Abstract
Rationale: Although obesity has been associated with asthma, body mass index is suboptimal to fully characterize adiposity.
Objectives: We examined the relation between adiposity and asthma in a large sample of the U.S. population, using indices defined by dual-energy X-ray absorptiometry.
Methods: We analyzed data from 8,886 children (aged 8–19 yr) and 12,795 adults (aged 20–69 yr) from the 2001 to 2006 National Health and Nutrition Examination Survey. In addition to body mass index, percent body fat, waist circumference, and waist-to-height ratio, dual-energy X-ray absorptiometry was used to calculate whole-body and local adiposity indices: fat mass index; total, trunk, and legs percent fat; and trunk-to-total fat mass ratio, legs-to-total fat mass ratio, and trunk-to-legs fat mass ratios. Logistic regression was used for the analysis of adiposity measures and asthma.
Results: Among children, general adiposity was significantly associated with asthma, with no major differences by sex. Results were driven by nonatopic children, in whom trunk-predominant (central) adiposity (assessed by waist circumference, waist-to-height ratio, trunk-to-total fat mass ratio, and trunk-to-legs fat mass ratio) was also associated with asthma. There were no significant associations among atopic children. Among adults, all adiposity indices were associated with asthma, with central adiposity significant only among women. The results in adults were driven by atopy, especially in women.
Conclusions: Adiposity measured by dual-energy X-ray absorptiometry provides similar information to that obtained by using anthropometric indices among children of both sexes and among adult men. However, dual-energy X-ray absorptiometry provides additional information in adult women, in whom dual-energy X-ray absorptiometry–measured central adiposity is significantly associated with asthma, particularly atopic asthma.
Keywords: obesity, asthma, adiposity, dual-energy X-ray absorptiometry
The worldwide prevalence of asthma and obesity has risen markedly in the last few decades (1, 2), and both diseases have become major public health concerns. Considerable evidence has shown an association between obesity and asthma in both children (3, 4) and adults (5). Several underlying mechanisms for this association—including enhanced systemic inflammation, changes in lung mechanics, increased oxidative stress, elevated adipokines, and epigenetic changes—have been studied but not well established (6).
“Obese asthma” has been proposed as a distinct asthma phenotype characterized by worsened symptoms and severity, decreased response to inhaled corticosteroids, and, in adults, late-onset and female predominance (7, 8). Epidemiological studies, however, have highlighted the heterogeneity of the effect of obesity on asthma risk and morbidity. Obesity seems to play a substantial role in patients with nonatopic asthma in both children (9) and adults (10), suggesting obesity influences asthma through noneosinophilic airway inflammation. Age of onset may modify the association between obesity and asthma (11), and sex differences in the association between adiposity and asthma have been reported in both children (12) and adults (13), albeit inconsistently.
General obesity, mostly measured by body mass index (BMI), has been associated with asthma (14) and worse asthma control (15, 16). However, BMI is unable to distinguish between fat mass and muscle mass or to predict abdominal fat predisposition, which is associated with pulmonary function (17), cytokine levels (18, 19), and the metabolic syndrome (20). Body percent fat, measured by dual-energy X-ray absorptiometry (DXA) (21) or bioelectric impedance (22), may be the preferred measure of adiposity in epidemiological studies of asthma in children (23, 24).
Whether DXA measures provide phenotypic information that differs from or adds to that obtained by measuring BMI for studies of asthma, however, is largely unknown. In this study, we examined the relation between adiposity indices (defined by both anthropometric and DXA measures) and asthma in a large sample of the U.S. population. We hypothesized that, compared with BMI alone, DXA-based measures would provide added information regarding asthma risk. We further hypothesized that the adiposity–asthma associations would differ by age group, sex, and atopy status.
Methods
Subject Recruitment
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional nationwide survey designed to assess the health and nutritional status of the noninstitutionalized U.S. population. Participants are selected by using a stratified multistage probability sampling. By design, persons 60 years and older and ethnic minorities (African Americans and Hispanics) are oversampled to increase the statistical power for data analysis and to represent the U.S. population of all ages after accounting for sampling weight. For this analysis, we included both children (8–19 years old) and adults (20–69 years old) who participated in the 2001 to 2002, 2003 to 2004, and 2005 to 2006 NHANES cycles with whole-body DXA examination. NHANES is approved by the Institutional Review Board of the National Center for Health Statistics of the U.S. Centers for Disease Control and Prevention. Informed consent is obtained from all participants. A proxy provided information for survey participants who were younger than 16 years of age and for subjects who could not answer the questions by themselves. Details of the methods, protocols, and definitions used in NHANES can be found on the study website (25).
Study Procedures
Current asthma was defined by a positive answer to both of the following questions: “Has a doctor or other health professional ever told you that you have asthma?” and “Do you still have asthma?” Participants who answered “no” to both questions were selected as control subjects. Those who answered “yes” to one question and “no” to the other were excluded.
Anthropometric measures were collected by trained health technicians on the basis of recommendations from the Anthropometric Standardization Reference Manual of NHANES (26). BMI was calculated by dividing weight in kilograms by height squared in meters. Obesity was defined as a BMI greater than or equal to 30 kg/m2 in adults and as a BMI greater than or equal to 95th percentile in children. Central obesity was defined as a waist circumference greater than or equal to 88 cm in women or waist circumference greater than or equal to 102 cm in men (27), and as a waist circumference greater than or equal to 95th percentile in children. For the analysis of BMI as a continuous variable, raw BMI (kg/m2) was used for adults, and BMI z scores were calculated based on the 2000 U.S. Centers for Disease Control and Prevention growth charts (28) for children. Similarly, raw waist circumference (in centimeters) was used for adults, and sample-specific waist circumference z score was used for children. Percent body fat was calculated from tricipital and subscapular skin folds (29, 30), and percent body fat z scores were calculated based on the reference equations for U.S. children and adolescents (31). Waist-to-height ratio was standardized by using the distribution of our study sample in children and adults separately.
The whole-body DXA examination was administered at the mobile examination center with a Hologic QDR-4500A fan-beam densitometer (Hologic, Inc.) by certified radiology technologists. DXA scans provide bone and soft tissue measurements for the total body, both arms and legs, and trunk. DXA scans were administered to eligible survey participants aged 8 to 69 years. Pregnant participants, individuals with self-reported use of radiographic contrast material (barium) in the prior week, or self-reported weight greater than 300 pounds or height greater than 6 feet 5 inches (DXA table limitations) were excluded. SAS-callable imputation and variance estimation software (IVEware) was developed to impute the missing or invalid DXA data (32) using the sequential regression imputation method (33). Five complete records containing valid and/or imputed values were created for each survey participant to allow the assessment of variability due to imputation. Further details of the DXA examination protocol are documented on the NHANES website (34) and elsewhere (35). DXA measures included in our analysis are: total percent fat, trunk percent fat, leg percent fat, and fat mass index (total fat mass divided by height squared, kg/m2). To further examine whether body fat distribution was associated with asthma, three indices were calculated: trunk-to-total fat ratio (trunk fat [grams]/total fat [grams]), legs-to-total fat ratio (sum of right and left leg fat [grams]/total fat [grams]), and trunk-to-legs ratio (trunk fat [grams]/sum of right and left leg fat [grams]).
Laboratory Studies
In NHANES 2005 to 2006, serum immunoglobulin E level for each of fifteen common aeroallergens was measured using the ImmunoCAP 1000 System (Pharmacia Diagnostics). For each allergen tested, a serum immunoglobulin E greater than or equal to 0.35 KU/L (the detection limit of the ImmunoCAP system) was considered positive. Atopy was defined as one or more positive specific immunoglobulin E results. In NHANES 2001 to 2006, fasting plasma glucose, serum insulin, high-density lipoprotein cholesterol, and total cholesterol were measured at a morning examination session in all NHANES participants 12 years or older, after fasting for 9 hours or more. Homeostasis model assessment-estimated insulin resistance (fasting insulin × fasting glucose [mmol/L]/22.5) was used as a measure of insulin resistance. Dyslipidemia was assessed by the total cholesterol/high-density lipoprotein cholesterol ratio.
Statistical Analysis
Primary sampling units and strata for the complex NHANES survey design were taken into account for data analysis. Sampling weights, stratification, and clusters provided in the NHANES data set were incorporated into the analysis to obtain proper estimates and their standard errors. Wald chi-square tests and t tests were used for bivariate analyses of binary and continuous variables, respectively. Pearson correlation coefficient (γ) was used to measure the correlation between anthropometric and DXA measures. Logistic regression was used for the multivariable analysis of adiposity indices and asthma. All models were adjusted for age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), annual household income, and serum levels of cotinine and C-reactive protein. All statistical analyses were conducted using the SAS SURVEY procedure and SAS 9.3 software (SAS Institute Inc.). All regression analyses were repeatedly performed for each of the five imputed data sets; using SAS MIANALYZE procedure, final results were generated by combining five sets of regression coefficients using Rubin’s combining rules (36). The MIANALYZE procedure reads parameter estimates and associated standard errors or covariance matrix computed by the standard statistical procedure for each imputed data set and derives valid univariate inferences for these parameters. For the subset of participants with laboratory studies, we investigated whether atopy modified the associations between obesity/adiposity and asthma (NHANES 2005–2006) and whether insulin resistance or dyslipidemia mediated these associations (NHANES 2001–2006). Figure E1 in the online supplement illustrates the subsets of NHANES participants who had each measure available for analysis.
Results
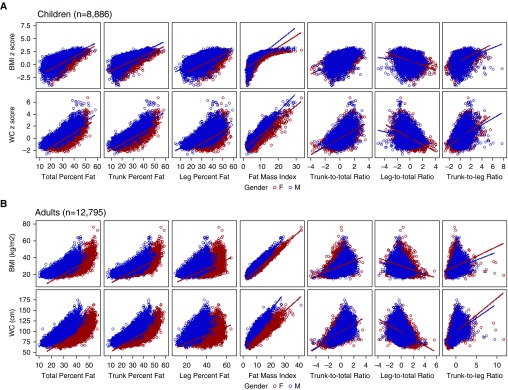
A total of 8,886 children and 12,795 adults from NHANES 2001 to 2006 who had DXA measures and data on asthma were included in the analysis (Table 1). The overall prevalence of current asthma among study participants was 9.9% in children and 7.6% in adults. Among participating children, 61.5% were non-Hispanic white, more than 81% had an annual household income greater than or equal to $20,000 or were covered by health insurance, 19.5% were obese, and 8.9% had central obesity defined by waist circumference. Mean total percent fat, trunk percent fat, and leg percent fat were 29.2%, 25.6%, and 34.7%, respectively. Among adult participants, 71.4% were non-Hispanic white, more than 82% had an annual household greater than or equal to $20,000 or were covered by health insurance, 32.4% were obese, and 50.8% had central obesity. Mean total percent fat, trunk percent fat, and leg percent fat were 33.9%, 33.4%, and 35.8%, respectively. Figure 1 shows the correlations between BMI, waist circumference, and DXA measures: BMI and waist circumference exhibited moderate to good correlation with most DXA indices (total percent fat, trunk percent fat, leg percent fat; γ, 50–97), and fair correlation with the DXA fat distribution indices (γ, 31–59). Scatter plots, however, show that these relationships are nonlinear. Overall, female participants—particularly adult women—had higher body fat indices than male participants.
Table 1.
Characteristics of study participants (National Health and Nutrition Examination Survey 2001–2006)
| Children, 8–19 yr (n = 8,886) | Adults, 20–69 yr, (n = 12,795) | |
|---|---|---|
| Age, yr | 13.42 (0.07) | 45.07 (0.29) |
| Male sex | 4,507 (51.58) | 6,466 (49.17) |
| Race | ||
| Non-Hispanic white | 2,449 (61.49) | 6,470 (71.44) |
| Non-Hispanic black | 2,984 (14.82) | 2,725 (11.27) |
| Hispanic | 3,062 (17.19) | 3,107 (12.18) |
| Other | 391 (6.50) | 493 (5.11) |
| Household income ≥ $20,000/yr | 6,207 (81.74) | 9,218 (83.86) |
| Covered by health insurance | 4,762 (85.64) | 7,327 (82.33) |
| Current asthma | 897 (9.86) | 896 (7.61) |
| Serum cotinine, ng/ml | 15.34 (1.32) | 67.37 (2.54) |
| C-reactive protein, mg/dl | 0.16 (0.01) | 0.42 (0.01) |
| ≥1 positive specific immunoglobulin E* | 1,309 (45.28) | 1,556 (44.51) |
| HOMA-IR† | 2.79 (0.08) | 2.96 (0.07) |
| TC/HDL-C† | 3.31 (0.03) | 4.03 (0.03) |
| Anthropometry | ||
| BMI‡ | 0.59 (0.03) | 28.29 (0.11) |
| Overweight subjects‡ | 1,499 (17.14) | 4,366 (33.87) |
| Obese subjects‡ | 1,902 (19.47) | 4,093 (32.36) |
| Waist circumference, cm | 76.8 (0.35) | 96.83 (0.30) |
| Centrally obese subjects§ | 861 (8.94) | 6,380 (50.75) |
| Waist-to-height ratio|| | −0.09 (0.02) | −0.09 (0.02) |
| Percent body fat¶ | 23.91 (0.20) | 31.28 (0.20) |
| Dual-energy X-ray absorptiometry | ||
| Total percent fat | 29.17 (0.17) | 33.94 (0.13) |
| Trunk percent fat | 25.57 (0.19) | 33.42 (0.15) |
| Leg percent fat | 34.07 (0.18) | 35.79 (0.13) |
| Fat mass index, kg/m2 | 6.78 (0.08) | 10.01 (0.08) |
| Trunk-to-total fat mass,|| g | −0.05 (0.02) | −0.06 (0.02) |
| Legs-to-total fat mass,|| g | 0.03 (0.02) | 0.08 (0.01) |
| Trunk-to-legs fat mass,|| g | −0.05 (0.02) | −0.07 (0.01) |
Definition of abbreviations: BMI = body mass index; HOMA-IR = homeostasis model assessment-estimated insulin resistance; TC/HDL-C = ratio of total cholesterol to high-density lipoprotein cholesterol.
Data are presented as mean (standard error) for continuous variables, and n (%) for binary variables. Numbers may vary because of missing data.
Available for NHANES (National Health and Nutrition Examination Survey) 2005 to 2006 only.
HOMA-IR and TC/HDL-C both measured at a morning examination session in all National Health and Nutrition Examination Survey participants aged 12 years and older after fasting at least 9 hours.
BMI z score for children, kg/m2 for adults. Overweight: BMI z score ≥ 1.036 for children, BMI ≥ 25 kg/m2 for adults. Obese: BMI z score ≥ 1.0645 for children, BMI ≥ 30 kg/m2 for adults.
Central obesity: waist circumference z score ≥ 1.0645 for children, waist circumference ≥ 88 cm for women or ≥102 cm for men.
z score calculated (separately) for both children and adults.
Percent body fat was calculated based on skin folds measured in National Health and Nutrition Examination Survey.
Figure 1.
Correlation between anthropometric measures and dual-energy X-ray absorptiometry measures in children and adults by sex. (A) Children (n = 8,886). (B) Adults (n = 12,795). BMI = body mass index; WC = waist circumference.
The multivariable analysis of adiposity measures and asthma is shown in Table 2. After adjusting for age, sex, race/ethnicity, annual household income, serum cotinine level, and C-reactive protein, each 1.0 z-score increase in anthropometric measures (BMI, waist circumference, waist-to-height ratio, percent body fat) was positively associated with ∼20% higher odds of asthma among children, whereas 1.0% increases in total percent fat, trunk percent fat, and leg percent fat were associated with a 2 to 5% higher odds of asthma. The associations for the anthropometric indices were stronger and more significant among girls than boys, except for central obesity, which was associated with ∼59% higher odds of asthma among boys and was nonsignificant among girls. Among adults, each 1.0-unit increase in general adiposity (BMI, fat mass index, total percent fat, trunk percent fat, and leg percent fat) was associated with 3 to 7% higher odds of asthma, whereas DXA-based trunk-predominant adiposity (trunk-to-total and trunk-to-legs fat mass ratios) was associated with 11 to 14% higher odds of asthma; conversely, lower extremity–predominant adiposity (legs-to-total fat mass ratio) was associated with lower odds of asthma (adjusted odds ratio [OR], 0.90; 95% confidence interval [CI], 0.81–0.99). When stratifying by sex, the association between trunk-predominant adiposity (higher trunk/total fat and trunk/legs fat, or lower legs/total fat) and asthma was found in adult women only. Overall, the general adiposity indices (both anthropometry and DXA based) were associated with higher odds of asthma, with a more consistent association among female participants. The regional adiposity indices were associated with asthma risk among adult women only.
Table 2.
Adiposity measures and current asthma in children and adults, by sex
| Children, 8–19 yr (n = 8,886) |
Adults, 20–69 yr, (n = 12,795) |
|||||
|---|---|---|---|---|---|---|
| All Asthma (n = 897) | Boys with Asthma (n = 468) | Girls with Asthma (n = 429) | All Asthma (n = 896) | Men with Asthma (n = 357) | Women with Asthma (n = 539) | |
| Anthropometry | ||||||
| Body mass index* | 1.21 (1.09–1.35)† | 1.13 (1.00–1.27)‡ | 1.34 (1.12–1.60)† | 1.04 (1.03–1.06)† | 1.04 (1.02–1.07)† | 1.04 (1.03–1.06)† |
| Obese§ | 1.50 (1.14–1.98)† | 1.36 (0.98–1.88) | 1.72 (1.13–2.63)‡ | 1.73 (1.45–2.05)† | 1.50 (1.10–2.05)‡ | 1.92 (1.51–2.42)† |
| Waist circumference* | 1.17 (1.05–1.30)† | 1.13 (0.99–1.29) | 1.25 (1.09–1.43)† | 1.02 (1.01–1.02)† | 1.02 (1.01–1.03)† | 1.02 (1.01–1.03)† |
| Central obese|| | 1.44 (1.04–2.01)‡ | 1.59 (1.02–2.47)‡ | 1.29 (0.74–2.27) | 1.33 (1.12–1.58)† | 1.21 (0.89–1.64) | 1.46 (1.18–1.81)† |
| Waist-to-height ratio¶ | 1.20 (1.07–1.34)† | 1.12 (0.98–1.28) | 1.24 (1.08–1.42)† | 1.33 (1.23–1.44)† | 1.33 (1.15–1.54)† | 1.35 (1.23–1.49)† |
| Percent body fat* | 1.20 (1.04–1.37)‡ | 1.21 (1.01–1.46)‡ | 1.20 (1.01–1.43)‡ | 1.01 (0.99–1.02) | 0.99 (0.97–1.02) | 1.01 (0.99–1.02) |
| Dural-energy X-ray absorptiometry | ||||||
| Total percent fat | 1.02 (1.01–1.04)† | 1.02 (1.00–1.04)‡ | 1.03 (1.00–1.05)‡ | 1.04 (1.03–1.06)† | 1.03 (1.01–1.06)† | 1.05 (1.03–1.07)† |
| Trunk percent fat | 1.02 (1.01–1.03)† | 1.02 (1.00–1.03)‡ | 1.02 (1.00–1.04)‡ | 1.03 (1.02–1.04)† | 1.02 (1.00–1.04)‡ | 1.04 (1.03–1.05)† |
| Leg percent fat | 1.02 (1.01–1.04)† | 1.02 (1.00–1.03)‡ | 1.02 (0.99–1.05) | 1.03 (1.02–1.05)† | 1.03 (1.01–1.06)† | 1.03 (1.01–1.06)† |
| Fat mass index | 1.05 (1.02–1.09)† | 1.05 (1.01–1.09)‡ | 1.06 (1.02–1.09)† | 1.07 (1.05–1.09)† | 1.08 (1.04–1.12)† | 1.07 (1.05–1.09)† |
| Trunk-to-total fat¶ | 1.10 (0.96–1.26) | 1.06 (0.88–1.28) | 1.15 (0.95–1.39) | 1.14 (1.05–1.25)† | 1.05 (0.90–1.22) | 1.21 (1.08–1.36)† |
| Legs-to-total fat¶ | 1.00 (0.88–1.14) | 1.07 (0.92–1.25) | 0.92 (0.74–1.13) | 0.90 (0.81–0.99)‡ | 1.01 (0.85–1.21) | 0.85 (0.75–0.97)† |
| Trunk-to-legs fat¶ | 1.05 (0.92–1.20) | 1.01 (0.83–1.23) | 1.12 (0.93–1.34) | 1.11 (1.01–1.22)‡ | 1.02 (0.87–1.19) | 1.19 (1.05–1.34)† |
Data presented as odds ratio (95% confidence interval) of asthma. All models adjusted for age, race, sex (for all), annual household income, serum cotinine, and serum C-reactive protein.
z score for children only (body mass index, waist circumference, percent body fat).
P < 0.01.
P < 0.05. Bold typeface used to highlight P < 0.05.
Compared with normal weight.
Compared with noncentral obese.
z score for children and adults, calculated separately for each age group (waist-to-height ratio, trunk-to-total fat mass ratio, legs-to-total fat mass ratio, trunk-to-legs fat mass ratio).
To evaluate whether these associations are partly driven by insulin resistance or dyslipidemia, we also included homeostasis model assessment-estimated insulin resistance or total cholesterol/high-density lipoprotein cholesterol in the models (NHANES 2005–2006 only). After additional adjustment for either measure, the association between adiposity and asthma remained significant in boys, but all associations became nonsignificant among girls (Table E1). Among adults, general adiposity associations with asthma remained significant both in men and women, but all regional adiposity indices (trunk-to-total fat mass ratio, trunk-to-legs fat mass ratio, and legs-to-total fat mass ratio) became nonsignificant among women.
We repeated the multivariable analysis after stratification by atopy, using data from NHANES 2005 to 2006 (Table 3). General adiposity (BMI, percent body fat) was associated with 79 to 84% increased odds of nonatopic asthma in children. Similarly, all the DXA indices (except for leg percent fat) were associated with nonatopic asthma in children. Contrary to the findings in children, general adiposity (BMI, fat mass index, total percent fat, trunk percent fat, and leg percent fat) and central or trunk-predominant adiposity (waist circumference, waist-to-height ratio, trunk-to-total fat mass ratio and trunk-to-legs fat mass ratio) were significantly associated with atopic asthma in adults. All significant results in adults were driven by atopic women (Table E2). There were no significant associations between general or localized adiposity and current asthma in children with atopic asthma or in adults with nonatopic asthma.
Table 3.
Adiposity measures and current asthma in children and adults, by atopy (National Health and Nutrition Examination Survey 2005–2006)
| Children, 8–19 yr |
Adults, 20–69 yr |
|||
|---|---|---|---|---|
| Nonatopic Asthma (n = 80) | Atopic Asthma (n = 200) | Nonatopic Asthma (n = 97) | Atopic Asthma (n = 170) | |
| Anthropometry | ||||
| Body mass index* | 1.79 (1.27–2.53)† | 0.99 (0.80–1.23) | 1.03 (0.99–1.07) | 1.06 (1.03–1.09)† |
| Obese‡ | 2.88 (1.38–5.99)† | 0.71 (0.38–1.32) | 1.63 (0.70–3.75) | 2.29 (1.54–3.40)† |
| Waist circumference* | 1.58 (1.18–2.12)† | 0.96 (0.77–1.20) | 1.01 (0.99–1.03) | 1.02 (1.01–1.03)† |
| Central obese§ | 2.21 (0.87–5.64) | 0.68 (0.31–1.50) | 1.32 (0.85–2.04) | 1.49 (0.96–2.33) |
| Waist-to-height ratio|| | 1.68 (1.23–2.30)† | 0.92 (0.72–1.19) | 1.12 (0.87–1.43) | 1.53 (1.28–1.82)† |
| Percent body fat* | 1.84 (1.30–2.62)† | 0.97 (0.74–1.27) | 1.03 (0.99–1.08) | 0.99 (0.98–1.02) |
| Dual-energy X-ray absorptiometry | ||||
| Total percent fat | 1.06 (1.01–1.11)¶ | 1.01 (0.98–1.03) | 1.04 (0.99–1.08) | 1.04 (1.02–1.07)† |
| Trunk percent fat | 1.05 (1.01–1.09)† | 1.00 (0.98–1.02) | 1.02 (0.99–1.06) | 1.04 (1.01–1.06)† |
| Leg percent fat | 1.05 (0.99–1.10) | 1.01 (0.99–1.03) | 1.04 (0.99–1.09) | 1.03 (1.00–1.06)¶ |
| Fat mass index | 1.13 (1.02–1.25)¶ | 1.00 (0.94–1.06) | 1.04 (0.98–1.11) | 1.08 (1.04–1.13)† |
| Trunk-to-total fat|| | 1.53 (1.03–2.26)¶ | 0.88 (0.64–1.20) | 1.00 (0.76–1.32) | 1.21 (1.03–1.41)¶ |
| Legs-to-total fat|| | 0.71 (0.51–0.99)¶ | 1.23 (0.90–1.66) | 1.10 (0.82–1.49) | 0.84 (0.71–0.98)¶ |
| Trunk-to-legs fat|| | 1.47 (1.08–1.99)¶ | 0.83 (0.59–1.17) | 0.92 (0.68–1.25) | 1.19 (1.01–1.42)¶ |
Data presented as odds ratio (95% confidence interval) of asthma. All models adjusted for age, sex, race, annual household income, serum cotinine, and serum C-reactive protein.
z score for children only (body mass index, waist circumference, percent body fat).
P < 0.01.
Compared with normal weight.
Compared with noncentral obese.
z score for children and adults, calculated separately for each age group (waist-to-height ratio, trunk-to-total fat mass ratio, legs-to-total fat mass ratio, trunk-to-legs fat mass ratio).
P < 0.05. Bold typeface used to highlight P < 0.05.
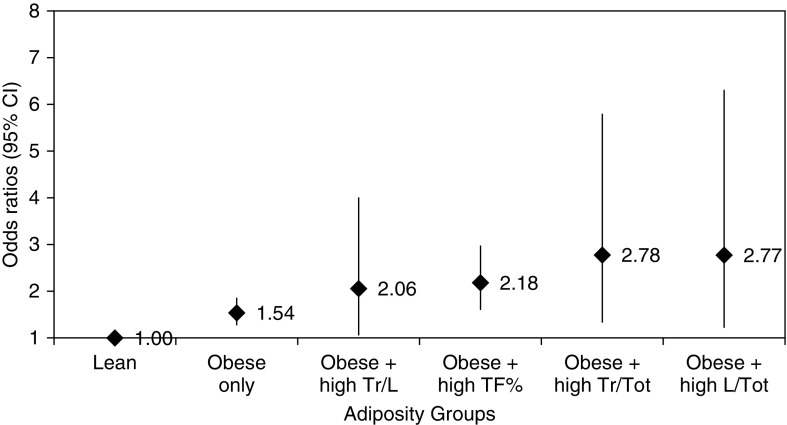
To assess whether the DXA adiposity indices contribute additional information to that obtained from BMI, we assessed “combined” models that included both obesity (defined by BMI) plus the DXA indices. Compared with women who were lean (e.g., normal BMI and normal DXA indices), those who were obese alone (BMI ≥ 30 kg/m2) had 1.54 times higher odds of asthma (95% CI, 1.27–1.86; Figure 2). The odds of current asthma further increased among those who were: obese with high trunk-to-legs fat mass ratio (OR, 2.06; 95% CI, 1.05–4.01), obese with high total percent fat (OR, 2.18; 95% CI, 1.60–2.98), obese with high trunk-to-total fat mass ratio (OR, 2.78; 95% CI, 1.33–5.80), or obese with high legs-to-total fat mass ratio (OR, 2.77; 95% CI, 1.22–6.31). After adding homeostasis model assessment-estimated insulin resistance to these “combined” models, the odds ratios for obesity plus the localized indices (trunk-to-legs fat mass ratio, trunk-to-total fat mass ratio, and legs-to-total fat mass ratio) became nonsignificant, whereas the combined model for obesity plus total percent fat remained unchanged (data not shown).
Figure 2.
Current asthma in adult women by adiposity groups (National Health and Nutrition Examination Survey 2001–2006). Adjusted odds ratios (95% confidence intervals [CIs]) of asthma for each group, using lean (normal body mass index and normal adiposity indices) as the reference. All models adjusted for age, sex, race, annual household income, serum cotinine, and serum C-reactive protein. L/Tot = legs-total fat mass ratio; TF% = total percent fat; Tr/L = trunk-to-legs fat mass ratio; Tr/Tot = trunk-to-total fat mass ratio.
Discussion
In this study of a large sample representative of the U.S. population, we found that adiposity, assessed either by anthropometry or by dual-energy X-ray absorptiometry, was associated with asthma in children—with the association driven by nonatopic children. Among adults, adiposity indices were also consistently associated with asthma but, contrary to the findings in children, all results were driven by atopic subjects—particularly by atopic women. To our knowledge, this is the first large-scale analysis of general and local adiposity assessed by dual-energy X-ray absorptiometry and asthma risk in U.S. children and adults.
Our results suggest that adiposity measured by dual-energy X-ray absorptiometry provides similar information to that obtained by using anthropometric indices among children of both sexes and among adult men. However, dual-energy X-ray absorptiometry measures on trunk-predominant adiposity provided additional value in relating adiposity to asthma in adult women: dual-energy X-ray absorptiometry central adiposity indices (trunk-to-total fat mass ratio, trunk-to-legs fat mass ratio, and legs-to-total fat mass ratio) were significantly associated with asthma only in this group. Furthermore, women with body mass index–defined obesity who also have high dual-energy X-ray absorptiometry indices of central obesity have higher odds of asthma than those with body mass index–defined obesity but without excess central adiposity, suggesting the assessment of both general and localized body fat compositions may provide a better estimate of asthma risk in women.
There have been several longitudinal epidemiological studies that have shown an association between obesity and incident asthma (7). As our understanding of what constitutes “obesity” has grown, so has the realization that body mass index may not suffice to characterize the disease or its complications (3). Rather, body fat composition and distribution may be a determining factor, and the type and characteristics of adipose tissue may play an important role in the effect of obesity on asthma. A large epidemiologic study in Norway recently reported that abdominal obesity (measured by waist circumference) is associated with asthma in women (5), but that study included only adults, did not assess dual-energy X-ray absorptiometry, and did not explore the role of atopy. Studies of obesity and asthma using dual-energy X-ray absorptiometry are scarce; they have reported an association in adult women and in children but have involved smaller sample sizes (37–39).
Similar to our findings, a number of studies have reported that the association between obesity and asthma varies with age and sex, although results are not consistent. In adults, higher body fat has been associated with allergic sensitization (40) and worse asthma control (41) in men but not in women. Conversely, obesity (42) and low serum adiponectin (43) have been associated with asthma among women but not men, whereas body mass index and body fat have been associated with worse asthma control, more asthma exacerbations, and lower forced expiratory volume in 1 second/forced vital capacity in adolescent girls but not in adolescent boys (44). A recent meta-analysis reported a dose–response relationship between overweight or obesity (defined by body mass index) and risk of asthma in children, with stronger effect seen in boys than in girls (12). These sex-specific associations are likely explained at least in part by sex differences in fat distribution, adipokine concentrations, and hormonal levels (45). Sex- and age-specific findings should be taken into account in future management approaches for obese asthma.
Whether atopy mediates the association between obesity and asthma is also unclear. Epidemiologic studies have reported conflicting findings on the association between different measures of adiposity and atopy, with some studies showing a positive association with allergic sensitization in all children (3), in girls (46, 47), in women (48), or in men only (40) and others reporting no significant association regardless of age or sex (49, 50). Our findings in children are consistent with prior reports that adiposity is associated with asthma risk among nonatopic children but not in children with atopy (51). However, our finding that the adiposity–asthma associations were driven exclusively by atopic adults, particularly in women, differs from those of previous studies that reported significant associations only in nonatopic adults (52). Estrogen and its receptors have been associated with allergic diseases through polarizing T-helper cell type 2 response, upregulating immunoglobulin E synthesis, and promoting the degranulation of mast cell/basophils (53). Although the role of estrogens in the etiology of obese asthma is unclear, our results suggest there may be a significant contribution of atopy to the sex-specific obesity–asthma relationship in adults. However, data on atopy were available only for National Health and Nutrition Examination Survey 2005 to 2006 participants, which included a smaller number of nonatopic adults with asthma, and thus our results should be interpreted cautiously.
Adipose tissue plays a role in innate immune responses, inflammatory processes, and production of cytokines and adipokines (54). Excess visceral/ectopic fat has been linked to insulin resistance, dyslipidemia, upregulated proinflammatory cytokines including tumor necrosis factor-α and interleukin-6, and decreased adiponectin (55). Insulin resistance has been reported as an effect modifier of the obesity–asthma association among U.S. children (56) and adults (57), and dyslipidemia has been associated with asthma symptoms and lower lung function among overweight and obese adults with asthma, independent from general or truncal adiposity (58). In our analysis, including homeostasis model assessment-estimated insulin resistance and/or total cholesterol/high-density lipoprotein cholesterol in the regression models rendered the association between dual-energy X-ray absorptiometry trunk-predominant adiposity and asthma among women nonsignificant. Similarly, the association between general obesity and asthma was not seen in girls after adjusting for homeostasis model assessment-estimated insulin resistance and total cholesterol/high-density lipoprotein cholesterol. Although the changes among adult women should be interpreted with caution, as the estimates changed only 3 to 5% and could be due to loss of statistical power from incorporating additional variables into the models, the results for girls were consistent, and most estimates changed by more than 10%. This suggests that in these groups, insulin resistance or dyslipidemia may explain at least part of the obesity–asthma association. Of particular interest, dual-energy X-ray absorptiometry local adiposity indices conferred extra asthma risk among women (Figure 2), but again that extra risk disappeared when homeostasis model assessment-estimated insulin resistance and total cholesterol/high-density lipoprotein cholesterol were included in the models; we hypothesize that, in women, atopy and metabolic dysregulation constitute independent pathways for asthma risk. However, future studies will be needed to confirm these findings.
The distinct strengths of this analysis include having a large representative sample of the U.S. population, as well as objective measurements of adiposity indices by trained personnel. Furthermore, we accounted for several potential confounders, including cigarette smoking, low-grade systematic inflammation (measured by C-reactive protein), insulin resistance (measured by homeostasis model assessment-estimated insulin resistance), and dyslipidemia (measured by total cholesterol/high-density lipoprotein cholesterol). We also acknowledge several limitations. The temporal relationship between obesity and asthma cannot be determined in this cross-sectional study. Although we report dual-energy X-ray absorptiometry–based truncal adiposity indices, direct measures of visceral abdominal fat were not available in the National Health and Nutrition Examination Survey. Visceral abdominal fat has been positively associated with fractional exhaled nitric oxide in children (23) and lung function in adults (59, 60). We lack data on factors that might influence adiposity or allergic sensitization, such as dietary pattern. Likewise, we did not have data on either pubertal stage or menopause and thus could not evaluate the potential influence of hormonal factors. We did not have data on asthma severity or control and thus could not evaluate the association between dual-energy X-ray absorptiometry–defined adiposity and these measures. Finally, we had limited statistical power to analyze whether the association between adiposity and nonatopic asthma differs by sex in children, because of the small number of children with asthma in that stratum.
In summary, our study supports the obesity–asthma link not only by anthropometric indices but also by dual-energy X-ray absorptiometry measurements. Whole-body adiposity indices or local adiposity ratios calculated by dual-energy X-ray absorptiometry were associated with asthma, but such association varied by age group, sex, and atopy. Future studies on obesity–asthma association will be enhanced by greater consideration of alternative measures of fat mass and body composition.
Supplementary Material
Footnotes
Supported by grants HL125666 from the U.S. National Institutes of Health (NIH) (E.F.), Children’s Hospital of Pittsburgh (E.F.), and the Klosterfrau Foundation (E.F.); grants HL079966, HL117191, and HL119952 from the U.S. NIH (J.C.C.), and The Heinz Endowments (J.C.C.). E.F. and Y.-Y.H. had full access to all of the data, and take responsibility for the integrity and accuracy of the analysis. None of the funding sponsors had any role in study design, data analysis, or manuscript preparation or approval.
Author Contributions: Study conception and design: E.F., Y.-Y.H., and J.C.C. Data interpretation: all authors. Data analysis and first draft of manuscript: E.F., Y.-Y.H., and J.C.C. Manuscript revision for important intellectual content: all authors. Approved the final version for submission: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forno E, Acosta-Pérez E, Brehm JM, Han YY, Alvarez M, Colon-Semidey A, et al. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol. 2014;133:1308–1314. doi: 10.1016/j.jaci.2013.09.041. 1314.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egan KB, Ettinger AS, Bracken MB. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. BMC Pediatr. 2013;13:121. doi: 10.1186/1471-2431-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brumpton B, Langhammer A, Romundstad P, Chen Y, Mai XM. General and abdominal obesity and incident asthma in adults: the HUNT study. Eur Respir J. 2013;41:323–329. doi: 10.1183/09031936.00012112. [DOI] [PubMed] [Google Scholar]
- 6.Ali Z, Ulrik CS. Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med. 2013;107:1287–1300. doi: 10.1016/j.rmed.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 8.Lang JE, Hossain J, Dixon AE, Shade D, Wise RA, Peters SP, et al. American Lung Association-Asthma Clinical Research Centers. Does age impact the obese asthma phenotype? Longitudinal asthma control, airway function, and airflow perception among mild persistent asthmatics. Chest. 2011;140:1524–1533. doi: 10.1378/chest.11-0675. [DOI] [PubMed] [Google Scholar]
- 9.Han YY, Forno E, Celedón JC. Adiposity, fractional exhaled nitric oxide, and asthma in U.S. children. Am J Respir Crit Care Med. 2014;190:32–39. doi: 10.1164/rccm.201403-0565OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE North West Adelaide Health Study Team. Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol. 2006;118:1284–1291. doi: 10.1016/j.jaci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–1493. doi: 10.1016/j.jaci.2011.03.036. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YC, Dong GH, Lin KC, Lee YL. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013;14:222–231. doi: 10.1111/j.1467-789X.2012.01055.x. [DOI] [PubMed] [Google Scholar]
- 13.Hancox RJ, Milne BJ, Poulton R, Taylor DR, Greene JM, McLachlan CR, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med. 2005;171:440–445. doi: 10.1164/rccm.200405-623OC. [DOI] [PubMed] [Google Scholar]
- 14.Brüske I, Flexeder C, Heinrich J. Body mass index and the incidence of asthma in children. Curr Opin Allergy Clin Immunol. 2014;14:155–160. doi: 10.1097/ACI.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 15.Borrell LN, Nguyen EA, Roth LA, Oh SS, Tcheurekdjian H, Sen S, et al. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;187:697–702. doi: 10.1164/rccm.201211-2116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatz M, Zeiger RS, Yang SJ, Chen W, Sajjan S, Allen-Ramey F, et al. Prospective Study on the Relationship of obesity to asthma impairment and risk. J Allergy Clin Immunol Pract. 2015;3:560–565. doi: 10.1016/j.jaip.2015.03.017. e1. [DOI] [PubMed] [Google Scholar]
- 17.Wehrmeister FC, Menezes AM, Muniz LC, Martínez-Mesa J, Domingues MR, Horta BL. Waist circumference and pulmonary function: a systematic review and meta-analysis. Syst Rev. 2012;1:55. doi: 10.1186/2046-4053-1-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capelo AV, da Fonseca VM, Peixoto MV, de Carvalho SR, Azevedo CM, Elsas MI, et al. Visceral adiposity is associated with cytokines and decrease in lung function in women with persistent asthma. Rev Port Pneumol. 2006;2016:255–261. doi: 10.1016/j.rppnen.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Ahangari F, Sood A, Ma B, Takyar S, Schuyler M, Qualls C, et al. Chitinase 3-like-1 regulates both visceral fat accumulation and asthma-like Th2 inflammation. Am J Respir Crit Care Med. 2015;191:746–757. doi: 10.1164/rccm.201405-0796OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 21.Yiallouros PK, Lamnisos D, Kolokotroni O, Moustaki M, Middleton N. Associations of body fat percent and body mass index with childhood asthma by age and gender. Obesity (Silver Spring) 2013;21:E474–E482. doi: 10.1002/oby.20284. [DOI] [PubMed] [Google Scholar]
- 22.Jeong A, Imboden M, Hansen S, Zemp E, Bridevaux PO, Lovison G, et al. Heterogeneity of obesity-asthma association disentangled by latent class analysis, the SAPALDIA cohort. Respir Med. 2017;125:25–32. doi: 10.1016/j.rmed.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 23.den Dekker HT, Ros KP, de Jongste JC, Reiss IK, Jaddoe VW, Duijts L. Body fat mass distribution and interrupter resistance, fractional exhaled nitric oxide, and asthma at school-age. J Allergy Clin Immunol. 2017;139:810–818. doi: 10.1016/j.jaci.2016.06.022. e6. [DOI] [PubMed] [Google Scholar]
- 24.Guibas GV, Manios Y, Xepapadaki P, Moschonis G, Douladiris N, Mavrogianni C, et al. The obesity-asthma link in different ages and the role of body mass index in its investigation: findings from the Genesis and Healthy Growth Studies. Allergy. 2013;68:1298–1305. doi: 10.1111/all.12245. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey [Updated 2017 Dec 28; accessed 2018 Jan 11] Available from: http://www.cdc.gov/nchs/nhanes.htm.
- 26.Lohman TG, Roche AF, Martorell R. Champaign, IL: Human Kinetics Books; 1988. Anthropometric standardization reference manual. [Google Scholar]
- 27.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults--the evidence report. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 28.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. National Center for Health Statistics. Vital Health Stat. 2002;11(246) [PubMed] [Google Scholar]
- 29.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 30.Faria FR, Faria ER, Cecon RS, Barbosa DA, Jr, Franceschini Sdo C, Peluzio Mdo C, et al. Body fat equations and electrical bioimpedance values in prediction of cardiovascular risk factors in eutrophic and overweight adolescents. Int J Endocrinol. 2013;2013:501638. doi: 10.1155/2013/501638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurson KR, Eisenmann JC, Welk GJ. Development of youth percent body fat standards using receiver operating characteristic curves. Am J Prev Med. 2011;41:S93–S99. doi: 10.1016/j.amepre.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: imputation and variance estimation software. Survey Research Center, Institute for Social Research, University of Michigan. 2002.
- 33.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger PW. A multivariate technique for multiply imputing missing values using a dequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 34.Centers for Disease Control and Prevention. 1999–2006 DXA multiple imputation data files. Atlanta, GA; [accessed 2018 Jan 11]. Available from: https://wwwn.cdc.gov/Nchs/Nhanes/Dxa/Dxa.aspx.
- 35.Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81:1018–1025. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- 36.Rubin DB. New York: John Wiley & Sons; 1987. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 37.Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, et al. Relationship between body composition, inflammation and lung function in overweight and obese asthma. Respir Res. 2012;13:10. doi: 10.1186/1465-9921-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sood A, Qualls C, Li R, Schuyler M, Beckett WS, Smith LJ, et al. CARDIA Investigators. Lean mass predicts asthma better than fat mass among females. Eur Respir J. 2011;37:65–71. doi: 10.1183/09031936.00193709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen ME, Gibson PG, Collins CE, Wood LG. Lean mass, not fat mass, is associated with lung function in male and female children with asthma. Pediatr Res. 2014;75:93–98. doi: 10.1038/pr.2013.181. [DOI] [PubMed] [Google Scholar]
- 40.Ouyang F, Kumar R, Pongracic J, Story RE, Liu X, Wang B, et al. Adiposity, serum lipid levels, and allergic sensitization in Chinese men and women. J Allergy Clin Immunol. 2009;123:940–948. doi: 10.1016/j.jaci.2008.11.032. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bafadhel M, Singapuri A, Terry S, Hargadon B, Monteiro W, Green RH, et al. Body mass and fat mass in refractory asthma: an observational 1 year follow-up study. J Allergy (Cairo) 2010;2010:251758. doi: 10.1155/2010/251758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sood A, Cui X, Qualls C, Beckett WS, Gross MD, Steffes MW, et al. Association between asthma and serum adiponectin concentration in women. Thorax. 2008;63:877–882. doi: 10.1136/thx.2007.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sood A, Qualls C, Schuyler M, Thyagarajan B, Steffes MW, Smith LJ, et al. Low serum adiponectin predicts future risk for asthma in women. Am J Respir Crit Care Med. 2012;186:41–47. doi: 10.1164/rccm.201110-1767OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sood A. Sex differences: implications for the obesity-asthma association. Exerc Sport Sci Rev. 2011;39:48–56. doi: 10.1097/JES.0b013e318201f0c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schachter LM, Peat JK, Salome CM. Asthma and atopy in overweight children. Thorax. 2003;58:1031–1035. doi: 10.1136/thorax.58.12.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alvarez Zallo N, Aguinaga-Ontoso I, Alvarez-Alvarez I, Guillén-Grima F, Azcona San Julian C. The influence of gender and atopy in the relationship between obesity and asthma in childhood. Allergol Immunopathol (Madr) 2017;45:227–233. doi: 10.1016/j.aller.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Vieira VJ, Ronan AM, Windt MR, Tagliaferro AR. Elevated atopy in healthy obese women. Am J Clin Nutr. 2005;82:504–509. doi: 10.1093/ajcn.82.3.504. [DOI] [PubMed] [Google Scholar]
- 49.Wang R, Custovic A, Simpson A, Belgrave DC, Lowe LA, Murray CS. Differing associations of BMI and body fat with asthma and lung function in children. Pediatr Pulmonol. 2014;49:1049–1057. doi: 10.1002/ppul.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Umławska W. Adipose tissue content and distribution in children and adolescents with bronchial asthma. Respir Med. 2015;109:200–207. doi: 10.1016/j.rmed.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Granell R, Henderson AJ, Evans DM, Smith GD, Ness AR, Lewis S, et al. Effects of BMI, fat mass, and lean mass on asthma in childhood: a Mendelian randomization study. PLoS Med. 2014;11:e1001669. doi: 10.1371/journal.pmed.1001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fenger RV, Gonzalez-Quintela A, Vidal C, Gude F, Husemoen LL, Aadahl M, et al. Exploring the obesity-asthma link: do all types of adiposity increase the risk of asthma? Clin Exp Allergy. 2012;42:1237–1245. doi: 10.1111/j.1365-2222.2012.03972.x. [DOI] [PubMed] [Google Scholar]
- 53.Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol. 2013;13:92–99. doi: 10.1097/ACI.0b013e32835a6dd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam YY, Mitchell AJ, Holmes AJ, Denyer GS, Gummesson A, Caterson ID, et al. Role of the gut in visceral fat inflammation and metabolic disorders. Obesity (Silver Spring) 2011;19:2113–2120. doi: 10.1038/oby.2011.68. [DOI] [PubMed] [Google Scholar]
- 55.Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 56.Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136:304–311. doi: 10.1016/j.jaci.2015.01.010. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardet JC, Ash S, Kusa T, Camargo CA, Jr, Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J. 2016;48:403–410. doi: 10.1183/13993003.00246-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rastogi D, Jung M, Strizich G, Shaw PA, Davis SM, Klein OL, et al. Association of systemic inflammation, adiposity, and metabolic dysregulation with asthma burden among Hispanic adults. Respir Med. 2017;125:72–81. doi: 10.1016/j.rmed.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thijs W, Alizadeh Dehnavi R, Hiemstra PS, de Roos A, Melissant CF, Janssen K, et al. Association of lung function measurements and visceral fat in men with metabolic syndrome. Respir Med. 2014;108:351–357. doi: 10.1016/j.rmed.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Lessard A, Alméras N, Turcotte H, Tremblay A, Després JP, Boulet LP. Adiposity and pulmonary function: relationship with body fat distribution and systemic inflammation. Clin Invest Med. 2011;34:E64–E70. doi: 10.25011/cim.v34i1.15102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.