Abstract
Heavy use of alcohol can lead to addictive behaviors and to eventual alcohol related tissue damage. While increased consumption of alcohol has been attributed to various factors including level of alcohol exposure and environmental factors such as stress, data from behavioral scientists and physiological researchers is revealing roles for the circadian rhythm in mediating the development of behaviors associated with alcohol use disorder as well as the tissue damage that drives physiological disease. In this work, we compile recent work on the complex mutually influential relationship that exists between the core circadian rhythm and the pharmacodynamics of alcohol. As we do so, we highlight implications of the relationship between alcohol and common circadian mechanisms of effected organs on alcohol consumption, metabolism, toxicity, and pathology.
Alcohol use in Modern Society
According to the Centers for Disease Control and Prevention (CDC), heavy alcohol use is constituted, in women, by a daily habit of 2 standard drinks (28g of alcohol) or by binging 4 drinks during a 2 hour period (Esser et al., 2014). In men, the standard is increased to 4 drinks daily or 5 drinks in less than 2 hours for a binge. These numbers are important because, in an alarming trend, they increasingly characterize the manner in which Americans consume alcohol. In 2012, a national study revealed that 8.2% of all Americans were considered heavy drinkers and 18.3% were binge drinkers, a nearly 10% increase over 2005 numbers (Dwyer-Lindgren et al., 2015). Over-consumption was highest (26%) in individuals 18–34 years of age. Characterized by the compulsive use of alcohol and anxiety associated with abstaining from alcohol, Alcohol use disorders like alcoholism and binge drinking have been linked by the World Health Organization to over 204 diseases in humans (WHO, 2014). Due in part to lost productivity, in 2010, the cost of Americans drinking to excess rose to a quarter of a trillion U.S. dollars with nearly 80% of these costs attributed to binge drinking (Control et al., 2016).
Byproducts of alcohol metabolism can result in detrimental biological effects. During alcohol metabolism by alcohol dehydrogenase and/or cytochrome P450 (CYP2E1) reactive oxygen species and other free radicals are generated that can react with critical biological molecules including amino acids, lipids, and nucleic acids to interfere with cellular mechanisms (Caro and Cederbaum, 2004). The resulting alcohol metabolism-derived oxidative stress can induce cytotoxicity, a systemic inflammatory response and damage to healthy tissues. Tissue damage resulting from heavy alcohol use, either chronically or through acute exposure, has broad medical ramifications. This is because the degree of injury, level of recovery, and possibility of clinical intervention to alcohol’s effects are affected by the degree of exposure. With documented pathological impact on the brain, heart, lungs, liver, pancreas, gastrointestinal tract and skeleton, the negative health effects of alcohol is now well established (Bruha et al., 2012; Haorah et al., 2005; Kershaw et al., 2008; Keshavarzian et al., 1999; Sampson, 1998).
An intriguing observation is that only 30% of alcoholics develop organ damage like intestinal leakiness to endotoxin and alcoholic liver disease (ALD) indicating that although excessive alcohol consumption is required it is not sufficient to cause tissue injury and additional factor(s) is required for end organ damage. Compelling studies provide evidence that disrupted circadian rhythm may be one factor that promotes alcohol-induced tissue injury in a subset of alcohol consumers. A better understanding of the underlying molecular and cellular mechanisms of alcohol-induced toxicity and tissue injury are essential because it will lead to identification of novel therapeutic target(s) that should result in more targeted and effective treatment strategies to mitigate the toxic effects of alcohol and prevent and/or treat alcohol-induced end organ damage. One of the biological systems that may be the key to understanding many of effects of alcohol on behavioral and physiology is the circadian rhythm. Significant bidirectional interactions between alcohol and circadian rhythms has been known for some time (Adan, 1994; Holloway et al., 1993). These human and rodent studies have shown that circadian machinery not only influences craving and alcohol consumption, but it can also modify alcohol-induced effects on behavior, physiology and tissue injury (Adan, 1994; Holloway et al., 1993). Below, we will provide an overview of bidirectional interaction of alcohol and circadian system and provide evidence that the disruption of circadian rhythms makes alcohol consumers susceptible to alcohol-induced effects, thus potentiating alcohol-induced tissue injury, organ dysfunction and end organ damage.
Circadian Rhythms
To organize biological activities of daily life, mammals have evolved a ‘master clock’ that resides above the optic chasm of the hypothalamus in the brain that functions as the primary regulator for all circadian-directed activities in the body (Klein et al., 1991). This small collection of about 10,000 cells, known as the suprachiasmatic nucleus (SCN), receives information about ambient light signals through the eye’s photosensitive receptors and the nerves of the retinohypothalamic tract, entraining the circadian system by utilizing the excitatory neurotransmitter glutamate (Kim et al., 2005; Klein et al., 1991). Through direct and indirect methods, the SCN presides over circadian clocks in cells and tissues of the brain, the gastrointestinal tract (GIT) and other organs (Buijs et al., 2001).
Behavior, the sleep cycle, food anticipation, hormone regulation, food metabolism, microbiota-gut interplay, and epithelial barrier function have been shown to be subject to circadian regulation (Malloy et al., 2012; Rosselot et al, 2016; Sadacca et al., 2011). Organ systems that are subordinate to the SCN, such as the intestine, may also use external or tissue-specific signals as temporal cues to influence circadian rhythms (Mohawk et al., 2012). These peripheral clocks can therefore have rhythms that are different from that of the master clock. An instance of this can be seen in the GIT and food consumption. Time of eating is a zeitgeber (time setter) for both the GIT and the liver, and a shifted eating schedule (e.g., consuming food at different times each day or consuming a majority of calories late in the day or at night) can result in a misaligned circadian rhythm of the digestive system from central circadian time (Shi and Zheng, 2013). The misalignment of central and peripheral clocks has been linked to metabolic dysfunction in humans and rodents (Morris et al., 2012).
Components of the Molecular Circadian Clock
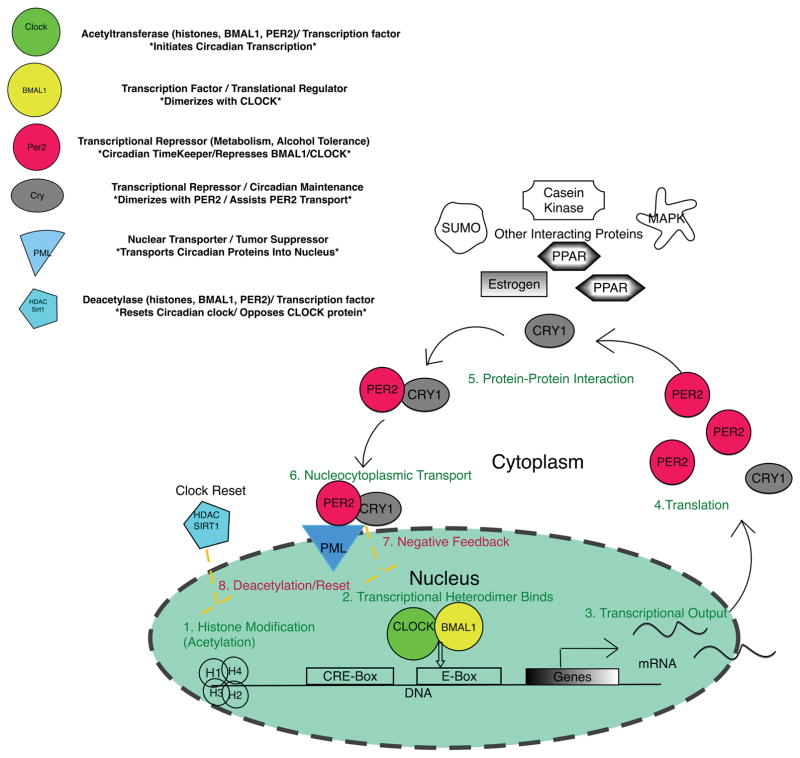
The primary driver of the molecular circadian clock that is present in virtually all cells as well as the SCN is a negative feedback mechanism involving the transcription and translation (Hut and Beersma, 2011; Tomita, 2005). The genes at the core of the circadian rhythm vary with taxonomy but in mammals, the basic clock includes genes that code for CLOCK, NPAS2, BMAL1, PER1-3, CRY1-2, ROR and REV-ERBA proteins (Bunger et al., 2000). The transcription factor CLOCK (Circadian Locomotor Output Cycles Kaput), is an important component of the circadian clock (Ko and Takahashi, 2006). Either by acting as a transcription factor, through intrinsic acetylation capacity, or through the recruitment of co-activator proteins with their own histone acetyltransferase (HAT) abilities, the CLOCK protein begins to control the circadian clock by participating in the remodeling of chromatin (Curtis et al., 2004; Doi et al., 2006). CLOCK-mediated DNA acetylation is followed by PAS-to-PAS domain binding with BMAL1 (Brain and Muscle ARNT-Like 1) to promote the removal of nucleosomes at DNA-binding sites. This is followed by the initiation of transcription of the core clock genes as well as large number of clock-controlled genes (CCGs) within the tissue transcriptome (Bozek et al., 2009; Etchegaray et al., 2003; Menet et al., 2014).
The CLOCK-BMAL1 heterodimer maintains circadian clock rhythmicity, binding to the enhancer box of core circadian genes including Period (Per1, 2, & 3) and Cryptochrome (CRY1&2) to induce transcription (Huang et al., 2012). NPAS2 (Neuronal PAS-domain containing protein 2) has its greatest importance in the CNS where under Clock-deficient conditions NPAS2 can substitute for CLOCK allowing the molecular cycle of the SCN to function normally, though peripheral tissues cannot maintain circadian rhythmicity without a functioning Clock gene (Debruyne, 2008; DeBruyne et al., 2007). Constitutively produced, CLOCK is important to some parts of the circadian cycle, however, the cellular availability of BMAL1, which is cyclically generated appears to be a driver of circadian transcription and behavior as the mutation of this gene in mice has been shown to result in the complete loss of circadian rhythmicity (Bunger et al., 2000; Stratmann et al., 2012). Phenotypically, Clock mutant mice reveal the scope and the critical nature of the circadian rhythm with chronic sleep reduction, altered locomotor activity, disrupted behavioral patterns, circadian dysregulation, arrhythmic transcription of core circadian genes and CCGs, as well as various symptoms traditionally associated with metabolic syndrome (obesity, insulin resistance, hypertriglyceridemia, liver hepatomegaly, steatosis, etc.) (Turek et al., 2005).
BMAL1 itself is a critical part of the circadian clock where it functions as a translation factor. Recent research reveals that BMAL1 associates with translational proteins in the cytoplasm to promote protein synthesis (Lipton et al., 2015). Critically the protein is rhythmically phosphorylated by kinases (mTOR-effector kinase, ribosomal S6 protein kinase 1 (S6K1)) to induce its participation in stimulate protein synthesis. Like NPAS can substitute for CLOCK, BMAL1 has been found to be conditionally dispensable with its paralog, Bmal2, becoming a viable substitute in its absence (Shi et al., 2010).
Once it is translated in the cytoplasm, PER2 protein activity within the core clock is enhanced by the CRY1 protein, which stabilizes PER2 and enhances the rate of PER2 nuclear transport (Vanselow et al., 2006). Inside the nucleus, the PER2-CRY1 heterodimer bind to the CLOCK-BMAL1 heterodimer closing the negative circadian feedback loop and inhibiting further transcription of core circadian genes. The RAR-related orphan receptors ROR-a and REV-ERB-A close another feedback loop by regulating the transcription of BMAL1. Forming the positive/negative arm respectively of the feedback cycle, nuclear receptors ROR-a and REV-ERB-A are trafficked in to the nucleus to compete against one another to either activate or repress BMAL1 transcription (Guillaumond et al., 2005). SIRTUIN1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent regulatory protein that effectively reverses the actions of CLOCK protein. SIRT1 deacetylates circadian proteins PER2 and BMAL1 to detach the CLOCK-BMAL1 dimer from E-boxes of circadian genes; it also removes acetyl groups from histones switching DNA to a closed conformation to reset the circadian cycle (Asher et al., 2008).
Dynamic trafficking of molecular clock components between the cytoplasm and the nucleus is required for proper functioning of the circadian clock making nuclear transport proteins critical to the maintenance of circadian rhythms (Herrero and Davis, 2012). Pharmacological inhibition of the nucleocytoplasmic shuttling proteins that aid in circadian protein PER2 trafficking leads to disruption of circadian regulation in vitro (ÖWe prop et al., 2014; Tamaru et al., 2003). A recent study analyzing the transcriptomes of 12 mouse organs found that, accounting for tissue specific variation, over 40% of gene transcription in the mouse showed rhythmic circadian expression (Zhang et al., 2014). Evaluation of circadian dynamics reveals that manipulation or inhibition of circadian protein kinetics can disrupt function or even abolish the rhythmic expression of circadian genes Aguilar-Arnal and Sassone-Corsi, 2015; Stratmann et al., 2012; Wallach et al., 2013).
It should be noted that, in order to maintain their respective roles in temporal regulation, circadian proteins interact with partner proteins outside of the core clock. Many of these proteins have been shown to assert influence within the circadian rhythm by fine-tuning the clock through multiple post-translational modifications (phosphorylation, ubiquitination, acetylation, SUMOylation) (Gallego and Virshup, 2007). Post-translational modifiers include: casein kinases (CSNK (or CK)I-2), Glycogen synthase kinase 3 (GSK3), adenosine monophosphate-activated protein kinase (AMPK), calcium/calmodulin-dependent protein kinase CAMK-1,2) mitogen activated protein kinase (MAPK1) protein phosphatases (PP1, PP2A) and others that have been shown to induce or alter circadian protein activity or binding (Reischl and Kramer, 2011; Um et al., 2011; Wang et al., 2011). Critical interacting proteins include receptors such as estrogen, peroxisome proliferator-activated receptor (PPAR), and others that link circadian rhythms to the reproductive, metabolic and other biological processes and have also been shown to exert influence the clock (Gery et al., 2007; Grimaldi et al., 2010; McElroy et al., 2009). These interactive partner proteins (e.g., CSNK1, AMPK, GSK, MAPK) could be the targets of future research and clinical interventions maximizing their influence on circadian regulation.
Effects of Alcohol on Elements of the Clock
Alcohol metabolism by CYP2E1 is induced with high levels of alcohol. Exposure of rat intestinal tissue to high concentrations of alcohol has been shown to drive the release of oxygen radicals compared to alcohol metabolism by the alcohol dehydrogenase pathway in HEPG2 in vitro liver cell lines (Cederbaum et al., 2001; Pronko, 2002). The reactive oxygen species generated by CYP2E1 can exceed cellular defense systems resulting in oxidative stress and various pathologic consequences for several systems including the circadian rhythm (Caro and Cederbaum, 2004). Many constituent proteins of the circadian core clock (CLOCK, BMAL1, PER1-3, CRY1-2) contain redox-sensing PAS domains it has been therefore suggested the alcohol-mediated oxidative stress is likely to have an effect on the circadian clock as well as downstream effects on the various biological systems that circadian rhythms regulate.
The expression of the core PAS-domain containing circadian proteins (CLOCK, BMAL1, PER1, PER2, CRY1, and CRY2) are affected by the presence of alcohol with the expression of each protein altered in the blood of human alcoholics compared to controls (Huang et al., 2010). An in vitro study revealed that alcohol metabolism mediated oxidative stress induces an increase in the circadian proteins CLOCK and PER2 and this aberrant increase in circadian proteins leads to further dysfunction downstream (Davis et al., 2017). It is through this mechanism of altering circadian expression and rhythmicity that alcohol may influence a myriad of modified circadian attributes such as altered photic phase-resetting within the SCN (Ruby et al., 2009).
The disruptive effect of alcohol on the circadian rhythms in the peripheral organs may be more powerful than its effect on the central rhythm in the SCN. In a 2013 study, the SCN and liver tissue were extracted from male Per2Luc mice and PER2 protein expression was examined ex vivo using luminescence. Analysis of bioluminescence as revealed that in the presence of alcohol the circadian rhythm in the SCN, as measured by PER2, was not disrupted, however, in the liver, alcohol induced a significant phase advance of core circadian and clock-controlled diurnal gene expression accompanied by altered lipid metabolism indicated by hepatic steatosis (Filiano et al., 2013).
More studies demonstrate an effect of alcohol on both central and peripheral circadian rhythms. Recently published work by our group monitored the blood, urine, and activity of healthy groups of day and nightshift workers who were social alcohol drinkers before and after 7 days of daily consumption of red wine (0.5 gram/kg, 1–2 glasses of wine) (Swanson et al., 2016). After wine consumption, nightshift workers had phase-delayed blood melatonin production (a marker of central circadian rhythm) and mononuclear blood cell circadian gene expression (marker of peripheral CR), effects not observed in day shift workers consuming alcohol, demonstrating that even moderate alcohol consumption can exacerbate central and peripheral circadian rhythm disruptions in subset of subjects who already have shifted circadian rhythms. This study confirmed the hypothesis that disrupted circadian rhythms (here due to shift work) can make the drinker susceptible to alcohol’s negative physiological effects.
Disruption or manipulation of the molecular circadian clock has a myriad of detrimental health effects including effects on behavior, cardiovascular disease, metabolic syndrome, and cancer, just to name a few. Comprehensive reviews of this literature are available elsewhere (Asher and Sassone-Corsi, 2015; Morris et al., 2012; Yu and Weaver, 2011), but in this review we are focusing on the effects of the manipulation of the circadian clock on alcohol consumption, alcohol metabolism, and alcohol-induced tissue injury and organ dysfunction..
Circadian Rhythms and Alcohol Consumption
The influence of alcohol over the circadian rhythm appears to be bilateral with research revealing that the circadian clock can affect alcohol preference, consumption, and dependence and each of these factors having an effect on circadian expression. In experiments to characterize whether the circadian rhythm alters alcohol intake, the free-running periods of rats and mice genetically predisposed to prefer alcohol differ in length from those of non-preferring animals (Hofstetter et al., 2003; Rosenwasser and Fixaris, 2013; Rosenwasser et al., 2005). Likewise, circadian rhythms can be manipulated through alterations in environmental cues (i.e., light, temperature), to induce changes in alcohol intake (Spanagel et al., 2005a).
The level of expression of core clock genes have been shown to influence alcohol intake and addiction. One group found that the selective reduction of mouse CLOCK expression in the brain’s ventral tegmental area (VTA) or the mutation of CLOCK result in significantly increased alcohol consumption (Ozburn et al., 2013). Multiple other studies have shown connections between the AUD of patients and the altered expression of core clock proteins (CLOCK, BMAL1 &2, CRY1&2, PER1&2) in peripheral blood cells (Huang et al., 2010; Kovanen et al., 2010; McCarthy et al., 2013). The strongest molecular link between circadian activity and alcohol consumption may be Per2, with mutations in Per2 linked to both circadian disturbance and increased alcohol intake in mice and in humans (Comasco et al., 2010; Spanagel et al., 2005b). The findings in this study have been buttressed by subsequent studies illustrating a critical involvement of Per1 in alcohol consumption, metabolism, and addiction (Dong et al., 2011; Gamsby et al., 2013). It should be noted that although there is strong evidence suggesting that PER influences alcohol consumption there has been conflicting literature ruling out PER1-related alcohol reinforcement (Zghoul et al., 2007).
One group analyzed the expression of core circadian genes in men to determine whether there is a connection between the circadian rhythm and alcohol use disorder (AUD) (Ando et al., 2010). A negative relationship was found to exist between alcohol consumption and levels of Per2. The results confirm the findings in other mouse studies identifying a relationship between PER2 and alcoholism (Huang et al., 2010; McCarthy et al., 2013; Sarkar, 2012; Sjoholm et al., 2010). Notably, there was also an inverse relationship between alcohol use and Bmal1 gene expression in blood cells linking another core circadian constituent to AUD and alcohol-induced suggesting impairment of circadian clock at a critical point in the cycle. The core clock is multifaceted and dynamic with contrasting differences across organ systems so further study on the effect of other components on alcohol consumption is required.
Circadian Rhythms and Alcohol Metabolism
While alcohol elimination was traditionally considered a zero-order process, it has become increasingly clear that the breakdown of alcohol is guided by the time-of-day-dependent oscillations of the circadian rhythm much like other aspects of metabolism (Cederbaum, 2012). Many of the enzymes responsible for alcohol metabolism (CYP2E1, ADH3, 4) have been observed to have a high Michaelis constant (Km) having a lower alcohol binding affinity and requiring a higher alcohol concentration to reach ½ of their maximum reaction rate (Vmax) (Holford, 1987; Ramchandani et al., 2001). These enzymes also have a diurnal variations in their activity levels (Baraldo, 2008; Gachon and Firsov, 2011) Taken together these mean concentration and time-dependent variations in alcohol metabolism that are influenced by elements of the circadian rhythm.
Circadian clocks regulate metabolism through the timed production of enzymes and the modulation of pathway specific endocrine factors (Bailey et al., 2014). This regulation includes the production of protective antioxidant enzymes necessary for the biological amelioration of certain re-dox states which have been linked to activity by of the CLOCK BMAL1 heterodimer (Kondratov et al., 2006, 2009). Oscillations in transcription and protein activity of ROS detoxifiers superoxide dismutase’s (SOD), catalase, glutathione peroxidase (GPx), glutathione (GSH), GSH-s-transferase, and glutamyl cysteine ligase (GCL) have been reported in mice and rats (Fonzo et al., 2009; Xu et al., 2012). These data indicate possible circadian-dependent susceptibilities to alcohol that need to be further characterized in future studies.
Circadian proteins CLOCK and BMAL1 are also associated with increased expression of Cyp2e11. Cyp2e1 mRNA was found to be increased in the livers of alcohol-exposed mice Per2 and Cry1 were linked to levels of CYP2E1 protein expression (Forsyth et al., 2013; Matsunaga et al., 2008) (Matsunaga et al., 2008). There appears to be a mutually influential relationship between the circadian system and the proteins it regulates because, in another mouse study, the siRNA knockdown of Cyp2e1 prevented the alcohol-mediated induction of CLOCK and PER2 proteins (Forsyth et al., 2013). Studies also reveal daily fluctuations in NAD+, a cofactor for CYP2E1-mediated alcohol metabolism, influencing circadian gene expression and alcohol metabolism through histone acetylation and methylation. In this way, alcohol induced depletion of NAD+ inhibits the deacetylase activity of SIRT1 (Etchegaray et al., 2003; Sahar et al., 2011; Thompson et al., 2015).
Studies into SIRT1 function have shown that inhibition by NAD+ depletion could lead to alterations in the rhythmic conversion of chromatin that permits binding of the CLOCK/BMAL1 heterodimer effectively changing the pace of the circadian cycle and likely the expression of circadian regulated enzymes and cofactors like NAD+ and CYP2E1 (Belden and Dunlap, 2008; Ripperger and Schibler, 2006). Using these direct (enzyme expression) and indirect (cofactor cycling) mechanisms the circadian rhythm asserts influence on the rate of alcohol metabolism, and by extension, the production of the damaging oxidative radicals that are produced through the breakdown of alcohol. Focusing attention on these time-dependent changes may allow researchers to highlight the amount of alcohol induced tissue damage that occur throughout the day and adjust the strength of interventions accordingly.
Circadian Rhythms and Alcohol Toxicity
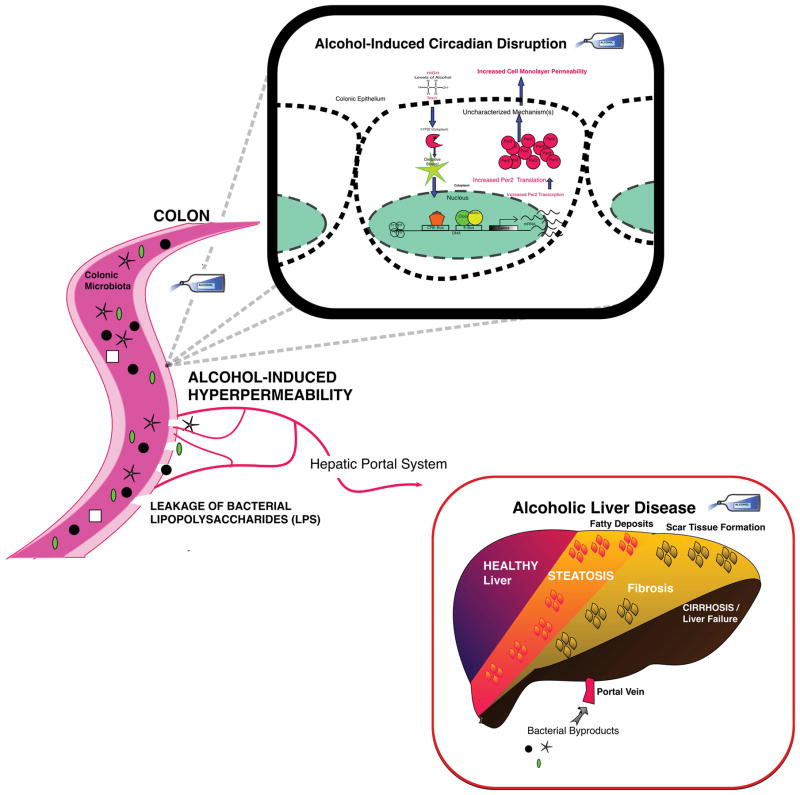
Chronic alcohol consumption has been shown in patients to induce damage in multiple tissue types in the body from the heart muscle (i.e., cardiomyopathy) to peripheral nerves (i.e., peripheral neuropathy) to the brain that reveals impeded neuronal growth, nutritional deficits, and shrinking white and gray matter under MRI with long-term heavy drinking (Ammendola et al., 2000; Haorah et al., 2005; Piano and Phillips, 2014; Rosenbloom et al., 2003) Currently however, most extensive research characterizing the role of circadian mechanisms in the development of tissue damage is takes place in the area of the liver and intestine; studies into the role of circadian mechanisms in the alcohol-induced damage seen in other organs would be valuable. Current evidence suggests that disruption of circadian rhythms either induced by alcohol, by genetic manipulation, or environmentally (e.g., alterations in light:dark cycles) is critical to increase the susceptibility of the colon and liver to alcohol-induced tissue injury and play a direct role in the severity of their alcohol induced pathology (Keshavarzian et al., 1999; Swanson et al., 2011).
Studies in mice demonstrate that disruption of circadian rhythms promote alcohol-induced effects on the GIT and liver. Wild-type C57BL6/J mice subjected to weekly circadian disruption induced via once weekly alterations in the light:dark cycle (i.e., 12:12hr inversion) have more intestinal hyperpermeability, endotoxemia, and liver steatosis compared to non-circadian rhythm disrupted controls (Summa et al., 2013). Similar results were observed in diurnally arrhythmic mice harboring a mutation in the Clock gene (i.e., ClockΔ19) (Summa et al., 2013). Indeed, both environmental and genetic circadian disruptions promote alcohol-induced effects on intestinal tight junctions. These disruptions allow the transfer of pro-inflammatory bacterial byproducts across the intestinal barrier, a critical step required for inflammation and liver pathology. Analysis of intestinal epithelial cells (i.e., Caco-2 cell model) reveals that disruption of the molecular circadian clock is vital for alcohol-induced effects on intestinal permeability. Specifically, siRNA knockdown of Clock or Per2 prevents the alcohol-mediated increase in intestinal permeability that is necessary for the development and progression through the stages of ALD (Steatosis, Fibrosis, Cirrhosis, then Liver Failure) (Forsyth et al., 2013; Swanson et al., 2011). These data point to a critical importance of an intact and proper functioning circadian clock in the intestine since disruption of the clock promotes alcohol-induced pathology.
Studies in humans are equally as compelling as those in mice. Several epidemiological and clinical studies suggest that disruptions to circadian homeostasis makes organs such as the liver and intestine more susceptible to alcohol toxicity. Consistent with binge studies in mice, multiple studies in human alcoholics have shown altered circadian gene expression that is delayed from their wake-sleep routine and blunted compared to healthy controls (Danel et al., 2003; Huang et al., 2010). These alcohol-mediated changes are usually attributed to an AUD and also shown to interfere with the ability of the person to limit consumption (Kovanen et al., 2010).
Reminiscent of mouse results these studies also reveal alcohol induced physiological changes as a result of circadian disruption. Healthy participants in one study with a reliable drinking pattern, working either a night or a day shift in nursing, filled out questionnaires and submitted blood and urine for analysis throughout the study. This work, published by our group, shows that participants working the night shift showed increases in alcohol induced intestinal barrier dysfunction, endotoxemia and elevated pro-inflammatory cytokine IL-6 compared to day shift workers without circadian rhythm disruption (Swanson et al., 2016). More work is necessary to establish the causal link between disrupted circadian rhythms and worsening of alcohol-induced pathology. Further investigation could include interventional studies to determine if the normalization of disrupted circadian rhythms by circadian-directed intervention (e.g., chronotherapeutics, pharmaceutical drugs, prebiotics, probiotics, etc) can prevent/mitigate alcohol-induced end organ damage like gut leakiness and steatohepatitis in man.
Conclusion
As a small, interactive molecule with almost unfettered access to every tissue alcohol has been linked to much pathology. Abstinence is the most effective means to prevent alcohol pathology; however, the promotion of abstinence through educational programs is difficult to achieve because of the craving that accompanies alcohol addiction. Circadian rhythms are linked to alcohol craving/addiction as well as alcohol consumption, therefore circadian-directed interventions such as light adaptation stimuli, chronobiotics (e.g. melatonin), dietary intake (the time of meal consumption and probiotics) or drugs targeting the circadian proteins may be opportunities to address alcohol addiction and increase adherence to alcohol abstinence. Observations of differential susceptibility to alcohol-mediated organ damage indicate that AUD is a requirement but insufficient to disease development and the circadian rhythm appears to be a critical factor.
The studies provided offer compelling evidence that the interplay between alcohol and the proteins of the circadian rhythm is key to understanding alcohol-induced pathologies. Again, this indicates the potential utility of circadian-directed interventions to normalize circadian misalignment a promising intervention to prevent and treat alcohol pathology. Further studies will be necessary to determine the ability of circadian-directed therapies to mitigate alcohol-mediated pathology. The ability of an intervention to override alcohol induced transcription, translation, transport and/or the activity of circadian components will be paramount. It may be necessary to investigate therapies that can redirect the reactive oxidative species associated with alcohol or adjuvants that can mediate downstream effects of this metabolic process.
Figure 1.
Main Protein Components of Core Circadian Clock
The core circadian clock is a transcriptional-translational feedback loop that is modulated Circadian rhythms are generated by intracellular transcriptional-transcriptional feedback loops that temporally synchronizes or separates biological processes through interaction, modification, and trafficking of proteins.
Figure 2.
Role of Core Circadian Clock in Alcohol-Induced Pathology: Alcohol mediates disruption of circadian rhythm mechanism(s) inducing the increased intestinal permeability that is critical to the development and progression of alcoholic liver disease.
Acknowledgments
The studies were supported by NIH grants: AA020216S1 (AK, BD), AA023417S2 (AK, BD) AA020216 (AK, CBF, RV), AA023417 (AK)
Contributor Information
Booker T Davis, IV, Rush University Medical Center, 1725 W Harrison St, Suite 206, Chicago IL 60612, 312-942-0721.
Robin M. Voigt, Rush University Medical Center, 1725 W Harrison St, Suite 206, Chicago IL 60612, 312-942-8973
Maliha Shaikh, Rush University Medical Center, 1725 W Harrison St, Suite 206, Chicago IL 60612, 312-942-8798
Christopher B. Forsyth, Rush University Medical Center, 1725 W Harrison St, Suite 206, Chicago IL 60612, 312-942-9009
Ali Keshavarzian, Josephine M. Dyrenforth Chair of Gastroenterology, Director, Division of Digestive Disease and Nutrition, Rush University Medical Center, Department of Internal Medicine, Section of Gastroenterology, 1725 W Harrison St, Suite 206, Chicago IL 60612, Phone: 312-563-3890 (Direct Line), Phone: 312-563-4175 (Admin. Ms. Denise Labedz), Fax: 312-563-3883
Bibliography
- Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction. 1994;89:455–462. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- Aguilar-Arnal L, Sassone-Corsi P. Chromatin landscape and circadian dynamics: Spatial and temporal organization of clock transcription. Proc Natl Acad Sci. 2015;112:6863–6870. doi: 10.1073/pnas.1411264111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola A, Gemini D, Iannaccone S, Argenzio F, Ciccone G, Ammendola E, Serio L, Ugolini G, Bravaccio F. Gender and peripheral neuropathy in chronic alcoholism: a clinical-electroneurographic study. Alcohol Alcohol Oxf Oxfs. 2000;35:368–371. doi: 10.1093/alcalc/35.4.368. [DOI] [PubMed] [Google Scholar]
- Ando H, Ushijima K, Kumazaki M, Eto T, Takamura T, Irie S, Kaneko S, Fujimura A. Associations of metabolic parameters and ethanol consumption with messenger RNA expression of clock genes in healthy men. Chronobiol Int. 2010;27:194–203. doi: 10.3109/07420520903398617. [DOI] [PubMed] [Google Scholar]
- Asher G, Sassone-Corsi P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell. 2015;161:84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Udoh US, Young ME. CIRCADIAN REGULATION OF METABOLISM. J Endocrinol. 2014;222:R75–R96. doi: 10.1530/JOE-14-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldo M. The influence of circadian rhythms on the kinetics of drugs in humans. Expert Opin Drug Metab Toxicol. 2008;4:175–192. doi: 10.1517/17425255.4.2.175. [DOI] [PubMed] [Google Scholar]
- Belden WJ, Dunlap JC. SIRT1 Is a Circadian Deacetylase for Core Clock Components. Cell. 2008;134:212–214. doi: 10.1016/j.cell.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozek K, Relógio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H. Regulation of Clock-Controlled Genes in Mammals. PLOS ONE. 2009;4:e4882. doi: 10.1371/journal.pone.0004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruha R, Dvorak K, Petrtyl J. Alcoholic liver disease. World J Hepatol. 2012;4:81–90. doi: 10.4254/wjh.v4.i3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–526. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 Is an Essential Component of the Master Circadian Pacemaker in Mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative Stress, Toxicology, and Pharmacology of Cyp2e1*. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI. ALCOHOL METABOLISM. Clin Liver Dis. 2012;16:667–685. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum AI, Wu D, Mari M, Bai J. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells 1, 2. Free Radic Biol Med. 2001;31:1539–1543. doi: 10.1016/s0891-5849(01)00743-2. [DOI] [PubMed] [Google Scholar]
- Comasco E, Nordquist N, Göktürk C, Åslund C, Hallman J, Oreland L, Nilsson KW. The clock gene PER2 and sleep problems: Association with alcohol consumption among Swedish adolescents. Ups J Med Sci. 2010;115:41–48. doi: 10.3109/03009731003597127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Control, C. for D., Prevention, and others. Excessive drinking is draining the US economy. Natl Cent Chronic Dis Prev Health Promot Div Popul Health. 2016 HttpwwwCdcGovfeaturescostsofdrinkingPubl. [Google Scholar]
- Curtis AM, Seo S, Westgate EJ, Rudic RD, Smyth EM, Chakravarti D, FitzGerald GA, McNamara P. Histone Acetyltransferase-dependent Chromatin Remodeling and the Vascular Clock. J Biol Chem. 2004;279:7091–7097. doi: 10.1074/jbc.M311973200. [DOI] [PubMed] [Google Scholar]
- Danel T, Jeanson R, Touitou Y. Temporal pattern in consumption of the first drink of the day in alcohol-dependent persons. Chronobiol Int. 2003;20:1093–1102. doi: 10.1081/cbi-120025533. [DOI] [PubMed] [Google Scholar]
- Davis BT, Voigt RM, Shaikh M, Forsyth CB, Keshavarzian A. CREB Mediates Alcohol-Induced Circadian Disruption and Intestinal Permeability. Alcohol Clin Exp Res. 2017 doi: 10.1111/acer.13513. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne JP. Oscillating perceptions: the ups and downs of the CLOCK protein in the mouse circadian system. J Genet. 2008;87:437–446. doi: 10.1007/s12041-008-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Hirayama J, Sassone-Corsi P. Circadian Regulator CLOCK Is a Histone Acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Desrivieres S, Clarke TK, Lourdusamy A, Smolka MN, et al. Effects of the Circadian Rhythm Gene Period 1 (Per1) on Psychosocial Stress-Induced Alcohol Drinking. Am J Psychiatry. 2011;168:1090–1098. doi: 10.1176/appi.ajp.2011.10111579. [DOI] [PubMed] [Google Scholar]
- Dwyer-Lindgren L, Flaxman AD, Ng M, Hansen GM, Murray CJL, Mokdad AH. Drinking Patterns in US Counties From 2002 to 2012. Am J Public Health. 2015;105:1120–1127. doi: 10.2105/AJPH.2014.302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of Alcohol Dependence Among US Adult Drinkers, 2009–2011. Prev Chronic Dis. 2014:11. doi: 10.5888/pcd11.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- Filiano AN, Millender-Swain T, Johnson R, Young ME, Gamble KL, Bailey SM. Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PloS One. 2013;8:e71684. doi: 10.1371/journal.pone.0071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo LSN, Golini RS, Delgado SM, Ponce IT, Bonomi MR, Rezza IG, Gimenez MS, Anzulovich AC. Temporal patterns of lipoperoxidation and antioxidant enzymes are modified in the hippocampus of vitamin A-deficient rats. Hippocampus. 2009;19:869–880. doi: 10.1002/hipo.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Voigt RM, Shaikh M, Tang Y, Cederbaum AI, Turek FW, Keshavarzian A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am J Physiol-Gastrointest Liver Physiol. 2013;305:G185–G195. doi: 10.1152/ajpgi.00354.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F, Firsov D. The role of circadian timing system on drug metabolism and detoxification. Expert Opin Drug Metab Toxicol. 2011;7:147–158. doi: 10.1517/17425255.2011.544251. [DOI] [PubMed] [Google Scholar]
- Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Gamsby JJ, Templeton EL, Bonvini LA, Wang W, Loros JJ, Dunlap JC, Green AI, Gulick D. The circadian Per1 and Per2 genes influence alcohol intake, reinforcement, and blood alcohol levels. Behav Brain Res. 2013;249:15–21. doi: 10.1016/j.bbr.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene. 2007;26:7916–7920. doi: 10.1038/sj.onc.1210585. [DOI] [PubMed] [Google Scholar]
- Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaumond F, Dardente H, Giguère V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Herrero E, Davis SJ. Time for a nuclear meeting: protein trafficking and chromatin dynamics intersect in the plant circadian system. Mol Plant. 2012;5:554–565. doi: 10.1093/mp/sss010. [DOI] [PubMed] [Google Scholar]
- Hofstetter JR, Grahame NJ, Mayeda AR. Circadian activity rhythms in high-alcohol-preferring and low-alcohol-preferring mice. Alcohol. 2003;30:81–85. doi: 10.1016/s0741-8329(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Holford NH. Clinical pharmacokinetics of ethanol. Clin Pharmacokinet. 1987;13:273–292. doi: 10.2165/00003088-198713050-00001. [DOI] [PubMed] [Google Scholar]
- Holloway FA, Miller JM, King DA, Bedingfield JB. Delayed ethanol effects on physiological and behavioral indices in the rat. Alcohol. 1993;10:511–519. doi: 10.1016/0741-8329(93)90075-y. [DOI] [PubMed] [Google Scholar]
- Huang MC, Ho CW, Chen CH, Liu SC, Chen CC, Leu SJ. Reduced expression of circadian clock genes in male alcoholic patients. Alcohol Clin Exp Res. 2010;34:1899–1904. doi: 10.1111/j.1530-0277.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- Huang N, Chelliah Y, Shan Y, Taylor CA, Yoo SH, Partch C, Green CB, Zhang H, Takahashi JS. Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science. 2012;337:189–194. doi: 10.1126/science.1222804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut RA, Beersma DGM. Evolution of time-keeping mechanisms: early emergence and adaptation to photoperiod. Philos Trans R Soc Lond B Biol Sci. 2011;366:2141–2154. doi: 10.1098/rstb.2010.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw CD, Guidot DM. Alcoholic lung disease. Alcohol Res Health. 2008;31:66. [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Kim DY, Choi HJ, Kim JS, Kim YS, Jeong DU, Shin HC, Kim MJ, Han HC, Hong SK, Kim YI. Voltage-gated calcium channels play crucial roles in the glutamate-induced phase shifts of the rat suprachiasmatic circadian clock. Eur J Neurosci. 2005;21:1215–1222. doi: 10.1111/j.1460-9568.2005.03950.x. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind’s Clock. Oxford University Press; 1991. [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging. 2009;1:979–987. doi: 10.18632/aging.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lönnqvist J, Partonen T. Circadian Clock Gene Polymorphisms in Alcohol Use Disorders and Alcohol Consumption. Alcohol Alcohol. 2010;45:303–311. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- Lipton JO, Yuan ED, Boyle LM, Ebrahimi-Fakhari D, Kwiatkowski E, Nathan A, Güttler T, Davis F, Asara JM, Sahin M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell. 2015;161:1138–1151. doi: 10.1016/j.cell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy JN, Paulose JK, Li Y, Cassone VM. Circadian rhythms of gastrointestinal function are regulated by both central and peripheral oscillators. Am J Physiol Gastrointest Liver Physiol. 2012;303:G461–73. doi: 10.1152/ajpgi.00369.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga N, Ikeda M, Takiguchi T, Koyanagi S, Ohdo S. The molecular mechanism regulating 24-hour rhythm of CYP2E1 expression in the mouse liver. Hepatol Baltim Md. 2008;48:240–251. doi: 10.1002/hep.22304. [DOI] [PubMed] [Google Scholar]
- McCarthy MJ, Fernandes M, Kranzler HR, Covault JM, Welsh DK. Circadian clock period inversely correlates with illness severity in cells from patients with alcohol use disorders. Alcohol Clin Exp Res. 2013;37:1304–1310. doi: 10.1111/acer.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy B, Zakaria A, Glass JD, Prosser RA. ETHANOL MODULATES MAMMALIAN CIRCADIAN CLOCK PHASE RESETTING THROUGH EXTRASYNAPTIC GABA RECEPTOR ACTIVATION. Neuroscience. 2009;164:842–848. doi: 10.1016/j.neuroscience.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet JS, Pescatore S, Rosbash M. CLOCK:BMAL1 is a pioneer-like transcription factor. Genes Dev. 2014;28:8–13. doi: 10.1101/gad.228536.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. CENTRAL AND PERIPHERAL CIRCADIAN CLOCKS IN MAMMALS. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Scheer FAJL. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–358. doi: 10.1016/B978-0-444-59427-3.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öllinger R, Korge S, Korte T, Koller B, Herrmann A, Kramer A. Dynamics of the circadian clock protein PERIOD2 in living cells. J Cell Sci. 2014;127:4322–4328. doi: 10.1242/jcs.156612. [DOI] [PubMed] [Google Scholar]
- Ozburn AR, Falcon E, Mukherjee S, Gillman A, Arey R, Spencer S, McClung CA. The Role of Clock in Ethanol-Related Behaviors. Neuropsychopharmacology. 2013;38:2393. doi: 10.1038/npp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano MR, Phillips SA. Alcoholic Cardiomyopathy: Pathophysiologic Insights. Cardiovasc Toxicol. 2014;14:291–308. doi: 10.1007/s12012-014-9252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronko P. EFFECT OF CHRONIC ALCOHOL CONSUMPTION ON THE ETHANOL- AND ACETALDEHYDE-METABOLIZING SYSTEMS IN THE RAT GASTROINTESTINAL TRACT. Alcohol Alcohol. 2002;37:229–235. doi: 10.1093/alcalc/37.3.229. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bosron WF, Li TK. Research advances in ethanol metabolism. Pathol Biol (Paris) 2001;49:676–682. doi: 10.1016/s0369-8114(01)00232-2. [DOI] [PubMed] [Google Scholar]
- Reischl S, Kramer A. Kinases and phosphatases in the mammalian circadian clock. FEBS Lett. 2011;585:1393–1399. doi: 10.1016/j.febslet.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Res Health J Natl Inst Alcohol Abuse Alcohol. 2003;27:146–152. [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC. Chronobiology of alcohol: studies in C57BL/6J and DBA/2J inbred mice. Physiol Behav. 2013;110–111:140–147. doi: 10.1016/j.physbeh.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW, Reed JD, Cotter SJN, Seggio JA. Circadian activity rhythms in selectively bred ethanol-preferring and nonpreferring rats. Alcohol. 2005;36:69–81. doi: 10.1016/j.alcohol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Rosselot AE, Hong CI, Moore SR. Rhythm and bugs: Circadian clocks, gut microbiota, and enteric infections. Curr Opin Gastroenterol. 2016;32:7–11. doi: 10.1097/MOG.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol - Regul Integr Comp Physiol. 2009;297:R729–R737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Nin V, Barbosa MT, Chini EN, Sassone-Corsi P. Altered behavioral and metabolic circadian rhythms in mice with disrupted NAD+ oscillation. Aging. 2011;3:794–802. doi: 10.18632/aging.100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson HW. Alcohol’s harmful effects on bone. Alcohol Health Res World. 1998;22:190–194. [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK. Circadian Genes, the Stress Axis, and Alcoholism. Alcohol Res Curr Rev. 2012;34:362–366. [PMC free article] [PubMed] [Google Scholar]
- Shi M, Zheng X. Interactions between the circadian clock and metabolism: there are good times and bad times. Acta Biochim Biophys Sin. 2013;45:61–69. doi: 10.1093/abbs/gms110. [DOI] [PubMed] [Google Scholar]
- Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian Clock Gene Bmal1 Is Not Essential; Functional Replacement with its Paralog, Bmal2. Curr Biol. 2010;20:316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoholm L, Kovanen L, Saarikoski S, Schalling M, Lavebratt C. CLOCK is suggested to associate with comorbid alcohol use and depressive disorders. J Circadian Rhythms. 2010:8. doi: 10.1186/1740-3391-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol Consumption and the Body’s Biological Clock. Alcohol Clin Exp Res. 2005a;29:1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005b;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Stratmann M, Suter DM, Molina N, Naef F, Schibler U. Circadian Dbp Transcription Relies on Highly Dynamic BMAL1-CLOCK Interaction with E Boxes and Requires the Proteasome. Mol Cell. 2012;48:277–287. doi: 10.1016/j.molcel.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, Vitaterna MH, Song S, Turek FW, Keshavarzian A. Disruption of the Circadian Clock in Mice Increases Intestinal Permeability and Promotes Alcohol-Induced Hepatic Pathology and Inflammation. PLoS ONE. 2013;8:e67102. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson G, Forsyth CB, Tang Y, Shaikh M, Zhang L, Turek FW, Keshavarzian A. Role of intestinal circadian genes in alcohol-induced gut leakiness. Alcohol Clin Exp Res. 2011;35:1305–1314. doi: 10.1111/j.1530-0277.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GR, Gorenz A, Shaikh M, Desai V, Kaminsky T, Berg JVD, Murphy T, Raeisi S, Fogg LF, Vitaterna MH, et al. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am J Physiol - Gastrointest Liver Physiol. 2016 doi: 10.1152/ajpgi.00087.2016. ajpgi.00087.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru T, Isojima Y, Van Der Horst GTJ, Takei K, Nagai K, Takamatsu K. Nucleocytoplasmic shuttling and phosphorylation of BMAL1 are regulated by circadian clock in cultured fibroblasts. Genes Cells. 2003;8:973–983. doi: 10.1046/j.1365-2443.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- Thompson KJ, Humphries JR, Niemeyer DJ, Sindram D, McKillop IH. The effect of alcohol on Sirt1 expression and function in animal and human models of hepatocellular carcinoma (HCC) Adv Exp Med Biol. 2015;815:361–373. doi: 10.1007/978-3-319-09614-8_21. [DOI] [PubMed] [Google Scholar]
- Tomita J. No Transcription-Translation Feedback in Circadian Rhythm of KaiC Phosphorylation. Science. 2005;307:251–254. doi: 10.1126/science.1102540. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JH, Pendergast JS, Springer DA, Foretz M, Viollet B, Brown A, Kim MK, Yamazaki S, Chung JH. AMPK Regulates Circadian Rhythms in a Tissue- and Isoform-Specific Manner. PLOS ONE. 2011;6:e18450. doi: 10.1371/journal.pone.0018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanselow K, Vanselow JT, Westermark PO, Reischl S, Maier B, Korte T, Herrmann A, Herzel H, Schlosser A, Kramer A. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS) Genes Dev. 2006;20:2660–2672. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach T, Schellenberg K, Maier B, Kalathur RKR, Porras P, Wanker EE, Futschik ME, Kramer A. Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions. PLOS Genet. 2013;9:e1003398. doi: 10.1371/journal.pgen.1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liang Y, Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett. 2011;585:986–994. doi: 10.1016/j.febslet.2010.11.047. [DOI] [PubMed] [Google Scholar]
- World Health Organization, (WHO) Global status report on alcohol and health - 2014. World Health Organization; 2014. [Google Scholar]
- Xu YQ, Zhang D, Jin T, Cai DJ, Wu Q, Lu Y, Liu J, Klaassen CD. Diurnal variation of hepatic antioxidant gene expression in mice. PloS One. 2012;7:e44237. doi: 10.1371/journal.pone.0044237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. 2011 doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R. Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1Brdm1 mutant mice. Psychopharmacology (Berl) 2007;190:13–19. doi: 10.1007/s00213-006-0592-z. [DOI] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]