Abstract
Candida albicans is a dimorphic commensal fungus that causes severe oral infections in immunodeficient patients. Invasion of C. albicans hyphae into oral epithelium is an essential virulence trait. IL-17 signaling is required for both innate and adaptive immunity to C. albicans. During the innate response, IL-17 is produced by γδ-T cells and a poorly understood population of innate-acting CD4+TCRαβ+ cells, but only the TCRαβ+ cells expand during acute infection. Confirming the innate nature of these cells, the TCR was not detectably activated during the primary response, evidenced by Nur77eGFP mice that report antigen-specific signaling through the TCR. Rather, expansion of innate TCRαβ+ cells was driven by both intrinsic and extrinsic IL-1R signaling. Unexpectedly, there was no requirement for CCR6/CCL20-dependent recruitment or prototypical fungal pattern recognition receptors. However, C. albicans mutants that cannot switch from yeast to hyphae showed impaired TCRαβ+ cell proliferation and Il17a expression. This prompted us to assess the role of Candidalysin, a hyphal-associated peptide that damages oral epithelial cells and triggers production of inflammatory cytokines including IL-1. Indeed, Candidalysin-deficient strains failed to upregulate Il17a or drive proliferation of innate TCRαβ+ cells. Moreover, Candidalysin signaled synergistically with IL-17, which further augmented expression of IL-1α/β and other cytokines. Thus, IL-17 and C. albicans, via secreted Candidalysin, amplify inflammation in a self-reinforcing feed-forward loop. These findings challenge the paradigm that hyphal formation per se is required for the oral innate response, and demonstrate that establishment of IL-1- and IL-17-dependent innate immunity is induced by tissue-damaging hyphae.
Introduction
The commensal fungus Candida albicans colonizes human mucosal surfaces. Changes in immune competency or oral mucosal barriers promote development of oropharyngeal candidiasis (OPC, thrush), an opportunistic infection prevalent in HIV/AIDS, iatrogenic immunosuppression, head-neck irradiation, Sjögren’s Sydnrome and infancy (1, 2). Patients with mutations in genes that impact Th17 cells or the IL-17R signaling pathway are extremely susceptible to chronic mucocutaneous candidiasis (CMC) (3). Neutralizing antibodies that occur in AIRE deficiency or as a result of biologic therapy for autoimmunity can also cause mucosal candidiasis (4). Mice with IL-17R signaling deficits are similarly susceptible to C. albicans infections (5, 6). Unlike humans, C. albicans is not a commensal microbe in rodents, and therefore mice are immunologically naïve to this fungus (7, 8). Nonetheless, during recall infections with C. albicans, mice mount vigorous Th17 responses that augment innate immunity, in keeping with humans where the memory response to C. albicans is Th17-dominated. During the naïve response, IL-17 is produced by several innate lymphocyte subsets, but the only cells that expand robustly upon infection belong to an oral-resident innate TCRαβ+ population, sometimes called ‘natural’ Th17 cells (9).
An essential virulence trait of C. albicans is its ability to transition from its commensal yeast form to an invasive and cell-damaging hyphal state. In the adaptive immune response, Dectin-1 expressed on myeloid cells recognizes β-glucan components of the fungal cell wall that are exposed during the hyphal transition. This leads to production of IL-6 and IL-23, which promote Th17 cell differentiation (10–12). Surprisingly, however, neither CARD9 nor IL-6 is required for the innate IL-17 response to OPC (9, 13). Therefore, it has been unclear how innate IL-17-expressing cells are activated during primary C. albicans infections, and why this only occurs in response to invasive, tissue-damaging hyphae.
The initiating event in OPC is exposure of oral epithelial cells (OEC) to C. albicans. Hyphae but not yeast cause lysis and danger responses in OECs, including production of cytokines and chemokines (IL-6, IL-1α/β, GM-CSF, G-CSF, CCL20), antimicrobial peptides (β-defensins) and DAMPs (IL-1α, S100A8/9) (14, 15). This OEC activation program is triggered by Candidalysin, an amphipathic pore-forming peptide derived from the hyphal-specific ECE1 (Extent of Cell Elongation 1) gene product (16). Many of the cytokines induced by Candidalysin are associated with Th17 responses or recruitment, e.g., IL-1α/β, IL-6 and CCL20, which led us to postulate that Candidalysin might influence generation of the early IL-17 response to infection.
Here we demonstrate that innate oral TCRαβ+ cells express IL-17 and proliferate in response to C. albicans infection without discernible activation of the TCR or a requirement from canonical fungal pattern recognition receptors. Instead, proliferation of innate IL-17+TCRαβ+ cells and expression of IL-17 and IL-1α/β were regulated by Candidalysin. Consistently, Il1r1−/− mice are susceptible to OPC, with redundant activities in hematopoietic and non-hematopoietic compartments. Moreover, Candidalysin and IL-17 signal synergistically in OECs to augment expression of antifungal response genes. Therefore, innate IL-17-induced responses are triggered specifically in response to Candidalysin secreted from hyphae, revealing surprising differences in how activation of innate versus adaptive IL-17-dependent immunity is controlled.
Results
C. albicans induces proliferative expansion of innate oral IL-17+TCRαβ + cells
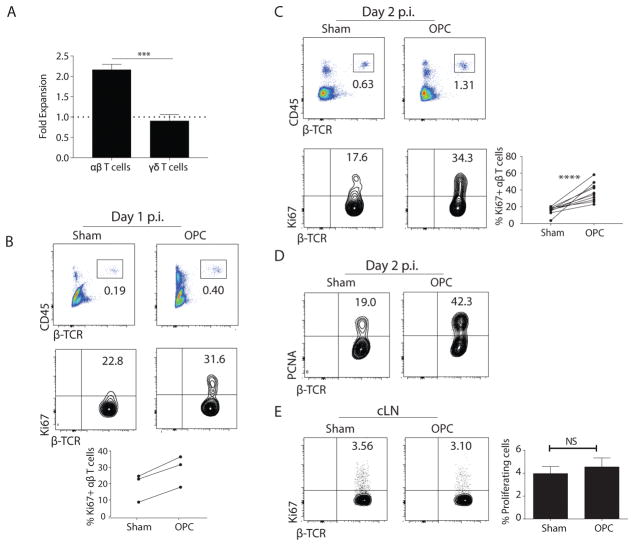
We previously showed that IL-17 is induced in the oral mucosa within 24 h of infection with C. albicans. Expression of IL-17 diminishes concomitantly with clearance, typically in 3 d, (5, 8). Using Il17aeYFP fate-tracking mice (17), we found that IL-17 produced during acute oral C. albicans challenge originates dominantly from tongue-resident γδ-T cells and an unconventional population of innate-like CD4+TCRαβ+ cells (9). IL-17 production by ILC3s has been reported in OPC (18), though their frequency is below the limit of detection in our hands. These IL-17+TCRαβ+ cells are sometimes termed ‘natural’ Th17 cells (9, 19, 20), but here we refer to them as ‘innate TCRαβ+ cells’ per Kashem et al. (21). In the oral cavity, the innate IL-17+TCRαβ+cells reproducibly expand ~2-fold following encounter with C. albicans, whereas the frequency of IL-17+γδ-T cells is low and does not change during infection (9) (Fig 1a). C. albicans-dependent expansion of oral TCRαβ+ cells was similarly observed in non-fate tracking mice, starting at 1 day p.i. and peaking at day 2 p.i. (Fig 1b, c, Fig S1).
Figure 1. Proliferation of oral TCRαβ+ cells following C. albicans infection.
(A) Il17aeYFP mice (17) were challenged sublingually with PBS (sham) or C. albicans. Homogenates were prepared from pooled tongues (n=2–3). YFP+TCRαβ+ or YFP+TCRγδ+ cells in the CD45+CD4+ gate were assessed by flow cytometry. Data show fold-increase versus sham, pooled from 3–4 independent experiments. (B–C) WT mice (C57BL/6J) were infected with C. albicans, and tongue homogenates prepared on days 1 or 2 p.i. Cells were gated on lymphocytes and staining of CD45 and TCRβ is shown (top). Proliferation of CD45+CD4+TCRβ+ cells was determined by staining for Ki67 (bottom). Data representative of 10 experiments. Graph in C: mean ± SEM of proliferating TCRαβ+ cells on days 1 and 2. (D) WT mice were infected with C. albicans and tongue homogenates prepared on day 2 p.i. Proliferation was determined by PCNA staining. Data representative of 3 experiments. (E) WT cervical LNs were harvested on day 2 p.i. Proliferation of CD45+CD4+TCRβ+ cells was determined by anti-Ki67 staining. Graph shows mean ± SEM of Ki67+ CD4+ cells in cLNs. Data are representative of 2 experiments. Statistical analyses: Student’s t test or 1-way ANOVA.
The expansion of innate TCRαβ+ cells could be due to proliferation, survival, recruitment or a combination. To assess proliferation, WT mice were infected orally and intracellular Ki67 was measured by flow cytometry. On day 1, Ki67+TCRβ+ cells were more frequent in the infected oral mucosa compared to sham controls (Fig 1b). More profound proliferation was observed at day 2, where we consistently saw a 2-fold increase in the percent and total cell number in C. albicans-infected mice compared to sham-infected controls harvested within the same experiment (Fig 1c). Proliferation was confirmed by intracellular staining for proliferating cell nuclear antigen (PCNA) (Fig 1d). The expansion of TCRαβ+ cells by C. albicans was similar in different vendors (Fig S2a), and the proliferating cells exhibited a diverse TCRvβ repertoire (Fig S2b). C. albicans-induced proliferation of TCRαβ+ cells was limited to the local site of infection (tongue), as there was no change in the baseline frequency of replicating CD4+TCRαβ+ cells in the draining cervical LN (cLN) (Fig 1e). These data confirm our previous findings that IL-17 is expressed in local oral tissue but not in cLN during a primary C. albicans infection (8). Thus, the 2-fold expansion of TCRαβ+ cells during OPC can be accounted for by local proliferation at the site of infection.
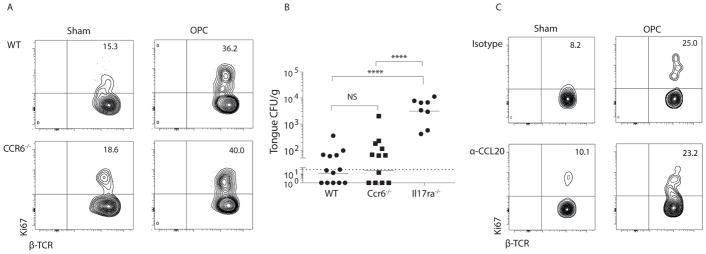
Oral-resident TCRαβ+ cells express CCR6, a marker of IL-17+ cells and receptor for the chemokine CCL20 (9). To determine if signaling through the CCL20/CCR6 axis was required for immunity to OPC, we analyzed responses in Ccr6−/− mice and mice given neutralizing anti- CCL20 Abs (22). Innate TCRαβ+ cells in Ccr6−/− mice showed a similar proliferation capacity to WT controls following C. albicans infection (Fig 2a, Fig S1). There was also no difference in the baseline population of TCRαβ+ cells in Ccr6−/− compared to WT mice (Fig S3). Resistance to OPC was similar in Ccr6−/− and WT mice, with low oral fungal burdens at 4 days p.i. (Fig 2b). Similar results were obtained when mice were administered anti-CCL20 Abs (Fig 2c). Accordingly, the baseline frequency and the C. albicans-induced expansion of TCRαβ+ cells in the oral mucosa is independent of CCL20 and CCR6, though we cannot rule out involvement of other chemotactic factors.
Figure 2. CCR6 is dispensable for expansion of innate TCRαβ+ cells in oral candidiasis.
(A) WT or Ccr6−/− mice were infected with C. albicans, and proliferation of oral TCRαβ+ cells was determined at day 2 p.i. Data representative of 3 independent experiments. (B) Indicated mice were infected orally with C. albicans, and fungal burden was assessed by CFU enumeration on day 4 p.i.. Bars = geometric mean. Each point represents an individual mouse. Dashed line = limit of detection (LOD, 30 CFU) (52). Data are compiled from 4 independent experiments. (C) WT mice were injected with 100 ug anti-CCL20 Abs or isotype controls on day -1 relative to infection. Proliferation of TCRαβ+ cells was determined on day 2 p.i. Data are representative of 2 experiments. Student’s t test or ANOVA used for statistics.
Oral-resident innate TCRαβ + cells drive anti-Candida immunity independently of TCR signaling or specificity
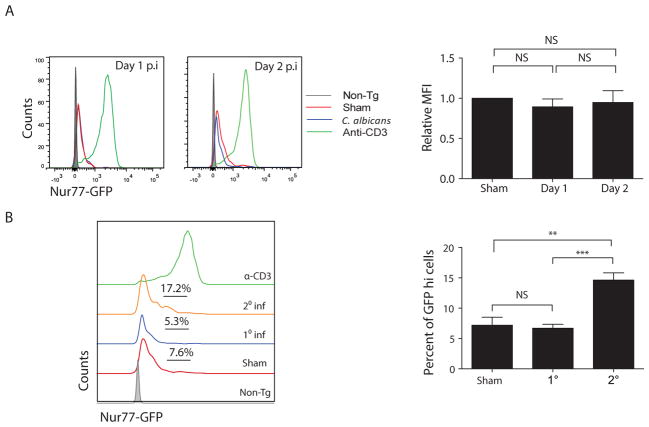
Mice are naïve to C. albicans, and animals lacking lymphocytes (e.g., Rag1−/−, Il7ra−/− mice) are highly susceptible to OPC (8, 9). To determine whether C. albicans-induced expansion of innate TCRβ+ cells requires antigen-specific signaling, we used Nur77eGFP reporter mice, which report the kinetics and magnitude of TCR signaling through expression of GFP driven by the promoter of the immediate-early gene Nr4a1 (Nur77) (23). First, to verify that TCR activation could be visualized in oral T cells, WT mice were given agonistic anti-CD3 Abs to activate the TCR nonspecifically; this treatment effectively induced GFP fluorescence in TCRβ+ cells from tongue (Fig 3a). Next, to determine if TCR signaling was activated during the innate response, Nur77eGFP mice were challenged orally with C. albicans or PBS (sham), and GFP fluorescence in oral TCRαβ+ cells was assessed at days 1 and 2 p.i. As expected, T cells from sham-infected mice showed a low but detectable baseline level of tonic GFP expression (23). In mice infected with C. albicans for 2 days (1° infection), there was the same baseline GFP fluorescence as seen in sham cohorts, indicating that there was no TCR signaling upon first encounter with C. albicans and confirming the innate nature of these cells (Fig 3a).
Figure 3. A primary C. albicans infection activates innate TCRαβ+ cells without engaging the TCR.
(A) Nur77GFP mice were sham-treated (red line) or infected with C. albicans and tongue homogenates prepared on days 1 or 2 p.i (blue line). Controls received anti-CD3 Abs (green line) to stimulate the TCR on all T cells. WT (“non-Tg”) mice were negative controls for GFP staining (grey line). Left: fluorescence intensity of GFP in oral CD45+CD4+TCRβ+ cells. Right: Relative mean fluorescence intensity (MFI) of GFP in CD45+CD4+TCRβ+ cells was assessed and normalized to sham. NS, not significant. Data from 3 independent experiments. (B) Nur77GFP mice were infected with C. albicans. Tongue homogenates were prepared 2 d p.i. (“1° Inf”). To induce C. albicans-specific TCR signaling (“2° Inf”), mice were infected orally, rested for 6 weeks, then re-challenged with a second oral infection (8). Left: GFP fluorescence in oral CD45+CD4+TCRβ+ cells, with % GFPhi cells indicated. Green line shows staining in mice administered agonistic anti-CD3 Abs, as in panel A. Right: Compiled percentage of GFPhi cells per cohort. Data representative of 3–4 independent experiments. Graphs show mean + SEM, analyzed by student’s t test or ANOVA.
The Nur77eGFP reporter system can also be used to compare TCR signaling strength, so we assessed the frequency of GFPhi cells (i.e., T cells with more potent TCR signaling) in mice given a primary (1°) or a secondary (2°) C. albicans infection. Again, there were no differences between sham-treated mice or those receiving a 2 day (1°) challenge (Fig 3b). To verify that C. albicans-specific signaling through the TCR could be observed if present, we generated a 2° response by subjecting mice to infection and then re-challenge after 6 weeks; this regimen induces an Ag-specific Th17 response that enhances fungal clearance (8). Indeed, there was an increased frequency of GFPhiTCRαβ+ cells in tongues from re-challenged mice, demonstrating that Ag-specific responses can be visualized with Nur77eGFP mice in the context of a recall response (Fig 3b). Therefore, consistent with the naïve state of mice with respect to C. albicans, TCR signaling appears not to be activated during expansion of TCRαβ+ cells in an innate infection.
Pattern recognition receptors required for the adaptive response to C. albicans are dispensable for activation of innate TCRαβ + cells
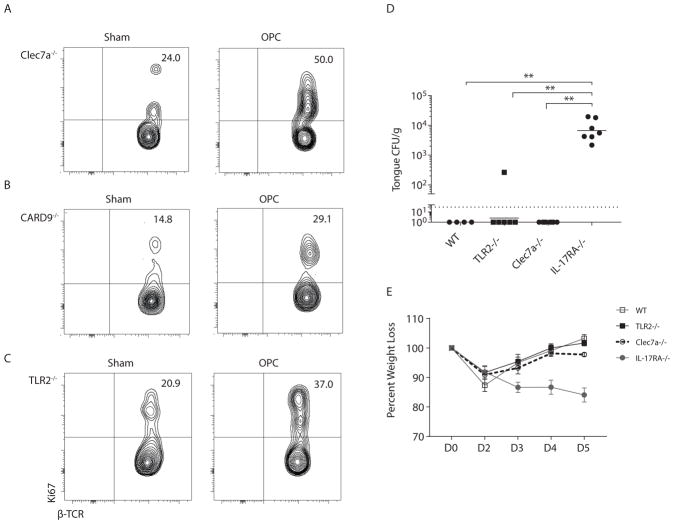
Dectin-1 (Clec7a) is a C-type lectin receptor (CLR) used by phagocytes to sense β-glucan carbohydrates that are exposed on C. albicans during filamentation. Dectin-1 induces IL-23 and IL-6 in APCs, skewing to a Th17 phenotype (11, 24). However, it was not known whether Dectin-1 signals similarly drive IL-17 production during the innate response. In Clec7a−/− mice there was rapid and robust proliferation of innate TCRαβ+ cells following oral C. albicans infection, indicating that Dectin-1 is not required for innate TCRαβ+ cell expansion (Fig 4a, Fig S1). A similar proliferative response occurred in mice lacking CARD9, a key adaptor downstream of Dectin-1 and other CLRs (Fig 4b, Fig S1) (25–27). TLR2 has also been implicated in recognition of C. albicans through engagement of hyphae (24, 28). However, there was robust proliferation of innate TCRβ+ cells in Tlr2−/− mice upon 1° C. albicans-challenge (Fig 4c, Fig S1).
Figure 4. TLR2 and Dectin-1 are dispensable for C. albicans-induced proliferation of innate TCRαβ+ cells.
(A–C) Indicated mice were infected with C. albicans and proliferation of CD45+CD4+TCRβ+ cells determined on day 2 p.i. Data representative of 2–3 independent experiments. (D) Indicated mice were infected and fungal burden quantified on day 5 p.i. Bars = geometric mean. Dashed line = LOD. Data from 2 independent experiments. Data analyzed by ANOVA, Mann Whitney correction. (E) Average % weight loss is shown.
To determine whether TLR2, Dectin-1 or CARD9 were necessary to clear C. albicans in acute oral infection, fungal loads were assessed 5 days p.i.. Clearance was not impaired in mice lacking Dectin-1 (Fig 4d–e), consistent with our prior report that CARD9 is dispensable for innate immunity to OPC (13). Similarly, resolution of C. albicans was not impaired in Tlr2−/− mice (Fig 4d–e). Hence, TLR2 or Dectin-1/CARD9 signaling are dispensable for expansion of innate TCRαβ+ cells during innate immunity to OPC.
The secreted peptide Candidalysin activates innate TCRαβ + cell expansion
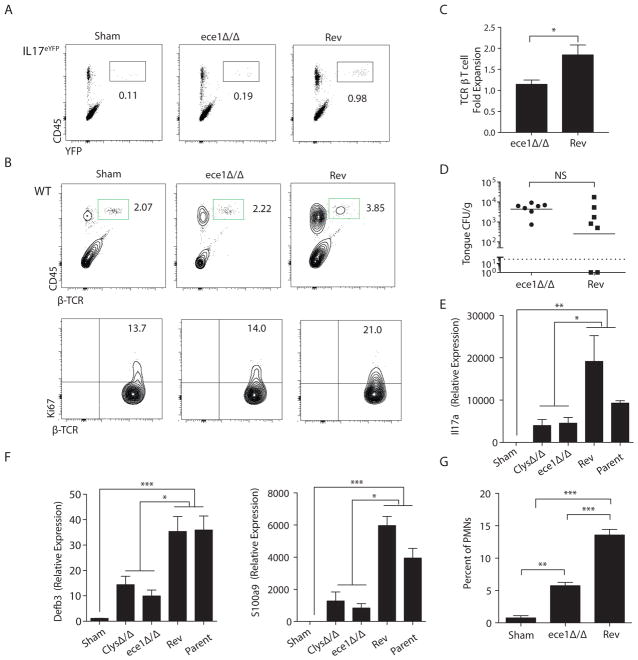
Hyphal formation is a key virulence trait for C. albicans. Consistently, a C. albicans mutant “locked” in the yeast phase (efg1Δ/Δ (29)) did not induce Il17a or expression of IL-17- dependent genes such as Defb3 (β-defensin 3), Il1b or Ccl20 (Fig S4a). In its hyphal state, C. albicans secretes Candidalysin, a short, amphipathic pore-forming peptide. Candidalysin, encoded by the ece1 gene, destabilizes epithelial membranes and triggers OEC production of cytokines such as IL-1α, IL-1β and IL-6 (16). Since these cytokines are linked to Th17 responses (30), we hypothesized that Candidalysin might serve as an activator of innate TCRαβ+ cell expansion and IL-17 production. Il17aeYFP reporter mice were infected with C. albicans strains lacking ECE1 (ece1Δ/Δ) or an ECE1-revertant control (“Rev”). Mice infected with ece1Δ/Δ exhibited reduced expansion of IL-17+TCRαβ+ cells at day 2 p.i. In contrast, mice challenged with the ECE1-Rev strain showed robust TCRαβ+ expansion (Fig 5a). Similar results were obtained in WT mice (Fig 5b, c). The diminished TCRαβ+ expansion in ece1Δ/Δ-infected mice correlated with reduced proliferation (Fig 5b, bottom), observed at both day 2 and day 3 p.i (Fig S4b). By day 5, the infection was resolved and the T cell proliferative response had returned to baseline. Importantly, at day 2 when cells were harvested, fungal loads were comparable, indicating that the impaired TCRαβ+ cell proliferation was not due to reduced exposure to fungal antigens (Fig 5d). Therefore, Candidalysin is required for expansion of innate TCRαβ+ cells during acute oral C. albicans infection.
Figure 5. Candidalysin drives proliferation of innate IL-17-producing TCRαβ+ cells.
(A) Il17aeYFP mice were infected with C. albicans (ece1Δ/Δ or Revertant “Rev”) and homogenates prepared 2 d p.i. Staining of CD45 and YFP in lymphocyte gate is shown. Data representative of 2 experiments. (B) WT mice were infected with the indicated strains of C. albicans, and expansion (top) and proliferation (bottom) of oral TCRαβ+ cells was analyzed at day 2 p.i.. Data representative of 3 experiments. (C) Fold-expansion of TCRβ+ cells following infection with ece1Δ/Δ or Rev strains. Data pooled from 4 experiments. (D) Fungal loads were assessed at day 2 p.i.. Bar = geometric mean. Data pooled from 2 experiments. (E–F) Tongue homogenates were prepared 2 days after infection with the indicated C. albicans strains. Total mRNA was subjected to qPCR normalized to Gapdh. Graphs show mean + SEM normalized to sham. Data compiled from 7–8 mice per group from 2 independent experiments. (G) Percentage of CD11b+Ly6Ghi cells in tongue was assessed at day 2 p.i. + SEM, compiled from 3 experiments. Statistics analyzed by student’s t test or ANOVA.
Consistent with the reduced TCRαβ+ cell proliferation, mice infected with strains lacking ECE1 or just the Candidalysin sequence (ClysΔ/Δ) showed impaired induction of Il17a mRNA expression (Fig 5e) as well as Defb3 and S100a9 (Fig 5f). Neutrophil mobilization to the tongue, which is regulated in part by IL-17 signaling (5, 31, 32), was also reduced in ece1Δ/Δ-challenged mice (Fig 5g). We verified activation that TCRαβ+ proliferation is induced in response to an unrelated C. albicans strain, HUN96 (33), a clinical isolate that expresses ECE1, induces c-Fos and damages OECs in vitro (Fig S4c). C. albicans secretes multiple virulence factors, particularly secreted aspartyl proteinases (SAPs). To determine if the innate IL-17 response was specific to Candidalysin, we evaluated TCRαβ+ proliferation following infection with fungal strains lacking the hypha-associated SAP genes (SAP4-6) (34, 35). Strikingly, there was no defect in TCRαβ+ proliferation in response to infection with sap4-6Δ/Δ strains compared to the parent strain (Fig S4d).
Innate TCRαβ + cell proliferation in the oral mucosa is dependent on IL-1α/β signaling
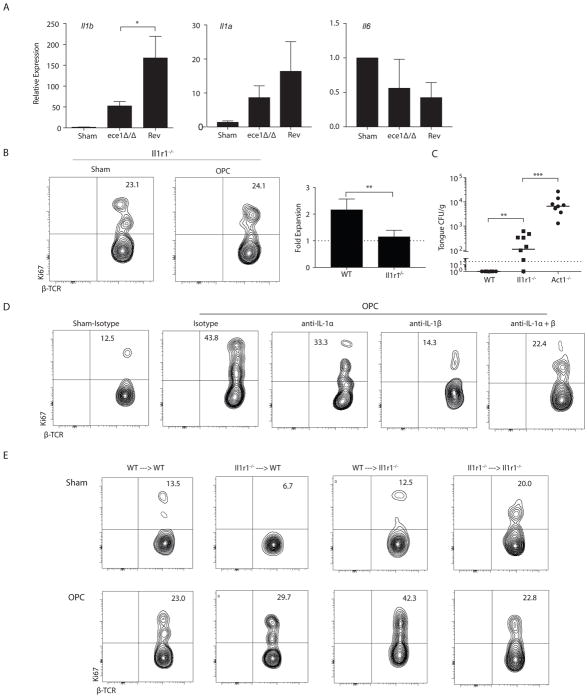
Candidalysin elicits production of several cytokines known to impact differentiation or proliferation of some IL-17-producing cells, such as IL-6, IL-1α and IL-1β (16). In tongues of mice subjected to 1° C. albicans infection, expression of Il1b mRNA was induced in an ECE1-dependent manner (Fig 6a). Expression of Il1a showed a similar trend, but Il6 was not induced in this time frame (Fig 6a). Il6−/− mice are resistant to acute OPC (9), and here we verified that proliferation of innate TCRαβ+ cells occurred normally in the absence of IL-6 (Fig S5). In contrast, there was no expansion or proliferation of oral innate TCRαβ+ cells in infected Il1r1−/− mice (Fig 6b, Fig S1). Consistently, Il1r1−/− mice were more susceptible to OPC than WT, although fungal burdens were not as high as in mice with an IL-17R signaling defect (here, Act1-deficiency (36)) (Fig 6c). We next used neutralizing Abs against either IL-1α or IL-1β (or both) to delineate the specific IL-1 family member needed to drive proliferation. As shown, blockade of either IL-1α or IL-1β impaired TCRβ+ cell proliferation, with a somewhat stronger effect under IL-1β blocking conditions (Fig 6d).
Figure 6. IL-1 activates innate TCRαβ+ T cell proliferation and anti-fungal immunity in a T cell intrinsic and T cell extrinsic manner.
(A) WT mice were infected with the indicated C. albicans strains and gene expression was measured on day 2 p.i.. Data show mean + SEM normalized to sham, from 7–8 mice/group in 2 experiments. (B) Expansion and proliferation of TCRαβ+ cells in Il1r1−/− mice at day 2 p.i. Data are from 3 experiments. (C) Fungal burdens in the indicated mice were quantified on day 5 p.i., from 2 experiments. (D) WT mice were administered anti-IL-1α, anti-IL-1β or isotype control Abs (1.0 mg/mouse used alone or 0.5 mg each when used together) on day -1 p.i.. Proliferation of oral TCRαβ+ cells was assessed at day 2 day p.i. Data representative of 2 experiments. (E) Reciprocal adoptive transfers of femoral BM were performed in WT or Il1r1−/− mice and proliferation of oral TCRαβ+ cells was determined. Experimental chimera results are representative of 2 experiments; control chimera data are from 1 experiment. Data analyzed by Student’s t test or ANOVA.
IL-1 signaling can occur on most cell types, including both hematopoietic and nonhematopoietic compartments. To identify the key cell type(s) that responded to IL-1, we irradiated congenically marked WT and Il1r1−/− mice and reconstituted them with the same or reciprocal bone marrow (BM). After 6 weeks, mice were infected orally with C. albicans and proliferation of donor TCRβ+ cells was assessed. As expected, Il1r1−/− mice given Il1r1−/− BM showed impaired proliferation compared to WT counterparts (Fig 6e). Surprisingly, however, regardless of the source of bone marrow, C. albicans infection induced TCRαβ+ cell proliferation in both experimental chimera conditions (i.e., WT → Il1r1−/− and Il1r1−/− → WT). There was some variation in the percent of Ki67+ cells at baseline (Sham) among cohorts, but in all cases there was a clear increase in proliferation after C. albicans infection. This result suggests that there are redundant IL-1R-dependent signals in radio-sensitive and radio-resistant compartments with respect to driving innate TCRαβ+ cell proliferation. To verify this unexpected finding, we created mixed chimeras, in which irradiated WT mice were reconstituted with a 50:50 mix of Il1r1−/− and WT bone marrow. Again, both WT and IL-1R-deficient cells proliferated robustly in response to infection (Fig S5b). As a third approach, we performed adoptive transfer experiments using BM from mice lacking Il1r1 specifically in TCRαβ+ cells (37). Again, TCRαβ+ cells proliferated following OPC (Fig S5c), indicating that IL-1 signals occur in both hematopoietic and non-hematopoietic cells. Collectively, these data suggest the existence of IL-1 responder cells in both compartments that indirectly drive TCRαβ+ cell proliferation. We also noted that the baseline Ki67 staining in innate TCRαβ+ cells was reduced in Il1r1−/− cells compared to WT, which was most apparent in the mixed BM chimera. These results suggested that IL-1R-driven signals may directly support T cell proliferation under homeostasis. Nonetheless, only when there is a global deficiency in the IL-1R is there a failure of TCRαβ+ cells to proliferate during C. albicans infection.
Candidalysin and IL-17 signal synergistically and amplify antifungal immunity in OECs
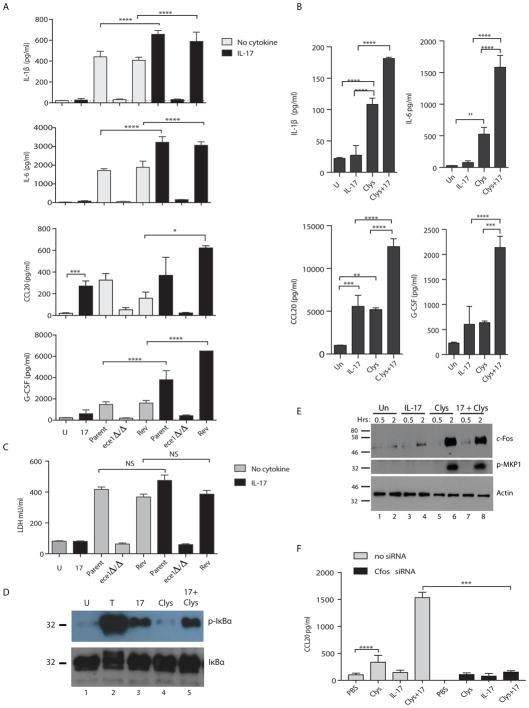
Candidalysin signaling in OECs upregulates inflammatory cytokines such as IL-6, IL-1β, G-CSF and CCL20. Many of these genes are also targets of IL-17 in OECs (32). IL-17 is generally a modest activator of signaling and gene expression compared to other inflammatory stimuli, and instead mediates its activities by signaling synergistically or additively with cytokines such as TNFα. Accordingly, we hypothesized that IL-17 and Candidalysin might signal cooperatively in OECs to drive antifungal immune responses. To test this, we infected human buccal epithelial cells (TR146 cell line) in vitro with C. albicans (WT parent strain, ece1Δ/Δ or Rev) in the presence or absence of IL-17. After 24 h, conditioned supernatants were assessed for Candidalysin-inducible cytokines and chemokines by Luminex. There was an additive or synergistic effect of IL-17 with Candidalysin in upregulating cytokines, including IL-1β, IL-6, CCL20 and G-CSF (Fig 7a). To determine whether this synergy was mediated by Candidalysin directly, cells were treated with sublytic concentrations of Candidalysin (15 μM) together with IL-17 (200 ng/ml) for 24 h. Indeed, there was a synergistic or additive induction of cytokines in the presence of IL-17 (Fig 7b). TNFα showed similar cooperation with Candidalysin (Fig S6). However, IL-22, which is also produced by Type 17 cells and is induced in the tongue during OPC (5), did not synergize with Candidalysin (Fig S6). Thus, IL-17, TNFα and Candidalysin cooperatively enhance inflammatory signaling in OECs.
Figure 7. Candidalysin and IL-17 signal synergistically or additively in oral epithelial cells.
(A) TR146 OECs were untreated (“U”, grey bars) or stimulated with IL-17 (200 ng/ml, black bars). Cells were infected with WT C. albicans (Bwp17+CIp30, “Parent”), ece1Δ/Δ or the Revertant (Rev) for 24 h. Supernatants were analyzed by Luminex (IL-1β, IL-6, G-CSF) or ELISA (CCL20). Graphs indicate mean + SEM. Data are representative of 2 experiments. (B) TR146 cells were untreated (“U”, grey bars) or treated with IL-17 (200 ng/ml, black bars) or Candidalysin peptide (Clys, 15 μM) for 24 h and analyzed as in panel A. (C) TR146 cells were incubated with C. albicans ± IL-17 (200 ng/ml). LDH in supernatants was evaluated after 24 h, representative of 3 experiments. (D) TR146 cells were treated with TNFα (20 ng/ml), IL-17 (200 ng/ml) or Candidalysin (15 μM) for 5 min. Lysates were immunoblotted for phospho-IκBα and total IκBα. (E) TR146 cells were incubated with TNFα (20 ng/ml), IL-17 (200 ng/ml) or Candidalysin (15 μM) for 30 min or 2 h. Lysates were immunoblotted for c-Fos, phospho-MKP1 or Actin. Data are representative of 2 experiments. (F) TR146 cells were transfected with c-Fos siRNA and stimulated for 24 h with PBS, Clys or IL-17. Supernatants were assessed for CCL20 by ELISA. Data are representative of 2 independent experiments. All data analyzed by ANOVA and Student’s t-test.
Another function of Candidalysin is to induce of cell damage, presumed to facilitate fungal access to nutrients and invasion into deep tissue (16). Conversely, IL-17 has been shown to induce tissue-protective/repair pathways in lung, renal and intestinal epithelia (38). Therefore, we postulated that IL-17 might offset the cell-damaging effects of Candidalysin. To address this issue, TR146 OECs were cultured with live C. albicans or lytic concentrations of Candidalysin (70 μM) with or without IL-17, and cell damage was measured by lactate dehydrogenase (LDH) activity in supernatants. There was no change in the extent of LDH induced by Candidalysin when cells were cultured with IL-17; and, as expected, a Candidalysin-deficient strain did not induce cell damage (Fig 7c). Thus, IL-17 neither contributes to nor protects against Candidalysin-induced cell damage.
To gain mechanistic insight into signaling cross-talk between IL-17 and Candidalysin, we assessed the downstream signaling pathways instigated by these factors. IL-17 activates NF-κB among other pathways (39), while Candidalysin-induced signaling is characterized by p38-MAPK/c-Fos activation and phosphorylation of the MKP1 (Dusp1) phosphatase (14, 16). In TR146 cells, treatment with IL-17 induced phosphorylation of IκBα, an early step in the canonical NF-κB pathway, albeit more weakly than TNFα (Fig 7d, left). Candidalysin did not activate phosphorylation of IκBα, nor was there an additive impact of co-stimulating cells with IL-17 and Candidalysin. While Candidalysin stimulated c-Fos upregulation and phosphorylation of MKP1, there was no synergistic activation of c-Fos or MKP-1 in the presence of IL-17 (Fig 7e). However, knockdown of c-Fos by RNA silencing blocked the synergistic activation of IL-17 and Candidalysin (Fig 7f), confirming cooperative activation of these pathways. Together, these data support a model in which secretion of Candidalysin by C. albicans hyphae during infection induces an innate cytokine response from OECs, which leads to the activation of resident innate TCRαβ+ cells through the IL-1 receptor. These innate TCRαβ+ cells respond by secreting IL-17, which signals through its receptor on OECs to further amplify expression of innate immune effector genes in a feed-forward amplification loop, ultimately resulting in the resolution of infection (Fig S7).
Discussion
OECs lining the tongue, palate and buccal mucosa are vital ‘first responders’ to acute microbial infection, and we recently found that IL-17R-dependent signals on keratin-13+ OECs are critical for immunity to oral candidiasis (32). Here, we identified a surprising mechanism by which OECs orchestrate IL-17-dependent immunity during a primary innate response OPC. When C. albicans hyphae invade oral epithelial barriers, they secrete the pore-forming peptide Candidalysin, which destabilizes membranes and provides access to host cell content and nutrients (16). Candidalysin signaling on OECs prompts release of IL-1α/β, which drive proliferation of innate IL-17+TCRαβ+ lymphocytes through both intrinsic and extrinsic mechanisms. Additionally, IL-17 synergizes with Candidalysin to further enhance proinflammatory signaling, establishing a feed-forward activation loop that mobilizes antifungal host defenses. This scenario ensures protective IL-17-driven responses only manifest in the presnce of tissue-damaging invasion of C. albicans hyphae (Fig S7).
In the mouth, innate TCRαβ+ cells and γδ-T cells constitute the main early sources of IL-17 (9). Although oral γδ-T cells evidently do not proliferate during OPC, their isolation is inefficient, so we cannot rule out the possibility that proliferation in these cells occurs at low levels. Moreover, γδ-T cells do contribute to the response; they can express large quantities of IL-17 on a per-cell basis (40) and mice lacking either γδ-T cells or αβ-T cells exhibit modestly increased susceptibility to OPC, suggesting redundancy of these cell types (9). Unlike humans, mice are naïve to C. albicans, and do not have C. albicans-specific T cells at baseline (7, 8, 41). Our data with Nur77GFP mice (which report ongoing TCR signaling) confirm that the proliferation of the TCRαβ+ cells is independent of Candida-specific antigens, at least within the detection limits of this system (Fig 4). In acute dermal candidiasis, IL-17 is also made by γδ-T cells and TCRαβ+ cells. However, here it is the γδ-T cells that proliferate and that are comparatively more important than TCRαβ+ cells (Fig 1) (9, 42). Although ILC3s have been implicated in OPC (18), Rag1−/− mice have high fungal loads after oral C. albicans infection (8, 9), and therefore contributions of this cell type appear to be negligible.
The prevailing paradigm in fungal immunology is that IL-17 responses are triggered upon sensing of hyphal cell wall carbohydrates through Dectin/CARD9 or TLR2 signaling (43). While true for adaptive responses, our data demonstrate that the acute IL-17 response is instead triggered by Candidalysin, which is responsible for cellular damage by invasive hyphae. Consequently, the host evidently exploits Candidalysin (ECE1) to discriminate between damaging and non-damaging hyphal tissue invasion. While Candidalysin-deficient strains fail to provoke efficient Type 17 responses (Fig 5), strains lacking ECE1 are less virulent in settings of immunodeficiency (16). Ece1Δ/Δ strains do not persist in immunocompetent mice (16), likely due to mechanical clearance by salivary flow and swallowing. We speculate that in healthy humans where C. albicans is a commensal microbe, non-damaging colonization provides a survival advantage to the fungus, as it does not set off host defense alarms through production of Candidalysin. A recent report evaluating different C. albicans strains found a similar, though imperfect, correlation of ECE1 levels with IL-17 production (44). C. albicans strains vary in cell wall composition or other properties, so it is possible that in some strains there are alternate virulence factors that trigger IL-17 responses. Nonetheless, the independent clinical isolate HUN96 (45) induced TCRαβ+ proliferation similarly to the CAF2-1 and Bwp17 strains that are more commonly used (both derived from the SC5314 lab strain) (Fig S4).
Adaptive T cells with innate properties have been identified in multiple barrier tissues. For example, gingiva-resident CD4+TCRαβ+IL-17+ cells are induced following mechanical damage upon mastication (46). Like the cells described here, gingival TCRαβ+ cells expand by local and rapid proliferation, are activated upon tissue damage and are present in mice from different vendors. However, these populations differ in their requirements for IL-6 and IL-1, and we previously saw that germ-free mice appear to lack baseline innate TCRαβ+ cells in the tongue (9). In skin, heterologous protection against C. albicans can be conferred by IL-17-secreting CD8+ T cells that are specific for commensal bacteria (47). Similarly, in the eye, γδ-T cells specific for an ocular commensal bacterium can provide protection from C. albicans through IL-17 production (48). Innate functions in pulmonary memory Th2 cells have been described that manifest effector responses without engaging the TCR (49), and memory T cells with innate-like functions have been reported in human mucosae and skin (50). These findings collectively indicate that tissue-resident T cells can be co-opted to function in an innate capacity. It is tempting to speculate that the ability of adaptive cells to function as innate effector cells may be an evolutionary remnant of their role in controlling invasive pathogens at barrier sites.
Materials and Methods
Mice
All mice were on the C57BL/6 background. Experiments were performed on both sexes with age- and sex-matched controls in each experiment. Il17ra−/− mice were provided by Amgen (Thousand Oaks, CA). Nur77GFP mice were from K. Hogquist (University of Minnesota) (23). Card9−/− mice were from X. Lin (MD Anderson). Act1−/− mice were from U. Siebenlist (NIH) (51). CD4CREIl1r1fl/fl mice were described (37). Il17aCreRosa26eYFP fate reporters (17) and other mice were from JAX (except as noted for Taconic Farms, Albany NY) and housed at the University of Pittsburgh for at least 7 d prior to experimentation. For adoptive transfers, mice were irradiated and given 106 femoral BM after 24 h. Mice were reconstituted for 6–9 weeks. Protocols were approved by the University of Pittsburgh IACUC. All efforts were made to minimize suffering, in accordance with the Guide for the Care and Use of Laboratory Animals of the NIH.
Oral Candidiasis and C. albicans strains
OPC was induced by sublingual inoculation with a cotton ball saturated in C. albicans for 75 min, as described (5). For re-challenge, mice were infected 6 weeks after the primary infection (8). Tongue homogenates were prepared on a GentleMACS (Miltenyi Biotec, Auburn, CA) and CFU determined by serial dilutions on YPD agar. Anti-CCL20 (R&D Systems, Minneapolis, MN), anti-IL-1α, anti-IL-1β or isotype control Abs (BioXCell, West Lebanon, NH) were administered on day -1 p.i. (100–1000 μg/mouse). CAF2-1 or Bwp17 C. albicans strains (derived from SC5314, www.candidagenome.org/Strains.shtml) were used as wild-type. Knockout strains (sap4-6Δ/Δ ece1Δ/Δ, efg1Δ/Δ) and HUN96 were described (16, 29, 34, 35).
Flow Cytometry
Flow cytometry was performed as described (9). Tongues were pooled (2 per sample) and cell suspensions prepared with the Tumor Dissociation kit (Miltenyi). Abs were from eBioscience, BD Biosciences or BioLegend. Proliferation was assessed using the Foxp3/Transcription Factor Buffer Kit (eBioscience) with Ki67-APC (BD Pharmigen) or PCNA-PE (eBioscience). Data acquired on an LSR Fortessa and analyzed with FlowJo (Ashland OR).
RNA and qPCR
Frozen tongue was homogenized in RLT buffer (RNAeasy kit; Qiagen) with a GentleMACS Dissociator (Miltenyi). cDNA was synthesized with a SuperScript III first-strand synthesis system (Invitrogen, Carlsbad, CA). Relative quantification of gene expression was determined by real-time PCR with SYBR green (Quanta BioSciences, Gaithersburg, MD) normalized to Gapdh. Primers were from SA Biosciences (Qiagen). Results were analyzed on a 7300 real-time PCR system (Applied Biosystems, Carlsbad, CA). Knockdown of c-Fos was performed as described in (14); briefly, 3×105 TR146 cells were serum starved for 24 h and transfected with 37 nM c-Fos siRNA in HiPerFect Reagent (Qiagen) for 2 d. Cells were treated with Clys or IL-17 for 24 h and supernatants analyzed by ELISA.
Cell culture, in vitro infections, cytokine stimulations, immunoblotting
TR146 cells (ECAAC10032305) were cultured in DMEM-F12/15% FBS as described (14). For infections in vitro, 3–5×105 cells were seeded overnight and cultured in serum-free DMEM with 1×105 CFU C. albicans yeast cells for 24 h. Recombinant human IL-17, TNFα and IL-22 (R&D Systems) were used at 200, 20 or 100 ng/ml, respectively. Candidalysin (SIIGIIMGILGNIPQVIQIIMSIVKAFKGNK) was from Peptide Protein Research Ltd (UK). Antibodies: Phospho-IκBα and IκBα (Upstate Biotechnology), c-Fos and phospho-MKP1 (Cell Signaling), Actin (clone C4, EMD Millipore).
Luminex, ELISAs and LDH assays
Conditioned media was analyzed by Luminex (IL-1α, IL-1β, IL-6, GM-CSF, G-CSF) or ELISA (CCL20), kits from R&D Systems. LDH assays were performed with CytoTox 96 Assay System reagents (Promega).
Statistics and study design
Data were analyzed on Prism (GraphPad Software), using ANOVA or Student’s t-test. Fungal load data are presented as geometric mean and evaluated by ANOVA with Mann-Whitney correction. All experiments were performed a minimum of twice in independently performed replicates.
Supplementary Material
Acknowledgments
We thank T. Hand, B. Klein, L. Monin, H. Conti, J. Kolls for helpful suggestions. Amgen provided Il17ra−/− mice. Act1−/− mice were from U. Siebenlist. Nur77GFP mice were from K. Hogquist. Ccr6−/− mice were from J. Farber or JAX.
Funding: SLG was supported by NIH (DE022550, DE023815 and AI107825), LPK by AI103022, MJM by AI110822 and PSB by DK104680. JRN was supported by the Medical Research Council (MR/M011372/1), Biotechnology & Biological Sciences Research Council (BB/N014677/1), the National Institute for Health Research at Guys and St Thomas’s NHS Foundation Trust, and King’s College London Biomedical Research Centre (IS-BRC-1215-20006). BH was supported by Deutsche Forschungsgemeinschaft (TR/CRC FungiNet Project C1), the Infect ERA-NET Program (FunComPath; BMBF 031L0001A) and by the H2020 – Marie Skłodowska-Curie Actions – European Training Networks (ETN) - Marie Sklodowska- Curie grant agreement No 642095 - “OPATHY”. ARH was supported by Medical College of Wisconsin Dept. of Pediatrics and Children’s Hospital of Wisconsin Research Institute. AW was supported by Deutsche Forschungsgemeinschaft (TR/CRC 128 and TR/CRC 156). This work benefitted from a BD LSR FORTESSATM grant 1S10-OD011925.
Abbreviations
- Clys
Candidalysin
- CMC
chronic mucosal candidiasis
- LDH
lactate dehydrogenase
- LOD
limit of detection
- OEC
oral epithelial cell
- OPC
oropharyngeal candidiasis
Footnotes
Author Contributions: AKV, SLG, JRN, LPK, DM, BH, PSB, MJM, DHK, IAM, CZ, AW, ARH designed experiments or conceptualized questions. AKH JPR, SLG, ARH, JH, KR, CZ, PSB and BMC performed experiments. AHV, JPR, JH, ARH, JRN, DM, CZ, SLG analyzed data. AHV and SLG wrote the manuscript, with input from JPR, DM, JH, ARH, KR, BMC, LPK, PSB, BH, and JRN.
Competing Interests: SLG has received grants from Novartis and Janssen. Candidalysin is the subject of a submitted patent to JRN, BH, DM, JPR: Naglik J, Moyes D, Tang S, Hube B, Wilson D, Hofs S, Richardson J (2013) Peptides and binding partners there for. WO 2014167335, Priority 2013-04-11.
References
- 1.Brown GD, et al. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Jabra-Rizk MA, et al. Candida albicans Pathogenesis: Fitting Within the “Host-Microbe Damage Response Framework”. Infect Immun. 2016;84:2724–2739. doi: 10.1128/IAI.00469-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conti HR, Gaffen SL. IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J Immunol. 2015;195:780–788. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milner J, Holland S. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat Rev Immunol. 2013;13:635–648. doi: 10.1038/nri3493. [DOI] [PubMed] [Google Scholar]
- 5.Conti H, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho A, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bär E, et al. A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol. 2012;188:5636–5643. doi: 10.4049/jimmunol.1200594. [DOI] [PubMed] [Google Scholar]
- 8.Hernández-Santos N, et al. Th17 cells confer long term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–910. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conti H, et al. Oral-resident ‘natural’ Th17 cells and γδ-T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobsen ID, et al. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther. 2012;10:85–93. doi: 10.1586/eri.11.152. [DOI] [PubMed] [Google Scholar]
- 11.Leibundgut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 12.Taylor PR, et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishu S, et al. CARD9 is required for adaptive but not innate immunity to oral mucosal Candida albicans infections. Infect Immun. 2014;82:1173–1180. doi: 10.1128/IAI.01335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyes DL, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moyes DL, et al. Protection against epithelial damage during Candida albicans infection is mediated by PI3K/Akt and mammalian target of rapamycin signaling. J Infect Dis. 2014;209:1816–1826. doi: 10.1093/infdis/jit824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moyes D, et al. Candidalysin: A fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirota K, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladiator A, Wangler N, Trautwein-Weidner K, Leibundgut-Landmann S. Cutting Edge: IL-17-Secreting Innate Lymphoid Cells Are Essential for Host Defense against Fungal Infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 19.Marks BR, et al. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuniga LA, Jain R, Haines C, Cua DJ. Th17 cell development: from the cradle to the grave. Immunol Rev. 2013;252:78–88. doi: 10.1111/imr.12036. [DOI] [PubMed] [Google Scholar]
- 21.Kashem S, et al. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity. 2015;43:515–526. doi: 10.1016/j.immuni.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedrick MN, et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest. 2009;119:2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennehy KM, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao XQ, et al. C-type lectin receptor dectin-3 mediates trehalose 6,6′-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-κB activation. J Biol Chem. 2014;289:30052–30062. doi: 10.1074/jbc.M114.588574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi L, et al. CARD9 mediates dectin-2-induced IκBα kinase ubiquitination leading to activation of NF-κB in response to stimulation by the hyphal form of Candida albicans. J Biol Chem. 2010;285:25969–25977. doi: 10.1074/jbc.M110.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson MJ, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hise AG, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo HJ, et al. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 30.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 31.Huppler AR, et al. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol. 2014;192:1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conti H, et al. IL-17RA signaling in oral epithelium is critical for protection against oropharyngeal candidiasis. Cell Host Microbe. 2016;20:606–617. doi: 10.1016/j.chom.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacCallum DM. Massive induction of innate immune response to Candida albicans in the kidney in a murine intravenous challenge model. FEMS Yeast Res. 2009;9:1111–1122. doi: 10.1111/j.1567-1364.2009.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naglik JR, et al. Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology. 2008;154:3266–3280. doi: 10.1099/mic.0.2008/022293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hube B, Monod M, Schofield DA, Brown AJ, Gow NA. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol. 1994;14:87–99. doi: 10.1111/j.1365-2958.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira MC, et al. Interleukin-17-induced protein lipocalin 2 is dispensable for immunity to oral candidiasis. Infect Immun. 2014;82:1030–1035. doi: 10.1128/IAI.01389-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mufazalov IA, et al. Generation of a Novel T Cell Specific Interleukin-1 Receptor Type 1 Conditional Knock Out Mouse Reveals Intrinsic Defects in Survival, Expansion and Cytokine Production of CD4 T Cells. PLoS One. 2016;11:e0161505. doi: 10.1371/journal.pone.0161505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 39.Gaffen SL, Jain R, Garg A, Cua D. IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Suegara N, Siegel JE, Savage DC. Ecological determinants in microbial colonization of the murine gastrointestinal tract: adherence of Torulopsis pintolopesii to epithelial surfaces. Infect Immun. 1979;25:139–145. doi: 10.1128/iai.25.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kashem SW, et al. Candida albicans Morphology and Dendritic Cell Subsets Determine T Helper Cell Differentiation. Immunity. 2015;42:356–366. doi: 10.1016/j.immuni.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drummond RA, Gaffen SL, Hise AG, Brown GD. Innate Defense against Fungal Pathogens. Cold Spring Harb Perspect Med. 2014;5 doi: 10.1101/cshperspect.a019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schonherr FA, et al. The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.2. in press. [DOI] [PubMed] [Google Scholar]
- 45.MacCallum DM, et al. Property differences among the four major Candida albicans strain clades. Eukaryot Cell. 2009;8:373–387. doi: 10.1128/EC.00387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutzan N, et al. On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity. 2017;46:133–147. doi: 10.1016/j.immuni.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naik S, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St Leger AJ, et al. An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal γδ T Cells. Immunity. 2017;47:148–158e145. doi: 10.1016/j.immuni.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo L, et al. Innate immunological function of TH2 cells in vivo. Nat Immunol. 2015;16:1051–1059. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmkvist P, et al. A major population of mucosal memory CD4+ T cells, coexpressing IL-18Rα and DR3, display innate lymphocyte functionality. Mucosal Immunol. 2015;8:545–558. doi: 10.1038/mi.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claudio E, et al. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whibley N, et al. Antibody blockade of IL-17-family cytokines in immunity to acute murine oral mucosal candidiasis. J Leukoc Biol. 2016;99:1153–1164. doi: 10.1189/jlb.4A0915-428R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.