KEY POINTS
Chronic kidney disease (CKD) stages 1 to 4 are asymptomatic health states. According to previously proposed definitions of disease, they don’t meet the criteria for “disease.”
Reduced levels of kidney function, currently called CKD, are not abnormal in older age groups.
The label “chronic kidney disease” can be misinterpreted by patients and can be an obstacle to communication.
Referring to the stages of “chronic kidney disease” as categories of “kidney age” could improve doctors’ ability to communicate, and patients’ understanding of, the degree of renal impairment and its implications for health.
Although a variety of conditions and syndromes may affect the kidneys over either chronic or acute time frames, the term “chronic kidney disease” (CKD) is used to describe a decrease in the filtration ability of the glomerular capillaries in the kidney. The most prevalent forms of CKD in health care systems are typically the asymptomatic stages conventionally termed CKD stage 4 or below.1,2 Should these asymptomatic stages be called “disease”?
What is meant by “chronic kidney disease”?
In 2002, the US National Kidney Foundation proposed that the term “chronic kidney disease” be applied for specific dysfunctions of the kidneys, defined primarily by the glomerular filtration rate. These guidelines proposed that glomerular filtration rate less than 60 mL/min/1.73m2 be considered CKD stage 3; glomerular filtration rate less than 30 mL/min/1.73m2 considered CKD stage 4; and less than 15 mL/min/1.73m2 considered CKD stage 5, with earlier stages1,2 dependent on other evidence of kidney damage, such as proteinuria.3 This approach has proved popular, arguably because it can be linked to clear clinical action plans.4 Subsequent guidelines in Canada, the United Kingdom, the United States and elsewhere have updated this scheme or proposed modifications for other countries.5 These revisions have often subdivided stage 3 with an additional threshold at 45 mL/min/1.73m2 or introduced a secondary classification by proteins in urine, but the emphasis on glomerular filtration rate, often estimated using markers in the blood, has remained constant.
High-quality observational evidence shows an association between the stages of CKD and cardiovascular disease as well as end-stage renal disease. A meta-analysis of large cohort studies showed that cardiovascular risk increases with each level of glomerular filtration rate (shown in Table 1), as does risk of future end-stage renal disease and risk of acute kidney injury.4,6 These risks also increase with albuminuria, at all levels of glomerular filtration rate except less than 15 mL/min/1.75m2 (stage 5). It has been argued that the utility of CKD classification is that it can be linked to a clear action plan,4 although the level of evidence for most of the recommended interventions is not strong.7
Table 1:
Definitions of chronic kidney disease according to glomerular filtration rate by the Kidney Disease Outcomes Quality Initiative of the US National Kidney Foundation
| Stage | Description | GFR (mL/min/1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or elevated GFR | ≥ 90 |
| 2 | Kidney damage with mild decrease in GFR | 60–89 |
| 3* | Moderate decrease in GFR | 30–59 |
| 4 | Severe decrease in GFR | 15–29 |
| 5 | Kidney failure | < 15 (or dialysis) |
Note: GFR = glomerular filtration rate.
Modified in 2014 to distinguish stage 3a, defined by GFR between 45 mL/min/1.73 m2 and 59 mL/min/1.73 m2, from stage 3b, defined by GFR between 30 and 44 mL/min/1.73m2.
What constitutes a disease?
Smart8 reviewed four prominent definitions of disease in the philosophy of medicine literature. We considered whether CKD, defined by current thresholds for glomerular filtration rate, is disease according to these definitions.9 We found that CKD stage 5 was disease by any definition, because it is associated with harm and is statistically uncommon in any age group. However, earlier stages of CKD are associated with harm as risk factors for further disease (renal or cardiovascular) rather than as disease itself, and whether they are statistically abnormal depends entirely on age.6
Is the label “chronic kidney disease” helpful to patients or clinicians?
Qualitative data from both clinicians10,11 and patients12 have shown that communicating a diagnosis of “chronic kidney disease” to patients can be uncomfortable and unsatisfactory for all concerned. As soon as the words “chronic” or “disease” are introduced at a consultation, primary care physicians face an uphill battle to retrieve the situation with reassurance.13 Our research14 found that the word “chronic” is often misinterpreted by patients as meaning serious, and “kidney disease” can trigger thoughts of dialysis and transplant, because people are usually unaware that earlier stages of kidney impairment exist before treatment becomes necessary (Box 1, quotes 1 and 2).
Box 1: Patient quotes (all from our own research14).
Quote 1: I couldn’t understand how I could be described as having chronic kidney disease when it had only just been discovered. “Chronic” to me means — well, maybe mistakenly — that I’ve had it for a long, long time … they keep using this word “chronic” and I think the word “chronic” makes it sound worse than what it is; to me, anyhow. (Eric, aged 79)
Quote 2: One of my older brothers — he had kidney problems when he was quite young. And then he had kidney failure completely and he had a transplant done, and that worries me a little bit … we’re both exactly the same build and everything. He had bowel cancer and it seems to me everything he got, I get. (Bill, aged 71)
Quote 3: I went for my normal routine check-up to have blood tests and blood pressure and water and that. And my diabetic nurse came in with the nurse that was doing the test to send away to have the results come back and she asked me if I’d like to join this program that you’re running. And I asked her what it was about and she explained in my language that it was due to kidney problems. And I went into defensive mode. I said, “No, I’ve never had kidney problems. I haven’t got kidney problems with diabetes. It’s, you know, I’ve been told everything was all right.” And then she told me that I had a kidney problem that was being monitored regularly and I asked her, “How long have I had it? Can you tell me when it was diagnosed?” And she said in 1997, which was an absolute shock. [Laughs] We had a short discussion because she had to go and see to other patients and she was under the impression I knew. But my daughter and I had been to the doctors when the results came through before, and I’d asked about kidneys and they’d always said, “No, they’re fine.” So this really was a bit of a … a shock. I laughed about it at first. I thought that it was a bit of a mix-up. But now I understand there isn’t but yet I haven’t had it explained to me quite what it entails or what it’s about. (Joan, aged 70)
Quote 4: I want to know why they’re failing, that’s what I want to know. I mean, I’m not a drinker, I don’t drink, so whether it is the medication or whether old age, well, she hasn’t explained. She just, as I say, she [general practitioner] has just said that my kidneys aren’t working as they should and I’m almost living on one kidney, but I wasn’t to worry because hundreds of people live on one kidney. But then it starts alarm bells ringing, doesn’t it? Why are your kidneys failing; what have I done to make them fail? Is it my lifestyle, is it tablets? I mean, it can’t be drink because I don’t drink; is it coffee? You don’t know, do you? (Elizabeth, aged 74)
Quote 5: But as far as an explanation of why the kidneys aren’t working perfectly, she [the GP] did, most recently, tell me that kidney function decreases as you get older, and I accept that as being the case like everything else. You get older, you lose your memory and things. (Eric, aged 79)
Clinicians may avoid using the term CKD with their patients or disclosing the diagnosis altogether. A qualitative study12 found that 19 of 26 patients with CKD interviewed had been told something about their kidney function, but only four had explicitly been given a diagnosis of CKD. A study of clinician views found a concern among general practitioners about possibly alarming patients by giving them a disease label when their kidney function was only mildly impaired.11 However, in our and the aforementioned qualitative study,12 nondisclosure of a CKD led to some patients finding out about it by accident, such as when consulting a clinician other than their usual one, who assumed they already knew. Such accidental disclosure could lead to shock, anger and upset (Box 1, quote 3). Patient participants reported having their kidney function described by doctors in euphemisms such as “borderline,” “under par” or “leaking kidneys,” rather than as a chronic disease.14 Another qualitative study13 found that general practitioners felt a need to downplay CKD when discussing it with patients.
Regardless of the terms that professionals used to describe early-stage CKD to their patients, where it is disclosed it is accompanied by efforts to reassure patients that it is nothing to worry about.13 In our research,14 when patients are told not to worry without an accompanying explanation or acknowledgement of their knowledge of other people with severe kidney failure, this often failed to provide sufficient reassurance to patients. Patients were left wanting more information about what might have caused their kidney impairment, its severity and what the test results meant, whether it was reversible and how quickly it might decline to a level where treatment would be needed, the kind of symptoms they should look out for, and whether they could do anything to prevent further decline (Box 1, quote 4).
However, when an explanation was offered, patients felt more reassured. Knowing that their kidneys were still functioning sufficiently well so as not to cause them any problems, that they were being regularly monitored and that their test results were satisfactory or stable were all sources of reassurance, along with the trust they had in their doctor. Many primary care doctors regard reduced but stable kidney function in older patients as a natural result of aging, and often use this as the basis for the explanation and reassurance they give their patients.11,13 Increasing age was the most common explanation for kidney impairment offered to our patient participants, and in most cases was successful in providing reassurance that they need not worry about it (Box 1, quote 5).
Is “normal” dependent on age?
Observational data support an age-related understanding of declining glomerular filtration rate. Population-based studies consistently show that prevalence of CKD depends strongly on age.15 For example, prevalence increases approximately 10-fold between young adulthood and middle age in the US Kidney Early Evaluation Program16 and continues to increase into old age.17 In the US Third National Health and Nutrition Examination Survey, Coresh and colleagues plotted estimated glomerular filtration rate against age.18 The median and the 5th and 95th centiles declined continuously with age across participants from age 20 to 90 years.
Kidney age, not kidney disease?
These observations suggest that declining kidney function could better be communicated to patients in the language of “kidney age” rather than “chronic kidney disease.” Similar terminology has been used previously to communicate current health or health risk to patients. Spiegelhalter has previously reviewed concepts of “heart age,” “brain age” and “lung age” that are based on risk of future disease.19 Groenewegen, in a review of proposed “heart age” and “vascular age” metrics, distinguished those based on multivariate prediction of future risk from those based on a single current indicator of vascular health such as carotid intima media thickness.20 In the latter approach, the vascular age of an individual with a given carotid intima media thickness value is the age at which the median carotid intima media thickness in a healthy population is this value.
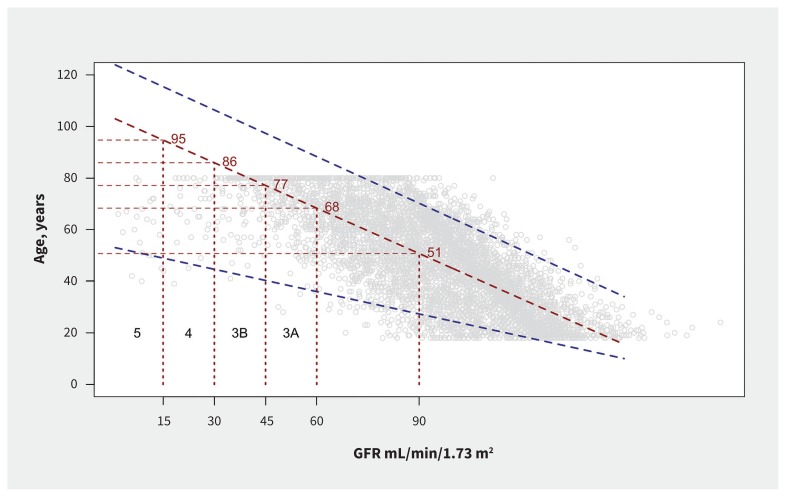
We replotted the graphs of Coresh and colleagues,18 using data from the most recent (2015/16) National Health and Nutrition Examination Survey (Figure 1), reversing the axes to propose a mapping from glomerular filtration rate as an indicator of renal health to age bands. CKD stage 3A, for example, could be communicated as “kidney age 68 to 77 years,” and stage 3B as “kidney age 77 to 86 years.” The term “chronic kidney disease” would be reserved for those with later stages: in particular, stage 5, or symptomatic stage 4.
Figure 1:
Percentiles of age by estimated glomerular filtration rate (GFR) (mL/min/1.73m2), from the National Health and Nutrition Examination Survey 2015 to 2016. Red line denotes median age for a given GFR and blue lines denote 5th and 95th centiles.
Previous authors have argued that failure to account for age in the definition of CKD stages is a “paradox” that leads to “persistent and serious criticism” of successive CKD classification systems.21 Rather than add complexity to definitions, we hypothesize that adjusting terminology would in many cases avoid unnecessary anxiety, while still signalling concern where appropriate. When estimated “kidney age” is approximately concordant with calendar age, we hope that patients will (as some already do) understand the decline as a natural aging process. Conversely, when “kidney age” is older than calendar age, this should be communicated to patients, with discussion about increased risk of future cardiovascular and renal disease. In either case, we hypothesize that the language of “kidney age” will reduce misunderstandings that arise from the jargon of “chronic” kidney “disease.”
We argue that the earlier stages (up to stage 4) of “chronic kidney disease” are better described as kidney aging, but we do not state that there is no such thing as kidney disease. Many pathologies affect the kidney and have existing nomenclature (e.g., nephrotic syndrome, polycystic kidneys) beyond the scope of our argument. Proteinuria can occur even with high glomerular filtration rate, and so a normal or low kidney age does not preclude the need to investigate urine protein when indicated (for example, by diabetes or hypertension). Conversely, a decreased glomerular filtration rate, communicated as “increasing kidney age,” remains an indication for further investigation and monitoring, and “kidney age” should be taken into account in management and prescribing decisions.
Next steps
Our proposal is only for a change in terminology, to overcome existing problems with describing declining glomerular filtration rate as “disease.” The limitations of existing methods for estimating glomerular filtration rate from serum creatinine apply equally whether the results are communicated to patients as “chronic kidney disease stage” or “kidney age.” The latter, however, is likely to have greater resonance with patients, as has the similar concept of “heart age” or “vascular age.” Usefully, existing clinical and prescribing guidelines, clinical action plans and approaches to managing cardiovascular risk would remain unchanged.
In our analysis of previous studies, we used data from a North American study for convenience; consideration should be given to whether kidney age should be defined separately in different populations; for example, in different ethnic groups. Given a candidate definition of kidney age, further work is then merited to test our hypothesis that this concept has advantages over existing terminology. Interviews or focus groups with patients and health care providers should investigate the face validity and acceptability of the “kidney age” concept, and explore other alternatives: “age-related” or “age-associated” kidney “dysfunction” or “decline,” for example. If results are encouraging, then intervention studies could examine the change in terminology as an intervention to improve patient–doctor communication in the first instance, and patient understanding as a consequence. Some hoped-for outcomes might be demonstrable on a large scale; for example, an increase in awareness among the proportion of patients on registers (such as the CKD register mandated by the UK Quality Outcomes Framework).
The required change in language would need to be widespread, ideally reaching clinical guidelines (and software) and information materials for the public and for patients, but it would be minor, in that it does not in principle require changes to practice, or to the content (as opposed to the language) of clinical guidelines. Potential advantages include better understanding in those with impaired but age-appropriate kidney function; similarly, readier understanding in those with age-inappropriate kidney function and, hence, greater readiness of primary care clinicians to discuss declining glomerular filtration rate with both groups; prevention of confusion or distress arising from the jargon term “chronic”; and reservation of the term “disease” for the stages of kidney dysfunction that are associated with direct harms — and for which treatment exists. Although these are consistent with the qualitative research findings discussed above, we list them as potential, rather than proven, advantages, and plan for further development and testing of the kidney age concept in the near future.
Acknowledgements
The authors thank the Stakeholder Group and Steering Committee of our NIHR Programme Grant for discussions that inspired this paper. We are grateful to Dr. Tim Holt and to the reviewers for suggestions that have improved the text.
Footnotes
This article has been peer reviewed.
Competing interests: Richard Stevens reports acting as an independent statistician on a trial data monitoring committee for Novartis, outside the submitted work. Rafael Perera reports that his department has received funding from the National Institute for Health Research Programme Grants for Applied Research, during the conduct of the study.
Contributors: Richard Stevens, Julie Evans, Elizabeth Holloway, Louise Locock, Marion Judd, Julie McLellan and Rafael Perera conceived the Analysis paper. Richard Stevens and Benjamin Smart designed and conducted the conceptual analysis. Julie Evans, Jeremy Horwood and Louise Locock designed and conducted the qualitative analysis. Richard Stevens and Jason Oke designed and conducted the quantitative analysis. F.D. Richard Hobbs provided clinical analysis and interpretation throughout. Richard Stevens and Julie Evans drafted the manuscript, and all authors contributed to further drafting and revision. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for the work.
Funding: This analysis arises from independent research funded by the National Institute for Health Research (NIHR)’s School for Primary Care Research (Project Reference No. NSPCR ID FR4.120) and the Programme Grant for Applied Research Programme (Ref. No. RP-PG-1210-12003). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The work of some authors (Hobbs, Locock, Perera) was partially supported by the NIHR Biomedical Research Centre, Oxford. F.D. Richard Hobbs acknowledges partial funding support by the NIHR School for Primary Care Research, and the NIHR Collaboration for Leadership in Applied Health Research and care, Oxford.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- 2.Arora P, Vasa P, Brenner D, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ 2013;185:E417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–266. [PubMed] [Google Scholar]
- 4.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- 5.Chronic kidney disease in adults: assessment and management. London (UK): National Institute for Health and Care Excellence; 2014:CG182 Available: www.nice.org.uk/guidance/cg182 (accessed 2017 Feb. 8). [PubMed] [Google Scholar]
- 6.Chronic Kidney Disease Prognosis Consortium Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–35. [DOI] [PubMed] [Google Scholar]
- 8.Smart B. Concepts and causes in the philosophy of disease. 1st ed Palgrave Pivot; 2015. [Google Scholar]
- 9.Do concepts of disease apply to “chronic kidney disease”? [presentation]. Proceedings of the conference on Too Much Medicine: Exploring the Relevance of Philosophy of Medicine to Medical Research and Practice; 2017 Apr. 19–20. [Google Scholar]
- 10.Crinson I, Gallagher H, Thomas N, et al. How ready is general practice to improve quality in chronic kidney disease? A diagnostic analysis. Br J Gen Pract 2010;60:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmonds R, Evans J, Feder G, et al. Understanding tensions and identifying clinician agreement on improvements to early-stage chronic kidney disease monitoring in primary care: a qualitative study. BMJ Open 2016;6:e010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daker-White G, Rogers A, Kennedy A, et al. Non-disclosure of chronic kidney disease in primary care and the limits of instrumental rationality in chronic illness self-management. Soc Sci Med 2015;131:31–9. [DOI] [PubMed] [Google Scholar]
- 13.Blakeman T, Protheroe J, Chew-Graham C, et al. Understanding the management of early-stage chronic kidney disease in primary care: a qualitative study. Br J Gen Pract 2012;62:e233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.People’s experiences: long term conditions — kidney health. 2017. Available: www.healthtalk.org/peoples-experiences/long-term-conditions/kidney-health/topics (accessed 2017 Mar. 10).
- 15.Zhang Q-L, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown WW, Peters RM, Ohmit SE, et al. Early detection of kidney disease in community settings: the kidney early evaluation program (KEEP). Am J Kidney Dis 2003;42:22–35. [DOI] [PubMed] [Google Scholar]
- 17.Garg AX, Papaioannou A, Ferko N, et al. Estimating the prevalence of renal insufficiency in seniors requiring long-term care. Kidney Int 2004;65:649–53. [DOI] [PubMed] [Google Scholar]
- 18.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:1–12. [DOI] [PubMed] [Google Scholar]
- 19.Spiegelhalter D. How old are you, really? Communicating chronic risk through “effective age” of your body and organs. BMC Med Inform Decis Mak 2016;16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groenewegen KA, Den Ruijter HM, Pasterkamp G, et al. Vascular age to determine cardiovascular disease risk: a systematic review of its concepts, definitions, and clinical applications. Eur J Prev Cardiol 2016;23:264–74. [DOI] [PubMed] [Google Scholar]
- 21.Glassock R, Delanaye P, El Nahas M. An age-calibrated classification of chronic kidney disease. JAMA 2015;314:559–60. [DOI] [PubMed] [Google Scholar]