Abstract
Activation of TGF-β1 initiates a program of temporary collagen accumulation important to wound repair in many organs. However, the outcome of temporary extracellular matrix strengthening all too frequently morphs into progressive fibrosis, contributing to morbidity and mortality worldwide. To avoid this maladaptive outcome, TGF-β1 signaling is regulated at numerous levels and intimately connected to feedback signals that limit accumulation. Here, we examine the current understanding of the core functions of TGF-β1 in promoting collagen accumulation, parallel pathways that promote physiological repair, and pathological triggers that tip the balance toward progressive fibrosis. Implicit in better understanding of these processes is the identification of therapeutic opportunities that will need to be further advanced to limit or reverse organ fibrosis.
Commonly affecting many organs including lung, heart, kidney, and liver, tissue fibrosis is a leading cause of morbidity and mortality worldwide (Friedman et al. 2013). Yet the capacity for rapid wound repair with collagen accumulation facilitates restoration of tissue strength as well as tissue integrity in many injury situations. For example, early cardiac fibrosis after myocardial infarction is important for protection against fatal cardiac rupture (Hofmann et al. 2012). In broader terms, the capacity of cells to secrete and adhere to a collagenous extracellular matrix (ECM) must be a strong evolutionary force for advantage because virtually all metazoans express genes for fibrillar collagens (Hynes 2012). In mammals, the potential role of transforming growth factor (TGF)-β1 in physiological repair and collagen accumulation was recognized soon after the discovery of TGF-β1 expression in cancer cell lines (Moses et al. 2016). TGF-β1 was found to promote fibronectin and collagen production by both epithelial and mesenchymal cells in culture, and to do so by transcriptional activation of the relevant genes (Ignotz and Massagué 1986). Additionally, subcutaneous injection of TGF-β1 was seen to strongly promote collagen accumulation and a fibrotic tissue response (Roberts et al. 1986). These observations and several subsequent lines of evidence emerged solidifying the role of TGF-β1 as a master regulator of ECM accumulation and consequently a potential key driver of fibrosis (Bartram and Speer 2004). TGF-β1 is also intricately involved in regulating inflammation (Shull et al. 1992; Kulkarni et al. 1993). Deletion of the Tgfb1 gene in mice results in rapid demise after birth because of overwhelming, systemic inflammation (Shull et al. 1992). Based on this and many other observations (Travis and Sheppard 2014), TGF-β1 is generally considered as a suppressor of excessive inflammation. Regulated inflammation itself is an important determinant of tissue repair, as resolution of provisional fibronectin-rich ECMs and elaboration of mediators that limit collagen accumulation after injury limit fibrosis. Inflammatory cells, especially macrophages, are important initiators of inflammation and mediators of inflammation resolution (Vannella and Wynn 2016; Minutti et al. 2017). This review will examine our current understanding of the core functions of TGF-β1 in promoting collagen accumulation and parallel pathways that help determine whether there is physiological repair or progressive fibrosis. Although increased levels of TGF-β2 and TGF-β3 have been observed in fibrotic disease (Burke et al. 2016), there is little evidence implicating either TGF-β2 or TGF-β3 with progressive tissue fibrosis, and therefore these TGF-β isoforms will not be discussed further here.
INTERPLAY BETWEEN INTEGRINS AND ECM STIFFNESS IN TGF-β1 ACTIVATION AND STROMAL EXPANSION
Newly translated TGF-β is processed by cleavage of an amino-terminal fragment, called the latency-associated peptide (LAP), from the polypeptide that homodimerizes to form the mature cytokine (Munger et al. 1997; Moses et al. 2016; Robertson and Rifkin 2016). The cleaved LAP also homodimerizes and associates noncovalently with mature TGF-β in a complex that prevents access of TGF-β to its receptors. In most cells, this “small latent complex” is disulfide linked to either a member of a family of latent TGF-β binding proteins (LTBPs) and stored in the ECM or to the transmembrane protein glycoprotein A repetitions predominant (GARP) for presentation on the cell surface (Stockis et al. 2009). Most organs of healthy adult mammals contain substantially more latent TGF-β than would be required, when activated, to cause extensive tissue fibrosis. Therefore, much of the regulation of TGF-β function in fibrotic diseases depends on the regulation of TGF-β activation rather than synthesis or secretion (Robertson and Rifkin 2016).
In vitro it is relatively easy to induce conformational change in latent TGF-β that leads to release of the active cytokine (i.e., TGF-β activation). For example, purified latent TGF-β can be activated by acidic or basic pH, heat denaturation, and oxidation by reactive oxygen species (ROS), incubation with thrombospondin 1, or a wide variety of proteases (Munger et al. 1997; Crawford et al. 1998). However, with the exception of thrombospondin 1, for which gene inactivation has identified some phenotypic features consistent with loss of TGF-β activation, and ROS in the setting of mammary gland irradiation (Barcellos-Hoff et al. 1994), the roles of these activation pathways in vivo have not been established. The most intensely studied activation mechanism with demonstrated relevance in vivo has been activation by interaction with a subset of integrins (Munger et al. 1999; Annes et al. 2002; Mu et al. 2002; Jaramillo et al. 2003; Henderson et al. 2013; Hinck et al. 2016).
The first indication that integrins could participate in the activation of TGF-β came from observations of the phenotype of mice lacking the β6 subunit of the epithelially restricted αvβ6 integrin (Munger et al. 1999). These mice show exaggerated inflammatory responses to normally trivial injurious stimuli in multiple epithelial organs (Huang et al. 1996). Despite these exaggerated responses, Itgb6−/− mice are dramatically protected from tissue fibrosis (Munger et al. 1999; Hahm et al. 2007). Tgfb1−/− mice have even more exaggerated tissue inflammation, reflecting the more limited tissue expression of Itgb6 relative to Tgfb1 (Kulkarni et al. 1993). Biochemical evidence has shown direct binding of the αvβ6 integrin to an arginine-glycine-aspartic acid (RGD) tripeptide within TGF-β1 LAP (Munger et al. 1999), and cell culture assays have shown that epithelial cells can use this integrin to bind and activate TGF-β1 and -β3 (Munger et al. 1999; Annes et al. 2002). Since these initial observations, several studies have shown reduced TGF-β activation in epithelial organs of Itgb6−/− mice, by cells lacking the αvβ6 integrin, and in response to blocking antibodies targeting this integrin (Munger et al. 1999; Weinreb et al. 2004; Jenkins et al. 2006; Aluwihare et al. 2009; Giacomini et al. 2012).
Because the inflammatory phenotype of mice lacking even one of the three isoforms of TGF-β (e.g., TGF-β1) is much more severe than the phenotype of mice lacking the αvβ6 integrin, additional mechanisms must regulate activation of TGF-β1 and -β3 in vivo. It is now clear that additional RGD-binding integrins are important contributors to this process. Cell culture studies with epithelial cells showed that the closely related integrin, αvβ8, can also potently activate TGF-β (Mu et al. 2002). Mice that lack integrin β8 also recapitulate some features of mice lacking TGF-β1, such as defects in vascular development, or TGF-β3 (e.g., cleft palate) (Zhu et al. 2002). When Itgb8−/− mice are crossed to an outbred genetic background that allows some mice to survive into early adulthood, treatment of these mice at birth with blocking antibody to the αvβ6 integrin results in a complete phenocopy of all developmental effects of loss of TGF-β1 and TGF-β3 (Aluwihare et al. 2009), suggesting that αvβ6 and αvβ8 together contribute to activation of all of the TGF-β1 and TGF-β3 required for normal development.
Additional RGD-binding integrins may play important roles in activating TGF-β to drive tissue pathology in adults. For example, Cre recombinase-mediated, tissue-restricted deletion of the RGD-binding integrins that share the αv subunit in cells expressing platelet-derived growth factor β (PDGFβ) protects mice from pathologic fibrosis in the lungs, liver and kidney (Henderson et al. 2013). Fibroblasts derived from αv-null mice following Pdgfb-Cre-mediated gene deletion have an impaired ability to bind and activate TGF-β1, and these mice show reduced evidence of TGF-β signaling in models of pathologic tissue fibrosis. The cells targeted by Pdgfb-Cre do not express the αvβ6 integrin, but express αvβ1, αvβ3, αvβ5, and αvβ8. However, global deletion of αvβ3 and/or αvβ5, by specific deletion of their β chains, has no effect on induced tissue fibrosis, and conditional deletion of αvβ8 in the same cells has no effect on liver fibrosis. These results suggest that one or more αv integrins, in addition to αvβ8 and αvβ6, can contribute to pathologic TGF-β activation in vivo. Accordingly, a small molecule inhibitor of the αvβ1 integrin that has minimal effects on other RGD-binding integrins was shown to inhibit TGF-β activation by primary lung and liver fibroblasts (Reed et al. 2015). Continuous subcutaneous administration of this inhibitor during the late phases of bleomycin-induced pulmonary fibrosis or carbon tetrachloride (CCl4)-induced hepatic fibrosis results in protection that is similar to that by a small molecule inhibitor of all αv integrins (Henderson et al. 2013). These data suggest that the fibroblast integrin responsible for TGF-β activation and progression of lung and liver fibrosis is αvβ1.
ROLE OF MECHANICAL FORCE IN INTEGRIN-MEDIATED TGF-β ACTIVATION
In the original description of αvβ6-mediated TGF-β activation, it was shown that sequences within the β6 subunit cytoplasmic domain required to link the integrin to the actin cytoskeleton were essential for TGF-β activation (Munger et al. 1999). Activation was also completely inhibited by cytochalasin D, an inhibitor of actin polymerization. These observations provided the first evidence that integrin-mediated TGF-β activation requires linkage to and active re-organization of the actin cytoskeleton. Subsequently, cell culture studies showed that contractile fibroblasts can use integrins to activate latent TGF-β, and that this process depends on actin–myosin interaction and generation of mechanical force (Wipff et al. 2007). Similar requirements were shown for αvβ6-mediated TGF-β activation by epithelial cells (Giacomini et al. 2012), although the role, if any, of mechanical force in TGF-β activation by αvβ8 is less clear (Mu et al. 2002). αvβ6-mediated TGF-β1 activation was also shown to require covalent disulfide linkage of LTBP-1 to TGF-β1 LAP (Annes et al. 2004). The effect of deletion of Ltbp1 could be rescued by expression of a short fusion protein composed of the region containing the cysteine residues responsible for disulfide linkage of LTBP-1 to LAP and the region required for direct binding to fibronectin in the ECM. These observations suggest that the requirement for LTBP-1 is explained by its role in physically tethering latent TGF-β.
The important role of mechanical force in integrin-mediated TGF-β activation fits very well with the crystal structures of latent TGF-β1 and of cocrystals of the αvβ6 integrin with TGF-β1 LAP (Shi et al. 2011; Dong et al. 2014, 2017; Hinck et al. 2016). The latent TGF-β1 structure showed a 180° axis between the exposed loop in LAP containing the integrin binding RGD sequence and the cysteine residues that form the disulfide linkage to LTBP-1, identifying how the force of cellular contraction could be transmitted through αvβ6 to deform physically constrained latent TGF-β. Furthermore, a single unstructured loop, called the latency lasso, was shown to make all of the critical contacts with active TGF-β within the latent complex and to be the likely weakest link that would be unfolded by mechanical force, thereby releasing free TGF-β from the complex. The αvβ6 structure identified critical residues, especially a hydrophobic pocket that provide a docking site for hydrophobic residues adjacent to the RGD sequence in TGF-β1 and -β3 LAP that help to explain the unusually high affinity of the interaction between αvβ6 and LAP.
To exert physical force on tethered latent TGF-β, cells must themselves be firmly attached to a relatively stiff substrate. Indeed, one of the principal methods used to show a requirement for physical force in integrin-mediated TGF-β activation has been plating cells on polyacrylamide gels of varying stiffness, and showing that cells plated on more deformable substrates have progressively impaired ability to activate TGF-β as the substrate deformability increases (Wipff et al. 2007; Giacomini et al. 2012). Integrins are one of the main families of receptors cells used to adhere tightly to components of the ECM, so it seems likely that inhibitors targeting integrins could inhibit TGF-β activation, even if the inhibited integrins do not directly bind tightly to TGF-β LAP. For example, a blocking antibody targeting the αvβ3 integrin can inhibit TGF-β activation by cultured renal fibroblasts but has no effect on adhesion of these cells to LAP. Rather, this inhibitory effect is completely dependent on the substrate on which these cells are plated, with marked inhibition when the cells are plated on the αvβ3 ligand or vitronectin, but no inhibition when the cells are plated on an irrelevant ligand, collagen 1 (Chang et al. 2017). Thus, integrin interactions with the ECM are intricately involved in how the ECM impacts TGF-β1 activation.
TGF-β1 SIGNALING AS A DRIVER OF COLLAGEN ACCUMULATION
TGF-β1 signals through its specific heterotetrameric receptor complex comprised of a pair of TβRI receptors that phosphorylate the receptor activated-Smads (R-Smads), termed Smad2 and Smad3, and a pair of TβRII, which contain the initial binding site for TGF-β1. These receptors are expressed on most cells in culture and their roles in TGF-β signaling have been discussed elsewhere (Feng and Derynck 2005; Heldin and Moustakas 2016). Cell-specific gene deletion by Cre-mediated recombination of either Tgfbr1 or Tgfbr2 revealed that TGF-β1 signaling, rather than TGF-β1 expression, in both epithelial cells and mesenchymal cells is required for bleomycin-induced lung fibrosis (Huang et al. 2002).
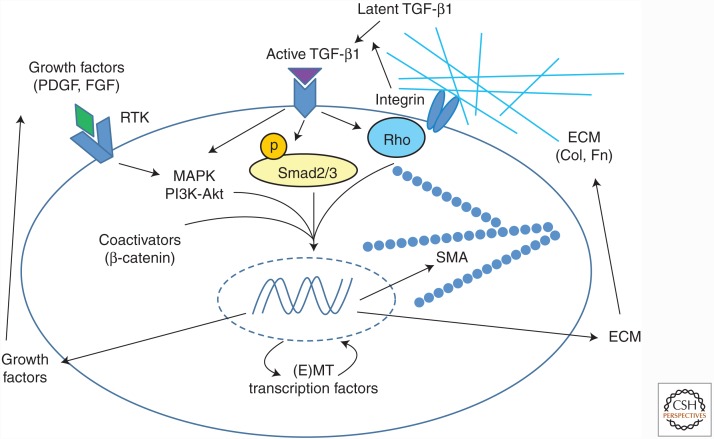
These findings indicate that TGF-β1-mediated signaling in both epithelial and fibroblastic cells is a common and critical feature of fibrogenesis. These findings also support the concept that TGF-β1 mediates fibrosis through multiple cell types and multiple interacting signaling pathways. The latter include not only coregulators of R-Smad-dependent transcription of TGF-β1 target genes such as AP-1 and Egr-1, specific negative regulators of R-Smad function such as Smad7, but also early response genes such as Nox4 and Tgfb1 itself further promote TGF-β1 signaling (Derynck and Zhang 2003; Feng and Derynck 2005). An altered profile of microRNAs (miRNAs) induced or suppressed in response to TGF-β1 also acts to stabilize translation of mesenchymal genes important to fibrosis and minimize antifibrotic pathways (Bowen et al. 2013). Finally, numerous other signaling pathways provide input that allows integration of Smad and non-Smad signaling immediately downstream from TGF-β receptor activation with contextual cues from the microenvironment that favor expansion of the ECM (Fig. 1). Integrins, in addition to mediating latent TGF-β1 activation, activate prosurvival and adhesive signals as a function of ECM composition and stiffness. Receptor tyrosine kinases respond to ligands after injury and promote proliferation and survival of fibroblasts. Hypoxia, acting through hypoxia inducible factors (HIFs), promotes epithelial–mesenchymal transition (EMT) and/or mesenchymal expansion with enhanced collagen expression (Falanga et al. 2002; Zhou et al. 2009; Chapman 2010; Kottmann et al. 2012). Several morphogen pathways, especially those induced by Wnt and Notch ligands, are activated in response to injury and have been implicated in promoting tissue fibrosis in multiple organs. The elaborate cellular response of stromal cells within injured tissues, directed in part by the influx of inflammatory cells, appears heavily oriented toward transient ECM accumulation accompanied by proliferation and migration of epithelial and stromal elements, followed by stabilization of the collagenous components of the ECM, and finally fibroblast apoptosis and removal of excess collagen to restore normal architecture (Beers and Morrisey 2011). TGF-β1 is a key cytokine participating in virtually all elements of the early tissue response to injury.
Figure 1.
Epithelial and mesenchymal cell fibrogenic activation by TGF-β1: costimulation by other signaling inputs and opportunities for feed-forward activation. Latent TGF-β is activated by αv integrins. Active TGF-β binds to its receptors leading to activation of canonical Smad signaling and Smad-independent signaling pathways, including MAPK pathways and small GTPases such as RhoA. Inputs from other signaling pathways converge on these pathways to regulate the TGF-β response. These are activated by other growth factors, such as PDGF, which generally signal through RTKs and through MAPK, integrin-mediated mechanotransduction mediated by Rho family GTPases, and coactivators such as β-catenin. These signaling factors lead to expression of profibrotic genes such as those encoding α-SMA, ECM proteins, and secreted cytokines and growth factors, which further modify the fibrogenic effector cell response. The expression of transcription factors involved in epithelial or mesenchymal cell transition into an activated state is also induced. PDGF, platelet-derived growth factor; FGF, fibroblast growth factor; RTK, receptor tyrosine kinase; MAPK, mitogen-activated protein kinase; (E)MT, (epithelial–) mesenchymal transition; Col, collagen; Fn, fibronectin; SMA, α-smooth muscle actin; ECM, extracellular matrix.
Collagens are comprised of three polypeptide chains highly organized into a triple helical conformation. Collagen monomers are not very soluble and, as discussed below, numerous posttranslational modifications are necessary for proper procollagen folding and secretion. There are 28 collagen proteins in mammals, the most abundant being collagen I, a major component of bone, most musculoskeletal structures, eye, lung, and the vasculature (Hulmes 2008). Collagen I belongs to the group of fibrillar collagens, including collagens I, II, III, and VI, that are largely triple helical structures and thus become organized into fibrils with very defined “packing” dependent on extensive covalent cross-linking of carboxyl terminus to amino terminus of adjacent collagen monomers. This elaborate organization results in high tensile strength for the collagen fibrils. This is in contrast to the much less extensively organized nonfibrillar collagens, such as collagen IV that is the major basement membrane collagen. After injury, collagens I and III are the dominant collagens whose expression is induced to restore tensile strength and tissue integrity. TGF-β1 is a required cytokine for the induction of these collagens after tissue injury.
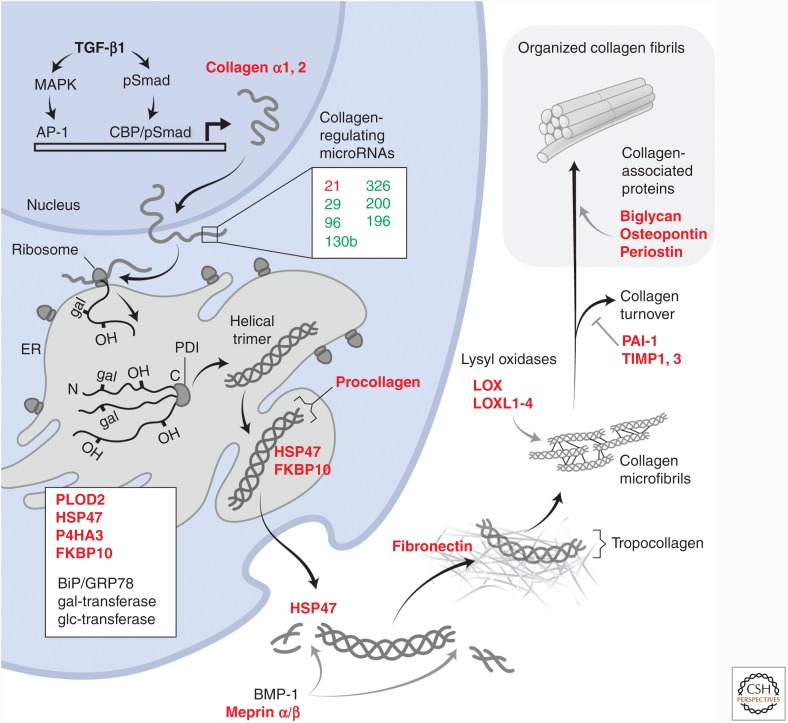
The intricate nature of TGF-β1 signaling in fibrogenesis is highlighted by the mechanisms engaged in expression and tissue accumulation of type I collagen. Type I procollagen is comprised of two polypeptide chains, pro-α1 and pro-α2, assembled in a 2:1 ratio (2α1/α2) leading to a heterotrimeric three-dimensional helical structure (Fig. 2). Following activation of latent TGF-β1 and TGF-β receptor engagement, initial transcription of COL1A2 gene (encoding type I collagen α2) is dependent on the nuclear translocation of Smad3 and Smad4, the DNA-binding transcription factors Sp1 and AP-1 (c-Jun/c-Fos), as well as CREB-binding protein (CBP) or p300 that act as coactivators and histone acetylases to facilitate chromatin relaxation and access of the transcription factors to the COL1A2 promoter (Zhang et al. 1998; Ghosh et al. 2000). The appearance of AP-1 in the nucleus in turn depends on activation of Smad-independent Erk or c-Jun amino-terminal kinase (JNK) mitogen-activated protein kinase (MAPK) signaling pathways by TGF-β1. Similar mechanisms operate to induce an early response of Egr-1 that has been shown to be critical for collagen I expression by fibroblasts in response to TGF-β1 (Chen et al. 2006). Numerous other factors coordinate with Smad2/3 signaling in the nucleus to promote COL1A1 and COL1A2 transcription. Once transcribed, the translation of collagen I mRNAs is also heavily regulated, in no small part by TGF-β1-induced repression of the expression of miRNAs known to target collagen mRNAs or mRNA for proteins required for collagen expression (Bowen et al. 2013). miR-29, for example, is known to repress the expression of heat shock protein 47 (HSP47) and lysyl oxidase like-2 (LOXL2), which both participate in the highly organized process of stable collagen accumulation in the ECM (discussed below). The expression of the miR-29 family is down-regulated in response to TGF-β1 in fibroblasts, and this repression corresponds to increased fibroblast collagen expression. Mice treated with miR-29 mimics have attenuated lung fibrotic responses to bleomycin (Yang et al. 2013). miR-96 and miR-130b also attenuate collagen accumulation and their expression is repressed in response to TGF-β1. Similarly, miR-326 has been shown to attenuate TGF-β1 translation (Das et al. 2014). Levels of miR-326 were found to be low in the bleomycin mouse model and in lungs of patients with idiopathic pulmonary fibrosis (IPF). Additionally, mice treated intranasally with a miR-326 mimic have attenuated fibrosis in this model. Collectively, the changes in miRNA expression by TGF-β1 signaling act to stabilize collagen protein expression, secretion, and stabilization in the ECM (Fig. 2).
Figure 2.
TGF-β1-induced collagen expression program. The schematic illustrates the proteins and microRNAs that are coordinately regulated by TGF-β1 to effect tissue collagen accumulation. Proteins whose expression is activated by TGF-β1 are highlighted in red, indicating that the expression of more than a dozen proteins is induced along with that of collagen and is required for substantial collagen expression and tissue accumulation. These proteins act at nearly every stage of collagen processing, from posttranslational proline and lysine hydroxylations by procollagen-lysine 2-oxogluterate 5-deoxygenase (PLOD2) and prolyl-4-hydroxylase (P4HA3), and glycosylation in the endoplasmic reticulum (ER), to required chaperones (HSP47 and FKBP10) to sustain the trimeric collagen structure and prevent premature fibril formation during passage through the secretory machinery. Additional, constitutively expressed proteins, such as protein disulfide isomerase (PDI), and quality control sensors, such as BiP/GRP78, are also important for collagen folding and subsequent expression. Once secreted, procollagen is proteolytically processed to generate tropocollagen that then spontaneously begins to self-associate into microfibrils. The final physical form and extent of stable, matrix fibrillar collagens depend heavily on additional TGF-β1-induced proteins such as fibronectin, the lysyl oxidases, and inhibitors of collagen turnover, plasminogen activator inhibitor 1 (PAI-1) and tissue inhibitor of metalloproteinases 1 and 3 (TIMP1, TIMP3). Finally, proteins such as biglycan and periostin associate with mature fibrils and control packing and organization. Collectively, this reprogramming of cells can be considered as the TGF-β1-induced collagen expression program.
Actual assembly and cellular trafficking of collagen I polypeptides through the cell secretory machinery is problematic because of the large size and tenuous solubility of the collagen chains. Extensive posttranslational modifications are required for the proper assembly of procollagens into very well ordered helical structures early in the secretory process (Fig. 2) (Hulmes et al. 2002; Bourhis et al. 2012; Ishikawa and Bachinger 2013). The primary amino acid sequences of fibrillar collagens underlie the propensity of these collagens to form a helical structure, with approximately 300 repeats of Gly-X-Y motifs providing sufficient glycines for tight chain folding. HSP47, whose expression is strongly induced in response to TGF-β1 in nearly all cells that express collagen I, acts as a key chaperone in collagen assembly, binding to collagen and promoting stability of both the unfolded collagen chains in the endoplasmic reticulum (ER) and the more assembled structures trafficking through the Golgi. Assembly into a properly folded procollagen also requires many distinct modifications. Immediately after synthesis in the rough ER, and possibly bound to its inner membrane, the unfolded collagen chains begin to undergo lysine and proline hydroxylations. The two key enzymes in this process are procollagen-lysine 2-oxogluterate 5-deoxygenase (PLOD2 or LH2) and prolyl-4-hydroxylase (P4HA3). The expression of both enzymes is strongly induced in response to TGF-β1. A variable fraction of the hydroxy-lysines are then glycosylated, and the remainder is further processed to very stable collagen cross-links once collagen is secreted. Like glycines, the hydroxyprolines are required for tight helical folding as the three collagen chains begin to assemble. The modified collagen chains are also substrate for a constitutively expressed protein disulfide isomerase (PDI) that mediates disulfide formation in the carboxy-terminal region. This is thought to place the chains in “register” for subsequent helical folding, beginning at the carboxyl terminus and proceeding toward the amino terminus, and full assembly of the procollagen molecule. Once the ordered, helical procollagen molecule assembles, there is a critical need for additional proteins to chaperone the trafficking of procollagen through the Golgi and secretory process without denaturation or premature fibril formation. HSP47 and a FK506 binding protein 10 (FKBP10) serve these functions. Their expression is strongly induced by TGF-β1 in fibroblasts (Ishida and Nagata 2011; Staab-Weijnitz et al. 2015).
Collagen is finally secreted as a procollagen with the extended carboxy- and amino-terminal nonhelical peptide chains (carboxy- and amino-terminal telopeptide extensions) that are then removed by proteolysis to generate the helical structure, tropocollagen (Fig. 2). A number of proteases appear to be capable of amino- and carboxy-terminal processing of procollagen and it is unclear if these are induced by TGF-β. The earliest described carboxy-terminal processing protease is bone morphogenetic protein-1 (BMP-1), a metalloproteinase that is critical to collagen maturation in bone (Li et al. 1996; Prockop et al. 1998; Asharani et al. 2012). Recently, meprin α and β metalloproteinases were shown to cleave both the amino- and carboxy-terminal peptides of procollagen, leading to fibril formation (Prox et al. 2015). These meprin levels are high at sites of collagen accumulation and fibrosis (Broder et al. 2013). Meprin expression is induced by pathways that lead to activation of nuclear AP-1, and thus may be induced by TGF-β1 (Biasin et al. 2014). Tropocollagen is thought to spontaneously form a polymeric collagen fibril, but collagen stabilization as a fibril (containing hundreds of tropocollagen molecules) requires lysyl (or hydroxyl-lysyl) oxidation to an aldehyde that is both necessary and sufficient for irreversible collagen cross-linking. Lysyl oxidases, a set of key enzymes that mediate collagen cross-linking, are all strongly induced by both TGF-β1 and HIF1α (Erler et al. 2006; Blaauboer et al. 2013). In addition to supporting fibril assembly, TGF-β1 concurrently induces the expression of protease inhibitors such as plasminogen activator inhibitor 1 (PAI-1) and tissue inhibitor of metalloproteinase 3 (TIMP3) that attenuate the breakdown of newly deposited tropocollagens that are vulnerable to proteases. The pattern of deposition of collagen is strikingly similar, and follows temporally that of fibronectin secretion, a prominent mesenchymal protein that is induced by TGF-β1 signaling (Hernnas et al. 1992; Muro et al. 2008). Extracellular fibronectin assembly, itself a complicated and integrin-dependent process, provides the suitable extracellular fibrillar substrate for organized deposition of collagen fibrils. The further organization of fibrils, depending perhaps on the needed tensile strength, is promoted by yet additional TGF-β1 target genes that encode collagen-interacting proteins such as osteopontin, periostin, and the proteoglycan biglycan (Oku et al. 2008; Farhat et al. 2012). Transglutaminase and nonenzymatic interactions among these proteins are thought to further enhance tissue stiffness over time (Cox and Erler 2011).
The evolutionary expansion of collagens from early metazoans to mammals has been accompanied by expansion of the family of monoamine oxidases, lysyl oxidases, needed to cross-link and stabilize ECM collagen (Kagan and Trackman 1991). The parent enzyme, LOX, is critically important to normal musculoskeletal development as deletion of LOX in mice is perinatal lethal with aortic aneurysms, defective skeletal structures, and defective airway development (Trackman 2005). In addition to LOX, there are four LOX-like enzymes (LOXL1–4) that share highly conserved catalytic domains but also are comprised of additional protein interaction domains. LOX and LOXL1 are closely related and expressed in a similar pattern. LOXL2, 3, and 4 have similar structures and, among these, LOXL2 is the most studied and widely expressed, although primary lung fibroblasts in culture express all of the LOX-like enzymes at variable levels. The expression of LOXL2 is strongly induced by TGF-β1 and hypoxia, and is reported to not only function extracellularly in collagen cross-linking but also intracellularly to promote Snail1 protein accumulation. Because of the strong positive impact of Snail1 on the expression of fibrillar collagens, the induction of LOXL2 expression in response to TGF-β1 can be viewed as a positive feedback loop to support ECM expansion after injury. Thus, LOXL2 levels are strongly linked to stable collagen accumulation, ECM stiffness, and fibrosis progression (Barry-Hamilton et al. 2010; Adamali and Maher 2012).
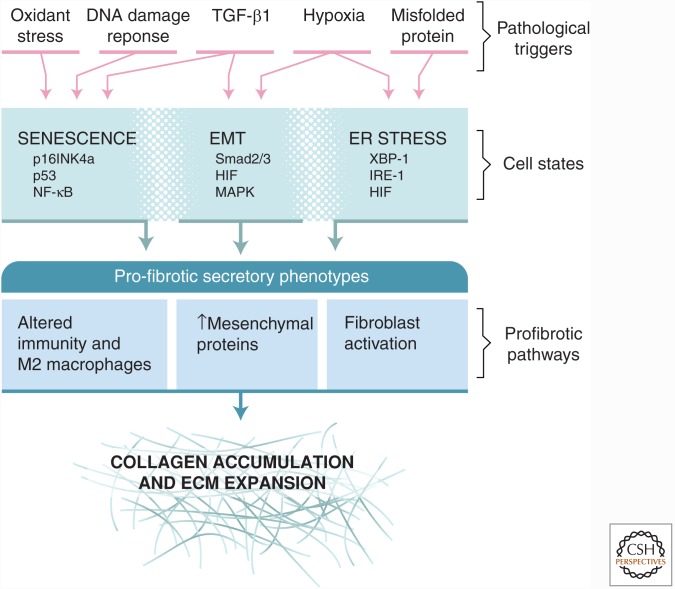
As might be predicted from the elaborate requirement for multiple posttranslational modifications to support fibrillar collagen folding and solubility in the ER, high-level expression of collagen renders the cell susceptible to ER stress from misfolded protein. Accordingly, TGF-β1 induces the expression of proteins that are linked to protection from the unfolded protein response (UPR) (e.g., BiP/GRP78, and HSP47) in parallel to expression of fibrillar collagens and modifiers of its primary structure (Fig. 2). Nonetheless, it has been reported that inositol-requiring enzyme-1α (IRE-1α), a key mediator of the UPR, is activated after TGF-β1-induced collagen expression in fibroblasts and in fibroblasts from patients with fibrotic disease (Heindryckx et al. 2016). The investigators implicated both increased X-box binding protein 1 (XBP-1) signaling, resulting in ER expansion and cleavage of miR-150 by the RNAase activity of activated IRE-1 in altering collagen expression, and fibrogenesis. Elevated miR-150 levels have been linked to protection from fibrosis (Honda et al. 2013). Hence, somewhat paradoxically, the activation of IRE-1, indicative of an activated UPR, promotes rather than suppresses collagen expression. Inhibition of IRE-1 in cell culture and in vivo attenuates the fibrotic response to TGF-β1 in liver and skin fibrosis models. These findings underscore the potential pathological operation of the UPR in the context of ER stress with a negative impact on tissue integrity (Fig. 3). This conclusion is supported by the findings that mutations in surfactant protein (SP) A2, a cause of familial pulmonary fibrosis, leads to type II alveolar epithelial cell ER stress accompanied by increased TGF-β1 secretion (Wang et al. 2009).
Figure 3.
Diverse pathways of epithelial responses to stress converge on profibrotic secretory phenotypes supporting extracellular matrix (ECM) expansion. The figure highlights three major epithelial states resulting from different pathological triggers: epithelial–mesenchymal transition (EMT), senescence, and endoplasmic reticulum (ER) stress with unfolded protein responses (UPRs). Each epithelial state has its characteristic transcriptional drivers. All of these states elicit secretory responses that share the potential to promote collagen accumulation. Distinctions among the secretory responses are still not completely defined and represent opportunities to better understand the connections between epithelial and mesenchymal biology in the context of cell stress. As discussed, TGF-β1 is a major driver of fibroblast activation and collagen secretion. Like epithelial cells, fibroblasts also respond to many of the same pathological triggers with activation of senescent or UPR signaling pathways, potentially further promoting fibrosis.
Collectively, it is clear that TGF-β1 drives a large, multifaceted reprogramming of both epithelial and mesenchymal cells to favor expression and extracellular accumulation of matrix proteins that provide rapid restoration of tissue integrity in the setting of injury and/or disruption of tissue barriers (Fig. 2). The phylogenetic origin of fibrillar collagen, and its complicated assembly process, dates to the earliest metazoans and has been highly conserved and further amplified for increased structural strength throughout the vertebrate family. The importance of collagen and numerous other ECM proteins in the injury response is highlighted not only by the highly coordinated induction by TGF-β1 of a large set of ECM structural proteins but also the parallel induction of numerous enzymes and chaperones that promote proper folding, secretion, and extracellular stabilization of collagen as well as down-regulation of miRNAs that specifically attenuate collagen expression and accumulation. It is hardly surprising then that such a robust and complicated cellular response system, driven after injury largely by a single master regulator, is subject to breakdown and disordered function leading to disease.
CONTRIBUTIONS OF INTEGRINS AND TGF-β1 TO TISSUE STIFFNESS ON PROGRESSION OF FIBROSIS
It is now abundantly clear that tissue stiffness can exert profound effects on cell differentiation and behavior. For example, mesenchymal stromal cells differentiate into fat cells, muscle cells, or bone cells when they are plated on substrates with stiffness mimicking that normally found in fat, muscle, or bone, respectively (Engler et al. 2006). Stiffness also profoundly affects the behavior of tissue fibroblasts, with increased stiffness favoring increased collagen production and expression of contractile proteins such as α-smooth muscle actin (SMA), and decreased stiffness favoring a more quiescent and noncontractile phenotype (Liu et al. 2010). As a consequence, the process of tissue fibrosis, which locally increases tissue stiffness, participates in a feed-forward loop, where the presence of local fibrosis increases fibroblast collagen production and force generation, further accelerating fibrosis. One of the mechanisms underlying this feed-forward loop is integrin-mediated TGF-β activation, because, as noted above, this process depends on cell contraction and is enhanced when cells are tethered to a stiff substrate. However, increased TGF-β activation does not fully explain this feed-forward loop. For example, tissue stiffness increases the sensitivity of cells to already activated TGF-β (Liu et al. 2010).
EPITHELIAL–MESENCHYMAL TRANSITION
An important potential contribution of EMT to tissue fibrosis was first proposed as early as the mid 1990s (Strutz et al. 1995). More insight has emerged with greater usage of fibrotic human tissue samples for biomedical research, the development of novel techniques for genetic modifications to mice, and a greater understanding of some of the prominent EMT signaling pathways (Kalluri and Neilson 2003; Teng et al. 2007; Kalluri and Weinberg 2009; Thiery et al. 2009; Flier et al. 2010; Lamouille et al. 2014). However, rather than establishing a definite role for EMT during fibrosis, these advances have led to increased controversy regarding the role or even the occurrence of EMT during fibrosis. Seemingly solid evidence both supports and refutes EMT as having a major contribution to fibrosis. Some of the controversies regarding EMT point to broader uncertainties and controversies in the fibrosis field at large.
Definition of EMT
Epithelial cells are characterized by a relatively sessile state with a defined shape (e.g., cuboidal, columnar, or squamous) forming tight cell–cell contacts, which establish an epithelial barrier that is important for the function of many organs. Epithelial cells have an apical–basal polarity and often produce factors that are secreted from the apical surface into a luminal space. In contrast, the mesenchymal cell phenotype provides front–rear polarity and enables migratory behavior. The cell–cell contacts are often transient and the cell shape is more dynamic and less defined. EMT can thus be defined as a process in which epithelial cells lose epithelial cell–cell contacts, undergo cytoskeletal changes and change polarity from apical–basal to anterior–posterior, all favoring acquisition of migration and invasion. These changes are accompanied by changes in gene and protein expression, including proteins that are involved in cell contact (e.g., loss of E-cadherin and gain of N-cadherin) and cytoskeletal proteins (e.g., loss of cytokeratin and gain of vimentin and SMA). There is also loss of proteins that are normally secreted into the luminal space (e.g., SPs, hormones) in favor of proteases and ECM proteins that promote migration and invasion. EMT can therefore be identified and defined by a set of biomarkers, reflecting loss of epithelial and gain of mesenchymal proteins (Zeisberg and Neilson 2009). EMT occurs during and is required for normal embryonic development. Roles for EMT during carcinogenesis and fibrosis have been suggested, but remain subjects of discussion and further research (Cardiff 2005; Tarin et al. 2005; Zeisberg and Duffield 2010). EMT during these different scenarios has overlapping features but also important differences leading some to classify EMT into type I (development and regeneration), type II (fibrosis) and type III (cancer) EMT, dependent on the physiological and pathological context (Kalluri and Weinberg 2009; Zeisberg and Neilson 2009). The transition of endothelial or endodermal cells into a mesenchymal phenotype has been labeled endothelial–mesenchymal transition (EndMT), but is often categorized within EMT, as these processes are similarly regulated and have many overlapping characteristics (Teng et al. 2007; Kokudo et al. 2008; Kovacic et al. 2012). EMT may simply represent a tunable, stereotypical epithelial response to the demand for dynamic cellular changes, as might occur during embryonic development, response to injury, and carcinogenesis. Frequently, the acquisition of such dynamic changes in epithelial behavior is reversible.
EMT Pathways
Features of EMT can be induced by many ligand–cell surface receptor interactions. TGF-β is the most extensively studied inducer of EMT. TGF-β binding to its receptors, TβRI and TβRII, activates Smad-dependent and Smad-independent pathways that can directly activate mesenchymal gene and suppress epithelial gene expression (Derynck et al. 2014; Lamouille et al. 2014). TGF-β–Smad signaling can also activate EMT through induction of the expression of several mesenchymal transcription factors, such as Snail1 and Twist, and these are therefore often referred to as EMT transcription factors. The TGF-β pathway intersects with a number of other pathways that may augment or attenuate the EMT response (Derynck et al. 2014). Hepatocyte growth factor (HGF), initially described as a “scatter factor,” and fibroblast growth factor (FGF) were among the earliest examples of a single factor capable of inducing EMT in cell culture (Stoker and Perryman 1985; Valles et al. 1990; Nusrat et al. 1994). Subsequently, many other factors have been shown to induce aspects of EMT through activation of cell surface receptors that ultimately lead to transcriptional changes. Among the better studied ligand–receptor combinations are Wnt factors that act through Frizzled receptors, which leads to stabilization of β-catenin, Sonic Hedgehog (Shh) ligand that acts through Patched receptors, which leads to Gli activation, and the Delta–Notch combination, which leads to cleavage and activation of the intracellular Notch domain. For example, β-catenin has emerged as an important Smad coactivator, so in addition to activating its own target genes through interactions with T-cell factor (TCF) or lymphoid enhancer-binding factor (LEF), β-catenin can regulate the EMT response through cooperation with the Smad complex. Among the most prominent Smad-independent EMT pathways is activation of Rho family GTPases, including RhoA, Rac1, and Cdc42. Rho GTPases are important regulators of cell morphology, adhesion, and movement. TGF-β signaling through the Rho GTPases allows convergence of signaling inputs from the ECM and cytoskeleton to control the EMT response to TGF-β, and allows TGF-β to coordinate transcriptional expression changes with cytoskeletal changes involved in mesenchymal transition. Activation of these GTPases leads to loss of adherens junction complexes, breakdown of the apical–basal polarity, and cytoskeletal rearrangement. Activation of small GTPases also promotes transcriptional changes through factors such as myocardin-related transcription factor (MRTF). Inhibition of several mediators of the Rho GTPase signaling pathway, including Rho kinase and MRTF, in genetically modified mice or with chemical inhibitors has been shown to attenuate EMT and fibrosis (Theriault et al. 2007; Bendris et al. 2012; Harris et al. 2013; Okada et al. 2015; Sisson et al. 2015). Other prominent pathways activated by TGF-β are the MAP kinase pathways and the phosphoinositide-3 kinase (PI3K)–Akt pathway. These pathways again allow for interaction and input from a number of different factors, such as FGF, epidermal growth factor (EGF), and tumor necrosis factor (TNF)-α, and correspondingly activated receptors. The extensive cross-talk between intracellular pathways directly activated by TGF-β and secondary pathways that modulate impact of proximal TGF-β signals on gene expression is discussed extensively in other reviews (Derynck et al. 2014).
EMT in Development
The role of EMT during development is discussed at length in many other reviews (Thiery et al. 2009), but is briefly mentioned here to provide context to studies exploring the potential role of EMT in fibrosis. Embryonic development has been proposed to involve multiple different EMT events that have been extensively studied (Acloque et al. 2009). EMT occurs early in embryogenesis during gastrulation, as epiblasts migrate to form the primitive streak, move to the interior of the blastocyte, and form the mesodermal germ layer (Nieto et al. 1994; Viebahn 1995; Viebahn et al. 1995; Nieto 2011). Later, a subset of neural tube cells detach and undergo EMT to form the neural crest cells that migrate and eventually give rise to many structures of mesenchymal origin (Duband et al. 1995; Sauka-Spengler and Bronner-Fraser 2008). EMT is also prominent at later stages of embryogenesis during organogenesis. Among the most studied are cardiac valve formation (Nakajima et al. 2000), palate closure (Fitchett and Hay 1989; Griffith and Hay 1992), and pancreatic development. During organogensis, EMT is often followed by a reversal of EMT or mesenchymal–epithelial transition (MET), highlighting the plasticity of cells during development (Davies 1996; Li et al. 2014). For example, a subset of cells from the developing pancreatic bud undergo EMT, dissociate, and migrate into the interstitium where they undergo MET to form the islets of Langerhans (Johansson and Grapin-Botton 2002). More durable or permanent EMT also occurs during organogenesis in later embryonic development. Recent studies of lung and gut development suggest that serosal mesothelial cells, which are squamous epithelial cells, are a major source of vascular smooth muscle cells, which are mesenchymal cells (Wilm et al. 2005; Dixit et al. 2013). Notably, there is no common epithelial cell progenitor during embryogenesis, as epithelial cells are derived from all three embryonic primordial germ layers. For example, lung airway and alveolar epithelial cells are derived from the endoderm, renal tubular epithelial cells are derived from mesoderm, and epidermal skin cells are derived from the ectoderm. Similarly, mesenchymal cells can derive from ectoderm (e.g., facial cartilage) and mesoderm (e.g., skeletal muscle) (Acloque et al. 2009; Thiery et al. 2009).
EMT in Fibrosis
The rationale for considering EMT during fibrosis arose from similarities in prominent EMT and fibrotic signaling pathways. TGF-β is the best defined profibrotic factor and, as mentioned above, a prominent inducer of EMT (Bartram and Speer 2004). Identification of aberrant Wnt/β-catenin signaling in IPF lung samples prompted interest in the possibility of EMT in lung fibrosis (Chilosi et al. 2003). Subsequently, almost every cytokine or intracellular signaling pathway that has been implicated in fibrosis or fibroblast activation has been shown to activate EMT in cell culture. Thus, the cytokine milieu in fibrotic tissue suggests a pro-EMT stimulus. Given the abundance of epithelial cells in tissues like the lung and kidney, EMT of even a small fraction of epithelial cells could potentially account for a significant number of activated fibroblasts. Further, the proposed myofibroblast function may promote further activation of other fibroblasts through the production of profibrotic factors and induction of mechanical forces. Thus, even transient EMT could initiate a process that leads to more sustained and robust fibroblast responses. As with cancer, the identification of cells that have undergone EMT in human disease in vivo has been challenging. Many studies have relied on co-immunostaining for epithelial and mesenchymal markers. Identification of costaining cells is suggestive of EMT, but has inherent limitations (Kakugawa et al. 2005; Willis et al. 2005; Kim et al. 2006). The co-immunostaining approach is at best descriptive, potentially identifying expression of two or more markers within a cell but lacking an assessment of the functional contribution of that cell. Even if an epithelial cell expresses procollagen I or SMA, to what extent is it contributing to fibrogenesis? The co-immunostaining approach likely underrepresents EMT because most studies of EMT show simultaneous decrease in epithelial markers and up-regulation of mesenchymal markers. EMT cells in early transition may have weak expression of mesenchymal markers, and EMT cells in late transition may have weak expression of epithelial markers. Co-immunostaining is also beset by difficulties in achieving specificity of antibodies and by the potential for artifactual staining of overlapping cells giving the appearance of a costained cell. Microscopic approaches, such as confocal or deconvolution microscopy, have been used to overcome this limitation (Willis et al. 2005; Rock et al. 2011). To partially address these issues, several groups have isolated cells by flow cytometry to unequivocally show expression of mesenchymal proteins in epithelial cells and expression of epithelial cell proteins in fibroblasts (Larsson et al. 2008; Marmai et al. 2011). To more directly track EMT during fibrogenesis, the alternative approach of fate mapping individual cells has emerged.
Cell Fate Mapping
The Cre-recombinase/lox P sequence system in mice is a powerful way to map and trace the fate of specific cell types in vivo (Akagi et al. 1997). The system involves a reporter gene (e.g., green fluorescent protein [GFP] or β-galactosidase) regulated by a strong constitutively active promoter but its expression is disrupted by a short sequence containing a stop codon. This sequence is flanked by loxP sites, enabling it to be permanently removed by Cre recombinase. A second transgene encodes the Cre recombinase under the control of a cell type–specific promoter, enabling cell type–specific removal of the stop codon sequence, and labeling those cells by expression of the reporter. Importantly, the labeling occurs at the level of the genomic DNA and is thus permanent. Thus, the Cre/lox system has been proposed to enable cell type–specific and permanent labeling of cells, making it an attractive way to map the fate of cells in response to injury, and to identify the origin of cells of interest, such as a myofibroblasts in fibrosis. A limitation of this approach is the potential for off-target expression of a constitutive Cre recombinase allele, either transiently during organ development or in response to new signals appearing after tissue injury. For example, a promoter specific to an adult epithelial cell may have been transiently expressed in a mesenchymal cell during organ develop, or only after tissue injury, resulting in spurious conclusions on the extent or even presence of EMT. To obviate this limitation, Cre recombinase alleles have been constructed that enable temporal as well as spatial control of Cre activity. Most early versions of this methodology used cell type–specific expression of a reverse tetracycline transactivator (rtTA) to regulate expression of Cre recombinase by a tetracyclin-response element (TRE), but this suffered from the requirement of three transgenes for a fully operational system and the problem that the rtTA function was not completely doxycycline-dependent. More recently, Cre recombinase activity has been temporally regulated through the use of a fusion protein comprised of Cre recombinase and a modified estrogen receptor “knocked in” to a cell-specific promoter. The estrogen receptor has been further modified such that it is much more sensitive to tamoxifen than estrogen (e.g., ERT2) (Feil et al. 2009, 2014). Virtually all expressed Cre recombinase remains in the cytoplasm unless tamoxifen is present to empower nuclear translocation and DNA recombination. Various Cre-dependent strategies have been used extensively to map the fate of epithelial cells in fibrosis, but with conflicting outcomes. In lung fibrosis, at least four different groups have identified significant numbers of lung epithelial–derived cells that coexpress mesenchymal markers in models of fibrosis (Table 1) (Kim et al. 2006, 2009; Tanjore et al. 2009; DeMaio et al. 2012; Balli et al. 2013). These groups used different promoters to drive expression of Cre (Nkx2.1 and surfactant protein C [SPC]), different reporter genes (GFP/LacZ), different techniques to quantify colabeled cells (immunohistochemistry, single cell suspension with cell sorting, cytospin staining, RNA quantification, and immunoblotting), and several models of fibrosis (bleomycin, overexpression of TGF-β, and radiation) (Kim et al. 2006, 2009; Balli et al. 2013). However, one report, also using the bleomycin model of fibrosis, found no evidence of EMT in mice using Sftpc promoter-driven CreERT2 expression to fate-map type II alveolar epithelial cells (Rock et al. 2011). Contradictory results on EMT cell fate mapping are not limited to lung fibrosis. Similar cell fate mapping studies in models of kidney and liver fibrosis found substantial evidence or lack of evidence of EMT. Contradictory results in cell fate mapping are not limited to EMT. Pericytes and fibrocytes, which have been proposed as sources of activated myofibroblasts, have cell fate mapping studies in favor of or against them serving as significant sources of myofibroblasts. For pericytes, the conflict is even more stark. For example, in the bleomycin model of lung fibrosis, one report concludes that more than two thirds of the myofibroblasts are derived from pericytes (Hung et al. 2013). In contrast, that same report concluded that there is no evidence of EMT and excludes pericytes and two epithelial cell populations at the origin of myofibroblasts (Rock et al. 2011). Thus, those favoring or opposing the possibility of EMT can point to cell fate-mapping publications supporting their conclusion.
Table 1.
Reports on fate mapping during fibrosis
| Report | Fate mapping system | Model | EMT | |
|---|---|---|---|---|
| Lung fibrosis | ||||
| Kim et al. 2006 | Sftpc-rtTA/tetO-Cre/RosaLacZ | AdTGF-β | Yes | |
| Kim et al. 2009 | Sftpc-rtTA/tetO-Cre/ZEG | Bleo | Yes | |
| Tanjore et al. 2009 | Sftpc-Cre/RosaLacZ | Bleo | Yes | |
| Hashimoto et al. 2010 | Tek-Cre/RosaLacZ | Bleo | Yes | |
| DeMaio et al. 2012 | Nkx2-1-Cre/RosaLacZ | Bleo | Yes | |
| Rock et al. 2011 | Sftpc-CreER/dTomato | Bleo | No | |
| Balli et al. 2013 | Sftpc-rtTA/tetO-Cre/RosaLacZ | XRT | Yes | |
| Kidney fibrosis | ||||
| Iwano et al. 2002 | Ggt1-Cre/RosaLacZ | UUO | Yes | |
| Zeisberg et al. 2008 | Tek-Cre/RosaYFP | UUO | Yes | |
| Humphreys et al. 2010 | Six2-Cre/RosaLacZ | UUO | No | |
| LeBleu et al. 2013 | Ggt1-Cre/RosaLacZ | UUO | Yes | |
| Cdh5-Cre/RosaYFP | UUO | Yes | ||
| Gastrointestinal and liver fibrosis | ||||
| Zeisberg et al. 2007b | Alb-Cre/RosaLacZ | CCl4 | Yes | |
| Flier et al. 2010 | Vil1-Cre/RosaLacZ | TNBS | Yes | |
| Taura et al. 2010 | Alb-Cre/RosaLacZ | CCl4 | No | |
| Chu et al. 2011 | Alb-Afp-Cre/RosaYFP | CCl4 | No | |
| Cardiac infarct | ||||
| Zhou and Pu 2011 | Wt1-CreER/tmGFP | Art ligation | Yes | |
| Zeisberg et al. 2007a | Tie1-Cre/Rosa26 | Ao banding | Yes | |
| Pericyte→Myofib | ||||
| Lung fibrosis | ||||
| Rock et al. 2011 | Cspg4-CreER/fGFP | Bleo | No | |
| Hung et al. 2013 | Foxd1-Cre/dTomato | Bleo | Yes | |
| Kidney fibrosis | ||||
| Humphreys et al. 2010 | Foxd1-Cre/Rosa26 | UUO | Yes | |
| LeBleu et al. 2013 | Cspg4-Cre/RosaYFP | UUO | No | |
| Pdgfrb-Cre/RosaYFP | UUO | No | ||
| BM→Myofib | ||||
| Lung fibrosis | ||||
| Hashimoto et al. 2004 | GFP BMT | Bleo | No | |
| Schmidt et al. 2003 | ex vivo labeling | OVA | Yes | |
| Madala et al. 2014 | GFP BMT | TGF-α | No | |
| Kidney Fibrosis | ||||
| Iwano et al. 2002 | S100a4-GFP BMT | UUO | Yes | |
| LeBleu et al. 2013 | Acta2-RFP BMT | UUO | Yes | |
| Liver fibrosis | ||||
| Higashiyama et al. 2009 | Col1a2-GFP BMT | CCl4 | No | |
| Kisseleva et al. 2006 | Col1a1-GFP BMT | BDL | No | |
EMT, epithelial–mesenchymal transition; AdTGF-β, adenovirus encoding active TGF-β1; bleo, bleomycin; XRT, X-ray treatment; OVA, ovalbumin; UUO, unilateral ureteral obstruction; TNBS, trinitrobenzene sulfonic acid; Pericyte→myofib, pericyte to myofibroblast transition; BM→myofib, bone marrow–derived cell to myofibroblast transition; art, arterial; Ao, aortic; BMT, bone marrow transplant; CCl4, carbon tetrachloride; BDL, bile duct ligation; GFP, green fluorescent protein.
Despite the promise of cell fate mapping, this approach has failed to resolve the controversy regarding the origin of activated myofibroblasts during fibrosis. This may result from some of the same limitations as referred to for the analysis of costained tissues. In addition, the Cre recombinase system has particular limitations. Cre-mediated recombination may be overly sensitive because of weak and transient activation of the cell-specific promoter in off-target cells leading to sufficient expression of Cre recombinase to permanently activate reporter gene expression, thus potentially confusing the cellular origin of the labeled cell (Vaughan et al. 2015). Additionally, tamoxifen, which is used to activate estrogen receptor fusion proteins, can persist in tissues or cells for much longer than previously appreciated (Reinert et al. 2012). Typical tamoxifen dosing and “chase” periods intended to allow loss of tamoxifen before injury may fail to eliminate functional tamoxifen levels during experimental injury and this may also result in spurious conclusions on the cells of origin of expanded populations after injury (Vaughan et al. 2015). Ultimately, new methods such as DNA barcoding will have to be applied to studies of cell fate to resolve these uncertainties. Even extremely accurate cell fate mapping may not reveal the functional contributions of subpopulations of epithelial or mesenchymal cells to fibrosis.
Definition of EMT Revisited
Much of the controversy regarding EMT in cancer, fibrosis, and even development stems from differences in the definition of EMT, and some have suggested the need to broaden beyond the original narrow definition of EMT (Nieto et al. 2016). On one extreme, epithelial cells might be defined, in part, as being derived from a parent epithelial cell, and mesenchymal cells as being derived from a parent mesenchymal cell. EMT is then defined as a process in which a fully differentiated epithelial cell loses all epithelial cell characteristics and gains all mesenchymal characteristics. This definition virtually excludes the possibility of EMT that is seen in vivo. On the other extreme, EMT might be defined as any deviation from a classic epithelial cell phenotype and protein expression pattern toward any acquisition of mesenchymal cell traits or proteins. By this loose definition, type II alveolar epithelial cell differentiation to type I alveolar epithelial cell, or epithelial cell migration to re-epithelialize after injury might be regarded as EMT. Indeed, in some circumstances EMT transcription factors are activated in re-epithelialization after injury (Savagner et al. 2005) in a way that is reminiscent of developmental organogenesis, in which ductal epithelial cells undergo EMT, migrate and invade, and then revert back to an epithelial cell phenotype through MET. Whether this is EMT or simply a transient gain of migratory behavior with accompanying changes in a few proteins is a focus of debate. There is increasing use of the terms “partial” and “full” EMT. But most, if not all, transcriptional responses and other cellular processes are partial. Furthermore, there is increasing recognition of unique functions attributed to the hybrid epithelial–mesenchymal phenotype (Jolly et al. 2016). Defining EMT during fibrosis has relied on lists of epithelial and mesenchymal markers (Zeisberg and Neilson 2009), but it remains unclear at what threshold loss of epithelial markers or gain of mesenchymal markers would be sufficient to achieve at least partial EMT and what threshold is required for full EMT. An alternative approach is to focus on the function of the cells of different origin. This is a departure from defining epithelial and mesenchymal cells as different lineages, but rather a focus on cell phenotype and function.
Similar to the controversy on defining EMT, there is a controversy on the definition of myofibroblasts. Some have argued that expression of SMA itself is not sufficient or required to define a myofibroblast but rather requires stress fiber formation and a contractile phenotype. Given their heterogeneity, some have suggested that the term myofibroblast may better define a phenotype rather than a specific cell type (Hinz 2013). Furthermore, although myofibroblasts are important fibrogenic effector cells, fibrosis is clearly a multicellular process. Also, myofibroblasts are an important source of type I collagen during fibrosis, but type I collagen expression is clearly not limited to myofibroblasts (Zhang et al. 1994). Furthermore, cell type–specific expression of a cytotoxic protein that can be activated, such as thymidine kinase or the diptheria toxin receptor, enables deletion of specific cell types and shows that M2 macrophages and myofibroblasts are necessary for fibrogenesis (Duffield et al. 2005; LeBleu et al. 2013). Further evidence shows a critical role for M2 polarized macrophages during fibrosis (Gibbons et al. 2011; Osterholzer et al. 2013).
Deletion of the TGF-β receptor in fibroblasts, epithelial cells, or bone marrow cells results in abrogated fibrosis (Hoyles et al. 2011; Li et al. 2011; LeBleu et al. 2013). TGF-β clearly elicits a phenotypic response in many cell types that might be viewed as an “activated” state (Katsuno et al. 2013; Derynck et al. 2014; Lamouille et al. 2014). Although the epithelial response to TGF-β might result in profibrotic effects other than EMT, epithelial-specific deletion of a gene encoding an EMT transcription factor leads to attenuated fibrosis. For example, hepatocyte-specific deletion of Snai1 attenuates fibrosis in the CCl4 model of liver fibrosis, and deletion of the gene encoding Forkhead box M1 (FoxM1) in alveolar epithelial cells blunts lung fibrosis after bleomycin or radiation (Rowe et al. 2011; Balli et al. 2013). In models of kidney fibrosis, epithelial-specific deletion of the genes encoding the EMT transcription factor Snail1 or Twist leads to attenuated fibrosis (Grande et al. 2015; Lovisa et al. 2015). These studies support a complex role of the EMT program beyond a simplistic view of EMT-derived cells contributing to a pool of activated myofibroblasts. Rather, cells in partial EMT may play a unique role necessary for fibrogenesis, through regulation of TGF-β-induced cell-cycle arrest and cross-talk with other cell types.
Some studies show that fibrosis can be attenuated by epithelial cell-specific deletion of secreted proteins that have been attributed to the function of activated fibroblasts. For example, connective tissue growth factor (CTGF) is a profibrotic matricellular protein, whose expression is induced by TGF-β. CTGF expression has been used as a marker of activated fibrotic fibroblasts, but several studies indicate that epithelial cells may be the major source of CTGF during fibrosis and deletion of CTGF within epithelial cells blocks fibrosis (Leask and Abraham 2003; Makino et al. 2013; Al-Alawi et al. 2014). Finally, production of type I collagen may be the most basic function of fibrogenic effector cells and deletion of the Col1a1 gene within lung epithelial cells leads to significant reduction in bleomycin-induced lung fibrosis (Rhim et al. 2014).
Thus, using a loss-of-function approach, there is strong evidence that epithelial cells contribute to fibrosis in ways that overlap with those of other fibrogenic effector cells. Whether this is sufficient to be labeled EMT depends on the precise definition of EMT. A broader question is whether fibrogenesis requires a differentiation event or whether cells can undergo significant phenotypic activation without necessarily differentiating into another cell type. There is increasing evidence that fibrosis is a multicellular process. The overlapping versus nonredundant functional contributions of these cell types during fibrosis remains unclear. Finally, attention to how activation, proliferation, and apoptosis of different cell types are orchestrated in response to injury or other fibrotic stimulus might reveal novel targets for intervention, with better effects and fewer side effects than targeting single cell types.
TGF-β REGULATION OF CELL PROLIFERATION AND APOPTOSIS
TGF-β activates the expression of a number of proteins involved in cell-cycle control, including regulators of cyclin-dependent kinases, and signaling by the MAP kinase and PI3K–Akt signaling pathways that promote cell proliferation. Depending on the context and cell type, TGF-β can have dramatic effects on cell proliferation, cell-cycle arrest, senescence, or apoptosis. Although these effects have mainly been studied in the context of cancer biology, these changes can have important consequences on the progression of fibrosis.
Regulation of Fibroblast Proliferation and Apoptosis
Fibrosis is characterized by accumulation of fibroblasts and myofibroblasts. The roles of TGF-β in fibroblast activation to a profibrotic phenotype are well described; however, the role of TGF-β in fibroblast proliferation as a way to account for the abundance of these cells remains unresolved (Bartram and Speer 2004). TGF-β was initially identified as an extracellular growth factor that enables anchorage-independent fibroblast proliferation (Roberts et al. 1980). Many early studies show TGF-β-induced proliferation of fibroblasts from multiple tissues (Hill et al. 1986; Moses et al. 1987, 1994; Schreier et al. 1993). However, other studies have found either no effect on proliferation (Fine and Goldstein 1987) or inhibition of fibroblast proliferation by TGF-β in cell culture and in vivo (Moses et al. 1990; McAnulty et al. 1997; Keerthisingam et al. 2001; Hostettler et al. 2008. Furthermore, immunostaining tissue sections from animal models of fibrosis or from lungs of patients with IPF for proliferation markers, such as Ki67, shows substantial proliferation of epithelial cells but seldom of myofibroblasts of fibroblast foci (El-Zammar et al. 2009; Lomas et al. 2012. These conflicting reports on the ability of TGF-β to induce fibroblast proliferation might be resolved by understanding the costimulatory activation of other signaling pathways that affect the cellular response to TGF-β (Grotendorst et al. 2004). TGF-β can induce the activation of several signaling pathways that promote cell proliferation (e.g., MAPK, PI3K, and Rho signaling). However, the activation of these pathways is not unique to TGF-β, and many other mitogens also promote cell proliferation. Therefore, the microenvironment with the cytokines that stimulate fibroblast proliferation may determine the proliferative response in fibrosis. Importantly, TGF-β can stimulate the expression and secretion of a number of growth factors and cytokines by fibroblasts or other cell types. Many of these TGF-β-induced factors, such as FGF2, PDGF, and CTGF, may secondarily act in autocrine or paracrine fashion to induce fibroblast proliferation (Kay et al. 1998; Strutz et al. 2001; Bartram and Speer 2004; Leask and Abraham 2004; Leask 2009; Xiao et al. 2012). Some studies argue that the TGF-β responses of fibroblasts toward activation and myofibroblast differentiation versus proliferation are mutually exclusive (Grotendorst et al. 2004).
Finally, there is evidence that fibroblasts from fibrotic tissue are resistant to apoptosis, and that TGF-β may confer resistance to apoptosis by classic death pathways such as the Fas-caspase cascade (Zhang and Phan 1999; Chodon et al. 2000; Tanaka et al. 2002; Thannickal and Horowitz 2006). However, the mechanism for their resistance to apoptosis is poorly characterized. It may be mediated through activation of signaling pathways, such as p38 MAPK and PI3K–Akt (Kulasekaran et al. 2009), and involve regulation of “inhibitor of apoptosis” (IAP) family members (Horowitz et al. 2012; Sisson et al. 2012). TGF-β may also promote fibroblast resistance to apoptosis through cell-cycle regulators such as p14ARF (Cisneros et al. 2012). Inhibition of fibroblast and myofibroblast apoptosis through these pathways may account for some of the accumulation of these cells during fibrosis.
TGF-β Regulation of Cell Senescence and Apoptosis
The response to TGF-β by epithelial and endothelial cells includes inhibition of growth, cell-cycle arrest, senescence, or apoptosis (Tucker et al. 1984; Heimark et al. 1986; Takehara et al. 1987). Various insults can lead to damaged cells or undamaged cells that have become essentially nonfunctional within the context of a damaged tissue, promoting either epithelial cell senescence or apoptosis with resulting initiation of fibrosis (Fig. 3).
Cellular senescence can be broadly defined as a process by which cells irreversibly cease to proliferate and typically acquire altered cell shapes and secretory profiles (Kuilman et al. 2010; Campisi 2013; Munoz-Espin and Serrano 2014) whereas apoptosis results from a process of programmed cell death (Campisi 2003). Senescence and apoptosis are adaptive necessary processes in response to injury. Senescence is characterized by the absence of markers of proliferation and increased expression of tumor suppressor proteins that function as cyclin-dependent kinase inhibitors including p16INK4a and p21Cip1. Strong mitogenic activators such as activated Ras or growth factor receptor activation can provoke a shut down in the cell cycle with induction of senescence (Blagosklonny 2003; Senturk et al. 2010).
Apoptosis can be initiated through an extrinsic or intrinsic pathway (Kuwano et al. 1998, 2004; Kuwano 2007). The extrinsic pathway involves engagement of an extracellular ligand to a cell surface death receptor. A well-studied interaction in this context is the activation of Fas by Fas ligand (FasL), but other ligands such as TNF-α can also initiate an apoptotic pathway. The intrinsic or mitochondrial pathway is initiated by cell stresses, such as oxidative stress. As noted above, this stress can lead to senescence or apoptosis through activation of pro-apoptotic Bcl-2 family members, or release of cytochrome C. The role of p21Cip1 may be particularly important in determining the epithelial cell response to stress toward senescence or apoptosis (Zhang et al. 2005).
TGF-β signaling through Smad-dependent and -independent pathways intersects with many senescence and apoptotic pathways (Munoz-Espin et al. 2013; Munoz-Espin and Serrano 2014). Smad complexes can directly activate transciption of p21Cip1 (Datto et al. 1995), which, as discussed above, is a critical inducer of cellular senescence (Munoz-Espin et al. 2013). Transcription of the gene encoding p21Cip1 is also regulated in response to PI3K and ROS, which are both downstream mediators from TGF-β signaling. ROS generation can also lead to activation of latent TGF-β potentially perpetuating senescence (Yu et al. 2009). In a more delayed fashion, TGF-β can also suppress the expression of transcription factors, such as Id (inhibitor of differentiation or DNA- binding) family members, which have been associated with cell proliferation and inhibition of senescence (Shibanuma et al. 1994; Di et al. 2006). Finally, TGF-β-induced secreted factors could indirectly contribute to epithelial cell senescence and apoptosis. As mentioned, ROS generation in response to TGF-β can initiate cell-cycle arrest or apoptosis. TGF-β can also induce expression and secretion of pro-apoptotic factors, including FasL and angiotensin (Wang et al. 1999). As with effects on proliferation, concurrent stimulation or activation by other signaling pathways has the potential to tune the degree of this TGF-β response.
Early studies in cell culture indicate that epithelial and endothelial cells undergo apoptosis in response to TGF-β. These observations correlate well with the increased epithelial cell apoptosis in fibrotic tissue and in animal models of fibrosis (Uhal et al. 1998; Barbas-Filho et al. 2001). Indeed, expression of activated TGF-β1 using transgenic approaches or adenoviral gene transfer is sufficient to induce epithelial cell apoptosis and subsequent fibrosis (Lee et al. 2004, 2006; Ask et al. 2008). Importantly, TGF-β1-induced fibrosis is attenuated by treating mice with a pan-caspase inhibitor, and in mice with inactivation of BH3-interacting domain death agonist (BID) or BCL-2-associated (BAX) expression, suggesting that apoptosis is critical for fibrosis (Lee et al. 2004; Kang et al. 2007).
The roles of senescence and apoptosis in injury, repair, and fibrosis depend on the context. Transient apoptosis or senescence may result in removal of unwanted cells and cell debris, and facilitate repair to reinstate a homeostatic architecture and function. Conversely, sustained or abnormal senescence or apoptosis could lead to a pathological wound repair response that progresses to fibrosis. Excessive senescence or apoptosis could also lead to delayed restoration of a damaged endothelial or epithelial barrier, thus promoting persistent leak of serum components into the lumen with continued activation of damage response pathways and sustained inflammation. Epithelial cell turnover and impaired proliferation may also delay production of antifibrotic factors, such as prostaglandin E2 (Moore et al. 2003). Activation of senescence leads to dramatic transcriptional changes that are mediated in large part by responses to activation of the NF-κB and C/EBP-β transcription factors (Campisi 2013; Campisi and Robert 2014). In particular, significant changes occur in the expression of many secreted factors, including cytokines, growth factors, and proteases in what has been called a “senescence-associated secretory phenotype” (SASP). The SASP thus allows the senescent cell to become an abundant source of injury and inflammatory mediators with potential important effects on promoting fibrosis (Fig. 3) (Aoshiba et al. 2003; Coppe et al. 2008, 2010; Aoshiba et al. 2013). Thus, the role of TGF-β in regulating fibrosis is mediated by divergent effects on both fibroblast and epithelial cell survival.
ROLE OF TGF-β1 IN DIVERSE FIBROTIC DISEASES
Tissue fibrosis is a common consequence of many different acute and chronic insults, which can severely impair the function of nearly every organ system. Fibrosis can also occur as a primary progressive process without an apparent inciting event. Collectively, fibrosis is a leading cause of death and morbidity in the developed world, accounting for up to 45% of all deaths by some estimates (Wynn 2007). There is considerable overlap among the different fibrotic conditions, with TGF-β1 signaling having a prominent role. Although progressive fibrosis, in general, is difficult to reverse or even slow down, understanding the role of TGF-β1 signaling in this process offers insight into potential novel therapeutic strategies. A review of the history and current thinking of the roles of TGF-β1 in pulmonary fibrosis serves to highlight this principle.
Evidence Linking TGF-β1 to Fibrosis in the Lung Parenchyma
As the promoting role of TGF-β1 in experimental fibrosis was well described soon after discovery of TGF-β1 (Roberts et al. 1986), the role of TGF-β1 in pulmonary fibrosis was examined decades ago. TGF-β1 expression was found to appear early in the bleomycin-induced model of pulmonary fibrosis, preceding collagen deposition, and to localize to sites of subsequent collagen accumulation in both a mouse model and in humans with fibrotic lung disease (Hoyt DG 1988). Active TGF-β1, when overexpressed in the lung using a viral vector, was found to be sufficient to cause severe lung fibrosis in rodents, requiring signaling through Smad3 (Sime et al. 1997; Gauldie et al. 1999). Interestingly, in an inducible model of TGF-β1 expression in lungs, reversal of TGF-β1 expression led to substantial resolution of the fibrotic process (Lee et al. 2004). TGF-β1 proved to be both necessary and sufficient for lung fibrosis. As discussed above, activation of endogenous, latent TGF-β1, via the integrin αvβ6, was found to be required for lung fibrosis in both bleomycin- and radiation-induced lung fibrosis models (Munger et al. 1999), as well as models of kidney fibrosis. The critical role of TGF-β1 in fibrosis was further validated by expression profiling of human fibrotic lungs. Microarray profiles reveal that ∼80% of the genes activated in fibrotic human lungs are known TGF-β1 target genes (Kaminski et al. 2000).
Epithelial Activation and Fibrogenesis
Genetic analysis of patients with familial interstitial pneumonitis point to type II alveolar epithelial dysfunction as a consequence of SP gene mutations and as a causal mechanism for pulmonary fibrosis (Nogee et al. 2001; Thomas et al. 2002; Wang et al. 2009; Garcia 2011). Mouse models expressing mutant SPs support this conclusion. This has led to the general concept that epithelial cells are both vulnerable to further injury and “activated” as a driver of fibrosis. There is evidence to support the development of at least three distinct dysfunctional states in the context of chronic injury and/or stress to the epithelium: TGF-β1-induced mesenchymal transition (EMT as defined above), senescence, and ER stress (Fig. 3). As discussed above, TGF-β1 may itself contribute to both senescence and ER stress. Each of these dysfunctional states have distinct biological features but all show evidence of enhanced secretory activity, at least some of which consists of profibrotic and/or pro-inflammatory cytokines and mediators. Frequently superimposed on these dysfunctional states are hypoxia, enhanced oxidant production, and exposure to environmental toxins such as smoke and viruses, further contributing to alveolar dysfunction. Collectively, these stresses reprogram the epithelium toward a secretory phenotype altering the immune milieu and promoting mesenchymal expansion (Fig. 3).
Progressive dysfunction of the alveolar epithelium can lead to apoptosis and alveolar collapse. Collapse of small distal airways (<2 mm diameter) and surrounding alveoli is prominent during parenchymal lung injury and repair (Seeger et al. 1993; Lutz et al. 2015). Active sites of fibrogenesis, termed fibrotic foci, in interstitial pneumonitis have been noted by many investigators to contain basement membrane within the interstitium, suggesting that the focus of fibrogenesis may emanate from hypoxic areas of small airway and alveolar collapse with incorporation of epithelial lining and inflammatory alveolar cells into a mesenchymal compartment (Myers and Katzenstein 1988). This phenomenon has been termed induration fibrosis and may be unique to lung architecture because incorporation of epithelial cells into a mesenchymal compartment has not been observed during either liver or kidney fibrogenesis (Lutz et al. 2015). Airway and alveolar collapse may promote direct epithelial–mesenchymal interactions, including activation and signaling by TGF-β1. The ultimate fate of such epithelial cells is uncertain but could be either apoptosis and/or persistent EMT, as discussed above. Hypoxia is known to promote collagen expression in fibroblasts and TGF-β1-dependent EMT in primary epithelial cells and within tumors (Falanga et al. 2002; Tzouvelekis et al. 2007; Zhou et al. 2009; Kottmann et al. 2012). Alveolar collapse also likely contributes to increased tissue stiffness that itself can propagate TGF-β1 activation as discussed above, raising the possibility that such collapse is an important trigger for disease progression.
TGF-β1 Signaling in Airway Fibrosis: Integration of Immunity and Stromal Signaling
The major lung airways are a frequent site of injury from environmental exposures. The most common chronic lung diseases, asthma and chronic obstructive pulmonary disease (COPD), are airway-centric inflammatory processes and in both cases there is repeated epithelial dysfunction and disordered ECM remodeling with collagen accumulation in the submucosa of airways (Aoshiba and Nagai 2004; Davies 2009; Hirota and Martin 2013). The development of fixed airway obstruction in some asthmatic patients over time is thought to result from airway wall remodeling in which airway collagen accumulation is a common and prominent feature. In some studies, high-resolution imaging of asthmatic airway wall thickness correlates with lung function and fixed airflow obstruction, cardinal features of airway disease (de Jong et al. 2005; Bosse et al. 2008). Airway remodeling, however, is not simply fibrotic tissue accumulation in the airway walls. Remodeling involves almost all elements of the airway wall: chronic inflammation involving innate and adaptive immune cells, epithelial goblet cell and subepithelial smooth muscle hyperplasia, as well as submucosal neovascularization in addition to thickening of the subepithelial lamina reticularis, one of the hallmarks of asthma histopathology (Postma and Timens 2006; Hirota and Martin 2013). Lying immediately below the basement membrane, the lamina reticularis is a membranous, collagenous airway structure found in large conducting airways and thickened in asthmatics by the accumulation of several ECM proteins, including the fibrillar collagens type I and type III. Collagen accumulation in the lamina reticularis in asthma is not as well organized into fibrillar bundles as fibrils in fibrotic tissues, but nonetheless collagen fibrils are commonly observed throughout these structures (Saglani et al. 2006). In addition, the airway submucosal collagens surrounding fibroblasts are frequently expanded in both animal models of chronic asthma and asthmatic patients. Similar changes in airway collagen accumulation occur to a somewhat lesser degree in COPD airways (Jeffery 2001; Aoshiba and Nagai 2004; Postma and Timens 2006). Such airway accumulation of fibrillar collagens may restrict airway lumen size, and this has been argued in rodent models. Because of the many elements involved in airway remodeling, it has not been determined to what degree collagen deposition per se contributes to fixed airway obstruction in longstanding active asthma. Nonetheless, airway remodeling involving accumulation of collagen is a prominent feature of asthma, and, as discussed below, accumulating evidence links this pathobiology to TGF-β1 activation (Makinde et al. 2007).
The dynamic interplay between activated epithelial cells and surrounding airway stromal elements in asthma has led to the concept of an epithelial–mesenchymal “trophic unit,” implying that airway homeostasis is maintained by an ongoing exchange of soluble mediators between the epithelium and surrounding mesenchymal cells (Holgate et al. 2004). In contrast, there is no evidence that activated epithelial cells cross the airway basement membranes to directly contact mesenchymal cells or become mesenchymal cells, in either asthma or COPD. The impact of signaling interactions between the epithelial lining cells and underlying stromal elements has largely been studied in the context of perturbed epithelial and immune cell function in inflammatory airway disease. Most studies point to mediators released by native and adaptive immune cells that activate epithelial cells to release cytokines impacting airway bronchoconstriction and remodeling (Boxall et al. 2006; Doherty et al. 2011; Halwani et al. 2011; Hirota and Martin 2013; Trian et al. 2015). Given the collagen accumulation as a prominent part of airway wall remodeling, it is not surprising that a major target of immune-mediated cytokine signaling is increased TGF-β1 expression and activation in both the epithelium and the airway walls of asthmatic and COPD patients. Epithelial–mesenchymal cross-talk has been studied in COPD, focusing on the squamous metaplasia commonly observed in large conducting airways of COPD patients (Araya et al. 2007). Squamous epithelial cells in culture, but not the airway basal cells from which they are derived, release cytokines, especially IL-1β, which then induce TGF-β1 activation and myofibroblast accumulation. Activated fibroblasts are thought to promote a positive feedback for this process by further releasing TGF-β1 to impair epithelial proliferation and promote the squamous phenotype. The squamous morphology of cultured bronchial epithelial cells and the high expression of involucrin and the cyclin-dependent kinase inhibitor p21Cip1 are suggestive of a senescent phenotype, a phenotype known to be promoted by stress and accompanied by release of pro-inflammatory cytokines, although this possibility has not been directly addressed in the lung (Rodier and Campisi 2011). Similar pathways are likely activated in asthma. In this case, the production of Th2 cytokines by innate and adaptive immune cells is increased, and their activities on epithelial cells, as well as epithelial injury, lead to the release of cytokines including TGF-β1, PDGF, and insulin-like growth factors by the epithelial cells. These then promote proliferation of fibroblasts and smooth muscle cells that is characteristic of airway remodeling in asthma (Holgate et al. 2004; Boxall et al. 2006; Davies 2009). As discussed below, a common and important element in altered fibroblast function in all these settings involves TGF-β1 activation, which is the likely driver of submucosal airway collagen accumulation.
Stromal elements involved in remodeling of asthmatic airways show enhanced sensitivity to immune Th2 cytokine signals. Primary human airway fibroblasts in culture respond to IL-13, a prominent Th2 cytokine implicated in asthma pathobiology, with activation of COL1A2 mRNA expression and collagen protein deposition. This response is greater in primary cells isolated from asthmatic patients when compared with cells of healthy individuals (Firszt et al. 2014). IL-13-induced collagen expression was found to depend on TGF-β1 activation and signaling by fibroblasts. IL-13 also promotes TGF-β1 expression and release from asthmatic epithelium. Earlier studies exploring the response of airway fibroblasts to IL-4 report similar findings, suggesting that in some way the ongoing inflammation and stimulation of stromal elements primes these airway fibroblasts for further response (Saito et al. 2003). In addition, smooth muscle cell proliferation has been reported to be hyperactive in asthmatic airways (Trian et al. 2015). Bronchial smooth muscle cells from asthmatic large airways express higher levels of the cysteinyl leukotriene receptor CysLTR1, a receptor for leukotriene C4, and display an increased proliferative response to the epithelial-derived leukotriene C4 compared with smooth muscle cells from normal airways (Trian et al. 2015). These findings underscore the paradigm that the epithelial-derived signals are in part responsive to cytokine stimulation from immune cells, and activate bidirectional signaling that leads to airway remodeling with airway fibrosis in both asthma and COPD.
The effector mechanisms of TGF-β1-dependent fibrogenesis in airways do not appear different from other sites of fibrosis driven by TGF-β1, but the mechanisms of latent TGF-β1 activation are distinct in the conducting airways. Whereas alveolar epithelial TGF-β1 activation depends on integrin αvβ6, large conducting airway epithelial TGF-β1 activation is mediated by both αvβ6 and αvβ8 integrins (Munger et al. 1999; Mu et al. 2002). The mechanism of activation of TGF-β1 by αvβ8 requires metalloproteinase (MMP)-mediated cleavage of latent TGF-β1, in contrast to cytoskeleton-mediated conformational strain on integrins in αvβ6-mediated TGF-β1 activation. Dendritic cells also require αvβ8 to activate surface TGF-β1 that in turn promotes differentiation of CD4 T cells to T cells that produce IL-17 along with IL-6, and to regulatory T cells (Tregs) (Travis and Sheppard 2014). Expansion of IL-17-producing CD4+ T cells enhances airway smooth muscle contraction in response to binding of IL-17 to its receptors that are expressed on smooth muscle cells, extending the well-known role of IL-17 as a pro-inflammatory cytokine (Kudo et al. 2012). Mice that lack expression of the dendritic cell integrin αvβ8, as a result of Cre recombinase expression from the Itgax promoter and Cre-mediated deletion of β8, spontaneously develop an autoimmune colitis at about six months of age, consistent with the well-known role of TGF-β1 in promoting differentiation of T cells toward an immunosuppressive Treg lineage (Wan and Flavell 2007). The anti-inflammatory and immune regulatory effects of TGF-β1, mediated in large part by dendritic cells, are prominent in asthma and are layered on top of the profibrotic pathways initiated in activated epithelial cells and fibroblasts. Hence, it may not be meaningful to attempt to correlate the overall levels of latent TGF-β1 expression or even TGF-β activity in the lung with the severity or progression of asthma and COPD. For example, inhibition of TGF-β1 signaling protects mice from airway remodeling in an allergen-induced model, but what affected cell type accounts for this protection is uncertain (McMillan et al. 2005). Mice deficient in periostin expression have enhanced bronchoconstrictor responses and higher allergen-induced IgE expression in an allergy model (Gordon et al. 2012). Periostin interacts with collagen and promotes its organization into fibrils. In the absence of periostin, TGF-β1 activation is reduced resulting in decreased Treg cell differentiation with an enhanced immune response. Thus, the pathogenic role of TGF-β1 in asthma pathobiology, and peri-airway fibrosis specifically, is complicated by the high activation level of the mucosal immune system in asthma. Indirect effects through changes in immune cell function rather than directly altered fibroblast collagen production could be a major mechanism for TGF-β1 effects in asthma and COPD.
THERAPEUTIC IMPLICATIONS
Integrin-mediated TGF-β activation has been shown to substantially contribute to tissue pathology in a number of disease models. For example, mice lacking the αvβ6 integrin are protected in murine models of lung, kidney, and biliary fibrosis (Munger et al. 1999; Jaramillo et al. 2003; Hahm et al. 2007). These mice are also protected in models of acute lung injury (Pittet et al. 2001), allergic asthma (Sugimoto et al. 2012; Jolly et al. 2014), and severe influenza infection (Jolly et al. 2014), and in each case, protection appeared to be explained by local inhibition of TGF-β activation. These results, together with evidence that αvβ6 integrin expression is dramatically increased in dysfunctional epithelial cells overlying regions of pulmonary fibrosis in humans (Horan et al. 2008), suggest that this integrin could be a promising therapeutic target. The development of potent blocking monoclonal antibodies that inhibit αvβ6-mediated TGF-β1 activation in multiple species has made it possible to evaluate their use in disease models after tissue pathology has already been established. The success of these antibodies in multiple disease models (Horan et al. 2008; Puthawala et al. 2008) has stimulated the development of a humanized monoclonal antibody that targets αvβ6 and is currently in a phase II clinical trial for treatment of IPF.
αvβ8-dependent TGF-β1 activation has also emerged as a promising therapeutic target. Mice lacking this integrin on dendritic cells are dramatically deficient in antigen-specific T helper 17 (Th17) cells, and are protected in experimental autoimmune encephalitis (Melton et al. 2010) and allergic asthma (Kudo et al. 2012). Mice lacking αvβ8-mediated TGF-β1 activation in fibroblasts are protected in models of airway inflammation, and studies in mice engineered to replace murine β8 with its human ortholog show that a monoclonal antibody against human αvβ8 that blocks TGF-β1 activation protects against allergic airway inflammation and remodeling induced by exposure to cigarette smoke (Minagawa et al. 2014).
As noted above, recent evidence suggests that the αvβ1 integrin on fibroblasts contributes to tissue fibrosis in multiple organs (Lawson et al. 2008, 2011; Tanjore et al. 2011, 2012, 2013; Torres-Gonzalez et al. 2012; Henderson et al. 2013; Reed et al. 2015). Therapeutic intervention with a broadly active αv integrin inhibitor or a highly specific αvβ1 small molecule inhibitor in mice with already established liver or pulmonary fibrosis causes a marked reduction in collagen accumulation, suggesting that αvβ1 could also be a therapeutic target for treatment of diseases characterized by excessive tissue fibrosis.
Besides the strong rationale for therapeutic agents directed at integrin-dependent TGF-β1 activation, elements of TGF-β1 signaling and other aspects of the pathophysiology of fibrogenesis are also potential therapeutic targets. As noted above, a number of profibrotic cytokines such as CTGF, FGF, and PDGF have been explored as potential targets for antifibrotic therapy, in large part because of their convergence with the TGF-β pathway. Studies of EMT in fibrosis began largely because of the strong parallels between signaling pathways of fibrosis and pathways of EMT in cell culture. One of the ironies then about the possible roles of EMT in fibrosis is that, from a clinical viewpoint, therapeutic agents directed at either pathways of EMT or pathways of fibroblast expansion and ECM secretion may almost completely overlap, making definitions and cells of origin of little importance. For example, the best-studied EMT transcription factors are Snail1, Slug/Snail2, Twist, ZEB1, and Sip1/ZEB2. Their expression can be induced by TGF-β1 in both epithelial and mesenchymal cells. Understanding these pathways and the epithelial cell response to TGF-β offers potential for therapeutic intervention in fibrosis, even if many of these pathways are not unique to EMT. In addition, expansion of the ECM impacts fibrogenesis downstream from TGF-β1 activation by exerting strong prosurvival signals as a result of integrin activation. Activated β1 integrins promote FAK and Akt activation leading to accumulation of anti-apoptotic factors that could counter the pro-apoptotic signaling in response to TGF-β1 in the epithelium, ER stress, and senescence (Horowitz et al. 2007; Thannickal et al. 2014). Moreover, recent studies point to ECM stiffness and integrin activation as a TGF-β1-independent pathway of epithelial Stat3 activation with subsequent further fibroblast activation and ECM accumulation (Prele et al. 2012; Duffield 2016). These studies highlight some additional sites of potential therapeutic intervention downstream from TGF-β1 activation that are studied in clinical and preclinical trials (Ahluwalia et al. 2014; Blackwell et al. 2014). The current rationales and status of trials directed at TGF-β signaling in fibrosis have recently been well summarized (Ahluwalia et al. 2014).
Emerging evidence indicating the prominence of ER stress and cell senescence in the pathobiology of fibrotic lung disease also offers new therapeutic possibilities (Lawson et al. 2008, 2011; Tanjore et al. 2011, 2012, 2013; Torres-Gonzalez et al. 2012). TGF-β1 signaling may contribute to this pathophysiology indirectly by the induction of enhanced collagen secretion in cells that are already stressed by the accumulation of misfolded proteins. Indeed, collagen itself is only marginally soluble and its complicated helical structure is prone to misfolding. Whether and how the UPR can be manipulated to attenuate the potentially profibrotic and self-destructive elements in its response to ER stress (Fig. 3) is an important avenue for further investigation. Likewise, the accumulation of senescent cells in the context of chronic and recurring injury, as well as aging itself, may offer new insights (Sanders et al. 2013; Thannickal 2013; Hecker and Thannickal 2016). For example, mice lacking caveolin 1 expression were found to be protected from fibrosis through decreased epithelial senescence after bleomycin-induced injury (Shivshankar et al. 2012). Further studies of telomerase activators have been suggested as a potential point of therapeutic intervention in senescence-initiated fibrosis (Le Saux et al. 2013). Similarly, targeting nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4), a TGF-β1-responsive gene, attenuates lung fibrosis at least in part through inhibition of NOX4- or ROS-mediated senescence (Hecker et al. 2009). The potentially profibrotic and pro-inflammatory character of the SASP secretome and regulators of senescence during fibrogenesis need better clarification (Kang et al. 2007). It remains unclear to what degree the SASP and the secretory profile of TGF-β1-induced EMT and ER stress (Fig. 3) share expression of common profibrotic mediators or exert their effects on fibrosis through distinct pathways. These uncertainties remain important to clarify as attempts to develop new therapeutics center around the early events that lead to epithelial dysfunction and a disordered repair process, not only in the lung but at all sites of fibrogenesis.
ACKNOWLEDGMENTS
We thank Justin A. Klein, CMI, for assistance with illustrations. This work was supported in part by the National Institutes of Health (NIH) grant PO1 HL108794.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
*Reference is also in this subject collection.
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. 2009. Epithelial–mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest 119: 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamali HI, Maher TM. 2012. Current and novel drug therapies for idiopathic pulmonary fibrosis. Drug Des Devel Ther 6: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia N, Shea BS, Tager AM. 2014. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med 190: 867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi K, Sandig V, Vooijs M, Van der Valk M, Giovannini M, Strauss M, Berns A. 1997. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res 25: 1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Alawi M, Hassan T, Chotirmall SH. 2014. Transforming growth factor β and severe asthma: A perfect storm. Respir Med 108: 1409–1423. [DOI] [PubMed] [Google Scholar]
- Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS. 2009. Mice that lack activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Rifkin DB, Munger JS. 2002. The integrin αVβ6 binds and activates latent TGFβ3. FEBS Lett 511: 65–68. [DOI] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB. 2004. Integrin αVβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J Cell Biol 165: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshiba K, Nagai A. 2004. Differences in airway remodeling between asthma and chronic obstructive pulmonary disease. Clin Rev Allergy Immunol 27: 35–43. [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Tsuji T, Nagai A. 2003. Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J 22: 436–443. [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Tsuji T, Kameyama S, Itoh M, Semba S, Yamaguchi K, Nakamura H. 2013. Senescence-associated secretory phenotype in a mouse model of bleomycin-induced lung injury. Exp Toxicol Pathol 65: 1053–1062. [DOI] [PubMed] [Google Scholar]
- Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, et al. 2007. Squamous metaplasia amplifies pathologic epithelial–mesenchymal interactions in COPD patients. J Clin Invest 117: 3551–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asharani PV, Keupp K, Semler O, Wang W, Li Y, Thiele H, Yigit G, Pohl E, Becker J, Frommolt P, et al. 2012. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet 90: 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ask K, Bonniaud P, Maass K, Eickelberg O, Margetts PJ, Warburton D, Groffen J, Gauldie J, Kolb M. 2008. Progressive pulmonary fibrosis is mediated by TGF-β isoform 1 but not TGF-β3. Int J Biochem Cell Biol 40: 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balli D, Ustiyan V, Zhang Y, Wang IC, Masino AJ, Ren X, Whitsett JA, Kalinichenko VV, Kalin TV. 2013. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. EMBO J 32: 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas-Filho JV, Ferreira MA, Sesso A, Kairalla RA, Carvalho CR, Capelozzi VL. 2001. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IFP)/usual interstitial pneumonia (UIP). J Clin Pathol 54: 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. 1994. Transforming growth factor-β activation in irradiated murine mammary gland. J Clin Invest 93: 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, et al. 2010. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med 16: 1009–1017. [DOI] [PubMed] [Google Scholar]
- Bartram U, Speer CP. 2004. The role of transforming growth factor β in lung development and disease. Chest 125: 754–765. [DOI] [PubMed] [Google Scholar]
- Beers MF, Morrisey EE. 2011. The three R’s of lung health and disease: Repair, remodeling, and regeneration. J Clin Invest 121: 2065–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendris N, Arsic N, Lemmers B, Blanchard JM. 2012. Cyclin A2, Rho GTPases and EMT. Small GTPases 3: 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasin V, Marsh LM, Egemnazarov B, Wilhelm J, Ghanim B, Klepetko W, Wygrecka M, Olschewski H, Eferl R, Olschewski A, et al. 2014. Meprin β, a novel mediator of vascular remodelling underlying pulmonary hypertension. J Pathol 233: 7–17. [DOI] [PubMed] [Google Scholar]
- Blaauboer ME, Emson CL, Verschuren L, van Erk M, Turner SM, Everts V, Hanemaaijer R, Stoop R. 2013. Novel combination of collagen dynamics analysis and transcriptional profiling reveals fibrosis-relevant genes and pathways. Matrix Biol 32: 424–431. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, Bar-Joseph Z, Bitterman P, Blackburn MR, Bradford W, et al. 2014. Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am J Respir Crit Care Med 189: 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. 2003. Cell senescence and hypermitogenic arrest. EMBO Rep 4: 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse Y, Pare PD, Seow CY. 2008. Airway wall remodeling in asthma: From the epithelial layer to the adventitia. Curr Allergy Asthma Rep 8: 357–366. [DOI] [PubMed] [Google Scholar]
- Bourhis JM, Mariano N, Zhao Y, Harlos K, Exposito JY, Jones EY, Moali C, Aghajari N, Hulmes DJ. 2012. Structural basis of fibrillar collagen trimerization and related genetic disorders. Nat Struct Mol Biol 19: 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen T, Jenkins RH, Fraser DJ. 2013. MicroRNAs, transforming growth factor β-1, and tissue fibrosis. J Pathol 229: 274–285. [DOI] [PubMed] [Google Scholar]
- Boxall C, Holgate ST, Davies DE. 2006. The contribution of transforming growth factor-β and epidermal growth factor signalling to airway remodelling in chronic asthma. Eur Respir J 27: 208–229. [DOI] [PubMed] [Google Scholar]
- Broder C, Arnold P, Vadon-Le Goff S, Konerding MA, Bahr K, Muller S, Overall CM, Bond JS, Koudelka T, Tholey A, et al. 2013. Metalloproteases meprin α and meprin β are C- and N-procollagen proteinases important for collagen assembly and tensile strength. Proc Natl Acad Sci 110: 14219–14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MA, Chang S, Wakimoto H, Gorham JM, Conner DA, Christodoulou DC, Parfenov MG, DePalma SR, Eminaga S, Konno T, et al. 2016. Molecular profiling of dilated cardiomyopathy that progresses to heart failure. JCI Insight 1: e86898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. 2003. Cellular senescence and apoptosis: How cellular responses might influence aging phenotypes. Exp Gerontol 38: 5–11. [DOI] [PubMed] [Google Scholar]
- Campisi J. 2013. Aging, cellular senescence, and cancer. Annu Rev Physiol 75: 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Robert L. 2014. Cell senescence: Role in aging and age-related diseases. Interdiscip Top Gerontol 39: 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff RD. 2005. Epithelial to mesenchymal transition tumors: Fallacious or snail’s pace? Clin Cancer Res 11: 8534–8537. [DOI] [PubMed] [Google Scholar]
- Chang Y JH, Reed NI, Wang Y, Atakilit A, DeGrado WF, Sheppard D. 2017. Fibroblast αvβ1 integrin is a key regulator of renal fibrosis and pharmacological blockage of αvβ1 ameliorates renal fibrosis in vivo. J Am Soc Nephrol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman HA. 2010. Epithelial–mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol 73: 413–435. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Ning H, Ishida W, Sodin-Semrl S, Takagawa S, Mori Y, Varga J. 2006. The early-immediate gene EGR-1 is induced by transforming growth factor-β and mediates stimulation of collagen gene expression. J Biol Chem 281: 21183–21197. [DOI] [PubMed] [Google Scholar]
- Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, et al. 2003. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162: 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodon T, Sugihara T, Igawa HH, Funayama E, Furukawa H. 2000. Keloid-derived fibroblasts are refractory to Fas-mediated apoptosis and neutralization of autocrine transforming growth factor-β1 can abrogate this resistance. Am J Pathol 157: 1661–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. 2011. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 53: 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros J, Hagood J, Checa M, Ortiz-Quintero B, Negreros M, Herrera I, Ramos C, Pardo A, Selman M. 2012. Hypermethylation-mediated silencing of p14ARF in fibroblasts from idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 303: L295–L303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6: 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. 2010. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu Rev Pathol 5: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Erler JT. 2011. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis Model Mech 4: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. 1998. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 93: 1159–1170. [DOI] [PubMed] [Google Scholar]
- Das S, Kumar M, Negi V, Pattnaik B, Prakash YS, Agrawal A, Ghosh B. 2014. MicroRNA-326 regulates profibrotic functions of transforming growth factor-β in pulmonary fibrosis. Am J Respir Cell Mol Biol 50: 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. 1995. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci 92: 5545–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JA. 1996. Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat 156: 187–201. [DOI] [PubMed] [Google Scholar]
- Davies DE. 2009. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc 6: 678–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PA, Muller NL, Pare PD, Coxson HO. 2005. Computed tomographic imaging of the airways: Relationship to structure and function. Eur Respir J 26: 140–152. [DOI] [PubMed] [Google Scholar]
- DeMaio L, Buckley ST, Krishnaveni MS, Flodby P, Dubourd M, Banfalvi A, Xing Y, Ehrhardt C, Minoo P, Zhou B, et al. 2012. Ligand-independent transforming growth factor-β type I receptor signalling mediates type I collagen-induced epithelial–mesenchymal transition. J Pathol 226: 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. 2003. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425: 577–584. [DOI] [PubMed] [Google Scholar]
- Derynck R, Muthusamy BP, Saeteurn KY. 2014. Signaling pathway cooperation in TGF-β-induced epithelial–mesenchymal transition. Curr Opin Cell Biol 31: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di K, Ling MT, Tsao SW, Wong YC, Wang X. 2006. Id-1 modulates senescence and TGF-β1 sensitivity in prostate epithelial cells. Biol Cell 98: 523–533. [DOI] [PubMed] [Google Scholar]
- Dixit R, Ai X, Fine A. 2013. Derivation of lung mesenchymal lineages from the fetal mesothelium requires Hedgehog signaling for mesothelial cell entry. Development 140: 4398–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, Norris PS, Choi H, Scheu S, Pfeffer K, et al. 2011. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med 17: 596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Hudson NE, Lu C, Springer TA. 2014. Structural determinants of integrin β-subunit specificity for latent TGF-β. Nat Struct Mol Biol 21: 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Zhao B, Iacob RE, Zhu J, Koksal AC, Lu C, Engen JR, Springer TA. 2017. Force interacts with macromolecular structure in activation of TGF-β. Nature 542: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband JL, Monier F, Delannet M, Newgreen D. 1995. Epithelium–mesenchyme transition during neural crest development. Acta Anat 154: 63–78. [DOI] [PubMed] [Google Scholar]
- Duffield JS. 2016. Beyond EMT: Epithelial STAT3 as a central regulator of fibrogenesis. J Am Soc Nephrol 27: 3502–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. 2005. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zammar O, Rosenbaum P, Katzenstein AL. 2009. Proliferative activity in fibrosing lung diseases: A comparative study of Ki-67 immunoreactivity in diffuse alveolar damage, bronchiolitis obliterans–organizing pneumonia, and usual interstitial pneumonia. Hum Pathol 40: 1182–1188. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. 2006. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440: 1222–1226. [DOI] [PubMed] [Google Scholar]
- Falanga V, Zhou L, Yufit T. 2002. Low oxygen tension stimulates collagen synthesis and COL1A1 transcription through the action of TGF-β1. J Cell Physiol 191: 42–50. [DOI] [PubMed] [Google Scholar]
- Farhat YM, Al-Maliki AA, Chen T, Juneja SC, Schwarz EM, O’Keefe RJ, Awad HA. 2012. Gene expression analysis of the pleiotropic effects of TGF-β1 in an in vitro model of flexor tendon healing. PLoS ONE 7: e51411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil S, Valtcheva N, Feil R. 2009. Inducible Cre mice. Methods Mol Biol 530: 343–363. [DOI] [PubMed] [Google Scholar]
- Feil S, Krauss J, Thunemann M, Feil R. 2014. Genetic inducible fate mapping in adult mice using tamoxifen-dependent Cre recombinases. Methods Mol Biol 1194: 113–139. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. 2005. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693. [DOI] [PubMed] [Google Scholar]
- Fine A, Goldstein RH. 1987. The effect of transforming growth factor-β on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem 262: 3897–3902. [PubMed] [Google Scholar]
- Firszt R, Francisco D, Church TD, Thomas JM, Ingram JL, Kraft M. 2014. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase-2 and transforming growth factor-β1 in airway fibroblasts in asthma. Eur Respir J 43: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitchett JE, Hay ED. 1989. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev Biol 131: 455–474. [DOI] [PubMed] [Google Scholar]
- Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. 2010. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem 285: 20202–20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Sheppard D, Duffield JS, Violette S. 2013. Therapy for fibrotic diseases: Nearing the starting line. Sci Transl Med 5: 167sr161. [DOI] [PubMed] [Google Scholar]
- Garcia CK. 2011. Idiopathic pulmonary fibrosis: Update on genetic discoveries. Proc Am Thorac Soc 8: 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J, Sime PJ, Xing Z, Marr B, Tremblay GM. 1999. Transforming growth factor-β gene transfer to the lung induces myofibroblast presence and pulmonary fibrosis. Curr Top Pathol 93: 35–45. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Yuan W, Mori Y, Varga J. 2000. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene 19: 3546–3555. [DOI] [PubMed] [Google Scholar]
- Giacomini MM, Travis MA, Kudo M, Sheppard D. 2012. Epithelial cells utilize cortical actin/myosin to activate latent TGF-β through integrin αvβ6-dependent physical force. Exp Cell Res 318: 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, van Rooijen N, Haslett C, Howie SE, Simpson AJ, et al. 2011. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med 184: 569–581. [DOI] [PubMed] [Google Scholar]
- Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, Conway SJ, Huang X, Locksley RM, Fahy JV. 2012. A protective role for periostin and TGF-β in IgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy 42: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande MT, Sanchez-Laorden B, Lopez-Blau C, De Frutos CA, Boutet A, Arevalo M, Rowe RG, Weiss SJ, Lopez-Novoa JM, Nieto MA. 2015. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21: 989–997. [DOI] [PubMed] [Google Scholar]
- Griffith CM, Hay ED. 1992. Epithelial–mesenchymal transformation during palatal fusion: Carboxyfluorescein traces cells at light and electron microscopic levels. Development 116: 1087–1099. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Rahmanie H, Duncan MR. 2004. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J 18: 469–479. [DOI] [PubMed] [Google Scholar]
- Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, et al. 2007. αvβ6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170: 110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. 2011. Role of transforming growth factor-β in airway remodeling in asthma. Am J Respir Cell Mol Biol 44: 127–133. [DOI] [PubMed] [Google Scholar]
- Harris WT, Kelly DR, Zhou Y, Wang D, MacEwen M, Hagood JS, Clancy JP, Ambalavanan N, Sorscher EJ. 2013. Myofibroblast differentiation and enhanced TGF-β signaling in cystic fibrosis lung disease. PLoS ONE 8: e70196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. 2004. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 113: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, Hasegawa Y. 2010. Endothelial–mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 43: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Thannickal VJ. 2016. Getting to the core of fibrosis: Targeting redox imbalance in aging. Ann Transl Med 4: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. 2009. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimark RL, Twardzik DR, Schwartz SM. 1986. Inhibition of endothelial regeneration by type-β transforming growth factor from platelets. Science 233: 1078–1080. [DOI] [PubMed] [Google Scholar]
- Heindryckx F, Binet F, Ponticos M, Rombouts K, Lau J, Kreuger J, Gerwins P. 2016. Endoplasmic reticulum stress enhances fibrosis through IRE1α-mediated degradation of miR-150 and XBP-1 splicing. EMBO Mol Med 8: 729–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Heldin C-H, Moustakas A. 2016. Signaling receptors for TGF-β family members. Cold Spring Harbor Perspect Biol 8: a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, et al. 2013. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19: 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernnas J, Nettelbladt O, Bjermer L, Sarnstrand B, Malmstrom A, Hallgren R. 1992. Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur Respir J 5: 404–410. [PubMed] [Google Scholar]
- Higashiyama R, Moro T, Nakao S, Mikami K, Fukumitsu H, Ueda Y, Ikeda K, Adachi E, Bou-Gharios G, Okazaki I, et al. 2009. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology 137: 1459–1466 e1451. [DOI] [PubMed] [Google Scholar]
- Hill DJ, Strain AJ, Elstow SF, Swenne I, Milner RD. 1986. Bi-functional action of transforming growth factor-β on DNA synthesis in early passage human fetal fibroblasts. J Cell Physiol 128: 322–328. [DOI] [PubMed] [Google Scholar]
- *.Hinck AP, Mueller TD, Springer TA. 2016. Structural biology and evolution of the TGF-β family. Cold Spring Harbor Perspect Biol 8: a022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. 2013. Matrix mechanics and regulation of the fibroblast phenotype. Periodontology 2000 63: 14–28. [DOI] [PubMed] [Google Scholar]
- Hirota N, Martin JG. 2013. Mechanisms of airway remodeling. Chest 144: 1026–1032. [DOI] [PubMed] [Google Scholar]
- Hofmann U, Bonz A, Frantz S, Hu K, Waller C, Roemer K, Wolf J, Gattenlohner S, Bauersachs J, Ertl G. 2012. A collagen α2(I) mutation impairs healing after experimental myocardial infarction. Am J Pathol 180: 113–122. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. 2004. Epithelial–mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc 1: 93–98. [DOI] [PubMed] [Google Scholar]
- Honda N, Jinnin M, Kira-Etoh T, Makino K, Kajihara I, Makino T, Fukushima S, Inoue Y, Okamoto Y, Hasegawa M, et al. 2013. miR-150 down-regulation contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin β3. Am J Pathol 182: 206–216. [DOI] [PubMed] [Google Scholar]
- Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, et al. 2008. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 177: 56–65. [DOI] [PubMed] [Google Scholar]
- Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. 2007. Combinatorial activation of FAK and AKT by transforming growth factor-β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 19: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JC, Ajayi IO, Kulasekaran P, Rogers DS, White JB, Townsend SK, White ES, Nho RS, Higgins PD, Huang SK, et al. 2012. Survivin expression induced by endothelin-1 promotes myofibroblast resistance to apoptosis. Int J Biochem Cell Biol 44: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostettler KE, Roth M, Burgess JK, Gencay MM, Gambazzi F, Black JL, Tamm M, Borger P. 2008. Airway epithelium-derived transforming growth factor-β is a regulator of fibroblast proliferation in both fibrotic and normal subjects. Clin Exp Allergy 38: 1309–1317. [DOI] [PubMed] [Google Scholar]
- Hoyles RK, Derrett-Smith EC, Khan K, Shiwen X, Howat SL, Wells AU, Abraham DJ, Denton CP. 2011. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor β receptor. Am J Respir Crit Care Med 183: 249–261. [DOI] [PubMed] [Google Scholar]
- Hoyt DG LJ. 1988. Alterations in pulmonary mRNA encoding procollagens, fibronectin, and transforming growth factor-β precede bleomycin-induced pulmonary fibrosis in mice. J Pharmacol Exp Ther 246: 765–771. [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV Jr., Sheppard D. 1996. Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 133: 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Sharma S, Zhu LX, Keane MP, Luo J, Zhang L, Burdick MD, Lin YQ, Dohadwala M, Gardner B, et al. 2002. IL-7 inhibits fibroblast TGF-β production and signaling in pulmonary fibrosis. J Clin Invest 109: 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulmes DJ. 2002. Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol 137: 2–10. [DOI] [PubMed] [Google Scholar]
- Hulmes DJ. 2008. Collagen diversity, synthesis, and assembly. In Collagen: Structure and mechanics (ed. Fratzl P), pp. 1–47. Springer Science+Business Media, New York. [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. 2010. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. 2013. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188: 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. 2012. The evolution of metazoan extracellular matrix. J Cell Biol 196: 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz RA, Massagué J. 1986. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 261: 4337–4345. [PubMed] [Google Scholar]
- Ishida Y, Nagata K. 2011. Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol 499: 167–182. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Bachinger HP. 2013. A molecular ensemble in the rER for procollagen maturation. Biochim Biophys Acta 1833: 2479–2491. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. 2002. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. 2003. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: A possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol 64: 521–529. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. 2001. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 164: S28–S38. [DOI] [PubMed] [Google Scholar]
- Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. 2006. Ligation of protease-activated receptor 1 enhances αvβ6 integrin-dependent TGF-β activation and promotes acute lung injury. J Clin Invest 116: 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson KA, Grapin-Botton A. 2002. Development and diseases of the pancreas. Clin Genet 62: 14–23. [DOI] [PubMed] [Google Scholar]
- Jolly L, Stavrou A, Vanderstoken G, Meliopoulos VA, Habgood A, Tatler AL, Porte J, Knox A, Weinreb P, Violette S, et al. 2014. Influenza promotes collagen deposition via αvβ6 integrin-mediated transforming growth factor β activation. J Biol Chem 289: 35246–35263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly MK, Tripathi SC, Jia D, Mooney SM, Celiktas M, Hanash SM, Mani SA, Pienta KJ, Ben-Jacob E, Levine H. 2016. Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget 10: 27067–27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan HM, Trackman PC. 1991. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol 5: 206–210. [DOI] [PubMed] [Google Scholar]
- Kakugawa T, Mukae H, Hayashi T, Ishii H, Nakayama S, Sakamoto N, Yoshioka S, Sugiyama K, Mine M, Mizuta Y, et al. 2005. Expression of HSP47 in usual interstitial pneumonia and nonspecific interstitial pneumonia. Respir Res 6: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. 2003. Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. 2009. The basics of epithelial–mesenchymal transition. J Clin Invest 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, Huang X, Sheppard D, Heller RA. 2000. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci 97: 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HR, Cho SJ, Lee CG, Homer RJ, Elias JA. 2007. Transforming growth factor (TGF)-β1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem 282: 7723–7732. [DOI] [PubMed] [Google Scholar]
- Katsuno Y, Lamouille S, Derynck R. 2013. TGF-β signaling and epithelial–mesenchymal transition in cancer progression. Curr Opin Onocol 25: 76–84. [DOI] [PubMed] [Google Scholar]
- Kay EP, Lee HK, Park KS, Lee SC. 1998. Indirect mitogenic effect of transforming growth factor-β on cell proliferation of subconjunctival fibroblasts. Invest Ophthalmol Vis Sci 39: 481–486. [PubMed] [Google Scholar]
- Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. 2001. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-β in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 158: 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. 2006. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci 103: 13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, Chapman HA. 2009. Integrin α3β1-dependent β-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol 184: 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA. 2006. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 45: 429–438. [DOI] [PubMed] [Google Scholar]
- Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. 2008. Snail is required for TGFβ-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci 121: 3317–3324. [DOI] [PubMed] [Google Scholar]
- Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, Honnons S, Jones C, Isern NG, Hu JZ, et al. 2012. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med 186: 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. 2012. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: From cardiovascular development to disease. Circulation 125: 1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, et al. 2012. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. 2010. The essence of senescence. Genes Dev 24: 2463–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasekaran P, Scavone CA, Rogers DS, Arenberg DA, Thannickal VJ, Horowitz JC. 2009. Endothelin-1 and transforming growth factor-β1 independently induce fibroblast resistance to apoptosis via AKT activation. Am J Respir Cell Mol Biol 41: 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. 1993. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci 90: 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano K. 2007. Epithelial cell apoptosis and lung remodeling. Cell Mol Immunol 4: 419–429. [PubMed] [Google Scholar]
- Kuwano K, Hagimoto N, Kawasaki M, Hara N. 1998. The roles of apoptosis in lung injury and fibrosis. Nihon Kokyuki Gakkai Zasshi 36: 739–744. [PubMed] [Google Scholar]
- Kuwano K, Hagimoto N, Nakanishi Y. 2004. The role of apoptosis in pulmonary fibrosis. Histol Histopathol 19: 867–881. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. 2014. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson O, Diebold D, Fan D, Peterson M, Nho RS, Bitterman PB, Henke CA. 2008. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS ONE 3: e3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, et al. 2008. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: Association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 294: L1119–L1126. [DOI] [PubMed] [Google Scholar]
- Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, et al. 2011. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci 108: 10562–10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. 2009. Signaling in fibrosis: Targeting the TGFβ, endothelin-1 and CCN2 axis in scleroderma. Front Biosci 1: 115–122. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. 2003. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol 81: 355–363. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. 2004. TGF-β signaling and the fibrotic response. FASEB J 18: 816–827. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R. 2013. Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19: 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. 2004. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor β1-induced pulmonary fibrosis. J Exp Med 200: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Kang HR, Homer RJ, Chupp G, Elias JA. 2006. Transgenic modeling of transforming growth factor-β1: Role of apoptosis in fibrosis and alveolar remodeling. Proc Am Thorac Soc 3: 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux CJ, Davy P, Brampton C, Ahuja SS, Fauce S, Shivshankar P, Nguyen H, Ramaseshan M, Tressler R, Pirot Z, et al. 2013. A novel telomerase activator suppresses lung damage in a murine model of idiopathic pulmonary fibrosis. PLoS ONE 8: e58423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ. 1996. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc Natl Acad Sci 93: 5127–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Krishnaveni MS, Li C, Zhou B, Xing Y, Banfalvi A, Li A, Lombardi V, Akbari O, Borok Z, et al. 2011. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest 121: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Gao HF, Wu YL. 2014. Targeting the MET pathway for potential treatment of NSCLC. Expert Opin Ther Targets 19: 663–674. [DOI] [PubMed] [Google Scholar]
- Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. 2010. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas NJ, Watts KL, Akram KM, Forsyth NR, Spiteri MA. 2012. Idiopathic pulmonary fibrosis: Immunohistochemical analysis provides fresh insights into lung tissue remodelling with implications for novel prognostic markers. Int J Clin Exp Pathol 5: 58–71. [PMC free article] [PubMed] [Google Scholar]
- Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC, Pentcheva-Hoang T, et al. 2015. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21: 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz D, Gazdhar A, Lopez-Rodriguez E, Ruppert C, Mahavadi P, Gunther A, Klepetko W, Bates JH, Smith B, Geiser T, et al. 2015. Alveolar derecruitment and collapse induration as crucial mechanisms in lung injury and fibrosis. Am J Respir Cell Mol Biol 52: 232–243. [DOI] [PubMed] [Google Scholar]
- Madala SK, Edukulla R, Schmidt S, Davidson C, Ikegami M, Hardie WD. 2014. Bone marrow-derived stromal cells are invasive and hyperproliferative and alter transforming growth factor-α-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 50: 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinde T, Murphy RF, Agrawal DK. 2007. The regulatory role of TGF-β in airway remodeling in asthma. Immunol Cell Biol 85: 348–356. [DOI] [PubMed] [Google Scholar]
- Makino K, Jinnin M, Hirano A, Yamane K, Eto M, Kusano T, Honda N, Kajihara I, Makino T, Sakai K, et al. 2013. The downregulation of microRNA let-7a contributes to the excessive expression of type I collagen in systemic and localized scleroderma. J Immunol 190: 3905–3915. [DOI] [PubMed] [Google Scholar]
- Marmai C, Sutherland RE, Kim KK, Dolganov GM, Fang X, Kim SS, Jiang S, Golden JA, Hoopes CW, Matthay MA, et al. 2011. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 301: L71–L78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnulty RJ, Hernandez-Rodriguez NA, Mutsaers SE, Coker RK, Laurent GJ. 1997. Indomethacin suppresses the anti-proliferative effects of transforming growth factor-β isoforms on fibroblast cell cultures. Biochem J 321: 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan SJ, Xanthou G, Lloyd CM. 2005. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-β antibody: Effect on the Smad signaling pathway. J Immunol 174: 5774–5780. [DOI] [PubMed] [Google Scholar]
- Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. 2010. Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest 120: 4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa S, Lou J, Seed RI, Cormier A, Wu S, Cheng Y, Murray L, Tsui P, Connor J, Herbst R, et al. 2014. Selective targeting of TGF-β activation to treat fibroinflammatory airway disease. Sci Transl Med 6: 241ra279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minutti CM, Knipper JA, Allen JE, Zaiss DM. 2017. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol 61: 3–11. [DOI] [PubMed] [Google Scholar]
- Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R III, Toews GB. 2003. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol 284: L342–L349. [DOI] [PubMed] [Google Scholar]
- Moses HL, Coffey RJ Jr., Leof EB, Lyons RM, Keski-Oja J. 1987. Transforming growth factor β regulation of cell proliferation. J Cell Physiol 133: 1–7. [DOI] [PubMed] [Google Scholar]
- Moses HL, Yang EY, Pietenpol JA. 1990. TGF-β stimulation and inhibition of cell proliferation: New mechanistic insights. Cell 63: 245–247. [DOI] [PubMed] [Google Scholar]
- Moses HL, Arteaga CL, Alexandrow MG, Dagnino L, Kawabata M, Pierce DF Jr, Serra R. 1994. TGFβ regulation of cell proliferation. Princess Takamatsu Symp 24: 250–263. [PubMed] [Google Scholar]
- *.Moses HL, Roberts AB, Derynck R. 2016. The discovery and early days of TGF-β: A historical perspective. Cold Spring Harbor Perspect Biol 8: a021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. 2002. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol 157: 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. 1997. Latent transforming growth factor-β: Structural features and mechanisms of activation. Kidney Int 51: 1376–1382. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. 1999. The integrin αvβ6 binds and activates latent TGFβ1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, Serrano M. 2014. Cellular senescence: From physiology to pathology. Nat Rev Mol Cell Biol 15: 482–496. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, et al. 2013. Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118. [DOI] [PubMed] [Google Scholar]
- Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, et al. 2008. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med 177: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JL, Katzenstein AL. 1988. Epithelial necrosis and alveolar collapse in the pathogenesis of usual interstitial pneumonia. Chest 94: 1309–1311. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. 2000. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: Roles of transforming growth factor (TGF)-β and bone morphogenetic protein (BMP). Anat Rec 258: 119–127. [DOI] [PubMed] [Google Scholar]
- Nieto MA. 2011. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 27: 347–376. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. 1994. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264: 835–839. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, Thiery JP. 2016. EMT: 2016. Cell 166: 21–45. [DOI] [PubMed] [Google Scholar]
- Nogee LM, Dunbar AE III, Wert SE, Askin F, Hamvas A, Whitsett JA. 2001. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 344: 573–579. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Parkos CA, Bacarra AE, Godowski PJ, Delp-Archer C, Rosen EM, Madara JL. 1994. Hepatocyte growth factor/scatter factor effects on epithelia. Regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J Clin Invest 93: 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Sinha S, Esposito I, Schiavon G, Lopez-Lago MA, Su W, Pratilas CA, Abele C, Hernandez JM, Ohara M, et al. 2015. The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT by restraining Ras-MAPK signalling. Nat Cell Biol 17: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku E, Kanaji T, Takata Y, Oshima K, Seki R, Morishige S, Imamura R, Ohtsubo K, Hashiguchi M, Osaki K, et al. 2008. Periostin and bone marrow fibrosis. Int J Hematol 88: 57–63. [DOI] [PubMed] [Google Scholar]
- Osterholzer JJ, Olszewski MA, Murdock BJ, Chen GH, Erb-Downward JR, Subbotina N, Browning K, Lin Y, Morey RE, Dayrit JK, et al. 2013. Implicating exudate macrophages and Ly-6Chigh monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol 190: 3447–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, et al. 2001. TGF-β is a critical mediator of acute lung injury. J Clin Invest 107: 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma DS, Timens W. 2006. Remodeling in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 434–439. [DOI] [PubMed] [Google Scholar]
- Prele CM, Yao E, O’Donoghue RJ, Mutsaers SE, Knight DA. 2012. STAT3: A central mediator of pulmonary fibrosis? Proc Am Thorac Soc 9: 177–182. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Sieron AL, Li SW. 1998. Procollagen N-proteinase and procollagen C-proteinase. Two unusual metalloproteinases that are essential for procollagen processing probably have important roles in development and cell signaling. Matrix Biol 16: 399–408. [DOI] [PubMed] [Google Scholar]
- Prox J, Arnold P, Becker-Pauly C. 2015. Meprin α and meprin β: Procollagen proteinases in health and disease. Matrix Biol 44–46: 7–13. [DOI] [PubMed] [Google Scholar]
- Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, et al. 2008. Inhibition of integrin αvβ6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 177: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, Sheppard D. 2015. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med 7: 288ra279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert RB, Kantz J, Misfeldt AA, Poffenberger G, Gannon M, Brissova M, Powers AC. 2012. Tamoxifen-induced Cre-loxP recombination is prolonged in pancreatic islets of adult mice. PLoS ONE 7: e33529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Thege FI, Santana SM, Lannin TB, Saha TN, Tsai S, Maggs LR, Kochman ML, Ginsberg GG, Lieb JG, et al. 2014. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 146: 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AB, Lamb LC, Newton DL, Sporn MB, De Larco JE, Todaro GJ. 1980. Transforming growth factors: Isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci 77: 3494–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AB SM, Assoian RK, Smith JM, Rocke NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS. 1986. Transforming growth factor β: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci 83: 4167–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Robertson IB, Rifkin DB. 2016. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harbor Perspect Biol 8: a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. 2011. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci 108: E1475–E1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J. 2011. Four faces of cellular senescence. J Cell Biol 192: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RG, Lin Y, Shimizu-Hirota R, Hanada S, Neilson EG, Greenson JK, Weiss SJ. 2011. Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol Cell Biol 31: 2392–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglani S, Molyneux C, Gong H, Rogers A, Malmstrom K, Pelkonen A, Makela M, Adelroth E, Bush A, Payne DN, et al. 2006. Ultrastructure of the reticular basement membrane in asthmatic adults, children and infants. Eur Respir J 28: 505–512. [DOI] [PubMed] [Google Scholar]
- Saito A, Okazaki H, Sugawara I, Yamamoto K, Takizawa H. 2003. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int Arch Allergy Immunol 132: 168–176. [DOI] [PubMed] [Google Scholar]
- Sanders YY, Liu H, Zhang X, Hecker L, Bernard K, Desai L, Liu G, Thannickal VJ. 2013. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox Biol 1: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. 2008. Evolution of the neural crest viewed from a gene regulatory perspective. Genesis 46: 673–682. [DOI] [PubMed] [Google Scholar]
- Savagner P, Kusewitt DF, Carver EA, Magnino F, Choi C, Gridley T, Hudson LG. 2005. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol 202: 858–866. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. 2003. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 171: 380–389. [DOI] [PubMed] [Google Scholar]
- Schreier T, Degen E, Baschong W. 1993. Fibroblast migration and proliferation during in vitro wound healing. A quantitative comparison between various growth factors and a low molecular weight blood dialysate used in the clinic to normalize impaired wound healing. Res Exp Med 193: 195–205. [DOI] [PubMed] [Google Scholar]
- Seeger W, Gunther A, Walmrath HD, Grimminger F, Lasch HG. 1993. Alveolar surfactant and adult respiratory distress syndrome. Pathogenetic role and therapeutic prospects. Clin Investig 71: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, Ozturk M. 2010. Transforming growth factor-β induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 52: 966–974. [DOI] [PubMed] [Google Scholar]
- Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. 2011. Latent TGF-β structure and activation. Nature 474: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibanuma M, Mashimo J, Kuroki T, Nose K. 1994. Characterization of the TGFβ1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J Biol Chem 269: 26767–26774. [PubMed] [Google Scholar]
- Shivshankar P, Brampton C, Miyasato S, Kasper M, Thannickal VJ, Le Saux CJ. 2012. Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice. Am J Respir Cell Mol Biol 47: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. 1992. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 359: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. 1997. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson TH, Maher TM, Ajayi IO, King JE, Higgins PD, Booth AJ, Sagana RL, Huang SK, White ES, Moore BB, et al. 2012. Increased survivin expression contributes to apoptosis-resistance in IPF fibroblasts. Adv Biosci Biotechnol 3: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, Kim KK, Keshamouni VG, White ES, Zhou Y, et al. 2015. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am J Pathol 185: 969–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab-Weijnitz CA, Fernandez IE, Knuppel L, Maul J, Heinzelmann K, Juan-Guardela BM, Hennen E, Preissler G, Winter H, Neurohr C, et al. 2015. FK506-binding protein 10 is a potential novel drug target for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 192: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockis J, Colau D, Coulie PG, Lucas S. 2009. Membrane protein GARP is a receptor for latent TGF-β on the surface of activated human Treg. Eur J Immunol 39: 3315–3322. [DOI] [PubMed] [Google Scholar]
- Stoker M, Perryman M. 1985. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci 77: 209–223. [DOI] [PubMed] [Google Scholar]
- Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. 1995. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V, van Kooten C, Muller G. 2001. TGF-β1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int 59: 579–592. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, LeBleu VS, Bosukonda D, Keck P, Taduri G, Bechtel W, Okada H, Carlson W Jr., Bey P, Rusckowski M, et al. 2012. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat Med 18: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K, LeRoy EC, Grotendorst GR. 1987. TGF-β inhibition of endothelial cell proliferation: Alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell 49: 415–422. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yoshimi M, Maeyama T, Hagimoto N, Kuwano K, Hara N. 2002. Resistance to Fas-mediated apoptosis in human lung fibroblast. Eur Respir J 20: 359–368. [DOI] [PubMed] [Google Scholar]
- Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, et al. 2009. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 180: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, Blackwell TS. 2011. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem 286: 30972–30980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjore H, Blackwell TS, Lawson WE. 2012. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 302: L721–L729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjore H, Lawson WE, Blackwell TS. 2013. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta 1832: 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin D, Thompson EW, Newgreen DF. 2005. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 65: 5996–6000. [DOI] [PubMed] [Google Scholar]
- Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, Brenner DA. 2010. Hepatocytes do not undergo epithelial–mesenchymal transition in liver fibrosis in mice. Hepatology 51: 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Zeisberg M, Kalluri R. 2007. Transcriptional regulation of epithelial–mesenchymal transition. J Clin Invest 117: 304–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ. 2013. Mechanistic links between aging and lung fibrosis. Biogerontology 14: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Horowitz JC. 2006. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc 3: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Henke CA, Horowitz JC, Noble PW, Roman J, Sime PJ, Zhou Y, Wells RG, White ES, Tschumperlin DJ. 2014. Matrix biology of idiopathic pulmonary fibrosis: A workshop report of the national heart, lung, and blood institute. Am J Pathol 184: 1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. 2007. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis 28: 1153–1162. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. 2009. Epithelial–mesenchymal transitions in development and disease. Cell 139: 871–890. [DOI] [PubMed] [Google Scholar]
- Thomas AQ, Lane K, Phillips J III, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, et al. 2002. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med 165: 1322–1328. [DOI] [PubMed] [Google Scholar]
- Torres-Gonzalez E, Bueno M, Tanaka A, Krug LT, Cheng DS, Polosukhin VV, Sorescu D, Lawson WE, Blackwell TS, Rojas M, et al. 2012. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol 46: 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trackman PC. 2005. Diverse biological functions of extracellular collagen processing enzymes. J Cell Biochem 96: 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Sheppard D. 2014. TGF-β activation and function in immunity. Annu Rev Immunol 32: 51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trian T, Allard B, Dupin I, Carvalho G, Ousova O, Maurat E, Bataille J, Thumerel M, Begueret H, Girodet PO, et al. 2015. House dust mites induce proliferation of severe asthmatic smooth muscle cells via an epithelium-dependent pathway. Am J Respir Crit Care Med 191: 538–546. [DOI] [PubMed] [Google Scholar]
- Tucker RF, Shipley GD, Moses HL, Holley RW. 1984. Growth inhibitor from BSC-1 cells closely related to platelet type β transforming growth factor. Science 226: 705–707. [DOI] [PubMed] [Google Scholar]
- Tzouvelekis A, Harokopos V, Paparountas T, Oikonomou N, Chatziioannou A, Vilaras G, Tsiambas E, Karameris A, Bouros D, Aidinis V. 2007. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1α in disease pathogenesis. Am J Respir Crit Care Med 176: 1108–1119. [DOI] [PubMed] [Google Scholar]
- Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M. 1998. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol 275: L1192–L1199. [DOI] [PubMed] [Google Scholar]
- Valles AM, Boyer B, Badet J, Tucker GC, Barritault D, Thiery JP. 1990. Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc Natl Acad Sci 87: 1124–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannella KM, Wynn TA. 2016. Mechanisms of organ injury and repair by macrophages. Annul Rev Physiol 79: 593–617. [DOI] [PubMed] [Google Scholar]
- Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, et al. 2015. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517: 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viebahn C. 1995. Epithelio-mesenchymal transformation during formation of the mesoderm in the mammalian embryo. Acta Anat 154: 79–97. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Mayer B, Miething A. 1995. Morphology of incipient mesoderm formation in the rabbit embryo: A light- and retrospective electron-microscopic study. Acta Anat 154: 99–110. [DOI] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. 2007. ‘Yin-Yang’ functions of transforming growth factor-β and T regulatory cells in immune regulation. Immunol Rev 220: 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ramos C, Joshi I, Zagariya A, Pardo A, Selman M, Uhal BD. 1999. Human lung myofibroblast-derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. Am J Physiol 277: L1158–L1164. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. 2009. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 84: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, Pearse BR, Yokota Y, Kawakatsu H, Atakilit A, et al. 2004. Function-blocking integrin αvβ6 monoclonal antibodies: Distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem 279: 17875–17887. [DOI] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. 2005. Induction of epithelial–mesenchymal transition in alveolar epithelial cells by transforming growth factor-β1: Potential role in idiopathic pulmonary fibrosis. Am J Pathol 166: 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. 2005. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 132: 5317–5328. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. 2007. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol 179: 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. 2007. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Du Y, Shen Y, He Y, Zhao H, Li Z. 2012. TGF-β1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Front Biosci 17: 2667–2674. [DOI] [PubMed] [Google Scholar]
- Yang T, Liang Y, Lin Q, Liu J, Luo F, Li X, Zhou H, Zhuang S, Zhang H. 2013. miR-29 mediates TGFβ1-induced extracellular matrix synthesis through activation of PI3K-AKT pathway in human lung fibroblasts. J Cell Biochem 114: 1336–1342. [DOI] [PubMed] [Google Scholar]
- Yu AL, Fuchshofer R, Kook D, Kampik A, Bloemendal H, Welge-Lussen U. 2009. Subtoxic oxidative stress induces senescence in retinal pigment epithelial cells via TGF-β release. Invest Ophthalmol Vis Sci 50: 926–935. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Duffield JS. 2010. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol 21: 1247–1253. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. 2009. Biomarkers for epithelial–mesenchymal transitions. J Clin Invest 119: 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. 2007a. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. 2007b. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 282: 23337–23347. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. 2008. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19: 2282–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Phan SH. 1999. Inhibition of myofibroblast apoptosis by transforming growth factor β1. Am J Respir Cell Mol Biol 21: 658–665. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rekhter MD, Gordon D, Phan SH. 1994. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol 145: 114–125. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R. 1998. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature 394: 909–913. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li J, Sejas DP, Pang Q. 2005. The ATM/p53/p21 pathway influences cell fate decision between apoptosis and senescence in reoxygenated hematopoietic progenitor cells. J Biol Chem 280: 19635–19640. [DOI] [PubMed] [Google Scholar]
- Zhou B, Pu WT. 2011. Epicardial epithelial-to-mesenchymal transition in injured heart. J Cell Mol Med 15: 2781–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI. 2009. Hypoxia-induced alveolar epithelial–mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol 297: L1120–L1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. 2002. β8 integrins are required for vascular morphogenesis in mouse embryos. Development 129: 2891–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]