Abstract
The human brain consumes 20% of the total basal oxygen (O2) budget to support ATP intensive neuronal activity. Without sufficient O2 to support ATP demands, neuronal activity fails, such that, even transient ischemia is neurodegenerative. While the essentiality of O2 to brain function is clear, how oxidative stress causes neurodegeneration is ambiguous. Ambiguity exists because many of the reasons why the brain is susceptible to oxidative stress remain obscure. Many are erroneously understood as the deleterious result of adventitious O2 derived free radical and non-radical species generation. To understand how many reasons underpin oxidative stress, one must first re-cast free radical and non-radical species in a positive light because their deliberate generation enables the brain to achieve critical functions (e.g. synaptic plasticity) through redox signalling (i.e. positive functionality). Using free radicals and non-radical derivatives to signal sensitises the brain to oxidative stress when redox signalling goes awry (i.e. negative functionality). To advance mechanistic understanding, we rationalise 13 reasons why the brain is susceptible to oxidative stress. Key reasons include inter alia unsaturated lipid enrichment, mitochondria, calcium, glutamate, modest antioxidant defence, redox active transition metals and neurotransmitter auto-oxidation. We review RNA oxidation as an underappreciated cause of oxidative stress. The complex interplay between each reason dictates neuronal susceptibility to oxidative stress in a dynamic context and neural identity dependent manner. Our discourse sets the stage for investigators to interrogate the biochemical basis of oxidative stress in the brain in health and disease.
Keywords: Mitochondria, Brain, Redox signalling, Oxidative stress, Neurodegeneration
Graphical abstract
Highlights
-
•
The brain deliberately produces reactive species to transmit redox signals.
-
•
Redox signalling regulates critical functions (e.g. synaptic plasticity).
-
•
The brain is susceptible to oxidative stress when redox signalling goes awry.
1. The brain and oxygen: locked in a lethal dance to the death
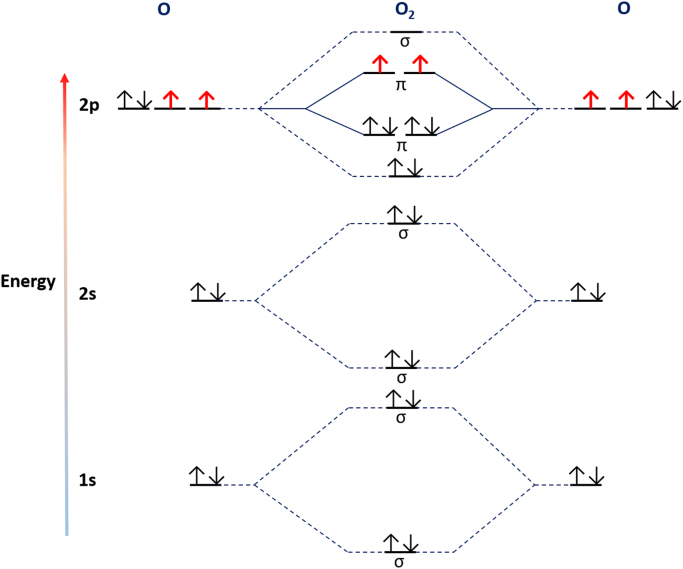
Despite weighing a mere ~1400 g the human brain voraciously consumes ~20% of the total basal oxygen (O2) budget to power its ~86 billion neurons and their unfathomably complex connectome spanning trillions of synapses [1], [2], [3]—abetted by ~250–300 billion glia [4], [5]. The brain must “breathe” to think—even transient ischemia heralds mass neurodegeneration [6]. Depriving the brain of O2 for just 30 min in ischemic stroke exacts a devastating toll: every minute ~1.9 million neurons and ~14 million synapses perish [6]. Neurons and their synapses perish because without sufficient O2, mitochondria are unable to reduce O2 to H2O to support ATP synthesis [7]. Yet, perversely, at least prima facia, the brain carefully regulates O2 use. For the simple biochemical reason that ground state molecular O2 is a di-radical and, therefore, a potentially toxic mutagenic gas. Fortuitously, the potential oxidising power of O2 is constrained by a chemical quirk: because the two lone electrons spin in parallel O2 can only accept one electron at a time [8], [9].
If spin restriction limits its reactivity, why is O2 considered toxic? The answer lies in its ability to give rise to free radical and non-radicals, notably superoxide anion (O2.-), hydrogen peroxide (H2O2) and hydroxyl (.OH) (their biochemistry is reviewed in [8], [10], [11]). Such species are usually considered to constitute the “dark side” of O2 biochemistry—the unavoidable cost of using O2 to respire [12]. It has long been assumed that their adventitious and unwanted generation sensitises the brain to “oxidative stress”. Indeed, oxidative stress is intimately tied to neurodegeneration [13], [14]. However, the simple dichotomy that O2 is good and its reactive progeny (e.g. O2.-) are bad, fails to explain why and how the brain is susceptible to oxidative stress because it is incorrect. To understand why and how the brain is susceptible to oxidative stress, one must abandon the dogma that O2 derived free radicals and non-radicals are just deleterious metabolic by-products and consider their nuances. For example, nestled within the brains sensitivity to hypoxia, resides an extraordinary molecular detail: mitochondrial O2.- signals beneficial adaptive responses [7]. Far from being an exception, such redox signalling is pervasive [15], [16]. Oxidative stress can arise when redox signalling goes awry (i.e. the “Janus” face of redox signalling). Redox nuances mean the brains susceptibility to oxidative stress is seldom rationalised, which hinders attempts to disambiguate the complex relationship between oxidative stress and neurodegeneration. To advance mechanistic understanding, we biochemically rationalise 13 reasons why the brain is susceptible to oxidative stress. To do so, we draw on the seminal work of Barry Halliwell and John Gutteridge [17], [18], [19].
1.1. Redox signalling: reactive species play useful biological roles
A singular and indeed often overlooked reason why the brain is susceptible to oxidative stress is because reactive species play useful biological roles [19], [20]. Two exemplars serve to illustrate the point. First, Chang's group [21] have shown that NADPH oxidase 2 (NOX2) derived O2.- and H2O2 regulate adult hippocampal progenitor cell growth via PI3K/Akt signalling. Their findings reveal a beneficial, homeostatic role for NOX2 derived O2.-/H2O2 in the maintenance of essential neural progenitors [21]. The expression of NOX2, a dedicated O2.- producing enzyme [22], [23], alone hints at an essential role for redox signalling. A related corollary is that NOX isoforms regulate hippocampal long term potentiation (LTP)—important for learning and memory [24]. Deleting NOX2 causes cognitive impairment in mice [25]. Second, Vriz's group, have identified beneficial roles for NOX derived H2O2 in axonal pathfinding and regeneration [26], [27]. Axonal pathfinding wires the developing brain [28], in part, via secreted chemoattractant and chemo-repellent cues that ensure correct target innervation. Pharmacologically inhibiting NOX2 mediated O2.-/H2O2 generation retards retinal ganglion cell axon outgrowth in vivo in larval zebrafish, placing H2O2 as an endogenous chemoattractant [26].
1.2. Calcium
Action potentials causes dramatic calcium (Ca2+) fluxes in pre-synaptic terminals, raising [Ca2+] by ~four orders of magnitude (from 0.01 to ~100 µM [29]). Ca2+ transients trigger neurotransmitter vesicle exocytosis [29]. Consequently, activity dependent Ca2+ transients control bidirectional synaptic plasticity [30]. Bidirectional synaptic plasticity is fundamental to brain function—being required for learning and memory to give just one prominent example [31], [32], [33]. The brains reliance on Ca2+ signalling [34] can cause oxidative stress: the nature of which is variable and context dependent owing to the complex relationship between Ca2+ and the intracellular redox environment [19]. The interested reader is referred elsewhere for a comprehensive review of Ca2+/ redox interplay [35], our discourse is confined to three points. First, Ca2+ transients stimulate neuronal nitric oxide synthase (nNOS) mediated nitric oxide (NO.) synthesis [36], provided sufficient O2 and NADPH are available for NO. synthesis [37]. Residually elevated intracellular [Ca2+] may, therefore, increase NO., which can inhibit mitochondrial respiration by binding to cytochrome c oxidase (COX) [38]. NO. reacts at a diffusion controlled rate with O2.- to yield peroxynitrite (ONOO-) [39]. ONOO- can lead to carbonate (CO3.-) and nitrogen dioxide (NO2.-) radical generation secondary to reaction with carbon dioxide (CO2) to yield peroxomonocarbonate [40]. CO3.- and NO2.- may contribute to neurodegeneration—for example, by nitrating heat shock protein 90 to induce apoptosis in amyotrophic lateral sclerosis (ALS) [41]. A related corollary: Ca2+ can increase phospholipase A2 activity [34]. Phospholipase A2 isoforms de-esterify membrane phospholipids—which can promote enzymatic (i.e. via LOX [42]) and non-enzymatic peroxidation of bis-allyic unsaturated lipids [43].
Second, intracellular Ca2+ release—important for synaptic plasticity [44]—is redox regulated [45], [46]. For example, Hajnoczky’ group [47] show that mitochondrial H2O2 nanodomains regulate Ca2+ transients. Ca2+ transients induce endoplasmic reticulum (ER) mitochondria contacts, termed ER associated mitochondria membranes (MAM [48], [49]), leading to mitochondrial Ca2+ uptake. Mitochondrial Ca2+ uptake amplifies ER Ca2+ release by inducing potassium uptake to thereby increase matrix volume and compress the MIS to concentrate matrix H2O2 at the MAM [47]. These authors suggest H2O2 induces ER Ca2+ release via the IP3 receptor, consistent with its redox regulation via cysteine oxidation [50]. Because the MAM regulates a host of mitochondrial functions (e.g. transport and biogenesis [48]) one can easily envisage how dysregulated inter-organelle communication can cause aberrant local Ca2+/H2O2 signalling associated oxidative stress [45]. To be sure, dysregulated MAM signalling is linked to neurodegeneration in AD and ALS [51]. For example, Stoica et al. [52] show that mutant TD43—a pathological trigger in ALS and frontotemporal dementia [53]—reduces MAM contacts and thereby disrupts Ca2+ homeostasis. (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6)
Fig. 1.
Molecular diagram of a ground state diatomic oxygen molecule (3∑g-O2). Left and right sides depict the electronic configuration of constituent oxygen atoms while the middle panel depicts bonding and antibonding orbitals within 3∑g-O2 by energy level. 3∑g-O2 is a di-radical because lone (i.e. single) electrons occupy the two degenerate π*2p antibonding orbitals (shown in red). The two lone electrons possess parallel spins—locking 3∑g-O2 in a spin restricted state. Spin restriction is fortuitous because it constrains the reactivity of 3∑g-O2.
Fig. 2.
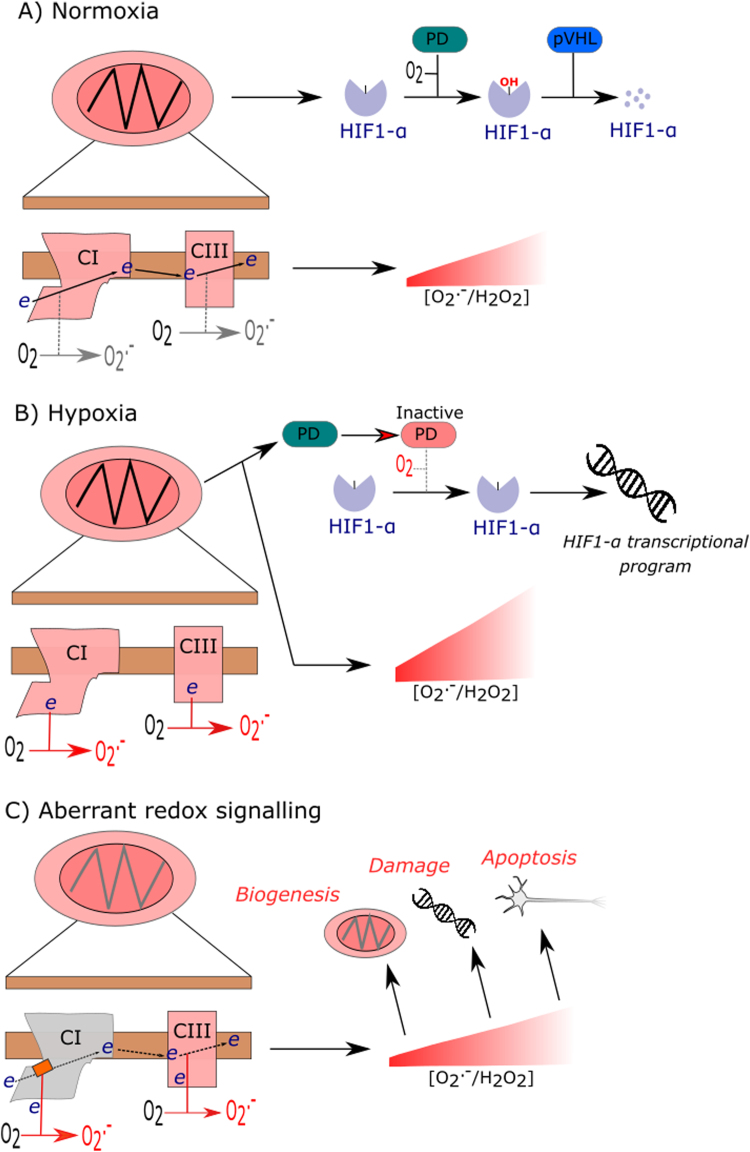
Mitochondrial redox signalling. A) In normoxia, mitochondrial O2.-/H2O2 release is depicted as being low, based on the assumption that mitochondria are generating ATP. Electron flux through CI and CIII is depicted with minimal O2.- generation. PD uses O2 to hydroxylate HIF1-α before pVHL degrades HIF1-α. B) In hypoxia, reduced CI and CIII generate O2.-/H2O2. O2.-/H2O2 inactivate PD, possible by liberating active site Fe2+. PD inhibition enables HIF1-α to enter the nucleus to transcribe hypoxia associated gene programs. C) Aberrant redox signalling. A misassembled CI owing to mito-nuclear mismatch is depicted (i.e. grey box over CI). CI mismatch diverts electrons to O2 to generate O2.-. Various signalling abnormalities may ensue including biogenesis, DNA damage responses and apoptosis owing to persistent mitochondrial O2.- generation are graphically depicted.
Fig. 3.
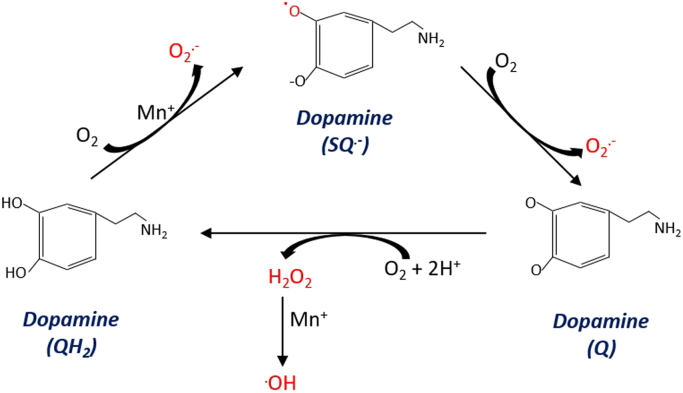
Neurotransmitter autoxidation. From left to right. A transition metal (Mn+) catalysed reaction is shown wherein the alpha hydroxyl group of dopamine is oxidised to the semi-quinone radical. The semi-quinone radical then reacts with O2 to generate O2.- and a quinone. O2 can re-oxidise the quinone to quinol to generate H2O2. Transition metals may react with H2O2 to yield indiscriminately reactive .OH.
Fig. 4.
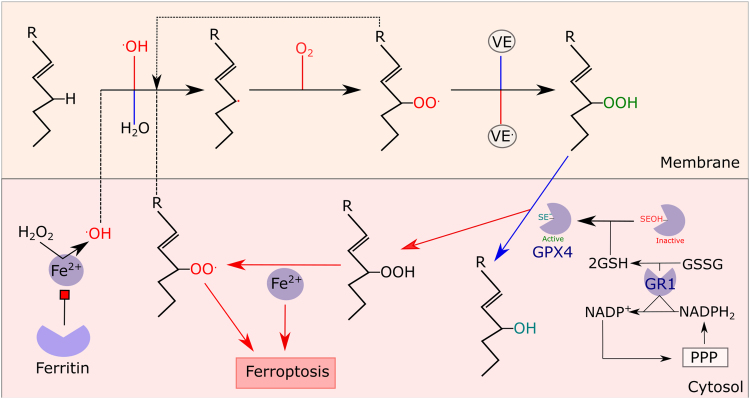
Lipid peroxidation. (1) Initiation..OH abstracts a H+ from an unsaturated lipid to yield a carbon radical. (2) Oxygenation. O2 reacts with the carbon radical to yield ROO. (3) Propagation. ROO. abstracts a H+ from an unsaturated lipid to yield ROOH and a carbon radical. (4). Termination. Alpha tocopherol (VE) terminates chain radicals by reacting with ROO. to yield ROOH. (5). GPX4. GPX4 converts ROOH to the corresponding alcohol. GPX4 is regenerated by 2GSH and the resultant GSSG is reduced by GR using PPP derived NADPH. 6. Fe2+. Fe2+ reacts with ROOH to yield ROO.. ROO. can initiate lipid peroxidation, which triggers ferroptosis when GPX4 activity is suppressed. In addition, Fe2+ can convert H2O2 to .OH (i.e. Fenton chemistry) to initiate lipid peroxidation. Ferritin can limit lipid peroxidation by ligating Fe2+. Certain steps (e.g. carbon radical re-arrangement) are omitted for clarity.
Fig. 5.
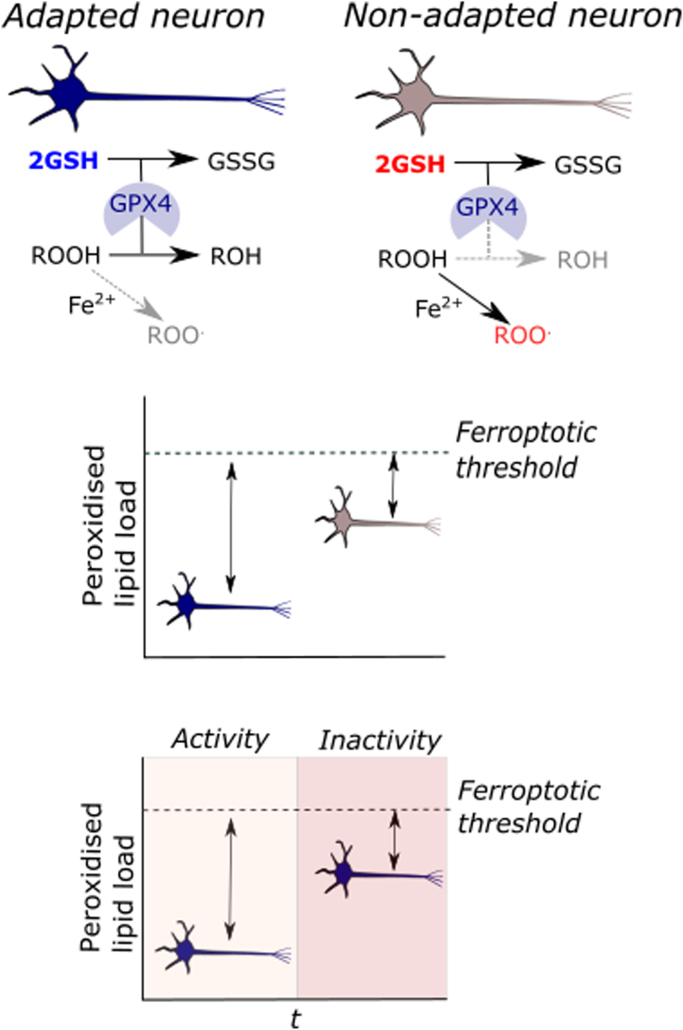
Use it or lose it. (1) Depicts GSH-linked GPX4 ROOH metabolism in an adapted (left) and non-adapted neuron (right). Neuronal activity induced GSH up-regulation supports GPX4 activity to decrease [ROOH] to avert Fe2+ catalysed radical reactions, which re-initiate lipid peroxidation. In the non-adapted neuron, low [GSH] increases the likelihood of Fe2+ reacting with ROOH owing to decreased GPX4 activity. (2) A theoretical threshold model wherein the adaptive history (described above) divergently modulates the peroxidised lipid load required to induct ferroptosis. An adapted neuron, can withstand a larger absolute rise in [ROOH] before undergoing ferroptosis. (3) A theoretical threshold model an adapted neuron loses its ferroptotic resistance with persistent inactivity. Hence, a lack of stimulus (e.g. neuronal activity) erodes the adaptation.
Fig. 6.
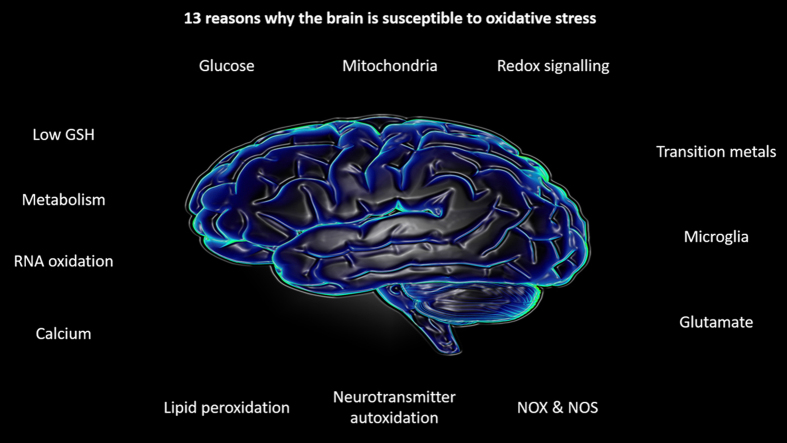
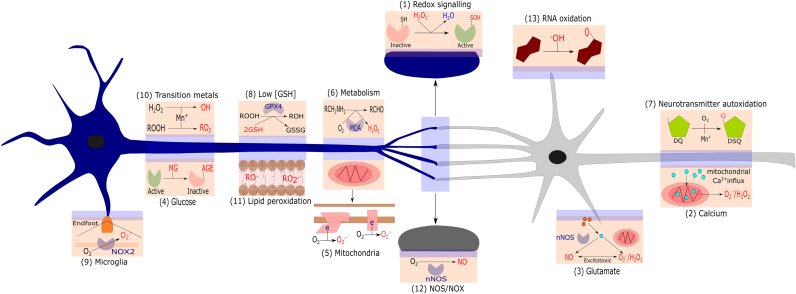
13 reasons why the brain is susceptible to oxidative stress. (1) Redox signalling. Depicts H2O2 induced activation of a signalling protein via sulphenic acid (SOH) formation. (2) Calcium. Depicts mitochondrial Ca2+ overload induced O2.-/H2O2 generation. (3) Glutamate. Depicts glutamate induced Ca2+ release inducing nNOS mediated NO. generation and mitochondrial O2.-/H2O2 generation, leading to ONOO- and excitotoxicity. (4). Glucose. Depicts protein inactivation via AGE formation. (5). Mitochondria. Depicts mitochondrial O2.- generation at CI and CIII. (6) Metabolism. Depicts MOA isoform catalysed H2O2 generation. (7) Neurotransmitter oxidation. Depicts redox active transition metal (Mn+) catalysed dopamine auto-oxidation to a semi-quinone radical. (8) Modest antioxidant defence. Depicts constrained GPX4 activity owing to low [GSH]. (9) Microglia. Depicts NOX2 mediated O2.- generation within an end-foot process. (10) Redox active transition metals. Depicts Mn+ catalysed ROO. and .OH generation. (11) Lipid peroxidation. Depicts RO. and ROO. within a neuronal cell membrane. (12) NOS/NOX expression. Depicts nNOS mediated NO. generation. (13). RNA oxidation. Depicts .OH mediated RNA oxidation (guanine is shown as an example).
A third related point of interplay: mitochondrial Ca2+ overload opens the mitochondrial permeability transition pore (mPTP) [54]. mPTP opening induces O2.-/H2O2 efflux and abolishes ATP synthesis [55], [56], [57]. Transient mPTP opening enables mitochondria to re-set matrix Ca2+ [54], [58], and is, perhaps, permissive for redox signalling by enabling O2.-/H2O2 to exit mitochondria to evade matrix metabolism [59] (a phenomenon that may be linked to mitochondrial contractions [60], [61]). Prolonged mPTP opening heralds necroptosis [62]. In addition, Ca2+ overload can regulate intrinsic apoptosis. Importantly, necroptosis and apoptosis are linked to neurodegeneration [63], [64]. Because mitochondrial Ca2+ uptake supports ATP synthesis [65], [66], [67], decreased mitochondrial [Ca2+] may cause oxidative stress by increasing [NADH] and concomitant O2.- generation at the FMN site in complex I [68], [69]. Cytochrome c could also use H2O2 to oxidise cardiolipin, an essential inner membrane phospholipid, to trigger intrinsic apoptosis [70], [71]. Unsurprisingly (1) the brain expends considerable ATP to maintain intracellular Ca2+ homeostasis and (2) neurodegenerative diseases are usually associated with disrupted Ca2+ homeostasis.
1.3. Glutamate
Excessive glutamate uptake (e.g. by N-methyl-D-aspartate receptors (NMDARs)) causes excitotoxicity [72], [73] secondary to aberrant Ca2+ signalling—for example, leading to sustained calpain signalling [74]. Glutamate excitotoxicity leads to Ca2+ overload linked mitochondrial [O2.-/H2O2] release associated cell death, typically via apoptosis and necrosis [17], [75], [76]. Ca2+ influx can activate nNOS: opening up the possibility that NO. inhibits COX to increase mitochondrial [O2.-/H2O2/ONOO-]. Consistent with pharmacological nNOS blockade protecting against excitotoxicity [77]. Necrotic cell death amplifies excitotoxicity by elevating extracellular [glutamate] [78]. Intriguingly, NMDAR mediated glutamate uptake may be subject to differential spatial regulation: extra-synaptic uptake causes excitotoxicity whereas synaptic uptake initiates adaptive responses [79], [80], [81], [82]. As Hardingham's group [79] show synaptic NMDAR mediated glutamergic neurotransmission up-regulates the peroxiredoxin-thioredoxin (PRDX-TRDX) enzyme system and down-regulates apoptotic signalling. Perhaps, spatial specificity underlies generator specific functionality wherein extra-synaptic NMDA linked mitochondrial O2.- generation is neurodegenerative whereas NOX2 linked synaptic NMDA receptor linked O2.- is protective [83]. Beyond receptors, glutamate can cause excitotoxicity by inhibiting the system Xc- transporter [84]—which exchanges intracellular glutamate for extracellular cystine [85]. Intracellular cystine is reduced to cysteine, which can be used by glutamate cysteine ligase for de novo glutathione (GSH) synthesis [86]. Inhibiting cystine uptake causes oxidative stress by depleting intracellular [GSH] [84], [87]. Depleting intracellular [GSH] is sufficient to trigger ferroptosis—iron and lipid peroxidation dependent cell death [88]—suggesting extracellular glutamate is an endogenous ferroptotic cue [89], [90]. However, as Cao & Dixon caution [90] despite redox commonalities glutamate excitotoxicity associated cell death and ferroptosis have distinctive elements, notably the involvement of apoptotic signalling in the former.
1.4. Glucose
The human brain consumes ~25% of circulating [glucose] to support neuronal activity [91] (corresponding to ~5.6 mg glucose per 100 g of brain tissue per min). The fate of glucose in the brain is complex and involves neuronal-glia metabolic coupling (reviewed in [3], [92]). Glia metabolise glucose to lactate before it is taken up, converted to pyruvate and oxidised by neuronal mitochondria to generate ATP [3]. Consistent with evidence suggesting neurons efficiently metabolise lactate [93], [94]. A related corollary: neurons constitutively degrade the rate-limiting glycolytic enzyme, phosphofructokinase (PFK), to preferentially use glucose to power the pentose phosphate pathway (PPP) [95]. From a redox perspective, transcellular metabolic coupling seems to compensate for the limited capacity of neurons to metabolise dicarbonyls owing to low glyoxylase 1 (GLO1) and glyoxylase 2 (GLO2) expression [96]. GLO isoforms metabolise methylglyoxyal (MG) [97]—a potentially toxic triose phosphate isomerase derived dicarbonly [98], [99]—in a GSH dependent manner. GLO1 metabolises GSH conjugated MG (i.e. hemithioacetal) to S-D-lactoylglutathione before GLO2 converts S-D-lactoylglutathione to D-lactate and GSH. Low GLO isoform content coupled to comparatively low [GSH] sensitises neurons to MG toxicity: 250 µM MG is sufficient to saturate neuronal MG metabolism whereas astrocytic metabolism remains intact at 2 mM [96]. With the caveat that “free” [MG] is typically 2–4 µM [100]. Notwithstanding, MG is reactive—50,000 fold more so than glucose—and readily forms Shiff bases to glycate proteins, RNA and DNA [100]. In particular, protein glycation underlies the formation of advanced end glycation products (AGE), which can cause oxidative stress by stimulating inflammation via their receptor, impairing protein and mitochondrial function [100], [101], [102]. AGEs can arise in absence of high glycolytic rates because lipid peroxidation can yield MG [101]. In sum, the brain is susceptible to glucose induced oxidative stress [97].
1.5. Mitochondria
Disproportionate O2 uptake supports oxidative phosphorylation to help fuel the brains extraordinary ATP demand [3]. Neurons expend ATP to maintain ionic gradients and support synaptic activity [103], [104]. The sheer energetic costs of synaptic activity are exemplified by neurotransmitter loaded vesicle release alone consuming 1.64 × 105 ATP per second per vesicle [104], [105]. Meeting neuronal ATP demands requires mitochondria, particularly synaptic mitochondria [106] owing to limited ATP diffusion. Neurons are especially reliant on mitochondria because they constitutively degrade PFK to limit glycolysis [95]—although glycosomes can temporarily support synaptic ATP synthesis [107]. Beyond oxidative phosphorylation, mitochondria are essential signalling hubs regulating a veritable plethora of essential processes, from Ca2+ homeostasis, Fe-S cluster synthesis to cell fate [55], [108], [109]. Neuronal mitochondria are a quintessential double-edged sword: endowing neurons with ATP and signalosomes while imparting intrinsic neurodegenerative vulnerability to their dysfunction [110].
Instead of propounding the somewhat prosaic view that O2.-/H2O2 are obligate, toxic by-products of mitochondrial respiration that cause oxidative damage, we interpret neuronal susceptibility to mitochondrial oxidative stress from a signalling perspective [111]. How mitochondria produce O2.-/H2O2 (see Murphy [68] for a comprehensive review) places them as sentinels of organelle health [112]. Their deliberate generation is intimately tied to adaptive redox signalling [113]. Hypoxia signalling is a cogent example. Mitochondria sense hypoxia (i.e. 0.3–3% O2) by generating complex I and complex III derived O2.-/H2O2 to activate hypoxia inducible factor one alpha (HIF1-α) via degrading propyl hydroxylase. HIF1-α initiates adaptive transcriptional responses [114], [115], [116], [117], [118]. Because mitochondrial O2.-/H2O2 production at a given site reflects: [O2] [reduced site] and the kinetics of the reaction [68], hypoxia reduces complex I and III to trigger O2.- generation (which may be abetted by reduced COX activity to increase local O2 availability). HIF1-α transcribes NDUFA4L2, an alternate complex I subunit, to suppress O2.- generation to conclude hypoxic signalling [119]. Aberrant redox signalling can be neurodegenerative. Failing to terminate mitochondrial O2.- generation could initiate redox regulated intrinsic apoptosis [120], [121]. In addition, misassembled respiratory chains owing to mito-nuclear mismatch could induce the signal (i.e. O2.-/H2O2) without the cue (i.e. hypoxia), leading to mal-adaptive responses [111]. If mutant mitochondria accumulate, they may cause dysfunction by clonal expanding their number because O2.-/H2O2 regulate mitochondrial biogenesis [111], [122], [123].
1.6. Endogenous neurotransmitter metabolism generates hydrogen peroxide
Endogenous amine based neurotransmitter (e.g. dopamine) metabolism generates mitochondrial H2O2 via monoamine oxidase enzymes. Monoamine oxidase A (MOA-A) and B (MOA-B) catalyse a deamination reaction: amine + O2 + H2O → aldehyde + H2O2 + NH3. While both enzymes metabolise dopamine, tyramine, tryptamine and noradrenaline, MOA-A preferentially metabolises 5-hydroxytryptamine whereas MOA-B prefers 2-phenylethylamine [124], [125]. During the catalytic cycle, amine oxidation to imine reduces a prosthetic flavin moiety, which reacts with O2 to yield H2O2 [126], [127]. Once the flavin is reduced, the rate of O2 binding controls H2O2 generation, with the implication that [O2] influences enzyme activity. The affinity of each isoform for O2 is 10 and 240 µM for MOA-A and MOA-B, respectively [127]. Under O2 saturated conditions, their capacity to produce H2O2 is considerable—Cadenas and colleagues [128] showed that tryamine demaination increases H2O2 levels by approximately 1 nmol/kg−1/min−1 in brain mitochondria. Axiomatically, the presence of a H2O2 generating enzyme together with neuronal activity induced substrate flux can cause oxidative stress [17], [18]. Particularly, when one considers that MOA-B is localised to the outer face mitochondrial inner membrane [129] because little endogenous capacity to metabolise H2O2 in the mitochondrial intramembrane space (MIS) exists. Glutathione peroxidase 4 (GPX4), the sole peroxidase in the MIS [59], preferentially reduces lipid hydroperoxides (ROOH) over H2O2 [130], [131]. MOA-A/B activity can trigger apoptosis in a Ca2+ sensitive fashion [132], [133], [134], which may link MOA/B derived H2O2 and neuronal cell death. Unsurprisingly, aberrant MOA-A/B activity has been linked to ageing [135] and related neurodegenerative disorders, notably Alzheimer's disease (AD) and Parkinson's disease (PD) [136], [137]. Spurring interest in the use of synthetic MOA-A/B inhibitors to treat neurodegeneration and indeed mood disorders [124], [125], [138].
An underappreciated aspect of MOA/B biology is by restricting O2.- induced neurotransmitter oxidation and subsequent redox cycling, they may limit H2O2 generation. For example, dopamine oxidation can yield multiple H2O2 molecules [139] whereas stoichiometric MOA-A/B metabolism produces a single H2O2 molecule. With the caveat that certain aldehydes products (e.g. 4-dihydroxyphenylacetaldehyde) can redox cycle [140], [141]. A related corollary is that by helping to terminate neuronal activity MOA isoforms may protect against excitotoxicity. MOA-A/B may also protect the brain from exogenous xenobiotics. Notwithstanding, electrophilic aldehydes can conjugate macromolecules to cause damage [142]. For example, 3,4-dihydroxyphenylacetaldehyde, a dopamine metabolite readily conjugates proteins and is toxic to neurons [143]—a rise from 2–3 to 6 µM is sufficient to cause cell death [144]. MOA isoform activity must, therefore, be counter-balanced with aldehyde dehydrogenase (ADH) activity to prevent toxicity. Because ADH2 [145] is localised to the mitochondrial matrix the MIS may be unable to remove aldehydes enzymatically, which would favour macromolecule conjugates—especially if electrical charge occludes passive diffusion. Perhaps, electrophilic aldehydes, as opposed to H2O2, underlie MOA induced oxidative stress. While speculative, H2O2 signalling may inform the nucleus that aldehydes are being formed. ADH inhibition contributes to PD [146]—which underscores the importance of counter-balancing MOA activity. In sum, MOA isoforms can cause oxidative stress in the brain.
1.7. Neurotransmitters can auto-oxidise
In their seminal works, Cohen and Heikkla [147], [148] showed that dopamine reacts with O2 to generate a dopamine semiquinone radical, which can then react with another O2 to generate O2.- and a dopamine quinone. While the initial rate of semiquinone radical formation is often slow [19], it can be accelerated by redox active transition metals [149]—which are abundant in the brain [17]. Dopamine quinones can combine to yield semiquinones [150]—which react with O2 to give O2.-, with the caveat that this reaction competes with a cyclisation reaction that averts redox cycling [151]. The mix of O2.-, H2O2 and .OH detected is indicative of hydroquinone, semi-quinone and quinone equilibria [150]. Dopamine oxidation products can also redox cycle [152]. For example, 6-hydroxydopamine can be reduced to a semiquinone radical which reacts with O2 to yield O2.-, in turn, O2.- reacts with another 6-hydroxydopamine to regenerate the semiquinone radical and H2O2. A situation that leads to further O2.- generation [139] and .OH generation, provided a fraction of the H2O2 generated reacts with redox active transition metals [11]. Ubiquitous superoxide dismutase (SOD) isoforms [153], [154], [155] rapidly remove O2.- (k ~2 × 109 M−1 s−1) to terminate radical chains [156]. The net influence of SOD isoforms is complex because they can also favour O2.- generation [139], [150]. By restricting [O2.-] to the picomolar range, SOD isoforms can shift equilibrium reactions of semiquinones towards their reaction with to O2 to generate O2.-; as is the case for 4-dihydroxyphenylacetaldehyde radical [140]. Importantly, serotonin and adrenaline also autoxidise, with adrenalin autoxidation being used to assay SOD activity [157]. Neurotransmitters with catechol groups, therefore, render the brain particularly sensitive to oxidative stress [17], [18]. For example, redox cycling of dopamine metabolites, in particular 6-hydroxydopamine, contributes to PD [158], [159]. In PD, dopamine oxidation [160] drives mitochondrial and lysosomal dysfunction, in part, via dopamine quinones abrogating glucocerebrosidase activity—a lysosomal enzyme implicated in PD pathogenesis [161]—and elevated mitochondrial [H2O2].
1.8. Modest endogenous antioxidant defence
As reviewed by Halliwell [17], [18], modest endogenous antioxidant defence sensitises the brain to oxidative stress. That is, comparatively low endogenous antioxidant defence relative to many tissues (e.g. liver) makes the brain susceptible to disrupted redox homeostasis. While low catalase content—neurons possess 50 times lower catalase content compared to hepatocytes [162]—is a frequently cited exemplar [163], the relative importance of catalase to steady-state H2O2 removal is questionable. Aside from catalase being largely restricted to peroxisomes, its reaction mechanism requires two H2O2 molecules [164]—which may restrict its activity at nanomolar H2O2 [165]. GSH, however, provides a cogent example. Cytosolic GSH is ~50% lower in neurons compared with other cells (e.g. ~5 mM in neurons compared with 10–11 mM in hepatocytes). Low cytosolic GSH reflects, in part, a reduced capacity for GSH synthesis owing to low γ-GCL content—a corollary of minimal Nrf-2 content and activity [81], [166]. Comparatively, low cytosolic GSH may restrict GPX4 activity [130], which may explain neuronal sensitivity to ferropotsis [167]. Low GSH may also limit the ability of neurons to metabolise electrophiles, particularly electrophilic aldehydes. From the discussion so far, it would seem the apparent defect relates to H2O2 metabolism [18], [19] because SOD isoform content and activity is normative (i.e. no defect in O2.- metabolism). Intact O2.- metabolism (i.e. SOD activity) is essential because neurons are unable to survive genetic deletion of MnSOD [168], [169], [170], the mitochondrial isoform [154]. In sum, GSH linked enzymatic systems are modest in neurons.
In 1994, Soo Goo Rhee's group identified the PRDX family as ubiquitous cysteine dependent H2O2 peroxidases [171]. PRDX isoforms are reduced by TRDX, oxidised TRDX is, in turn, reduced by thioredoxin reductase at the expense of NADPH [172], [173], [174], [175], [176]. Their discovery has important implications for neuronal H2O2 metabolism because neurons express PRDX-TRDX isoforms [162], [177]. A functional PRRX-TRDX system may enable neurons to metabolise H2O2—particularly when it is considered that PRDX isoforms are abundant and distributed throughout the cell [171], [175], [178]. We are unaware of any report to the effect that PRDX-TRDX activity is comparatively modest in neurons. On the contrary, PRDX activity may be comparatively high in neurons as they preferentially funnel glucose into the NADPH generating PPP [95]. However, enzymes (e.g. NOS) use NADPH to generate O2.- and NO. [179], [180], so it is unwise to assume NADPH exclusively fuels “antioxidants” [181]. However, PRDX6 prefers GSH as a reductant [182] so comparatively low GSH may limit its activity. It is remiss to consider PRDX-TRDX as “only” H2O2 “neutralisers” because compelling biochemical evidence suggests PRDX-TRDX transduce redox signals [16], [172], [183], [184], [185], [186], [187]. As Flohé et al. [188] elegantly enunciate nature is unlikely to have evolved over ten peroxidases just to remove H2O2. Lu, Holmgren and co-workers [162], suggest that PRDX-TRDX endow neurons with the capacity to harness their relative “oxidative stress” to transduce redox signals. If so, such a state of affairs is perilous, if PRDX isoforms become over-oxidised when [H2O2] rises to high nanomolar levels that seem to herald cell death [45], [180]. Particularly, given the modest capacity of neuronal GSH linked enzyme systems [81], [166]. PRDX-TRDX provide a means to remove, as well as, harness H2O2 for cell signalling but the possibility remains that beyond a critical threshold elevated [H2O2] easily short circuits this system to cause oxidative stress.
1.9. Microglia
Microglia are specialised, resident immune cells [189], [190] that perpetually scan their local niche for homeostatic threats [191], [192]. Microglia deploy extended processes to survey synapse health by monitoring neuronal activity [193]. By monitoring neuronal activity, microglia play an important role in removing unhealthy cells, neuronal wiring during development and activity dependent synaptic plasticity [194], [195], [196], [197]. The ground breaking work of Bernard Babior [198], showed that active immune cells produce O2.- via NOX isoforms (principally NOX2 [23]). The role of O2.- in bacterial killing was one of the first examples of a biologically useful role for free radicals [199]. It is unsurprising, therefore, that microglia generate O2.- and related reactive progeny during phaogcytoysis [200]. However, one should note that because O2.- production depends on O2, microglia activity will be extremely sensitive to local O2 bioavailability—their O2 use may even be one way to remove synapses by consuming O2 to power O2.-, as opposed to ATP, synthesis. It is unlikely that facile (e.g. .OH) or anionic species (i.e. O2.-) exit phagocytic endosomes to harm neighbouring neurons (if anions did escape their entry is charge restricted in any event), uncharged H2O2 and NO. may diffuse to cause damage or amplify local inflammation by attracting more microglia. Niethammer's and Amaya's groups have shown that H2O2 acts as a chemoattractant in wound healing and limb regeneration [201], [202]. Patrolling microglia may “sense” H2O2 to induce their activation and proliferation [203], which provides a mechanism whereby neuronal H2O2 release attracts microglia. In support, Lyn, a tyrosine kinase, detects nerve derived H2O2 and primes microglia for chemotaxis via F-actin [204]. How Lyn detects H2O2 is unclear but may involve H2O2 linked phosphatase inactivation [15], [16]. Self-amplifying inflammatory loops exist owing to cytokine and peroxidised lipid induced microglia activity [205], [206]. While essential for normal brain development and function, unabated microglia activity can cause oxidative stress [206]. For example, in AD, microglia prune synapses to drive neurodegeneration [207], [208]. Whether aberrant pruning requires oxidative stress is unclear., Perhaps peroxidised lipid metabolies [209](e.g. 4-HNE) attract microglia by modifying protein cysteine residues via Michael addition [210], [211].
1.10. Redox active transition metals
Redox active transition metals (i.e. Fe2+ and Cu+) are enriched in the brain [17], [18]. The relative abundance of transition metals in the brain is underlined by their 10,000 enrichment relative to neurotransmitters [212], [213]. Chelated [Fe2+] alone can reach mM levels. Nature harnesses the rich biochemistry of Fe2+ and Cu+ to accelerate chemical reactions. For example, Fe2+ can bind electron dense O and N groups in organic molecules [214]. Accordingly, Fe2+ (and Fe3+) is required to ensure the catalytic activity of several enzymes, including aconitase, fumarase and cytochrome P450 [214]. In addition, Fe2+ is essential for myelin synthesis [215], [216] acting as a co-factor for essential de novo lipid synthesis enzymes. Neurons also harbour a loosely chelated Fe pool, termed the labile iron pool (LIP), which depending on dietary Fe intake is ≈20 µM in most tissues [167], [217]. Fe enrichment means neurons must tightly control [O2.-/H2O2] to avoid the perils of mis-metallation—a corollary of FeS displacement [218]—and Fenton chemistry, which yields indiscriminately reactive .OH (Fe2+ + H2O2 → OH- + .OH) [19]. Even with abundant and kinetically rapid SOD enzymes maintaining steady-state [O2.-] in the picomolar range, Imlay [219] estimates the half-time for mononuclear enzyme damage is still ~20 min owing to favourable kinetics (k ~106 M−1 s−1). For example, Fe2+ loss inactivates the PPP enzyme ribulose-5-phosphate 3-epimerase [220]. Such inactivation may only be transient as compensatory mechanisms exist (e.g. Mg2+ insertion, or LIP mediated re-metallation [220]). Notwithstanding, ablated PPP activity owing to ribulose-5-phosphate 3-epimerase inactivity could compromise neuronal function by restricting nucleotide synthesis [221]. Of particular interest, Fe2+ regulates ferropotosis—a novel Fe2+ and lipid peroxidation dependent form of cell death [88], [167]. Fe2+ contributes to ferroptosis by catalysing peroxyl (ROO.) and aloxyl (RO.) radical generation from ROOH in a reaction is kinetically favoured (k ~1.3 × 103 M−1 s−1) compared to Fenton reaction(k ~76 M−1 s−1) [19], [222], [223]. The influence of Fe2+ is complex because Fe2+ can inhibit lipid peroxidation by scavenging ROO. and RO. in kinetically faster reactions [222] (e.g. RO. + H+ + Fe2+ → ROH + Fe3+, k ~3.0 × 108 M−1 s−1). However, low [ROO./RO.] means Fe2+ is more likely to react with ROOH. For these reasons, the brain is susceptible to dysregulated Fe homeostasis [224]. The pathological susceptibility of the brain to dysregulated Fe2+ homeostasis is underscored by the observation that iron-amyloid beta complexes contribute to plaque deposits in AD [225].
Analogous to Fe2+, neurons contain a “labile” Cu+ pool [226] that seems to be important for cell signalling and neuronal excitability [227], [228]. For example, neuronal activity redistributes the loosely chelated Cu+ pool from the soma to dendrites, which regulates spontaneous neuronal activity [227], [229], [230]. In addition, Cu+ is an essential co-factor for enzymes [212], prominent examples being COX and copper zinc SOD (CuZnSOD) [153], [231], [232]. Neuronal Cu+ enrichment (0.1 mM, up to 1.3 mM in certain regions) predisposes to Cu2+ catalysed Fenton chemistry and H2O2 assisted protein oxidation [212]. The potential perils of dysregulated Cu+ homeostasis are exemplified in ALS. Specifically, mutated CuZnSOD variants contribute to both familial and sporadic ALS [233]. How CuZnSOD causes neurodegeneration is incompletely understood [234], [235] but may relate to a toxic gain of function involving protein aggregates, peroxidase activity, which can generate CO3.- generation via HOOCO2 [231], [236], and thiol oxidase activity [237], [238]. In addition, reduced CuZnSOD activity can also increase [ONOO-] [239].
1.11. Unsaturated lipid enrichment
The brain is the major sink for polyunsaturated n-3 fatty acids [240], notably DHA. Given their ATP demands, one would expect neurons to oxidise lipids to generate ATP, particularly since the ATP yield is greater: 106 ATP per mol palmitic acid vs 32 ATP per mol glucose [241], and only 14–17 ATP per mol lactate [3]. However, compared with other metabolically active tissues (e.g. skeletal muscle [242]), beta oxidation is limited in the brain [241]. Perhaps, to conserve O2—oxidising palmitc acid consumes 15% more O2—and, in light of modest catalase activity [163], to limit preoxisomal enzyme induced H2O2 generation [243], [244]. Recalcitrance to oxidising lipids to generate ATP may stem from using peroxidised lipids to signal [19]. For example, DHA can be metabolised to anti-inflammatory resolvins [245]. Beyond DHA, myelin synthesis requires fatty acids being enriched with cholesterol—its importance being reflected by the brain accounting for ~20% of total cholesterol [19]. Cholesterol auto-oxidises by free radical and non-radical mechanisms [246]. High unsaturated lipid content defines a cause of oxidative stress because of their susceptibility to lipid peroxidation and indeed may be the biological cost of using peroxidised lipids to signal [89].
Lipid peroxidation (reviewed in [43], [247], [248], [249], [250]) involves the initial generation of a carbon radical following an addition or abstraction reaction by a sufficiently reactive species (e.g. .OH) on a methylene group. As an aside, .OH may be dispensable for initiating lipid peroxidation, hyper-valent Fe-O species (e.g. FeIV=O) may be key [222]. Carbon radicals rapidly react with O2 to yield ROO., which can abstract a bis-allyic H+ from another methleyne group to propagate the radical chain by yielding ROOH and a carbon radical [43], [247], [248], [249], [250]. Alpha tocopherol (α-TOC) terminates radical chain propagation to yield ROOH and a resonance stabilised α-TOC radical [249]. ROOH can react with redox active transition metals to re-generate ROO. and RO. [222]. GPX4 rapidly removes ROOH to yield ROH [251], before the inactive enzyme is regenerated using GSH, which is, in turn, regenerated using NADPH dependent glutathione reductase [130], [131], [188]. Ferroptosis [90], [252] explains why genetically deleting GPX4 is embryonically lethal [253], [254] because GPX4 regulates ferroptosis by removing ROOH [223], [255]—gain and loss of GPX activity is sufficient to disable and activate ferroptosis [256], respectively. Modest [GSH] may render neurons particularly susceptible to ferropotsis confirmed by the observation that conditionally deleting GPX4 is lethal to neurons [257], [258], [259]. Consistent with lipid peroxidation contributing to the pathogenesis of neurodegenerative diseases (e.g. AD [209], [211]).
1.12. The brain uses NOS and NOX for signalling
The brain harnesses nNOS and NOX isoforms to achieve essential functions. First, nNOS uses O2, NADPH and L-arginine to catalytically synthesise NO. [37]. The affinity of nNOS for O2 is 300 µM, mean brain [O2] is ~20 µM, which may limit NO. synthesis [260]. NO. regulates essential physiological processes, including LTP [261], [262], axon growth [263] and pruning [264]. As discussed, NO. can underlie oxidative stress—especially when O2.- is co-generated. nNOS biochemistry means that O2.- and NO. can be spatially co-generated making [ONOO-] generation likely [265]. Co-fluxes occur when nNOS is uncoupled. Uncoupling typically arises when essential co-factors (e.g. tetrahydrobiopterin) become oxidised or unbound. Second, NOX isoforms use prosthetic redox groups to oxidise NADPH to reduce O2 to O2.- [22], [23]. NOX isoforms are important in the brain (reviewed in [266]) to support microglia and LTP to give just two examples [24]. Because NOX isoform mediated O2.- generation is far from adventitious being regulated at several levels [22], [23], [267], NOX isoform associated oxidative stress likely stems from the unwanted and continued presence of activating cues coupled to a sustained supply of NADPH and O2 to support enzyme activity. Such a scenario may manifest in neuronal inflammation [206] wherein cytokines provoke and sustain microglia NOX2 associated O2.- generation [205].
1.13. RNA oxidation
RNA oxidation is a seldom appreciated reason why the brain is susceptible to oxidative stress [268]. Beyond essential messenger RNA, the brain heavily relies on non-coding RNAs, particularly long non-coding RNAs and microRNAs (reviewed in [269], [270], [271]). From a biochemical perspective, RNA is equally susceptible to oxidation as DNA, undergoing analogous reactions [272]. For example, 8-oxo-guanine is a principal outcome of both DNA and RNA oxidation [273]. Owing to its single-stranded nature, RNA is also vulnerable to oxidation and indeed alkylation [274] at Watson-Crick interfaces. RNA also lacks protective histones and nuclear compartment in axons and synapses. Although local protein synthesis is essential to synaptic function [275], [276], the possibility that RNA oxidation perturbs local protein synthesis is unexplored. Oxidised RNA associated coding errors stall ribosomal protein synthesis [277], which can if left unrepaired produce truncated, mutated and mis-folded proteins [278]. The spatial positioning of mRNA close to mitochondria and the temporal dynamics of RNA oxidation (order of seconds) compared with translation (order of hours) make RNA oxidation likely—especially in neurons with divalent redox active transition metals present to catalyse Fenton chemistry [279], [280], [281]. The mandate to consider mRNA oxidation as a cause of oxidative stress associated neurodegeneration is strengthened by the observation that oxidised CuZnSOD mRNA is an early pre-clinical feature of ALS [282]. As a number of excellent reviews [268], [272] surmise further work is required to understand oxidised RNA recognition, turnover and repair [283]. Only with a better understanding of each process can one appraise the neuronal susceptibility to RNA oxidation because [oxidised RNA] is a function of formation and removal over time.
2. Perspectives
We wish to propose an overarching perspective for interpreting why redox signalling leads to oxidative stress in the brain. The ultimate price of using redox signalling to inform brain function is innate susceptibility to oxidative stress when signals go awry—as seems to be the case in disease. Few neuroscientists would deny the central importance of neuronal activity. Based on how mitochondria produce O2.-/H2O2 [68], neuronal activity should divergently regulate mitochondrial O2.-/H2O2 generation. At an active synapse, ATP demands—provided they can be met—should reduce net mitochondrial O2.-/H2O2 generation. Whereas at an inactive synapse, low ATP demands and a reduced respiratory chain should favour mitochondrial O2.-/H2O2 generation, potentially placing mitochondrial O2.-/H2O2 as synaptic activity sentinels. If so, one can rationalise how synaptic inactivity induced mitochondrial O2.-/H2O2 release triggers long-term depotentation (LTD) and even synapse pruning—especially if the same pathway is used reiteratively [284]. Mitochondrial apoptosis regulates LTD and pruning [285], [286], [287]. Mitochondrial inactivity associated O2.-/H2O2 release may induce local sub-lethal intrinsic apoptosis to induct LTD and pruning. Perhaps, redox regulated apoptosis enables the developing brain to prune synapses—the essential prelude to a complex connectome and mandatory requirement for continued sculpting in adulthood [284]. Placing mitochondria with their hands on the proverbial shears renders the brain vulnerable to unwanted synapse loss. If mitochondria are unable to meet ATP demands or if O2 is limiting resultant O2.-/H2O2 release may recapitulate the “pruning” signal to cause unwanted synapse loss. Biological precedent exists: unwanted reactivation of developmental pruning signalling contributes to synapse loss in AD [207].
In biochemically rationalising 13 reasons why the brain is susceptible to oxidative stress, we deliberately adopted a global view focusing on “neurons” as a collective for the purposes of a general primer. Apt parallels between the monolithic umbrella terms neurons and reactive species exist [20]. Reactive species subsumes chemically heterogeneous species, that can differ in their rate of reaction with a given substance by orders of magnitude (e.g. for guanine .OH reacts at a diffusion controlled rate whereas O2.- leaves guanine unscathed owing to low reactivity). Analogous to reactive species, neurons are heterogeneous being ill-served by global monikers because they can widely differ in many key parameters, including function, location, connectivity, myelination and axon length. Neuronal heterogeneity informs differential susceptibility to oxidative stress both within a neuron (i.e. soma vs. synapse), subdomain (i.e. synaptic mitochondria vs. synaptic membranes) and between neuronal populations. Dopaminergic neurons in the substantia nigra pars compacta exemplify differential vulnerability: they experience residual (i.e. without additional homeostatic perturbation) oxidative stress because an L-type Ca2+ channel defined mitochondrial O2.-/H2O2 axis controls their autonomous pace-making capacity [288]. Teetering on the edge of an oxidative breakpoint, even minor unchecked shifts in the intracellular redox environment—perhaps related to dopamine metabolism [160]—seem sufficient to herald their demise.
Neuronal sensitivity to oxidative stress oscillates. Just as steady state [O2.-] reflects its dynamic rate of generation and removal in a given compartment [68], a myriad of interconnected factors dynamically set neuronal sensitivity to oxidative stress over time. We briefly consider Fe2+ mediated ROOH reduction to ROO. as a topical example relevant to ferroptosis [223]. The second order bimolecular elementary reaction is informed by the rate constant, [ROOH]ss and [Fe2+]ss. Reactant availability at a given time governs the probability of ROO. generation—with GPX4 catalysed ROOH metabolism and ferritin mediated Fe2+ chelation being prominent examples. If a xenobiotic conjugates GSH [19] to abrogate local [GSH] to compromise GPX4 activity, ROO. generation may be favoured. That the “history” of the neuron influences susceptibility to a redox challenge adds complexity. For example, synaptic activity associated sub-lethal redox challenges herald co-ordinated transcellular neuronal-glia adaptive responses that increase neuronal [GSH] (reviewed in [81]). In our example, an adapted neuron is better able to buffer the xenobiotic mediated GSH conjugation to abrogate ROO. generation to thereby raise the peroxidised lipid load required for ferroptosis [89]. As a cautionary note, adaption requires frequent stimulus because [GSH] is transcription dependent at multiple levels. An intriguing parallel with the exercise physiology axiom “use it or lose it” emerges: activity dependent beneficial adaptive redox responses persist with continued activity but progressively decay with inactivity.
From a translational perspective, the sheer complexity of neuronal redox homeostasis helps rationalise the failure of nutritional antioxidants to treat neurodegenerative diseases [289]. Bioavailability concerns aside, their failure relates to kinetic and spatial constraints (reviewed in [181], [290], [291], [292], [293]). The probability of any one compound possessing sufficient biochemical versatility to significantly modify each reason simultaneously is low. Above all, the failure of nutritional antioxidants reinforces their inherent biochemical strictures—being insufficient evidence to dismiss a causative role for oxidative stress. Much brain redox homeostasis in health and disease remains opaque. Only when basic research unmasks the mechanistic details can one rationally design redox active therapeutics for neurodegenerative diseases.
3. Conclusion
A complex interconnected myriad of reasons render the brain susceptible to oxidative stress; just 13 (many more exist [17], [18]) reasons include unsaturated lipid enrichment, glucose, mitochondria, calcium, glutamate, modest antioxidant defence, redox active transition metals, neurotransmitter auto-oxidation and RNA oxidation. The brain is susceptible to oxidative stress because it harness chemically diverse reactive species to perform heterogeneous signalling functions. From using lipid radicals to trigger ferroptosis when lipid signalling fails, NO. to fine-tune synaptic plasticity or mitochondrial O2.-/H2O2 to signal hypoxia. The balance between species specific useful and harmful biochemistry is a fine one, which, in the brain, means the relationship is bittersweet: exquisite redox signalling functionality easily gives rise to oxidative stress when electrons go awry.
Acknowledgements
We are indebted to Professor Barry Halliwell (Department of Biochemistry, National University of Singapore) for kindly providing critical insight. D.M.B is supported by a Royal Society Wolfson Research Fellowship (#WM170007).
Acknowledgments
Conflict of interest
The authors declare that no conflicts of interest exist.
References
- 1.Mink J., Blumenschine J., Adams D. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 1981;241:R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M.S., Hawrylycz M., Miller J.A., Snyder A.Z., Raichle M.E. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magistretti P.J., Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Araque A., Parpura V., Sanzgiri R.P., Haydon P.G. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 5.Nedergaard M., Ransom B., Goldman S.A. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Saver J.L. Time is brain - quantified. Stroke. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 7.Bailey D.M., Bärtsch P., Knauth M., Baumgartner R.W. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: from the molecular to the morphological. Cell. Mol. Life Sci. 2009;66:3583–3594. doi: 10.1007/s00018-009-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuto J.M., Carrington S.J., Tantillo D.J., Harrison J.G., Ignarro L.J., Freeman B.A., Chen A., Wink D.A. Small molecule signaling agents: the integrated chemistry and biochemistry of nitrogen oxides, oxides of carbon, dioxygen, hydrogen sulfide, and their derived species. Chem. Res. Toxicol. 2012;25:769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawyer D., Valentine J. How super is superoxide? Acc. Chem. Res. 1981;14:393–400. [Google Scholar]
- 10.Winterbourn C.C. The Biological Chemistry of Hydrogen Peroxide. 1st ed. Elsevier Inc; 2013. [DOI] [PubMed] [Google Scholar]
- 11.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 12.Lane N. Oxford University Press; Oxford: 2002. Oxygen: The Molecule That Made the World. [Google Scholar]
- 13.Halliwell B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007;35:1147–1151. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 14.Andersen J.K. Oxidative stress in neurodegeneration: cause or consequence? Nat. Rev. Neurosci. 2004;10:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 15.Janssen-Heininger Y.M.W., Mossman B.T., Heintz N.H., Forman H.J., Kalyanaraman B., Finkel T., Stamler J.S., Rhee S.G., van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology & Medicine. Fifth edition. Oxford University Press; 2015. [Google Scholar]
- 20.Murphy M.P., Holmgren A., Larsson N.-G., Halliwell B., Chang C.J., Kalyanaraman B., Rhee S.G., Thornalley P.J., Partridge L., Gems D., Nyström T., Belousov V., Schumacker P.T., Winterbourn C.C. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickinson B.C., Peltier J., Store D., Schaffer D.V., Chang C.J. Nox2 redox signaling maintains essential cell populations in the brain. Nat. Chem. Biol. 2011;7:106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 23.Bedard K., Krause K. The NOX family of ROS-Generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 24.Massaad C., Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal. 2011;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishida K.T., Hoeffer C.A., Hu D., Pao M., Holland S.M., Klann E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol. Cell. Biol. 2006;26:5908–5920. doi: 10.1128/MCB.00269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauron C., Meda F., Dupont E., Albadri S., Quenech’Du N., Ipendey E., Volovitch M., Del Bene F., Joliot A., Rampon C., Vriz S. Hydrogen peroxide (H2O2) controls axon pathfinding during zebrafish development. Dev. Biol. 2016;414:133–141. doi: 10.1016/j.ydbio.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Meda F., Gauron C., Rampon C., Teillon J., Volovitch M., Vriz S. Nerves control redox levels in mature tissues through schwann cells and hedgehog signalling. Antioxid. Redox Signal. 2016;24:299–311. doi: 10.1089/ars.2015.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasterkamp R.J. Getting neural circuits into shape with semaphorins. Nat. Rev. Neurosci. 2012;13:605–618. doi: 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- 29.Zucker R.S. Calcium- and activity-dependent synaptic plasticity. Curr. Opin. Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler D., Randall A., Tisen R. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 31.Bliss T.V.P., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 32.Kim S.J., Linden D.J. Ubiquitous Plasticity and Memory Storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Ganguly K., Poo M. ming. Activity-dependent neural plasticity from bench to bedside. Neuron. 2013;80:729–741. doi: 10.1016/j.neuron.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Carafoli E., Krebs J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016;291:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipton S.A., Choi Y.-B., Pan Z.-H., Lei S.Z., Chen H.-S.V., Sucher N.J., Loscalzo J., Singel D.J., Stamler J.S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 37.Thomas D.D. Breathing new life into nitric oxide signaling: a brief overview of the interplay between oxygen and nitric oxide. Redox Biol. 2015;5:225–233. doi: 10.1016/j.redox.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown G.C. Nitric oxide and mitochondrial respiration. Biochim. Biophys. Acta (BBA)-Bioenerg. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 39.Carballal S., Bartesaghi S., Radi R. Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim. Biophys. Acta - Gen. Subj. 2014;1840:768–780. doi: 10.1016/j.bbagen.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Augusto O., Bonini M.G., Amanso A.M., Linares E., Santos C.C.X., De Menezes S.L. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic. Biol. Med. 2002;32:841–859. doi: 10.1016/s0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 41.Franco M.C., Ye Y., Refakis C.A., Feldman J.L., Stokes A.L., Basso M., Melero Fernandez de Mera R.M., Sparrow N.A., Calingasan N.Y., Kiaei M., Rhoads T.W., Ma T.C., Grumet M., Barnes S., Beal M.F., Beckman J.S., Mehl R., Estevez A.G. Nitration of Hsp90 induces cell death. Proc. Natl. Acad. Sci. 2013;110:E1102–E1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhn H., Banthiya S., Van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2015;1851:308–330. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reis A., Spickett C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta - Biomembr. 2012;1818:2374–2387. doi: 10.1016/j.bbamem.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Rose C.R., Konnerth A. Stores not just for storage. Neuron. 2001;31:519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- 45.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raturi A., Gutiérrez T., Ortiz-Sandoval C., Ruangkittisakul A., Herrera-Cruz M.S., Rockley J.P., Gesson K., Ourdev D., Lou P.H., Lucchinetti E., Tahbaz N., Zaugg M., Baksh S., Ballanyi K., Simmen T. TMX1 determines cancer cell metabolism as a thiol based modulator of ER-mitochondria Ca2+ flux. J. Cell Biol. 2016;214:433–444. doi: 10.1083/jcb.201512077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Booth D.M., Enyedi B., Geiszt M., Varnai P., Hajnoczky G. Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Mol. Cell. 2016;63:240–248. doi: 10.1016/j.molcel.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowland A.A., Voeltz G.K. Endoplasmic reticulum–mitochondria contacts: function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizzuto R. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 50.Bánsághi S., Golenár T., Madesh M., Csordás G., RamachandraRao S., Sharma K., Yule D.I., Joseph S.K., Hajnóczky G. Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J. Biol. Chem. 2014;289:8170–8181. doi: 10.1074/jbc.M113.504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paillusson S., Stoica R., Gomez-suaga P., Lau D.H.W., Mueller S., Miller T., Miller C.C.J. There ’ s something wrong with my MAM; the ER – mitochondria axis and neurodegenerative diseases. Trends Neurosci. 2016;39:146–157. doi: 10.1016/j.tins.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoica R., De Vos K.J., Paillusson S., Mueller S., Sancho R.M., Lau K.-F., Vizcay-Barrena G., Lin W.-L., Xu Y.-F., Lewis J., Dickson D.W., Petrucelli L., Mitchell J.C., Shaw C.E., Miller C.C.J. ER–mitochondria associations are regulated by the VAPB–PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun. 2014;5 doi: 10.1038/ncomms4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B., Ackerley S. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernardi P., Krauskopf A., Basso E., Petronilli V., Blalchy-Dyson E., Di Lisa F., Forte M.A. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 55.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 56.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baines C. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:626–629. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 58.Bernardi P., Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy M.P. Mitochondrial Thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid. Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 60.Breckwoldt M.O., Pfister F.M.J., Bradley P.M., Marinković P., Williams P.R., Brill M.S., Plomer B., Schmalz A., St Clair D.K., Naumann R., Griesbeck O., Schwarzländer M., Godinho L., Bareyre F.M., Dick T.P., Kerschensteiner M., Misgeld T. Multiparametric optical analysis of mitochondrial redox signals during neuronal physiology and pathology in vivo. Nat. Med. 2014;20:555–560. doi: 10.1038/nm.3520. [DOI] [PubMed] [Google Scholar]
- 61.Breckwoldt M., Kurz F., Breckwoldt M.O., Armoundas A.A., Aon M.A., Bendszus M. Mitochondrial redox and pH signaling occurs in axonal and synaptic organelle clusters Mitochondrial redox and pH signaling occurs in axonal and synaptic organelle clusters. Sci. Rep. 2016;6:23251. doi: 10.1038/srep23251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petronilli V., Penzo D., Scorrano L., Bernardi P., Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J. Biol. Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- 63.Vila M., Przedborski S. Neurological diseases: targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 64.Mattson M. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 65.Jouaville L.S., Pinton P., Bastianutto C., a Rutter G., Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cárdenas C., Miller R.A., Smith I., Bui T., Molgó J., Müller M., Vais H., Cheung K.H., Yang J., Parker I., Thompson C.B., Birnbaum M.J., Hallows K.R., Foskett J.K. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balaban R.S. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta - Bioenerg. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pryde K.R., Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J. Biol. Chem. 2011;286:18056–18065. doi: 10.1074/jbc.M110.186841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kagan V.E., Tyurin V.A., Jiang J., Tyurina Y.Y., Ritov V.B., Amoscato A.A., Osipov A.N., Belikova N.A., Kapralov A.A., Kini V., Vlasova I.I., Zhao Q., Zou M., Di P., Svistunenko D.A., Kurnikov I.V., Borisenko G.G. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 71.Maguire J.J., Tyurina Y.Y., Mohammadyani D., Kapralov A.A., Anthonymuthu T.S., Qu F., Amoscato A.A., Sparvero L.J., Tyurin V.A., Planas-Iglesias J., He R.R., Klein-Seetharaman J., Bayır H., Kagan V.E. Known unknowns of cardiolipin signaling: the best is yet to come. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2017;1862:8–24. doi: 10.1016/j.bbalip.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curtis D., Phillis J., Watkins J. Chemical excitation of spinal neurons. Nature. 1959;183:611–612. doi: 10.1038/183611a0. [DOI] [PubMed] [Google Scholar]
- 73.Choi D.W. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardingham G.E., Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reynolds I.J., Hastings T.G. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coyle J., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 77.Schulz J.B., Matthews R.T., Jenkins B.G., Ferrante R.J., Siwek D., Henshaw D.R., Ben Cipolloni P., Mecocci P., Kowall N.W., Rosen B.R. Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo. J. Neurosci. 1995;15:8419–8429. doi: 10.1523/JNEUROSCI.15-12-08419.1995. (doi:8613773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X., Michaelis E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010;2:1–13. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papadia S., Soriano F.X., Léveillé F., Martel M.-A., a Dakin K., Hansen H.H., Kaindl A., Sifringer M., Fowler J., Stefovska V., McKenzie G., Craigon M., Corriveau R., Ghazal P., Horsburgh K., a Yankner B., a Wyllie D.J., Ikonomidou C., Hardingham G.E. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baxter P.S., Bell K.F.S., Hasel P., Kaindl A.M., Fricker M., Thomson D., Cregan S.P., Gillingwater T.H., Hardingham G.E. Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat. Commun. 2015;6:6761. doi: 10.1038/ncomms7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baxter P.S., Hardingham G.E. Adaptive regulation of the brain's antioxidant defences by neurons and astrocytes. Free Radic. Biol. Med. 2016;100:147–152. doi: 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hardingham G.E., Fukunaga Y., Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 83.Brennan A.M., Won Suh S., Joon Won S., Narasimhan P., Kauppinen T.M., Lee H., Edling Y., Chan P.H., Swanson R.A. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat. Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy T., Miyamoto M., Sastre A., Schnaar R., Coyle J. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 85.Bridges R.J., Natale N.R., Patel S.A. System x c- cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012;165:20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H., Forman H.J. Glutathione synthesis and its role in redox signaling. Semin. Cell Dev. Biol. 2012;23:722–728. doi: 10.1016/j.semcdb.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shih A.Y., Erb H., Sun X., Toda S., Kalivas P.W., Murphy T.H. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Angeli P.F., Stockwell B.R., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gasco S., Linkermann A., Murphy M.E., Overholtzer M., Oyagi A., Pagnussat G.C., Primer Ferroptosis A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on Astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 92.Allaman I., Bélanger M., Magistretti P.J. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 93.Itoh Y., Esaki T., Shimoji K., Cook M., Law M.J., Kaufman E., Sokoloff L. Dichloroacetate effects on glucose and lactate oxidation by neurons and astroglia in vitro and on glucose utilization by brain in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:4879–4884. doi: 10.1073/pnas.0831078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mächler P., Wyss M.T., Elsayed M., Stobart J., Gutierrez R., Von Faber-Castell A., Kaelin V., Zuend M., San Martín A., Romero-Gómez I., Baeza-Lehnert F., Lengacher S., Schneider B.L., Aebischer P., Magistretti P.J., Barros L.F., Weber B. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 2016;23:94–102. doi: 10.1016/j.cmet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 95.Herrero-Mendez A., Almeida A., Fernández E., Maestre C., Moncada S., Bolaños J.P. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat. Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 96.Belanger M., Yang J., Petit J.-M., Laroche T., Magistretti P.J., Allaman I. Role of the glyoxalase system in astrocyte-mediated neuroprotection. J. Neurosci. 2011;31:18338–18352. doi: 10.1523/JNEUROSCI.1249-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allaman I., Bélanger M., Magistretti P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015;9:1–12. doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richard J. Kinetic parameters for the elimination reaction catalyzed by triosephosphate isomerase and an estimation of the reaction's physiological significanc. Biochemistry. 1991;30:4581–4585. doi: 10.1021/bi00232a031. [DOI] [PubMed] [Google Scholar]
- 99.Richard J. Mechanism for the formation of methylglyoxal from triosephosphates. Biochem. Soc. Trans. 1993;21:549–553. doi: 10.1042/bst0210549. [DOI] [PubMed] [Google Scholar]
- 100.Rabbani N., Thornalley P.J. Dicarbonyls linked to damage in the powerhouse: glycation of mitochondrial proteins and oxidative stress. Biochem. Soc. Trans. 2008;36:1045–1050. doi: 10.1042/BST0361045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pun P.B.L., Murphy M.P. Pathological significance of mitochondrial glycation. Int. J. Cell Biol. 2012;2012 doi: 10.1155/2012/843505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pun P.B.L., Logan A., Darley-Usmar V., Chacko B., Johnson M.S., Huang G.W., Rogatti S., Prime T.A., Methner C., Krieg T., Fearnley I.M., Larsen L., Larsen D.S., Menger K.E., Collins Y., James A.M., Kumar G.D.K., Hartley R.C., Smith R.A.J., Murphy M.P. A mitochondria-targeted mass spectrometry probe to detect glyoxals: implications for diabetes. Free Radic. Biol. Med. 2014;67:437–450. doi: 10.1016/j.freeradbiomed.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alle H., Roth A., Geiger J.R. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- 104.Harris J.J., Jolivet R., Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 105.Attwell D., Laughlin S. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 106.Vos M., Lauwers E., Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front. Synaptic Neurosci. 2010;2:1–10. doi: 10.3389/fnsyn.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jang S., Nelson J.C., Bend E.G., Underwood K., Jorgensen E.M., Tueros F.G., Cartagenova L. Glycolytic enzymes localize to synapses under energy stress to support synaptic function article glycolytic enzymes localize to synapses under energy stress to support synaptic function. Neuron. 2016;90:278–291. doi: 10.1016/j.neuron.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ye H., Rouault T.A. Human iron-sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry. 2010;49:4945–4956. doi: 10.1021/bi1004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tait S.W.G., Green D.R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 110.Schon E.A., Przedborski S. Mitochondria: the next (Neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lane N. Mitonuclear match: optimizing fitness and fertility over generations drives ageing within generations. BioEssays. 2011;33:860–869. doi: 10.1002/bies.201100051. [DOI] [PubMed] [Google Scholar]
- 112.Latorre-Pellicer A., Moreno-Loshuertos R., Lechuga-Vieco A.V., Sánchez-Cabo F., Torroja C., Acín-Pérez R., Calvo E., Aix E., González-Guerra A., Logan A., Bernad-Miana M.L., Romanos E., Cruz R., Cogliati S., Sobrino B., Carracedo Á., Pérez-Martos A., Fernández-Silva P., Ruíz-Cabello J., Murphy M.P., Flores I., Vázquez J., Enríquez J.A. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature. 2016;535:561–565. doi: 10.1038/nature18618. [DOI] [PubMed] [Google Scholar]
- 113.Collins Y., Chouchani E.T., James A.M., Menger K.E., Cocheme H.M., Murphy M.P. Mitochondrial redox signalling at a glance. J. Cell Sci. 2012;125 doi: 10.1242/jcs.098475. (1837–1837) [DOI] [PubMed] [Google Scholar]
- 114.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–166. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bell E.L., Klimova T.A., Eisenbart J., Moraes C.T., Murphy M.P., Budinger G.R.S., Chandel N.S. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guzy R.D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K.D., Simon M.C., Hammerling U., Schumacker P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 117.Reczek C.R., Chandel N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chandel N.S., Maltepe E., Goldwasser E., Mathieu C.E., Simon M.C., Schumacker P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tello D., Balsa E., Acosta-Iborra B., Fuertes-Yebra E., Elorza A., Ordóñez Á., Corral-Escariz M., Soro I., López-Bernardo E., Perales-Clemente E., Martínez-Ruiz A., Enríquez J.A., Aragonés J., Cadenas S., Landázuri M.O. Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting complex i activity. Cell Metab. 2011;14:768–779. doi: 10.1016/j.cmet.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 120.Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 121.Ricci J.E., Muñoz-Pinedo C., Fitzgerald P., Bailly-Maitre B., Perkins G.A., Yadava N., Scheffler I.E., Ellisman M.H., Green D.R. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 122.Noack H., Bednarek T., Heidler J., Ladig R., Holtz J., Szibor M. TFAM-dependent and independent dynamics of mtDNA levels in C2C12 myoblasts caused by redox stress. Biochim. Biophys. Acta - Gen. Subj. 2006;1760:141–150. doi: 10.1016/j.bbagen.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 123.St-Pierre J., Drori S., Uldry M., Silvaggi J.M., Rhee J., Jäger S., Handschin C., Zheng K., Lin J., Yang W., Simon D.K., Bachoo R., Spiegelman B.M. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 124.Youdim M.B.H., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 125.Mousseau D.D., Baker G.B. Recent developments in the regulation of monoamine oxidase form and function: is the current model restricting our understanding of the breadth of contribution of monoamine oxidase to brain [dys]function? Curr. Top. Med. Chem. 2012;12:2163–2176. doi: 10.2174/156802612805219969. (〈 http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L368517640%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=15680266&id=doi:&atitle=Recent+developments+in+the+regulation+of+monoamine+oxidase+form+and+function%3A+Is+the+current+〉. [DOI] [PubMed] [Google Scholar]
- 126.Edmondson D.E., Binda C., Wang J., Upadhyay A.K. Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases. Biochemistry. 2009;48:4220–4230. doi: 10.1021/bi900413g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Edmondson D. Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: biological implications. Curr. Pharm. Des. 2014;20:155–160. doi: 10.2174/13816128113190990406. [DOI] [PubMed] [Google Scholar]
- 128.Hauptmann N., Grimsby J., Shih J.C., Cadenas E. The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Arch. Biochem. Biophys. 1996;335:295–304. doi: 10.1006/abbi.1996.0510. [DOI] [PubMed] [Google Scholar]
- 129.Binda C., Newton-Vinson P., Hubálek F., Edmondson D.E., Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002;9:22–26. doi: 10.1038/nsb732. [DOI] [PubMed] [Google Scholar]
- 130.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta - Gen. Subj. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 131.Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 132.Cao X., Wei Z., Gabriel G.G., Li X., Mousseau D.D. Calcium-sensitive regulation of monoamine oxidase-A contributes to the production of peroxyradicals in hippocampal cultures: implications for Alzheimer disease-related pathology. BMC Neurosci. 2007;8:73. doi: 10.1186/1471-2202-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yi H., Akao Y., Maruyama W., Chen K., Shih J., Naoi M. Type A monoamine oxidase is the target of an endogenous dopaminergic neurotoxin, N-methyl(R)salsolinol, leading to apoptosis in SH-SY5Y cells. J. Neurochem. 2006;96:541–549. doi: 10.1111/j.1471-4159.2005.03573.x. [DOI] [PubMed] [Google Scholar]
- 134.Cohen G., Kesler N. Monoamine oxidase and mitochondrial respiration. J. Neurochem. 1999;73:2310–2315. doi: 10.1046/j.1471-4159.1999.0732310.x. [DOI] [PubMed] [Google Scholar]
- 135.Weinreb O., Badinter F., Amit T., Bar-Am O., Youdim M.B.H. Effect of long-term treatment with rasagiline on cognitive deficits and related molecular cascades in aged mice. Neurobiol. Aging. 2015;36:2628–2636. doi: 10.1016/j.neurobiolaging.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 136.Gal S., Zheng H., Fridkin M., Youdim M.B.H. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases. In vivo selective brain monoamine oxidase inhibition and prevention of MPTP-induced striatal dopamine depletion. J. Neurochem. 2005;95:79–88. doi: 10.1111/j.1471-4159.2005.03341.x. [DOI] [PubMed] [Google Scholar]
- 137.Cohen G., Farooqui R., Kesler N. Parkinson disease: a new link between monoamine oxidase and mitochondrial electron flow. Proc. Natl. Acad. Sci. USA. 1997;94:4890–4894. doi: 10.1073/pnas.94.10.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Castrén E. Is mood chemistry? Nat. Rev. Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 139.Buettner G.R., Ng C.F., Wang M., Rodgers V.G.J., Schafer F.Q., New A. Paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic. Biol. Med. 2006;41:1338–1350. doi: 10.1016/j.freeradbiomed.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anderson D.G., Mariappan S.V.S., Buettner G.R., Doorn J.A. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an ortho-quinone. J. Biol. Chem. 2011;286:26978–26986. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li S.W., Lin T.S., Minteer S., Burke W.J. 3,4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson's disease pathogenesis. Mol. Brain Res. 2001;93:1–7. doi: 10.1016/s0169-328x(01)00120-6. [DOI] [PubMed] [Google Scholar]
- 142.Rees J.N., Florang V.R., Eckert L.L., Doorn J.A. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem. Res. Toxicol. 2009;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Plotegher N., Berti G., Ferrari E., Tessari I., Zanetti M., Lunelli L., Greggio E., Bisaglia M., Veronesi M., Girotto S., Dalla Serra M., Perego C., Casella L., Bubacco L. DOPAL derived alpha-synuclein oligomers impair synaptic vesicles physiological function. Sci. Rep. 2017;7:40699. doi: 10.1038/srep40699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kristal B.S., Conway A.D., Brown A.M., Jain J.C., Ulluci P.A., Li S.W., Burke W.J. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic. Biol. Med. 2001;30:924–931. doi: 10.1016/s0891-5849(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 145.Marchitti S.A., Deitrich R.A., Vasiliou V. Neurotoxicity and metabolism of the catecholamine- derived 3, 4-dihydroxyphenylacetaldehyde and the role of aldehyde dehydrogenase. Pharmacol. Rev. 2007;59:125–150. doi: 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- 146.Fitzmaurice A.G., Rhodes S.L., Lulla A., Murphy N.P., Lam H.A., O’Donnell K.C., Barnhill L., Casida J.E., Cockburn M., Sagasti A., Stahl M.C., Maidment N.T., Ritz B., Bronstein J.M. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc. Natl. Acad. Sci. USA. 2013;110:636–641. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heikkila R.E., Cohen G. 6-Hydroxydopamine: evidence for superoxide radical as an oxidative intermediate. Science. 1973;181:456–457. doi: 10.1126/science.181.4098.456. [DOI] [PubMed] [Google Scholar]
- 148.Cohen G., Heikkila R.E. Generation of hydrogen-peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J. Biol. Chem. 1974;249:2447–2452. [PubMed] [Google Scholar]
- 149.Miller D., Buettner G.R., Aust S. Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med. 1990;8:95–108. doi: 10.1016/0891-5849(90)90148-c. [DOI] [PubMed] [Google Scholar]
- 150.Song Y., Buettner G.R. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic. Biol. Med. 2010;49:919–962. doi: 10.1016/j.freeradbiomed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li G., Zhang H., Sader F., Vadhavkar N., Njus D. Oxidation of 4-methylcatechol: implications for the oxidation of catecholamines. Biochemistry. 2007;46:6978–6983. doi: 10.1021/bi061699+. [DOI] [PubMed] [Google Scholar]
- 152.Kuhn D.M., Arthur R. Dopamine inactivates tryptophan hydroxylase and forms a redox-cycling quinoprotein: possible endogenous toxin to serotonin neurons. J. Neurosci. 1998;18:7111–7117. doi: 10.1523/JNEUROSCI.18-18-07111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McCord J., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 154.Keele B., McCord J., Fridovich I. Superoxide dismutase from Escherichia coli B: a new manganese-containing enzyme. J. Biol. Chem. 1970;245:6175–6181. [PubMed] [Google Scholar]
- 155.Fridovich I. Superoxide anion radical (O·̄2), superoxide dismutases, and related matters. Biochemistry. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 156.Winterbourn C.C., French J., Claridge R. Superoxide dismutase as an inhibitor of semiquinone radicals. FEBS Lett. 1978;94:269–272. doi: 10.1016/0014-5793(78)80953-3. [DOI] [PubMed] [Google Scholar]
- 157.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase the role of superoxide anion in the epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. (doi:4623845) [PubMed] [Google Scholar]
- 158.Dauer W., Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 159.Przedborski S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017;18:251–259. doi: 10.1038/nrn.2017.25. [DOI] [PubMed] [Google Scholar]
- 160.Burbulla L.F., Song P., Mazzulli J.R., Zampese E., Wong Y.C., Jeon S., Santos D.P., Blanz J., Obermaier C.D., Strojny C., Savas J.N., Kiskinis E., Zhuang X., Krüger R., Surmeier D.J., Krainc D. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson's disease. Science. 2017;9080:1–12. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mazzulli J.R., Xu Y.H., Sun Y., Knight A.L., McLean P.J., Caldwell G.A., Sidransky E., Grabowski G.A., Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ren X., Zou L., Zhang X., Branco V., Wang J., Carvalho C., Holmgren A., Lu J. Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid. Redox Signal. 2017;27:989–1010. doi: 10.1089/ars.2016.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sinet P., Heikkila R.E., Cohen G. Hydrogen peroxide production by rat brain in vivo. J. Neurochem. 1980;34:1421–1428. doi: 10.1111/j.1471-4159.1980.tb11222.x. [DOI] [PubMed] [Google Scholar]
- 164.Chance B. The enzyme-substrate compounds of catalase and peroxides. Nature. 1948;161:914–917. doi: 10.1038/161914a0. [DOI] [PubMed] [Google Scholar]
- 165.Kirkman H.N., Gaetani G.F. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem. Sci. 2007;32:44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 166.Bell K.F.S., Al-Mubarak B., Martel M.-A., McKay S., Wheelan N., Hasel P., Márkus N.M., Baxter P., Deighton R.F., Serio A., Bilican B., Chowdhry S., Meakin P.J., Ashford M.L.J., Wyllie D.J.A., Scannevin R.H., Chandran S., Hayes J.D., Hardingham G.E. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat. Commun. 2015;6:7066. doi: 10.1038/ncomms8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 168.Li Y., Huang T., Carlson E.J., Melov S., Ursell P., Olson L., Nobel L., Yoshimura M., Berger C., Chan P., Wallace D.C., Epstein C.J. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 169.Lebovitz R.M., Zhang H., Vogel H., Cartwright J., Dionne L., Lu N., Huang S., Matzuk M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Flynn J.M., Melovn S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 2013;62:4–12. doi: 10.1016/j.freeradbiomed.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chae H.Z., Robison K., Poole L.B., Church G., Storz G., Rhee S.G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Perkins A., Nelson K.J., Parsonage D., Poole L.B., Karplus P.A. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015;40:435–445. doi: 10.1016/j.tibs.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rhee S.G., Woo H.A. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid. Redox Signal. 2011;15:781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 174.Lu J., Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 175.Karplus P.A. A primer on peroxiredoxin biochemistry. Free Radic. Biol. Med. 2015;80:183–190. doi: 10.1016/j.freeradbiomed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nagy P., Karton A., Betz A., Peskin A.V., Pace P., O’Reilly R.J., Hampton M.B., Radom L., Winterbourn C.C. Model for the exceptional reactivity of peroxiredoxins 2 and 3 with hydrogen peroxide: a kinetic and computational study. J. Biol. Chem. 2011;286:18048–18055. doi: 10.1074/jbc.M111.232355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim M.-S., Pinto S., Getnet D., Nirujogi R., Manda S., Chaerkady R., Madugundu A., Kelkar D., Isserlin R., Jain S., Thomas J., Muthusamy B., Pamela L.-R., Kumar P., Sahasrabuddhe N., Balakrishnan L., Advani J., George B., Renuse S., Selvan L., Patil A., Nanjappa V., Radhakrishnan A., Prasad S., Subbannayya T., Raju R., Kumar M., Sreenivasamurthy S., Marimuthu A., Sathe G., Chavan S., Datta K., Subbannayya Y., Sahu A., Yelamanchi S., Jayaram S., Rajagopalan P., Sharma J., Murthy K., Syed N., Goel R., Khan A., Ahmad S., Dey G., Mudgal K., Chatterjee A., Huang T.-C., Zhong J., Wu X., Shaw P., Freed D., Zahari M., Mukherjee K., Shankar S., Mahadevan A., Lam H., Mitchell C., Shankar S., Satishchandra P., Schroeder J., Sirdeshmukh R., Maitra A., Leach S., Drake C., Halushka M., Prasad T., Hruban R., Kerr C., Bader G., Christine I.-D., Gowda H., Pandey A. A draft map of the human proteome. Nature. 2014;509:575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chang T.S., Cho C.S., Park S., Yu S., Sang W.K., Sue G.R. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J. Biol. Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 179.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jones D.P., Sies H. The redox code. Antioxid. Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cobley J.N., McHardy H., Morton J.P., Nikolaidis M.G., Close G.L. Influence of vitamin C and vitamin E on redox signalling: implications for exercise adaptations. Free Radic. Biol. Med. 2015;84:65–76. doi: 10.1016/j.freeradbiomed.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 182.Fisher A.B. Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch. Biochem. Biophys. 2017;617:68–83. doi: 10.1016/j.abb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sobotta M.C., Liou W., Stöcker S., Talwar D., Oehler M., Ruppert T., Scharf A.N.D., Dick T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015;11:64–70. doi: 10.1038/nchembio.1695. [DOI] [PubMed] [Google Scholar]
- 184.Winterbourn C.C., Hampton M.B. Redox biology: signaling via a peroxiredoxin sensor. Nat. Chem. Biol. 2014;11:5–6. doi: 10.1038/nchembio.1722. [DOI] [PubMed] [Google Scholar]
- 185.Rhee S.G., Kil I.S. Mitochondrial H2O2 signaling is controlled by the concerted action of peroxiredoxin III and sulfiredoxin: linking mitochondrial function to circadian rhythm. Free Radic. Biol. Med. 2016;100:73–80. doi: 10.1016/j.freeradbiomed.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 186.Woo H.A., Yim S.H., Shin D.H., Kang D., Yu D.Y., Rhee S.G. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 187.Wood Z.A., Poole L.B., Karplus P.A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 188.Flohé L., Toppo S., Cozza G., Ursini F. A comparison of thiol peroxidase mechanisms. Antioxid. Redox Signal. 2011;15:763–780. doi: 10.1089/ars.2010.3397. [DOI] [PubMed] [Google Scholar]
- 189.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., Samokhvalov I.M., Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;701:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gomez Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L., Garner H., Trouillet C., de Bruijn M.F., Geissmann F., Rodewald H.-R. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2014;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1319. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 192.Davalos D., Grutzendler J., Yang G., Kim J.V., Zuo Y., Jung S., Littman D.R., Dustin M.L., Gan W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 193.Wake H., Moorhouse A.J., Jinno S., Kohsaka S., Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., Sher A., Litke A.M., Lambris J.D., Smith S.J., John S.W.M., Barres B. a. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 195.Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., Ragozzino D., Gross C.T. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 198.Babior B.M., Lambeth J.D., Nauseef W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 199.Winterbourn C.C., Kettle A. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 2013;18:642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 200.Colton C.A., Gilbert D.L. Production of superoxide anion by a CNS macrophage, the microglia. FEBS Lett. 1987;223:284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- 201.Niethammer P., Grabher C., Look a.T., Mitchison T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Love N.R., Chen Y., Ishibashi S., Kritsiligkou P., Lea R., Koh Y., Gallop J.L., Dorey K., Amaya E. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat. Cell Biol. 2013;15:222–228. doi: 10.1038/ncb2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mander P.K., Jekabsone A., Brown G.C. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J. Immunol. 2006;176:1046–1052. doi: 10.4049/jimmunol.176.2.1046. [DOI] [PubMed] [Google Scholar]
- 204.Wang S., Chu C.-H., Stewart T., Ginghina C., Wang Y., Nie H., Guo M., Wilson B., Hong J.-S., Zhang J. α-Synuclein, a chemoattractant, directs microglial migration via H2O2 -dependent Lyn phosphorylation. Proc. Natl. Acad. Sci. 2015;112:E1926–E1935. doi: 10.1073/pnas.1417883112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Brown G.C., Vilalta A. How microglia kill neurons. Brain Res. 1628;2015:288–297. doi: 10.1016/j.brainres.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 206.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hong S., Beja-Glasser V.F., Nfonoyim B.M., Frouin A., Li S., Ramakrishnan S., Merry K.M., Shi Q., Rosenthal A., Barres B., Lemere C.A., Selkoe D.J., Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hong S., Dissing-Olesen L., Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Curr. Opin. Neurobiol. 2016;36:128–134. doi: 10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sultana R., Perluigi M., Butterfield D.A. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 2013;62:157–169. doi: 10.1016/j.freeradbiomed.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang H., Forman H.J. Signaling by 4-hydroxy-2-nonenal: exposure protocols, target selectivity and degradation. Arch. Biochem. Biophys. 2017;617:145–154. doi: 10.1016/j.abb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Di Domenico F., Tramutola A., Butterfield D.A. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med. 2017;111:253–261. doi: 10.1016/j.freeradbiomed.2016.10.490. [DOI] [PubMed] [Google Scholar]
- 212.Que E.L., Domaille D.W., Chang C.J. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 2008;39:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- 213.Domaille D.W., Que E.L., Chang C.J. Synthetic fluorescent sensors for studying the cell biology of metals. Nat. Chem. Biol. 2008;4:168–175. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]
- 214.Imlay J.A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006;59:1073–1082. doi: 10.1111/j.1365-2958.2006.05028.x. [DOI] [PubMed] [Google Scholar]
- 215.Connor J., Menzies S. Relationship of iron to oligodendrocytes and myelination. Glia. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 216.Todorich B., Pasquini J.M., Garcia C.I., Paez P.M., Connor J.R. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 217.Petrat F., De Groot H., Sustmann R., Rauen U. The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 2002;383:489–502. doi: 10.1515/BC.2002.051. [DOI] [PubMed] [Google Scholar]
- 218.Imlay J.A. The mismetallation of enzymes during oxidative stress. J. Biol. Chem. 2014;289:28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Imlay J.A. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sobota J.M., Imlay J.A. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc. Natl. Acad. Sci. USA. 2011;108:5402–5407. doi: 10.1073/pnas.1100410108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stincone A., Prigione A., Cramer T., Wamelink M.M.C., Campbell K., Cheung E., Olin-Sandoval V., Grüning N.M., Krüger A., Alam M. Tauqeer, Keller M.A., Breitenbach M., Brindle K.M., Rabinowitz J.D., Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015;90:927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cheng Z., Li Y. What is responsible for the initiating chemistry of iron-mediated lipid peroxidation: an update. Chem. Rev. 2007;107:2165. doi: 10.1021/cr040077w. [DOI] [PubMed] [Google Scholar]
- 223.Maiorino M., Conrad M., Ursini F. GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid. Redox Signal. 2017;0 doi: 10.1089/ars.2017.7115. [DOI] [PubMed] [Google Scholar]
- 224.Zecca L., Youdim M.B.H., Riederer P., Connor J.R., Crichton R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 225.Telling N.D., Everett J., Collingwood J.F., Dobson J., van der Laan G., Gallagher J.J., Wang J., Hitchcock A.P. Iron biochemistry is correlated with amyloid plaque morphology in an established mouse model of Alzheimer's disease. Cell Chem. Biol. 2017;24:1205–1215. doi: 10.1016/j.chembiol.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 226.Banci L., Bertini I., Ciofi-Baffoni S., Kozyreva T., Zovo K., Palumaa P. Affinity gradients drive copper to cellular destinations. Nature. 2010;465:645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 227.Chang C.J. Searching for harmony in transition-metal signaling. Nat. Chem. Biol. 2015;11:744–747. doi: 10.1038/nchembio.1913. [DOI] [PubMed] [Google Scholar]
- 228.Kardos J., Kovas I., Hajos F., Kalman M., Simonyi M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci. Lett. 1989;103:139–144. doi: 10.1016/0304-3940(89)90565-x. [DOI] [PubMed] [Google Scholar]
- 229.Dodani S.C., Domaille D., Nam C.I., Miller E.W., Finney L., Vogt S., Chang C.J. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescent microscopy. Proc. Natl. Acad. Sci. 2011;108:5980–5985. doi: 10.1073/pnas.1009932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dodani S.C., Firl A., Chan J., Nam C.I., Aron A.T., Onak C.S., Ramos-Torres K.M., Paek J., Webster C.M., Feller M.B., Chang C.J. Copper is an endogenous modulator of neural circuit spontaneous activity. Proc. Natl. Acad. Sci. 2014;111:16280–16285. doi: 10.1073/pnas.1409796111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Abreu I. a., Cabelli D.E. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim. Biophys. Acta - Proteins Proteom. 2010;1804:263–274. doi: 10.1016/j.bbapap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 232.Sheng Y., Abreu I. a., Cabelli D.E., Maroney M.J., Miller A.F., Teixeira M., Valentine J.S. Superoxide dismutases and superoxide reductases. Chem. Rev. 2014;114:3854–3918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 234.Renton A.E., Chiò A., Traynor B.J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 2013;17:17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kiernan M.C., Vucic S., Cheah B.C., Turner M.R., Eisen A., Hardiman O., Burrell J.R., Zoing M.C. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 236.Zhang H., Joseph J., Gurney M., Becker D., Kalyanaraman B. Bicarbonate enhances peroxidase activity of Cu,Zn-superoxide dismutase: role of carbonate anion radical and scavenging of carbonate anion radical by metalloporphyrin antioxidant enzyme mimetics. J. Biol. Chem. 2002;277:1013–1020. doi: 10.1074/jbc.M108585200. [DOI] [PubMed] [Google Scholar]
- 237.Winterbourn C.C., Peskin A.V., Parsons-Mair H.N. Thiol oxidase activity of copper,zinc superoxide dismutase. J. Biol. Chem. 2002;277:1906–1911. doi: 10.1074/jbc.M107256200. [DOI] [PubMed] [Google Scholar]
- 238.Karunakaran C., Zhang H., Joseph J., Antholine W.E., Kalyanaraman B. Thiol oxidase activity of copper, zinc superoxide dismutase stimulates bicarbonate-dependent peroxidase activity via formation of a carbonate radical. Chem. Res. Toxicol. 2005;18:494–500. doi: 10.1021/tx049747j. [DOI] [PubMed] [Google Scholar]
- 239.Barber S.C., Mead R.J., Shaw P.J. Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim. Biophys. Acta - Gen. Subj. 2006;1762:1051–1067. doi: 10.1016/j.bbadis.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 240.Bazinet R.P., Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 241.Schönfeld P., Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? - Reflections on disadvantages of the use of free fatty acids as fuel for brain. J. Cereb. Blood Flow Metab. 2013;33:1493–1499. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cobley J.N., Sakellariou G.K., Owens D.J., Murray S., Waldron S., Gregson W., Fraser W.D., Burniston J.G., Iwanejko L.A., McArdle A., Morton J.P., Jackson M.J., Close G.L. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic. Biol. Med. 2014;70:23–32. doi: 10.1016/j.freeradbiomed.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 243.Río L.A. Del. Peroxisomes as a cellular source of reactive nitrogen species signal molecules. Arch. Biochem. Biophys. 2011;506:1–11. doi: 10.1016/j.abb.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 244.Trompier D., Vejux A., Zarrouk A., Gondcaille C., Geillon F., Nury T., Savary S., Lizard G. Brain peroxisomes. Biochimie. 2014;98:102–110. doi: 10.1016/j.biochi.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 245.Farooqui A.A., Horrocks L.A., Farooqui T. Modulation of inflammation in brain: a matter of fat. J. Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 246.Zerbinati C., Iuliano L. Cholesterol and related sterols autoxidation. Free Radic. Biol. Med. 2017;111:151–155. doi: 10.1016/j.freeradbiomed.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 247.Niki E., Yoshida Y., Saito Y., Noguchi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005;338:668–676. doi: 10.1016/j.bbrc.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 248.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic. Biol. Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 249.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic. Biol. Med. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 250.Sousa B.C., Pitt A.R., Spickett C.M. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 2017;111:294–308. doi: 10.1016/j.freeradbiomed.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 251.Ursini F., Maiorino M., Valente M., Ferri L., Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta. 1982;710:197–211. doi: 10.1016/0005-2760(82)90150-3. [DOI] [PubMed] [Google Scholar]
- 252.Yang W.S., Stockwell B.R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yant L.J., Ran Q., Rao L., Van Remmen H., Shibatani T., Belter J.G., Motta L., Richardson A., Prolla T.A. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 254.Imai H., Hirao F., Sakamoto T., Sekine K., Mizukura Y., Saito M., Kitamoto T., Hayasaka M., Hanaoka K., Nakagawa Y. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 2003;305:278–286. doi: 10.1016/s0006-291x(03)00734-4. [DOI] [PubMed] [Google Scholar]
- 255.Seiler A., Schneider M., Förster H., Roth S., Wirth E.K., Culmsee C., Plesnila N., Kremmer E., Rådmark O., Wurst W., Bornkamm G.W., Schweizer U., Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 256.Yang W.S., Sriramaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., Brown L.M., Girotti A.W., Cornish V.W., Schreiber S.L., Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chen L., Hambright W.S., Na R., Ran Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 2015;290:28097–28106. doi: 10.1074/jbc.M115.680090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hambright W.S., Fonseca R.S., Chen L., Na R., Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17. doi: 10.1016/j.redox.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yoo S.E., Chen L., Na R., Liu Y., Rios C., Van Remmen H., Richardson A., Ran Q. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic. Biol. Med. 2012;52:1820–1827. doi: 10.1016/j.freeradbiomed.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Toledo J., Augusto O. Connecting the Chemical and Biological Properties of Nitric Oxide. Chem. Res. Toxicol. 2012;25:975–989. doi: 10.1021/tx300042g. [DOI] [PubMed] [Google Scholar]
- 261.Schuman E., Madison D. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- 262.Izumi Y., Clifford D., Zorumski C. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- 263.Rabinovich D., Yaniv S.P., Alyagor I., Schuldiner O., Rabinovich D., Yaniv S.P., Alyagor I., Schuldiner O. Nitric oxide as a switching mechanism between axon degeneration and regrowth during developmental remodeling article nitric oxide as a switching mechanism between axon degeneration and regrowth during developmental remodeling. Cell. 2016;164:170–182. doi: 10.1016/j.cell.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang T., Xie Z., Lu B. Nitric oxide mediates activity-dependent synaptic suppression at developing neuromuscular synapses. Nature. 1995;374:262–266. doi: 10.1038/374262a0. [DOI] [PubMed] [Google Scholar]
- 265.Ferrer-Sueta G., Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem. Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 266.Nayernia Z., Jaquet V., Krause K.-H. New insights on NOX enzymes in the central nervous system. Antioxid. Redox Signal. 2014;20:2815–2837. doi: 10.1089/ars.2013.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.DeCoursey T.E., Ligeti E. Regulation and termination of NADPH oxidase activity. Cell. Mol. Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Poulsen H.E., Specht E., Broedbaek K., Henriksen T., Ellervik C., Mandrup-Poulsen T., Tonnesen M., Nielsen P.E., Andersen H.U., Weimann A. RNA modifications by oxidation: a novel disease mechanism? Free Radic. Biol. Med. 2012;52:1353–1361. doi: 10.1016/j.freeradbiomed.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 269.Briggs J.A., Wolvetang E.J., Mattick J.S., Rinn J.L., Barry G. Mechanisms of long non-coding RNAs in mammalian nervous system development, plasticity, disease, and evolution. Neuron. 2015;88:861–877. doi: 10.1016/j.neuron.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 270.Hwang J.-Y., Aromolaran K.A., Zukin R.S. The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat. Rev. Neurosci. 2017;18:347–361. doi: 10.1038/nrn.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Salta E., Strooper B. De. Noncoding RNAs in neurodegeneration. Nat. Rev. Neurosci. 2017;18:627–640. doi: 10.1038/nrn.2017.90. [DOI] [PubMed] [Google Scholar]
- 272.Simms C.L., Zaher H.S. Quality control of chemically damaged RNA. Cell. Mol. Life Sci. 2016;73:3639–3653. doi: 10.1007/s00018-016-2261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hofer T., Badouard C., Bajak E., Ravanat J.L., Mattsson Å., Cotgreave I.A. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol. Chem. 2005;386:333–337. doi: 10.1515/BC.2005.040. [DOI] [PubMed] [Google Scholar]
- 274.Aas P.A., Otterlei M., Falnes P.Ø., Vågbø C.B., Skorpen F., Akbari M., Sundheim O., Bjørås M., Slupphaug G., Seeberg E., Krokan H.E. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature. 2003;421:859–863. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- 275.Jung H., Yoon B.C., Holt C.E. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat. Rev. Neurosci. 2012;13 doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Martin K.C., Zukin R.S. RNA trafficking and local protein synthesis in dendrites: an overview. J. Neurosci. 2006;26:7131–7134. doi: 10.1523/JNEUROSCI.1801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tanaka M., Chock P.B., Stadtman E.R. Oxidized messenger RNA induces translation errors. Proc. Natl. Acad. Sci. 2006;104:66–71. doi: 10.1073/pnas.0609737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Shan X., Chang Y., Lin C.-l.G. Messenger RNA oxidation is an early event preceding cell death and causes reduced protein expression. FASEB J. 2007;21:2753–2764. doi: 10.1096/fj.07-8200com. [DOI] [PubMed] [Google Scholar]
- 279.Nunomura A., Perry G., Pappolla M.A., Wade R., Hirai K., Chiba S., Smith M.A. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J. Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Liu J., Head E., Gharib A.M., Yuan W., Ingersoll R.T., Hagen T.M., Cotman C.W., Ames B.N. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R- -lipoic acid. Proc. Natl. Acad. Sci. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ding Q., Dimayuga E., Keller J.N. Oxidative stress alters neuronal RNA- and protein-synthesis: implications for neural viability. Free Radic. Res. 2007;41:903–910. doi: 10.1080/10715760701416996. [DOI] [PubMed] [Google Scholar]
- 282.Chang Y., Kong Q., Shan X., Tian G., Ilieva H., Cleveland D.W., Rothstein J.D., Borchelt D.R., Wong P.C., Lin C.L.G. Messenger RNA oxidation occurs early in disease pathogenesis and promotes motor neuron degeneration in ALS. PLoS One. 2008;3:e2849. doi: 10.1371/journal.pone.0002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Trewick S.C., Henshaw T.F., Hausinger R.P., Lindahl T., Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature. 2002;419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 284.Piochon C., Kano M., Hansel C. LTD-like molecular pathways in developmental synaptic pruning. Nat. Neurosci. 2016;19:1299–1310. doi: 10.1038/nn.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Williams D.W., Kondo S., Krzyzanowska A., Hiromi Y., Truman J.W. Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 2006;9:1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- 286.Li Z., Jo J., Jia J.M., Lo S.C., Whitcomb D.J., Jiao S., Cho K., Sheng M. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wang J.Y., Chen F., Fu X.Q., Ding C.S., Zhou L., Zhang X.H., Luo Z.G. Caspase-3 cleavage of dishevelled induces elimination of postsynaptic structures. Dev. Cell. 2014;28:670–684. doi: 10.1016/j.devcel.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 288.Guzman J.N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E., Schumacker P.T., Surmeier D.J. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bjelakovic G., Nikolova D., Gluud L., Simonetti R., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012;3:CD007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Forman H.J., Davies K.J. a., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Murphy M.P. Antioxidants as therapies: can we improve on nature? Free Radic. Biol. Med. 2014;66:20–23. doi: 10.1016/j.freeradbiomed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 292.Day B.J. Antioxidant therapeutics: pandoras box. Free Radic. Biol. Med. 2014;66:58–64. doi: 10.1016/j.freeradbiomed.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gutteridge J.M.C., Halliwell B. Antioxidants: molecules, medicines, and myths. Biochem. Biophys. Res. Commun. 2010;393:561–564. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]