Abstract
Background
Postoperative delirium is a common preventable complication experienced by older adults undergoing elective surgery. In this systematic review and meta-analysis, we identified prognostic factors associated with the risk of postoperative delirium among older adults undergoing elective surgery.
Methods
Medline, EMBASE, CINAHL, Cochrane Central Register of Controlled Trials, and AgeLine were searched for articles published between inception and April 21, 2016. A total of 5692 titles and abstracts were screened in duplicate for possible inclusion. Studies using any method for diagnosing delirium were eligible. Two reviewers independently completed all data extraction and quality assessments using the Cochrane Risk-of-Bias Tool for randomized controlled trials (RCTs) and the Newcastle-Ottawa Scale (NOS) for cohort studies. Random effects meta-analysis models were used to derive pooled effect estimates.
Results
Forty-one studies (9384 patients) reported delirium-related prognostic factors. Among our included studies, the pooled incidence of postoperative delirium was 18.4% (95% confidence interval [CI] 14.3–23.3%, number needed to follow [NNF] = 6). Geriatric syndromes were important predictors of delirium, namely history of delirium (odds ratio [OR] 6.4, 95% CI 2.2–17.9), frailty (OR 4.1, 95% CI 1.4–11.7), cognitive impairment (OR 2.7, 95% CI 1.9–3.8), impairment in activities of daily living (ADLs; OR 2.1, 95% CI 1.6–2.6), and impairment in instrumental activities of daily living (IADLs; OR 1.9, 95% CI 1.3–2.8). Potentially modifiable prognostic factors such as psychotropic medication use (OR 2.3, 95% CI 1.4–3.6) and smoking status (OR 1.8 95% CI 1.3–2.4) were also identified. Caregiver support was associated with lower odds of postoperative delirium (OR 0.69, 95% CI 0.52–0.91).
Discussion
Though caution must be used in interpreting meta-analyses of non-randomized studies due to the potential influence of unmeasured confounding, we identified potentially modifiable prognostic factors including frailty and psychotropic medication use that should be targeted to optimize care.
Electronic supplementary material
The online version of this article (10.1007/s11606-017-4204-x) contains supplementary material, which is available to authorized users.
KEY WORDS: delirium, elective surgery, perioperative medicine, older adults, prognostic factors
INTRODUCTION
As our population ages, there will be a greater need for elective surgeries among older adults; however, elective surgeries can be associated with significant harm.1 , 2 Delirium is one of the most common complications of surgery among older adults, and has been associated with prolonged hospitalization, death, and admission to long-term care.2 – 4 Multifactorial interventions with a focus on optimizing mobility, vision, hearing, hydration, cognition, and sleep have proven to be effective delirium prevention strategies in medical and surgical settings.5 – 7 Therefore, identifying patients at risk of delirium is important because clinicians can develop plans to mitigate this risk. Although older adults are seen in the preoperative medicine clinic for cardiovascular and respiratory risk optimization before an elective surgery, often not enough consideration is given to risk stratification for adverse outcomes that are more common in older adults, such as delirium—despite evidence to aid in its assessment and of effective interventions for its prevention.6 , 8
Understanding delirium risk factors may help clinicians, patients, and caregivers in targeting non-pharmacological and pharmacological interventions aimed at lessening its burden. The purpose of our systematic review is to identify preoperative patient characteristics of older adults undergoing elective surgery that either predispose them to or protect them from developing postoperative delirium. We also present the pooled prevalence of postoperative delirium and the risk of delirium-related adverse outcomes from these studies. This information can be used by clinicians and patients to enhance medical decision-making and by researchers to study possible interventions aimed at lowering the incidence of postoperative delirium among older adults undergoing elective surgery.
METHODS
This study was reported in accordance with both the PRISMA statement for reporting systematic reviews and meta-analyses and the MOOSE statement for reporting meta-analysis of observational studies in epidemiology.9 , 10 This systematic review and meta-analysis has a companion report under review at the time of publication that focuses on complications of elective surgery among older adults, excluding delirium.
Eligibility Criteria
Prospective studies (e.g., RCTs, non-randomized controlled trials, prospective cohort studies) were eligible if they included older adults undergoing elective surgery (all patients ≥60 years old and mean patient age ≥65 years) and reported prognostic factors associated with postoperative delirium or delirium-related adverse outcomes. We limited our search to prospective studies because delirium is not well-captured in patient charts or electronic databases.11 Studies using any method for diagnosing postoperative delirium were eligible, and all definitions of each prognostic factor were included. Studies that included patients ≥60 years old were selected to align with definitions from the United Nations and the World Health Organization.12 , 13 Studies reporting only clinical, laboratory, or imaging investigations that are not conducted as part of routine clinical practice (i.e., measuring serum interleukin levels) were excluded, as were studies disseminated in languages other than English.
Information Sources and Search Strategy
An experienced librarian searched MEDLINE (OVID interface, 1948 to April week 3, 2016), EMBASE (OVID interface, 1980 to April week 3, 2016), CINAHL (EBSCO interface, 1994 to April 21, 2016), Cochrane Central Register of Controlled Trials (issue 4, April 2016), and AgeLine (EBSCO interface, 1968 to April 21, 2016) for potentially relevant studies. The full search strategy for MEDLINE (eSearch 1 in Supplement 1) was modified as necessary for the other databases (full searches available upon request). The electronic search was supplemented by scanning the reference lists of included studies, searching the authors’ personal files, and contacting authors of conference proceedings.
Study Selection
Two levels of screening were completed independently by two reviewers using Synthesi.SR (proprietary online software developed by the Knowledge Translation Program, Toronto, Canada): (1) screening of titles and abstracts and (2) full-text screening of articles. Initially, each reviewer independently screened 10% of a random sample of citations to ensure adequate inter-rater agreement. Study authors were contacted for further information if it was unclear whether the study met inclusion criteria. Disagreements concerning article inclusion were resolved through discussion; otherwise, a third reviewer was available to make a final decision.
Data Abstraction
Two reviewers abstracted data independently from studies retained from level 2 screening. Study characteristics (e.g., study design) and prognostic factors associated with postoperative delirium were abstracted from included studies. Definitions operationalized by study authors for individual prognostic factors were also abstracted, where appropriate (eTable 1 in Supplement 1). Conflicts regarding the abstracted data were resolved through discussion. Authors were contacted for further information when the data were not clearly reported. When multiple studies reported data from the same source, the publication with the longest duration of follow-up was considered the major publication. If the duration of follow-up was the same for two or more publications, the study published first was considered the major publication.
Methodological Quality Assessment
Two reviewers independently completed the quality assessment for each study using the Cochrane Risk-of-Bias Tool for RCTs or the Newcastle-Ottawa Scale (NOS) for cohort studies.14 , 15 We planned to assess other study designs with the Cochrane Effective Practice and Organization Care (EPOC) Risk-of-Bias Tool.16
Statistical Methods
We calculated odds ratios (ORs), mean differences (MDs), or standardized mean differences (SMDs) to quantify the relative risk of prognostic factors associated with postoperative delirium and delirium-related complications. Whenever only continuous effect measures, such as MDs (e.g., age, body mass index) were reported by study authors, these effect measures were transformed to OR estimates, if needed, to derive an overall effect estimate that combined both dichotomous and continuous study-level effect estimates.17 For studies that reported multiple options with which to derive the effect estimate (e.g., 2 × 2 tables, adjusted and unadjusted ORs, MDs), the order of preference for selecting the source data is described in eTable 2 in Supplement 1.
Random effects models were used to pool incidences of delirium and derive overall effect estimates with 95% CIs when two or more studies reported extractable effect estimates. The NNF was calculated as 1/pooled incidence of delirium. Information regarding data imputation methods to approximate mean and standard deviation values is found in eMethods 1 in Supplement 1. Between-study statistical heterogeneity was quantitatively assessed with the I2 statistic and thresholds for its interpretation were consistent with those reported in the Cochrane Handbook for Systematic Reviews of Interventions.18 Subgroup analyses were conducted to explore sources of substantial between-study heterogeneity based on patient age and the type of elective surgery. A prognostic factor was considered significantly associated with postoperative delirium at a two-tailed p-value <0.05.
Prognostic factors that were reported in at least 10 studies were assessed for publication bias by visual inspection of funnel plots for asymmetry and with the Egger’s test.18 , 19 Where there was evidence of significant publication bias (p < 0.05), the trim-and-fill method was used to estimate the number of missing studies and to derive the combined effect estimates, adjusting for publication bias.20 All statistical analyses were conducted in R, version 3.2.4, using the metafor and meta packages.21 , 22
RESULTS
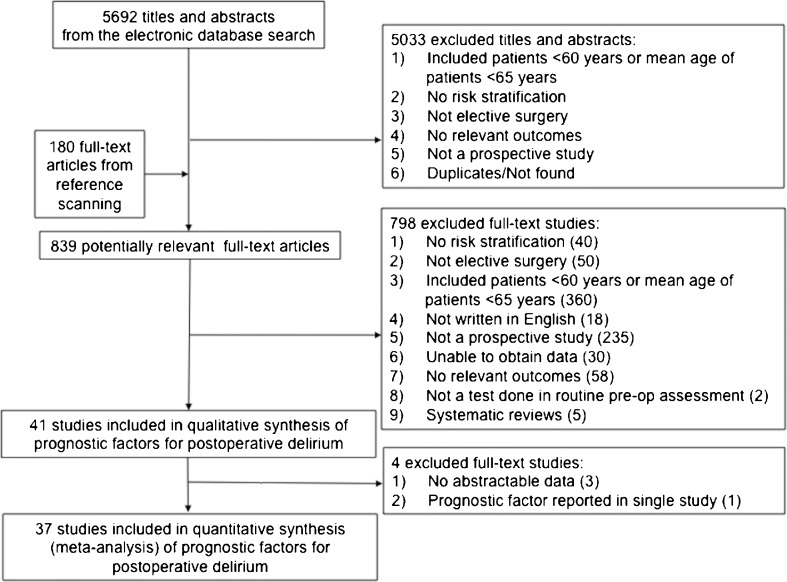
Of the 5692 titles and abstracts that were screened for inclusion, we identified 41 studies (9384 patients) that reported prognostic factors associated with the risk of developing postoperative delirium and its consequences (Fig. 1 and Table 1). Of these, 37 studies (8557 patients) were included in meta-analyses.1 – 3 , 23 – 47 , 49 – 52 , 56 – 60 In the four studies not included in our meta-analyses, data relating to prognostic factors were either not reported in an abstractable format or were reported in only one study.61 – 64 One additional study was excluded from analysis as there were data inconsistencies that precluded calculation of effect measures and we were not able to obtain further information from the study authors (see eMethods 2 in Supplement 1). One RCT was included, which was at moderate to high risk of bias (eTable 3 in Supplement 1). Overall, the included cohort studies were of moderate to high methodological quality (eTable 4 in Supplement 1). The most common biases were the adequacy of follow-up of cohorts and the failure to demonstrate that delirium was not present prior to surgery. Delirium was diagnosed by the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria (48.8%), the Confusion Assessment Method (CAM) (24.4%), both the DSM and CAM criteria (4.9%), and other methods. The method of diagnosis was not stated in 4 studies (Table 1).65 , 66
Figure 1.
Study flow diagram outlining the number of studies 1) retrieved in our literature search, 2) excluded from our systematic review at each level of screening, and 3) included in our qualitative and quantitative synthesis of preoperative prognostic factors associated with developing postoperative delirium.
Table 1.
Characteristics of Prospective Studies Reporting Risk Factors for Postoperative Delirium Among Older Adults Undergoing Elective Surgery
| Study | Number of patients | Age range (years) | % Female | Type of surgery | Criteria for delirium | Length of follow-up (weeks) | Cases of incident delirium | |
|---|---|---|---|---|---|---|---|---|
| No. | % | |||||||
| Rogers, 198923 | 46 | ≥60 | 67.4 | Orthopedic | Psychiatric interview for DSM-III criteria for delirium | 0.57 | 13 | 28.3 |
| Fisher, 199524 | 80 | ≥60 | 53.8 | Orthopedic | CAM | 0.57 | 14 | 17.5 |
| Rolfson, 199925 | 71 | ≥65 | 21.3 | Cardiac | CAM | 0.57 | 23 | 32.4 |
| Eriksson, 200226 | 52 | ≥60 | 23.1 | Cardiac | OBS and CAM | 0.71 | 12 | 23.1 |
| Kudoh, 200427 | 328 | 65–80 | 81.1 | Orthopedic | CAM | 1 | 49 | 14.9 |
| Santos, 200428 | 220 | 61–87 | 35.4 | Cardiac | DSM-IV criteria for delirium | 0.71 | 74 | 33.6 |
| Freter, 200529 | 132 | >65 | 65.2 | Orthopedic | CAM | Until hospital discharge | 18 | 13.6 |
| Fukuse, 200530 | 120 | 60–84 | 40 | Thoracic | NR | Until hospital discharge | 3 | 2.5 |
| Olin, 200531 | 51 | ≥65 | 39.2 | Abdominal | CAM and medical records | Until hospital discharge | 26 | 51 |
| Papaioannou, 200532 | 47 | ≥60 | 36.2 | Orthopedic, urological, vascular, gynecological | DSM-III criteria for delirium | 0.43 | 9 | 19.2 |
| Rudolph, 200733 | 1161 | ≥60 | 46.8 | Orthopedic, thoracic, abdominal, vascular, other noncardiac | DSM-III criteria for delirium | 1 | 99 | 8.5 |
| Veliz-Reissmuller, 20072 | 104 | ≥60 | 38.3 | Cardiac | CAM | NR | 25 | 23.4 |
| Priner, 200834 | 101 | ≥60 | 57.4 | Orthopedic | CAM | NR | 15 | 14.8 |
| Dasgupta, 200935 | 125 | 70–92 | 58 | Orthopedic, vascular, abdominal, neurosurgical | Chart abstraction method | NR | 15 | 12 |
| Hattori, 200922 | 160 | >75 | NR | Gastrointestinal, orthopedic, vascular | NEECHAM score < 20 | 1.43 | 87 | 54.7 |
| Koebrugge, 200936 | 71 | ≥65 | 47.9 | Abdominal | DOS and DSM-IV criteria | NR | 17 | 23.9 |
| Morimoto, 200937 | 20 | >65 | 30 | Abdominal | DRS ≥ 12 and DSM-IV criteria for delirium | 1 | 5 | 25 |
| Brouquet, 201038 | 118 | 75–98 | 52.5 | Abdominal | CAM | 0.57 | 28 | 23.7 |
| Kristjansson, 201039 | 178 | ≥70 | 57 | Colorectal | NR | 4 | 14 | 7.9 |
| Cerejeira, 201140 | 101 | 60–89 | 50.5 | Orthopedic | CAM and DSM-IV-TR confirmation | 0.43 | 37 | 36.6 |
| Clement, 201141 | 1343 | 80–93 | 59.2 | Orthopedic | NR | NR | 17 | 1.3 |
| Jankowski, 201142 | 418 | ≥65 | 50.7 | Orthopedic | CAM | 12 | 42 | 10 |
| Patti, 20113 | 100 | >65 | 60 | Colorectal | CAM | Until hospital discharge | 18 | 18 |
| Tognoni, 201143 | 90 | 66–93 | 10 | Urological | CAM | 1 | 8 | 8.9 |
| Bakker, 20121 | 201 | ≥70 | 39.8 | Cardiac | CAM-ICU | 1 or until hospital discharge | 63 | 31.3 |
| Flink, 201244 | 106 | ≥65 | 55.7 | Orthopedic | CAM and DSM-IV criteria | 0.43 | 27 | 25.5 |
| Robinson, 201245 | 186 | ≥65 | 4.3 | Abdominal, cardiac, non-cardiac thoracic, vascular | CAM-ICU | NR | 102 | 54.8 |
| Sasajima, 201246 | 299 | 61–91 | 12.7 | Vascular | CAM | NR | 88 | 29.4 |
| Gani, 201347 | 640 | ≥65 | 3.9 | Urological | CAM | NR | 166 | 25.9 |
| Kim, 201348 | 141 | NR | 39.9 | General, urological, gynecological, thoracic, breast, ophthalmologic, ENT | DSM-IV criteria | NR | 14 | 9.9 |
| Large, 201349 | 49 | ≥65 | 18.4 | Urological | CAM | 1 | 14 | 28.6 |
| Leung, 201350 | 581 | 65–96 | 50.2 | Orthopedic, urological, gynecological, vascular, thoracic, ENT, plastic, general | CAM | 0.28 | 234 | 40.3 |
| Otomo, 201351 | 153 | ≥60 | 28.8 | Cardiac | DRS and DSM-IV criteria for delirium | 1 post-extubation | 16 | 10.4 |
| Kosar, 201452 | 459 | ≥70 | 59.7 | Orthopedic | CAM or validated chart-based method | Until hospital discharge | 106 | 23.1 |
| Suh, 201453 | 60 | 70–85 | 100 | Gynecological, general | >2/5 criteria: disorientation, inappropriate behavior, inappropriate words, delusion/hallucination, delayed psychomotor activity | 4 | 4 | 6.7 |
| Blakoe, 201554 | 79 | ≥70 | NR | Cardiac | NR | NR | NR | NR |
| Min, 201555 | 62 | 65–90 | 29 | Cardiac | New symptoms of confusion, agitation, and/or altered mental status, or new need for antipsychotic medications | NR | 9 | 14.5 |
| Raats, 201556 | 83 | 65–89 | 42.2 | Colorectal | DOS | NR | 15 | 18.1 |
| Visser, 201557 | 463 | ≥60 | 23.1 | Vascular | DOS and DSM-IV criteria | NR | 22 | 5 |
| Hempenius, 201658 | 227 | >65 | 61.9 | Abdominal, gynecological, ENT, maxillofacial | DOS and DSM-IV criteria | 13 | 26 | 10 |
| Xue, 201659 | 358 | ≥65 | 0 | Urological | CAM | 1 | 28 | 7.8 |
Abbreviations: CAM, Confusion Assessment Method; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; DOS, Delirium Observation Scale; DRS, Delirium Rating Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; NEECHAM, Neelon and Champagne Confusion Scale; NR, not reported; OBS, Organic Brain Syndrome Scale
Incidence of Postoperative Delirium
The pooled incidence of postoperative delirium among included studies was 18.7% (95% CI 14.6–23.5%, 40 studies, 9305 patients, I2 = 95.6%, NNF = 6). The pooled incidence of delirium varied by surgical type: cardiac (23.6%, 95% CI 17–31.9%, 7 studies, I2 = 81.5%, NNF = 5), orthopedic (15.2%, 95% CI 8.6–25.5%, 10 studies, I2 = 95.3%, NNF = 7), general (26.6%, 95% CI 19.4–35.2%, 7 studies, I2 = 75.2%, NNF = 4), vascular (15.8%, 95% CI 7.7–29.5%, 4 studies, I2 = 94.2%, NNF = 7), and urological surgery (12.7%, 95% CI 1.8–53.8%, 2 studies, I2 = 98.6%, NNF = 8).
Prognostic Factors
A history of delirium, frailty, and cognitive impairment were most strongly associated with developing postoperative delirium (Table 2 and eFigure 1 in Supplement 1). Other factors associated with developing postoperative delirium included psychotropic medication use, smoking status, older age, ASA status, and impairment in ADLs and IADLs. The availability of caregiver support was associated with a lower odds of postoperative delirium.
Table 2.
Prognostic Factors for the Development of Postoperative Delirium Among Older Adults Undergoing Elective Surgery
| Prognostic factor | Number of studies | Number of patients | Odds ratio (95% CI) | Heterogeneity (I2) |
|---|---|---|---|---|
| History of delirium | 3 | 242 | 6.4 (2.2–17.9) | 0 |
| Frailty | 2 | 303 | 4.1 (1.4–11.7) | 0 |
| Cognitive impairment | 22 | 3982 | 2.7 (1.9–3.8) | 72.9 |
| Renal insufficiency | 5 | 1336 | 2.3 (1.1–4.8) | 70.9 |
| Psychotropic medication use | 6 | 1041 | 2.3 (1.4–3.6) | 0 |
| Psychiatric history | 5 | 1512 | 2.2 (1.5–3.1) | 0 |
| Older age | 27 | 6200 | 2.2 (1.6–3.2) | 94.8 |
| ADL impairment | 9 | 1881 | 2.1 (1.6–2.6) | 0 |
| IADL impairment | 2 | 699 | 1.9 (1.3–2.8) | 5.2 |
| Cerebrovascular disease | 7 | 2205 | 1.8 (1.2–2.7) | 0 |
| Smoking status | 9 | 2467 | 1.8 (1.3–2.4) | 0 |
| ASA status | 10 | 2482 | 1.7 (1.2–2.4) | 29.3 |
| Low education | 8 | 1973 | 1.5 (1.1–2.0) | 19.6 |
| Diabetes mellitus | 11 | 2924 | 1.4 (1.0–2.0) | 36.6 |
| Neurological disease | 3 | 981 | 1.4 (1.0–1.9) | 0 |
| Charlson comorbidity index | 7 | 1588 | 0.40 (0.07–0.7)* | 82.5 |
| Caregiver support | 6 | 1378 | 0.69 (0.52–0.9) | 0 |
| Male sex | 25 | 5430 | 1.1 (0.76–1.4) | 62.6 |
| Alcohol consumption | 12 | 3198 | 0.84 (0.57–1.2) | 45.1 |
| Dyslipidemia | 5 | 784 | 0.71 (0.50–1.0) | 0 |
| Hypertension | 9 | 2759 | 1.3 (0.86–1.9) | 48.9 |
| Coronary artery disease | 6 | 1770 | 1.2 (0.78–1.8) | 17.0 |
| Myocardial infarction | 5 | 1739 | 1.2 (0.70–1.9) | 56.7 |
| Obstructive lung disease | 3 | 790 | 0.84 (0.44–1.6) | 13.2 |
| Hypoalbuminemia | 2 | 218 | 4.0 (1–14.3) | 73.6 |
| Depression scale score | 9 | 1846 | 1.4 (0.99–1.8) | 25.6 |
| Greater number of drugs | 5 | 484 | 1.4 (0.95–2.1) | 0 |
| Heart failure | 4 | 1504 | 1.4 (0.90–2.1) | 0 |
| Body mass index (BMI) | 10 | 1869 | 0.10 (−0.45, 0.6)† | 36.1 |
| General anesthesia | 10 | 2568 | 1.1 (0.80–1.4) | 19.6 |
| Pre-hospitalization | 3 | 390 | 1.3 (0.77–2.2) | 0 |
Abbreviations: ADL, activities of daily living; ASA, American Society of Anesthesiologists; CI, confidence interval; IADL, instrumental activities of daily living
*Reported as a standardized mean difference (SMD)
†Reported as a mean difference (MD)
Subgroup Analyses of Prognostic Factors
The odds of developing postoperative delirium among patients aged ≥80 years were significantly increased (Table 3).25 , 32 , 36 , 43 – 45 , 49 , 58 Age was a significant prognostic factor among patients undergoing non-cardiac surgery3 , 24 – 29 , 37 , 38 , 41 – 45 , 49 – 51 , 56 , 58 , 59; however, it was not a significant prognostic factor among patients undergoing cardiac surgery.1 , 2 , 31 , 33 , 34 , 36 Because of the substantial heterogeneity among the studies conducted in the non-cardiac surgery population, subgroup analyses were conducted based on the type of surgery. A statistically significant association between age and the odds of developing postoperative delirium remained for each of the following surgical populations: orthopedic surgery, vascular surgery, urological surgery, and general surgery.
Table 3.
Subgroup Analyses Exploring the Influence of Effect Modifiers on the Association Between Prognostic Factors and Postoperative Delirium
| Subgroup | Number of studies | Number of patients | Odds ratio (95% CI) | Heterogeneity (I2) |
|---|---|---|---|---|
| a) Older age | ||||
| Aged ≥80 years | 8 | 3161 | 2.3 (1.2–4.3) | 56.1 |
| Cardiac surgery | 6 | 801 | 1.3 (0.96–1.96) | 44.1 |
| Non-cardiac surgery | 21 | 5399 | 2.5 (1.4–3.8) | 93.6 |
| Orthopedic surgery | 7 | 1829 | 1.3 (1.1–1.5) | 0 |
| Vascular surgery | 2 | 762 | 5.7 (2.9–11.2) | 0 |
| Urological surgery | 4 | 1078 | 2.8 (1.4–5.6) | 51.9 |
| General surgery | 5 | 392 | 7.5 (1.5–37.8) | 94.9 |
| b) Cognitive impairment | ||||
| Cardiac surgery | 4 | 529 | 2.3 (1.3–4.0) | 0 |
| Non-cardiac surgery | 17 | 3267 | 2.5 (1.7–3.8) | 75 |
| Orthopedic surgery | 6 | 938 | 2.4 (1.1–5.0) | 72.2 |
| Vascular surgery | 2 | 762 | 6.4 (1.6–26.5) | 66.1 |
| Urological surgery | 3 | 497 | 2.6 (1.6–4.2) | 13.6 |
| General surgery | 3 | 209 | 1.2 (0.45–3.0) | 68 |
Cognitive impairment was a significant risk factor for postoperative delirium in patients undergoing cardiac or non-cardiac surgery.24 – 27 , 29 , 32 , 37 , 38 , 41 – 44 , 50 – 52 , 56 , 59 Cognitive impairment was also a risk factor for delirium in patients undergoing orthopedic surgery, vascular surgery, and urological surgery; however, cognitive impairment among patients undergoing general surgery was not associated with development of postoperative delirium.
In a subgroup analysis of patients who reported a history of alcohol abuse, there was no significant effect on the development of postoperative delirium (OR 1.00, 95% CI 0.56–1.8, 4 studies, I2 = 0%); however, among a subgroup of current smokers, there was an association between smoking and postoperative delirium (OR 2.8, 95% CI 1.5–5.3, 3 studies, I2 = 0%).
Sensitivity Analyses
Sensitivity analyses were conducted that included studies reporting ORs adjusted for potentially important confounders. Among studies reporting a significant association in the original meta-analyses, only renal insufficiency was no longer associated with increased odds of postoperative delirium (OR 2.0, 95% CI 0.44–9.6, 2 studies, I2 = 87.3%); however, the two studies included in this summary effect measure were clinically heterogeneous. The study by Bakker et al. reported renal insufficiency as a measure of serum creatinine level and found no significant association between renal insufficiency and postoperative delirium, whereas the study by Sasajima et al., reported the number of patients with end-stage renal disease, which was significantly associated with postoperative delirium.1 , 59 Following sensitivity analysis, a significant association remained for both age (OR 2.0, 95% CI 1.3–3.3, 9 studies, I2 = 96.1%) and cognitive impairment (OR 2.9, 95% CI 1.6–5.3, 10 studies, I2 = 78.9%). Additionally, when only those studies reporting the age-adjusted odds of postoperative delirium among older adults with cognitive impairment were included in the sensitivity analysis, there was still a significant association (OR 3.5,95% CI 1.5–7.9, 8 studies, I2 = 86.5%). Among the prognostic factors that were not significantly associated with postoperative delirium in the original meta-analyses, only the association between hypertension and postoperative delirium was statistically significant (OR 3.1, 95% CI 1.4–7.0, 2 studies, I2 = 6.1%). There were a number of studies that reported only unadjusted ORs, which precluded sensitivity analysis (eTable 5 in Supplement 1).
Assessment for Publication Bias
There was evidence suggesting small study bias among those studies reporting cognitive impairment (p = 0.02). It was estimated that five studies with non-significant results may have been suppressed, although even after adjusting for these potentially missing studies, cognitive impairment remained a significant risk factor (OR 2.2, 95% CI 1.5–3.2, I2 = 76.3%, p < 0.001; eFigure 2 in Supplement 1).
Delirium-Related Adverse Outcomes
Among studies reporting preoperative prognostic factors associated with developing postoperative delirium, postoperative delirium was significantly associated with postoperative mortality, postoperative complications, length of hospitalization, and discharge to an institution or care facility. The association between postoperative delirium and subsequent hospital readmission was reported in two studies (Table 4 and eFigure 1 in Supplement 1).26 , 44
Table 4.
Risk of Adverse Outcomes in Patients Who Developed Postoperative Delirium
| Outcome | Number of studies | Number of patients | Odds ratio (95% CI) | Heterogeneity (I2) |
|---|---|---|---|---|
| Postoperative mortality | 8 | 951 | 4.0 (2.0–8.1) | 14.7 |
| Postoperative complications | 7 | 929 | 3.1 (1.6–5.7) | 35.0 |
| Non-home discharge | 2 | 656 | 8.7 (3.9–19.4) | 27.7 |
| Length of hospitalization | 7 | 743 | 2.8 (0.7–4.9)* | 73.7 |
| Hospital readmission | 2 | 276 | 2.2 (0.1–44.6) | 88.7 |
Abbreviations: CI, confidence interval
*Reported as a mean difference
DISCUSSION
Our systematic review demonstrated that postoperative delirium occurs frequently in the setting of elective surgery among older adults (NNF = 6). We also identified prognostic factors associated with the risk of postoperative delirium in this patient population. Common geriatric syndromes, including cognitive and functional impairment, were shown to be important prognostic factors that are identifiable during the preoperative assessment of older adults. Findings from our systematic review support the targeting of potentially modifiable (e.g. smoking, frailty) and protective (caregiver support) factors to optimize care. Furthermore, we showed that delirium is associated with adverse outcomes that can severely impact both the quality and quantity of life experienced by older adults following elective surgery.
In addition to non-pharmacological delirium prevention strategies that have demonstrated effectiveness in both the medical and surgical settings, other potential targets for intervention were identified in this study that warrant further research.6 , 7 For example, smoking status, frailty, and the use of psychotropic medications are potentially modifiable prognostic factors that were associated with developing postoperative delirium. Interventions for preoperative smoking cessation have been associated with a lower risk of postoperative complications.67 Direct patient education has also been effective at reducing benzodiazepine prescriptions.68
Although some would argue that there are no effective interventions for the treatment of frailty, researchers have shown that the progression of frailty is potentially preventable.69 , 70 Indeed, multicomponent interventions aimed at improving nutrition, physical fitness, and cognition have shown promise in reversing frailty.71 Even exercise-based interventions alone have been shown to improve frailty status.72 , 73 The application of an exercise-based intervention in the preoperative setting, termed “prehabilitation”, is currently being studied to determine whether frail patients in the perioperative setting can derive similar benefit.74 If such an intervention proves successful, frailty could be identified in the preoperative clinic in order to offer patients prehabilitation in anticipation of their elective surgery.
Importantly, the availability of a caregiver in the perioperative period was shown to be protective against developing postoperative delirium. This is consistent with a randomized trial in older adults with hip fractures, which showed that a non-pharmacological multicomponent intervention provided by caregivers in the perioperative setting was associated with a decreased incidence of delirium.75 Previous work on the subjective experience and satisfaction of patients during the perioperative period has also highlighted the provision of information and education as very important to improving patient care.54 This information should be communicated to patients and their caregivers as part of their preoperative clinic visit, and this finding should potentially be added to clinical practice guidelines concerning the perioperative care of older adults.48
In contrast to recent systematic reviews and meta-analyses on adverse postoperative outcomes among older adults, which have included patients with various indications for surgery such as hip fracture or other emergency procedures, we targeted older adults undergoing elective surgery because of the potential to intervene in the preoperative setting to improve patient outcomes.53 , 55 Contrary to our finding that smoking status was associated with developing postoperative delirium, Scholz et al. found no such association among patients aged 50 years and older undergoing any elective or emergency gastrointestinal surgery; however, their result was based on just two studies and 218 patients, while our meta-analysis included nine studies and 2467 patients.53 There are several potential explanations for the association between smoking status and postoperative delirium, including nicotine withdrawal during hospitalization, or perhaps these patients have a higher burden of cardiometabolic disease with as yet undiagnosed vascular cognitive impairment. For example, greater agitation was reported in a study of intensive care unit patients experiencing nicotine withdrawal; however, the association between smoking and delirium remains unclear in the broader body of literature outside the perioperative setting.76 , 77 Similar to our current study, a meta-analysis by Witlox et al. found that patients who developed postoperative delirium experienced increased postoperative mortality and institutionalization.4
There were limitations in our study’s review process. First, only studies that were published in English were included in this review to increase feasibility, but our findings are likely generalizable given the number of geographical regions represented in our systematic review. Second, there was substantial heterogeneity among studies for some outcomes, which could not always be adequately explored given a limited number of studies and a lack of individual patient-level data. There were also limitations in the studies themselves. The methodological quality assessment demonstrated that a number of studies reported varying lengths and intensity of follow-up, which may have impacted the incidence of delirium reported. Although some studies reported only unadjusted effect measures, which limited their ability to account for possible confounders, our sensitivity analyses demonstrated that our findings were largely consistent when only study-level effect estimates adjusted for important confounders were included in the meta-analyses.
Our study had a number of strengths. Forty-one studies and over 9000 patients were included in our systematic review and meta-analysis, which allowed us to investigate a number of possible protective and predisposing factors. The hypothesis-generating nature of this study allowed for the identification of important prognostic factors, including the availability of caregiver support, which has potentially important clinical and policy implications for patient care.
In summary, postoperative delirium is a common (NNF = 6), yet preventable, complication experienced by older adults undergoing elective surgery that can lead to prolonged hospitalization, the inability to return home, and death. This systematic review and meta-analysis identified protective and modifiable prognostic factors, including smoking, frailty, and psychotropic medication use, which should be further studied to develop interventions aimed at mitigating potential harm.
Electronic supplementary material
(DOCX 1580 kb)
(DOCX 28 kb)
Acknowledgments
Contributors
We would like to thank Laure Perrier and Alissa Epworth for conducting the literature searches, Bianca Petrut and Lubna Al-Ansary for helping with article screening, and Susan Le for formatting the manuscript.
Funders
JW is funded by the Canadian Institutes of Health Research Frederick Banting and Charles Best Canada Graduate Scholarship (Master’s Award) and the Eliot Phillipson Clinician Scientist Training Program. ACT is funded by a Tier 2 Canada Research Chair in Knowledge Synthesis. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation. There was no funding for this study. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Compliance with Ethical Standards
Prior Presentations
The contents of this manuscript have been presented three times: (1) 36th Annual Canadian Geriatrics Society Annual Scientific Meeting, (2) 2016 Society for General Internal Medicine Meeting, and (3) 2016 Institute of Health Policy, Management and Evaluation Annual Research Day.
Conflict of Interest
The authors declare no competing interests.
Data Availability
The full data set is available from the corresponding author upon reasonable request.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s11606-017-4204-x) contains supplementary material, which is available to authorized users.
Contributor Information
Jennifer Watt, Email: jennifer.watt@mail.utoronto.ca.
Andrea C. Tricco, Email: triccoa@smh.ca.
Catherine Talbot-Hamon, Email: catherine.talbot-hamon@mail.mcgill.ca.
Ba’ Pham, Email: ba.pham@theta.utoronto.ca.
Patricia Rios, Email: riosp@smh.ca.
Agnes Grudniewicz, Email: agnes.grudniewicz@telfer.uottawa.ca.
Camilla Wong, Email: wongcam@smh.ca.
Douglas Sinclair, Email: sinclaird@smh.ca.
Sharon E. Straus, Phone: 416-864-3068, Email: sharon.straus@utoronto.ca.
References
- 1.Bakker RC, Osse RJ, Tulen JHM, Kappetein AP, Bogers AJJC. Preoperative and operative predictors of delirium after cardiac surgery in elderly patients. Eur J Cardiothorac Surg. 2012;41:544–549. doi: 10.1093/ejcts/ezr031. [DOI] [PubMed] [Google Scholar]
- 2.Veliz-Reissmuller G, Agüero Torres H, van der Linden J, Lindblom D, Eriksdotter Jonhagen M. Pre-operative mild cognitive dysfunction predicts risk for post-operative delirium after elective cardiac surgery. Aging Clin Exp Res. 2007;19:172–177. doi: 10.1007/BF03324686. [DOI] [PubMed] [Google Scholar]
- 3.Patti R, Saitta M, Cusumano G, Termine G, Di Vita G. Risk factors for postoperative delirium after colorectal surgery for carcinoma. Eur J Oncol Nurs. 2011;15:519–523. doi: 10.1016/j.ejon.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 5.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340(9):669–676. [DOI] [PubMed]
- 6.Chen C, Li H, Liang J, et al. Effect of a modified hospital elder life program on delirium and length of stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg. 2017;152(9):827–834. [DOI] [PMC free article] [PubMed]
- 7.Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016; 10.1002/14651858.CD005563.pub3. [DOI] [PMC free article] [PubMed]
- 8.Mohanty S, Rosenthal RA, Russell MM, et al. Optimal perioperative management of the geriatric patient: best practices guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg. 2016;222(5):930–947. [DOI] [PubMed]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Juurlink D, Preyra C, Croxford R, et al. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 12.Tousignant M, Corriveau H, Roy PM, Desrosiers J, Dubuc N, Hebert R. Efficacy of supervised Tai Chi exercises versus conventional physical therapy exercises in fall prevention for frail older adults: a randomized controlled trial. Disabil Rehabil. 2013;35(17):1429–1435. doi: 10.3109/09638288.2012.737084. [DOI] [PubMed] [Google Scholar]
- 13.Gianoudis J, Bailey CA, Ebeling PR, et al. Effects of a targeted multimodal exercise program incorporating high-speed power training on falls and fracture risk factors in older adults: a community-based randomized controlled trial. J Bone Miner Res. 2014;29(1):182–191. doi: 10.1002/jbmr.2014. [DOI] [PubMed] [Google Scholar]
- 14.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, Ontario: Ottawa Hospital Research Institute; 2008. [Google Scholar]
- 15.Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochrane Effective Practice and Organization Care Group. EPOC risk of bias tool. 2011.
- 17.Sanchez-Meca J, Marin-Martinez F, Chacon-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol Methods. 2003;8(4):448–467. doi: 10.1037/1082-989X.8.4.448. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J, Green SE. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available from www.handbook.cochrane.org.
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Viechtbauer W. Conducting meta-analyses in R with the metafor package. 2010;36(3):48.
- 22.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 23.Olin K, Eriksdotter-Jonhagen M, Jansson A, Herrington MK, Kristiansson M, Permert J. Postoperative delirium in elderly patients after major abdominal surgery. Br J Surg. 2005;92(12):1559–1564. doi: 10.1002/bjs.5053. [DOI] [PubMed] [Google Scholar]
- 24.Priner M, Jourdain M, Bouche G, Merlet-Chicoine I, Chaumier JA, Paccalin M. Usefulness of the short IQCODE for predicting postoperative delirium in elderly patients undergoing hip and knee replacement surgery. Gerontology. 2008;54(2):116–119. doi: 10.1159/000117574. [DOI] [PubMed] [Google Scholar]
- 25.Freter SH, Dunbar MJ, MacLeod H, Morrison M, MacKnight C, Rockwood K. Predicting post-operative delirium in elective orthopaedic patients: the Delirium Elderly At-Risk (DEAR) instrument. Age Ageing. 2005;34(2):169–171. doi: 10.1093/ageing/afh245. [DOI] [PubMed] [Google Scholar]
- 26.Large MC, Reichard C, Williams JT, et al. Incidence, risk factors, and complications of postoperative delirium in elderly patients undergoing radical cystectomy. Urology. 2013;81(1):123–128. doi: 10.1016/j.urology.2012.07.086. [DOI] [PubMed] [Google Scholar]
- 27.Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology. 2012;116:788–796. doi: 10.1097/ALN.0b013e31824b94fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers MP, Liang MH, Daltroy LH, Eaton H, Peteet J, Wright E, Albert M. Delirium after elective orthopedic surgery: risk factors and natural history. Int J Psychiatry Med. 1989;19:109–121. doi: 10.2190/2Q3V-HYT4-NN49-BPR4. [DOI] [PubMed] [Google Scholar]
- 29.Jankowski CJ, Trenerry MR, Cook DJ, et al. Cognitive and functional predictors and sequelae of postoperative delirium in elderly patients undergoing elective joint arthroplasty. Anesth Analg. 2011;112(5):1186–1193. doi: 10.1213/ANE.0b013e318211501b. [DOI] [PubMed] [Google Scholar]
- 30.Kudoh A, Takase H, Takahira Y, Takazawa T. Postoperative confusion increases in elderly long-term benzodiazepine users. Anesth Analg. 2004;99:1674–1678. doi: 10.1213/01.ANE.0000136845.24802.19. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson M, Samuelsson E, Gustafson Y, Aberg T, Engstrom KG. Delirium after coronary bypass surgery evaluated by the organic brain syndrome protocol. Scand Cardiovasc J. 2002;36(4):250–255. doi: 10.1080/14017430260180436. [DOI] [PubMed] [Google Scholar]
- 32.Fukuse T, Satoda N, Hijiya K, Fujinaga T. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest. 2005;127:886–891. doi: 10.1378/chest.127.3.886. [DOI] [PubMed] [Google Scholar]
- 33.Otomo S, Maekawa K, Goto T, Baba T, Yoshitake A. Pre-existing cerebral infarcts as a risk factor for delirium after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2013;17(5):799–804. doi: 10.1093/icvts/ivt304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolfson DB, McElhaney JE, Rockwood K, et al. Incidence and risk factors for delirium and other adverse outcomes in older adults after coronary artery bypass graft surgery. Can J Cardiol. 1999;15(7):771–776. [PubMed] [Google Scholar]
- 35.Kristjansson SR, Nesbakken A, Jordhøy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–217. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Santos FS, Velasco IT, Fraguas R, Jr Risk factors for delirium in the elderly after coronary artery bypass graft surgery. Int Psychogeriatr. 2004;16(2):175–193. [PubMed]
- 37.Koebrugge B, Koek HL, van Wensen RJ, Dautzenberg PL, Bosscha K. Delirium after abdominal surgery at a surgical ward with a high standard of delirium care: incidence, risk factors and outcomes. Dig Surg. 2009;26(1):63–68. doi: 10.1159/000194947. [DOI] [PubMed] [Google Scholar]
- 38.Morimoto Y, Yoshimura M, Utada K, Setoyama K, Matsumoto M, Sakabe T. Prediction of postoperative delirium after abdominal surgery in the elderly. J Anesth. 2009;23(1):51–56. doi: 10.1007/s00540-008-0688-1. [DOI] [PubMed] [Google Scholar]
- 39.Papaioannou A, Fraidakis O, Michaloudis D, Balalis C, Askitopoulou H. The impact of the type of anaesthesia on cognitive status and delirium during the first postoperative days in elderly patients. Eur J Anesthesiol. 2005;22:492–499. doi: 10.1017/S0265021505000840. [DOI] [PubMed] [Google Scholar]
- 40.Kosar CM, Tabloski PA, Travison TG, et al. Effect of preoperative pain and depressive symptoms on the risk of postoperative delirium: a prospective cohort study. Lancet Psychiatry. 2014;1:431–436. doi: 10.1016/S2215-0366(14)00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung JM, Sands LP, Lim E, Tsai TL, Kinjo S. Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium? Am J Geriatr Psychiatry. 2013;21(10):946–956. doi: 10.1016/j.jagp.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tognoni P, Simonato A, Robutti N, et al. Preoperative risk factors for postoperative delirium (POD) after urological surgery in the elderly. Arch Gerontol Geriatr. 2011;52:e166–e169. doi: 10.1016/j.archger.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Hattori H, Kamiya J, Shimada H, et al. Assessment of the risk of postoperative delirium in elderly patients using E-PASS and the NEECHAM Confusion Scale. Int J Geriatr Psychiatry. 2009;24(11):1304–1310. doi: 10.1002/gps.2262. [DOI] [PubMed] [Google Scholar]
- 44.Visser L, Prent A, Van Der Laan MJ, et al. Predicting postoperative delirium after vascular surgical procedures. J Vasc Surg. 2015;62:185–189. doi: 10.1016/j.jvs.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 45.Clement ND, MacDonald D, Howie CR, Biant LC. The outcome of primary total hip and knee arthroplasty in patients aged 80 years or more. J Bone Joint Surg Br. 2011;93(9):1265–1270. doi: 10.1302/0301-620X.93B9.25962. [DOI] [PubMed] [Google Scholar]
- 46.Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807–813. doi: 10.1016/j.amjmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 47.Robinson TN, Wu DS, Pointer LF, Dunn CL, Moss M. Preoperative cognitive dysfunction is related to adverse postoperative outcomes in the elderly. J Am Coll Surg. 2012;215(1):12–17. doi: 10.1016/j.jamcollsurg.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF. Optimal perioperative management of the geriatric patient: best practices guideline from ACS NSQIP/American Geriatrics Society. J Am Coll Surg. 2016;222(5):930–947. doi: 10.1016/j.jamcollsurg.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 49.Gani H, Domi R, Kodra N, et al. The incidence of postoperative delirium in elderly patients after urologic surgery. Med Arch. 2013;67(1):45–47. doi: 10.5455/medarh.2013.67.45-47. [DOI] [PubMed] [Google Scholar]
- 50.Cerejeira J, Batista P, Nogueira V, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. Low preoperative plasma cholinesterase activity as a risk marker of postoperative delirium in elderly patients. Age Ageing. 2011;40:621–626. doi: 10.1093/ageing/afr053. [DOI] [PubMed] [Google Scholar]
- 51.Brouquet A, Cudennec T, Benoist S, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251(4):759–765. doi: 10.1097/SLA.0b013e3181c1cfc9. [DOI] [PubMed] [Google Scholar]
- 52.Fisher BW, Flowerdew G. A simple model for predicting postoperative delirium in older patients undergoing elective orthopedic surgery. J Am Geriatr Soc. 1995;43(2):175–178. doi: 10.1111/j.1532-5415.1995.tb06385.x. [DOI] [PubMed] [Google Scholar]
- 53.Scholz AFM, Oldroyd C, McCarthy K, Quinn TJ, Hewitt J. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg. 2016;103:e21–e28. doi: 10.1002/bjs.10062. [DOI] [PubMed] [Google Scholar]
- 54.Rhodes L, Miles G, Pearson A. Patient subjective experience and satisfaction during the perioperative period in the day surgery setting: a systematic review. Int J Nurs Pract. 2006;12:178–192. doi: 10.1111/j.1440-172X.2006.00575.x. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y, Zhao X, Dong T, Yang Z, Zhang Q, Zhang Y. Risk factors for postoperative delirium following hip fracture repair in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res. 2016:1–12. [DOI] [PubMed]
- 56.Xue P, Wu Z, Wang K, Tu C, Wang X. Incidence and risk factors of postoperative delirium in elderly patients undergoing transurethral resection of prostate: a prospective cohort study. Neuropsychiatr Dis Treat. 2016;12:137–142. doi: 10.2147/NDT.S97249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hempenius L, Slaets JPJ, van Asselt D, de Bock TH, Wiggers T, van Leeuwen BL. Long term outcomes of a geriatric liaison intervention in frail elderly cancer patients. PLoS One. 2016;11:e0143364. doi: 10.1371/journal.pone.0143364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raats JW, Steunenberg SL, Crolla RMPH, Wijsman JHH, te Slaa A, van der Laan L. Postoperative delirium in elderly after elective and acute colorectal surgery: a prospective cohort study. Int J Surg. 2015;18:216–219. doi: 10.1016/j.ijsu.2015.04.080. [DOI] [PubMed] [Google Scholar]
- 59.Sasajima Y, Sasajima T, Azuma N, et al. Factors related to postoperative delirium in patients with lower limb ischaemia: a prospective cohort study. Eur J Vasc Endovasc Surg. 2012;44(4):411–415. doi: 10.1016/j.ejvs.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 60.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48:78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Blakoe M, Greve H. Frailty - preoperative assessment and implications for nursing practice following heart surgery. Paper presented at: EuroHeartCare 2015; Dubrovnik, Croatia.
- 62.Min L, Mazzurco L, Gure TR, et al. Longitudinal functional recovery after geriatric cardiac surgery. J Surg Res. 2015;194:25–33. doi: 10.1016/j.jss.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suh DH, Kim J-W, Kim HS, Chung HH, Park NH, Song YS. Pre- and intra-operative variables associated with surgical complications in elderly patients with gynecologic cancer: the clinical value of comprehensive geriatric assessment. J Geriatr Oncol. 2014;5:315–322. doi: 10.1016/j.jgo.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Kim K-I, Park K-H, Koo K-H, Han H-S, Kim C-H. Comprehensive geriatric assessment can predict postoperative morbidity and mortality in elderly patients undergoing elective surgery. Arch Gerontol Geriatr. 2013;56:507–512. doi: 10.1016/j.archger.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. a new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 66.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington (VA): American Psychiatric Association; 2013.
- 67.Thomsen T, Villebro N, Moller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. 2014(3):Cd002294. [DOI] [PMC free article] [PubMed]
- 68.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890–898. doi: 10.1001/jamainternmed.2014.949. [DOI] [PubMed] [Google Scholar]
- 69.Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muscedere J, Andrew MK, Bagshaw SM, et al. Screening for frailty in Canada’s health care system: a time for action. Can J Aging. 2016;35(3):281–297. doi: 10.1017/S0714980816000301. [DOI] [PubMed] [Google Scholar]
- 71.Ng T, Feng L, Nyunt M, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med. 2015;128(11):1225–1236. doi: 10.1016/j.amjmed.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 72.Singh NA, Quine S, Clemson LM, et al. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2012;13:24–30. doi: 10.1016/j.jamda.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;569194. [DOI] [PMC free article] [PubMed]
- 74.Abdullah HR, Lien VP, Ong HK, et al. Protocol for a single-centre, randomised controlled study of a preoperative rehabilitation bundle in the frail and elderly undergoing abdominal surgery. BMJ Open. 2017;e016815. [DOI] [PMC free article] [PubMed]
- 75.Martinez FT, Tobar C, Beddings CI, Vallejo G, Fuentes P. Preventing delirium in an acute hospital using a non-pharmacological intervention. Age Ageing. 2012;41(5):629–634. doi: 10.1093/ageing/afs060. [DOI] [PubMed] [Google Scholar]
- 76.Hsieh SJ, Shum M, Lee AN, Hasselmark F, Gong MN. Cigarette smoking as a risk factor for delirium in hospitalized and intensive care unit patients: a systematic review. Ann Am Thorac Soc. 2013;10(5):496–503. doi: 10.1513/AnnalsATS.201301-001OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lucidarme O, Seguin A, Daubin C, et al. Nicotine withdrawal and agitation in ventilated critically ill patients. Crit Care. 2010;14:R58. doi: 10.1186/cc8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1580 kb)
(DOCX 28 kb)
Data Availability Statement
The full data set is available from the corresponding author upon reasonable request.