Abstract
Nephrolithiasis, or stones, is one of the oldest urological diseases, with descriptions and treatment strategies dating back to ancient times. Despite the enormous number of patients affected by stones, a surprising lack of conceptual understanding of many aspects of this disease still exists. This lack of understanding includes mechanisms of stone formation and retention, the clinical relevance of different stone compositions and that of formation patterns and associated pathological features to the overall course of the condition. Fortunately, a number of new tools are available to assist in answering such questions. New renal endoscopes enable kidney visualization in much higher definition than was previously possible, while micro-CT imaging is the optimal technique for assessment of stone microstructure and mineral composition in a nondestructive fashion. Together, these tools have the potential to provide novel insights into the aetiology of stone formation that might unlock new prevention and treatment strategies, and enable more effective management of patients with nephrolithiasis.
Nephrolithiasis is the most costly urological disease1 and warrants intensive research efforts directed towards both prevention and treatment. Greater insight into the mechanisms of stone formation is critical, and an improved understanding of the aetiology of stone development and growth would enable researchers and clinicians to develop novel ways of slowing, and possibly even preventing this process. A number of novel tools have been introduced over the past several years with the potential to improve our understanding of the aetiology of nephrolithiasis, most notably, high-definition renal endoscopy and micro-CT. In this Perspectives article, we describe the development and clinical use of these technologies, focusing in particular on how they might complement existing modalities used to study kidney stones and to classify stone formers.
Current limitations in classification
Analysis of stone mineral composition and assessments of the presence of these same minerals and metabolites, such as calcium, oxalate and uric acid in 24-h urine samples are currently the two most commonly used tools for the characterization of stone formers. Both of these approaches are recommended in the evaluation of stone formers with a high risk of recurrence2,3 and both have considerable limitations and potential for inaccuracies. The main limitation of stone analysis is that current methods, such as Fourier transform infrared spectroscopy (FT–IR), require destruction of the stone. Thus, this type of analysis denies investigators the opportunity to investigate the morphology of the stone, or the distribution of mineral subtypes once it has been analysed. Other analysis methods also exist, and these vary in their utility, costs and reliability. Such methods include radiographic powder diffraction, Raman spectroscopy, scanning electron microscopy and thermal analysis4–8. Given the large number of techniques available, each with their own limitations, a surprising lack of consensus statements or guidelines advocating the optimal approach towards stone analysis currently exists.
FT–IR is currently the favoured stone analysis modality at most large medical centres owing to the generally high reliability of this technique; however, investigators often fail to appreciate that this technique is only performed on a representative stone fragment as opposed to the entire specimen supplied to the laboratory. This approach would not be of any concern if most stones were of a homogenous composition; however, this is rarely the case. In fact, among >10,000 stones analysed in France by Daudon et al.9 only 7% were found to be composed of a single mineral9. As a result, analysis of a stone fragment and not the entire stone creates a considerable risk of sampling bias, depending upon the part of the stone that is analysed. Krambeck et al.10 tested this hypothesis by fragmenting 25 different stones and sending representative pieces to five different commercial stone analysis laboratories in the USA. These investigators found that results of the analyses of the composition of pure stones, consisting of a single substance, were highly reproducible; however, considerable discrepancies in the reported composition of different fragments of the same stone were observed following the analyses of stones of a mixed composition. Furthermore, this study demonstrated particularly poor reproducibility in correctly identifying struvite (struvite was universally identified in only two of four known struvite-containing stones) and apatite (apatite was not detected in 20% of fragments from known apatite-containing stones)10.
Chemical analyses of 24-h urine samples are similarly problematic as a tool for appropriately classifying stone formers. Generating a 24-h urine sample requires a high degree of patient compliance. As a result of this need for patient compliance, estimates published in 2015 show that samples might be inappropriately collected as often as 50% of the time in non-research settings11. Even when performed correctly, results can be widely variable depending upon patients’ diet, hydration status, physical activity, medication use and other factors specific to that 24-h period12. For this reason, considerable debate exists within the urology and nephrology communities as to whether a single collection should be considered sufficient for the comprehensive clinical workup of a patient with nephrolithaisis12–16.
The inherent flaws of these two widely used analysis methodologies makes the subclassification of stone formers very challenging and also presents a limitation to future stone research. As our level of insight into the aetiology and pathogenesis of stones continues to expand, investigators are increasingly becoming aware that stones are a common end point of a number of unique pathophysiological processes and diseases that are likely to require unique treatment strategies17–19. The identification of these unique pathologies associated with stone formation, followed by attempts to individualize treatments to the specific disease subtypes, will be critical next steps in stone research. New tools such as high-definition renal endoscopy and micro-CT have the potential to help achieve this goal.
High-definition renal endoscopy
The introduction of smaller endoscopes with high-definition digital imaging capabilities has been one of the greatest advances in the endoscopic treatment of renal stones. Such endoscopes not only offer unparalleled access to the renal collecting system, thus enabling visualization of each individual papilla, but also provide superior optics, wider fields of view, higher resolution and, overall, an improved ability to examine the tissue compared with that of conventional endoscopes20–23.
Technological advances
The development of charge-coupled devices (CCD) and complementary metal oxide semiconductor (CMOS) sensors, which convert optical light to a digital signal, has been critical to the development of high-definition endoscopes23,24. Before the availability of this technology, older-generation fibre-optic ureteroscopes were used, which process optical images from a lens located at the distal tip of the instrument followed by relaying of the image through the instrument to a camera head, where it is processed and projected onto a video monitor. The numerous interfaces and cables required for this process introduce the potential for interference and distortion of the image25. Newer-generation digital ureteroscopes, however, feature CCD and/or CMOS chips on the tip of the endoscope, which enable immediate digitization of the image and direct transmission to a video display, therefore eliminating the interfaces at which a loss of image quality most commonly occurs26. (FIG. 1) Besides these improvements in image resolution, these ‘chip on a stick’ advances have enabled a greater extent of image magnification, use of greater light intensity, and improved image capture and video recording20,21. Furthermore, the benefits of improved optics have not come at the expense of increased scope size or a loss of manoeuverability, hence, these modern scopes retain the ability to access the entirety of the renal collecting system27.
Figure 1. Fibre-optic versus digital ureteroscopic images.
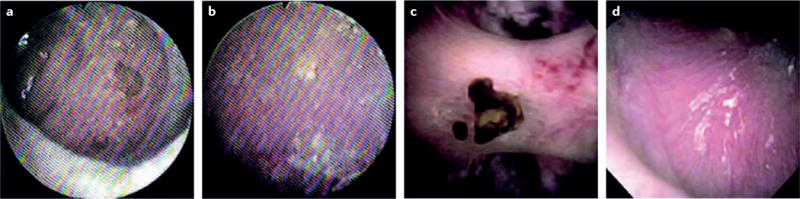
Images of the renal papilla acquired using a,b | a fibre-optic ureteroscope (KARL STORZ FLEX-X2, KARL STORZ, Tuttlingen, Germany) and c,d | a digital ureteroscope (ACMI/Olympus Invisio DUR-D, Gyrus ACMI, Massachusetts, USA) The enhanced view provided by the digital scope allows superior visualization of the papilla including both an attached stone as well as nascent mineral which might ultimately become a stone or be related to stone formation. The ability to identify such details opens new doors to study associations between papillary appearance and stone disease23. Reproduced with permission obtained from Elsevier Ltd © Humphreys, M. R. et al., A new world revealed: early experience with digital ureteroscopy. J. Urol. 179, 970–975 (2008).
Papillary inspection
Inspection of the renal papillae, performed at the time of stone removal either using a ureteroscope during flexible ureteroscopy or a nephroscope during percutaneous nephrolithotomy is an often underappreciated aspect of renal endoscopy that might provide clues as to why that patient is forming stones. Normal papillae have entirely smooth, rounded, surfaces and are conical in shape except when compound or fused with an adjacent papilla. Barely visible openings to the ducts of Bellini and minimal-to-no interstitial mineral deposition are common features of the renal papillae of individuals with no history of nephrolithiasis (FIG. 2). However, papillary appearance in stone formers often varies from this ‘classical’ description and includes unique findings, such as mineral deposition and changes in papillary architecture and shape18,28. These changes are generally easy to identify with modern digital scopes but difficult to describe18,29,30.
Figure 2. Digital ureteroscopic image of a classic renal papilla.
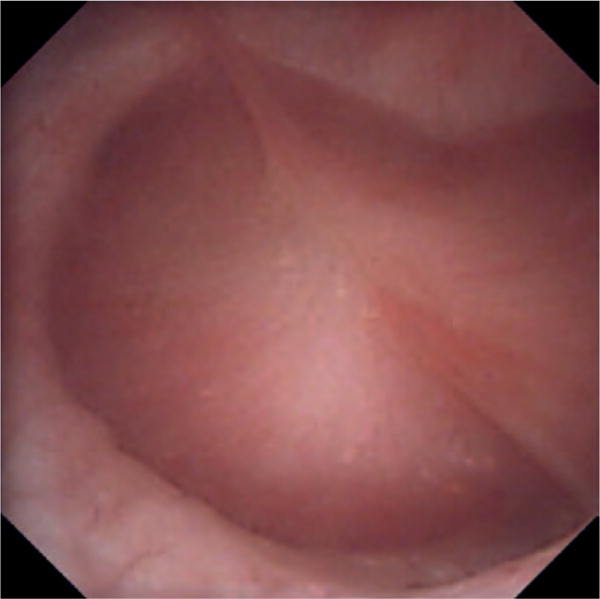
The surface of the papilla is entirely smooth, round and conical in shape and no mineral deposition can be observed.
Presumably, the ‘abnormal’ appearance of the papillae in stone formers is a direct consequence of stone formation, given the general consensus among experts that the renal papilla is the site of origin of many, if not most stones. This consensus is especially true of patients who form calcium stones18. Idiopathic calcium oxalate stones, defined as those predominantly composed of calcium oxalate in the absence of systemic disease, are not only the most abundant type of stone but also the type with the greatest degree of evidence supporting the papilla as a site of stone formation. In such instances, papillary mineralization (Randall plaque) has been demonstrated to act as a nidus for calcium oxalate stone overgrowth on both endoscopy and biopsy sample analysis, thus supporting a link between endoscopic papillary pathology and stone formation31–33. Papillary appearance might also provide unique information in instances where the stones are not believed to originate from the papilla. For example, papillary appearance in stone formers that form struvite34 or uric acid stones35 is distinct from that of individuals who do not form stones. Currently, our understanding of the clinical significance of papillary abnormalities remains limited, although the advent of high-definition renal endoscopy opens many opportunities for future investigations in this area.
To date, high-definition renal endoscopy has been used in the characterization of a number of unique stone-forming diseases, including, among many others, primary hyperparathyroidism and distal renal tubular acidosis36–45. A number of distinct mechanisms of stone formation have been identified in the process of investigating these diseases, with visual correlates that can be observed grossly at the level of the papilla. The two most commonly identified abnormalities of this type are Randall plaques and ductal plugs, each of which is a distinct pathway towards the growth of calcium stones18,19.
Investigations in this area date back to work by Alexander Randall, who first described papillary mineral deposition in the 1930s after examining >1,000 human kidneys at autopsy46. In the years since then, two distinct types of papillary mineralization patterns have been identified. The most well studied are Randall plaques, which begin as apatite mineral deposition in the thin descending limbs of the loop of Henle that subsequently extend outwards into the surrounding interstitium and ultimately erode into the urinary space. Once in contact with urine, this type of mineral deposition forms a site of stone overgrowth through a mechanism that remains largely unclear41. Classically, stones that grow on Randall plaques are composed of calcium oxalate. Visually, Randall plaques appear as white suburothelial mineral deposits that can be either focused around the tip of the papilla or scattered across its surface. To date, the amount of Randall plaques identified at the time of nephroscopy has been shown to directly correlate with the extent of stone-forming activity47, thus adding to the potential clinical utility of observing and noting papillary appearance during renal endoscopic procedures.
The other type of papillary mineralization, ductal plugs, are also most commonly composed of apatite, but instead of being white, are distinctly yellow in appearance. These yellow mineral deposits occur within the lumina and ducts of the renal collecting system as opposed to within the tissue itself18 and are identifiable as large yellow mineral deposits extending from the ducts of Bellini into the urinary space. Such ductal plugs can frequently be seen under the surface of the urothelium as yellow mineral deposits within the inner medullary collecting ducts that have not yet reached the distal end at the level of the urinary space. These mineral deposits often extend in a ‘spoke-wheel’ pattern from the tip of the papilla to the calyceal fornix. Ductal plugs are well described in renal biopsy specimens, particularly from patients with certain types of stone composition (brushite or hydroxyapatite), or stone-forming diseases (renal tubular acidosis and primary hyperparathyroidism); however, their ability to directly contribute to clinical stone formation remains speculative18,36,39,42,44. The presence of ductal plugs, however, can be easily visualized using high-definition renal endoscopy and these are morphologically distinct from Randall plaques. The presence of this mineralization pattern, compared with Randall plaques, is also indicative of different underlying patterns of stone formation.
In a study comparing patients with idiopathic calcium oxalate stones (ICS) to those with idiopathic hydroxyapatite stones (HAS), the relative degrees of Randall plaque and ductal plug formation were demonstrated to enable greater discrimination between these two groups of patients than chemical analyses of urine content alone. Patients with ICS had an average of 8% coverage of the papillary surface by Randall plaques compared with <1% among patients in the HAS cohort44. Conversely, the HAS cohort had a much greater number of ductal plugs (12 per mm3) within the papilla compared with no plugging in the ICS cohort44. The overall significance of the presence of ductal plugs remains an active area of current research, particularly whether or not their presence indicates the existence of an underlying systemic stone-forming disease.
Micro-CT imaging
Micro-CT is a nondestructive, in vitro laboratory method that enables 3D visualization of small structures (<10 cm in diameter) to a resolution 1,000 times finer than is possible using a clinical CT scanner48. Different minerals in urinary stones generally possess distinct radiographic attenuation values49, these minerals can be easily detected and identified using micro-CT in most kinds of urinary stones, including those of a heterogeneous composition48,50. Like most other forms of CT imaging, micro-CT yields a 3D representation of the object. Thus, the micro-CT reconstruction of a stone can be rendered to reveal the surface features of the stone, or sliced in any plane to reveal the internal structure. The 3D image can also be quantitatively segmented for measurement of the volumes occupied by different mineral types.
Micro-CT of stones
Data from micro-CT imaging enables reconstruction of a fragment retrieved during percutaneous removal of a renal pelvic stone (FIG. 3). The scan for this fragment took 20 min, followed by 18 s for graphics-card-accelerated tomographic reconstruction. The system we use is capable of scanning such a specimen to voxel sizes as small as 0.9 μm, but such a high-resolution scan would require >3 h to complete, and the resulting image stack would be >50 GB in size, making it impractical to visualize on most computer systems.
Figure 3. Micro-CT imaging and reconstruction of a stone fragment.
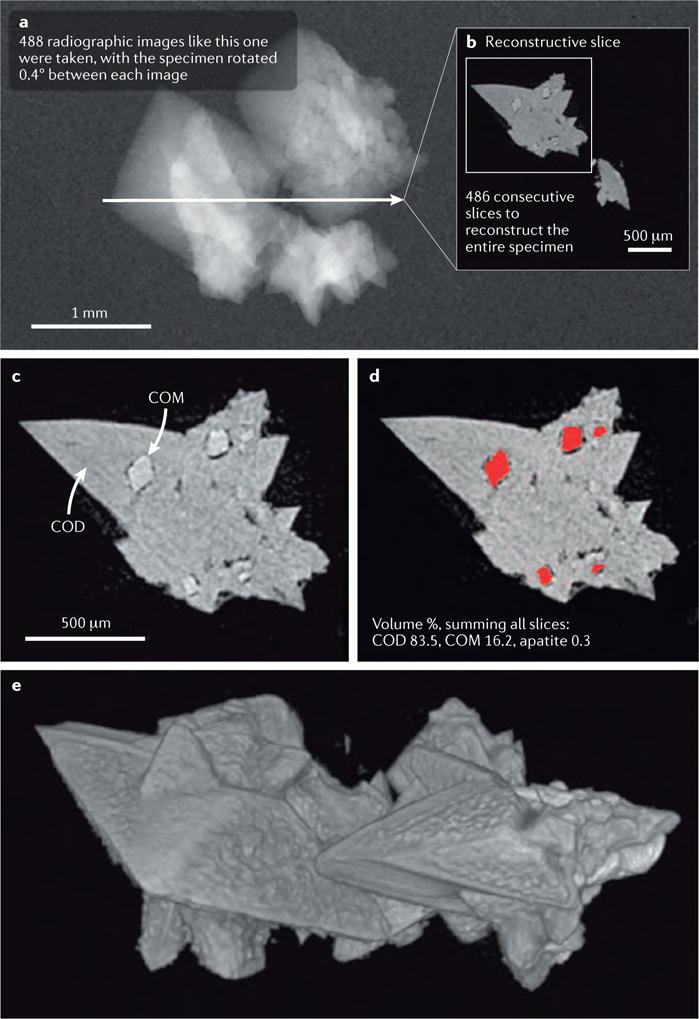
a | First, the specimen is imaged using a radiation source and an x-ray camera, with a small degree of rotation accomplished between consecutive images. In the example shown here, the specimen was rotated 0.4° between each image, for a total rotational range of slightly more than 180°. The computer then used this series of radiographic images to reconstruct the 3D structure of the specimen. b | A single reconstructed slice taken through the specimen depicted in part a. c | A portion of the same slice as depicted in part b; note that the regions of transformation of the calcium oxalate dihydrate (COD) to monohydrate (COM) are quite small, in the order of 100 μm in size, but these can be easily observed. Imaging of transformed regions within COD crystals has also previously been described, but only by physically cutting sections of the crystals65,66. The COM absorbs radiation with a slightly greater avidity than that of COD, therefore the image could be easily segmented to measure the portion of the stone occupied by COM. d | Segmentation of COM. When all image slices are similarly segmented, the volume percentages of the minerals can be easily calculated. e | Surface rendering of the fragment used in this example, note the clarity with which the polyhedral crystals of COD are shown.
Several characteristics make micro-CT a valuable technique for the study of urinary stones: micro-CT enables microscopic imaging of entire stones, and in a nondestructive manner, so that the stone can also be subjected to more conventional methods of analysis after micro-CT, if desired. Micro-CT also enables most minerals in the stone to be distinguished by their distinctive radiographic absorption properties and can enable the visualization of crystal structures, thus providing additional information that can be helpful in identifying stone minerals51. Micro-CT provides images with a very high level of resolution, thus enabling imaging of structures of only a few microns in size. When minerals are segmented based upon radiographic attenuation values, micro-CT can be used to easily quantitate the percentages of the total volume of the stone composed of each type of mineral. Finally, because micro-CT produces 3D image stacks that are isotropic (that is, the image voxels are cubic in shape), the 3D image can be resliced in any desired plane. This possibility enables the investigator to minutely examine the structures within a stone in exquisite detail.
Micro-CT offers some unique benefits when compared with FT-IR, which is one of the most widely used and accepted means of assessing the mineral content of kidney stones4. One particular advantage of micro-CT is the nondestructive nature and ability to define microstructural aspects of the stone being analysed. This new approach would be limited if it was unable to accurately characterize stone mineral composition; however, micro-CT compares favourably to FT-IR in this regard.
Our group has been utilizing micro-CT as a complement to FT-IR for much of the past two decades. In unpublished data from our institution comparing FT-IR and micro-CT analysis of 472 unique human kidney stones, the two modalities demonstrated similar accuracy. Analysis using FT-IR correctly identified the mineral composition of 87.5% of specimens, and analysis using micro-CT correctly identified the composition of 86.4% of specimens52,53. More than half of the stones that were misidentified using micro-CT were misidentified owing to the presence of uncommon stone mineral subtypes (such as whitlockite, urates or drug-related minerals). The remaining misclassifications reflected false identification of, or a failure to identify struvite. This limitation is probably attributable to the fact that struvite is commonly observed as an admixture among stones also containing several other mineral components, which can make interpretations of data from micro-CT imaging challenging. Interestingly, FT-IR was also found to miss minor stone components such as high-density apatite, a component that was accurately identified using micro-CT, thus further illustrating the complementary nature of these two modalities. The future of this technology holds promise, although several limitations should also be considered including the substantial upfront costs of the micro-CT scanner itself, the increased time demands to properly process specimens, as well as the lack of a standardized nomenclature to describe microstructural mineralization patterns and findings.
Practical utilization
As part of an ongoing NIH-sponsored research study, our group has meticulously characterized stone formation in >300 patients54. Our techniques have evolved over the course of this investigation and now include high-definition renal endoscopy and micro-CT as key elements of the optimal assessment of these patients. Our standard practice for a patient receiving treatment as part of our research programme includes several components: after providing detailed medical, surgical and stone-specific histories, patients undergo metabolic evaluation including analysis of the mineral content of serum samples and two 24-h urine sample collections performed while not receiving any medications that could potentially affect baseline physiology. At the time of surgical stone removal, ‘renal mapping’ is performed, whereby a high-definition endoscopic video of each papilla is recorded and matched with a fluoroscopic depiction of calyceal location (FIG. 4). Unique papillary features are described and recorded, including the presence of dilated ducts of Bellini, papillary erosion, papillary contour and the presence of mineral deposits (Randall plaques or ductal plugs).
Figure 4. Renal mapping of a right kidney using high-definition renal endoscopy.
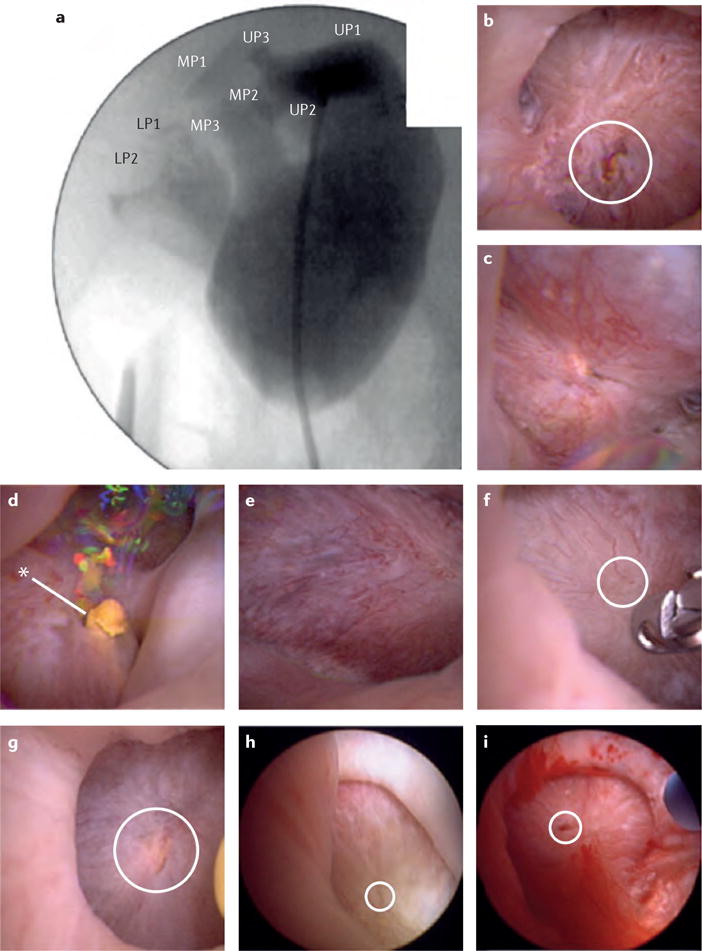
a | Calyceal location and number is denoted on fluoroscopic imaging. b-d | Endoscopic images of the upper-pole papillae (UP)s 1-3. e-g | Endoscopic images of the interpolar papillae (MP)s. h,i | Endoscopic images of the lower-pole papillae (LP)s. Dilated ducts of Bellini are circled in white, asterisk indicates the presence of a yellow ductal plug, no Randall plaques are visualized.
Stone basket extraction is an important element of our technique, whereby we attempt to remove all stones in the least destructive way possible, such that stone microstructure is retained, enabling patterns of stone growth and retention to be studied using micro-CT. Use of a ureteral access sheath is necessary to accomplish the goal of stone preservation as alternative mechanisms of stone treatment, such as ‘dusting’, do not provide sufficient stone samples to enable micro-CT analysis. All stones identified are removed and labelled individually, including information on the location of each stone within the kidney, as well as whether the stone was attached, freely floating or required fragmentation. Upon completion of stone removal, a papillary biopsy sample is obtained. Individually labelled stones then undergo evaluation of their composition and structure using micro-CT, and papillary biopsy samples are processed and examined for the presence of histopathological abnormalities and mineral content.
Distinguishing plaque-based from plug-based disease
Endoscopic appearances of the renal papillae often reflect stone formation characteristics, particularly in patients who form ICS or HAS stones44 (FIG. 5). Patients with ICS generally have a greater coverage of Randall plaques on the papillary surface, which are visible as superficial white mineral deposits, as opposed to the more dense yellow mineral deposits commonly seen in those with HAS (FIG. 5). These distinct papillary appearances often also correlate with differences in stone microstructure. For example, two attached stones of similar size were taken from different patients. Both of these stones were attached to the renal papilla, but the mode of attachment — and the underlying process of stone formation — was quite different between the two18 (FIGS 6,7).
Figure 5. Common renal papillary abnormalities observed in stone formers.
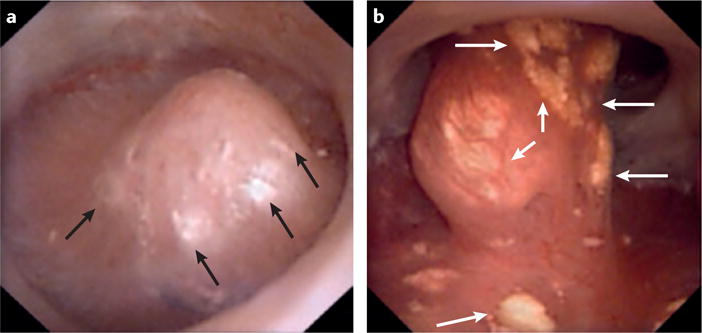
Digital endoscopic images showing the papillary appearance of two different patients. a | Randall plaquesseen commonly in patients that form idiopathic calcium oxalate stones and b | ductal plugs seen commonly in patients who form idiopathic hydroxyapatite stones. In these images Randall plaques and ductal plugs are distinguishable by their colour (white versus yellow, respectively). Arrows indicate the presence of these abnormalities.
Figure 6. Comparisons of stones anchored to papillary tissue in two different ways.
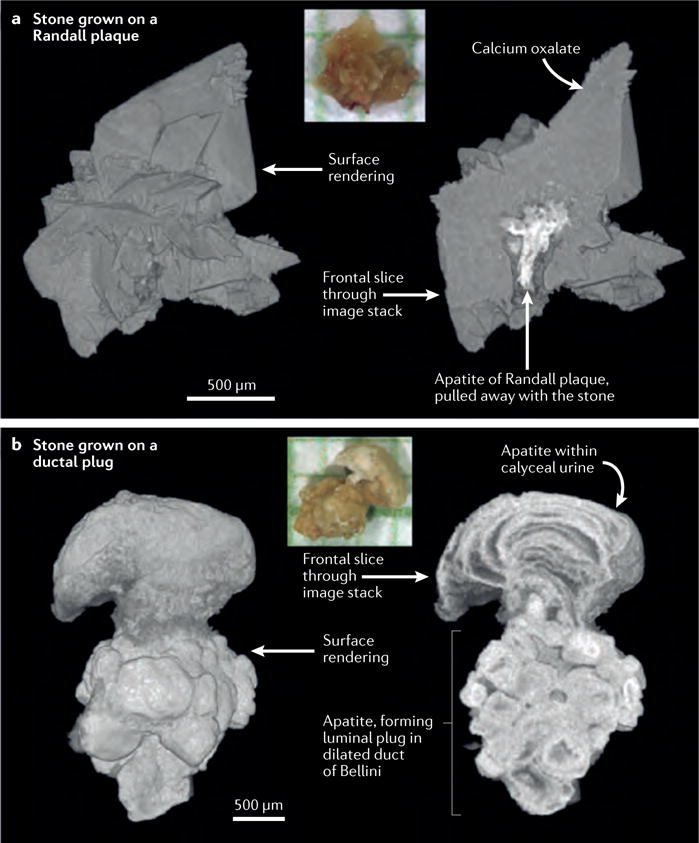
a | Digital reconstruction of a calcium oxalate stone that accumulated on a Randall plaque. The colour inset shows a photograph of the stone on mm-grid paper. Surface rendering and sliced stack analyses both reveal the presence of calcium oxalate (grey). The colour of the Randall plaque (white) indicates a high level of radiographic attenuation, reflecting the presence of apatite. Note the sparse and thin distribution of the apatite comprising the plaque itself b | Digital reconstruction of an apatite stone that accumulated on a ductal plug. In this image the apatite ductal plug anchoring the stone to the papilla is more substantial, thicker and denser compared to that of the Randall plaque indicating a distinct mechanism of formation and retention within the kidney at the level of the papilla.
Figure 7. Direct comparison of reconstructions of stones formed on a Randall plaque or on a ductal plug.
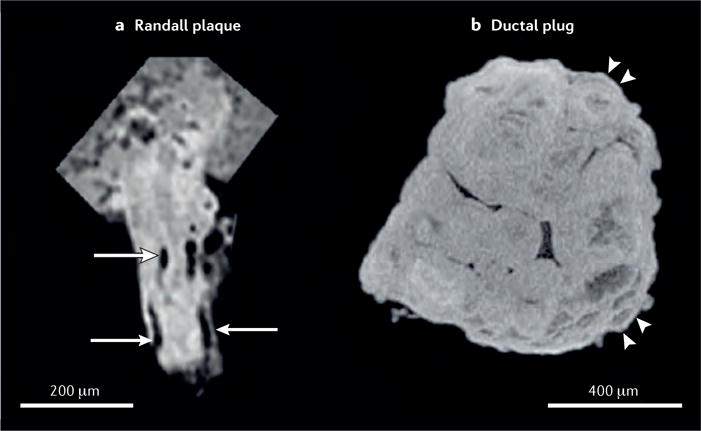
a | Stone formed on a Randall plaque showing lumina of tubules and/or vessels (as indicated by arrows), demonstrating that this apatite region is interstitial. In Randall plaques, apatite accumulates in the papillary interstitium, without any deposition into tubular lumina. By contrast, the stone formed on a ductal plug. b | conforms to the shape of the dilated duct in which it formed, and shows signs of accretion by layering (as indicated by arrowheads). This ductal plug seems to have been formed from multiple, small spheres of apatite.
The first of these two patients had 17 unique calcium oxalate stones removed during bilateral ureteroscopy. The majority of these stones were attached to the papillae and all showed evidence, on endoscopic or micro-CT, of having grown on Randall plaques (FIG. 6a). Examination of the kidneys during this procedure found all papillae to be normal in shape and appearance, with the exception of Randall plaques, which were evident on the majority of papillae (FIG. 5a). A metabolic workup did not reveal any underlying systemic disease and the patient was thus characterized as an idiopathic calcium oxalate stone former.
The second patient also had numerous bilateral calculi, although this patient also had a known diagnosis of primary hyperparathyroidism. Several stones were found to be attached to the papillae, although no evidence of Randall plaques was detected on either endoscopic examination or micro-CT. The papillae in this patient had a distinct appearance with extensive evidence of yellow ductal plugs and dilated ducts of Bellini, similar to those shown in the presented image (FIG. 5b). Findings of micro-CT analysis of the attached stones were also distinct with a wide, dense stalk of apatite over which the stone had grown, consistent with the appearance of a ductal plug (FIGS 6,7).
A variety of morphological differences between apatite found on Randall plaques, and apatite found within the lumina of a duct of Bellini can be observed using micro-CT (FIG. 7). The stone formed upon a Randall plaque contains spaces that are roughly cylindrical in shape, when viewed in 3D, and with typical diameters of 10–30 μm. These spaces likely reflect the presence of tubules, around which the apatite deposits probably formed, and are consistent with stone formation being an interstitial, as opposed to intratubular or ductal process. The apatite within the Randall plaque does not show alternating radiographically dense and lucent layers, as is typical of apatite stones51. Instead, the radiographic density of the apatite varies within the plaque, being the most dense in the middle and least dense toward the periphery of the plaque. In our own experience, rings of apatite can sometimes be observed at the edges of a region of the renal papilla containing a high density of Randall plaques, which probably indicates the mineralized thin limbs of the plaques protruding towards the basement membrane, as has been described for the early stages of Randall plaque formation55.
In contrast to apatite accumulation in Randall plaques, the apatite in a ductal plug usually shows signs of having accumulated by accretion of layers. The shape of the plug is typically cylindrical or oblong, with tapered ends. Sometimes ductal plugs show evidence of having formed from multiple spherical or ovoid shapes (FIG. 7), however, some ductal plugs have been observed to be more monolithic, but still with signs of having grown by addition of layers44. These two stones provide anecdotal examples of the ways that micro-CT imaging of stones, combined with examinations of the renal papillae, can add insight into mechanisms of retention and growth of calculi. The diversity of mechanisms of urinary stone formation is one of the important lessons that we are learning from studies of patients with stones published within the past 6 years19,56.
Taken together, information gained from considerations of papillary pathology and stone microstructure on micro-CT hold promise as a way of improving upon current methods of classifying stone formers. Using this approach, routinely commenting upon mechanisms of stone formation in addition to mineral composition alone might soon become possible. As an example, patients with ICSF and primary hyperoxaluria both form calcium oxalate stones and would be indistinguishable based upon the results of FT-IR analysis of their stones alone. However, patients that form one of these two types of stones have quite distinct papillary appearances with Randall plaques commonly observed in ICSF and rarely, or never seen in those with primary hyperoxaluria18. Consideration of these distinctions can, therefore, be used to help distinguish the underlying pathophysiology of these patients. Similarly, stones commonly have mixed compositions and often contain different amounts of apatite and calcium oxalate. As such, FT-IR analysis is prone to error depending on the mineral composition of the stone fragment being sampled. Therefore the addition of information on microstructural patterns of stone formation (plaques versus plugs) could provide more information than mineral analysis alone.
At this point in time, greater efforts are being made to improve the conceptual understanding of how papillary pathology is associated with stone microstructure and/or mineral composition. Ultimately, improving the ability to properly classify patients on the basis of the unique underlying mechanism through which stones are formed has the potential to dramatically improve the management of patients with this condition. This concept is not new. In fact, mineral analysis alone has revolutionized the medical management of stone disease, leading to guidelines such that patients with particular types of stones receive directed treatment2. However, much still remains to be learned and the precision with which stone formers are currently classified still has the potential for vast improvement, which ultimately could lead to more effective care.
Classification of papillary abnormalities
The potential of papillary abnormalities to act as a surrogate marker of the underlying disease has been proven among carefully selected patients whose clinical features are analysed by a team with experience in this area, although whether or not this approach is applicable to the wider urological community currently remains unclear. Over the past 10–20 years, minimally invasive approaches to the treatment of stone disease have entirely replaced open surgical methods57. As such, the majority of urological surgeons now have high degrees of comfort, skill and experience in using an endoscope to examine the kidney. Despite this change in approach, such procedures almost entirely focus on treatment and removal of the stone, with renal papillary inspection remaining an almost entirely unappreciated aspect of the procedure. This lack of implementation of papillary inspection could possibly be a result of the widespread use of older-generation instruments that do not provide the high level of image resolution provided by modern scopes; although, this deficit more likely reflects the fact that, even if papillary abnormalities were commonly appreciated, the relevance of these abnormalities is entirely unclear. Hence, the current revolution in scope optics is an opportunity, upon which new insights from the wider urological community will be necessary in achieving a consensus regarding the normal, abnormal and clinically relevant features of the renal papilla. The creation of a common language, with which researchers and clinicians alike are able to describe papillary findings using the same descriptive terminology, will be a critical element of accomplishing this goal.
Our research team has introduced a grading scale for the purpose of identifying and quantifying the presence, extent and type of papillary abnormalities across four distinct papillary domains (ductal plugs, ‘pitting’, ‘loss of contour’ and Randall plaques)28. Ductal plugs and Randall plaques have been previously described. ‘Pitting’ refers to focal erosion or crater-like areas of the papillary surface, which should normally be entirely smooth. ‘Loss of contour’ refers to the global flattening of the papilla, which should normally have the appearance of a tall hill or mountain peak.
Reproducibility of such a grading system is a critical component in ensuring its implementation as a meaningful clinical tool. Early investigation in this regard has elucidated the fact that the ability to appreciate the presence and magnitude of such abnormalities is not intuitive, but improves with experience and education. In the initial description of this system, reproducibility was most favourable when applied by the two urologists that were most familiar with using the grading system, thus demonstrating exact inter-investigator agreement across all four measured domains in nearly 75% of patients studied28. Subsequent efforts examining the reproducibility of this approach among the wider urological community have shown that this concept is new even for those who are familiar with renal endoscopy. In an unpublished experience, 16 endourologists with no prior familiarity using such a grading system were asked to grade nine separate videos of unique papillae, each having variable amounts of papillary abnormality. Using a weighted κ statistic to quantify interobserver agreement, agreement across all observers was found to be moderate for ductal plugs (0.42) and pitting (0.43), but only fair for loss of contour (0.27) and Randall plaques (0.20). However, reproducibility might improve with appropriate education of clinicians and increased familiarity with the concepts behind the scoring system. In a separate analysis58, a senior and junior urologist, neither of whom had previously encountered the grading system, were provided with a 1-h dedicated training session including example images of various papillae58, and opportunities to clarify any questions. Subsequently, videos of a single papilla from 50 separate patients were examined by these two urologists, in addition to another senior and junior urologist who were both already familiar with the grading scale. Videos were viewed twice to assess the level of intra-investigator agreement. In this study, both the intra-investigator and inter-investigator levels of agreement were considerably higher across all domains regardless of experience, with substantial to near-perfect agreement for assessments of all measured variables (weighted κ-statistic for level of agreement >0.6) excluding Randall plaques, for which a moderate level of inter-investigator agreement (0.52) was observed58.
This renal papillary grading system is the composite end point of years of experience and attention directed towards this purpose by a team of interested individuals; yet, we readily acknowledge the potential for such a system to evolve further. In fact, improved recognition of papillary disease is the entire purpose of creating such a grading system and might provide a novel tool for the consideration of stone disease on a much larger scale, thus enabling widespread collaboration across the urological community. Development and utilization of such a system would hardly be unique in medicine. Many established examples of comparable grading scales exist, and are used for the similar purposes of characterizing disease pathology and guiding treatment. Some of these scales even form the cornerstones of disease diagnosis and classification including the Bethesda System for Pap smear grading to predict cervical cancer59, the Braden Scale assessments to predict the risk of pressure ulcers60, and the Kellgren & Lawrence system for classification of knee osteoarthritis61. In fact, the field of urology also already has several similar scales including the Bosniak classification system to predict the likelihood of malignancy based upon the complexity of renal cysts62 and the renal trauma grading scale to assess damage and guide intervention for traumatic renal injuries63.
If a similar papillary grading system for the purposes of subclassifying stone formers was to be fully validated, the potential research and clinical utility would probably be enormous. Such a grading system would enable researchers to identify patients with specific stone-forming pathophysiologies and in the process potentially develop better disease-specific treatments across multiple clinical domains including optimal dietary, metabolic, pharmacological and surgical approaches towards stone prevention and treatment.
Micro-CT also has a promising future. Our previous example focused on using micro-CT as a tool to analyse and distinguish papillary stones, however no good reason exists to suggest this technique cannot also be used to study alternative mechanisms of stone formation, including the assessment of concretions formed in animal models to determine how much these resemble human stones. Further research using this technique might help to elucidate the mechanisms by which certain stones in humans grow in free solution, help determine the potential for calcification of stents and nephrostomy tubes and even provide insight into the stone–tissue relationship itself. For example, we have previously performed micro-CT analysis of renal biopsy samples whereby the pattern of mineral distribution within the renal tissue itself becomes more evident64. Currently, this technology is too costly and demanding of resources to be offered as anything more than a research tool. However, as experience, familiarity and awareness continue to grow, the possibility emerges that novel ways of streamlining costs and practical usage could open up opportunities for utilization of this tool on a wider scale. In fact, in many ways a stone removed from a kidney is no different from a tissue biopsy sample taken to diagnose other diseases for which advanced and expensive techniques, including tissue microdissection, flow cytometry and genetic sequencing, which were once seen as radical research tools, are now used routinely.
Conclusions
A variety of underlying pathologies can result in urinary stones, and identification of these pathologies and possible treatments for them, will be important to accomplish advances in the treatment of patients with stones in the coming years. High-definition renal endoscopy and micro-CT imaging of stone specimens enables further enhancement of our ability to discriminate between stone formers that have distinctly different underlying causes of their disease, and will undoubtedly be an important aspect of attempts to improve the classification of stones in the future.
Acknowledgments
J.C.W., A.P.E. and J.E.L. gratefully acknowledge funding from the National Institutes of Health P01 DK056788/DK/NIDDK NIH HHS/United States.
Footnotes
Author contributions
All authors made substantial contributions to researching data for this article, discussions of content, writing and editing and reviewing of this manuscript prior to submission.
Competing interests statement
J.E.L. is the owner and scientific director of Beck Analytical and has acted as a consultant for Boston Scientific. The other authors declare no competing interests.
Contributor Information
Michael S. Borofsky, Department of Urology, Indiana University Health at Methodist Hospital, 1801 Senate Boulevard #220, Indianapolis, Indianapolis 46202, USA
Casey A. Dauw, Department of Urology, Indiana University Health at Methodist Hospital, 1801 Senate Boulevard #220, Indianapolis, Indianapolis 46202, USA
Andrew Cohen, Department of Urology, University of Chicago Hospital, 5841 S Maryland Avenue, Mc 6038, Chicago, Illinois 60637, USA.
James C. Williams, Jr, Department of Anatomy and Cell Biology, Indiana University School of Medicine, 635 Barnhill Drive, VanNuys Medical Science Building, Indianapolis, Indiana 46202, USA.
Andrew P. Evan, Department of Anatomy and Cell Biology, Indiana University School of Medicine, 635 Barnhill Drive, VanNuys Medical Science Building, Indianapolis, Indiana 46202, USA
James E. Lingeman, Department of Urology, Indiana University Health at Methodist Hospital, 1801 Senate Boulevard #220, Indianapolis, Indianapolis 46202, USA
References
- 1.Kirkali Z, Rasooly R, Star RA, Rodgers GP. Urinary Stone disease: progress, status, and needs. Urology. 2015;86:651–653. doi: 10.1016/j.urology.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearle MS, et al. Medical management of kidney stones: AUA guideline. J Urol. 2014;192:316–324. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Turk C, et al. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. 2015;69:475–482. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Cloutier J, Villa L, Traxer O, Daudon M. Kidney stone analysis: “Give me your stone, I will tell you who you are!”. World J Urol. 2015;33:157–169. doi: 10.1007/s00345-014-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma RN, Shah I, Gupta S, Sharma P, Beigh AA. Thermogravimetric analysis of urinary stones. Br J Urol. 1989;64:564–566. doi: 10.1111/j.1464-410x.1989.tb05308.x. [DOI] [PubMed] [Google Scholar]
- 6.Fazil Marickar YM, Lekshmi PR, Varma L, Koshy P. EDAX versus FTIR in mixed stones. Urol Res. 2009;37:271–276. doi: 10.1007/s00240-009-0202-8. [DOI] [PubMed] [Google Scholar]
- 7.Bastian PJ, Lorken M, Euler H, Lummen G, Bastian HP. Results of the evaluation of 85337 urinary stone analyses. Aktuelle Urol. 2008;39:298–304. doi: 10.1055/s-2007-993039. (in German) [DOI] [PubMed] [Google Scholar]
- 8.Daudon M, Protat MF, Reveillaud RJ, Jaeschke-Boyer H. Infrared spectrometry and Raman microprobe in the analysis of urinary calculi. Kidney Int. 1983;23:842–850. doi: 10.1038/ki.1983.104. [DOI] [PubMed] [Google Scholar]
- 9.Daudon M, et al. Sex- and age-related composition of 10 617 calculi analyzed by infrared spectroscopy. Urol Res. 1995;23:319–326. doi: 10.1007/BF00300021. [DOI] [PubMed] [Google Scholar]
- 10.Krambeck AE, et al. Inaccurate reporting of mineral composition by commercial stone analysis laboratories: implications for infection and metabolic stones. J Urol. 2010;184:1543–1549. doi: 10.1016/j.juro.2010.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuire BB, et al. Predicting patients with inadequate 24- or 48-hour urine collections at time of metabolic stone evaluation. J Endourol. 2015;29:730–735. doi: 10.1089/end.2014.0544. [DOI] [PubMed] [Google Scholar]
- 12.Healy KA, Hubosky SG, Bagley DH. 24-hour urine collection in the metabolic evaluation of stone formers: is one study adequate? J Endourol. 2013;27:374–378. doi: 10.1089/end.2012.0216. [DOI] [PubMed] [Google Scholar]
- 13.Castle SM, Cooperberg MR, Sadetsky N, Eisner BH, Stoller ML. Adequacy of a single 24-hour urine collection for metabolic evaluation of recurrent nephrolithiasis. J Urol. 2010;184:579–583. doi: 10.1016/j.juro.2010.03.129. [DOI] [PubMed] [Google Scholar]
- 14.Pak CY, Peterson R, Poindexter JR. Adequacy of a single stone risk analysis in the medical evaluation of urolithiasis. J Urol. 2001;165:378–381. doi: 10.1097/00005392-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Parks JH, Goldfisher E, Asplin JR, Coe FL. A single 24-hour urine collection is inadequate for the medical evaluation of nephrolithiasis. J Urol. 2002;167:1607–1612. [PubMed] [Google Scholar]
- 16.Nayan M, Elkoushy MA, Andonian S. Variations between two 24-hour urine collections in patients presenting to a tertiary stone clinic. Can Urol Assoc J. 2012;6:30–33. doi: 10.5489/cuaj.11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evan AP, Lingeman JE, McAteer JA, Williams JC., Jr Introduction to special issue on the Proceedings of the 3rd International Urolithiasis Research Symposium held in Indianapolis. Urol Res. 2010;38:237. doi: 10.1007/s00240-010-0301-6. [DOI] [PubMed] [Google Scholar]
- 18.Evan AP, Worcester EM, Coe FL, Williams J, Jr, Lingeman JE. Mechanisms of human kidney stone formation. Urolithiasis. 2015;43(Suppl. 1):19–32. doi: 10.1007/s00240-014-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coe FL, Evan AP, Lingeman JE, Worcester EM. Plaque and deposits in nine human stone diseases. Urol Res. 2010;38:239–247. doi: 10.1007/s00240-010-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rassweiler J, Rassweiler MC, Klein J. New technology in ureteroscopy and percutaneous nephrolithotomy. Curr Opin Urol. 2016;26:95–106. doi: 10.1097/MOU.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 21.Borofsky MS, Shah O. Advances in ureteroscopy. Urol Clin North Am. 2013;40:67–78. doi: 10.1016/j.ucl.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Andonian S, Okeke Z, Smith AD. Digital ureteroscopy: the next step. J Endourol. 2008;22:603–606. doi: 10.1089/end.2008.0017. [DOI] [PubMed] [Google Scholar]
- 23.Humphreys MR, et al. A new world revealed: early experience with digital ureteroscopy. J Urol. 2008;179:970–975. doi: 10.1016/j.juro.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 24.Zilberman DE, et al. The digital flexible ureteroscope: in vitro assessment of optical characteristics. J Endourol. 2011;25:519–522. doi: 10.1089/end.2010.0206. [DOI] [PubMed] [Google Scholar]
- 25.Flachenecker G, Fastenmeier K. High frequency current effects during transurethral resection. J Urol. 1979;122:336–341. doi: 10.1016/s0022-5347(17)56395-8. [DOI] [PubMed] [Google Scholar]
- 26.Somani BK, Al-Qahtani SM, de Medina SD, Traxer O. Outcomes of flexible ureterorenoscopy and laser fragmentation for renal stones: comparison between digital and conventional ureteroscope. Urology. 2013;82:1017–1019. doi: 10.1016/j.urology.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Multescu R, Geavlete B, Georgescu D, Geavlete P. Improved durability of flex-Xc digital flexible ureteroscope: how long can you expect it to last? Urology. 2014;84:32–35. doi: 10.1016/j.urology.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Borofsky MS, et al. A proposed grading system to standardize the description of renal papillary appearance at the time of endoscopy in patients with nephrolithiasis. J Endourol. 2016;30:122–127. doi: 10.1089/end.2015.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low RK, Stoller ML. Endoscopic mapping of renal papillae for Randall’s plaques in patients with urinary stone disease. J Urol. 1997;158:2062–2064. doi: 10.1016/s0022-5347(01)68153-9. [DOI] [PubMed] [Google Scholar]
- 30.Linnes MP, et al. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int. 2013;84:818–825. doi: 10.1038/ki.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evan A, Lingeman J, Coe FL, Worcester E. Randall’s plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006;69:1313–1318. doi: 10.1038/sj.ki.5000238. [DOI] [PubMed] [Google Scholar]
- 32.Miller NL, et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall’s plaque. BJU Int. 2009;103:966–971. doi: 10.1111/j.1464-410X.2008.08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matlaga BR, et al. Endoscopic evidence of calculus attachment to Randall’s plaque. J Urol. 2006;175:1720–1724. doi: 10.1016/S0022-5347(05)01017-7. discussion 1724. [DOI] [PubMed] [Google Scholar]
- 34.Jaeger CD, et al. Endoscopic and pathologic characterization of papillary architecture in struvite stone formers. Urology. 2016;90:39–44. doi: 10.1016/j.urology.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viers BR, et al. Endoscopic and histologic findings in a cohort of uric acid and calcium oxalate stone formers. Urology. 2015;85:771–776. doi: 10.1016/j.urology.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evan AE, et al. Histopathology and surgical anatomy of patients with primary hyperparathyroidism and calcium phosphate stones. Kidney Int. 2008;74:223–229. doi: 10.1038/ki.2008.161. [DOI] [PubMed] [Google Scholar]
- 37.Evan AP, et al. Renal intratubular crystals and hyaluronan staining occur in stone formers with bypass surgery but not with idiopathic calcium oxalate stones. Anat Rec. 2008;291:325–334. doi: 10.1002/ar.20656. [DOI] [PubMed] [Google Scholar]
- 38.Evan AP, et al. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006;69:2227–2235. doi: 10.1038/sj.ki.5000268. [DOI] [PubMed] [Google Scholar]
- 39.Evan AP, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 2007;71:795–801. doi: 10.1038/sj.ki.5002113. [DOI] [PubMed] [Google Scholar]
- 40.Evan AP, et al. Intra-tubular deposits, urine and stone composition are divergent in patients with ileostomy. Kidney Int. 2009;76:1081–1088. doi: 10.1038/ki.2009.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evan AP, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evan AP, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 43.Evan AP, et al. Renal histopathology and crystal deposits in patients with small bowel resection and calcium oxalate stone disease. Kidney Int. 2010;78:310–317. doi: 10.1038/ki.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evan AP, et al. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxy apatite, brushite, or calcium oxalate stones. Anat Rec. 2014;297:731–748. doi: 10.1002/ar.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evan AP, et al. Biopsy proven medullary sponge kidney: clinical findings, histopathology, and role of osteogenesis in stone and plaque formation. Anat Rec. 2015;298:865–877. doi: 10.1002/ar.23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randall A. The origin and growth of renal calculi. Ann Surg. 1937;105:1009–1027. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SC, et al. Stone formation is proportional to papillary surface coverage by Randall’s plaque. J Urol. 2005;173:117–119. doi: 10.1097/01.ju.0000147270.68481.ce. discussion 119. [DOI] [PubMed] [Google Scholar]
- 48.Williams JC, Jr, McAteer JA, Evan AP, Lingeman JE. Micro-computed tomography for analysis of urinary calculi. Urol Res. 2010;38:477–484. doi: 10.1007/s00240-010-0326-x. [DOI] [PubMed] [Google Scholar]
- 49.Qu M, et al. Dual-energy dual-source CT with additional spectral filtration can improve the differentiation of non-uric acid renal stones: an ex vivo phantom study. Am J Roentgenol. 2011;196:1279–1287. doi: 10.2214/AJR.10.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zarse CA, et al. Nondestructive analysis of urinary calculi using micro computed tomography. BMC Urol. 2004;4:15. doi: 10.1186/1471-2490-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pramanik R, Asplin JR, Jackson ME, Williams JC., Jr Protein content of human apatite and brushite kidney stones: significant correlation with morphologic measures. Urol Res. 2008;36:251–258. doi: 10.1007/s00240-008-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zarse CA, et al. Helical computed tomography accurately reports urinary stone composition using attenuation values: in vitro verification using high-resolution micro-computed tomography calibrated to fourier transform infrared microspectroscopy. Urology. 2004;63:828–833. doi: 10.1016/j.urology.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 53.Williams JC, et al. Progress in the use of helical CT for imaging urinary calculi. J Endourol. 2004;18:937–941. doi: 10.1089/end.2004.18.937. [DOI] [PubMed] [Google Scholar]
- 54.Kuo RL, et al. Endoscopic renal papillary biopsies: a tissue retrieval technique for histological studies in patients with nephrolithiasis. J Urol. 2003;170:2186–2189. doi: 10.1097/01.ju.0000096065.61481.35. [DOI] [PubMed] [Google Scholar]
- 55.Evan AP, Lingeman J, Coe FL, Worcester E. Randall’s plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006;69:1313–1318. doi: 10.1038/sj.ki.5000238. [DOI] [PubMed] [Google Scholar]
- 56.Williams JC, Jr, McAteer J. A Retention and growth of urinary stones—insights from imaging. J Nephrol. 2013;26:25–31. doi: 10.5301/jn.5000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borofsky MS, Lingeman JE. The role of open and laparoscopic stone surgery in the modern era of endourology. Nat Rev Urol. 2015;12:392–400. doi: 10.1038/nrurol.2015.141. [DOI] [PubMed] [Google Scholar]
- 58.Borofsky MS, et al. 33rd World Congress of Endourology. London: 2015. A grading system for papillary injury in stone formers. [Google Scholar]
- 59.Grapsa D, Ekaterini P. Standardized categorical reporting of cytopathology results: the strengths and weaknesses of a constantly evolving and expanding system. Diagnost Cytopathol. 2013;41:917–921. doi: 10.1002/dc.22927. [DOI] [PubMed] [Google Scholar]
- 60.Pancorbo-Hidalgo PL, Garcia-Fernandez FP, Lopez-Medina IM, Alvarez-Nieto C. Risk assessment scales for pressure ulcer prevention: a systematic review. J Adv Nurs. 2006;54:94–110. doi: 10.1111/j.1365-2648.2006.03794.x. [DOI] [PubMed] [Google Scholar]
- 61.Ajuied A, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42:2242–2252. doi: 10.1177/0363546513508376. [DOI] [PubMed] [Google Scholar]
- 62.McGuire BB, Fitzpatrick JM. The diagnosis and management of complex renal cysts. Curr Opin Urol. 2010;20:349–354. doi: 10.1097/MOU.0b013e32833c7b04. [DOI] [PubMed] [Google Scholar]
- 63.Shenfeld OZ, Gnessin E. Management of urogenital trauma: state of the art. Curr Opin Urol. 2011;21:449–454. doi: 10.1097/MOU.0b013e32834b4a9e. [DOI] [PubMed] [Google Scholar]
- 64.Williams JC, et al. Micro-CT imaging of Randall’s plaques. Urolithiasis. 2015;43(Suppl 1):13–17. doi: 10.1007/s00240-014-0702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cifuentes Delatte L, Hidalgo A, Bellanato J, Santos M. In: Urinary Calculi: Recent Advances in Aetiology, Stone Structure and Treatment. Cifuentes Delatte L, Rapado A, Hodgkinson A, editors. S. Karger; 1973. pp. 220–230. [Google Scholar]
- 66.Schubert G, Brien G. Crystallographic investigations of urinary calcium oxalate calculi. Int Urol Nephrol. 1981;13:249–260. doi: 10.1007/BF02082422. [DOI] [PubMed] [Google Scholar]


