Abstract
Bone morphogenetic proteins (BMPs) constitute the largest subdivision of the transforming growth factor (TGF)-β family of ligands and exert most of their effects through the canonical effectors Smad1, 5, and 8. Appropriate regulation of BMP signaling is critical for the development and homeostasis of numerous human organ systems. Aberrations in BMP pathways or their regulation are increasingly associated with diverse human pathologies, and there is an urgent and growing need to develop effective approaches to modulate BMP signaling in the clinic. In this review, we provide a wide perspective on diseases and/or conditions associated with dysregulated BMP signal transduction, outline the current strategies available to modulate BMP pathways, highlight emerging second-generation technologies, and postulate prospective avenues for future investigation.
SIGNAL TRANSDUCTION IN THE BONE MORPHOGENETIC PROTEIN PATHWAY
Bone morphogenetic proteins (BMPs) constitute the largest subdivision of the transforming growth factor (TGF)-β family of ligands with nearly thirty distinct human proteins bearing the BMP name. Important differences exist among these molecules with regard to pathway mechanics and effects on cell behavior. Two of the first BMPs to be cloned, BMP-1 and BMP-3, are not signaling molecules in the classical sense; BMP-1 is a metalloprotease that promotes BMP signaling (Kessler et al. 1996; Li et al. 1996), whereas BMP-3 is a nonsignaling receptor antagonist (Gamer et al. 2005). The nomenclature that accompanied the discovery of BMPs is most often based on sequence homology and may be confusing when discussing BMP effects. Clarification comes, however, by focusing on the downstream pathways activated by each BMP ligand. For instance, as will be discussed below, it is now known that the intracellular signaling effectors Smad1, Smad5, and Smad8 actuate autoinduction of bone at extraskeletal sites, which is the original function attributed to the BMP pathway (Urist 1965; Wozney et al. 1988). We contend, then, that proteins that elicit activation of Smads 1, 5, and 8 are bona fide components of the canonical BMP signaling cascade. We use this narrow definition of BMP signaling in this review and, on this basis, identify approximately 12 bona fide BMP ligands in humans (Table 1).
Table 1.
Components of the canonical bone morphogenetic protein (BMP)-induced Smad signaling pathway
| Ligands | BMP-2 (BMP-2A, BDA-2A) BMP-4 (BMP-2B, BMP-2B1, MCOPS6, OFC11, ZYME) BMP-5 BMP-6 (VGR, VGR1) BMP-7 (OP-1) BMP-8A BMP-8B (OP-2) BMP-9 (GDF-2, HHT5) BMP-10 GDF-5 (BMP-14, OS5, LAP4, BDA1C, CDMP1, SYM1B, SYNS2) GDF-6 (BMP-13, KFM, KFS, KFS1, KFSL, SGM1, CDMP2, LCA17, MCOP4, SCDO4, MCOPCB6) GDF-7 (BMP-12) |
| Type I receptors | ALK-1 (ACVRL1) ALK-2 (ACVR1, ActRI) ALK-3 (BMPRIA) ALK-6 (BMPRIB) |
| Type II receptors | BMPRII ActRII (ActRIIA, ACVR2, ACVR2A) ActRIIB (ACVR2B) |
| R-Smad | Smad1 Smad5 Smad8 (Smad9) |
| Co-Smad | Smad4 |
Alternative names are in parentheses.
BMP ligands are generally portrayed as homodimers of two identical subunits that are related by twofold rotational symmetry around the intermolecular disulfide bond through a cysteine knot, a hallmark of this ligand family (Hinck 2012). BMPs are synthesized as large precursor molecules, consisting of a signal peptide, a large prodomain, and a carboxy-terminal region of 100 to 125 amino acids and upon secretion from the cell are further processed to their mature forms. The ability of BMPs to form heterodimers with each other has been established through in vitro studies and genetic studies in model organisms, and multiple BMPs are often coexpressed in tissues, suggesting heterodimer formation may occur in vivo. However, to date, only homodimeric BMPs have been purified from harvested human tissue. Interest in the formation of heterodimers continues as it represents a fairly simple way to alter the functionality of BMP ligands. For example, producing heterodimers of BMP-2/7, BMP-2/6, and BMP-4/7 leads to enhanced activity (Aono et al. 1995; Israel et al. 1996; Xu et al. 2009; Isaacs et al. 2010; Valera et al. 2010; Buijs et al. 2012; Zheng et al. 2012; Bi et al. 2013; Krase et al. 2014; Dang et al. 2015; Morimoto et al. 2015; Neugebauer et al. 2015), although the reason for this remains to be determined.
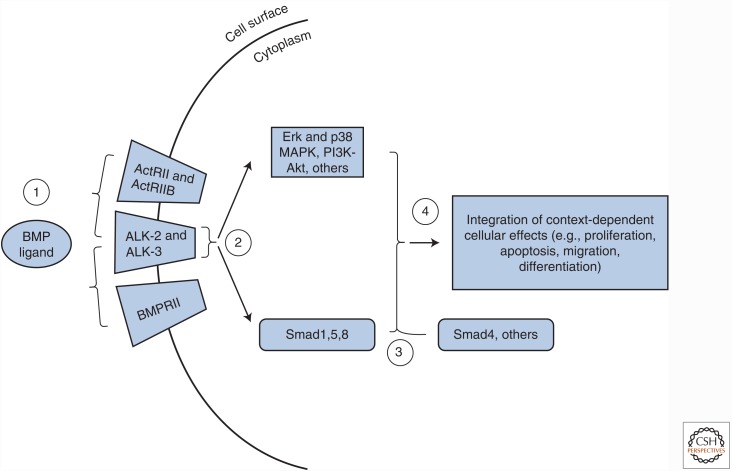
BMP ligands activate signaling by complexing with receptor kinases with dual specificity that are present at the cell surface (Fig. 1). These receptors are classified into type I and II receptors, of which there are seven and five, respectively. Four type I receptors (i.e., ALK-1, ALK-2, ALK-3/BMPRIA, and ALK-6/BMPRIB) and three type II receptors (i.e., BMPRII, ActRII, and ActRIIB) serve as BMP signal transducers (Table 1). In the classical (i.e., the canonical) Smad pathway, ligand binding brings a pair of constitutively active type II receptors into close proximity with a pair of type I receptors, allowing receptor trans-phosphorylation to occur. The activated type I receptors phosphorylate the carboxyl termini of the aforementioned Smads 1, 5, and 8, thus activating them (Fig. 1). The receptor-activated Smads, or R-Smads, can then form complexes with the transcription factor Smad4 and translocate into the nucleus to influence gene regulation (Katagiri and Watabe 2016).
Figure 1.
The bone morphogenetic protein (BMP) pathway and potential strategies for therapeutic modulation. (1) Activation of the BMP pathway occurs via interaction between dimeric BMP ligands and complexes of type I (e.g., ALK-2 and ALK-3) and type II receptors (BMPRII, ActRII, or ActRIIB). This step may be inhibited by delivery of extracellular ligand traps such as naturally occurring antagonists, receptor decoys, or neutralizing antibodies. Alternatively, BMP ligand availability may be enhanced through delivery of exogenous ligands or inhibiting endogenous extracellular BMP antagonists by neutralizing antibodies or small molecules. (2) Ligand binding leads to activation of the type I receptors by type II receptors and subsequent phosphorylation of the receptor-activated Smads 1, 5, and 8 (R-Smads) along with other pathways including extracellular signal-regulated kinase (Erk) and p38 mitogen-activated protein kinase (MAPK), and PI3K-Akt. The kinase activities of the type I receptors may be blocked by small molecule inhibitors such as LDN-193189. The BMP pathway inhibitors FKBP12 and casein kinase 2 endogenously limit the activities of the type I receptors and may be inactivated by delivery of FK506 and CK2.3, respectively, to increase BMP signal transduction. (3) R-Smads may perform Smad4-independent activities such as regulating microRNA processing or associate with Smad4 or other transcription factors to control gene regulatory networks. Persistence of BMP signaling may be modulated by regulating the Smurf1-mediated ubiquitylation of Smad effector proteins by disrupting Smurf1 interaction with R-Smads by small molecule inhibitors or by increasing Smurf1 protein levels. (4) R-Smad-dependent and R-Smad-independent events are integrated in a context-dependent manner to control cellular activity.
It should be noted that Smad4-independent BMP activities have also been reported (Fig. 1), consistent with the finding that several noncanonical signaling pathways such as p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (Erk), and Akt, and microRNA processing (Davis et al. 2008; Zhang 2009) are also regulated by BMP ligands. In fact, a proteomic study indicates that the phosphorylation status of nearly 400 proteins changes within thirty minutes of stimulation by BMP-2, suggesting that these modifications do not depend on Smad activation and Smad-mediated signaling and transcription (Kim et al. 2009). One proposed mechanism for how Smad versus non-Smad signaling occurs stems from biochemical analyses that show the presence of preformed BMP receptor oligomers on the cell surface before ligand binding. In this model, preformed receptor complexes containing one type I BMP receptor and one type II BMP receptor are proposed to participate in canonical BMP signaling, whereas ligand-induced receptor complex formation between homodimeric type I and type II receptors are proposed to segregate with noncanonical p38 MAPK BMP signaling (Nohe et al. 2002; Hassel et al. 2003). More recently, a number of high-resolution microscopy techniques have refined this idea (Guzman et al. 2012). It appears that preformed heteromeric BMP receptor complexes are highly dynamic and transient in the absence of ligand, and, once ligand is added to the complex, canonical signaling is quickly initiated and completed. In contrast, noncanonical BMP pathway activation requires greater stability to initiate and complete signaling and takes place in specialized membrane microdomains that enhance type I and type II receptor interactions after ligand association via cytoskeletal elements and membrane scaffolding proteins. It is important to point out that the exact biological significance of distinctions in receptor complex formation has yet to be determined in vivo.
BMP pathway activity is regulated at many levels (Walsh et al. 2010; Huang and Chen 2012). Extracellular antagonists, such as noggin and gremlin, function to sequester ligands upstream of receptor binding, preventing pathway activation. The inhibitory Smads, Smad6 and Smad7, block R-Smad activation at the type I receptor level, and prevent R-Smad interaction with Smad4. R-Smad and receptor degradation is promoted by E3 ubiquitin ligases such as Smurf1 (Smad ubiquitination regulatory factor 1). Additionally, transcriptional regulation by Smads can be blocked by interaction with inhibitory proteins such as c-Ski or the c-Ski-like proto-oncogene product SnoN (SKIL) (Hill 2016; Miyazawa and Miyazono 2017).
CURRENT STRATEGIES TO MODULATE BMP PATHWAY ACTIVITY
Aberrations in BMP signal transduction—both overactivation and underactivation—are implicated in a variety of clinically relevant settings. A later section of this review will detail the rationale for and provide evidence of successful BMP-based therapeutics. Here, we wish to provide a brief outline of strategies currently available to modulate the BMP pathway in vivo, starting from upstream of receptor engagement and moving downstream from receptor and effector activity (Table 2). A greatly expanded discussion of this topic with detailed applications has been published recently (Lowery et al. 2016).
Table 2.
General overview of current strategies to modulate bone morphogenetic protein (BMP) signal transduction
| Extracellular | Overexpression of ligand via gene transfer | Overexpression of extracellular antagonist via gene transfer |
| Delivery of recombinant ligand | Delivery of recombinant extracellular antagonist or decoy receptor | |
| Neutralization of extracellular antagonists by antibody, decoy ligand, or small molecule | Neutralization of ligand by antibody | |
| RNA interference–mediated silencing of endogenous expression of extracellular antagonists or microRNAs | RNA interference–mediated silencing of expression of endogenous ligands | |
| Intracellular | Overexpression or induced expression of BMP receptors by gene transfer, pharmacological agent, or RNA interference–mediated silencing of microRNAs | RNA interference–mediated silencing of expression of receptors or effectors |
| Delivery of CK2.3 peptide or FK506 to alleviate BMP receptor inhibition | Delivery of BMP receptor kinase inhibitors | |
| Stabilizing effector turnover by RNA interference–mediated silencing or small molecule inhibition of Smurf1 |
Modulation of BMP Activity in the Extracellular Environment
The United States Food and Drug Administration (FDA) has approved the use of recombinant BMP-2 and BMP-7, which are marketed as InFuse Bone Graft and OP-1, respectively, in several orthopedic and oral and maxillofacial applications. Significant off-label use of these products has been noted, however, and ongoing or upcoming clinical trials seek to evaluate the usefulness of recombinant BMP-2 and/or BMP-7 in additional orthopedic or dental applications. Along with naturally occurring BMP ligands, numerous engineered biomimetic versions have been generated for optimized expression in Escherichia coli (Saito et al. 2003; Seol et al. 2006; He et al. 2008; Lee et al. 2008, 2009; Bergeron et al. 2009; Lin et al. 2010; Zouani et al. 2010; Allendorph et al. 2011; Sugimoto et al. 2012; Tang et al. 2012; Kang et al. 2013; Suarez-Gonzalez et al. 2014; Kuo et al. 2014; Lauzon et al. 2014; Beauvais et al. 2015; Falcigno et al. 2015; Liu et al. 2015; Ma et al. 2015; Zhang et al. 2015; Zhou et al. 2015). Additionally, several BMP-inspired ligands have been developed with enhanced signaling ability compared with naturally occurring BMP ligands (Table 3). Specific applications involve engineered BMP ligands and are highlighted in a recent review (Lowery et al. 2016). Delivery of cDNAs encoding these natural or engineered BMP ligands for synthesis in vivo has also been achieved in numerous settings (Lowery et al. 2016). Additionally, several FDA approved drugs regulate expression of BMP ligands or potentiate the BMP pathway, including the statin drugs lovastatin and simvastatin (Sugiyama et al. 2000; Maeda et al. 2001; Song et al. 2003; Bradley et al. 2007; Kodach et al. 2007; Zhang and Lin 2008), the Rho-kinase inhibitor fasudil (Kanazawa et al. 2009, 2010), and the phosphodiesterase inhibitors pentoxifylline, rolipram, and sildenafil (Horiuchi et al. 2001; Horiuchi et al. 2002; Rondelet et al. 2010; Tokuhara et al. 2010; Yen et al. 2010; Munisso et al. 2012; Yang et al. 2013b).
Table 3.
Engineered bone morphogenetic protein (BMP) ligands
| Category | Name | Comment(s) | Selected references |
|---|---|---|---|
| BMP-2-based | B2A (B2A2-K-NS) | BMP-2-based peptide with heparin-binding domain that augments the activity of BMP-2 but has no intrinsic signaling ability | Lin et al. 2005, 2007, 2012; Smucker et al. 2008; Cunningham et al. 2009; Liu et al. 2012; Sardar et al. 2015 |
| BMP2-L51P | BMP-2 mutant that augments the activity of BMP-2 but has no intrinsic signaling ability | Albers et al. 2012; Sebald et al. 2012; Khattab et al. 2014 | |
| BMP2_108 | BMP-2-based peptide; mimics the activity of BMP-2 | Zhang et al. 2015 | |
| mBMP | BMP-2-based peptide with mineral-binding domain; mimics the activity of BMP-2 | Suarez-Gonzalez et al. 2014 | |
| OPD | BMP-2-based peptide; mimics or presumed to mimic the activity of BMP-2 | Lee et al. 2009 | |
| P1 | Lee et al. 2008 | ||
| P2 | |||
| P24 | Lin et al. 2010; Tang et al. 2012 | ||
| PEP7 | Kang et al. 2013 | ||
| Unnamed | Saito et al. 2003; Seol et al. 2006; He et al. 2008; Zouani et al. 2010; Falcigno et al. 2015; Liu et al. 2015; Ma et al. 2015; Zhou et al. 2015 | ||
| BMP-2/activin A chimerae | AB204 | Segmental chimera of BMP-2 and activin A with enhanced activity over BMP-2; noggin-resistant | Allendorph et al. 2011; Ahn et al. 2014; Yoon et al. 2014, 2015a,b; Kim et al. 2015 |
| AB204-I103Y | Variant of AB204; enhanced activity over BMP-2 and AB204 | Yoon et al. 2014 | |
| AB211 | Segmental chimera of BMP-2 and activin A with enhanced activity over BMP-2; noggin-resistant | Allendorph et al. 2011 | |
| AB215 | Allendorph et al. 2011; Jung et al. 2014 | ||
| BMP-2/BMP-9 chimera | BB29 | Segmental chimera of BMP-2 and BMP-9 with enhanced folding when produced in Escherichia coli | Allendorph et al. 2011 |
| BMP-6/BMP-7 chimera | 80-1 | Segmental chimera of BMP-6 and BMP-7 with reduced noggin binding when compared with BMP-7 | Schwaerzer et al. 2012 |
| BMP-7-based | BMP7-E60K | BMP-6-informed mutant with reduced noggin binding | Schwaerzer et al. 2012 |
| THR-123 | BMP-7-based peptide | Sugimoto et al. 2012 | |
| Unnamed | BMP-7-based peptide; mimics activity of BMP-7 | Zouani et al. 2010 | |
| BMP-9-based | MB109 | BMP-9-based peptide optimized for production in E. coli | Kuo et al. 2014 |
| pBMP9 | BMP-9-based peptide with enhanced activity over BMP-9 | Bergeron et al. 2009; Lauzon et al. 2014; Beauvais et al. 2015 | |
| SpBMP9 | Beauvais et al. 2015 | ||
| Unnamed | BMP-9-based peptide; mimics activity of BMP-9 | Zouani et al. 2010 | |
| GDF-5-based | GDF5-S94N | Naturally occurring mutant with enhanced activity due to decreased inhibition by noggin | Schwaerzer et al. 2012 |
| GDF5-N445K | Seemann et al. 2009 | ||
| GDF5-N445T | Seemann et al. 2009; Degenkolbe et al. 2015 | ||
| GDF5-V453/V456 | BMP-2-informed variant of GDF-5; enhanced activity over GDF-5 and BMP-2 | Kasten et al. 2010; Kleinschmidt et al. 2014 | |
| BMP heterodimers | BMP-2/6 | Heterodimer with enhanced activity over BMP-2 and BMP-6 | Isaacs et al. 2010; Valera et al. 2010 |
| BMP-2/7 | Heterodimer with enhanced activity over BMP-2 and BMP-7 | Xu et al. 2009; Buijs et al. 2012; Zheng et al. 2012; Bi et al. 2013; Dang et al. 2015; Morimoto et al. 2015 | |
| BMP-4/7 | Heterodimer with enhanced activity over BMP-4 and BMP-7 | Aono et al. 1995; Krase et al. 2014; Neugebauer et al. 2015 |
BMP signal transduction can be blocked by preventing ligands in the extracellular space from interacting with receptors embedded in the plasma membrane. One method to achieve this blockade is through the use of soluble decoy receptors, composed of the ligand-binding domains of individual BMP receptors. This approach takes advantage of defined ligand affinities for particular receptors. A successful example of this strategy is an ALK-1 decoy, marketed as dalantercept, which preferentially sequesters BMP-9 and BMP-10 (Cunha et al. 2010; Mitchell et al. 2010; Larrivee et al. 2012; Ricard et al. 2012; Bendell et al. 2014; Hawinkels et al. 2016). This molecule is currently in clinical trials as an anti-angiogenic cancer therapy (see clinicaltrials.org registry numbers NCT01458392, NCT01642082, NCT01720173, NCT01727336, and NCT02024087). This approach parallels BMP regulation in vivo as BMP ligands are sequestered upstream of receptor engagement by a large number of naturally occurring soluble antagonists (Walsh et al. 2010). Several of these, most notably noggin and gremlin, either delivered as recombinant proteins or synthesized in vivo by gene transfer, have shown efficacy in modulating BMP signaling (Lowery et al. 2016). Conversely, several neutralizing antibodies have been developed to block the activity of naturally occurring BMP antagonists in the extracellular environment (Hampton et al. 2007a,b; Ciuclan et al. 2013). It is possible that the interaction between noggin and its target BMP ligand could be disrupted by small molecules (Ahmed et al. 2010).
Modulating Receptor and Effector Activities
BMP signal transduction may be blunted by small molecules that block the BMP receptor protein kinase pocket. The first such compound was dorsomorphin (Yu et al. 2008b), which served as a guide for subsequent generations of analogs, such as LDN-193189, with enhanced specificity and efficacy (Table 4). Specific applications involving engineered BMP ligands are summarized in a recent review (Lowery et al. 2016). Conversely, the peptide CK2.3 leads to increased BMP signal transduction by disrupting the inhibitory interaction between casein kinase (CK) 2 and BMP type I receptors (Akkiraju et al. 2015), whereas the FDA approved immunosuppressant FK506 activates BMP signaling by inhibiting FKBP12. The BMP pathway can also be modulated downstream from receptor activity by stabilizing Smads 1, 5, and 8 through silencing the expression of the E3 ubiquitin ligase Smurf1 or, potentially, by preventing Smurf1 interaction with these Smads (Okada et al. 2009; Kato et al. 2011; Cao et al. 2014).
Table 4.
Bone morphogenetic protein (BMP) receptor kinase inhibitors
| Name | Comment(s) | Selected references |
|---|---|---|
| 1LWY | Dramatically enhanced selectivity for ALK-2 versus other type I BMP receptors (approximate order of selectivity: ALK-2>ALK-3>ALK-6); greatly reduced off-target effects compared with DM and LDN | Tsugawa et al. 2014 |
| DMH1 | Pan-type I BMP receptor inhibitor (approximate order of selectivity: ALK-3>ALK-1>ALK-6>ALK-2); reduced off-target effects compared with DM and LDN | Hao et al. 2010; Ao et al. 2012; Engers et al. 2013; Mohedas et al. 2013; Sheng et al. 2014; Alsamarah et al. 2015; Owens et al. 2015; Lin et al. 2016; Sanders et al. 2016 |
| DMH2 | Pan-type I BMP receptor inhibitor (approximate order selectivity: ALK-6>ALK-3>ALK-2); notable off-target effects, including BMPRII, TβRII, ALK-4, ALK-5, AMPK, VEGFR2) | Hao et al. 2010; Langenfeld et al. 2013; Tsugawa et al. 2014 |
| DMH3 | Presumed pan-type I BMP receptor inhibitor; reduced off-target effects compared with DM and LDN | Hao et al. 2010 |
| Dorsomorphin (DM) | Pan-type I BMP receptor inhibitor (approximate order of selectivity: ALK-2>ALK-3>ALK-1>ALK-6); notable off-target effects, including BMPRII, ActRII, ActRIIB, TβRII, ALK-5, AMPK, VEGFR2, and PDGFRβ | Hao et al. 2008, 2010; Yu et al. 2008b; Bai et al. 2010; Boergermann et al. 2010; Kim et al. 2010; Vogt et al. 2011; Ao et al. 2012; Hamasaki et al. 2012; Engers et al. 2013; Mohedas et al. 2013; Shanmugam and Cherayil 2013; Chang et al. 2014; Garulli et al. 2014; Horbelt et al. 2015 |
| K02288 | Modestly enhanced selectivity for ALK-1 and ALK-2 versus other type I BMP receptors (approximate order of selectivity: ALK-2>ALK-1>ALK-6>ALK-3); reduced off-target effects compared with DM and LDN | Mohedas et al. 2013; Sanvitale et al. 2013; Kerr et al. 2015 |
| LDN-193189 (LDN) | Pan-type I BMP receptor inhibitor (approximate order of selectivity: ALK-1∼ALK-2>ALK-3>ALK-6); notable off-target effects, including BMPRII, ActRII, ActRIIB, TβRII, ALK-5, AMPK, VEGFR2, and PDGFRβ | Cuny et al. 2008; Yu et al. 2008a; Boergermann et al. 2010; Lee et al. 2011; Steinbicker et al. 2011; Vogt et al. 2011; Hamasaki et al. 2012; Saeed et al. 2012; Balboni et al. 2013; Engers et al. 2013; Helbing et al. 2013; Komatsu et al. 2013; Mohedas et al. 2013; Sanvitale et al. 2013; Peterson et al. 2014; Tsugawa et al. 2014; Horbelt et al. 2015; Kajimoto et al. 2015; Malhotra et al. 2015; Mayeur et al. 2015 |
| LDN-212854 | Significantly enhanced selectivity for ALK-1 and ALK-2 versus other type I BMP receptors (approximate order of selectivity: ALK-2>ALK-1>ALK-3); reduced off-target effects compared with DM and LDN-193189 | Mohedas et al. 2013; Dey et al. 2016 |
| LDN-214117 | Dramatically enhanced selectivity for ALK-2 versus other type I BMP receptors (approximate order of selectivity: ALK-1, ALK-2>ALK-3); greatly reduced off-target effects compared with DM and LDN-193189 | Mohedas et al. 2014 |
| ML-347 | Dramatically enhanced selectivity for ALK-1 and ALK-2 versus other type I BMP receptors (approximate order of selectivity: ALK-2>ALK-1>>ALK-3); reduced off-target effects compared with DM and LDN-193189 | Engers et al. 2010, 2013 |
| VU5350 | Pan-type 1 BMP receptor inhibitor (approximate order selectivity: ALK3>ALK2>ALK6); notable off-target effects, including BMPRII, TβRII, AMPK, VEGFR2) | Tsugawa et al. 2014 |
CLINICAL RELEVANCE OF BMP-BASED THERAPEUTICS
Orthopedic and Craniofacial Settings
More than 50 million people in the United States alone have osteoporosis or osteopenia (Wright et al. 2014), and this number is expected to increase as the population ages. Thus, understanding the mechanisms that regulate bone growth and remodeling is an important goal. It is widely accepted that BMP signaling is required for normal skeletal development and patterning (Salazar et al. 2016). However, in comparison to the information available regarding the embryonic role of BMP signaling in skeletogenesis, relatively little is known about the roles of the BMP pathway in the postnatal skeleton, and many of the available data are merely correlative. For instance, although BMP signaling levels correlate with bone mineral density (Szweras et al. 2002; Yan et al. 2009; Nallamshetty et al. 2013; Shen et al. 2013; Guemes et al. 2014; Kureel et al. 2014), and aberrations in the expression of BMP pathway components or BMP-induced effects are observed in bone marrow stromal cells (BMSCs) from aged (Moerman et al. 2004) or osteoporotic (Prall et al. 2013; Haasters et al. 2014) subjects, respectively. These correlative findings raise the possibility of a causative relationship. Other results are controversial and/or inconsistent between studies. Two studies have linked a single nucleotide polymorphism (SNP) in the BMP2 gene (rs2273073, c.109T>G, Ser37Ala) with lumbar spine bone mineral density and osteoporotic fractures in an international cohort and an Icelandic cohort, respectively (Reneland et al. 2005; Styrkarsdottir et al. 2003). However, this SNP is not associated with bone parameters in studies of Dutch (Medici et al. 2006), Swedish (McGuigan et al. 2007), or American Caucasian populations (Ichikawa et al. 2006). Similarly, a SNP in BMP4 (rs17563, c.538C>T, Val147Ala) is linked to bone mineral density in Australian Caucasian women (Ramesh Babu et al. 2005) and possibly Taiwanese women (Lin et al. 2008) but not in Italian women (Semprini et al. 2000). It should be noted that associations between bone mineral density and two other SNPs in BMP2 or one SNP in BMP4 were found in Korean males (Choi et al. 2006), and we are not aware of reports corroborating or contradicting these findings.
Beyond these correlative findings, several studies show that systemic administration of recombinant BMP-2, BMP-6, or BMP-7 or alleviating inhibition of the BMP receptor ALK-3 using a synthetic peptide improve bone mass and associated parameters (Turgeman et al. 2002; Simic et al. 2006; Dumic-Cule et al. 2014; Akkiraju et al. 2015). These anabolic effects are likely due to increased osteoblastogenesis and/or an enhanced bone formation rate in vivo, which is supported by the high-bone-mass phenotype seen by 4 months of age in transgenic mice with constitutively activated canonical BMP signaling in osteoblasts (Zhang et al. 2009). These studies suggest that augmenting BMP signaling in individuals with low bone mass may hold therapeutic benefit, and a phase II clinical trial is examining this possibility through injection of recombinant BMP-2 into the hip (NCT00752557). Although results are not yet available for this study, the rationale is reminiscent of the numerous reports detailing the ability of recombinant BMP ligands to promote local bone growth in maxillofacial applications, including the FDA approved use for recombinant BMP-2 in sinus lift and alveolar ridge augmentation procedures. Building on these results, an ongoing clinical trial examines the possible benefit of coating dental implants with recombinant BMP-2 (NCT00422279). Augmenting BMP signaling has also been used with considerable success in the healing of recalcitrant fractures. Although most fractures heal without intervention, ∼10% result in nonunions, increasing patient morbidity owing to infection and requiring increased hospital stay. To date, recombinant BMP-2 and BMP-7 have received FDA approval as adjunct therapies for the treatment of nonunion fractures, in which the benefits of treatment include accelerated healing and lower infection rates (Ali and Brazil 2014). Augmenting BMP signaling by recombinant ligand administration has also shown efficacy in procedures that require bone grafts, such as skeletal defects resulting from severe trauma, tumor resection, pathological degeneration, and congenital malformation. In these circumstances, BMPs can be combined with autografts that are harvested from the patient’s own skeleton, allografts harvested from cadavers, or synthetic bone substitutes. The best examples of success in using exogenous BMPs are in the area of spine fusion surgery, where BMP-2 and BMP-7 have shown efficacy equal to that of using autograft for establishing bone union (Burkus et al. 2005). Concerns relating to the dose of exogenous BMP required for healing, the mode of BMP delivery and the potential for unwanted heterotopic ossification (HO) at neighboring sites have led to the ongoing development of novel BMP molecules that show greater potency and would be predicted to have enhanced efficacy and safety when delivered at lower doses (Cahill et al. 2015). Alternatively, the use of agents that can modulate the production of endogenous BMPs would offer substantial benefit although the clinical usage of this approach remains to be uncovered.
Heterotopic Ossification
The studies described above show that BMP signaling is a potent inducer of de novo bone formation. Thus, it is not altogether surprising that BMP signaling is implicated in the pathogenesis of HO, a common acquired disorder in which bone forms at extraskeletal sites, and, once formed, may impair mobility and cause chronic pain. HO is often associated with the soft tissue trauma during joint replacement or other major reconstructive surgeries. HO is also an unfortunate and troublesome complication seen in severely wounded soldiers, amputees, or paralyzed individuals. Current treatments for nongenetic forms of HO include nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit the production of prostaglandins at the injury site, and local irradiation to block the recruitment of skeletal stem cells to the site of injury. Although both can be somewhat effective in dampening the HO response, each treatment has severe side effects. NSAID use has been associated with gastrointestinal distress, renal toxicity, and reduced platelet function. Radiation, although preventing HO, destroys healthy tissue. In severe cases, surgical removal of the HO has been performed, but this practice often increases hospital stay, and there remains the potential for recurrence of HO because of the trauma induced by tissue resection. A new treatment option that has shown promise in animal models is the delivery of synthetic retinoid agonists that potently inhibit the early BMP-mediated chondrogenic stage of HO (Sinha et al. 2016). Retinoid agonist therapy is currently being examined in patients with a rare, genetic form of HO (see below). BMP receptor kinase inhibitors may also have utility in treating HO. Combinatorial approaches that combine retinoid agonists with BMP receptor kinase antagonists have also been suggested as potential therapies as they might be able to be tailored to the patient.
Unlike acquired HO, fibrodysplasia ossificans progressiva (FOP) is a rare and highly disabling skeletal disease characterized by seemingly spontaneous episodes of HO that often begin in early childhood. The crippling accumulation of extraskeletal bone tissue in FOP results in skeletal deformities, chronic pain, and joint ankylosis, and eventually encompasses much of the body (Huning and Gillessen-Kaesbach 2014). FOP is caused by missense mutations in the ACVR1/ALK2 gene that alter the tertiary structure of this type I BMP receptor and enable activins, ligands that do not normally trigger BMP signaling, to induce BMP signals (Shore et al. 2006; Hatsell et al. 2015; Hino et al. 2015). Current treatments for FOP, such as steroidal and nonsteroidal NSAIDs, are palliative but do not prevent the progression of HO. Clinical trials with synthetic retinoid agonists are ongoing (NCT02190747, EudraCT 2014-001453-17, EudraCT 2014-002496-28), based on the notion that FOP and HO have common pathogenic mechanisms that converge on activation of BMP signaling. Unlike HO, blocking activin signaling may be an effective treatment for FOP patients. Systemic blockade of activins has been shown to ameliorate cancer-induced cachexia, raising the possibility that similar agents might successfully control the BMP signaling caused by activins in patients with FOP (Zhou et al. 2010). As apparent from the Regeneron Pharmaceuticals website, clinical trials examining safety of this approach have begun. Additionally, given that a single, recurrent mutation underlies most FOP cases, strategies such as allele-specific RNA interference may prove useful in reducing expression of mutant ALK-2 through gene therapy approaches (Lowery and Rosen 2012).
Vascular Disease
Related to the notion that aberrant BMP pathway activation leads to HO, evidence also indicates that elevated BMP signaling plays a major role in vascular calcification (Cai et al. 2012; Garcia de Vinuesa et al. 2015; Morrell et al. 2016). For instance, genetic loss of the BMP pathway antagonists matrix-Gla protein, which binds to and inhibits BMP ligands (Zebboudj et al. 2002; Yao et al. 2006), or Smad6 leads to widespread vascular calcification in mice (Luo et al. 1997; Galvin et al. 2000). As such, strategies designed to reduce BMP pathway activation, including RNA interference of individual BMPs and delivery of recombinant BMP antagonists or small molecule inhibitors of BMP signaling, diminish vascular inflammation and reactive oxygen species formation, and/or limit the degree of vessel calcification (Derwall et al. 2012; Saeed et al. 2012; Koga et al. 2013; Zhang et al. 2014; Kajimoto et al. 2015; Malhotra et al. 2015). Together, these studies suggest that therapies aimed at reducing BMP signaling in the vasculature may be beneficial in patients at high risk for calcification such as those with end-stage renal disease; however, we are unaware of completed or ongoing clinical trials examining this possibility.
In contrast to the logical connection between BMP-induced extracellular matrix formation and vascular calcification, little was known about the critical role of BMP signaling in maintaining integrity of the pulmonary vasculature before the finding that the vast majority of patients with heritable pulmonary arterial hypertension (HPAH) carry heterozygous mutations in the BMPR2 gene (Deng et al. 2000; Lane et al. 2000). HPAH is a rare form of the relatively common disease pulmonary hypertension (PH), in which the small, resistance-level arterioles of the lung undergo structural remodeling to become thicker and less compliant. These changes increase the load on the right ventricle and lead to right ventricular hypertrophy and, ultimately, right-sided heart failure (Simonneau et al. 2009). Given that the pathology in all forms of pulmonary hypertension (PH) show strikingly similar pathology, that ∼20% of patients with sporadic pulmonary arterial hypertension (PAH) also carry BMPR2 mutations, and that BMP signal transduction and pathway components are down-regulated in the lungs of PH patients (Lowery and de Caestecker 2010), it is likely that adequate levels of BMP signaling are required for proper regulation of the pulmonary vasculature. However, the specific roles of BMP signaling in this context remain unclear. Numerous mechanisms have been proposed for the dysregulated BMP signaling with varying degrees of experimental support, including anti-inflammatory and/or antimitogenic effects, direct deregulation of vascular tone, deregulation of endothelial cell apoptosis and integrity of the tunica intima, and anti-oxidant actions by repressing reactive oxygen species formation (Lowery and de Caestecker 2010).
Clearly, an increased understanding of the endogenous actions of BMP signaling in the pulmonary vasculature could contribute to developing targeted therapies in the future. Even without a detailed understanding of the downstream actions, animal models suggest that several strategies aimed at generally increasing BMP signal transduction in the pulmonary vasculature may be beneficial in PAH. Indeed, the phosphodiesterase-5 inhibitor sildenafil, which is FDA approved for the treatment of PAH, prevents disease development in a toxin-induced model of PAH in rats and this is associated with increased BMP signaling in the lung (Yang et al. 2013b). Similarly, the FDA approved antimalarial chloroquine attenuates PAH development in rats with an associated increase in BMPRII expression (Long et al. 2013). More direct evidence comes from the observations that increasing BMPRII expression using direct gene transfer or by repressing the action of miR-20a reduces the severity of PAH development (Reynolds et al. 2007; Brock et al. 2014). Moreover, increasing BMPRII expression has been reported to reverse the pathological changes associated with PAH in mice (Reynolds et al. 2012; Feng et al. 2016), suggesting that increasing the availability of BMPRII alone may be sufficient to provide therapeutic benefit in PAH, although this has not been observed in every study (McMurtry et al. 2007). Alternatively, beneficial outcomes are apparent when BMP signal transduction is increased in established PAH by neutralizing the action of gremlin (Ciuclan et al. 2013), administering recombinant BMP-9 (Long et al. 2015), or alleviating the FKBP12-mediated inhibition of BMP type I receptors using the FDA approved small molecule FK506 (Spiekerkoetter et al. 2013). Of note, a clinical trial examining the safety and efficacy of FK506 in patients with sporadic or heritable PAH (NCT01647945) was initiated but then terminated because of limited funding and/or slow recruitment of subjects.
It is striking to point out that the vascular abnormalities characteristic of PAH have generally been considered restricted to the pulmonary vascular bed (Fares 2014). This is especially intriguing when considering that several BMP ligands are present in human and rodent serum at biologically active concentrations (David et al. 2008; Herrera and Inman 2009) and that BMP pathway components are expressed by vascular cells derived from other locations in the body (Lowery and de Caestecker 2010). Indeed, emerging evidence suggests that PAH patients likely experience vascular manifestations in the systemic circulation, including endothelial dysfunction and/or structural anomalies of capillaries (Fares 2014). The latter is consistent with genetic studies in animals that reveal the developmental requirement of BMP signaling in normal embryonic angiogenesis and vessel maturation (Lowery and de Caestecker 2010). Furthermore, most patients with hereditary hemorrhagic telangiectasia (HHT), which is characterized by structurally weak arteriovenous malformations (AVMs) that may appear in numerous vascular beds, inherit mutations in genes encoding the BMP receptors ALK-1 or endoglin (Cai et al. 2012; Garcia de Vinuesa et al. 2015). Treatment for AVMs varies by location and suspected severity, but generally involves coagulation therapy, surgical removal, or occlusion. It is unclear at present if BMP pathway modulation will be useful in the treatment of HHT and we are not aware of any clinical trials evaluating this possibility.
Tissue Fibrosis
Given that increased BMP pathway activation is implicated in calcification and ossification of soft tissue, it is somewhat surprising that activating the BMP pathway has been identified as a potential therapy for tissue fibrosis. In particular, the ability of BMP signaling to oppose TGF-β-induced fibrosis and promote tissue recovery has been shown in several clinically relevant contexts (Hudnall et al. 2016). For example, endogenous BMP signaling plays a critical role in recovery after obstructive uropathies and treatment of mice with exogenous BMP-7 enhances renal recovery after unilateral ureteral obstruction, in which TGF-β promotes glomerular fibrosis (Manson et al. 2011b). Activation of BMP signaling also has beneficial effects on TGF-β-induce fibrosis of cardiomyocytes (Wang et al. 2012b), ocular burn injuries (Saika et al. 2006), and silica-induced or allergen-induced pulmonary fibrosis (Myllarniemi et al. 2008; Yang et al. 2013a, 2016; Stumm et al. 2014). These studies serve as substantial proof-of-concept for the notion that activation of BMP signaling may hold broad therapeutic benefit in other contexts of fibrosis. Support for this hypothesis comes from preclinical studies examining the utility of recombinant BMP-7 or BMP-7-based gene therapy in bone marrow fibrosis (Gonzalez et al. 2002), corneal fibrosis (Saika et al. 2005; Tandon et al. 2013), hepatic fibrosis (Kinoshita et al. 2007; Hao et al. 2012; Zhong et al. 2013; Wang et al. 2014), lens fibrosis (Saika et al. 2006), prosthesis-related fibrosis (Tan et al. 2013), cardiac fibrosis (Zeisberg et al. 2007; Urbina and Singla 2014), and numerous models of renal fibrosis (Vukicevic et al. 1998; Klahr and Morrissey 2003; Zeisberg et al. 2003; Sugimoto et al. 2007; Manson et al. 2011a,b; Zhen-Qiang et al. 2012; Li et al. 2015). Although much of the research thus far has focused on BMP-7, it should be noted that other strategies aimed at generally increasing BMP signal transduction have been reported to be beneficial in models of tissue fibrosis. These include administration of recombinant BMP-2 or gene therapy-based BMP-2 expression (Yang et al. 2009; Wang et al. 2012b), administration of a BMP-related peptide (Sugimoto et al. 2012) or the small molecule FK506 (Qi et al. 2014), reducing expression of the BMP antagonist gremlin using siRNA (Zhang et al. 2010), or delivery of the downstream BMP target genes Id1 and Id3 (Saika et al. 2006).
Hemochromatosis and Iron Deficiency Anemia
The body has a complex system to regulate iron homeostasis (Gangat and Wolanskyj 2013). Iron is essential to make the hemoglobin necessary for red blood cells to carry oxygen; anemia occurs when iron levels are inadequate. Iron excess, however, is toxic, and as there is no known mechanism for regulated iron excretion, systemic iron homeostasis must be maintained by tightly balancing intestinal iron absorption and iron release by macrophages and hepatocytes (Babitt et al. 2007). Iron release into the circulation occurs through the iron exporter ferroportin. This export process is regulated by the iron regulator protein hepcidin, a 25-amino-acid peptide produced by the liver (Zhao et al. 2013). Hepcidin levels are sensitive to the iron status in the body through mechanisms that involve canonical BMP–Smad signaling (Parrow and Fleming 2014). Hepatocyte-specific deletion of Smad4 produces mice with a severe iron overload phenotype, whereas mutations in the HFE2 gene, which encodes a BMP coreceptor hemojuvelin, result in juvenile hemochromatosis, a disease characterized by severe iron overload (Babitt et al. 2006). Further evidence of the importance of BMP signaling in iron homeostasis comes from analysis of the physiological role of BMP-6. Bmp6 mRNA expression correlates with body iron stores in mice, and mice lacking BMP-6 show low hepcidin expression and severe iron overload, which can be corrected by increasing BMP-6 levels (Andriopoulos et al. 2009). Most recently, BMP-2 has been implicated in iron homeostasis with effects independent of BMP-6 (Koch et al. 2017).
These and additional experimental data support the notion that decreasing BMP might be beneficial in treating disorders of iron deficiency. For instance, neutralizing antibodies targeting BMP-6 have been shown to increase serum iron levels in mice (Andriopoulos et al. 2009; Meynard et al. 2011; Wang et al. 2012a). It should be noted that, although this provides compelling evidence implicating BMP-6 as the predominant BMP ligand in iron homeostasis in vivo, it does not rule out strategies targeting the BMP–Smad signaling pathway in general. Consistent with this, reducing BMP signaling through several means, including ALK-3 and hemojuvelin decoys, or the kinase inhibitors dorsomorphin and LDN-193189, increase serum iron levels in models of iron deficiency anemia (Babitt et al. 2007; Yu et al. 2008b; Andriopoulos et al. 2009; Steinbicker et al. 2011; Theurl et al. 2011; Wang et al. 2012a). Data from completed or ongoing clinical trials examining these strategies in humans are not yet available, but the possible use of LDN-193189 for treating anemia of inflammation is a focus of the Bridging Interventional Gaps (BrIDGs) Program of the National Institutes of Health (NIH) National Center for Advancing Translational Sciences.
Central Nervous System Ischemia-Related Injury
Bmp7 expression increases following ischemic injury to the cerebrum (Chang et al. 2003), raising the possibility that BMP signaling exerts protective or reparative actions in this tissue, and that augmenting its effect may be beneficial in the treatment of stroke. Support for this assertion comes from reports that administration of recombinant BMP-7 in a rodent model of transient ischemia before or at the onset of reperfusion leads to neuroprotection and decreased cerebral apoptosis through reduced activation of NF-κB, caspase 3, caspase 8, and caspase 9 (Pei et al. 2013; Xu et al. 2013). The translational potential of this strategy was further advanced by the showing that intracisternal administration of recombinant BMP-7 one day after reperfusion leads to rapid improvement of neurological function (Liu et al. 2001). This finding was accompanied by increased glucose usage and local cerebral blood flow in the affected region (Liu et al. 2001). The therapeutic window for BMP pathway activation appears to be early as BMP-7 administration 2 weeks following ischemia-reperfusion did not lead to functional improvement (Shin et al. 2014).
Collectively, the results discussed above suggest that exogenous activation of BMP signaling may exert protective effects following stroke. It should be noted that administration of the BMP inhibitor noggin has also been reported to promote functional recovery following stroke injury. For example, recombinant noggin delivered by intraventricular implantation of an osmotic pump 2 weeks after ischemia-reperfusion promotes functional recovery and tissue repair (Shin et al. 2014). The timing of noggin delivery in this study leaves open the possibility that the protective actions of endogenous BMP signaling occur early after ischemia-reperfusion injury. That said, transplantation of noggin-expressing BMSCs 6 hours following reperfusion also leads to improved neurological function (Chen et al. 2011). We suggest that these seemingly conflicting results may be attributed to the difference in augmenting endogenous repair mechanisms versus actuating artificial, exogenous repair mechanisms that introduce numerous unknown variables to the injury site. Head-to-head comparison of these different therapeutic strategies is required to determine which is more appropriate in the clinical setting; however, we are not aware of any completed or ongoing clinical trial evaluating modulation of BMP signaling in stroke.
Spinal Cord Injury
In vitro and in vivo evidence point to a role for BMP signaling in regulating neural lineage determination, with a seeming predilection for astrocytic differentiation over neuronal or oligodendrocytic fates (Mabie et al. 1997; Setoguchi et al. 2004; Hampton et al. 2007a; Xiao et al. 2010). Several groups have attempted to promote regeneration of the central nervous system following injury by modulating BMP signaling. For instance, expression of BMP-2 and BMP-7 increase rapidly with compressive injury of the spinal cord in rodents (Matsuura et al. 2008; Xiao et al. 2010) and intrathecal administration of recombinant noggin improves locomotor function 10 weeks after injury (Matsuura et al. 2008). Increased regrowth of the corticospinal tract is also observed with noggin administration in this model (Matsuura et al. 2008). These findings suggest that inhibition of endogenous BMP signaling may be beneficial in improving motor function caused by spinal cord injury (SCI), and this conclusion is further supported by results obtained when neural precursor cells expressing noggin are delivered to the site of injury (Setoguchi et al. 2004). However, it should be noted that, in a different rodent SCI model involving severing of the spinal cord, administration of a neutralizing antibody against noggin also increases axonal sprouting and neural plasticity (Hampton et al. 2007a,b). The impact of noggin inhibition on motor function following SCI was not reported in the short follow-up period of these studies. We are not aware of any completed or ongoing clinical trials evaluating BMP pathway modulation for treatment of SCI.
Myocardial Infarction
Loss of cardiomyocytes and cardiac function is seen in several kinds of myocardial injury including myocardial infarction, viral myocarditis, and ischemia-reperfusion. Current treatments are not effective at replenishing cardiomyocyte numbers and strategies to reduce apoptosis or increase proliferation may improve cardiac function. In vitro studies indicate that BMP-2 treatment reduces cardiomyocyte apoptosis in response to hypoxia or oxidative stress (Ebelt et al. 2013), and systemic administration of a single dose of recombinant BMP-2 limits infarct size and reduces cardiomyocyte apoptosis after acute myocardial ischemia in mice (Ebelt et al. 2013); however, no long-term benefit on cardiac function is observed during the follow-up period of 3 weeks postinfarction. It is possible that benefit could occur with a lengthier follow-up period. This is, in fact, the case when recombinant BMP-10 is released from an implanted sponge; cardiac function improves beginning ∼6 weeks postinfarction and continues for at least 12 weeks postinfarction (Sun et al. 2014). BMP-10 administration is also associated with increased cardiomyocyte proliferation and reduced infarct size (Sun et al. 2014). These findings must be balanced, however, with results showing that infarct size is substantially reduced by heterozygous loss of Bmp4 when ischemia is followed by reperfusion (Pachori et al. 2010). Consistent with this, administration of recombinant noggin or dorsomorphin thirty minutes before reperfusion reduces infarct size (Pachori et al. 2010). In addition, administration of recombinant follistatin-like 1 during reperfusion reduces the activation of Smad1, 5, and 8, and improves cardiac function at an early time point of 24 hours postinjury (Ogura et al. 2012).
These collective findings raise the possibility that BMP signaling exerts biphasic effects in the injured myocardium. BMP signaling may promote cardiomyocyte apoptosis immediately following injury, and cardiomyocyte proliferation and function after a lag period. This complex picture of BMP action creates uncertainty for the therapeutic rationale of modulating the BMP pathway as a means to improve cardiac function following myocardial infarction. Characterizing the currently unknown mechanism that mediates this shift is an important area for future investigation. We are not aware of any clinical trials evaluating the ability of BMP signaling to improve cardiac function following myocardial injury.
Other Pathologies
In addition to the applications above that have substantial experimental and clinical underpinnings, preclinical studies also suggest that increasing BMP pathway activation may be beneficial in other clinically relevant scenarios, such as retinal injury, in which recombinant BMP-4 treatment reduces retinal ganglion cells death (Ueki and Reh 2012), strabismus, in which recombinant BMP-4 reduces force generation and size of the superior rectus muscle (Anderson et al. 2011), infertility, in which administration of recombinant BMP-6 or BMP-7 in vivo improves oocyte quality and folliculogenesis (Lee et al. 2001; Park et al. 2012), and diet-induced obesity, in which recombinant BMP-7 treatment improves blood lipids and hyperglycemia (Boon et al. 2013). Additionally, two simultaneous and independent reports suggest that augmenting BMP signaling may be beneficial in promoting skeletal muscle growth and inhibiting muscle wasting by opposing the effects of myostatin (also known as growth and differentiation factor 8, GDF-8) and related molecules (Sartori et al. 2013; Winbanks et al. 2013). The ligand GDF-5 appears to be predominantly responsible for the endogenous antagonism of Smad2/3-mediated effects in this context (Sartori et al. 2013), although artificially increasing BMP-7 expression can exert the same or similar effects (Winbanks et al. 2013). It is worth noting that direct delivery of BMP ligands to the skeletal muscle environment is well documented to promote ectopic bone formation and is the defining characteristic for bona fide BMP ligands. Thus, capitalizing on findings that suggest that increasing local BMP activity improves muscle repair will likely be challenging and requires clever strategies to activate BMP signaling using intracellular means. Indeed, both landmark studies show that adenoviral-based expression of a constitutively active version of ALK-3 leads to substantial myofiber hypertrophy (Sartori et al. 2013; Winbanks et al. 2013).
Conversely, preclinical studies suggest that decreasing BMP pathway activation may be beneficial in varied clinically relevant scenarios, such as Duchenne’s muscular dystrophy, in which Noggin gene delivery improves skeletal muscle histology and markers of myogenesis (Shi et al. 2011), intraventricular hemorrhage of the brain, in which recombinant noggin treatment restores cellular morphology, myelination, and motor function (Dummula et al. 2011), regeneration of the liver, in which small molecule BMP type I receptor inhibitors, including LDN-193189, DMH2, or VU5350, increase hepatocyte proliferation following partial hepatectomy and LDN-193189 restores liver mass (Tsugawa et al. 2014), acute lung injury, in which LDN-193189 preserves epithelial barrier integrity after bleomycin-induced injury (Helbing et al. 2013), and rhytid, in which an ALK-3 decoy reduces wrinkle formation (Yoon et al. 2013).
FINAL PERSPECTIVES
The evidence discussed above strongly indicates that modulation of BMP signaling may be beneficial in treating human disease and improving patient quality of life. It is striking, then, how few tools are currently available to target this pathway in the clinical setting. The only FDA approved use at present is that of recombinant ligands delivered in relatively few clinical scenarios, such as open or nonunion fractures, vertebral fusion, and maxillofacial bone augmentation. The large quantities of ligand that must be used in these settings have created major concerns over both the cost and the potential for adverse events such as inflammation and HO. We are encouraged by the significant advances that have been made in addressing both of these limitations. For instance, numerous groups have generated short BMP-inspired biomimetic peptides that can be synthesized and are less expensive to generate than recombinant proteins, and, in some cases, have enhanced activity over the naturally occurring BMP ligands (Table 3). Two promising examples of this approach are the BMP-9-based peptide pBMP-9 (Bergeron et al. 2009; Lauzon et al. 2014; Beauvais et al. 2015) and the chimeric activin A/BMP-2 protein (AB204) (Allendorph et al. 2011; Ahn et al. 2014; Yoon et al. 2014; Kim et al. 2015; Yoon et al. 2015a,b). It is possible that drugs aimed at blocking the action of BMP antagonists could further reduce the quantity of ligand or peptide required for clinical benefit (Okada et al. 2009; Ahmed et al. 2010; Kato et al. 2011; Cao et al. 2014). Additionally, considerable advancement has been made toward the development of a carrier system that stabilizes BMP ligands at the site of implantation, and reducing the diffusion of BMP ligands from the delivery site, which would improve the ability to enact local activation of BMP signaling while reducing the likelihood of adverse events at distant sites (Agrawal and Sinha 2016). Although we are unaware of completed or ongoing clinical trials examining these next-generation BMP signaling activation strategies, recent progress allows us to envision a future in which augmentation of the BMP pathway can be accomplished using extremely small quantities of material.
Some disease conditions or particular patient populations, however, may not be well suited for broad BMP signaling activation strategies. For instance, although preclinical models suggest that correcting deficient BMP signaling by ligand delivery might help in treating PAH (Long et al. 2015), the ability of BMP signaling to promote vascular calcification raises concern regarding the systemic administration of BMP signaling activators. Such scenarios may call for more nuanced strategies, such as the one built on the observation that the BMP antagonist gremlin is elevated in PAH and that delivery of an antigremlin neutralizing antibody improves disease pathology (Ciuclan et al. 2013). Extension of this methodology to other disease states may reveal new, targeted strategies that modulate BMP signaling in defined physiological contexts by inhibiting aberrantly expressed antagonists or ligands.
Given that BMP signaling typically controls a large network of genes and cellular outcomes, it is highly likely that only a small portion of these directly relates to disease pathology, and that partial restoration would provide clinical benefit. Again using PAH as an example, defects in BMPRII-dependent signaling lead to changes in numerous signaling pathways in the pulmonary vasculature (Lowery and de Caestecker 2010), only one of which is impaired nitric oxide signaling (Frank et al. 2008). However, drugs that potentiate or promote nitric oxide action such as sildenafil are effective in improving pulmonary hemodynamics (Abrams et al. 2000; Prasad et al. 2000; Galie et al. 2005). To us, this signifies that the future of BMP-based therapeutics must take into account the downstream events that are controlled by BMP signaling in any given pathological context, thus allowing for targeted therapies—perhaps increasing the expression of even a single BMP target gene like Id1 or Id3 (Saika et al. 2006).
Finally, we wish to draw attention to the need for drugs that discriminate between individual type I BMP receptors. As discussed above, altered activity of the type I BMP receptor ALK-2 causes FOP (Shore et al. 2006), and pharmacological inhibition of BMP signaling reduces ectopic bone formation in preclinical models (Yu et al. 2008a; Peterson et al. 2014). Although compelling and groundbreaking, the drug used in these studies, LDN-193189, also potently inhibits other type I BMP receptors in addition to ALK-2 and has notable off-target effects on AMP kinase, vascular endothelial growth factor (VEGF) receptor 2 and platelet-derived growth factor (PDGF) receptor β, which must be taken into consideration (Lowery et al. 2016). As outlined in Table 4, this has prompted several groups to develop new type I BMP receptor inhibitors with dramatically enhanced selectivity for ALK-2 (and the closely related ALK-1) over the other type I receptors (Engers et al. 2013; Mohedas et al. 2013, 2014; Tsugawa et al. 2014). We are unaware of completed or ongoing clinical trials data comparing the safety or efficacy of these compounds, although one of these, LDN-212854, has been shown to block ectopic bone formation in a mouse model of BMP-dependent HO (Mohedas et al. 2013). The significant interest in drugs for treating FOP may also be translatable to another human disease, diffuse intrinsic pontine glioma (DIPG), which is also caused by activating mutations in the ACVR1/ALK2 gene (Buczkowicz et al. 2014; Fontebasso et al. 2014; Taylor et al. 2014; Wu et al. 2014). Furthermore, the intensity of this line of investigation may develop a toolbox to help delineate the effects of specific type I receptors in vivo; for example, differential type I receptor targeting proved beneficial in building an understanding of how BMP signaling promotes liver regeneration in a rodent model (Tsugawa et al. 2014).
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
*Reference is also in this subject collection.
- Abrams D, Schulze-Neick I, Magee AG. 2000. Sildenafil as a selective pulmonary vasodilator in childhood primary pulmonary hypertension. Heart 84: E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal V, Sinha M. 2016. A review on carrier systems for bone morphogenetic protein-2. J Biomed Mater Res B Appl Biomater 10.1002/jbm.b.33599. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Metpally RP, Sangadala S, Reddy BV. 2010. Virtual screening and selection of drug-like compounds to block noggin interaction with bone morphogenetic proteins. J Mol Graph Model 28: 670–682. [DOI] [PubMed] [Google Scholar]
- Ahn C, Maslennikov I, Choi JY, Oh H, Cheong C, Choe S. 2014. Characterization of Activin/BMP2 chimera, AB204, formulated for preclinical studies. Protein Pept Lett 21: 426–433. [DOI] [PubMed] [Google Scholar]
- Akkiraju H, Bonor J, Olli K, Bowen C, Bragdon B, Coombs H, Donahue LR, Duncan R, Nohe A. 2015. Systemic injection of CK2.3, a novel peptide acting downstream of bone morphogenetic protein receptor BMPRIa, leads to increased trabecular bone mass. J Orthop Res 33: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers CE, Hofstetter W, Sebald HJ, Sebald W, Siebenrock KA, Klenke FM. 2012. L51P—A BMP2 variant with osteoinductive activity via inhibition of noggin. Bone 51: 401–406. [DOI] [PubMed] [Google Scholar]
- Ali IHA, Brazil DP. 2014. Bone morphogenetic proteins and their antagonists: Current and emerging clinical uses. Br J Pharmacol 171: 3620–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorph GP, Read JD, Kawakami Y, Kelber JA, Isaacs MJ, Choe S. 2011. Designer TGFβ superfamily ligands with diversified functionality. PLoS ONE 6: e26402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsamarah A, LaCuran AE, Oelschlaeger P, Hao J, Luo Y. 2015. Uncovering molecular bases underlying bone morphogenetic protein receptor inhibitor selectivity. PLoS ONE 10: e0132221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BC, Daniel ML, Kendall JD, Christiansen SP, McLoon LK. 2011. Sustained release of bone morphogenetic protein-4 in adult rabbit extraocular muscle results in decreased force and muscle size: Potential for strabismus treatment. Invest Ophthalmol Vis Sci 52: 4021–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, et al. 2009. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet 41: 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao A, Hao J, Hopkins CR, Hong CC. 2012. DMH1, a novel BMP small molecule inhibitor, increases cardiomyocyte progenitors and promotes cardiac differentiation in mouse embryonic stem cells. PLoS ONE 7: e41627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y. 1995. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun 210: 670–677. [DOI] [PubMed] [Google Scholar]
- Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, et al. 2006. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet 38: 531–539. [DOI] [PubMed] [Google Scholar]
- Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. 2007. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest 117: 1933–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Gao Y, Arzigian M, Wojchowski DM, Wu WS, Wang ZZ. 2010. BMP4 regulates vascular progenitor development in human embryonic stem cells through a Smad-dependent pathway. J Cell Biochem 109: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni AL, Hutchinson JA, DeCastro AJ, Cherukuri P, Liby K, Sporn MB, Schwartz GN, Wells WA, Sempere LF, Yu PB, et al. 2013. ΔNp63α-mediated activation of bone morphogenetic protein signaling governs stem cell activity and plasticity in normal and malignant mammary epithelial cells. Cancer Res 73: 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais S, Drevelle O, Lauzon MA, Daviau A, Faucheux N. 2015. Modulation of MAPK signalling by immobilized adhesive peptides: Effect on stem cell response to BMP-9-derived peptides. Acta Biomater 31: 241–251. [DOI] [PubMed] [Google Scholar]
- Bendell JC, Gordon MS, Hurwitz HI, Jones SF, Mendelson DS, Blobe GC, Agarwal N, Condon CH, Wilson D, Pearsall AE, et al. 2014. Safety, pharmacokinetics, pharmacodynamics, and antitumor activity of dalantercept, an activin receptor-like kinase-1 ligand trap, in patients with advanced cancer. Clin Cancer Res 20: 480–489. [DOI] [PubMed] [Google Scholar]
- Bergeron E, Senta H, Mailloux A, Park H, Lord E, Faucheux N. 2009. Murine preosteoblast differentiation induced by a peptide derived from bone morphogenetic proteins-9. Tissue Eng Part A 15: 3341–3349. [DOI] [PubMed] [Google Scholar]
- Bi W, Gu Z, Zheng Y, Zhang X, Guo J, Wu G. 2013. Heterodimeric BMP-2/7 antagonizes the inhibition of all-trans retinoic acid and promotes the osteoblastogenesis. PLoS ONE 8: e78198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boergermann JH, Kopf J, Yu PB, Knaus P. 2010. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int J Biochem Cell Biol 42: 1802–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon MR, van den Berg SA, Wang Y, van den Bossche J, Karkampouna S, Bauwens M, De Saint-Hubert M, van der Horst G, Vukicevic S, de Winther MP, et al. 2013. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS ONE 8: e74083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JD, Cleverly DG, Burns AM, Helm NB, Schmid MJ, Marx DB, Cullen DM, Reinhardt RA. 2007. Cyclooxygenase-2 inhibitor reduces simvastatin-induced bone morphogenetic protein-2 and bone formation in vivo. J Periodontal Res 42: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, et al. 2014. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J 35: 3203–3211. [DOI] [PubMed] [Google Scholar]
- Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, Morrison A, Lewis P, Bouffet E, Bartels U, et al. 2014. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 46: 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs JT, van der Horst G, van den Hoogen C, Cheung H, de Rooij B, Kroon J, Petersen M, van Overveld PG, Pelger RC, van der Pluijm G. 2012. The BMP2/7 heterodimer inhibits the human breast cancer stem cell subpopulation and bone metastases formation. Oncogene 31: 2164–2174. [DOI] [PubMed] [Google Scholar]
- Burkus JK, Sandhu HS, Gornet MF, Longley MC. 2005. Use of rhBMP-2 in combination with structural cortical allografts: Clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am 87: 1205–1212. [DOI] [PubMed] [Google Scholar]
- Cahill KS, McCormick PC, Levi AD. 2015. A comprehensive assessment of the risk of bone morphogenetic protein use in spinal fusion surgery and postoperative cancer diagnosis. J Neurosurg Spine 23: 86–93. [DOI] [PubMed] [Google Scholar]
- Cai J, Pardali E, Sanchez-Duffhues G, ten Dijke P. 2012. BMP signaling in vascular diseases. FEBS letters 586: 1993–2002. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wang C, Zhang X, Xing G, Lu K, Gu Y, He F, Zhang L. 2014. Selective small molecule compounds increase BMP-2 responsiveness by inhibiting Smurf1-mediated Smad1/5 degradation. Sci Rep 4: 4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, Chen HL, Hoffer BJ, Wang Y. 2003. Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke 34: 558–564. [DOI] [PubMed] [Google Scholar]
- Chang HM, Cheng JC, Taylor E, Leung PC. 2014. Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells. Mol Hum Reprod 20: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Cheng Y, Chen J. 2011. Transfection of noggin in bone marrow stromal cells (BMSCs) enhances BMSC-induced functional outcome after stroke in rats. J Neurosci Res 89: 1194–1202. [DOI] [PubMed] [Google Scholar]
- Choi JY, Shin CS, Hong YC, Kang D. 2006. Single-nucleotide polymorphisms and haplotypes of bone morphogenetic protein genes and peripheral bone mineral density in young Korean men and women. Calcif Tissue Int 78: 203–211. [DOI] [PubMed] [Google Scholar]
- Ciuclan L, Sheppard K, Dong L, Sutton D, Duggan N, Hussey M, Simmons J, Morrell NW, Jarai G, Edwards M, et al. 2013. Treatment with anti-Gremlin 1 antibody ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension in mice. Am J Pathol 183: 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha SI, Pardali E, Thorikay M, Anderberg C, Hawinkels L, Goumans MJ, Seehra J, Heldin CH, ten Dijke P, Pietras K. 2010. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med 207: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham BW, Atkinson BL, Hu N, Kikkawa J, Jenis L, Bryant J, Zamora PO, McAfee PC. 2009. Ceramic granules enhanced with B2A peptide for lumbar interbody spine fusion: An experimental study using an instrumented model in sheep. J Neurosurg Spine 10: 300–307. [DOI] [PubMed] [Google Scholar]
- Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. 2008. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett 18: 4388–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, Jing L, Shi W, Qin P, Li Y, Diao A. 2015. Expression and purification of active recombinant human bone morphogenetic 7-2 dimer fusion protein. Protein Expr Purif 115: 61–68. [DOI] [PubMed] [Google Scholar]
- David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. 2008. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res 102: 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. 2008. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolbe E, Schwarz C, Ott CE, Konig J, Schmidt-Bleek K, Ellinghaus A, Schmidt T, Lienau J, Ploger F, Mundlos S, et al. 2015. Improved bone defect healing by a superagonistic GDF5 variant derived from a patient with multiple synostoses syndrome. Bone 73: 111–119. [DOI] [PubMed] [Google Scholar]
- Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. 2000. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. 2012. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol 32: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D, Bagarova J, Hatsell SJ, Armstrong KA, Huang L, Ermann J, Vonner AJ, Shen Y, Mohedas AH, Lee A, et al. 2016. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci Transl Med 8: 366ra163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumic-Cule I, Brkljacic J, Rogic D, Bordukalo Niksic T, Tikvica Luetic A, Draca N, Kufner V, Trkulja V, Grgurevic L, Vukicevic S. 2014. Systemically available bone morphogenetic protein two and seven affect bone metabolism. Int Orthop 38: 1979–1985. [DOI] [PubMed] [Google Scholar]
- Dummula K, Vinukonda G, Chu P, Xing Y, Hu F, Mailk S, Csiszar A, Chua C, Mouton P, Kayton RJ, et al. 2011. Bone morphogenetic protein inhibition promotes neurological recovery after intraventricular hemorrhage. J Neurosci 31: 12068–12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebelt H, Hillebrand I, Arlt S, Zhang Y, Kostin S, Neuhaus H, Muller-Werdan U, Schwarz E, Werdan K, Braun T. 2013. Treatment with bone morphogenetic protein 2 limits infarct size after myocardial infarction in mice. Shock 39: 353–360. [DOI] [PubMed] [Google Scholar]
- Engers DW, Frist AY, Lindsley CW, Hong CH, Hopkins CR. 2010. Development of a potent and ALK2 selective bone morphogenetic protein receptor (BMP) inhibitor. In Probe reports from the NIH molecular libraries program, National Center for Biotechnology Information (US), Bethesda, MD. [PubMed] [Google Scholar]
- Engers DW, Frist AY, Lindsley CW, Hong CC, Hopkins CR. 2013. Synthesis and structure-activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a]pyrimidine scaffold of dorsomorphin: The discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe. Bioorg Med Chem Lett 23: 3248–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcigno L, D’Auria G, Calvanese L, Marasco D, Iacobelli R, Scognamiglio PL, Brun P, Danesin R, Pasqualin M, Castagliuolo I, et al. 2015. Osteogenic properties of a short BMP-2 chimera peptide. J Pept Sci 21: 700–709. [DOI] [PubMed] [Google Scholar]
- Fares WH. 2014. The other vascular beds in pulmonary arterial hypertension. Surrogates or associated? Ann Am Thorac Soc 11: 596–597. [DOI] [PubMed] [Google Scholar]
- Feng F, Harper RL, Reynolds PN. 2016. BMPR2 gene delivery reduces mutation-related PAH and counteracts TGF-β-mediated pulmonary cell signalling. Respirology 21: 526–532. [DOI] [PubMed] [Google Scholar]
- Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, Nikbakht H, Gerges N, Fiset PO, Bechet D, Faury D, De Jay N, Ramkissoon LA, et al. 2014. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet 46: 462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DB, Lowery J, Anderson L, Brink M, Reese J, de Caestecker M. 2008. Increased susceptibility to hypoxic pulmonary hypertension in Bmpr2 mutant mice is associated with endothelial dysfunction in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 294: L98–L109. [DOI] [PubMed] [Google Scholar]
- Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, et al. 2005. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA Jr, et al. 2000. A role for Smad6 in development and homeostasis of the cardiovascular system. Nat Genet 24: 171–174. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Nove J, Levin M, Rosen V. 2005. BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in Xenopus embryos. Dev Biol 285: 156–168. [DOI] [PubMed] [Google Scholar]
- Gangat N, Wolanskyj AP. 2013. Anemia of chronic disease. Semin Hematol 50: 232–238. [DOI] [PubMed] [Google Scholar]
- Garcia de Vinuesa A, Abdelilah-Seyfried S, Knaus P, Zwijsen A, Bailly S. 2015. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev 27: 65–79. [DOI] [PubMed] [Google Scholar]
- Garulli C, Kalogris C, Pietrella L, Bartolacci C, Andreani C, Falconi M, Marchini C, Amici A. 2014. Dorsomorphin reverses the mesenchymal phenotype of breast cancer initiating cells by inhibition of bone morphogenetic protein signaling. Cell Signal 26: 352–362. [DOI] [PubMed] [Google Scholar]
- Gonzalez EA, Lund RJ, Martin KJ, McCartney JE, Tondravi MM, Sampath TK, Hruska KA. 2002. Treatment of a murine model of high-turnover renal osteodystrophy by exogenous BMP-7. Kidney Int 61: 1322–1331. [DOI] [PubMed] [Google Scholar]
- Guemes M, Garcia AJ, Rigueur D, Runke S, Wang W, Zhao G, Mayorga VH, Atti E, Tetradis S, Peault B, et al. 2014. GATA4 is essential for bone mineralization via ERα and TGFβ/BMP pathways. J Bone Miner Res 29: 2676–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman A, Zelman-Femiak M, Boergermann JH, Paschkowsky S, Kreuzaler PA, Fratzl P, Harms GS, Knaus P. 2012. SMAD versus non-SMAD signaling is determined by lateral mobility of bone morphogenetic protein (BMP) receptors. J Biol Chem 287: 39492–39504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasters F, Docheva D, Gassner C, Popov C, Bocker W, Mutschler W, Schieker M, Prall WC. 2014. Mesenchymal stem cells from osteoporotic patients reveal reduced migration and invasion upon stimulation with BMP-2 or BMP-7. Biochem Biophys Res Commun 452: 118–123. [DOI] [PubMed] [Google Scholar]
- Hamasaki M, Hashizume Y, Yamada Y, Katayama T, Hohjoh H, Fusaki N, Nakashima Y, Furuya H, Haga N, Takami Y, et al. 2012. Pathogenic mutation of ALK2 inhibits induced pluripotent stem cell reprogramming and maintenance: Mechanisms of reprogramming and strategy for drug identification. Stem Cells 30: 2437–2449. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Asher RA, Kondo T, Steeves JD, Ramer MS, Fawcett JW. 2007a. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci 26: 3024–3035. [DOI] [PubMed] [Google Scholar]
- Hampton DW, Steeves JD, Fawcett JW, Ramer MS. 2007b. Spinally upregulated noggin suppresses axonal and dendritic plasticity following dorsal rhizotomy. Exp Neurol 204: 366–379. [DOI] [PubMed] [Google Scholar]
- Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. 2008. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE 3: e2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. 2010. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol 5: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao ZM, Cai M, Lv YF, Huang YH, Li HH. 2012. Oral administration of recombinant adeno-associated virus-mediated bone morphogenetic protein-7 suppresses CCl4-induced hepatic fibrosis in mice. Mol Ther 20: 2043–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel S, Schmitt S, Hartung A, Roth M, Nohe A, Petersen N, Ehrlich M, Henis YI, Sebald W, Knaus P. 2003. Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. J Bone Joint Surg Am 85-A: 44–51. [DOI] [PubMed] [Google Scholar]
- Hatsell SJ, Idone V, Wolken DMA, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, et al. 2015. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med 7: 303ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawinkels LJ, de Vinuesa AG, Paauwe M, Kruithof-de Julio M, Wiercinska E, Pardali E, Mezzanotte L, Keereweer S, Braumuller TM, Heijkants RC, et al. 2016. Activin receptor-like kinase 1 ligand trap reduces microvascular density and improves chemotherapy efficiency to various solid tumors. Clin Cancer Res 22: 96–106. [DOI] [PubMed] [Google Scholar]
- He X, Ma J, Jabbari E. 2008. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir 24: 12508–12516. [DOI] [PubMed] [Google Scholar]
- Helbing T, Herold EM, Hornstein A, Wintrich S, Heinke J, Grundmann S, Patterson C, Bode C, Moser M. 2013. Inhibition of BMP activity protects epithelial barrier function in lung injury. J Pathol 231: 105–116. [DOI] [PubMed] [Google Scholar]
- Herrera B, Inman GJ. 2009. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol 10: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hill CS. 2016. Transcriptional control by the SMADs. Cold Spring Harb Perspect Biol 8: a022079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck AP. 2012. Structural studies of the TGF-βs and their receptors––Insights into evolution of the TGF-β superfamily. FEBS Lett 586: 1860–1870. [DOI] [PubMed] [Google Scholar]
- Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M, Sekiguchi K, Shibata M, Nagata S, Matsuda S, et al. 2015. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc Natl Acad Sci 112: 15438–15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbelt D, Boergermann JH, Chaikuad A, Alfano I, Williams E, Lukonin I, Timmel T, Bullock AN, Knaus P. 2015. Small molecules dorsomorphin and LDN-193189 inhibit myostatin/GDF8 signaling and promote functional myoblast differentiation. J Biol Chem 290: 3390–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, Saito N, Kinoshita T, Wakabayashi S, Tsutsumimoto T, Takaoka K. 2001. Enhancement of bone morphogenetic protein-2-induced new bone formation in mice by the phosphodiesterase inhibitor pentoxifylline. Bone 28: 290–294. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Saito N, Kinoshita T, Wakabayashi S, Yotsumoto N, Takaoka K. 2002. Effect of phosphodiesterase inhibitor-4, rolipram, on new bone formations by recombinant human bone morphogenetic protein-2. Bone 30: 589–593. [DOI] [PubMed] [Google Scholar]
- Huang F, Chen YG. 2012. Regulation of TGF-β receptor activity. Cell Biosci 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudnall AM, Arthur JW, Lowery JW. 2016. Clinical relevance and mechanisms of antagonism between the BMP and activin/TGF-β signaling pathways. J Am Osteopath Assoc 116: 452–461. [DOI] [PubMed] [Google Scholar]
- Huning I, Gillessen-Kaesbach G. 2014. Fibrodysplasia ossificans progressiva: Clinical course, genetic mutations and genotype-phenotype correlation. Mol Syndromol 5: 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Johnson ML, Koller DL, Lai D, Xuei X, Edenberg HJ, Hui SL, Foroud TM, Peacock M, Econs MJ. 2006. Polymorphisms in the bone morphogenetic protein 2 (BMP2) gene do not affect bone mineral density in white men or women. Osteoporos Int 17: 587–592. [DOI] [PubMed] [Google Scholar]
- Isaacs MJ, Kawakami Y, Allendorph GP, Yoon BH, Izpisua Belmonte JC, Choe S. 2010. Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Mol Endocrinol 24: 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM. 1996. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 13: 291–300. [DOI] [PubMed] [Google Scholar]
- Jung JW, Ahn C, Shim SY, Gray PC, Kwiatkowski W, Choe S. 2014. Regulation of FSHβ induction in LβT2 cells by BMP2 and an Activin A/BMP2 chimera, AB215. J Endocrinol 223: 35–45. [DOI] [PubMed] [Google Scholar]
- Kajimoto H, Kai H, Aoki H, Uchiwa H, Aoki Y, Yasuoka S, Anegawa T, Mishina Y, Suzuki A, Fukumoto Y, et al. 2015. BMP type I receptor inhibition attenuates endothelial dysfunction in mice with chronic kidney disease. Kidney Int 87: 128–136. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. 2009. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. Am J Physiol Endocrinol Metab 296: E139–146. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. 2010. Fasudil hydrochloride induces osteoblastic differentiation of stromal cell lines, C3H10T1/2 and ST2, via bone morphogenetic protein-2 expression. Endocr J 57: 415–421. [DOI] [PubMed] [Google Scholar]
- Kang EJ, Kim SK, Eom TG, Choi KO, Lee TH. 2013. Evaluation of the osteogenic activity of the BMP-2 mimetic peptide, PEP7, in vitro and in vivo. Int J Oral Maxillofac Implants 28: 749–756. [DOI] [PubMed] [Google Scholar]
- Kasten P, Beyen I, Bormann D, Luginbuhl R, Ploger F, Richter W. 2010. The effect of two point mutations in GDF-5 on ectopic bone formation in a β-tricalciumphosphate scaffold. Biomaterials 31: 3878–3884. [DOI] [PubMed] [Google Scholar]
- *.Katagiri T, Watabe T. 2016. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol 8: a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Sangadala S, Tomita K, Titus L, Boden SD. 2011. A synthetic compound that potentiates bone morphogenetic protein-2-induced transdifferentiation of myoblasts into the osteoblastic phenotype. Mol Cell Biochem 349: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr G, Sheldon H, Chaikuad A, Alfano I, von Delft F, Bullock AN, Harris AL. 2015. A small molecule targeting ALK1 prevents Notch cooperativity and inhibits functional angiogenesis. Angiogenesis 18: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. 1996. Bone morphogenetic protein-1: The type I procollagen C-proteinase. Science 271: 360–362. [DOI] [PubMed] [Google Scholar]
- Khattab HM, Ono M, Sonoyama W, Oida Y, Shinkawa S, Yoshioka Y, Maekawa K, Tabata Y, Sugama K, Sebald W, et al. 2014. The BMP2 antagonist inhibitor L51P enhances the osteogenic potential of BMP2 by simultaneous and delayed synergism. Bone 69: 165–173. [DOI] [PubMed] [Google Scholar]
- Kim BG, Lee JH, Ahn JM, Park SK, Cho JH, Hwang D, Yoo JS, Yates JR III, Ryoo HM, Cho JY. 2009. ‘Two-stage double-technique hybrid (TSDTH)’ identification strategy for the analysis of BMP2-induced transdifferentiation of premyoblast C2C12 cells to osteoblast. J Proteome Res 8: 4441–4454. [DOI] [PubMed] [Google Scholar]
- Kim DS, Lee JS, Leem JW, Huh YJ, Kim JY, Kim HS, Park IH, Daley GQ, Hwang DY, Kim DW. 2010. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev 6: 270–281. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim JI, Kim JB, Choe S. 2015. The activinβA/BMP-2 chimera AB204 is a strong stimulator of adipogenesis. J Tissue Eng Regen Med 10.1102/term.2050. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Iimuro Y, Otogawa K, Saika S, Inagaki Y, Nakajima Y, Kawada N, Fujimoto J, Friedman SL, Ikeda K. 2007. Adenovirus-mediated expression of BMP-7 suppresses the development of liver fibrosis in rats. Gut 56: 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr S, Morrissey J. 2003. Obstructive nephropathy and renal fibrosis: The role of bone morphogenic protein-7 and hepatocyte growth factor. Kidney Int Suppl 87: S105–112. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt K, Wagner-Ecker M, Bartek B, Holschbach J, Richter W. 2014. Superior angiogenic potential of GDF-5 and GDF-5(V453/V456) compared with BMP-2 in a rabbit long-bone defect model. J Bone Joint Surg Am 96: 1699–1707. [DOI] [PubMed] [Google Scholar]
- Koch PS, Olsavszky V, Ulbrich F, Sticht C, Demory A, Leibing T, Henzler T, Meyer M, Zierow J, Schneider S, et al. 2017. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood 129: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodach LL, Bleuming SA, Peppelenbosch MP, Hommes DW, van den Brink GR, Hardwick JC. 2007. The effect of statins in colorectal cancer is mediated through the bone morphogenetic protein pathway. Gastroenterology 133: 1272–1281. [DOI] [PubMed] [Google Scholar]
- Koga M, Engberding N, Dikalova AE, Chang KH, Seidel-Rogol B, Long JS, Lassegue B, Jo H, Griendling KK. 2013. The bone morphogenic protein inhibitor, noggin, reduces glycemia and vascular inflammation in db/db mice. Am J Physiol Heart Circ Physiol 305: H747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Yu PB, Kamiya N, Pan H, Fukuda T, Scott GJ, Ray MK, Yamamura K, Mishina Y. 2013. Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. J Bone Miner Res 28: 1422–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krase A, Abedian R, Steck E, Hurschler C, Richter W. 2014. BMP activation and Wnt-signalling affect biochemistry and functional biomechanical properties of cartilage tissue engineering constructs. Osteoarthritis Cartilage 22: 284–292. [DOI] [PubMed] [Google Scholar]
- Kuo MM, Nguyen PH, Jeon YH, Kim S, Yoon SM, Choe S. 2014. MB109 as bioactive human bone morphogenetic protein-9 refolded and purified from E. coli inclusion bodies. Microb Cell Fact 13: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureel J, Dixit M, Tyagi AM, Mansoori MN, Srivastava K, Raghuvanshi A, Maurya R, Trivedi R, Goel A, Singh D. 2014. miR-542-3p suppresses osteoblast cell proliferation and differentiation, targets BMP-7 signaling and inhibits bone formation. Cell Death Dis 5: e1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, Loyd JE, Nichols WC, Trembath RC. 2000. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet 26: 81–84. [DOI] [PubMed] [Google Scholar]
- Langenfeld E, Hong CC, Lanke G, Langenfeld J. 2013. Bone morphogenetic protein type I receptor antagonists decrease growth and induce cell death of lung cancer cell lines. PLoS ONE 8: e61256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrivee B, Prahst C, Gordon E, del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A. 2012. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell 22: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon MA, Marcos B, Faucheux N. 2014. Effect of initial pBMP-9 loading and collagen concentration on the kinetics of peptide release and a mathematical model of the delivery system. J Control Release 182: 73–82. [DOI] [PubMed] [Google Scholar]
- Lee WS, Otsuka F, Moore RK, Shimasaki S. 2001. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod 65: 994–999. [DOI] [PubMed] [Google Scholar]
- Lee JY, Choo JE, Park HJ, Park JB, Lee SC, Lee SJ, Park YJ, Chung CP. 2008. Synthetic peptide-coated bone mineral for enhanced osteoblastic activation in vitro and in vivo. J Biomed Mater Res A 87: 688–697. [DOI] [PubMed] [Google Scholar]
- Lee JY, Choo JE, Choi YS, Suh JS, Lee SJ, Chung CP, Park YJ. 2009. Osteoblastic differentiation of human bone marrow stromal cells in self-assembled BMP-2 receptor-binding peptide-amphiphiles. Biomaterials 30: 3532–3541. [DOI] [PubMed] [Google Scholar]
- Lee YC, Cheng CJ, Bilen MA, Lu JF, Satcher RL, Yu-Lee LY, Gallick GE, Maity SN, Lin SH. 2011. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res 71: 5194–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ. 1996. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc Natl Acad Sci 93: 5127–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RX, Yiu WH, Wu HJ, Wong DW, Chan LY, Lin M, Leung JC, Lai KN, Tang SC. 2015. BMP7 reduces inflammation and oxidative stress in diabetic tubulopathy. Clin Sci (London) 128: 269–280. [DOI] [PubMed] [Google Scholar]
- Lin X, Zamora PO, Albright S, Glass JD, Pena LA. 2005. Multidomain synthetic peptide B2A2 synergistically enhances BMP-2 in vitro. J Bone Miner Res 20: 693–703. [DOI] [PubMed] [Google Scholar]
- Lin X, Elliot JJ, Carnes DL, Fox WC, Pena LA, Campion SL, Takahashi K, Atkinson BL, Zamora PO. 2007. Augmentation of osseous phenotypes in vivo with a synthetic peptide. J Orthop Res 25: 531–539. [DOI] [PubMed] [Google Scholar]
- Lin GT, Tseng HF, Chang CK, Chuang LY, Liu CS, Yang CH, Tu CJ, Wang EC, Tan HF, Chang CC, et al. 2008. SNP combinations in chromosome-wide genes are associated with bone mineral density in Taiwanese women. Chin J Physiol 51: 32–41. [PubMed] [Google Scholar]
- Lin ZY, Duan ZX, Guo XD, Li JF, Lu HW, Zheng QX, Quan DP, Yang SH. 2010. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J Control Release 144: 190–195. [DOI] [PubMed] [Google Scholar]
- Lin X, Guo H, Takahashi K, Liu Y, Zamora PO. 2012. B2A as a positive BMP receptor modulator. Growth Factors 30: 149–157. [DOI] [PubMed] [Google Scholar]
- Lin T, Wang XL, Zettervall SL, Cai Y, Guzman RJ. 2016. Dorsomorphin homologue 1, a highly selective small-molecule bone morphogenetic protein inhibitor, suppresses medial artery calcification. J Vasc Surg 10.1016/j.jvs.2016.03.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Belayev L, Zhao W, Busto R, Saul I, Alonso O, Ginsberg MD. 2001. The effect of bone morphogenetic protein-7 (BMP-7) on functional recovery, local cerebral glucose utilization and blood flow after transient focal cerebral ischemia in rats. Brain Res 905: 81–90. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lin X, Takahashi K, Zamora PO. 2012. B2A, a receptor modulator, increases the growth of pluripotent and preosteoblast cells through bone morphogenetic protein receptors. Growth Factors 30: 410–417. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tang Y, Kang T, Rao M, Li K, Wang Q, Quan C, Zhang C, Jiang Q, Shen H. 2015. Synergistic effect of HA and BMP-2 mimicking peptide on the bioactivity of HA/PMMA bone cement. Colloids Surf B Biointerfaces 131: 39–46. [DOI] [PubMed] [Google Scholar]
- Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW. 2013. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 112: 1159–1170. [DOI] [PubMed] [Google Scholar]
- Long L, Ormiston ML, Yang X, Southwood M, Graf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, et al. 2015. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 21: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery JW, de Caestecker MP. 2010. BMP signaling in vascular development and disease. Cytokine Growth Factor Rev 21: 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery JW, Rosen V. 2012. Allele-specific RNA interference in FOP silencing the FOP gene. Gene Ther 19: 701–702. [DOI] [PubMed] [Google Scholar]
- Lowery JW, Brookshire B, Rosen V. 2016. A survey of strategies to modulate the bone morphogenetic protein signaling pathway: Current and future perspectives. Stem Cells Int 2016: 7290686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. 1997. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386: 78–81. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhang Z, Liu Y, Li H, Wang N, Liu W, Li W, Jin L, Wang J, Chen S. 2015. Nanotubes functionalized with BMP2 knuckle peptide improve the osseointegration of titanium implants in rabbits. J Biomed Nanotechnol 11: 236–244. [DOI] [PubMed] [Google Scholar]
- Mabie PC, Mehler MF, Marmur R, Papavasiliou A, Song Q, Kessler JA. 1997. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci 17: 4112–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Matsunuma A, Kawane T, Horiuchi N. 2001. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun 280: 874–877. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Burke MF, Martyn T, Shakartzi HR, Thayer TE, O’Rourke C, Li P, Derwall M, Spagnolli E, Kolodziej SA, et al. 2015. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency. PLoS ONE 10: e0117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson SR, Niederhoff RA, Hruska KA, Austin PF. 2011a. The BMP-7–Smad1/5/8 pathway promotes kidney repair after obstruction induced renal injury. J Urol 185: 2523–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson SR, Niederhoff RA, Hruska KA, Austin PF. 2011b. Endogenous BMP-7 is a critical molecular determinant of the reversibility of obstruction-induced renal injuries. Am J Physiol Renal Physiol 301: F1293–F1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura I, Taniguchi J, Hata K, Saeki N, Yamashita T. 2008. BMP inhibition enhances axonal growth and functional recovery after spinal cord injury. J Neurochem 105: 1471–1479. [DOI] [PubMed] [Google Scholar]
- Mayeur C, Kolodziej SA, Wang A, Xu X, Lee A, Yu PB, Shen J, Bloch KD, Bloch DB. 2015. Oral administration of a bone morphogenetic protein type I receptor inhibitor prevents the development of anemia of inflammation. Haematologica 100: e68–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan FE, Larzenius E, Callreus M, Gerdhem P, Luthman H, Akesson K. 2007. Variation in the BMP2 gene: Bone mineral density and ultrasound in young adult and elderly women. Calcif Tissue Int 81: 254–262. [DOI] [PubMed] [Google Scholar]
- McMurtry MS, Moudgil R, Hashimoto K, Bonnet S, Michelakis ED, Archer SL. 2007. Overexpression of human bone morphogenetic protein receptor 2 does not ameliorate monocrotaline pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 292: L872–L878. [DOI] [PubMed] [Google Scholar]
- Medici M, van Meurs JB, Rivadeneira F, Zhao H, Arp PP, Hofman A, Pols HA, Uitterlinden AG. 2006. BMP-2 gene polymorphisms and osteoporosis: The Rotterdam study. J Bone Miner Res 21: 845–854. [DOI] [PubMed] [Google Scholar]
- Meynard D, Vaja V, Sun CC, Corradini E, Chen S, Lopez-Otin C, Grgurevic L, Hong CC, Stirnberg M, Gutschow M, et al. 2011. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood 118: 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D, Pobre EG, Mulivor AW, Grinberg AV, Castonguay R, Monnell TE, Solban N, Ucran JA, Pearsall RS, Underwood KW, et al. 2010. ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol Cancer Ther 9: 379–388. [DOI] [PubMed] [Google Scholar]
- *.Miyazawa K, Miyazono K. 2017. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol 9: a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. 2004. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging cell 3: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohedas AH, Xing X, Armstrong KA, Bullock AN, Cuny GD, Yu PB. 2013. Development of an ALK2-biased BMP type I receptor kinase inhibitor. ACS Chem Biol 8: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohedas AH, Wang Y, Sanvitale CE, Canning P, Choi S, Xing X, Bullock AN, Cuny GD, Yu PB. 2014. Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. J Med Chem 57: 7900–7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T, Kaito T, Matsuo Y, Sugiura T, Kashii M, Makino T, Iwasaki M, Yoshikawa H. 2015. The bone morphogenetic protein-2/7 heterodimer is a stronger inducer of bone regeneration than the individual homodimers in a rat spinal fusion model. Spine J 15: 1379–1390. [DOI] [PubMed] [Google Scholar]
- Morrell NW, Bloch DB, ten Dijke P, Goumans MJ, Hata A, Smith J, Yu PB, Bloch KD. 2016. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol 13: 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munisso MC, Kang JH, Tsurufuji M, Yamaoka T. 2012. Cilomilast enhances osteoblast differentiation of mesenchymal stem cells and bone formation induced by bone morphogenetic protein 2. Biochimie 94: 2360–2365. [DOI] [PubMed] [Google Scholar]
- Myllarniemi M, Lindholm P, Ryynanen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. 2008. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med 177: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallamshetty S, Wang H, Rhee EJ, Kiefer FW, Brown JD, Lotinun S, Le P, Baron R, Rosen CJ, Plutzky J. 2013. Deficiency of retinaldehyde dehydrogenase 1 induces BMP2 and increases bone mass in vivo. PLoS ONE 8: e71307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer JM, Kwon S, Kim HS, Donley N, Tilak A, Sopory S, Christian JL. 2015. The prodomain of BMP4 is necessary and sufficient to generate stable BMP4/7 heterodimers with enhanced bioactivity in vivo. Proc Natl Acad Sci U S A 112: E2307–E2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. 2002. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem 277: 5330–5338. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Ouchi N, Ohashi K, Shibata R, Kataoka Y, Kambara T, Kito T, Maruyama S, Yuasa D, Matsuo K, et al. 2012. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation 126: 1728–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Sangadala S, Liu Y, Yoshida M, Reddy BV, Titus L, Boden SD. 2009. Development and optimization of a cell-based assay for the selection of synthetic compounds that potentiate bone morphogenetic protein-2 activity. Cell Biochem Funct 27: 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens P, Pickup MW, Novitskiy SV, Giltnane JM, Gorska AE, Hopkins CR, Hong CC, Moses HL. 2015. Inhibition of BMP signaling suppresses metastasis in mammary cancer. Oncogene 34: 2437–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachori AS, Custer L, Hansen D, Clapp S, Kemppa E, Klingensmith J. 2010. Bone morphogenetic protein 4 mediates myocardial ischemic injury through JNK-dependent signaling pathway. J Mol Cell Cardiol 48: 1255–1265. [DOI] [PubMed] [Google Scholar]
- Park SS, Park MJ, Joo BS, Joo JK, Son JB, Lee KS. 2012. Improvement of ovarian response and oocyte quality of aged female by administration of bone morphogenetic protein-6 in a mouse model. Reprod Biol Endocrinol 10: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrow NL, Fleming RE. 2014. Bone morphogenetic proteins as regulators of iron metabolism. Annu Rev Nutr 34: 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H, Cao D, Guo Z, Liu G, Guo Y, Lu C. 2013. Bone morphogenetic protein-7 ameliorates cerebral ischemia and reperfusion injury via inhibiting oxidative stress and neuronal apoptosis. Int J Mol Sci 14: 23441–23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, De La Rosa S, Eboda O, Cilwa KE, Agarwal S, Buchman SR, Cederna PS, Xi C, Morris MD, Herndon DN, et al. 2014. Treatment of heterotopic ossification through remote ATP hydrolysis. Sci Transl Med 6: 255ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall WC, Haasters F, Heggebo J, Polzer H, Schwarz C, Gassner C, Grote S, Anz D, Jager M, Mutschler W, et al. 2013. Mesenchymal stem cells from osteoporotic patients feature impaired signal transduction but sustained osteoinduction in response to BMP-2 stimulation. Biochem Biophys Res Commun 440: 617–622. [DOI] [PubMed] [Google Scholar]
- Prasad S, Wilkinson J, Gatzoulis MA. 2000. Sildenafil in primary pulmonary hypertension. N Engl J Med 343: 1342. [DOI] [PubMed] [Google Scholar]
- Qi R, Li W, Yu S. 2014. FK506 inhibits the mice glomerular mesangial cells proliferation by affecting the transforming growth factor-β and Smads signal pathways. Ren Fail 36: 589–592. [DOI] [PubMed] [Google Scholar]
- Ramesh Babu L, Wilson SG, Dick IM, Islam FM, Devine A, Prince RL. 2005. Bone mass effects of a BMP4 gene polymorphism in postmenopausal women. Bone 36: 555–561. [DOI] [PubMed] [Google Scholar]
- Reneland RH, Mah S, Kammerer S, Hoyal CR, Marnellos G, Wilson SG, Sambrook PN, Spector TD, Nelson MR, Braun A. 2005. Association between a variation in the phosphodiesterase 4D gene and bone mineral density. BMC Med Genet 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AM, Xia W, Holmes MD, Hodge SJ, Danilov S, Curiel DT, Morrell NW, Reynolds PN. 2007. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 292: L1182–L1192. [DOI] [PubMed] [Google Scholar]
- Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. 2012. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J 39: 329–343. [DOI] [PubMed] [Google Scholar]
- Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. 2012. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood 119: 6162–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondelet B, Dewachter L, Kerbaul F, Dewachter C, Hubloue I, Fesler P, Franck S, Remmelink M, Brimioulle S, Naeije R. 2010. Sildenafil added to sitaxsentan in overcirculation-induced pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 299: H1118–H1123. [DOI] [PubMed] [Google Scholar]
- Saeed O, Otsuka F, Polavarapu R, Karmali V, Weiss D, Davis T, Rostad B, Pachura K, Adams L, Elliott J, et al. 2012. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol 32: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Flanders KC, Nakajima Y, Miyamoto T, Ohnishi Y, Kao WW, Muragaki Y, Ooshima A. 2005. Therapeutic effects of adenoviral gene transfer of bone morphogenic protein-7 on a corneal alkali injury model in mice. Lab Invest 85: 474–486. [DOI] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Flanders KC, Ohnishi Y, Nakajima Y, Muragaki Y, Ooshima A. 2006. Adenoviral gene transfer of BMP-7, Id2, or Id3 suppresses injury-induced epithelial-to-mesenchymal transition of lens epithelium in mice. Am J Physiol Cell Physiol 290: C282–C289. [DOI] [PubMed] [Google Scholar]
- Saito A, Suzuki Y, Ogata S, Ohtsuki C, Tanihara M. 2003. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim Biophys Acta 1651: 60–67. [DOI] [PubMed] [Google Scholar]
- Salazar VS, Gamer LW, Rosen V. 2016. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol 12: 203–221. [DOI] [PubMed] [Google Scholar]
- Sanders LN, Schoenhard JA, Saleh MA, Mukherjee A, Ryzhov S, McMaster WG Jr, Nolan K, Gumina RJ, Thompson TB, Magnuson MA, et al. 2016. BMP antagonist gremlin-2 limits inflammation after myocardial infarction. Circ Res 119: 434–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvitale CE, Kerr G, Chaikuad A, Ramel MC, Mohedas AH, Reichert S, Wang Y, Triffitt JT, Cuny GD, Yu PB, et al. 2013. A new class of small molecule inhibitor of BMP signaling. PLoS ONE 8: e62721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar Z, Alexander D, Oxner W, du Plessis S, Yee A, Wai EK, Anderson DG, Jarzem P. 2015. Twelve-month results of a multicenter, blinded, pilot study of a novel peptide (B2A) in promoting lumbar spine fusion. J Neurosurg Spine 22: 358–366. [DOI] [PubMed] [Google Scholar]
- Sartori R, Schirwis E, Blaauw B, Bortolanza S, Zhao J, Enzo E, Stantzou A, Mouisel E, Toniolo L, Ferry A, et al. 2013. BMP signaling controls muscle mass. Nat Genet 45: 1309–1318. [DOI] [PubMed] [Google Scholar]
- Schwaerzer GK, Hiepen C, Schrewe H, Nickel J, Ploeger F, Sebald W, Mueller T, Knaus P. 2012. New insights into the molecular mechanism of multiple synostoses syndrome (SYNS): Mutation within the GDF5 knuckle epitope causes noggin-resistance. J Bone Miner Res 27: 429–442. [DOI] [PubMed] [Google Scholar]
- Sebald HJ, Klenke FM, Siegrist M, Albers CE, Sebald W, Hofstetter W. 2012. Inhibition of endogenous antagonists with an engineered BMP-2 variant increases BMP-2 efficacy in rat femoral defect healing. Acta Biomater 8: 3816–3820. [DOI] [PubMed] [Google Scholar]
- Seemann P, Brehm A, Konig J, Reissner C, Stricker S, Kuss P, Haupt J, Renninger S, Nickel J, Sebald W, et al. 2009. Mutations in GDF5 reveal a key residue mediating BMP inhibition by NOGGIN. PLoS Genet 5: e1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semprini S, Mango R, Brancati F, Dallapiccola B, Becherini L, Novelli G, De Lorenzo A, Brandi ML, Gennari L. 2000. Absence of correlation between BMP-4 polymorphism and postmenopausal osteoporosis in Italian women. Calcif Tissue Int 67: 93–94. [DOI] [PubMed] [Google Scholar]
- Seol YJ, Park YJ, Lee SC, Kim KH, Lee JY, Kim TI, Lee YM, Ku Y, Rhyu IC, Han SB, et al. 2006. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J Biomed Mater Res A 77: 599–607. [DOI] [PubMed] [Google Scholar]
- Setoguchi T, Nakashima K, Takizawa T, Yanagisawa M, Ochiai W, Okabe M, Yone K, Komiya S, Taga T. 2004. Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Exp Neurol 189: 33–44. [DOI] [PubMed] [Google Scholar]
- Shanmugam NK, Cherayil BJ. 2013. Serum-induced up-regulation of hepcidin expression involves the bone morphogenetic protein signaling pathway. Biochem Biophys Res Commun 441: 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen ZJ, Hu J, Ali A, Pastor J, Shiizaki K, Blank RD, Kuro-o M, Malter JS. 2013. Pin1 null mice exhibit low bone mass and attenuation of BMP signaling. PLoS ONE 8: e63565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Sun B, Guo WT, Liu X, Wang YC, Xie X, Xiao XL, Li N, Dong DL. 2014. (4-[6-(4-isopropoxyphenyl)pyrazolo [1,5-a]pyrimidin-3-yl] quinoline) is a novel inhibitor of autophagy. Br J Pharmacol 171: 4970–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hoogaars WM, de Gorter DJ, van Heiningen SH, Lin HY, Hong CC, Kemaladewi DU, Aartsma-Rus A, ten Dijke P, ’t Hoen PA. 2011. BMP antagonists enhance myogenic differentiation and ameliorate the dystrophic phenotype in a DMD mouse model. Neurobiol Dis 41: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JA, Lim SM, Jeong SI, Kang JL, Park EM. 2014. Noggin improves ischemic brain tissue repair and promotes alternative activation of microglia in mice. Brain Behav Immun 40: 143–154. [DOI] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, et al. 2006. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38: 525–527. [DOI] [PubMed] [Google Scholar]
- Simic P, Culej JB, Orlic I, Grgurevic L, Draca N, Spaventi R, Vukicevic S. 2006. Systemically administered bone morphogenetic protein-6 restores bone in aged ovariectomized rats by increasing bone formation and suppressing bone resorption. J Biol Chem 281: 25509–25521. [DOI] [PubMed] [Google Scholar]
- Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, et al. 2009. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54: S43–54. [DOI] [PubMed] [Google Scholar]
- Sinha S, Uchibe K, Usami Y, Pacifici M, Iwamoto M. 2016. Effectiveness and mode of action of a combination therapy for heterotopic ossification with a retinoid agonist and an anti-inflammatory agent. Bone 90: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucker JD, Bobst JA, Petersen EB, Nepola JV, Fredericks DC. 2008. B2A peptide on ceramic granules enhance posterolateral spinal fusion in rabbits compared with autograft. Spine 33: 1324–1329. [DOI] [PubMed] [Google Scholar]
- Song C, Guo Z, Ma Q, Chen Z, Liu Z, Jia H, Dang G. 2003. Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem Biophys Res Commun 308: 458–462. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, et al. 2013. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 123: 3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbicker AU, Sachidanandan C, Vonner AJ, Yusuf RZ, Deng DY, Lai CS, Rauwerdink KM, Winn JC, Saez B, Cook CM, et al. 2011. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood 117: 4915–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm CL, Halcsik E, Landgraf RG, Camara NO, Sogayar MC, Jancar S. 2014. Lung remodeling in a mouse model of asthma involves a balance between TGF-β1 and BMP-7. PLoS ONE 9: e95959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styrkarsdottir U, Cazier JB, Kong A, Rolfsson O, Larsen H, Bjarnadottir E, Johannsdottir VD, Sigurdardottir MS, Bagger Y, Christiansen C, et al. 2003. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol 1: E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Gonzalez D, Lee JS, Diggs A, Lu Y, Nemke B, Markel M, Hollister SJ, Murphy WL. 2014. Controlled multiple growth factor delivery from bone tissue engineering scaffolds via designed affinity. Tissue Eng Part A 20: 2077–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Grahovac G, Zeisberg M, Kalluri R. 2007. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 56: 1825–1833. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, LeBleu VS, Bosukonda D, Keck P, Taduri G, Bechtel W, Okada H, Carlson W Jr., Bey P, Rusckowski M, et al. 2012. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat Med 18: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M, Kodama T, Konishi K, Abe K, Asami S, Oikawa S. 2000. Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun 271: 688–692. [DOI] [PubMed] [Google Scholar]
- Sun L, Yu J, Qi S, Hao Y, Liu Y, Li Z. 2014. Bone morphogenetic protein-10 induces cardiomyocyte proliferation and improves cardiac function after myocardial infarction. J Cell Biochem 115: 1868–1876. [DOI] [PubMed] [Google Scholar]
- Szweras M, Liu D, Partridge EA, Pawling J, Sukhu B, Clokie C, Jahnen-Dechent W, Tenenbaum HC, Swallow CJ, Grynpas MD, et al. 2002. α-2-HS glycoprotein/fetuin, a transforming growth factor-β/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J Biol Chem 277: 19991–19997. [DOI] [PubMed] [Google Scholar]
- Tan HC, Poh CK, Cai Y, Wang W. 2013. Anti-fibrosis effect of BMP-7 peptide functionalization on cobalt chromium alloy. J Orthop Res 31: 983–990. [DOI] [PubMed] [Google Scholar]
- Tandon A, Sharma A, Rodier JT, Klibanov AM, Rieger FG, Mohan RR. 2013. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS ONE 8: e66434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Zhao J, Xu S, Li J, Teng Y, Quan D, Guo X. 2012. Bone induction through controlled release of novel BMP-2-related peptide from PTMC11-F127-PTMC11 hydrogels. Biomed Mater 7: 015008. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Mackay A, Truffaux N, Butterfield YS, Morozova O, Philippe C, Castel D, Grasso CS, Vinci M, Carvalho D, et al. 2014. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46: 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, Eller K, Wolf D, Seifert M, Sun CC, et al. 2011. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood 118: 4977–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhara Y, Wakitani S, Imai Y, Nomura C, Hoshino M, Yano K, Taguchi S, Kim M, Kadoya Y, Takaoka K. 2010. Local delivery of rolipram, a phosphodiesterase-4-specific inhibitor, augments bone morphogenetic protein-induced bone formation. J Bone Miner Metab 28: 17–24. [DOI] [PubMed] [Google Scholar]
- Tsugawa D, Oya Y, Masuzaki R, Ray K, Engers DW, Dib M, Do N, Kuramitsu K, Ho K, Frist A, et al. 2014. Specific activin receptor-like kinase 3 inhibitors enhance liver regeneration. J Pharmacol Exp Ther 351: 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeman G, Zilberman Y, Zhou S, Kelly P, Moutsatsos IK, Kharode YP, Borella LE, Bex FJ, Komm BS, Bodine PV, et al. 2002. Systemically administered rhBMP-2 promotes MSC activity and reverses bone and cartilage loss in osteopenic mice. J Cell Biochem 86: 461–474. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Reh TA. 2012. Activation of BMP–Smad1/5/8 signaling promotes survival of retinal ganglion cells after damage in vivo. PLoS ONE 7: e38690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina P, Singla DK. 2014. BMP-7 attenuates adverse cardiac remodeling mediated through M2 macrophages in prediabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 307: H762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist MR. 1965. Bone: formation by autoinduction. Science 150: 893–899. [DOI] [PubMed] [Google Scholar]
- Valera E, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S. 2010. BMP-2/6 heterodimer is more effective than BMP-2 or BMP-6 homodimers as inductor of differentiation of human embryonic stem cells. PLoS ONE 5: e11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt J, Traynor R, Sapkota GP. 2011. The specificities of small molecule inhibitors of the TGFβ and BMP pathways. Cell Signal 23: 1831–1842. [DOI] [PubMed] [Google Scholar]
- Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, et al. 1998. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest 102: 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DW, Godson C, Brazil DP, Martin F. 2010. Extracellular BMP-antagonist regulation in development and disease: Tied up in knots. Trends Cell Biol 20: 244–256. [DOI] [PubMed] [Google Scholar]
- Wang L, Trebicka E, Fu Y, Ellenbogen S, Hong CC, Babitt JL, Lin HY, Cherayil BJ. 2012a. The bone morphogenetic protein-hepcidin axis as a therapeutic target in inflammatory bowel disease. Inflamm Bowel Dis 18: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sun A, Li L, Zhao G, Jia J, Wang K, Ge J, Zou Y. 2012b. Up-regulation of BMP-2 antagonizes TGF-β1/ROCK-enhanced cardiac fibrotic signalling through activation of Smurf1/Smad6 complex. J Cell Mol Med 16: 2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Dong JZ, Xiong LJ, Shi KQ, Zou ZL, Zhang SN, Cao ST, Lin Z, Chen YP. 2014. BMP-7 attenuates liver fibrosis via regulation of epidermal growth factor receptor. Int J Clin Exp Pathol 7: 3537–3547. [PMC free article] [PubMed] [Google Scholar]
- Winbanks CE, Chen JL, Qian H, Liu Y, Bernardo BC, Beyer C, Watt KI, Thomson RE, Connor T, Turner BJ, et al. 2013. The bone morphogenetic protein axis is a positive regulator of skeletal muscle mass. J Cell Biol 203: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. 1988. Novel regulators of bone formation: Molecular clones and activities. Science 242: 1528–1534. [DOI] [PubMed] [Google Scholar]
- Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. 2014. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29: 2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, Zhu X, Qu C, Chen X, Zhang J, et al. 2014. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Du Y, Wu W, Yip HK. 2010. Bone morphogenetic proteins mediate cellular response and, together with noggin, regulate astrocyte differentiation after spinal cord injury. Exp Neurol 221: 353–366. [DOI] [PubMed] [Google Scholar]
- Xu J, Li X, Lian JB, Ayers DC, Song J. 2009. Sustained and localized in vitro release of BMP-2/7, RANKL, and tetracycline from FlexBone, an elastomeric osteoconductive bone substitute. J Orthop Res 27: 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JH, Zhang TZ, Zhao YY, Wang JK, Yuan ZG. 2013. Protective effects of recombinant human bone morphogenetic protein-7 on focal cerebral ischemia-reperfusion injury. Int J Neurosci 123: 375–384. [DOI] [PubMed] [Google Scholar]
- Yan Y, Tang D, Chen M, Huang J, Xie R, Jonason JH, Tan X, Hou W, Reynolds D, Hsu W, et al. 2009. Axin2 controls bone remodeling through the β-catenin–BMP signaling pathway in adult mice. J Cell Sci 122: 3566–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Liu YS, Chuang LY, Guh JY, Lee TC, Liao TN, Hung MY, Chiang TA. 2009. Bone morphogenetic protein-2 antagonizes renal interstitial fibrosis by promoting catabolism of type I transforming growth factor-β receptors. Endocrinology 150: 727–740. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhu Z, Wang Y, Gao A, Niu P, Tian L. 2013a. Bone morphogenetic protein-7 inhibits silica-induced pulmonary fibrosis in rats. Toxicol Lett 220: 103–108. [DOI] [PubMed] [Google Scholar]
- Yang J, Li X, Al-Lamki RS, Wu C, Weiss A, Berk J, Schermuly RT, Morrell NW. 2013b. Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol 33: 34–42. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhu Z, Wang Y, Gao A, Niu P, Chen L, Tian L. 2016. Bone morphogenetic protein 7 attenuates epithelial–mesenchymal transition induced by silica. Hum Exp Toxicol 35: 69–77. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. 2006. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem 281: 33921–33930. [DOI] [PubMed] [Google Scholar]
- Yen CH, Leu S, Lin YC, Kao YH, Chang LT, Chua S, Fu M, Wu CJ, Sun CK, Yip HK. 2010. Sildenafil limits monocrotaline-induced pulmonary hypertension in rats through suppression of pulmonary vascular remodeling. J Cardiovasc Pharmacol 55: 574–584. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Jeon YH, Hwang B, Kwon H, Choe S, Yang Z. 2013. Anti-wrinkle effect of bone morphogenetic protein receptor 1a-extracellular domain (BMPR1a-ECD). BMB Rep 46: 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Esquivies L, Ahn C, Gray PC, Ye SK, Kwiatkowski W, Choe S. 2014. An activin A/BMP2 chimera displays bone healing properties superior to those of BMP2. J Bone Miner Res 29: 1950–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Lee JH, Na K, Ahn C, Cho J, Ahn HC, Choi J, Oh H, Kim BM, Choe S. 2015a. The effects of a single intravenous injection of novel activin A/BMP-2 (AB204) on toxicity and the respiratory and central nervous systems. Drug Chem Toxicol 39: 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BH, Lee JH, Na K, Cho J, Choe S. 2015b. The toxicological evaluation of repetitive 2- and 4-week intravenous injection of activin A/BMP-2 chimera (AB204) into rats. Regul Toxicol Pharmacol 73: 1–8. [DOI] [PubMed] [Google Scholar]
- Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, et al. 2008a. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med 14: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. 2008b. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebboudj AF, Imura M, Boström K. 2002. Matrix GLA Protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem 277: 4388–4394. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Bottiglio C, Kumar N, Maeshima Y, Strutz F, Muller GA, Kalluri R. 2003. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol 285: F1060–F1067. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. 2007. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961. [DOI] [PubMed] [Google Scholar]
- Zhang YE. 2009. Non-Smad pathways in TGF-β signaling. Cell Res 19: 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lin CY. 2008. Simvastatin stimulates chondrogenic phenotype of intervertebral disc cells partially through BMP-2 pathway. Spine 33: E525–E531. [DOI] [PubMed] [Google Scholar]
- Zhang F, Qiu T, Wu X, Wan C, Shi W, Wang Y, Chen JG, Wan M, Clemens TL, Cao X. 2009. Sustained BMP signaling in osteoblasts stimulates bone formation by promoting angiogenesis and osteoblast differentiation. J Bone Miner Res 24: 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Shi Y, Wada J, Malakauskas SM, Liu M, Ren Y, Du C, Duan H, Li Y, Li Y, et al. 2010. In vivo delivery of gremlin siRNA plasmid reveals therapeutic potential against diabetic nephropathy by recovering bone morphogenetic protein-7. PLoS ONE 5: e11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu J, Tian XY, Wong WT, Chen Y, Wang L, Luo J, Cheang WS, Lau CW, Kwan KM, et al. 2014. Inhibition of bone morphogenic protein 4 restores endothelial function in db/db diabetic mice. Arterioscler Thromb Vasc Biol 34: 152–159. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shuang Y, Fu H, Zhou W, Qian L, Dai J, Miron RJ. 2015. Characterization of a shorter recombinant polypeptide chain of bone morphogenetic protein 2 on osteoblast behaviour. BMC Oral Health 15: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Zhang AS, Enns CA. 2013. Iron regulation by hepcidin. J Clin Invest 123: 2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wang L, Zhang X, Zhang X, Gu Z, Wu G. 2012. BMP2/7 heterodimer can modulate all cellular events of the in vitro RANKL-mediated osteoclastogenesis, respectively, in different dose patterns. Tissue Eng A 18: 621–630. [DOI] [PubMed] [Google Scholar]
- Zhen-Qiang F, Bing-Wei Y, Yong-Liang L, Xiang-Wei W, Shan-Hong Y, Yuan-Ning Z, Wei-Sheng J, Wei C, Ye G. 2012. Localized expression of human BMP-7 by BM-MSCs enhances renal repair in an in vivo model of ischemia-reperfusion injury. Genes Cells 17: 53–64. [DOI] [PubMed] [Google Scholar]
- Zhong L, Wang X, Wang S, Yang L, Gao H, Yang C. 2013. The anti-fibrotic effect of bone morphogenic protein-7(BMP-7) on liver fibrosis. Int J Med Sci 10: 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, et al. 2010. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142: 531–543. [DOI] [PubMed] [Google Scholar]
- Zhou X, Feng W, Qiu K, Chen L, Wang W, Nie W, Mo X, He C. 2015. BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Appl Mater Interfaces 7: 15777–15789. [DOI] [PubMed] [Google Scholar]
- Zouani OF, Chollet C, Guillotin B, Durrieu MC. 2010. Differentiation of pre-osteoblast cells on poly(ethylene terephthalate) grafted with RGD and/or BMPs mimetic peptides. Biomaterials 31: 8245–8253. [DOI] [PubMed] [Google Scholar]