly multifactorial. However, acute and/or chronic hypoxia-ischemia and infection/inflammation may act independently or synergistically to induce injury (Galinsky et al., 2018).
Hypoxia-ischemia
Acute, profound asphyxia at birth, with early onset hypoxic-ischemic encephalopathy (HIE) occurs more frequently in premature infants (< 37 weeks of gestation) than at term (37–42 weeks of gestation), and is highly associated with adverse outcomes (Low et al., 2003; Manuck et al., 2016). In term infants, Low et al. (2003) reported that the overall incidence of fetal asphyxia was 25/1,000 live births, the majority of these cases were categorized as mild HIE; moderate to severe HIE was seen in approximately 4/1,000 live births. In a cohort of 115,502 deliveries in the USA, from 2008 to 2011, Manuck et al. (2016) reported that 37.3/1,000 infants born before 37 weeks of gestation had moderate to severe HIE, with a disproportionate number of cases in infants born before 30 weeks of gestation. Indeed, infants born before 28 weeks of gestation had an overall rate of HIE of 120/1,000. Previous smaller studies also suggest that late preterm infants have much higher risk than at term, but less than extremely preterm infants, with reported rates of HIE of 1.4 to 9/1,000 (Salhab and Perlman, 2005; Schmidt and Walsh, 2010; Chalak et al., 2012). Broadly consistent with these reports, in the Canadian CP Registry, 16% of children with CP who were born at 32 to 35 weeks of gestation had a history suggestive of acute birth asphyxia (Garfinkle et al., 2017); rates in extremely preterm infants were not reported. These data show that although HIE occurs in a minority of preterm births (Low et al., 2003), when it is present, it is a substantial contributor to severe disability.
Although it is important, acute, severe asphyxia is not the whole story. The multicenter Extremely Low Gestational Age Newborns (ELGAN) study, for example, reported that the combination of severe intrauterine growth restriction plus placental vascular thrombosis, consistent with long-standing, mild to moderate prenatal hypoxia, were associated with impaired neurodevelopmental outcome at 2 years of age (Helderman et al., 2012). Thus, even mild to moderate prenatal hypoxia can contribute to adverse outcomes after preterm birth.
Infection/inflammation
Many clincal studies now provide compelling evidence that exposure to infection/inflammation is strongly associated with brain injury in preterm and term infants. For example, in 327 term/near term infants, Wu et al. (2003) reported that clinical chorioamnionitis, defined by maternal fever, leukocytosis, tachycardia, uterine tenderness and preterm rupture of membranes, was associated with a 4-fold increase in the risk of CP at 2-years of age. In preterm infants, subclinical/histological chorioamnionitis, defined by inflammation of the placenta and fetal membranes (Galinsky et al., 2013), was associated with impaired cognition, memory and learning ability at 5 years of age (Ylijoki et al., 2016). In recent cohorts, the presence of histological and clinical chorioamnionitis was associated with an increased risk of impaired cognition compared to histological chorioamnionitis alone (Pappas et al., 2014). Furthermore, systemic upregulation of pro-inflammatory cytokines, including tumor necrosis factor (TNF; formerly known as TNF-α) and interleukin (IL)-1β, in preterm infants are associated with impaired neural function within the first 72 hours of life and cognitive impairment at 2–3 years corrected age (Bartha et al., 2004; Wikstrom et al., 2008).
Approximately 50–60% of extremely preterm infants will develop cognitive and behavioral impairments (Marlow et al., 2005; Serenius et al., 2013). Neuro-cognitive problems persist in to adulthood. For example, the Bavarian Longitudinal Study of at-risk children born in 1985/1986 found that extremely preterm infants had intelligence quotient (IQ) scores 1.16 standard deviations below controls, with multiple other cognitive problems (Eryigit Madzwamuse et al., 2015). In that cohort, there was no narrowing of the deficit from age 6 to 26 years. In the ELGAN study, at 10 years of age children had high risks of cognitive, neurologic and behavioral deficits (Kuban et al., 2016). Overall, 28% of boys and 21% of girls exhibited moderate-to-severe impairment of cognitive abilities. Impaired behavioral executive function is found in nearly half of extremely preterm survivors at 18 years of age, and is associated with poorer academic outcomes (Costa et al., 2017). It is concerning that in Australia, more recent cohorts show a trend to higher rates of impaired executive function at 7 to 8 years of age, suggesting that improved neonatal care is not addressing the triggers of this long-lasting impairment (Burnett et al., 2018). Moreover, although overall attention span increased from childhood to adulthood, very preterm born children remained at increased risk of attention deficit in adulthood (Breeman et al., 2016).
Timing of injury
Early imaging and postmortem data indicate that cerebral injury occurs in the immediate perinatal period in approximately two thirds of cases, while an appreciable number of cases occur before the onset of labor. By contrast, injury after the early neonatal period represents approximately 15% of cases (de Vries et al., 1998; Bell et al., 2005). Strongly consistent with this, acute electroencephalography (EEG) abnormalities are reported in the early perinatal period and are highly predictive of long-term developmental outcome (Kubota et al., 2002; Kidokoro et al., 2010; Wikstrom et al., 2012; Pichler et al., 2013). However, this does not necessarily mean that early onset injury was due to insults during birth itself.
The pattern of perinatal brain injury
Postmortem case series of infants dying in the early neonatal period show that the most severely affected infants have significant neuronal loss (Bell et al., 2005; Takizawa et al., 2006). Historically, magnetic resonance imaging (MRI) of preterm infants exposed to severe perinatal hypoxia demonstrate a consistent pattern of acute subcortical damage involving the hippocampus, thalamus and basal ganglia, and cerebellar infarction combined with diffuse periventricular white matter injury, but sparing of the cortex (Barkovich and Sargent, 1995). An essentially identical pattern is seen after acute severe asphyxia in fetal sheep (George et al., 2004; Galinsky et al., 2017c; Lear et al., 2017).
By contrast, modern cohorts of preterm infants predominantly show gliosis and diffuse, non-cystic white matter injury without overt neuronal loss (Back et al., 2012). MRI studies show preterm infants have reduced cortical and subcortical (e.g., striatal and thalamic) volumes, without evidence of gross pathology, compared with term born controls (Rathbone et al., 2011; Meng et al., 2016). The reduced volumes persist into adulthood and are strongly predictive of cognitive deficit (Rathbone et al., 2011). Preterm human post mortem studies show that reduced cortical and subcortical growth is associated with impaired dendritic arborization (Mrzljak et al., 1992), and that diffuse white matter injury involves microscopic gliosis and acute cell death, but rapid regeneration of pre-oligodendrocytes. However, these newly generated cells fail to differentiate into mature myelinating oligodendrocytes and thus fail to myelinate axons (Buser et al., 2012). Oligodendrocytes play an integral role in axonal development and function (Lee et al., 2012), suggesting the possibility that mild diffuse loss of white matter reduces the functional integrity of neuronal axons and contributes to impaired neuronal growth, development and function after birth.
In preterm fetal sheep, pure hypoperfusion injury is associated with a similar pattern of gliosis and gray and white matter dysmaturation, as shown by reduced arborization of cortical and striatal neurons without evidence of overt neuronal loss (Dean et al., 2013; McClendon et al., 2014), and by microscopic gliosis and acute death of pre-oligodendrocytes, regenerative proliferation and later dysmaturation (Riddle et al., 2011). Reduced neuronal arborization was the major factor underlying impaired maturation of cortical and subcortical neuronal microstructure, as shown by a delayed decline in fractional anisotropy (FA). Normal cortical and subcortical grey maturation is associated with a decline in MRI measurements of FA due to increased morphological complexity of cortical and subcortical neuronal arbor. This was also associated with reduced cortical and striatal volumes on high field ex vivo MRI (Dean et al., 2013; McClendon et al., 2014). Similarly, human survivors of preterm birth show a delayed decline in cortical and subcortical FA (Ball et al., 2013).
A similar pattern of gliosis and cellular dysmaturation has been reported after exposure to the pro-inflammatory cytokines IL-1β and TNF. For example, in preterm equivalent rodents, direct exposure to IL-1β inhibited oligodendrocyte maturation and led to long-lasting deficits in axonal myelination and cognitive deficits without overt cell loss (Favrais et al., 2011). Furthermore, IL-1β receptor blockade restored oligodendrocyte maturation and myelination after lipopolysaccharide-induced injury (Pang et al., 2003). In vitro studies show that TNF inhibits oligodendrocyte maturation (Bonora et al., 2014) and dendritic arborization and that this can be reversed with TNF receptor blockade (Neumann et al., 2002). Collectively, these data demonstrate that the pattern of gray and white matter injury, and subsequent oligodendrocyte and neuronal injury in modern postmortem series and survivors of preterm birth is replicated in preclinical studies.
Connexin hemichannels in perinatal brain injury
One of the most striking features of perinatal encephalopathy is the evolving nature of injury. This involves the spread of damage into previously uninjured regions over many hours to days after the insult (Fleiss and Gressens, 2012). The mechanisms responsible for the spread of injury are not fully understood; however, there is increasing evidence that undocked connexin hemichannels (that when docked make up gap junctions) play a key role. As previously reviewed (Davidson et al., 2013b), gap junctions are intercellular channels that link the cytoplasm of neighboring cells, allowing the exchange of small molecules and ions. Under normal conditions, gap junctions function in an open state, but the undocked hemichannels primarily remain closed (Decrock et al., 2009). However, after multiple types of insults, including ischemia, inflammation, oxygen glucose deprivation, metabolic stress or low extracellular calcium ions levels, unopposed connexin hemichannels can open (Li et al., 1996; Kondo et al., 2000; Contreras et al., 2002; Orellana et al., 2010; Davidson et al., 2012), leading to disruption of resting membrane potential, extracellular release of cytotoxic levels of glutamate (Ye et al., 2003) and adenosine triphosphate (ATP) (Kang et al., 2008) and excessive intracellular calcium accumulation, cell swelling and rupture (Quist et al., 2000; Rodriguez-Sinovas et al., 2007; Mallard et al., 2014).
There are 21 known connexin hemichannel isoforms. However, connexin 43 (Cx43), predominantly found on astrocytes, plays a key role in the pathogenesis of oligodendrocyte and neuronal injury and is one of the key therapeutic targets for research into neuroprotection (Schulz et al., 2015; Gajardo-Gomez et al., 2016). In preterm and near term fetal sheep, blockade of Cx43 hemichannels after hypoxia-ischemia was associated with reduced electrographic seizures, improved EEG activity and reduced gliosis and oligodendrocyte loss, and an intermediate reduction in cortical neuronal injury (Davidson et al., 2012, 2013a, 2014). Subsequent analysis confirmed that Cx43 hemichannel blockade after hypoxia ischemia was associated with reduced subcortical neuronal loss. Furthermore, longer duration of peptide infusion was associated with a graded improvement in neural recovery, such that acute (1 hour) blockade of Cx43 hemichannels after hypoxia-ischemia was not associated with improved survival of cortical or striatal neurons, but instead improved neurophysiological recovery. Acute blockade was associated with reduced electrographic seizure burden, faster return of sleep state cycling and improved recovery of EEG activity (Davidson et al., 2012; Galinsky et al., 2017a) compared to vehicle, all of which are associated with improved clinical outcomes (Miller et al., 2002; van Rooij et al., 2005; Tekgul et al., 2006; Murray et al., 2009; Thoresen et al., 2010; Drury et al., 2014). By contrast, prolonged blockade of Cx43 hemichannels reduced striatal neuronal injury and improved neurophysiological recovery. These data demonstrate that the relationship between neuronal survival and brain activity is complex and not purely dependent on improved cell survival. Speculatively, acute blockade of Cx43 hemichannels may have improved maturation of the dendritic arbor and myelination, which in turn led to improved functionality of surviving neurons and glia. This remains an important area of research and requires further investigation. Although the precise mechanisms underlying how connexin hemichannels contribute to neuronal and oligodendrocyte injury are not fully understood, below we discuss potential pathways that are likely to be involved.
Intracellular calcium handling
During and/or after hypoxia-ischemia or inflammation, impaired intracellular calcium handling has been implicated in the pathogenesis of mitochondrial dysfunction and cellular apoptosis and necrosis in the adult (Ankarcrona et al., 1995; Schild et al., 2003) and developing brain (Puka-Sundvall et al., 2000; Mallard et al., 2014). Blockade of Cx43 hemichannels after hypoxia-ischemia reduced injury to striatal neurons that expressed the calcium binding proteins, calretinin, calbindin and parvalbumin (Galinsky et al., 2017a). Excessive intracellular calcium accumulation, possibly through open connexin hemichannels, has been implicated in the pathogenesis of mitochondrial dysfunction due to mitochondrial calcium accumulation, depolarization and cytoplasmic release of cytochrome c, which triggers apoptosis, necrosis and impaired development of neurons and oligodendrocytes (Ankarcrona et al., 1995; Puka-Sundvall et al., 2000; Schild et al., 2003; Mallard et al., 2014). Thus, blockade of Cx43 hemichannels may improve cellular survival and integrity by improving intracellular calcium homeostasis. Furthermore, these data suggest that subcortical neurons expressing calcium binding proteins show greater vulnerability to metabolic stressors, such as hypoxia-ischemia or inflammation. Supporting this concept, striatal mitochondria show greater sensitivity to disturbances in calcium homeostasis compared to cortical neurons (Pickrell et al., 2011).
Blood-brain barrier (BBB) integrity
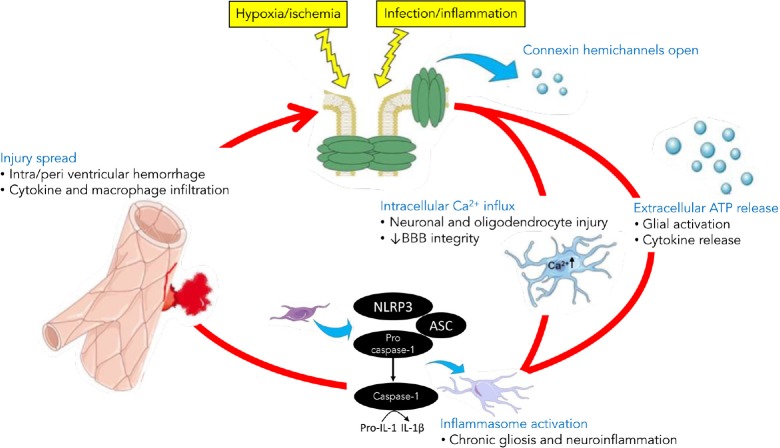
Intra-and/or peri-ventricular hemorrhage commonly leads to poor neurodevelopmental outcomes in preterm and term infants (Kluckow and Evans, 2000). Compromised BBB integrity primarily occurs because of a combination of periods of low or unstable cerebral perfusion, a developmentally immature cerebral vascular bed that is susceptible to vascular leak and impaired autoregulatory capacity (Polglase et al., 2014). The endothelium of the BBB expresses several connexin hemichannel isoforms, including Cx43 (De Bock et al., 2013). Pairs of Cx43 hemichannels are expressed on pericyte and endothelial cell membranes and at the astrocyte-endothelial and astrocyte-neuronal interfaces, respectively, forming gap junctions within the neurovascular unit (Winkler et al., 2011). BBB injury compromises vascular integrity and causes the vascular endothelium to become permeable to plasma proteins and macrophages after the insult (Barzo et al., 1996), which may augment regional neuroinflammation and injury. Vascular leak is caused by impaired endothelial calcium handling. A rise in intracellular calcium concentration causes a drop in transendothelial electrical resistance which enables circulating macrophages to penetrate the BBB (De Bock et al., 2013). Peptidoglycan-induced inflammation leads to opening of endothelial Cx43 hemichannels (Robertson et al., 2010). Endothelial calcium permeability is increased during exposure to the inflammatory peptide bradykinin, and can be rapidly inhibited by blocking Cx43 using the mimetic peptide, Gap26 (De Bock et al., 2013). Furthermore, blocking Cx43 mediated vascular leak with another mimetic peptide, Peptide5, was associated with reduced neurodegeneration and inflammation at 7 and 21 days after retinal ischemia–reperfusion in adult rats (Danesh-Meyer et al., 2012). Collectively, these data strongly suggest connexin hemichannels, in particular Cx43, mediate neurovascular health by regulating BBB integrity (Figure 1).
Figure 1.
Schema of the hypothesized role of opening of astrocyte hemichannels in sustaining chronic brain inflammation well after the initial injury.
The inflammasomes are innate immune systems that regulates activation of caspase-1 and inflammation. Connexin hemichannels mediate intracellular Ca2+ influx and adenosine triphosphate (ATP) release in to the extracellular space, which trigger inflammasome activation and injury spread during the evolution of brain injury. BBB: Blood-brain barrier; NLRP3: NOD-like receptor protein-3; ASC: adaptor protein; IL: interleukin.
Purinergic receptor signaling
Neurons and oligodendrocytes express purinergic receptors and are susceptible to ATP released in to the extracellular space from open hemichannels (Amadio et al., 2002, 2007). In peritraumatic areas, extracellular ATP can activate neuronal and glial purinergic receptors and induce cellular injury by promoting extracellular calcium influx through the receptor channel (Peng et al., 2009). Intracellular calcium accumulation augments connexin hemichannel opening and leads to ‘ATP-induced ATP release’ (Stout et al., 2002; Baroja-Mazo et al., 2013), which potentiates the spread of calcium waves through the astrocytic syncytium and, in turn, increases the potential for ATP-mediated neurotoxicity (Cotrina et al., 1998; Stout et al., 2002). Thus connexin hemichannel mediated release of ATP is likely to play a key role in the evolution of perinatal encephalopathy (Figure 1).
Inflammasome activation
There is emerging evidence to support a key role for inflammasome activation in the pathogenesis of developmental brain injury (Hagberg et al., 2015; Kim et al., 2016). Inflammasomes are multimeric protein complexes that assemble in the cytosol in response to exogenous and/or endogenous stress detected by pattern recognition receptors (PRR) (Guo et al., 2015). Several families of PRRs have been shown to play an important role in inflammasome activation. However, one of the most widely studied and strongly implicated PRRs is NOD-like receptor protein-3 (NLRP3) (Wen et al., 2013). In addition to calcium wave propagation (described above), ATP activates the NLRP3 inflammasome (Bours et al., 2011; de Rivero Vaccari et al., 2014). NLRP3 triggers the recruitment of adaptor protein (ASC) and pro-caspase-1 to form the oligomeric inflammasome complex. The inflammasome converts pro-caspase-1 to caspase-1, enabling cleavage of proinflammatory cytokines, notably IL-1β, for release from the cell. Cx43 hemichannels mediate ATP release, including ATP induced ATP release (Suadicani et al., 2006; Bennett et al., 2012; Mallard et al., 2014), and thus are likely to be instrumental in inflammasome activation in the brain (Kim et al., 2016; Mugisho et al., 2017). Extracellular release of ATP from open astrocytic connexin hemichannels can augment the pro-inflammatory function of activated microglia by binding to microglial P2RX7 receptors and triggering secretion of IL-1β (as well as pyroptosis) (Ferrari et al., 1997; Di Virgilio et al., 2001). Furthermore, TNF is capable of upregulating NLRP3 and inducing caspase-1 activation within the inflammasome (Furuoka et al., 2016). Thus, a self-sustaining, chronic inflammatory cycle may be established with connexin hemichannel mediated ATP release amplifying and perpetuating gliosis, inflammatory cytokine release (Mugisho et al., 2017; Tonkin et al., 2018) and injury spread (Figure 1).
The link between connexin hemichannels and inflammation-induced brain injury
There is extensive evidence from preclinical studies that the central nervous system (CNS) immune response is implicated in the pathogenesis of perinatal brain injury, as previously reviewed by Hagberg et al. (2015). Similarly, clinical studies and human post mortem series show a strong association between chronic upregulation of systemic and CNS cytokines and gliosis with adverse neurological outcomes (Savman et al., 1998; Bartha et al., 2004; Buser et al., 2012). For example, upregulation of TNF and IL-1β in preterm infants is associated with impaired neural function in the first 72 hours of life and cognitive impairment at 2–3 years corrected age (Bartha et al., 2004; Wikstrom et al., 2008). Furthermore, microglial infiltration, astrogliosis and upregulation of TNF and IL-1β are identified between 24 hours and 2 months after birth in white and grey matter structures in post mortem brain tissue from preterm infants with white matter injury (Kadhim et al., 2001, 2003; Buser et al., 2012). Importantly, cerebral inflammation plays a key role in connexin hemichannel activation. Release of IL-1β and TNF from activated microglia increased astrocytic Cx43 hemichannel activity and astrocyte membrane permeability (Retamal et al., 2007), whereas blockade of Cx43 hemichannels abrogated membrane permeability (Retamal et al., 2007). Furthermore, increased astrocytic hemichannel opening has also been observed in a mouse model of Staphylococcus aureus-induced brain injury, as shown by increased ethidium bromide uptake in the injured regions (Karpuk et al., 2011). Enhanced ethidium bromide uptake was reduced with pharmacological blockade of Cx43 using the mimetic peptide Gap26. Collectively, these data strongly support a key role for connexin hemichannels in promulgating and perpetuating the spread of injury during cerebral inflammation.
A role for pannexin hemichannels?
Pannexins share 20% sequence homology with the invertebrate innexin proteins that form invertebrate gap junctions, but have no homology with connexins (Panchin et al., 2000; Yen and Saier, 2007). However, connexin and pannexin membrane topology is very similar and both channels are blocked by a number of commonly used compounds, such as carbenoxolone and flufenamic acid, leading to controversy regarding their relative contributions to cerebral homeostasis (Bruzzone et al., 2005). It has been suggested that in vertebrates pannexins cannot form gap junctions, as interaction between pannexin hemichannels is prevented by their extensive glycosylation and therefore they exist only in the hemichannel form (Boassa et al., 2007). Pannexin1 and Pannexin2 are expressed on neurons throughout the brain, including in the cortex, striatum, olfactory bulb, hippocampus, thalamus and cerebellum (Bruzzone et al., 2003).
Similar to connexin hemichannels, pannexin hemichannels are thought to be involved in purinergic signaling under physiological conditions. Knockdown of Pannexin1 hemichannels has been shown to significantly reduce ATP release from astrocytes in response to 3-O-(4-benzoyl)benzoyl adenosine triphosphate (BzATP), a P2X7 agonist (Iglesias et al., 2009). However, this concept has recently been challenged by evidence that ATP release was no different in Pannexin1/Pannexin2 deficient astrocytes compared to wild type in response to BzATP stimulation (Bargiotas et al., 2011). This release of ATP was blocked by carbenoxolone, an inhibitor of both pannexin and connexin hemichannels, suggesting that connexin hemichannels were mediating the release of ATP from these astrocytes.
Supporting a role for pannexin hemichannels in ischemic brain injury, oxygen-glucose deprivation has been shown to open Pannexin1 hemichannels in isolated hippocampal neurons (Thompson et al., 2006). In an in vivo study in mice, double knockout of Pannexin1/Pannexin2 improved neurological deficits and reduced infarct size after ischemic stroke compared to wild type or mice with a single knockout of either Pannexin1 or Pannexin2 (Bargiotas et al., 2011, 2012).
By contrast, Madry and colleagues found that pannexin hemichannels did not contribute significantly to the generation of early anoxic depolarization nor the later uptake of dye during ischemia in CA1 pyramidal cells in hippocampal slices (Madry et al., 2010). Further, hemichannel activity induced by hypoxia in cortical astrocytes was blocked by Cx43 mimetic peptide but not by mimetic peptides targeting pannexin hemichannels (Froger et al., 2010). Iwabuchi and colleagues have proposed that pannexin hemichannels are part of a complex negative feedback loop, whereby ATP released through Pannexin1 hemichannels early after the onset of ischemia acts via P2X7 receptors to induce closure of Pannexin1 hemichannels. This process has been called ‘ATP-induced suppression of ATP release’ (Iwabuchi and Kawahara, 2011).
Conclusion
The spread of damage into previously uninjured regions over weeks to months after the insult is one of the most striking features of perinatal encephalopathy. Although the precise mechanisms responsible for the spread of injury are not fully understood, Cx43 hemichannels play a key role in the evolution of oligodendrocyte and neuronal injury. Likely mechanisms include Cx43-mediated modulation of intracellular calcium handling, blood brain barrier integrity, purinergic receptor signaling and inflammasome pathway activation. Collectively, these cellular processes are likely to initiate a vicious cycle of excessive ATP release, which propagates activation of purinergic receptors on microglia and astrocytes to trigger inflammation-induced injury of neurons and oligodendroglia. Many studies of perinatal neuroprotection have been limited by failure to prevent iatrogenic neuroprotective hypothermia (Galinsky et al., 2017b), and by a paucity of information on when key events occur and therefore when it might be possible to target them to protect the brain. Thus, developing new neuroprotective strategies for perinatal encephalopathy will benefit from further methodologically sound studies that will provide us with a more detailed understanding of connexin hemichannel and glial responses during the evolution of perinatal encephalopathy.
Footnotes
Funding: The study was supported by the Health Research Council of New Zealand (grant 17/601), the Auckland Medical Research Foundation, National Health and Medical Research Council CJ Martin Early Career Fellowship (grant No.1090890 to RG) and the Victorian Government Operational Infrastructure Support Program.
Conflicts of interest: CRG is an inventor on patents related to hemichannel regulation for treatment of injuries and disease and a founder of the company (OcuNexus Therapeutics, Inc., USA) holding rights to those patents. The other authors have no conflicts of interest to declare.
Financial support: The study was supported by the Health Research Council of New Zealand (grant 17/601), the Auckland Medical Research Foundation, National Health and Medical Research Council CJ Martin Early Career Fellowship (RG: 1090890) and the Victorian Government Operational Infrastructure Support Program.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review reports:
Reviewer 1: Creed Stary, Stanford University, USA.
Comments to authors: In the present study, the authors provide a review of astrocytic gap junction hemichannels in injury outcomes following perinatal encephalopathy. The review is comprehensive and topical. The manuscript can be improved by expanding the discussion of astrocytes in neuroprotection and maintenance, and considering the role of astrocyte-derived exosomes and non-coding RNAs in regulation of Cx43 expression.
Reviewer 2: Wenhui Hu, Temple University, USA.
Comments to authors: Connexins are well known to form gap junction for many important functions such as electrical synapses, ionic and neurotransmitter buffering, calcium wave propagation, myelin stabilization, etc. Connexins also form non-juxtaposed hemichannels that share properties with pannexins. Connexin hemichannels play an important role in the inflammatory response after various types of CNS injury and disease. This review updates the role of astrocytic connexin, in particular the non-juxtaposed hemichannels in the spread of neural injury underlying the perinatal encephalopathy. After comprehensive analysis and discussion, the authors concluded the key role of Cx43 hemichannels in mediating oligodendrocyte and neuronal injuries. They also discussed the potential mechanisms underlying Cx43 hemichannels' role, including intracellular calcium overload, blood-brain barrier impairment, and activation of purinergic receptor signaling and inflammasome pathway. Thus, therapeutic strategies to target connexin hemichannels might provide neuroprotective benefits for patients with perinatal encephalopathy.
References
- 1.Amadio S, Montilli C, Picconi B, Calabresi P, Volonte C. Mapping P2X and P2Y receptor proteins in striatum and substantia nigra: An immunohistological study. Purinergic Signal. 2007;3:389–398. doi: 10.1007/s11302-007-9069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadio S, D'Ambrosi N, Cavaliere F, Murra B, Sancesario G, Bernardi G, Burnstock G, Volonte C. P2 receptor modulation and cytotoxic function in cultured CNS neurons. Neuropharmacology. 2002;42:489–501. doi: 10.1016/s0028-3908(01)00197-6. [DOI] [PubMed] [Google Scholar]
- 3.Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 4.Back SA, Riddle A, Dean J, Hohimer AR. The instrumented fetal sheep as a model of cerebral white matter injury in the premature infant. Neurotherapeutics. 2012;9:359–370. doi: 10.1007/s13311-012-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball G, Srinivasan L, Aljabar P, Counsell SJ, Durighel G, Hajnal JV, Rutherford MA, Edwards AD. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci U S A. 2013;110:9541–9546. doi: 10.1073/pnas.1301652110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bargiotas P, Krenz A, Monyer H, Schwaninger M. Functional outcome of pannexin-deficient mice after cerebral ischemia. Channels. 2012;6:453–456. doi: 10.4161/chan.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A. 2011;108:20772–20777. doi: 10.1073/pnas.1018262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barkovich AJ, Sargent SK. Profound asphyxia in the premature infant: imaging findings. AJNR Am J Neuroradiol. 1995;16:1837–1846. [PMC free article] [PubMed] [Google Scholar]
- 9.Baroja-Mazo A, Barbera-Cremades M, Pelegrin P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta. 2013;1828:79–93. doi: 10.1016/j.bbamem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Bartha AI, Foster-Barber A, Miller SP, Vigneron DB, Glidden DV, Barkovich AJ, Ferriero DM. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr Res. 2004;56:960–966. doi: 10.1203/01.PDR.0000144819.45689.BB. [DOI] [PubMed] [Google Scholar]
- 11.Barzo P, Marmarou A, Fatouros P, Corwin F, Dunbar J. Magnetic resonance imaging-monitored acute blood-brain barrier changes in experimental traumatic brain injury. J Neurosurg. 1996;85:1113–1121. doi: 10.3171/jns.1996.85.6.1113. [DOI] [PubMed] [Google Scholar]
- 12.Bell JE, Becher JC, Wyatt B, Keeling JW, McIntosh N. Brain damage and axonal injury in a Scottish cohort of neonatal deaths. Brain. 2005;128:1070–1081. doi: 10.1093/brain/awh436. [DOI] [PubMed] [Google Scholar]
- 13.Bennett MV, Garre JM, Orellana JA, Bukauskas FF, Nedergaard M, Saez JC. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012;1487:3–15. doi: 10.1016/j.brainres.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–31743. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- 15.Bonora M, De Marchi E, Patergnani S, Suski JM, Celsi F, Bononi A, Giorgi C, Marchi S, Rimessi A, Duszynski J, Pozzan T, Wieckowski MR, Pinton P. Tumor necrosis factor-alpha impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ. 2014;21:1198–1208. doi: 10.1038/cdd.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bours MJ, Dagnelie PC, Giuliani AL, Wesselius A, Di Virgilio F. P2 receptors and extracellular ATP: a novel homeostatic pathway in inflammation. Front Biosci (Schol Ed) 2011;3:1443–1456. doi: 10.2741/235. [DOI] [PubMed] [Google Scholar]
- 17.Breeman LD, Jaekel J, Baumann N, Bartmann P, Wolke D. Attention problems in very preterm children from childhood to adulthood: the Bavarian Longitudinal Study. J Child Psychol Psychiatry. 2016;57:132–140. doi: 10.1111/jcpp.12456. [DOI] [PubMed] [Google Scholar]
- 18.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 19.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnett AC, Anderson PJ, Lee KJ, Roberts G, Doyle LW, Cheong JLY, Victorian Infant Collaborative Study Group Trends in executive functioning in extremely preterm children across 3 birth eras. Pediatrics. 2018;141 doi: 10.1542/peds.2017-1958. e20171958. [DOI] [PubMed] [Google Scholar]
- 21.Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalak LF, Rollins N, Morriss MC, Brion LP, Heyne R, Sanchez PJ. Perinatal acidosis and hypoxic-ischemic encephalopathy in preterm infants of 33 to 35 weeks' gestation. J Pediatr. 2012;160:388–394. doi: 10.1016/j.jpeds.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheong JLY, Anderson PJ, Burnett AC, Roberts G, Davis N, Hickey L, Carse E, Doyle LW. Victorian Infant Collaborative Study Group (2017) Changing neurodevelopment at 8 years in children born extremely preterm since the 1990s. Pediatrics. 139 doi: 10.1542/peds.2016-4086. e20164086. [DOI] [PubMed] [Google Scholar]
- 24.Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa DS, Miranda DM, Burnett AC, Doyle LW, Cheong JLY, Anderson PJ. Executive function and academic outcomes in children who were extremely preterm. Pediatrics. 2017;140:e20170257. doi: 10.1542/peds.2017-0257. [DOI] [PubMed] [Google Scholar]
- 26.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danesh-Meyer HV, Kerr NM, Zhang J, Eady EK, O'Carroll SJ, Nicholson LF, Johnson CS, Green CR. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain. 2012;135:506–520. doi: 10.1093/brain/awr338. [DOI] [PubMed] [Google Scholar]
- 28.Davidson JO, Green CR, Nicholson LF, Bennet L, Gunn AJ. Connexin hemichannel blockade is neuroprotective after but not during global cerebral ischemia in near-term fetal sheep. Exp Neurol. 2013a;248:301–308. doi: 10.1016/j.expneurol.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Davidson JO, Drury PP, Green CR, Nicholson LF, Bennet L, Gunn AJ. Connexin hemichannel blockade is neuroprotective after asphyxia in preterm fetal sheep. PLoS One. 2014;9:e96558. doi: 10.1371/journal.pone.0096558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson JO, Green CR, Nicholson LF, O'Carroll SJ, Fraser M, Bennet L, Gunn AJ. Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann Neurol. 2012;71:121–132. doi: 10.1002/ana.22654. [DOI] [PubMed] [Google Scholar]
- 31.Davidson JO, Green CR, Bennet L, Nicholson LF, Danesh-Meyer H, Carroll SJ, Gunn AJ. A key role for connexin hemichannels in spreading ischemic brain injury. Current Drug Targets. 2013b;14:36–46. doi: 10.2174/138945013804806479. [DOI] [PubMed] [Google Scholar]
- 32.De Bock M, Wang N, Decrock E, Bol M, Gadicherla AK, Culot M, Cecchelli R, Bultynck G, Leybaert L. Endothelial calcium dynamics connexin channels and blood-brain barrier function. Prog Neurobiol. 2013;108:1–20. doi: 10.1016/j.pneurobio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 33.de Rivero Vaccari JP, Dietrich WD, Keane RW. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab. 2014;34:369–375. doi: 10.1038/jcbfm.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vries LS, Eken P, Groenendaal F, Rademaker KJ, Hoogervorst B, Bruinse HW. Antenatal onset of haemorrhagic and/or ischaemic lesions in preterm infants: prevalence and associated obstetric variables. Arch Dis Child Fetal Neonatal Ed. 1998;78:F51–56. doi: 10.1136/fn.78.1.f51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dean JM, McClendon E, Hansen K, Azimi-Zonooz A, Chen K, Riddle A, Gong X, Sharifnia E, Hagen M, Ahmad T, Leigland LA, Hohimer AR, Kroenke CD, Back SA. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci Transl Med. 2013;5:168ra167. doi: 10.1126/scitranslmed.3004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decrock E, De Vuyst E, Vinken M, Van Moorhem M, Vranckx K, Wang N, Van Laeken L, De Bock M, D'Herde K, Lai CP, Rogiers V, Evans WH, Naus CC, Leybaert L. Connexin 43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death Differ. 2009;16:151–163. doi: 10.1038/cdd.2008.138. [DOI] [PubMed] [Google Scholar]
- 37.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 38.Drury PP, Gunn ER, Bennet L, Gunn AJ. Mechanisms of hypothermic neuroprotection. Clin Perinatol. 2014;41:161–175. doi: 10.1016/j.clp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Eryigit Madzwamuse S, Baumann N, Jaekel J, Bartmann P, Wolke D. Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. J Child Psychol Psychiatry. 2015;56:857–864. doi: 10.1111/jcpp.12358. [DOI] [PubMed] [Google Scholar]
- 40.Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, Lacaud A, Saliba E, Dammann O, Gallego J, Sizonenko S, Hagberg H, Lelievre V, Gressens P. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70:550–565. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 2012;11:556–566. doi: 10.1016/S1474-4422(12)70058-3. [DOI] [PubMed] [Google Scholar]
- 43.Froger N, Orellana JA, Calvo CF, Amigou E, Kozoriz MG, Naus CC, Saez JC, Giaume C. Inhibition of cytokine-induced connexin43 hemichannel activity in astrocytes is neuroprotective. Mol Cell Neurosci. 2010;45:37–46. doi: 10.1016/j.mcn.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Furuoka M, Ozaki K, Sadatomi D, Mamiya S, Yonezawa T, Tanimura S, Takeda K. TNF-alpha induces caspase-1 activation independently of simultaneously induced NLRP3 in 3T3-L1 cells. J Cell Physiol. 2016;231:2761–2767. doi: 10.1002/jcp.25385. [DOI] [PubMed] [Google Scholar]
- 45.Gajardo-Gomez R, Labra VC, Orellana JA. Connexins and pannexins: new insights into microglial functions and dysfunctions. Front Mol Neurosci. 2016;9:86. doi: 10.3389/fnmol.2016.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJ. The consequences of chorioamnionitis: preterm birth and effects on development. J Pregnancy. 2013;2013:412831. doi: 10.1155/2013/412831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galinsky R, Davidson JO, Lear CA, Bennet L, Green CR, Gunn AJ. Connexin hemichannel blockade improves survival of striatal GABA-ergic neurons after global cerebral ischaemia in term-equivalent fetal sheep. Sci Rep. 2017a;7:6304. doi: 10.1038/s41598-017-06683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galinsky R, Dean JM, Lear CA, Davidson JO, Dhillon S, Wassink G, Bennet L, Gunn AJ. In the era of therapeutic hypothermia, how well do studies of perinatal neuroprotection control temperature? Dev Neurosci. 2017b;39:7–22. doi: 10.1159/000452859. [DOI] [PubMed] [Google Scholar]
- 49.Galinsky R, Draghi V, Wassink G, Davidson JO, Drury PP, Lear CA, Gunn AJ, Bennet L. Magnesium sulfate reduces EEG activity but is not neuroprotective after asphyxia in preterm fetal sheep. J Cereb Blood Flow Metab. 2017c;37:1362–1373. doi: 10.1177/0271678X16655548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galinsky R, Lear CA, Dean JM, Wassink G, Dhillon SK, Fraser M, Davidson JO, Bennet L, Gunn AJ. Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Dev Med Child Neurol. 2018;60:126–133. doi: 10.1111/dmcn.13629. [DOI] [PubMed] [Google Scholar]
- 51.Garfinkle J, Wintermark P, Shevell MI, Oskoui M. Children born at 32 to 35 weeks with birth asphyxia and later cerebral palsy are different from those born after 35 weeks. J Perinatol. 2017;37:963–968. doi: 10.1038/jp.2017.23. [DOI] [PubMed] [Google Scholar]
- 52.George S, Gunn AJ, Westgate JA, Brabyn C, Guan J, Bennet L. Fetal heart rate variability and brainstem injury after asphyxia in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;287:R925–933. doi: 10.1152/ajpregu.00263.2004. [DOI] [PubMed] [Google Scholar]
- 53.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, Gressens P. The role of inflammation in perinatal brain injury. Nat Rev Neurol. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helderman JB, O'Shea TM, Kuban KC, Allred EN, Hecht JL, Dammann O, Paneth N, McElrath TF, Onderdonk A, Leviton A. Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics. 2012;129:494–502. doi: 10.1542/peds.2011-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Honeycutt A, Dunlap L, Chen H, al Homsi G, Grosse S, Schendel D. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment: United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:57–59. [PubMed] [Google Scholar]
- 57.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwabuchi S, Kawahara K. Functional significance of the negative-feedback regulation of ATP release via pannexin-1 hemichannels under ischemic stress in astrocytes. Neurochem Int. 2011;58:376–384. doi: 10.1016/j.neuint.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Kadhim H, Tabarki B, De Prez C, Sebire G. Cytokine immunoreactivity in cortical and subcortical neurons in periventricular leukomalacia: are cytokines implicated in neuronal dysfunction in cerebral palsy? Acta Neuropathol. 2003;105:209–216. doi: 10.1007/s00401-002-0633-6. [DOI] [PubMed] [Google Scholar]
- 60.Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sebire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- 61.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karpuk N, Burkovetskaya M, Fritz T, Angle A, Kielian T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J Neurosci. 2011;31:414–425. doi: 10.1523/JNEUROSCI.5247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kidokoro H, Kubota T, Hayashi N, Hayakawa M, Takemoto K, Kato Y, Okumura A. Absent cyclicity on aEEG within the first 24 h is associated with brain damage in preterm infants. Neuropediatrics. 2010;41:241–245. doi: 10.1055/s-0030-1270479. [DOI] [PubMed] [Google Scholar]
- 64.Kim Y, Davidson JO, Gunn KC, Phillips AR, Green CR, Gunn AJ. Role of hemichannels in CNS inflammation and the inflammasome pathway. Adv Protein Chem Struct Biol. 2016;104:1–37. doi: 10.1016/bs.apcsb.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2000;82:F188–194. doi: 10.1136/fn.82.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 67.Kuban KC, Joseph RM, O'Shea TM, Allred EN, Heeren T, Douglass L, Stafstrom CE, Jara H, Frazier JA, Hirtz D, Leviton A. Girls and boys born before 28 weeks gestation: risks of cognitive, behavioral, and neurologic outcomes at age 10 years. J Pediatr. 2016;173:l69–75. doi: 10.1016/j.jpeds.2016.02.048. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kubota T, Okumura A, Hayakawa F, Kato T, Itomi K, Kuno K, Watanabe K. Combination of neonatal electroencephalography and ultrasonography: sensitive means of early diagnosis of periventricular leukomalacia. Brain Dev. 2002;24:698–702. doi: 10.1016/s0387-7604(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 69.Lear CA, Davidson JO, Mackay GR, Drury PP, Galinsky R, Quaedackers JS, Gunn AJ, Bennet L. Antenatal dexamethasone before asphyxia promotes cystic neural injury in preterm fetal sheep by inducing hyperglycemia. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17703124. doi: 10.1177/0271678X17703124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Low JA, Killen H, Derrick EJ. Antepartum fetal asphyxia in the preterm pregnancy. Am J Obstet Gynecol. 2003;188:461–465. doi: 10.1067/mob.2003.37. [DOI] [PubMed] [Google Scholar]
- 73.Madry C, Haglerod C, Attwell D. The role of pannexin hemichannels in the anoxic depolarization of hippocampal pyramidal cells. Brain. 2010;133:3755–3763. doi: 10.1093/brain/awq284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mallard C, Davidson JO, Tan S, Green CR, Bennet L, Robertson NJ, Gunn AJ. Astrocytes and microglia in acute cerebral injury underlying cerebral palsy associated with preterm birth. Pediatr Res. 2014;75:234–240. doi: 10.1038/pr.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, Thorp JM, Caritis SN, Prasad M, Tita AT, Saade GR, Sorokin Y, Rouse DJ, Blackwell SC, Tolosa JE, Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215:103.e1–103.e14. doi: 10.1016/j.ajog.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 77.McClendon E, Chen K, Gong X, Sharifnia E, Hagen M, Cai V, Shaver DC, Riddle A, Dean JM, Gunn AJ, Mohr C, Kaplan JS, Rossi DJ, Kroenke CD, Hohimer AR, Back SA. Prenatal cerebral ischemia triggers dysmaturation of caudate projection neurons. Ann Neurol. 2014;75:508–524. doi: 10.1002/ana.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meng C, Bauml JG, Daamen M, Jaekel J, Neitzel J, Scheef L, Busch B, Baumann N, Boecker H, Zimmer C, Bartmann P, Wolke D, Wohlschlager AM, Sorg C. Extensive and interrelated subcortical white and gray matter alterations in preterm-born adults. Brain Struct Funct. 2016;221:2109–2121. doi: 10.1007/s00429-015-1032-9. [DOI] [PubMed] [Google Scholar]
- 79.Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, Newton N, Partridge JC, Glidden DV, Vigneron DB, Barkovich AJ. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- 80.Mrzljak L, Uylings HB, Kostovic I, van Eden CG. Prenatal development of neurons in the human prefrontal cortex. II. A quantitative Golgi study. J Comp Neurol. 1992;316:485–496. doi: 10.1002/cne.903160408. [DOI] [PubMed] [Google Scholar]
- 81.Mugisho OO, Green CR, Kho DT, Zhang J, Graham ES, Acosta ML, Rupenthal ID. The inflammasome pathway is amplified and perpetuated in an autocrine manner through connexin43 hemichannel mediated ATP release. Biochim Biophys Acta. 2017;1862:385–393. doi: 10.1016/j.bbagen.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 82.Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124:e459–467. doi: 10.1542/peds.2008-2190. [DOI] [PubMed] [Google Scholar]
- 83.Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde YA. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J Neurosci. 2002;22:854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orellana JA, Hernandez DE, Ezan P, Velarde V, Bennett MV, Giaume C, Saez JC. Hypoxia in high glucose followed by reoxygenation in normal glucose reduces the viability of cortical astrocytes through increased permeability of connexin 43 hemichannels. Glia. 2010;58:329–343. doi: 10.1002/glia.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 86.Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 87.Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, Walsh MC, Das A, Hale EC, Newman NS, Higgins RD. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014;168:137–147. doi: 10.1001/jamapediatrics.2013.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pichler G, Avian A, Binder C, Zotter H, Schmolzer GM, Morris N, Muller W, Urlesberger B. aEEG and NIRS during transition and resuscitation after birth: promising additional tools; an observational study. Resuscitation. 2013;84:974–978. doi: 10.1016/j.resuscitation.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 90.Pickrell AM, Fukui H, Wang X, Pinto M, Moraes CT. The striatum is highly susceptible to mitochondrial oxidative phosphorylation dysfunctions. J Neurosci. 2011;31:9895–9904. doi: 10.1523/JNEUROSCI.6223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierrat V, Marchand-Martin L, Arnaud C, Kaminski M, Resche-Rigon M, Lebeaux C, Bodeau-Livinec F, Morgan AS, Goffinet F, Marret S, Ancel PY, the EPIPAGE-2 writing group Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks' gestation in France in 2011: EPIPAGE-2 cohort study. BMJ. 2017;358:j3448. doi: 10.1136/bmj.j3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Polglase GR, Miller SL, Barton SK, Kluckow M, Gill AW, Hooper SB, Tolcos M. Respiratory support for premature neonates in the delivery room: effects on cardiovascular function and the development of brain injury. Pediatr Res. 2014;75:682–688. doi: 10.1038/pr.2014.40. [DOI] [PubMed] [Google Scholar]
- 93.Puka-Sundvall M, Gajkowska B, Cholewinski M, Blomgren K, Lazarewicz JW, Hagberg H. Subcellular distribution of calcium and ultrastructural changes after cerebral hypoxia-ischemia in immature rats. Brain Res Dev Brain Res. 2000;125:31–41. doi: 10.1016/s0165-3806(00)00110-3. [DOI] [PubMed] [Google Scholar]
- 94.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rathbone R, Counsell SJ, Kapellou O, Dyet L, Kennea N, Hajnal J, Allsop JM, Cowan F, Edwards AD. Perinatal cortical growth and childhood neurocognitive abilities. Neurology. 2011;77:1510–1517. doi: 10.1212/WNL.0b013e318233b215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Saez PJ, Saez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riddle A, Dean J, Buser JR, Gong X, Maire J, Chen K, Ahmad T, Cai V, Nguyen T, Kroenke CD, Hohimer AR, Back SA. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol. 2011;70:493–507. doi: 10.1002/ana.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robertson J, Lang S, Lambert PA, Martin PE. Peptidoglycan derived from Staphylococcus epidermidis induces Connexin43 hemichannel activity with consequences on the innate immune response in endothelial cells. Biochem J. 2010;432:133–143. doi: 10.1042/BJ20091753. [DOI] [PubMed] [Google Scholar]
- 99.Rodriguez-Sinovas A, Cabestrero A, Lopez D, Torre I, Morente M, Abellan A, Miro E, Ruiz-Meana M, Garcia-Dorado D. The modulatory effects of connexin 43 on cell death/survival beyond cell coupling. Prog Biophys Mol Biol. 2007;94:219–232. doi: 10.1016/j.pbiomolbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 100.Salhab WA, Perlman JM. Severe fetal acidemia and subsequent neonatal encephalopathy in the larger premature infant. Pediatr Neurol. 2005;32:25–29. doi: 10.1016/j.pediatrneurol.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 101.Savman K, Blennow M, Gustafson K, Tarkowski E, Hagberg H. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr Res. 1998;43:746–751. doi: 10.1203/00006450-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 102.Schild L, Huppelsberg J, Kahlert S, Keilhoff G, Reiser G. Brain mitochondria are primed by moderate Ca2+ rise upon hypoxia/reoxygenation for functional breakdown and morphological disintegration. J Biol Chem. 2003;278:25454–25460. doi: 10.1074/jbc.M302743200. [DOI] [PubMed] [Google Scholar]
- 103.Schmidt JW, Walsh WF. Hypoxic-ischemic encephalopathy in preterm infants. J Neonatal Perinatal Med. 2010;3:277–284. [Google Scholar]
- 104.Schulz R, Gorge PM, Gorbe A, Ferdinandy P, Lampe PD, Leybaert L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther. 2015;153:90–106. doi: 10.1016/j.pharmthera.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serenius F, Kallen K, Blennow M, Ewald U, Fellman V, Holmstrom G, Lindberg E, Lundqvist P, Marsal K, Norman M, Olhager E, Stigson L, Stjernqvist K, Vollmer B, Stromberg B. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309:1810–1820. doi: 10.1001/jama.2013.3786. [DOI] [PubMed] [Google Scholar]
- 106.Smithers-Sheedy H, McIntyre S, Gibson C, Meehan E, Scott H, Goldsmith S, Watson L, Badawi N, Walker K, Novak I, Blair E. A special supplement: findings from the Australian Cerebral Palsy Register, birth years 1993 to 2006. Dev Med Child Neurol 58 Suppl. 2016;2:5–10. doi: 10.1111/dmcn.13026. [DOI] [PubMed] [Google Scholar]
- 107.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 108.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takizawa Y, Takashima S, Itoh M. A histopathological study of premature and mature infants with pontosubicular neuron necrosis: neuronal cell death in perinatal brain damage. Brain Res. 2006;1095:200–206. doi: 10.1016/j.brainres.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 110.Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, Volpe J, Bourgeois B, du Plessis AJ. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–1280. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- 111.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 112.Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–139. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 113.Tonkin RS, Bowles C, Perera CJ, Keating BA, Makker PGS, Duffy SS, Lees JG, Tran C, Don AS, Fath T, Liu L, O'Carroll SJ, Nicholson LFB, Green CR, Gorrie C, Moalem-Taylor G. Attenuation of mechanical pain hypersensitivity by treatment with Peptide5, a connexin-43 mimetic peptide, involves inhibition of NLRP3 inflammasome in nerve-injured mice. Exp Neurol. 2018;300:1–12. doi: 10.1016/j.expneurol.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 114.van Rooij LG, Toet MC, Osredkar D, van Huffelen AC, Groenendaal F, de Vries LS. Recovery of amplitude integrated electroencephalographic background patterns within 24 hours of perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2005;90:F245–251. doi: 10.1136/adc.2004.064964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wikstrom S, Ley D, Hansen-Pupp I, Rosen I, Hellstrom-Westas L. Early amplitude-integrated EEG correlates with cord TNF-alpha and brain injury in very preterm infants. Acta Paediatr. 2008;97:915–919. doi: 10.1111/j.1651-2227.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- 117.Wikstrom S, Pupp IH, Rosen I, Norman E, Fellman V, Ley D, Hellstrom-Westas L. Early single-channel aEEG/EEG predicts outcome in very preterm infants. Acta Paediatr. 2012;101:719–726. doi: 10.1111/j.1651-2227.2012.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 120.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yen MR, Saier MH., Jr Gap junctional proteins of animals: the innexin/pannexin superfamily. Prog Biophys Mol Biol. 2007;94:5–14. doi: 10.1016/j.pbiomolbio.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ylijoki M, Lehtonen L, Lind A, Ekholm E, Lapinleimu H, Kujari H, Haataja L. Chorioamnionitis and five-year neurodevelopmental outcome in preterm infants. Neonatology. 2016;110:286–295. doi: 10.1159/000446236. [DOI] [PubMed] [Google Scholar]