Abstract
Purpose
Early detection of gastric cancer is important to improve prognosis. Early detection enables local treatment, such as endoscopic submucosal dissection (ESD). Therefore, we investigated whether early detection of gastric cancer could reduce healthcare costs by comparison according to stage and treatment modalities.
Materials and Methods
Medical care costs were investigated according to tumor stage and initial treatment modality in 1,188 patients newly diagnosed with gastric cancer at 7 medical institutions from December 2011 to June 2012. Total medical care costs during the first-year after diagnosis (total first-year costs) were examined, including the costs of initial treatment, post-initial treatment, and inpatient and outpatient visits.
Results
Stage I (75.3%) was the most common cancer stage. ESD was the second most common treatment following surgery. Total first-year costs increased significantly from stages I to IV. The costs of initial treatment and post-initial treatment were lowest in patients with stage I cancer. Among patients with stage I cancer, total first-year costs were significantly lower when treated by ESD; in particular, initial ESD treatment costs were much lower than others.
Conclusions
The cost of healthcare has increased significantly with increasing cancer stages. ESD can greatly reduce medical care costs of gastric cancer. Thus, early detection of gastric cancer is important to reduce healthcare costs.
Keywords: Stomach neoplasms, Endoscopic submucosal dissection, Healthcare costs, Cancer staging, Early detection of cancer
INTRODUCTION
Early detection of gastric cancer is very important to improve prognosis. Furthermore, early detection enables local treatments to preserve the stomach, such as endoscopic submucosal dissection (ESD). ESD can be curative for selected cases of early gastric cancer (EGC) [1].
The economic burden of cancer care is important for patients with cancer. Cancer accounts for 4%–10% of total healthcare costs worldwide, and the rate of inflation of these costs is widely viewed as unsustainable from a societal perspective [2]. Cancer care costs can be divided into direct and indirect costs [3]. Direct costs include medical care costs, such as treatment costs, and indirect costs are opportunity costs lost due to contracting a disease and death [3,4]. Direct costs differ by cancer characteristics, including stage and treatment options [2].
Cancer care cost analyses are usually performed in 3 phases to reflect clinical and cost-related dynamics, including an initial phase (time following diagnosis, usually up to 1 year), a continuing phase (time between initial and final phases), and a final phase (usually 1 year before death) [5,6]. Previous studies have reported that a large proportion of cancer care costs are incurred during the initial phase [7,8].
Early detection of gastric cancer, and especially treatment modality, can affect healthcare costs. However, the effect of ESD on healthcare costs has not been evaluated for gastric cancer. Therefore, we investigated whether early detection of gastric cancer could reduce healthcare costs by comparison according to stage and treatment modalities.
MATERIALS and methods
Study subjects
The medical records of 1,188 patients newly diagnosed with gastric cancer at 7 medical institutions (Seoul St. Mary's Hospital, Asan Medical Center, Yeouido St. Mary's Hospital, Gangnam Severance Hospital, St. Vincent Hospital, Uijeongbu St. Mary's Hospital, and Severance Hospital) from December 2011 to June 2012 were analyzed retrospectively, including initial diagnostic route, initial treatment modality, and tumor stage. The initial diagnostic routes included “screening” and “due to symptoms.” Screening was either individualized or through national organized gastric cancer screening. Tumor stage was based on pathological and clinical findings. Pathological stage was defined using the 7th tumor, node, metastasis (TNM) staging system.
Generally, treatments according to the stage are as follows: ESD or surgery for stage I; surgery or surgery with adjuvant chemotherapy for stages II and III, and palliative chemotherapy or conservative care for stage IV. After treatment, surveillance is conducted every 3–6 months for the first-year by medical examination, blood test, endoscopy, and computed tomography.
If several treatments were employed, the initial treatment modality was considered the first option as curative treatment. For example, if additional surgery was performed because of non-curative resection after ESD, the surgery was considered the initial treatment modality for the patient. Thus, initial treatment modalities were categorized into ESD, surgery, surgery with adjuvant chemotherapy, palliative chemotherapy, and conservative care.
Exclusion criteria included neoadjuvant chemo- or radiotherapy; follow-up duration <1 year after diagnosis, except death; combined with other malignancies within the past 5 years; or severe co-morbidities.
Cost definition and estimate
Cancer care costs are divided into direct and indirect costs [3]. Direct costs are medical and non-medical care costs. Medical care costs are costs for medical procedures and services associated with treatment and cancer care, including costs for hospitalization, outpatient visits, and prescription drugs [9]. Medical care costs for medical procedures associated with treatment included all of the costs related to tests, medical procedures, and services after diagnosis of gastric cancer. For example, if additional surgery was performed because of non-curative resection after ESD, the cost of ESD was considered as medical care costs for medical procedures associated with treatment. Thus, the cost of initial treatment is the same between surgery only and ESD with additional surgery. However, total medical care costs were different; the cost of patients who underwent ESD with additional surgery was higher than that of those undergoing surgery only.
Non-medical costs include costs of transportation to healthcare providers, costs associated with care, and costs for complementary and alternative medicine [9]. Indirect costs are the opportunity costs lost by contracting a disease and death [4].
Medical care costs during the first-year after diagnosis (total first-year costs) were compared according to tumor stage and initial treatment modality. Total first-year costs included payments for insurance-covered services (insurer payments) and payments for co-insurance and uncovered services (patient payments). Cost of initial treatment, the cost after initial treatment, and the number of inpatient and outpatient visits were determined. Cost of initial treatment only included the cost related to the treatment, and not the cost spent on the staging of the cancer.
The cost for initial palliative chemotherapy treatment was defined as the cost for the first palliative chemotherapy session; later sessions were included in costs after initial treatment. The cost for initial conservative care treatment was defined as the cost for conservative cancer treatment during the first admission.
All estimated costs are represented using the 2012 annual exchange rate of 1,100 Korean won to 1 USD.
Statistical analyses
The χ2 test was used to examine associations among categorical variables, and 1-way analysis of variance was used for non-categorical variables. A P-value <0.05 was considered significant. All analyses were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Baseline clinical characteristics of the subjects
Table 1 shows the subjects' clinicopathological features. Most initial diagnoses involved upper endoscopy. Stage I was the most common cancer stage (75.3% of all the patients). ESD was the second most common initial treatment following surgery. Laparoscopic gastrectomy was more common than open or robot-assisted gastrectomy among surgical procedures.
Table 1. Baseline clinical characteristics of the subjects.
| Characteristics | Values | ||
|---|---|---|---|
| Male | 798 (67.2) | ||
| Age (yr) | 61.6±12.0 | ||
| Initial diagnostic method | |||
| Upper endoscopy | 1,183 (99.6) | ||
| Barium series | 2 (0.2) | ||
| Others | 3 (0.3) | ||
| Initial diagnostic route | |||
| Organized screening | 191 (16.1) | ||
| Individualized screening | 460 (38.7) | ||
| Due to symptoms | 332 (27.9) | ||
| Unknown | 205 (17.3) | ||
| Stage | |||
| I/II/III/IV | 894/103/105/86 (75.3/8.7/8.8/7.2) | ||
| Initial treatment | |||
| ESD | 380 (32.0) | ||
| Surgery | 527 (44.4) | ||
| Laparoscopic | 334 (28.1) | ||
| Open | 166 (14.0) | ||
| Robot | 27 (2.3) | ||
| Surgery with chemotherapy | 188 (15.8) | ||
| Chemotherapy | 76 (6.4) | ||
| Conservative care | 17 (1.4) | ||
Data shown are number (%) or mean±standard deviation.
ESD = endoscopic submucosal dissection.
Table 2 shows clinical features according to tumor stage. Organized screening was the most common initial diagnostic route for stage I cancer, whereas “due to symptoms” was the most common for stage IV. About 42% of stage I cases underwent ESD as an initial gastric cancer treatment.
Table 2. Clinical characteristics of the subjects according to the tumor stage.
| Stages | I (n=894) | II (n=103) | III (n=105) | IV (n=86) | P-value | |
|---|---|---|---|---|---|---|
| Male | 68.9 | 62.1 | 59.0 | 65.1 | 0.127 | |
| Age (yr) | 61.7±11.4 | 62.7±13.8 | 62.2±13.5 | 58.5±14.0 | 0.158 | |
| Initial diagnostic route | <0.001 | |||||
| Organized screening | 19.0 | 8.7 | 11.4 | 0.0 | ||
| Individualized screening | 38.6 | 43.7 | 39.0 | 33.7 | ||
| Due to symptoms | 22.3 | 33.0 | 41.9 | 64.0 | ||
| Initial treatment | <0.001 | |||||
| ESD | 42.5 | 0.0 | 0.0 | 0.0 | ||
| Surgery | 50.2 | 47.6 | 24.8 | 3.5 | ||
| Surgery with CTx | 7.2 | 48.5 | 66.7 | 4.7 | ||
| CTx | 0.0 | 1.0 | 6.7 | 79.1 | ||
| Conservative care | 0.1 | 2.9 | 1.9 | 12.8 | ||
Data shown are percentage (%) or mean±standard deviation.
ESD = endoscopic submucosal dissection; CTx = chemotherapy.
Medical care costs according to cancer stage
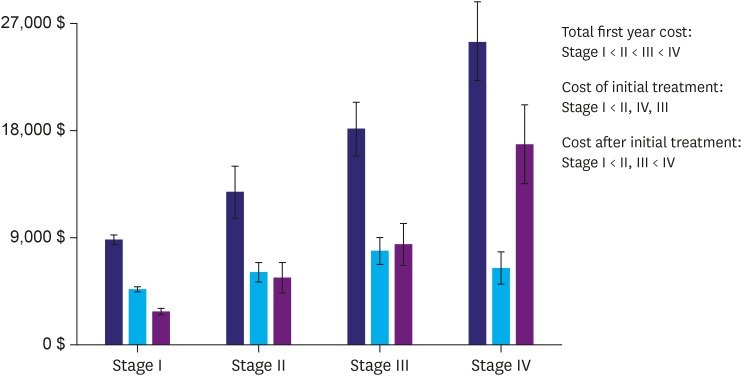
Total first-year costs increased significantly from stage I through stage IV (Fig. 1). The costs of initial treatment and post-initial treatment were the lowest for stage I (Fig. 1). Detailed costs according to tumor stage are shown in Table 3. Most first-year costs increased from stage I through stage IV, except patient and initial treatment costs, which were highest in stage III.
Fig. 1.
Medical care costs according to cancer stage. Total first-year costs increased significantly from stage I through stage IV. Initial treatment and post-initial treatment costs were the lowest for stage I (“<” indicates a significant difference).
Table 3. Medical care costs according to the stage.
| Variables | Stage I | Stage II | Stage III | Stage IV | P-value |
|---|---|---|---|---|---|
| Total first-year costs ($) | 8,907±5,790 | 12,956±10,988 | 18,277±11,489 | 25,753±15,520 | <0.001 |
| Insurer payments | 4,412±3,453 | 7,768±8,886 | 11,476±7,881 | 19,559±10,885 | <0.001 |
| Patient payments | 2,482±2,529 | 3,463±3,576 | 5,549±6,183 | 4,158±4,537 | <0.001 |
| Cost of initial treatment | 4,700±3,489 | 6,157±4,161 | 7,974±5,631 | 6,478±6,439 | <0.001 |
| Cost after initial treatment | 2,807±3,317 | 5,682±6,736 | 8,510±9,091 | 17,012±15,577 | <0.001 |
| No. of outpatient visits | 10.0±7.2 | 14.0±9.0 | 17.0±12.0 | 20.5±17.6 | <0.001 |
| No. of admissions | 1.3±1.1 | 2.9±2.8 | 5.5±5.3 | 8.7±7.5 | <0.001 |
Data shown are mean±standard deviation.
Medical care costs according to initial treatment of stage I cancer
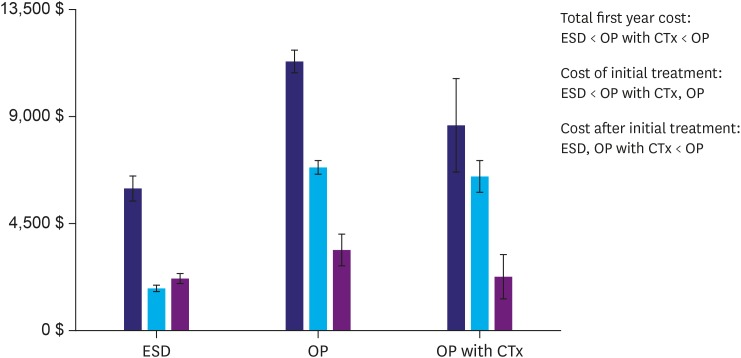
Initial treatment modalities for stage I cancer included ESD, surgery, and surgery with adjuvant chemotherapy. Total first-year costs for stage I cancer were significantly lower for ESD and much lower for initial ESD treatment than for other modalities (Fig. 2). Costs were detailed according to initial treatment modality for stage I cancer in Table 4. Most costs were significantly lower in stage I, except the number of admissions, which did not differ among initial stage I treatments.
Fig. 2.
Medical care costs according to initial treatment modality in stage I cases. Initial stage I treatment modalities included ESD, OP, and OP with adjuvant CTx. Total first-year costs for stage I cancer were significantly lower for ESD, and, particularly, initial ESD treatment costs were lower than those of the other treatment modalities (“<” indicates a significant difference).
ESD = endoscopic submucosal dissection; OP = surgery; CTx = chemotherapy.
Table 4. Medical care costs according to initial treatment modalities in stage I.
| Variables | ESD | Surgery | Surgery with CTx | P-value |
|---|---|---|---|---|
| Total first-year costs ($) | 6,028±5,092 | 11,391±4,778 | 8,715±7,852 | <0.001 |
| Insurer payments | 2,113±1,655 | 5,708±3,455 | 6,388±4,698 | <0.001 |
| Patient payments | 1,133±1,246 | 3,788±2,646 | 1,363±2,648 | <0.001 |
| Cost of initial treatment | 1,795±1,292 | 6,906±3,046 | 6,534±2,687 | <0.001 |
| Cost after initial treatment | 2,194±1,992 | 3,411±3,995 | 2,254±3,649 | <0.001 |
| No. of outpatient visits | 8.9±5.2 | 10.7±7.9 | 11.8±11.1 | <0.001 |
| No. of admissions | 1.2±0.8 | 1.3±1.1 | 1.6±1.9 | >0.050 |
Data shown are mean±standard deviation.
ESD = endoscopic submucosal dissection; CTx = chemotherapy.
Discussion
Gastric cancer is one of the most common cancers in Korea as 33,000 new patients are diagnosed annually [10]. The Department of Health and Human Services in Korea conducts cancer screening and recommends that adults ≥40 years old should undergo free mandatory upper endoscopy or gastrography every 2 years to detect EGC. This recommendation contributes to the recent increase in rates of discovery of EGC in Korea. According to our data, screening as an initial diagnostic route is very important for diagnosing EGC. The proportion of “due to symptoms” as the initial diagnostic route increased significantly from stage I through stage IV cancer.
ESD represents a major treatment for EGC in Korea; according to our data, 42.5% of patients with stage I cancer underwent ESD. The treatment modalities used by the National Medical Insurance System can affect costs. The Korean Health Insurance Review and Assessment Service began to reimburse for ESD of the stomach in November 2011. Thus, our study enrolled patients newly diagnosed with gastric cancer since December 2011.
Our data indicate that total first-year costs increased significantly from stage I through stage IV cancer. That is, cancer stage was one of the most important factors affecting cancer care costs, in agreement with a previous report [11]. Our data show that early detection of gastric cancer reduces medical care costs. All total first-year cost categories were lowest in patients with stage I disease. However, patient and initial treatment costs were highest in stage III, not stage IV, which was probably due to differences in the proportions of treatment modalities used. The proportion of combined treatments, such as surgery with chemotherapy, was higher in stage III cases than others, whereas costs after initial treatment were much higher for patients with stage IV cancer. That is, treatment type and cancer stage were the major factors affecting cancer care costs. Thus, early detection of gastric cancer can reduce medical care costs, particularly total first-year costs.
Treatment modality was the most important factor affecting medical care costs within the same cancer stage. One study in patients with stage III colorectal cancer showed that financial burden was highest in patients who used adjuvant chemotherapy [12]. Among stage I cases, which accounted for the largest fraction of patients in our study, ESD had the lowest total first-year costs compared to those of other treatment modalities. In particular, initial treatment costs were much lower for ESD. Therefore, ESD treatment for stage I gastric cancer can reduce medical care costs by decreasing initial treatment costs. Our data show that ESD for EGC provides a curative local treatment with a low financial burden.
In this study, the proportion of stage I was higher than in other Korean data [13,14]. This is probably due to short duration and limited institutions of enrollment. Thus, our results must be validated in the future. In addition, we only analyzed direct medical costs because these costs are closely associated with cancer characteristics, such as cancer stage and treatment options [2]. Some studies have evaluated direct medical costs to investigate trends in initial cancer treatment costs [6,15]. A survey, such as the Medical Expenditure Panel Survey, which consists of indicators of productivity loss, should be performed to properly investigate indirect costs [16,17,18]. However, our study was retrospective, so a survey was not possible. Nonetheless, we infer that, if indirect costs were analyzed, ESD would be associated with much lower indirect and direct medical costs than other treatment modalities.
In conclusion, early detection of gastric cancer is important to reduce healthcare costs. Especially, ESD can greatly reduce medical care costs of gastric cancer. Thus, screening should be done for early detection of gastric cancer.
Footnotes
Funding: This work was supported by the Korean College of Helicobacter and Upper Gastrointestinal Research Foundation Grant.
- Conceptualization: K.S.S., P.S.H.
- Data curation: K.J.H.
- Formal analysis: K.J.H.
- Funding acquisition: P.S.H.
- Investigation: K.J.H., K.S.S., L.J.H., J.D.H., C.D.Y., C.W.C.
- Methodology: K.J.H.
- Project administration: K.J.H., K.S.S.
- Resources: K.J.H., K.S.S., L.J.H., J.D.H., C.D.Y., C.W.C., P.S.H.
- Software: K.J.H., K.S.S., L.J.H., J.D.H., C.D.Y., C.W.C., P.S.H.
- Supervision: K.S.S.
- Validation: K.J.H., K.S.S., L.J.H., J.D.H., C.D.Y., C.W.C., P.S.H.
- Visualization: K.J.H., K.S.S.
- Writing - original draft: K.J.H.
- Writing - review & editing: K.J.H., K.S.S.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Goto O, Fujishiro M, Kodashima S, Ono S, Omata M. Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy. 2009;41:118–122. doi: 10.1055/s-0028-1119452. [DOI] [PubMed] [Google Scholar]
- 2.Peppercorn J. The financial burden of cancer care: do patients in the US know what to expect? Expert Rev Pharmacoecon Outcomes Res. 2014;14:835–842. doi: 10.1586/14737167.2014.963558. [DOI] [PubMed] [Google Scholar]
- 3.Guy GP, Jr, Ekwueme DU, Yabroff KR, Dowling EC, Li C, Rodriguez JL, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31:3749–3757. doi: 10.1200/JCO.2013.49.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haga K, Matsumoto K, Kitazawa T, Seto K, Fujita S, Hasegawa T. Cost of illness of the stomach cancer in Japan - a time trend and future projections. BMC Health Serv Res. 2013;13:283. doi: 10.1186/1472-6963-13-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker MS, Kessler LG, Urban N, Smucker RC. Estimating the treatment costs of breast and lung cancer. Med Care. 1991;29:40–49. doi: 10.1097/00005650-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Li TY, Hsieh JS, Lee KT, Hou MF, Wu CL, Kao HY, et al. Cost trend analysis of initial cancer treatment in Taiwan. PLoS One. 2014;9:e108432. doi: 10.1371/journal.pone.0108432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stump TK, Eghan N, Egleston BL, Hamilton O, Pirollo M, Schwartz JS, et al. Cost concerns of patients with cancer. J Oncol Pract. 2013;9:251–257. doi: 10.1200/JOP.2013.000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinan MA, Robinson TJ, Zagar TM, Scales CD, Jr, Curtis LH, Reed SD, et al. Changes in initial treatment for prostate cancer among Medicare beneficiaries, 1999–2007. Int J Radiat Oncol Biol Phys. 2012;82:e781–e786. doi: 10.1016/j.ijrobp.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SG, Hahm MI, Choi KS, Seung NY, Shin HR, Park EC. The economic burden of cancer in Korea in 2002. Eur J Cancer Care (Engl) 2008;17:136–144. doi: 10.1111/j.1365-2354.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 10.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res Treat. 2014;46:124–130. doi: 10.4143/crt.2014.46.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SY, Kim SG, Park JH, Park EC. Costs of initial cancer care and its affecting factors. J Prev Med Public Health. 2009;42:243–250. doi: 10.3961/jpmph.2009.42.4.243. [DOI] [PubMed] [Google Scholar]
- 12.Veenstra CM, Regenbogen SE, Hawley ST, Griggs JJ, Banerjee M, Kato I, et al. A composite measure of personal financial burden among patients with stage III colorectal cancer. Med Care. 2014;52:957–962. doi: 10.1097/MLR.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macalindong SS, Kim KH, Nam BH, Ryu KW, Kubo N, Kim JY, et al. Effect of total number of harvested lymph nodes on survival outcomes after curative resection for gastric adenocarcinoma: findings from an eastern high-volume gastric cancer center. BMC Cancer. 2018;18:73. doi: 10.1186/s12885-017-3872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SG, Seo HS, Lee HH, Song KY, Park CH. Comparison of the differences in survival rates between the 7th and 8th editions of the AJCC TNM staging system for gastric adenocarcinoma: a single-institution study of 5,507 patients in Korea. J Gastric Cancer. 2017;17:212–219. doi: 10.5230/jgc.2017.17.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekwueme DU, Yabroff KR, Guy GP, Jr, Banegas MP, de Moor JS, Li C, et al. Medical costs and productivity losses of cancer survivors--United States, 2008–2011. MMWR Morb Mortal Wkly Rep. 2014;63:505–510. [PMC free article] [PubMed] [Google Scholar]
- 17.Guy GP, Jr, Yabroff KR, Ekwueme DU, Smith AW, Dowling EC, Rechis R, et al. Estimating the health and economic burden of cancer among those diagnosed as adolescents and young adults. Health Aff (Millwood) 2014;33:1024–1031. doi: 10.1377/hlthaff.2013.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowling EC, Chawla N, Forsythe LP, de Moor J, McNeel T, Rozjabek HM, et al. Lost productivity and burden of illness in cancer survivors with and without other chronic conditions. Cancer. 2013;119:3393–3401. doi: 10.1002/cncr.28214. [DOI] [PMC free article] [PubMed] [Google Scholar]