Abstract
Methanobactins (Mbns) are ribosomally produced, post-translationally modified natural products that bind copper with high affinity and specificity. Originally identified in methanotrophic bacteria, which have a high need for copper, operons encoding these compounds have also been found in many non-methanotrophic bacteria. The proteins responsible for Mbn biosynthesis include several novel enzymes. Mbn transport involves export through a multidrug efflux pump and re-internalization via a TonB-dependent transporter. Release of copper from Mbn and the molecular basis for copper regulation of Mbn production remain to be elucidated. Future work is likely to result in the identification of new enzymatic chemistry, opportunities for bioengineering and drug targeting of copper metabolism, and an expanded understanding of microbial metal homeostasis.
Keywords: metal homeostasis, copper transport, metalloprotein, siderophore, bacterial genetics, chalkophore, copper transport, metallophore, methanobactin, RiPPs
Introduction
Transition metals are key cofactors in metabolically important enzymes across all kingdoms of life (1). Nevertheless, careful control of cellular metal levels is required; a cellular surplus can limit viability due to oxidative stress (2), but metal starvation can also be fatal. Investigations of metal influx during conditions of metal scarcity have often been limited to iron, which is poorly bioavailable under aerobic conditions (3). Iron-chelating natural products (siderophores) are secreted by many species, and iron from siderophores is incorporated into the cellular iron pool after re-internalization (4). Although efflux has historically dominated studies of non-iron homeostasis, there is increasing evidence that similar systems exist for uptake of other metal ions (5, 6). One of the best-understood examples is methanobactin (Mbn),2 a natural product involved in copper homeostasis in methanotrophic bacteria.
Methanotrophic bacteria oxidize methane to methanol in the first step of their metabolism (7). Two unrelated metalloenzymes catalyze aerobic methane oxidation (8): the cytoplasmic iron enzyme soluble methane monooxygenase (sMMO) and the more widespread copper enzyme particulate methane monooxygenase (pMMO), a integral inner membrane protein. Some methanotrophic bacteria can produce both enzymes, but whenever sufficient copper is present, sMMO is down-regulated and pMMO is preferred (9). In the presence of copper, methanotrophs produce extensive intracytoplasmic membranes (10, 11). These membranes contain large quantities of pMMO, representing up to a fifth of the cellular protein mass (12). pMMO activity is copper-dependent (13), and methanotrophs thus have several systems for copper influx alongside the better-understood efflux systems of other microbes (14). Some methanotrophs secrete the post-translationally modified protein MopE to bind extracellular copper (15), whereas other methanotrophs use the copper-binding “chalkophore” (from the Greek chalko-, copper) Mbn to mediate copper uptake into the intracellular copper pool (16, 17). Mbns are ribosomally produced, post-translationally modified natural products (RiPPs) (18). Operons encoding Mbn precursor peptides along with proteins involved in Mbn biosynthesis, transport, and regulation have been identified in a range of bacteria, including non-methanotrophs, in which Mbn is increasingly believed to play a similar role in copper homeostasis (19, 20). Here, we summarize the current state of knowledge regarding Mbns.
Mbn structures
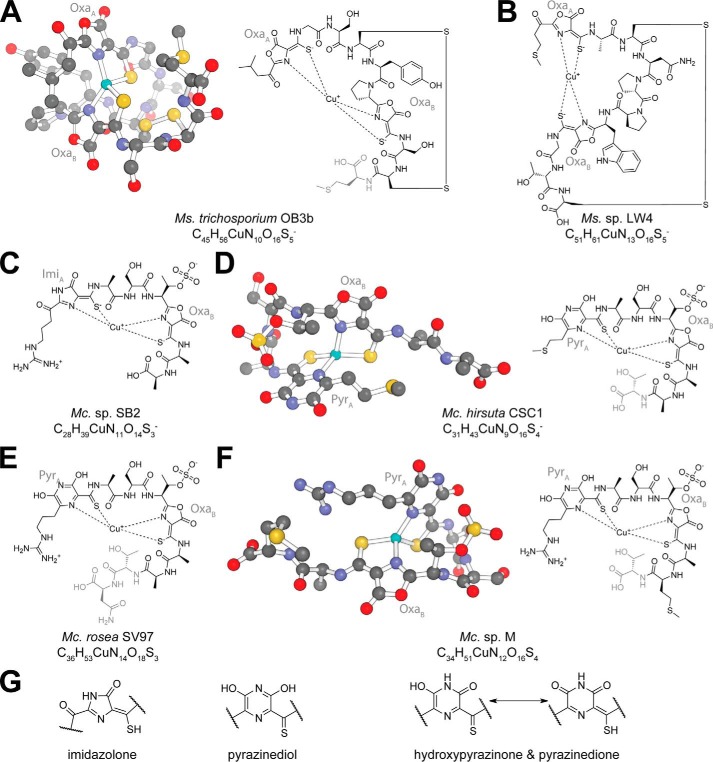
The crystal structure of copper-loaded Mbn (CuMbn) from Methylosinus (Ms.) trichosporium OB3b was assigned as N-2-isopropylester–(4-thionyl-5-hydroxy-imidazole)–Gly1–Ser2–Cys3–Tyr4–pyrrolidine–(4-hydroxy-5-thionyl-imidazole)–Ser5–Cys6–Met7, with a disulfide bridge between the two cysteine residues (17). In this structure, two hydroxyimidazolate rings and neighboring thioamide groups coordinate a copper ion in a distorted tetrahedral geometry. Re-analysis by NMR provided two key corrections: the heterocycles are instead oxazolone rings, and the “N-terminal” group is actually a 3-methylbutanoyl group (Fig. 1A) (21). These oxazolone rings (and in some circumstances other nitrogen-containing heterocycles) and neighboring enethiol/thioamide groups are the core Mbn post-translational modifications. Oxazolone rings contain an acid-labile lactone moiety, and Mbn is thus susceptible to acid-catalyzed methanolysis (21) and hydrolysis (22). The C-terminal methionine is sometimes absent (23), although it is unclear when and how this residue loss occurs. The structure of a second Methylosinus Mbn, Ms. sp. LW4 Mbn, was predicted based on its Mbn operon content; despite an otherwise divergent peptidic backbone, this Mbn has two oxazolone/thioamide pairs, an internal disulfide bond, and an N-terminal ketone group, as observed in Ms. trichosporium OB3b Mbn (Fig. 1B) (24).
Figure 1.
Structures of copper-bound Mbns. In all structures, residues that are sometimes absent are denoted in gray. Additional C-terminal residues appear to be lost in all Methylocystis Mbns (20). A, Ms. trichosporium OB3b CuMbn crystal structure and chemical structure. Both heterocycles are oxazolones (labeled OxaA and OxaB). B, Ms. sp. LW4 CuMbn chemical structure. As with Ms. trichosporium OB3b CuMbn, both heterocycles are oxazolones (labeled OxaA and OxaB). C, Mc. sp. SB2 CuMbn chemical structure. Heterocycle A has been described as an imidazolone (labeled ImiA), whereas heterocycle B is an oxazolone (labeled OxaB). D, Mc. hirsuta CSC1 CuMbn crystal structure and chemical structure. Heterocycle A has been depicted as a pyrazinediol (labeled PyrA), whereas heterocycle B is an oxazolone (labeled OxaB). E, Mc. rosea SV97 CuMbn chemical structure. Heterocycle A has been depicted as a pyrazinediol (labeled PyrA), whereas heterocycle B is an oxazolone (labeled OxaB). F, Mc. sp. M CuMbn crystal structure and chemical structure. Heterocycle A has been depicted as a pyrazinediol (labeled PyrA), whereas heterocycle B is an oxazolone (labeled OxaB). G, possible identities for heterocycle A in Methylocystis Mbns. Hydroxypyrazinone and pyrazinedione tautomers are potentially consistent with the observed crystal structures and NMR data.
CuMbn from Methylocystis (Mc.) sp. SB2, characterized by NMR, was reported to have a divergent peptidic backbone, no cysteine-derived disulfide bond, and a sulfonated threonine as well as two heterocycle/thioamide moieties (Fig. 1C) (22). Several C-terminal residues are lost in the characterized compound (20). The first “N-terminal” heterocycle (heterocycle A) was deemed an imidazolone ring based on an NMR-detectable secondary amine embedded in that heterocycle (22). The second heterocycle (heterocycle B) is an oxazolone, as in Ms trichosporium OB3b Mbn. Two additional Mbns from the Methylocystis species have been characterized via X-ray crystallography and a third closely related Mbn via mass spectrometry (Fig. 1, D–F). Although these Mbns differ from Mc. sp. SB2 Mbn by only one or two residues, heterocycle A is clearly a six-membered ring, depicted as a pyrazinediol group (25). Given that the Methylocystis species are closely related, the structural discrepancy in heterocycle A is puzzling (20). One explanation is that Methylocystis Mbns may actually contain a hydroxypyrazinone or pyrazinedione tautomer (Fig. 1G), which would contain a heterocyclic secondary amine, as observed by NMR, and the six-membered rings observed via X-ray crystallography. Supporting this notion, the non-copper-chelating nitrogen in heterocycle A in the Methylocystis Mbn crystal structures appears to be protonated (24). Thioamide/enethiol tautomerization may also occur, depending on ionic state, copper chelation, and the identity of the neighboring heterocycle.
The paired heterocycles and thioamides found in all these Mbns have characteristic spectral features. Oxazolone B absorbs at 340–342 nm, whereas heterocycle A absorbs at 388–394 nm (22, 24–26). Fluorescence is observed at 375–475 nm with excitation at the heterocycle-associated absorbance maxima (26, 27). Absorbance features from tyrosines or tryptophans are also observed for the two Methylosinus compounds, and a feature at 254 nm may be related to the thioamide/enethiol groups. Major spectral shifts occur upon copper binding (22, 27), and oxazolone-derived fluorescence is mostly abolished (26–28).
Mbns as metallophores
Mbns have a high affinity for copper in both oxidation states. Values vary significantly by measurement technique (28), but the broad consensus is that characterized Mbns have Cu(I)-binding constants of at least 1020–1021 m−1 (23, 25, 28, 29). Structural modifications beyond the first coordination sphere such as loss of C-terminal residues or desulfonation of the threonine in Methylocystis Mbns slightly affect copper affinity (23, 25). The Cu(I) affinity is high enough that Mbn can liberate bio-unavailable copper from sources ranging from humic acids (30) to minerals (31) to borosilicate glass (32, 33). Although Mbns bind Cu(II) with lower affinity, generally calculated to be 1011–1014 m−1 (25), binding is reductive, with conversion to Cu(I) within the first 10 min via an unknown mechanism, as confirmed by electron paramagnetic resonance and X-ray absorption spectroscopies (27, 29, 34, 35). Under superstoichiometric copper conditions, a second copper binds, albeit with lower affinity and without reduction (29). Other stoichiometries are also observed under some conditions (29).
Like other metallophores, Mbns can bind additional metal ions. Harder metals, including Cd(II), Co(II), Fe(III), Mn(II), Ni(II), and Zn(II), bind Mbn poorly and sometimes as bischelates or as dimetallated compounds, and no reductive binding is observed (36). Softer metals such as Ag(I), Au(III), Hg(II), Pb(II), and U(VI) bind single Mbn molecules with a 1:1 stoichiometry. Recent ion-mobility mass spectrometry experiments complicate this classification of Mbn–metal interactions, although further spectroscopic analysis may be necessary to confirm these results (37). Nevertheless, binding of softer metals is consistently of higher affinity, results in spectral features resembling those of CuMbn, and can be reductive for at least Au(III), Ag(I), and Hg(II) (36). Relative binding affinities show some pH dependence (36, 37). However, reported binding constants for these metals are approximately 5 orders of magnitude lower than that for Cu(II) and 15 orders of magnitude lower than that for Cu(I) (36). Despite this lower affinity, spectroscopic data suggest that bound Au(III), Ag(I), and Hg(II) are not readily displaced by copper (38, 39). Metal binding has only been investigated extensively for Ms. trichosporium OB3b and Mc. sp. SB2 Mbns, but there are indications that relative affinities for metals other than copper may vary by Mbn (39).
Mbn operons
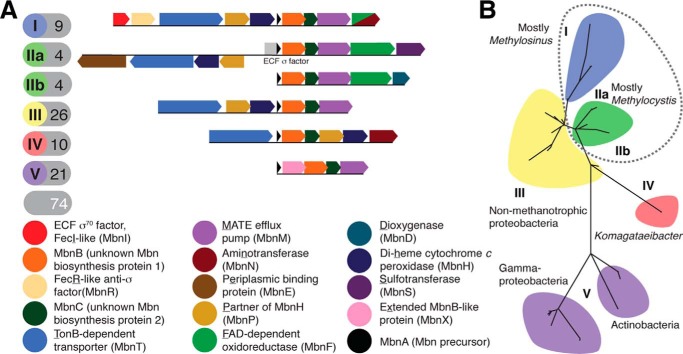
The peptidic Mbn backbone was originally thought to be the result of non-ribosomal peptide synthesis (40, 41). However, a short open reading frame encoding a 30-amino acid peptide with 11 C-terminal residues resembling the Mbn backbone was identified in the Ms. trichosporium OB3b genome, suggesting a ribosomal origin (22), and disruption of this open reading frame abrogated Mbn production (42). Bioinformatic analyses identified related genes with similar genomic neighborhoods in other species (43), with 18 operons identified in 16 species by 2013 (19). To date, 74 Mbn operons have been found in 71 species, with Mbn operons in methanotrophs forming a minority (Fig. 2A) (20). No Mbn operons are found in γ-proteobacterial methanotrophs; their reported Mbns may be other metallophores (19, 35).
Figure 2.
Mbn operons. A, schematics and content of typical operons from the common groups. B, phylogenetic tree of Mbn operons, based on MbnB protein sequences; similar results were obtained using MbnA and MbnC protein sequences. Subgroups containing methanotrophs are circled with a dotted line.
Mbn operon content varies considerably. Only three genes are found in all operons: mbnA encoding the precursor peptide, and mbnB and mbnC encoding hypothetical proteins with proposed roles in Mbn biosynthesis (19). Genes for membrane proteins related to Mbn import (TonB-dependent transporters) and export (MATE multidrug export proteins) are found in many but not all operons. Beyond MbnB and MbnC, several groups of operons encode other biosynthesis proteins, including aminotransferases, predicted dioxygenases, flavoenzymes, sulfotransferases, and distant MbnB homologues, although none of these genes are widespread among Mbn operons.
Phylogenetic analysis of MbnA, MbnB, and MbnC yields six major Mbn subgroups (Fig. 2B); these groups are also easily distinguished by their varying operon content (19) (Fig. 2A). Groups I, IIa, and IIb are found exclusively in α-proteobacterial methanotrophs belonging to the Methylosinus and Methylocystis genera, and groups III–V are found in non-methanotrophs (Fig. 2B). Group III operons are present in various proteobacteria, particularly Cupriavidus and Pseudomonas species. Group IV operons are found exclusively in Komagataeibacter/Gluconacetobacter and related genera. Group V operons are found in a diverse range of species, including non-methanotrophic proteobacteria as well as Gram-positive Streptomyces species and even a Chlamydiales strain (20).
Biosynthetic pathway of Mbns
RiPPs originate as larger precursor peptides, containing both a “core” peptide, which is the basis for the final natural product, as well as “leader” peptide sequences that mediate interactions with biosynthetic enzymes and are lost during maturation (18). The MbnA precursor peptides are 22–35 amino acids in length (19, 20). Almost all MbnA leader peptides contain several positively charged residues and a hydrophobic patch; group V MbnAs also have negatively charged residues. The core peptides are more variable. The copper-binding oxazolones and thioamides derive from post-translationally modified cysteines (22), and only these cysteines are universal in core peptides (19). Not all cysteines in MbnAs are modified. A specific peptide sequence triggers modification: the target cysteine is followed by a small, often hydrophobic residue (alanine, glycine, or occasionally serine) and then a slightly larger, mildly hydrophilic residue (particularly serine and threonine) (19). Unmodified cysteines may form disulfide bonds, as in Ms. trichosporium OB3b and Ms. sp. LW4 Mbns (17, 24).
The primary candidate proteins for oxazolone and thioamide biosynthesis are MbnB and MbnC (19). Neither belongs to a characterized protein family, although both are likely to be cytoplasmic, and MbnB is predicted to be a TIM barrel protein, with the closest characterized families comprising xylose isomerases and endonuclease IV enzymes. In some RiPP biosynthesis enzymes, a conserved RiPP recognition element (RRE) mediates enzyme–peptide interactions (44), but no RRE elements are found in MbnB, MbnC, or any other putative Mbn biosynthesis enzyme. Nevertheless, genes encoding MbnB and MbnC are present in all Mbn operons (Fig. 2A) as a translationally coupled pair (20). No other biosynthesis proteins are universally present, and although a handful of other natural products contain oxazolone or thioamide groups, no homologues for any genes involved in biosynthesis of those compounds can be found in Mbn operons.
Genes in some Mbn operons have other predicted biosynthetic roles, and all appear to encode cytoplasmic proteins. Genes encoding PLP-dependent aminotransferases (annotated mbnN) are present in some group I and all group IV Mbn operons (Fig. 2A), although the aminotransferases in the two groups are not closely related (19, 20). When mbnN is disrupted in Ms. trichosporium OB3b, no wild-type Mbn production is observed (45). A smaller compound is present, with a mass equivalent to that of the apo compound altered by C-terminal methionine loss, an N-terminal primary amine rather than a ketone, and acid hydrolysis of one of the two labile oxazolone/thioamide moieties. This compound was proposed to result from incomplete oxazolone A formation without MbnN. Given that all Mbns characterized thus far have oxazolone B (21, 22, 24, 25), despite the absence of an aminotransferase in all Methylocystis Mbn operons (19), it is unclear why oxazolone A would require MbnN in the two Methylosinus species. Increased acid lability resulting in hydrolysis of oxazolone A is an alternative interpretation.
As with MbnN, the role of a cytoplasmic 3′-phosphoadenosine-5′-phosphosulfate-dependent sulfotransferase (MbnS) is relatively straightforward. It is found only in group IIa Mbn operons (19), which encode Mbns containing a sulfonated threonine (22, 25), and is predicted to perform that post-translational modification. An NAD(P)H-dependent flavoenzyme (MbnF) may play a role in heterocycle biosynthesis in some group I and II Mbns (19, 20), catalyzing the transformation of the oxazolone A to a (hydroxy)pyrazin(edi)one. The closest characterized relatives are monooxygenases that carry out hydroxylation reactions (46), but it is unclear whether MbnF modifies Mbn intermediates similarly. Alternatively, MbnF may be involved in an oxidation step in oxazolone biosynthesis (47), although Ms. trichosporium OB3b and Ms. sp. LW4 Mbn operons lack MbnFs, but produce oxazolone-containing Mbns (19, 21, 24).
Two cytoplasmic biosynthesis proteins have unpredicted roles. A gene annotated as a dioxygenase (mbnD) follows mbnF in group IIb operons, but no Mbns from these operons have been characterized so its role in Mbn biosynthesis is unclear (19). A distant relative of MbnB, MbnX, is encoded in group V operons, with mbnX immediately following mbnA and translationally coupled with mbnB, mbnC, and mbnM (19). In the absence of characterized Mbns encoded by these operons, the role of MbnX is unclear. Notably, no identifiable protease is conserved in Mbn operons. Cytoplasmic enzymes such as MbnN and MbnF are predicted to carry out modifications requiring prior leader peptide loss, meaning leader peptide loss must occur in the cytoplasm, but it is unknown when and how this loss occurs.
Finally, a pair of proteins encoded in many Mbn operons, MbnH, a di-heme cytochrome c peroxidase, and MbnP, a tryptophan-rich protein from no identifiable family, have also been proposed to play a role in oxazolone biosynthesis (47). However, these proteins have genes more closely associated with Mbn import machinery (19, 48), are periplasmic unlike all other cytoplasmic biosynthesis enzymes (20), and are not present in the operon of at least one characterized Mbn (24). Their function has not been investigated experimentally.
Mbn transport
Uptake of intact CuMbn was demonstrated using isotopic and fluorescent labeling, and competition experiments with apo Mbn provided evidence for the existence of a specific transporter (49). This transporter was hypothesized to belong to the TonB-dependent transporter (TBDT) family (41), members of which import siderophores and other compounds across bacterial outer membranes and into the periplasm (50), powered by the proton-motive force (51). Experiments using spermine as a passive transport inhibitor and carbonyl cyanide m-chlorophenylhydrazone as an active transport inhibitor confirmed that CuMbn is taken up actively via a process distinct from the passive and likely porin-dependent uptake of soluble copper compounds such as CuCl2 and CuSO4, although copper uptake via either pathway can increase cellular copper and affect copper-dependent gene regulation (49).
TonB-dependent transporters are present in four of the five Mbn operon groups (19). The exceptions are group V operons, which are predicted to produce divergent Mbns that may have roles other than copper uptake. TBDTs in Mbn operons belong to three distinct phylogenetic groups, of which none belong to known TBDT subfamilies (48). Group I Mbn operons encode MbnT1s with an N-terminal extension involved in trans-periplasmic interactions with inner-membrane anti-σ factors (52). Analogous to the FecIRA system, in which uptake of iron citrate through FecA (the TBDT) triggers an interaction with FecR (the anti-σ factor), which then activates FecI (the σ factor) to increase the expression of the FecIRA (and other) genes (53), MbnT1-mediated transport and regulation may be coupled (Fig. 3). MbnT1s are found primarily in methanotrophs and ammonia oxidizers, including many species that lack Mbn operons, suggesting that Mbn piracy may occur. The MbnT2s encoded in group II Mbn operons and the MbnT3s encoded in group III and IV operons lack N-terminal extensions and are not associated with regulatory components (48).
Figure 3.
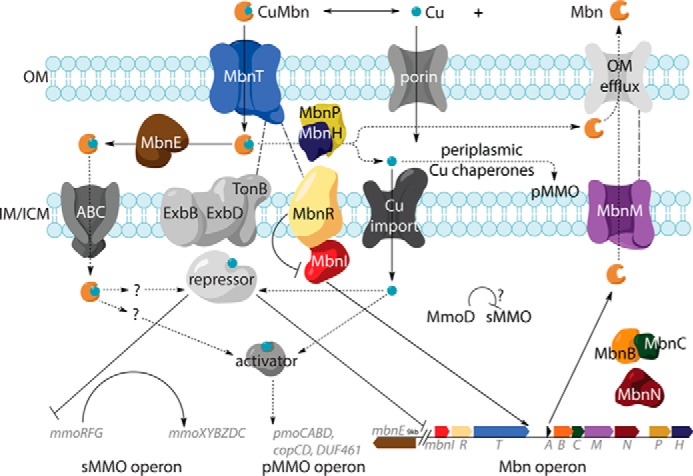
Schematic for Mbn biosynthesis, transport, and regulation in Ms. trichosporium OB3b.
MbnT function has been verified experimentally. Disruption of the Ms. trichosporium OB3b mbnT gene effectively eliminates import of CuMbn, but not soluble copper (48, 54), and heterologous expression of MbnT in E. coli enables these bacteria to take up CuMbn (48). Two other copper-repressed non-operon mbnIRTPH clusters are present in the Ms. trichosporium OB3b genome, but their regulatory patterns differ from the operon mbnIRTPH genes, and their products do not appear to substitute for the Mbn operon mbnIRT. Multiple MbnT homologues may offer separate uptake paths for non-native Mbns as in siderophore piracy. However, surface plasmon resonance experiments indicate that non-native Mbns can bind (if not necessarily be transported by) MbnTs (48).
Some group I and II Mbn operons encode periplasmic binding proteins (PBPs), termed MbnEs, that interact with periplasmic CuMbns (48). MbnEs are related to oligopeptide-binding PBPs like OppA and AppA, which are members of solute-binding protein family 5 (55) and are part of oligopeptide ABC transport systems, conveying peptides to inner membrane import systems after their initial uptake across the outer membrane and into the periplasm. Unlike genes for related PBPs such as yejA, whose product binds the peptidic natural product microcin C7 (56), mbnEs are copper-regulated like the Mbn operon, even if they are not in its immediate genomic proximity (48). The crystal structure of Mc. parvus OBBP MbnE (48) exhibits a substrate-binding cavity as large as that of the nonapeptide-binding AppA (57), consistent with a role in Mbn binding. Multiple heterologously expressed and immobilized MbnEs bind native Mbns, but unlike MbnTs, no binding of non-native Mbns is observed (48). Because mbnEs lack neighboring ABC transporter genes, it is unclear whether MbnEs share an ABC transport system with oligopeptide-binding PBPs or whether they play a role unrelated to cytoplasmic uptake of intact CuMbn.
The uncertainty regarding the fate of internalized Mbn extends to Mbn copper release. Some siderophores are degraded during metal release (58), but many siderophores are recycled, including pyoverdine, carboxymycobactin, and ferrichrome (59–61). For some siderophores, iron reduction allows proteins with higher Fe(II) affinities to remove the metal without metallophore modification (62). In Mbns, bound copper could conceivably be oxidized to Cu(II). Periplasmic copper proteins commonly encoded by Mbn operons, like CopC (63) and DUF461 (64), might ultimately bind the oxidized copper but are unlikely to be the oxidases. However, most Mbn group I–IV Mbn operons contain mbnH and mbnP (19). Their association with mbnT genes suggests a role related to Mbn import (19, 20) that could possibly involve copper release.
Mbn export is not yet well-characterized. Four of the five Mbn operon groups, including the divergent group V operons, contain genes encoding inner membrane efflux pumps, MbnMs, belonging to the multidrug and toxic compound extrusion (MATE) family (19). These H+/Na+ antiporters mediate the efflux of cationic xenobiotic compounds across the inner membrane and into the periplasm (65, 66), but are poorly understood, and their outer membrane partners are unidentified. A role for MATE proteins in the export of native natural products such as Mbns would be new but is suggested by the conservation of MbnM in the large majority of Mbn operons.
Regulation of Mbn in methanotrophs
In methanotroph copper homeostasis, sMMO and pMMO are reciprocally regulated by copper (the copper switch) (67). Expression and proteomic analysis of multiple species support significant down-regulation of sMMO in the presence of copper, whereas most studies show that pMMO is mildly up-regulated (67–71). Because Mbn secretion was first observed at low copper in wild-type methanotrophs (72, 73) or in variant strains with a constitutively copper-starved phenotype (74, 75), and because there should be no need for Mbn production in the presence of abundant bioavailable copper, the Mbn operon was expected to be copper-repressed. An extensive set of qRT-PCR experiments, involving several time points after the addition of copper to copper-starved Ms. trichosporium OB3b cells, confirmed that the entire Mbn operon in that species is copper-regulated, along with the mbnE gene, despite separation from the main operon (71). Furthermore, the Mbn and sMMO operons are co-regulated, with swift co-repression of the regulatory genes followed by a slower decrease in main operon transcription, perhaps as existing regulatory proteins degrade and are not replaced. Additional studies identified significant but non-identical copper down-regulation patterns in non-operon mbnIRTPH gene clusters (48). Recent RNA-sequencing studies under low- and high-copper conditions lack the time resolution of the qRT-PCR studies but support these expression patterns (76).
The regulatory protein(s) that repress the Mbn and sMMO operons in the presence of copper have not been identified. CuMbns may be a direct signaling factor in species with MbnT1s, but the copper switch occurs in organisms lacking Mbn operons, ruling out involvement of anything Mbn-related (including MbnI and Mbn/CuMbn itself (47)) as the copper switch regulator. MmoD, a protein of unknown function encoded in the sMMO operon, has also been suggested as a regulator, potentially in tandem with Mbn/CuMbn (42, 47). However, biological support for this hypothesis relies on knockouts of most of the sMMO operon (42), or of MmoD alone (77), and in vitro biochemical evidence suggests that MmoD interacts with and affects the activity of sMMO (78). Knockout of major metabolic enzymes can cause significant metabolic rewiring (79), so the extent to which phenotypic effects reflect copper switch perturbation versus disruption of sMMO activity remains unclear. There is also no evidence that MmoD binds DNA, copper, or Mbn/CuMbn (71, 80). Despite claims to the contrary (47), MmoD transcription is strongly copper-repressed in several species (70, 71, 76), which is incompatible with several regulatory schemes. Finally, the overlap between species producing sMMO, pMMO, and Mbn is quite small (20). A yet-to-be-identified copper-responsive regulator remains the most likely candidate for the copper switch in methanotrophs (Fig. 3). Regulation of Mbn operons in non-methanotrophs has yet to be investigated.
Broader roles for Mbns
Mbn research has historically focused on its role in methanotroph copper homeostasis. However, most Mbn operons are found in non-methanotrophs and remain unstudied. Nevertheless, the content of non-methanotrophic group III and IV Mbn operons supports a role in copper homeostasis (20). CopC (a periplasmic copper-binding protein) and CopD (an inner-membrane copper transporter) are involved in copper uptake (81) and are encoded in many Mbn operons. Genes encoding other periplasmic copper-binding proteins are also frequently present, including Sco1 (commonly involved in cytochrome c oxidase copper loading (82)) as well as the poorly understood DUF461 (83) and DUF2946 proteins, the latter of which is TBDT-associated. The presence of so many genes encoding periplasmic copper-binding proteins in Mbn operons is suggestive of a role in copper homeostasis for non-methanotrophs. By contrast, group V operons lack both importers and copper-binding proteins (19), suggesting that these Mbns may have a completely different function, perhaps acting as antibiotics.
Mbns might also play a role in protection against toxicity of metal ions other than copper. Copper binding by the siderophore yersiniabactin is believed to shield pathogens from copper toxicity during infection (84). Mbn chelation of Hg(II) and Au(III) has been proposed to have a similar function (39, 85). However, it is unclear whether this broader-spectrum metal binding is biologically relevant for most methanotrophs, which live in a wide range of environments, most of which are not contaminated with heavy metals. Similar caveats apply to methylmercury demethylation, in which Mbn has been proposed to play a role, possibly replacing MerA via reductive binding (86). Mbn-mediated production of gold nanoparticles has also been reported (87–89). Sequestration of toxic gold via nanoparticle production is observed in several species (90) and can be mediated by the natural product delftibactin in Delftia avidovorans (91). It is conceivable that Mbn-derived nanoparticle production is a means of defense against unwanted metals. Finally, CuMbn has been reported to exhibit superoxide dismutase, oxidase, and hydrogen peroxide reductase activities (92). Because bacterial secretion of superoxide can be a source of environmental oxidative stress (93), extracellular superoxide dismutase activity mediated by secreted natural products may be biologically relevant.
In terms of potential applications, CuMbn from Ms. trichosporium OB3b has been reported to exhibit antibiotic activity against Gram-positive bacteria (94). Mbn has also been investigated as a treatment for Wilson disease, a human disorder of impaired copper efflux and toxic copper accumulation (95). Existing treatments are limited, and most have significant side effects, fail to liberate some bound forms of copper, or bind problematic amounts of other biologically relevant metals (96). In a rat model, Mbn reversed the acute liver failure associated with copper overload (97). Mbns or Mbn analogues are thus of significant interest as copper-chelating drug candidates. Once it is better understood, the modular Mbn RiPP biosynthetic machinery can be deployed to produce non-natural Mbns, using rational design and high-throughput screening to adjust chemical properties in a search for bioactive compounds. Similar techniques have already been used in other RiPP systems, including the cyanobactins (98).
Conclusions
Mbns play a key role in methanotroph copper homeostasis, and efforts to elucidate that role are important for attempts to bioengineer these organisms. However, it is clear that copper uptake mediated by these compounds is relevant far beyond methanotrophs. Future Mbn research will require a reassessment of bacterial copper homeostasis, both in the broader environment and at the host–pathogen interface. Characterization of Mbns from a wider range of species will yield additional dividends as new post-translational modifications and biosynthetic mechanisms are identified, and any resulting bioactive compounds are investigated as drug candidates and ultimately re-engineered for increased activity. The history of Mbns may be defined by methanotrophy, but their future lies in the broader bacterial world.
This work was supported by National Institutes of Health Grant GM118035 and Department of Energy Grant DE-SC0016284 (to A. C. R.). This is the first article in the Thematic Minireview series “Metals in Biology 2018: Copper homeostasis and utilization in redox enzymes.” The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and Department of Energy.
- Mbn
- methanobactin
- CuMbn
- copper-loaded Mbn
- sMMO
- soluble methane monooxygenase
- pMMO
- particulate methane monooxygenase
- RiPP
- ribosomally produced, post-translationally modified natural product
- RRE
- RiPP recognition element
- TBDT
- TonB-dependent transporter
- PBP
- periplasmic binding protein
- MATE
- multidrug and toxic compound extrusion
- qRT-PCR
- quantitative RT-PCR.
References
- 1. Thomson A. J., and Gray H. B. (1998) Bio-inorganic chemistry. Curr. Opin. Chem. Biol. 2, 155–158 10.1016/S1367-5931(98)80056-2 [DOI] [PubMed] [Google Scholar]
- 2. Valko M., Morris H., and Cronin M. T. (2005) Metals, toxicity and oxidative stress. Curr. Med. Chem. 12, 1161–1208 10.2174/0929867053764635 [DOI] [PubMed] [Google Scholar]
- 3. Raymond K. N., and Carrano C. J. (1979) Coordination chemistry and microbial iron transport. Acc. Chem. Res. 12, 183–190 10.1021/ar50137a004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miethke M., and Marahiel M. A. (2007) Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71, 413–451 10.1128/MMBR.00012-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnstone T. C., and Nolan E. M. (2015) Beyond iron: non-classical biological functions of bacterial siderophores. Dalton Trans. 44, 6320–6339 10.1039/C4DT03559C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Springer S. D., and Butler A. (2016) Microbial ligand coordination: Consideration of biological significance. Coord. Chem. Rev. 306, 628–635 10.1016/j.ccr.2015.03.013 [DOI] [Google Scholar]
- 7. Hanson R. S., and Hanson T. E. (1996) Methanotrophic bacteria. Microbiol. Rev. 60, 439–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sazinsky M. H., and Lippard S. J. (2015) Methane monooxygenase: functionalizing methane at iron and copper. Met. Ions Life Sci. 15, 205–256 [DOI] [PubMed] [Google Scholar]
- 9. Semrau J. D., DiSpirito A. A., and Yoon S. (2010) Methanotrophs and copper. FEMS Microbiol. Rev. 34, 496–531 10.1111/j.1574-6976.2010.00212.x [DOI] [PubMed] [Google Scholar]
- 10. Scott D. C., Brannan J., and Higgins I. J. (1981) The effect of growth conditions on intracytoplasmic membranes and methane mono-oxygenase activities in Methylosinus trichosporium OB3b. J. Gen. Microbiol. 125, 63–72 10.1099/00221287-125-1-63 [DOI] [Google Scholar]
- 11. Prior S. D., and Dalton H. (1985) The effect of copper ions on membrane content and methane monooxygenase activity in methanol-grown cells of Methylococcus capsulatus (Bath). Microbiology 131, 155–163 10.1099/00221287-131-1-155 [DOI] [Google Scholar]
- 12. Martinho M., Choi D. W., Dispirito A. A., Antholine W. E., Semrau J. D., and Münck E. (2007) Mössbauer studies of the membrane-associated methane monooxygenase from Methylococcus capsulatus Bath: evidence for a diiron center. J. Am. Chem. Soc. 129, 15783–15785 10.1021/ja077682b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balasubramanian R., Smith S. M., Rawat S., Yatsunyk L. A., Stemmler T. L., and Rosenzweig A. C. (2010) Oxidation of methane by a biological dicopper centre. Nature 465, 115–119 10.1038/nature08992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Festa R. A., and Thiele D. J. (2011) Copper: An essential metal in biology. Curr. Biol. 21, R877–R883 10.1016/j.cub.2011.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karlsen O. A., Berven F. S., Stafford G. P., Larsen Ø., Murrell J. C., Jensen H. B., and Fjellbirkeland A. (2003) The surface-associated and secreted MopE protein of Methylococcus capsulatus (Bath) responds to changes in the concentration of copper in the growth medium. Appl. Environ. Microbiol. 69, 2386–2388 10.1128/AEM.69.4.2386-2388.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kraemer S. M., Duckworth O. W., Harrington J. M., and Schenkeveld W. D. (2014) Metallophores and trace metal biogeochemistry. Aquat. Geochem. 21, 159–195 10.1007/s10498-015-9263-1 [DOI] [Google Scholar]
- 17. Kim H. J., Graham D. W., DiSpirito A. A., Alterman M. A., Galeva N., Larive C. K., Asunskis D., and Sherwood P. M. (2004) Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science 305, 1612–1615 10.1126/science.1098322 [DOI] [PubMed] [Google Scholar]
- 18. Arnison P. G., Bibb M. J., Bierbaum G., Bowers A. A., Bugni T. S., Bulaj G., Camarero J. A., Campopiano D. J., Challis G. L., Clardy J., Cotter P. D., Craik D. J., Dawson M., Dittmann E., Donadio S., et al. (2013) Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160 10.1039/C2NP20085F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kenney G. E., and Rosenzweig A. C. (2013) Genome mining for methanobactins. BMC Biol. 11, 17 10.1186/1741-7007-11-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dassama L. M., Kenney G. E., and Rosenzweig A. C. (2017) Methanobactins: from genome to function. Metallomics 9, 7–20 10.1039/c6mt00208k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Behling L. A., Hartsel S. C., Lewis D. E., DiSpirito A. A., Choi D. W., Masterson L. R., Veglia G., and Gallagher W. H. (2008) NMR, mass spectrometry and chemical evidence reveal a different chemical structure for methanobactin that contains oxazolone rings. J. Am. Chem. Soc. 130, 12604–12605 10.1021/ja804747d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krentz B. D., Mulheron H. J., Semrau J. D., Dispirito A. A., Bandow N. L., Haft D. H., Vuilleumier S., Murrell J. C., McEllistrem M. T., Hartsel S. C., and Gallagher W. H. (2010) A comparison of methanobactins from Methylosinus trichosporium OB3b and Methylocystis strain SB2 predicts methanobactins are synthesized from diverse peptide precursors modified to create a common core for binding and reducing copper ions. Biochemistry 49, 10117–10130 10.1021/bi1014375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El Ghazouani A., Baslé A., Firbank S. J., Knapp C. W., Gray J., Graham D. W., and Dennison C. (2011) Copper-binding properties and structures of methanobactins from Methylosinus trichosporium OB3b. Inorg. Chem. 50, 1378–1391 10.1021/ic101965j [DOI] [PubMed] [Google Scholar]
- 24. Kenney G. E., Goering A. W., Ross M. O., DeHart C. J., Thomas P. M., Hoffman B. M., Kelleher N. L., and Rosenzweig A. C. (2016) Characterization of methanobactin from Methylosinus sp. LW4. J. Am. Chem. Soc. 138, 11124–11127 10.1021/jacs.6b06821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El Ghazouani A., Baslé A., Gray J., Graham D. W., Firbank S. J., and Dennison C. (2012) Variations in methanobactin structure influences copper utilization by methane-oxidizing bacteria. Proc. Natl. Acad. Sci. U.S.A. 109, 8400–8404 10.1073/pnas.1112921109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim H. J., Galeva N., Larive C. K., Alterman M., and Graham D. W. (2005) Purification and physical-chemical properties of methanobactin: a chalkophore from Methylosinus trichosporium OB3b. Biochemistry 44, 5140–5148 10.1021/bi047367r [DOI] [PubMed] [Google Scholar]
- 27. Choi D. W., Zea C. J., Do Y. S., Semrau J. D., Antholine W. E., Hargrove M. S., Pohl N. L., Boyd E. S., Geesey G. G., Hartsel S. C., Shafe P. H., McEllistrem M. T., Kisting C. J., Campbell D., Rao V., et al. (2006) Spectral, kinetic, and thermodynamic properties of Cu(I) and Cu(II) binding by methanobactin from Methylosinus trichosporium OB3b. Biochemistry 45, 1442–1453 10.1021/bi051815t [DOI] [PubMed] [Google Scholar]
- 28. Bandow N., Gilles V. S., Freesmeier B., Semrau J. D., Krentz B., Gallagher W., McEllistrem M. T., Hartsel S. C., Choi D. W., Hargrove M. S., Heard T. M., Chesner L. N., Braunreiter K. M., Cao B. V., Gavitt M. M., et al. (2012) Spectral and copper binding properties of methanobactin from the facultative methanotroph Methylocystis strain SB2. J. Inorg. Biochem. 110, 72–82 10.1016/j.jinorgbio.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 29. Pesch M.-L., Christl I., Hoffmann M., Kraemer S. M., and Kretzschmar R. (2012) Copper complexation of methanobactin isolated from Methylosinus trichosporium OB3b: pH-dependent speciation and modeling. J. Inorg. Biochem. 116, 55–62 10.1016/j.jinorgbio.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 30. Pesch M.-L., Hoffmann M., Christl I., Kraemer S. M., and Kretzschmar R. (2013) Competitive ligand exchange between Cu-humic acid complexes and methanobactin. Geobiology 11, 44–54 10.1111/gbi.12010 [DOI] [PubMed] [Google Scholar]
- 31. Knapp C. W., Fowle D. A., Kulczycki E., Roberts J. A., and Graham D. W. (2007) Methane monooxygenase gene expression mediated by methanobactin in the presence of mineral copper sources. Proc. Natl. Acad. Sci. U.S.A. 104, 12040–12045 10.1073/pnas.0702879104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kulczycki E., Fowle D. A., Knapp C. W., Graham D. W., and Roberts J. A. (2007) Methanobactin-promoted dissolution of Cu-substituted borosilicate glass. Geobiology 5, 251–263 10.1111/j.1472-4669.2007.00102.x [DOI] [Google Scholar]
- 33. Kulczycki E., Fowle D. A., Kenward P. A., Leslie K., Graham D. W., and Roberts J. A. (2011) Stimulation of methanotroph activity by Cu-substituted borosilicate glass. Geomicrobiol. J. 28, 1–10 10.1080/01490451003614971 [DOI] [Google Scholar]
- 34. Hakemian A. S., Tinberg C. E., Kondapalli K. C., Telser J., Hoffman B. M., Stemmler T. L., and Rosenzweig A. C. (2005) The copper chelator methanobactin from Methylosinus trichosporium OB3b binds copper(I). J. Am. Chem. Soc. 127, 17142–17143 10.1021/ja0558140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi D. W., Bandow N. L., McEllistrem M. T., Semrau J. D., Antholine W. E., Hartsel S. C., Gallagher W., Zea C. J., Pohl N. L., Zahn J. A., and DiSpirito A. A. (2010) Spectral and thermodynamic properties of methanobactin from γ-proteobacterial methane oxidizing bacteria: a case for copper competition on a molecular level. J. Inorg. Biochem. 104, 1240–1247 10.1016/j.jinorgbio.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 36. Choi D. W., Do Y. S., Zea C. J., McEllistrem M. T., Lee S.-W., Semrau J. D., Pohl N. L., Kisting C. J., Scardino L. L., Hartsel S. C., Boyd E. S., Geesey G. G., Riedel T. P., Shafe P. H., Kranski K. A., et al. (2006) Spectral and thermodynamic properties of Ag(I), Au(III), Cd(II), Co(II), Fe(III), Hg(II), Mn(II), Ni(II), Pb(II), U(IV), and Zn(II) binding by methanobactin from Methylosinus trichosporium OB3b. J. Inorg. Biochem. 100, 2150–2161 10.1016/j.jinorgbio.2006.08.017 [DOI] [PubMed] [Google Scholar]
- 37. McCabe J. W., Vangala R., and Angel L. A. (2017) Binding selectivity of methanobactin from Methylosinus trichosporium OB3b for copper(I), silver(I), zinc(II), nickel(II), cobalt(II), manganese(II), lead(II), and iron(II). J. Am. Soc. Mass Spectrom. 28, 2588–2601 [DOI] [PubMed] [Google Scholar]
- 38. Kalidass B., Ul-Haque M. F., Baral B. S., DiSpirito A. A., and Semrau J. D. (2015) Competition between metals for binding to methanobactin enables expression of soluble methane monooxygenase in the presence of copper. Appl. Environ. Microbiol. 81, 1024–1031 10.1128/AEM.03151-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baral B. S., Bandow N. L., Vorobev A., Freemeier B. C., Bergman B. H., Herdendorf T. J., Fuentes N., Ellias L., Turpin E., Semrau J. D., and DiSpirito A. A. (2014) Mercury binding by methanobactin from Methylocystis strain SB2. J. Inorg. Biochem. 141, 161–169 10.1016/j.jinorgbio.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 40. DiSpirito A. A., Zahn J. A., Graham D. W., Kim H. J., Alterman M. A., and Larive C. K. (April 3, 2007) Methanobactin: a copper binding compound having antibiotic and antioxidant activity isolated from methanotrophic bacteria, U. S. Patent 7199099 B2 [Google Scholar]
- 41. Balasubramanian R., and Rosenzweig A. C. (2008) Copper methanobactin: a molecule whose time has come. Curr. Opin. Chem. Biol. 12, 245–249 10.1016/j.cbpa.2008.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Semrau J. D., Jagadevan S., DiSpirito A. A., Khalifa A., Scanlan J., Bergman B. H., Freemeier B. C., Baral B. S., Bandow N. L., Vorobev A., Haft D. H., Vuilleumier S., and Murrell J. C. (2013) Methanobactin and MmoD work in concert to act as the “copper-switch” in methanotrophs. Environ. Microbiol. 15, 3077–3086 10.1111/1462-2920.12150 [DOI] [PubMed] [Google Scholar]
- 43. Haft D. H., Selengut J. D., Richter R. A., Harkins D., Basu M. K., and Beck E. (2013) TIGRFAMs and genome properties in 2013. Nucleic Acids Res. 41, D387–D395 10.1093/nar/gks1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burkhart B. J., Hudson G. A., Dunbar K. L., and Mitchell D. A. (2015) A prevalent peptide-binding domain guides ribosomal natural product biosynthesis. Nat. Chem. Biol. 11, 564–570 10.1038/nchembio.1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gu W., Baral B. S., DiSpirito A. A., and Semrau J. D. (2017) An aminotransferase is responsible for the deamination of the N-terminal leucine and required for formation of oxazolone ring A in methanobactin of Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 83, e02619–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Groom K., Bhattacharya A., and Zechel D. L. (2011) Rebeccamycin and staurosporine biosynthesis: insight into the mechanisms of the flavin-dependent monooxygenases RebC and StaC. ChemBioChem 12, 396–400 10.1002/cbic.201000580 [DOI] [PubMed] [Google Scholar]
- 47. DiSpirito A. A., Semrau J. D., Murrell J. C., Gallagher W. H., Dennison C., and Vuilleumier S. (2016) Methanobactin and the link between copper and bacterial methane oxidation. Microbiol. Mol. Biol. Rev. 80, 387–409 10.1128/MMBR.00058-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dassama L. M., Kenney G. E., Ro S. Y., Zielazinski E. L., and Rosenzweig A. C. (2016) Methanobactin transport machinery. Proc. Natl. Acad. Sci. U.S.A. 113, 13027–13032 10.1073/pnas.1603578113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Balasubramanian R., Kenney G. E., and Rosenzweig A. C. (2011) Dual pathways for copper uptake by methanotrophic bacteria. J. Biol. Chem. 286, 37313–37319 10.1074/jbc.M111.284984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schauer K., Rodionov D. A., and de Reuse H. (2008) New substrates for TonB-dependent transport: do we only see the 'tip of the iceberg'? Trends Biochem. Sci. 33, 330–338 10.1016/j.tibs.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 51. Noinaj N., Guillier M., Barnard T. J., and Buchanan S. K. (2010) TonB-dependent transporters: regulation, structure, and function. Annu. Rev. Microbiol. 64, 43–60 10.1146/annurev.micro.112408.134247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koebnik R. (2005) TonB-dependent trans-envelope signalling: the exception or the rule? Trends Microbiol. 13, 343–347 10.1016/j.tim.2005.06.005 [DOI] [PubMed] [Google Scholar]
- 53. Ochs M., Angerer A., Enz S., and Braun V. (1996) Surface signaling in transcriptional regulation of the ferric citrate transport system of Escherichia coli: mutational analysis of the alternative sigma factor FecI supports its essential role in fec transport gene transcription. Mol. Gen. Genet. 250, 455–465 [DOI] [PubMed] [Google Scholar]
- 54. Gu W., Farhan Ul Haque M., Baral B. S., Turpin E. A., Bandow N. L., Kremmer E., Flatley A., Zischka H., DiSpirito A. A., and Semrau J. D. (2016) A TonB-dependent transporter is responsible for methanobactin uptake by Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 82, 1917–1923 10.1128/AEM.03884-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Berntsson R. P., Smits S. H., Schmitt L., Slotboom D.-J., and Poolman B. (2010) A structural classification of substrate-binding proteins. FEBS Lett. 584, 2606–2617 10.1016/j.febslet.2010.04.043 [DOI] [PubMed] [Google Scholar]
- 56. Novikova M., Metlitskaya A., Datsenko K., Kazakov T., Kazakov A., Wanner B., and Severinov K. (2007) The Escherichia coli Yej transporter is required for the uptake of translation inhibitor microcin C. J. Bacteriol. 189, 8361–8365 10.1128/JB.01028-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levdikov V. M., Blagova E. V., Brannigan J. A., Wright L., Vagin A. A., and Wilkinson A. J. (2005) The structure of the oligopeptide-binding protein, AppA, from Bacillus subtilis in complex with a nonapeptide. J. Mol. Biol. 345, 879–892 10.1016/j.jmb.2004.10.089 [DOI] [PubMed] [Google Scholar]
- 58. Schalk I. J., and Guillon L. (2013) Fate of ferrisiderophores after import across bacterial outer membranes: different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways. Amino Acids 44, 1267–1277 10.1007/s00726-013-1468-2 [DOI] [PubMed] [Google Scholar]
- 59. Imperi F., Tiburzi F., and Visca P. (2009) Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 106, 20440–20445 10.1073/pnas.0908760106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jones C. M., Wells R. M., Madduri A. V., Renfrow M. B., Ratledge C., Moody D. B., and Niederweis M. (2014) Self-poisoning of Mycobacterium tuberculosis by interrupting siderophore recycling. Proc. Natl. Acad. Sci. U.S.A. 111, 1945–1950 10.1073/pnas.1311402111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hannauer M., Barda Y., Mislin G. L., Shanzer A., and Schalk I. J. (2010) The ferrichrome uptake pathway in Pseudomonas aeruginosa involves an iron release mechanism with acylation of the siderophore and recycling of the modified desferrichrome. J. Bacteriol. 192, 1212–1220 10.1128/JB.01539-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miethke M., Hou J., and Marahiel M. A. (2011) The siderophore-interacting protein YqjH acts as a ferric reductase in different iron assimilation pathways of Escherichia coli. Biochemistry 50, 10951–10964 10.1021/bi201517h [DOI] [PubMed] [Google Scholar]
- 63. Lawton T. J., Kenney G. E., Hurley J. D., and Rosenzweig A. C. (2016) The CopC family: structural and bioinformatic insights into a diverse group of periplasmic copper binding proteins. Biochemistry 55, 2278–2290 10.1021/acs.biochem.6b00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Thompson A. K., Gray J., Liu A., and Hosler J. P. (2012) The roles of Rhodobacter sphaeroides copper chaperones PCuAC and Sco (PrrC) in the assembly of the copper centers of the aa3-type and the cbb3-type cytochrome c oxidases. Biochim. Biophys. Acta 1817, 955–964 10.1016/j.bbabio.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Omote H., Hiasa M., Matsumoto T., Otsuka M., and Moriyama Y. (2006) The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol. Sci. 27, 587–593 10.1016/j.tips.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 66. Kuroda T., and Tsuchiya T. (2009) Multidrug efflux transporters in the MATE family. Biochim. Biophys. Acta 1794, 763–768 10.1016/j.bbapap.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 67. Nielsen A. K., Gerdes K., and Murrell J. C. (1997) Copper-dependent reciprocal transcriptional regulation of methane monooxygenase genes in Methylococcus capsulatus and Methylosinus trichosporium. Mol. Microbiol. 25, 399–409 10.1046/j.1365-2958.1997.4801846.x [DOI] [PubMed] [Google Scholar]
- 68. Nielsen A. K., Gerdes K., Degn H., and Murrell J. C. (1996) Regulation of bacterial methane oxidation: transcription of the soluble methane mono-oxygenase operon of Methylococcus capsulatus (Bath) is repressed by copper ions. Microbiology 142, 1289–1296 10.1099/13500872-142-5-1289 [DOI] [PubMed] [Google Scholar]
- 69. Kao W.-C., Chen Y.-R., Yi E. C., Lee H., Tian Q., Wu K.-M., Tsai S.-F., Yu S. S., Chen Y.-J., Aebersold R., and Chan S. I. (2004) Quantitative proteomic analysis of metabolic regulation by copper ions in Methylococcus capsulatus (Bath). J. Biol. Chem. 279, 51554–51560 10.1074/jbc.M408013200 [DOI] [PubMed] [Google Scholar]
- 70. Larsen Ø., and Karlsen O. A. (2016) Transcriptomic profiling of Methylococcus capsulatus (Bath) during growth with two different methane monooxygenases. MicrobiologyOpen 5, 254–267 10.1002/mbo3.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kenney G. E., Sadek M., and Rosenzweig A. C. (2016) Copper-responsive gene expression in the methanotroph Methylosinus trichosporium OB3b. Metallomics 8, 931–940 10.1039/C5MT00289C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Téllez C. M., Gaus K. P., Graham D. W., Arnold R. G., and Guzman R. Z. (1998) Isolation of copper biochelates from Methylosinus trichosporium OB3b and soluble methane monooxygenase mutants. Appl. Environ. Microbiol. 64, 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. DiSpirito A. A., Zahn J. A., Graham D. W., Kim H. J., Larive C. K., Derrick T. S., Cox C. D., and Taylor A. (1998) Copper-binding compounds from Methylosinus trichosporium OB3b. J. Bacteriol. 180, 3606–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Phelps P. A., Agarwal S. K., Speitel G. E., and Georgiou G. (1992) Methylosinus trichosporium OB3b mutants having constitutive expression of soluble methane monooxygenase in the presence of high levels of copper. Appl. Environ. Microbiol. 58, 3701–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fitch M. W., Graham D. W., Arnold R. G., Agarwal S. K., Phelps P., Speitel G. E. Jr., and Georgiou G. (1993) Phenotypic characterization of copper-resistant mutants of Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 59, 2771–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gu W., and Semrau J. D. (2017) Copper and cerium-regulated gene expression in Methylosinus trichosporium OB3b. Appl. Microbiol. Biotechnol. 101, 8499–8516 10.1007/s00253-017-8572-2 [DOI] [PubMed] [Google Scholar]
- 77. Yan X., Chu F., Puri A. W., Fu Y., and Lidstrom M. E. (2016) Electroporation-based genetic manipulation in Type I methanotrophs. Appl. Environ. Microbiol. 82, 2062–2069 10.1128/AEM.03724-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Merkx M., and Lippard S. J. (2002) Why OrfY? Characterization of MMOD, a long overlooked component of the soluble methane monooxygenase from Methylococcus capsulatus (Bath). J. Biol. Chem. 277, 5858–5865 10.1074/jbc.M107712200 [DOI] [PubMed] [Google Scholar]
- 79. Long C. P., Gonzalez J. E., Feist A. M., Palsson B. O., and Antoniewicz M. R. (2018) Dissecting the genetic and metabolic mechanisms of adaptation to the knockout of a major metabolic enzyme in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 115, 222–227 10.1073/pnas.1716056115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kalidass B. (2015) Investigating the Impact of Metals and Methanobactin on Gene Expression in Methylosinus trichosporium OB3b. Ph.D. thesis, University of Michigan, Ann Arbor, MI [Google Scholar]
- 81. Chillappagari S., Miethke M., Trip H., Kuipers O. P., and Marahiel M. A. (2009) Copper acquisition is mediated by YcnJ and regulated by YcnK and CsoR in Bacillus subtilis. J. Bacteriol. 191, 2362–2370 10.1128/JB.01616-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lohmeyer E., Schröder S., Pawlik G., Trasnea P.-I., Peters A., Daldal F., and Koch H.-G. (2012) The ScoI homologue SenC is a copper binding protein that interacts directly with the cbb3-type cytochrome oxidase in Rhodobacter capsulatus. Biochim. Biophys. Acta 1817, 2005–2015 10.1016/j.bbabio.2012.06.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ekici S., Pawlik G., Lohmeyer E., Koch H.-G., and Daldal F. (2012) Biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. Biochim. Biophys. Acta 1817, 898–910 10.1016/j.bbabio.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chaturvedi K. S., Hung C. S., Crowley J. R., Stapleton A. E., and Henderson J. P. (2012) The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat. Chem. Biol. 8, 731–736 10.1038/nchembio.1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vorobev A., Jagadevan S., Baral B. S., Dispirito A. A., Freemeier B. C., Bergman B. H., Bandow N. L., and Semrau J. D. (2013) Detoxification of mercury by methanobactin from Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 79, 5918–5926 10.1128/AEM.01673-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lu X., Gu W., Zhao L., Farhan Ul., Haque M., DiSpirito A. A., Semrau J. D., and Gu B. (2017) Methylmercury uptake and degradation by methanotrophs. Sci. Adv. 3, e1700041 10.1126/sciadv.1700041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Xin J.-Y., Cheng D.-D., Zhang L.-X., Lin K., Fan H.-C., Wang Y., and Xia C.-G. (2013) Methanobactin-mediated one-step synthesis of gold nanoparticles. Int. J. Mol. Sci. 14, 21676–21688 10.3390/ijms141121676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xin J.-Y., Lin K., Wang Y., and Xia C.-G. (2014) Methanobactin-mediated synthesis of gold nanoparticles supported over Al2O3 toward an efficient catalyst for glucose oxidation. Int. J. Mol. Sci. 15, 21603–21620 10.3390/ijms151221603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xin J.-Y., Zhang L.-X., Chen D.-D., Lin K., Fan H.-C., Wang Y., and Xia C.-G. (2015) Colorimetric detection of melamine based on methanobactin-mediated synthesis of gold nanoparticles. Food Chem. 174, 473–479 10.1016/j.foodchem.2014.11.098 [DOI] [PubMed] [Google Scholar]
- 90. Reith F., Etschmann B., Grosse C., Moors H., Benotmane M. A., Monsieurs P., Grass G., Doonan C., Vogt S., Lai B., Martinez-Criado G., George G. N., Nies D. H., Mergeay M., Pring A., et al. (2009) Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc. Natl. Acad. Sci. U.S.A. 106, 17757–17762 10.1073/pnas.0904583106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Johnston C. W., Wyatt M. A., Li X., Ibrahim A., Shuster J., Southam G., and Magarvey N. A. (2013) Gold biomineralization by a metallophore from a gold-associated microbe. Nat. Chem. Biol. 9, 241–243 10.1038/nchembio.1179 [DOI] [PubMed] [Google Scholar]
- 92. Choi D. W., Semrau J. D., Antholine W. E., Hartsel S. C., Anderson R. C., Carey J. N., Dreis A. M., Kenseth E. M., Renstrom J. M., Scardino L. L., Van Gorden G. S., Volkert A. A., Wingad A. D., Yanzer P. J., McEllistrem M. T., et al. (2008) Oxidase, superoxide dismutase, and hydrogen peroxide reductase activities of methanobactin from types I and II methanotrophs. J. Inorg. Biochem. 102, 1571–1580 10.1016/j.jinorgbio.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 93. Diaz J. M., Hansel C. M., Voelker B. M., Mendes C. M., Andeer P. F., and Zhang T. (2013) Widespread production of extracellular superoxide by heterotrophic bacteria. Science 340, 1223–1226 10.1126/science.1237331 [DOI] [PubMed] [Google Scholar]
- 94. Johnson C. L. (2006) Methanobactin: a potential novel biopreservative for use against the foodborne pathogen Listeria monocytogenes. Ph.D. thesis, Iowa State University, Ames, IA [Google Scholar]
- 95. Summer K. H., Lichtmannegger J., Bandow N., Choi D. W., DiSpirito A. A., and Michalke B. (2011) The biogenic methanobactin is an effective chelator for copper in a rat model for Wilson disease. J. Trace Elem. Med. Biol. 25, 36–41 10.1016/j.jtemb.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 96. Delangle P., and Mintz E. (2012) Chelation therapy in Wilson's disease: from d-penicillamine to the design of selective bioinspired intracellular Cu(I) chelators. Dalton Trans. 41, 6359–6370 10.1039/c2dt12188c [DOI] [PubMed] [Google Scholar]
- 97. Lichtmannegger J., Leitzinger C., Wimmer R., Schmitt S., Schulz S., Kabiri Y., Eberhagen C., Rieder T., Janik D., Neff F., Straub B. K., Schirmacher P., DiSpirito A. A., Bandow N., Baral B. S., et al. (2016) Methanobactin reverses acute liver failure in a rat model of Wilson disease. J. Clin. Invest. 126, 2721–2735 10.1172/JCI85226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sardar D., Lin Z., and Schmidt E. W. (2015) Modularity of RiPP enzymes enables designed synthesis of decorated peptides. Chem. Biol. 22, 907–916 10.1016/j.chembiol.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]