Abstract
Early growth is associated with blood pressure measured on one occasion, but whether early life growth patterns are associated with longitudinal blood pressure trajectories is under-researched. Therefore, we sought to examine the association between early growth and blood pressure trajectories from childhood to adulthood. Blood pressure was measured on seven occasions between ages 5 and 18 years in the Birth to Twenty cohort study and conditional variables for growth in infancy and mid-childhood were computed from anthropometric measures (n=1937, 52% girls). We used a group-based trajectory modeling approach to identify distinct height-adjusted blood pressure trajectories and then tested their association with growth between birth and mid-childhood adjusting for several covariates.
Three trajectory groups were identified for systolic and diastolic blood pressure: ‘lower’, ‘middle’ and ‘upper’ in boys and girls, separately. In boys, predictors of the middle or upper systolic blood pressure trajectories versus the lower trajectory were in birth weight (odds ratio [OR] 0.75[95% CI 0.58–0.96] per SD) and relative weight gain in infancy (4.11[1.25–13.51] per SD). In girls, greater relative weight gain and linear growth in both infancy and mid-childhood were consistently associated with an almost 2-fold higher likelihood of being in the upper versus lower systolic blood pressure trajectory. The associations for the diastolic blood pressure trajectories were inconsistent. These findings emphasize the importance of identifying children at risk of progression to high blood pressure. Accelerated growth in infancy and mid-childhood may be a key target for early life intervention in prevention of elevated blood pressure progression.
Keywords: Blood pressure, trajectories, childhood, adolescence, growth
Introduction
Hypertension remains a major cause of morbidity and premature mortality, and a leading risk factor for the global burden disease worldwide.1 High blood pressure (BP) in adulthood will affect three quarters of the population in low and middle income countries (LMICs) by 2025.2 Of great concern is the emergence of hypertension in pediatric populations concomitant with the rising epidemic of obesity.3 We recently showed that BP tracks from early childhood to late adolescence in a black South African population; one in three children with elevated BP at age 5 years (>90th percentile for age, height and sex) remain so at age 18 years.4 Since children with elevated BP may show early signs of cardiovascular damage, renal injury and are likely to have high BP in adulthood, 5,6 identifying children with atypical BP patterns and risk factors may be crucial for early life intervention to modify progression to hypertension in adulthood.
Substantial evidence suggests that early life growth predicts elevated BP, particularly in children who are small at birth but experience accelerated growth in childhood.7–9 Conventional growth modelling techniques have been used to explore how early life growth relates to mean levels or changes in BP in children and adolescents. Those studies use a single mean growth curve to describe BP trajectories in a given population and assume that covariates influence the growth curve in a similar way.10 Yet BP is a highly variable physiological characteristic, hence describing a population using a single estimated trajectory oversimplifies the complexity of BP tracking patterns that characterize children and adolescents. In addition, height is a known marker of early life environment and a major correlate of BP in childhood and adolescence,11 and thus should be incorporated in longitudinal modelling of BP.
Recently, investigators have begun to use finite mixture models to identify distinct clusters of individuals following similar patterns of BP change over time.12,13 One recent study identified four SBP trajectory groups between ages 7 and 38 years using group-based trajectory modeling (GBTM) on data from the Dunedin longitudinal birth cohort. That study reported that low birth weight was associated with higher odds of being in the hypertensive trajectory group.14 In support of those findings, a UK birth cohort reported two to three BP trajectory groups in midlife (36 to 53 years) and confirmed that individuals with higher birth weight were likely to be in the normative BP group than in the ‘BP increaser’ groups.15 Trajectories of BP in black children and adolescents in South Africa have not been identified, and are complicated by more ambiguous definitions of elevated BP in children than in adults. GBTM may be useful in identifying children with atypical patterns of SBP and DBP and their associated risk factors.
To our knowledge, no study in Africa within a LMIC scenario has explored the association between early life growth and trajectories of BP in children and adolescents, which is likely to be important because of the increasing burden of hypertension on this continent. We hypothesized that early growth, in particular lower birth weight and rapid postnatal weight gain is associated with a higher height-adjusted BP trajectory in a Black South African pediatric population. The aim of this study was to use Birth to Twenty longitudinal birth cohort data (Bt20) to (1) identify distinct trajectory groups for BP between 5 and 18 years of birth, (2) examine the association between early growth and BP trajectories and (3) assess the influence of height on the association between early growth and BP trajectories.
Methods
Study population
The data analyzed here are from the Bt20 cohort study of singleton children born within a 7-week period in Soweto, Johannesburg in 1990 (n=3273). Details of recruitment and selection criteria for the cohost study are described elsewhere. 16 All parents and caregivers of the children provided written informed consent, and the University of Witwatersrand Committee granted ethical approval for Research on Human Subjects (certificate number: M130556). The study sample was restricted to black participants (approximately 78% of the cohort), who had not been pregnant during adolescence and had anthropometric measurements, SBP and DBP measurements at two or more of the following mean ages: 5years (n=1046;49.0% boys), 8years (1175;48.8%), 10years (n=695;48.6%), 13years (n=1355;47.5%), 14years (n=1392;47.4%), 16years (n=1657;48.1%) and 18years (n=1592;48.2%) . The overall sample size was n=1937 (1005 girls and 932 boys).
Assessment of blood pressure
BP was measured in a seated position in triplicate: after a five minute rest and a two minute interval between measurements, using Dinamap Signs monitor 1846SX (Critikon, USA) at 5years and an Omron M6 (Omron, Kyoto, Japan) at 8 to 18years. All the measurements were taken by a trained research assistant using an appropriate cuff size. Hypertension status at age 5 years was classified as greater than 95th percentile for age, sex and height using the Fourth report on National High Blood pressure program in children and adolescence.17
Assessment of growth
Birth weight and gestational age were obtained from birth notification records entered at birth. Subsequently, weight was measured using a digital scale to the nearest 0.1kg and height was measured using a calibrated stadiometer. Birth weight, weight-for-age and height-for-age z-scores were computed using WHO growth standards.18. To deal with high correlation of repeated weight and height measures in longitudinal data, conditional weight independent of height (relative weight gain) and relative height gain independent of weight (relative linear growth) were computed as standardized residuals derived from sex-specific linear regressions of a current growth measure on a prior one in infancy (0–2years) and mid-childhood (2–5 years).19,20
Assessment of covariates
Maternal and infant characteristics at birth were identified using standard questionnaires. Socio-economic status (SES) in infancy was represented as a count of household assets reported by the mother or caregiver.
Statistical analysis
Group based trajectory modeling (GBTM) was performed using a STATA plug in program of SAS Proc Traj to estimate model parameters using maximum likelihood estimation in STATA 11.21 We used a censored normal model for continuous variables, firstly without covariates, then including height as a time varying covariate (preliminary analyses). To identify the groups, two-class models were specified for SBP and DBP for each sex, then classes were added sequentially until parsimony was attained for linear, quadratic and cubic terms (Table S1). To test the model with the best fit we used the following criteria: low Bayesian Information Criterion (BIC), high posterior probabilities (above 0.7), not less than 1% of total sample in a trajectory group and prior knowledge from the literature.22 Thereafter, the groups defined from the best-fitting models were used as categorical outcome variables in multinomial logistic regression models with covariates (time varying and time invariant) yielding the log-odds estimates, standard errors and p values, from which odd ratios and 95% confidence intervals (95% CI) were computed.
Not all participants had measures at all occasions; hence we compared those who were included in the sample and those with missing BP measures with respect to key variables to inform whether missingness was random (Table S2). Descriptive statistics were conducted to characterize average BP per occasion by BP class membership. ANOVA was used to describe continuous study sample characteristics for each BP trajectory and to validate the trajectory groups using BP status at age 5 years of age and ϰ2 test was used for categorical study variables. Multinomial logistic regressions were conducted in traj and the estimates and standard errors converted to odds ratios and 95% confidence intervals
Results
1005 girls and 932 boys had BP measurements on at least two occasions. There were no significant differences between the analyzed sample and those not included (n=631) with regards to birth weight, weight in infancy and mid-childhood, height in infancy and childhood, but the two samples differed with regards to SES in infancy. Those in the included sample had lower number of household assets compared to those excluded (p<0.0001).
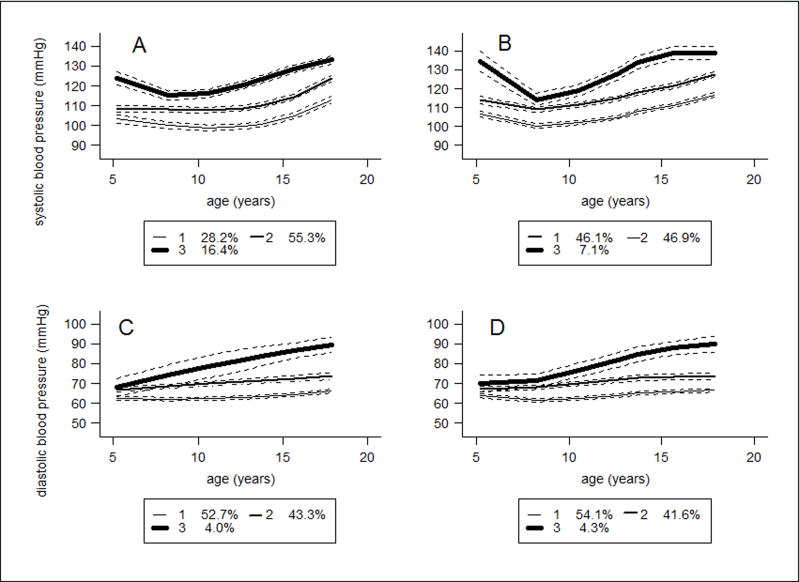
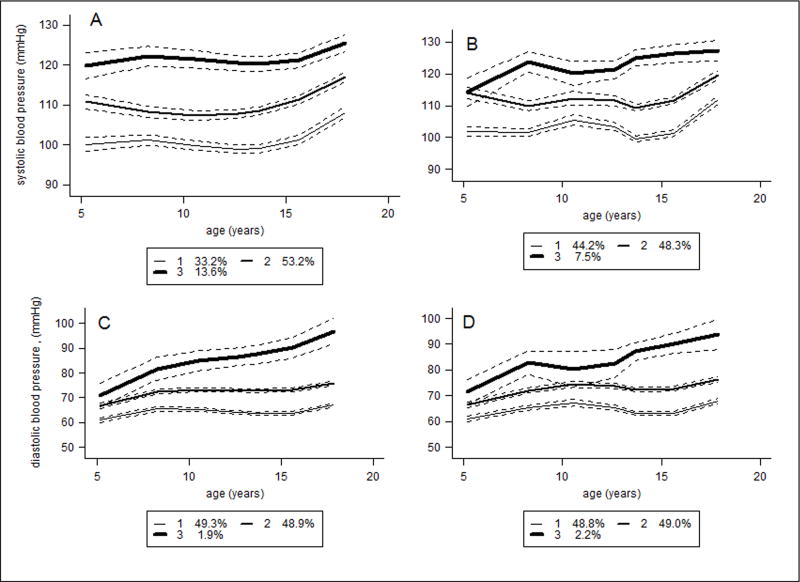
In each sex, the best-fitting models included three distinct trajectories for SBP and DBP with cubic terms (Table S1). For descriptive purposes, the BP trajectories were labelled as ‘lower’, ‘middle’ or ‘upper’. The lower trajectory was considered as the reference group in all regression analyses (Figure 1 and 2). The BP trajectories represent mean BP based on the posterior probabilities of being assigned to a particular class. In boys (Figure 1 A-D), the upper SBP trajectory group (16.4%) started at 125.0mmHg at age 5 years and increased to 132.4mmHg by age 18 years. The middle SBP trajectory group (55.3%) started at 107.3mmHg and increased to 122.9mmHg. The lower SBP trajectory group (28.2%) started at 101.9mmHg and increased to 115.2 mmHg. In girls (figure 2A-D), the upper SBP trajectory group (13.6%) started at 120.5 mmHg and increased to 125.7mmHg, the middle SBP group (53.2%) started at 110.4mmHg and increased to 116.9mmHg; and the lower SBP group (33.2%) started at 99.0mmHg and increased to 107.5mmHg (Table 1).
Figure 1.
Trajectories of blood pressure for boys between 5 and 18 years of age from group based trajectory models. A. Systolic blood pressure over time. B. Systolic blood pressure over time adjusted for height as a time varying covariate. C. Diastolic blood pressure over time. D. Diastolic blood pressure over time accounting for height per time point
Figure 2.
Girls’ trajectories of blood pressure for between 5 and 18 years of age from group based trajectory models. A. Systolic blood pressure changes with age. B. Systolic blood pressure over time adjusted for height at each time point. C. Diastolic blood pressure over time. D. Diastolic blood pressure over time accounting for height per time point.
Table 1.
Sex -specific Mean BP by trajectory group membership
| Mean Age(years) | SBP trajectory group membership | DBP trajectory group membership | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Boys | Girls | Boys | Girls | |||||||||
| Low | Middle | Upper | Low | Middle | Upper | Low | Middle | Upper | Low | Middle | Upper | |
| 5 | 101.9(10.8) | 107.3(10.4) | 125.0(12.0) | 99.0(8.6) | 110.4(9.9) | 120.5(13.3) | 60.5(7.5) | 65.8(7.8) | 69.9(8.3) | 60.5(6.9) | 67.2(8.7) | 70.0(8.6) |
| 8 | 101.7(7.5) | 110.8(8.6) | 119.0(10.9) | 101.7(7.0) | 110.3(8.4) | 125.5(10.0) | 65.9(7.0) | 73.1(7.5) | 75.1(6.8) | 66.1(7.0) | 73.8(7.5) | 85.6(12.0) |
| 10 | 98.5(9.1) | 106.8(9.4) | 117.9(14.7) | 99.1(8.5) | 107.0(9.6) | 119.2(13.2) | 63.8(7.4) | 69.7(7.8) | 76.4(12.0) | 64.2(7.5) | 71.0(7.5) | 80.3(12.8) |
| 13 | 96.9(6.7) | 107.2(6.6) | 119.1(9.2) | 98.0(6.6) | 107.5(6.9) | 119.3(8.8) | 61.0(5.9) | 69.7(6.4) | 80.6(11.5) | 62.8(5.7) | 72.3(6.6) | 83.3(7.4) |
| 14 | 98.6(6.9) | 109.1(7.3) | 122.6(9.9) | 99.0(6.8) | 108.5(7.5) | 120.3(9.7) | 63.0(5.9) | 72.7(6.7) | 86.7(8.5) | 65.1(5.9) | 75.1(6.9) | 91.0(7.2) |
| 16 | 105.3(8.0) | 117.3(9.0) | 132.3(11.3) | 100.0(8.3) | 112.7(9.1) | 124.1(10.6) | 62.8(6.4) | 72.3(1.1) | 90.9(13.1) | 62.9(6.7) | 73.2(7.3) | 93.4(12.6) |
| 18 | 111.4(7.6) | 122.9(8.2) | 132.4(10.4) | 107.5(7.0) | 116.9(8.1) | 125.7(9.2) | 66.8(6.4) | 75.3(7.2) | 88.1(7.8) | 67.2(6.2) | 76.6(6.9) | 97.5(12.2) |
| SBP trajectory group membership-height adjusted | DBP trajectory group membership-height adjusted | |||||||||||
| 5 | 103.7(10.4) | 110.7(12.0) | 130.9(10.4) | 100.6(9.1) | 113.6(10.9) | 113.1(13.6) | 61.4(7.8) | 64.9(8.1) | 76.7(9.8) | 60.4(7.0) | 70.0(8.2) | 70.0(8.2) |
| 8 | 104.7(8.3) | 113.7(9.5) | 118.0(10.3) | 102.9(7.4) | 113.2(9.2) | 126.9(11.0) | 66.7(7.2) | 73.6(7.8) | 73.9(5.1) | 66.1(6.9) | 85.3(11.0) | 85.3(11.0) |
| 10 | 101.4(9.8) | 109.6(10.9) | 117.8(15.3) | 100.0(8.4) | 110.0(10.2) | 118.9(17.3) | 64.2(7.5) | 70.3(8.1) | 76.1(10.6) | 63.8(7.4) | 80.4(11.3) | 80.4(11.3) |
| 13 | 100.6(7.8) | 110.0(7.9) | 121.0(10.9) | 100.0(7.0) | 109.1(7.4) | 122.8(9.9) | 61.6(6.0) | 70.6(6.9) | 80.8(13.4) | 62.7(5.7) | 84.0(7.1) | 84.0(7.1) |
| 14 | 102.2(7.9) | 112.2(8.5) | 128.2(10.4) | 100.3(7.2) | 110.3(7.3) | 124.9(9.9) | 63.6(6.2) | 73.5(7.0) | 86.8(9.7) | 65.0(5.9) | 89.5(7.4) | 89.5(7.4) |
| 16 | 109.1(9.1) | 121.2(9.9) | 137.2(12.5) | 102.0(8.8) | 114.7(9.1) | 128.0(11.6) | 63.5(6.7) | 72.9(7.9) | 96.3(11.9) | 62.6(6.5) | 91.2(12.2) | 91.2(12.2) |
| 18 | 115.2(8.9) | 125.7(8.3) | 134.0(13.5) | 109.2(7.4) | 118.3(7.7) | 130.4(11.9) | 67.2(6.4) | 76.5(7.7) | 87.4(7.9) | 67.0(6.1) | 96.0(11.8) | 96.0(11.8) |
Summary statistics showing mean blood pressure and standard deviations across the trajectory group memberships of blood pressure between ages 5 and 18 on seven time points depicted by mean ages.
Adding child’s height between 5 and 18 years as a time varying covariate reduced by half the proportion of individuals in the upper SBP trajectory in both boys (7.1%) and girls (7.5%) (Figure 1B and 2 B) but made no major differences for DBP (Figure 1D and 2D). Table S3 describes the association of age terms and height, and BP trajectories for the time varying models. Height between ages 5 and 18 was positively associated with all BP trajectories in boys and girls, except an apparent protective effect on the upper SBP trajectory in girls (est:−0.25, SE: 0.10, p=0.0125). The DBP trajectory for girls was not associated with height between ages 5 and 18.
Hypertension status at age 5 years was highly predictive of height-adjusted BP trajectory (Table S4). The relative risk of being in the upper and middle SBP trajectory given a BP status above the 95th percentile for age sex and height was 3.85 and 21.80 times higher compared to the lower trajectory in boys. In girls, the relative risk ratios were almost six and seven fold greater for the middle and upper relative to the lower SBP trajectory for a BP measure above the 95th percentile for age, height and sex. With respect to DBP trajectories, BP status at age 5 years was highly predictive of the BP trajectory groups with higher risk for the upper and middle trajectory groups compared to the lower one in both sexes.
SBP trajectory group membership in boys was associated with relative linear growth in infancy and childhood, and SES in infancy, while in girls it was associated with relative weight and relative height gain in infancy and childhood, parity, maternal age and household assets in infancy and SBP at age 5 years. With respect to DBP, rapid relative weight gain in infancy, rapid linear growth in mid-childhood, DBP at age 5 years and SES in infancy was significantly associated with the girls’ trajectory group membership (Table 2). For the height-adjusted BP trajectories, weight gain in infancy and childhood, parity, BP at 5 years of age and SES between 0–2 years of age remained as determinants of class membership for the girls, while in boys, relative linear growth in mid-childhood and BP at age 5 years were associated with BP trajectory groups.
Table 2.
Study sample characteristics by sex and BP trajectory groups
| Study characteristics |
SBP trajectory group | DBP trajectory group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Boys | Girls | Boys | Girls | |||||||||
| Lower | Middle | Upper | Lower | Middle | Upper | Lower | Middle | Upper | Lower | Middle | Upper | |
|
|
||||||||||||
| Gestational age(weeks) | 38.0(1.5) | 37.9(1.9) | 38.0(2.0) | 37.9(1.8) | 38.0(1.8) | 37.8(1.9) | 37.9(1.9) | 38.0(1.6) | 37.8(2.9) | 37.9(1.9) | 37.9(1.6) | 38.1(2.2) |
| Birth weight(g) | 3110.4(0.5) | 3132.2(0.5) | 3116.1(0.5) | 3023.8(0.5) | 3027.7(0.5) | 3061.3(0.4) | 3125.2(0.5) | 3132.4(0.5) | 3078.6(0.6) | 3025.4(0.5) | 3034.7(0.5) | 3121.4(0.4) |
| Relative weight gain infancy | −0.05(0.9) | 0.14(1.0) | 0.17(1.0) | −02(1.0) | 0.20(1.0) | 0.30(1.0)** | 0.05(1.0) | 0.17(1.0) | 0.01(1.1) | 0.07(1.0) | 0.20(0.9) | 0.78(0.8)* |
| Relative weight gain mid-childhood | −0.14(1.0) | 0.02(1.0) | 0.07(1.2) | −0.16(1.0) | −0.08(1.0) | 0.32(1.0)** | −0.06(1.0) | 0.04(1.1) | 0–0.13(1.5) | −0.14(1.1) | 0.001(1.0) | 0.44(1.0) |
| Relative linear growth infancy | −0.31(0.8) | −0.05(1.0) | 0.19(0.9)*** | −0.31(0.9) | 0.01(1.0) | 0.14(1.0)*** | −0.13(1.0) | −0.03(1.0) | 0.16(1.0) | −0.12(1.0) | −0.01(1.0) | −0.31(1.2) |
| Relative linear growth mid-childhood | −0.20(1.1) | 0.04(1.0) | 0.04(0.9)* | −0.19(1.0) | 0.03(1.0) | 0.40(0.9)*** | −0.13(1.0) | 0.11(1.0) | 0.30(1.1)* | −0.07(1.0) | 0.07(1.0) | 0.64(1.0)* |
| SBP at 5 years of age/mmHg | 103.1(11.9) | 108.4(11.1) | 124.7(14.0)*** | 104.0(1.4) | 115.6(11.4) | 118.7(16.0)*** | - | - | ||||
| DBP at 5 years of age/mmHg | - | - | 60.2(7.7) | 63.8(7.9) | 71.7(7.9)*** | 60.4(6.8) | 66.7(8.7) | 69.4(10.7)*** | ||||
| Parity: ≤2 | 92(41.4) | 298(33.8) | 51(35.7) | 149(48.5) | 301(33.6) | 40(33.6) | 278(33.8) | 156(39.5) | 7(25.0) | 304(36.7) | 182(38.0) | 4(28.6) |
| >2 | 130(58.6) | 583(66.1) | 92(64.3) | 158(51.5) | 595(66.4) | 79(66.4)*** | 545(66.2) | 239(60.5) | 21(75.0) | 525(63.3) | 297(62.0) | 10(71.4) |
| SES in infancy(household assets) | 4(2) | 3(2) | 3(2)** | 4(2) | 3(2) | 3(2)* | 3(2) | 3(2) | 3(1) | 3(2) | 3(2) | 3(1)** |
|
|
||||||||||||
| SBP trajectory groups-height adjusted | DBP trajectory groups-height adjusted | |||||||||||
|
|
||||||||||||
| Gestational age | 38.0(1.7) | 37.9(2.0) | 37.9(2.0) | 37.9(1.8) | 37.9(1.9) | 37.9(1.5) | 37.9(1.8) | 38.0(1.7) | 37.6(3.1) | 37.8(2.0) | 38.0(1.7) | 38.0(2.1) |
| Birth weight | 3160.0(0.5) | 3077.2(0.5) | 3070.0(0.5) | 3031.1(0.5) | 3026.1(0.5) | 3074.1(0.4) | 3140.0(0.5) | 3090.7(0.5) | 3133.3(0.5) | 3009.6(0.5) | 3040.0(0.5) | 3111.8(0.4) |
| Relative weight gain infancy | 0.11(1.0) | 0.10(1.0) | 0–0.02(1.1) | −02(0.9) | 0.25(1.0) | 0.26(0.9)** | 0.08(1.0) | 0.15(1.0) | 0–0.19(1.0) | 0.02(1.0) | 0.23(1.0) | 0.81(0.8)*** |
| Relative weight gain mid-childhood | 0.01(1.1) | 0–0.07(1.0) | 0.03(1.2) | −0.19(1.0) | −0.02(1.0) | 0.19(1.0)* | −0.06(1.0) | 0.07(1.1) | 0–0.03(1.0) | −0.17(1.1) | 0.02(1.0) | 0.44(1.0)* |
| Relative linear growth infancy | −0.09(0.9) | −0.06(1.0) | 0–0.33(0.9) | −0.15(1.0) | 0.01(1.0) | 0–0.09(1.0) | −0.04(1.0) | −0.18(0.9) | 0–0.04(1.0) | −0.06(1.0) | 0–0.06(1.0) | −0.51(1.2) |
| Relative linear growth mid-childhood | −0.09(1.0) | 0.05(1.1) | 0.24(0.8)* | −0.07(1.0) | 0.04(1.0) | 0.24(0.8) | −0.04(1.0) | 0.005(1.0) | 0.12(0.9)* | −0.06(1.0) | 0.05(1.0) | 0.40(0.9) |
| SBP at 5 years of age/mmHg | 110(12.0) | 103.7(8.1) | 130.9(10.4)*** | 100.6(9.1) | 113.6(10.9) | 113.1(13.7)*** | - | - | ||||
| DBP at 5 years of age/mmHg | - | - | 61.4(7.8) | 64.9(8.1) | 76.7(9.8)*** | 60.4(7.0) | 67.0(8.6) | 69.6(8.2)*** | ||||
| Parity : ≤2 | 162(36.8) | 262(35.1) | 17(28.3) | 206(47.8) | 265(31.8) | 19(32.8)*** | 305(34.2) | 130(38.8) | 6(28.6) | 207(43.1) | 278(33.7) | 5(29.4)** |
| >2 | 278(63.2) | 484(64.9) | 43(71.7) | 225(52.2) | 568(68.2) | 39(67.2) | 585(65.7) | 205(61.2) | 15(71.4) | 273(56.9) | 547(66.3) | 12(70.6) |
| SES in infancy | 3(2) | 3(2) | 3(2) | 3(2) | 3(2) | 3(2) | 3(2) | 3(2) | 3(1) | 3(2) | 3(2) | 3(1)* |
Model showing the summary statistics of factors associated with the BP trajectories with level of significance:
p<0.001,
p<0.01,
p<0.05
Analysis of variance for continuous exposures (gestational age, birth weight, weight and linear growth between 0–2yrs, 2–5yrs, BP at 5yrs and asset-based SES score) and chi square test for categorical variable, parity, associated with the BP trajectories
Relative weight gain in infancy and rapid linear growth between birth and mid-childhood was associated with SBP trajectory group membership in boys after adjusting for time invariant covariates (Table 3). However, in height-adjusted models, a unit increase in birth weight reduced the risk of being in the middle trajectory by 25% (OR: 0.75; 95%CI 0.58–0.96, p=0.0223) while rapid weight gain in infancy was associated with almost a 4-fold higher risk (OR: 4.11; 1.25–13.51; p=0.0202) of being in the upper trajectory. In girls, relative weight gain and linear growth in infancy were associated with higher odds of being in the middle and upper relative to the lower SBP trajectory in height-adjusted models.
Table 3.
Association of birth weight relative weight gain and linear growth in infancy and mid-childhood, and SBP trajectory groups between 5 and 18 years (OR, 95%CI, p value)
| A. BOYS | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Middle vs Lower trajectory group | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value |
|
|
||||||||||||
| Birth weight(z score) | 1.02 | 0.76–1.38 | 0.8736 | 0.99 | 0.73–1.35 | 0.9738 | 0.77 | 0.6–0.98 | 0.0307 | 0.75 | 0.58–0.96 | 0.0223 |
| Relative weight gain in infancy | 1.54 | 1.14–2.09 | 0.0050 | 1.65 | 1.2–2.26 | 0.0021 | 1.21 | 0.95–1.55 | 0.1237 | 1.23 | 0.96–1.59 | 0.1046 |
| Relative weight gain in mid-childhood | 1.20 | 0.94–1.54 | 0.1522 | 1.28 | 0.96–1.7 | 0.0918 | 1.25 | 0.99–1.57 | 0.0653 | 1.26 | 0.98–1.62 | 0.0712 |
| Relative linear growth in infancy | 1.76 | 1.28–2.43 | 0.0005 | 1.72 | 1.26–2.35 | 0.0007 | 0.84 | 0.64–1.11 | 0.2157 | 0.84 | 0.64–1.11 | 0.2299 |
| Relative linear growth in mid-childhood | 1.65 | 1.24–2.18 | 0.0005 | 1.58 | 1.19–2.11 | 0.0018 | 0.85 | 0.67–1.09 | 0.2021 | 0.89 | 0.7–1.15 | 0.3774 |
| Upper vs Lower trajectory group | ||||||||||||
| Birth weight(z score) | 1.03 | 0.7–1.51 | 0.8705 | 1.02 | 0.66–1.56 | 0.9453 | 0.69 | 0.31–1.55 | 0.3652 | 0.67 | 0.28–1.61 | 0.3685 |
| Relative weight gain in infancy | 1.58 | 1.08–2.33 | 0.0190 | 1.67 | 1.09–2.56 | 0.0196 | 3.74 | 1.43–9.83 | 0.0074 | 4.11 | 1.25–13.51 | 0.0202 |
| Relative weight gain in mid-childhood | 1.34 | 0.95–1.89 | 0.0950 | 1.36 | 0.92–2.02 | 0.1221 | 1.21 | 0.59–2.48 | 0.6088 | 1.20 | 0.54–2.65 | 0.6604 |
| Relative linear growth in infancy | 2.15 | 1.41–3.28 | 0.0004 | 2.18 | 1.39–3.43 | 0.0007 | 1.62 | 0.67–3.93 | 0.2847 | 1.98 | 0.71–5.48 | 0.1912 |
| Relative linear growth in mid-childhood | 1.44 | 1–2.09 | 0.0513 | 1.53 | 1.02–2.3 | 0.0400 | 1.02 | 0.44–2.34 | 0.9645 | 1.12 | 0.39–3.2 | 0.8392 |
| B.GIRLS | ||||||||||||
| Middle vs Lower trajectory group | ||||||||||||
| Birth weight(z score) | 1.23 | 0.93–1.63 | 0.1490 | 1.12 | 0.85–1.48 | 0.4309 | 1.09 | 0.84–1.42 | 0.5190 | 0.97 | 0.73–1.28 | 0.8223 |
| Relative weight gain in infancy | 1.39 | 1.04–1.86 | 0.0276 | 1.37 | 1.02–1.85 | 0.0350 | 1.33 | 1.02–1.75 | 0.0378 | 1.33 | 1–1.76 | 0.0492 |
| Relative weight gain in mid-childhood | 1.00 | 0.75–1.32 | 0.9802 | 1.09 | 0.81–1.46 | 0.5669 | 1.11 | 0.85–1.44 | 0.4507 | 1.22 | 0.93–1.59 | 0.1546 |
| Relative linear growth in infancy | 2.11 | 1.51–2.94 | 0.0000 | 2.07 | 1.48–2.91 | 0.0000 | 1.60 | 1.15–2.21 | 0.0053 | 1.58 | 1.15–2.17 | 0.0046 |
| Relative linear growth in mid-childhood | 1.14 | 0.87–1.49 | 0.3394 | 1.12 | 0.85–1.48 | 0.4209 | 1.03 | 0.79–1.36 | 0.8098 | 1.01 | 0.75–1.35 | 0.9578 |
| Upper vs Lower trajectory group | ||||||||||||
| Birth weight(z score) | 1.34 | 0.93–1.94 | 0.1123 | 1.31 | 0.89–1.92 | 0.1682 | 1.35 | 0.94–1.94 | 0.1043 | 1.27 | 0.86–1.88 | 0.2246 |
| Relative weight gain in infancy | 1.67 | 1.14–2.44 | 0.0085 | 1.59 | 1.06–2.37 | 0.0236 | 1.69 | 1.14–2.5 | 0.0084 | 1.63 | 1.08–2.46 | 0.0211 |
| Relative weight gain in mid-childhood | 1.65 | 1.18–2.31 | 0.0038 | 1.76 | 1.22–2.55 | 0.0026 | 1.69 | 1.2–2.38 | 0.0026 | 1.77 | 1.22–2.56 | 0.0025 |
| Relative linear growth in infancy | 2.35 | 1.59–3.48 | 0.0000 | 2.23 | 1.48–3.35 | 0.0001 | 1.98 | 1.33–2.96 | 0.0008 | 1.90 | 1.27–2.86 | 0.0019 |
| Relative linear growth in mid-childhood | 1.95 | 1.37–2.78 | 0.0002 | 1.99 | 1.36–2.91 | 0.0004 | 2.03 | 1.38–2.99 | 0.0003 | 2.12 | 1.39–3.23 | 0.0005 |
The multinomial logistic regression showing odds ratios, 95% confidence intervals and the p values from GBTM showing:
Model 1: Multinomial logistic regression of growth variables and SBP trajectory classes adjusted for gestational age (time-invariant covariate-a proxy for intrauterine environment).
Model 2: Multinomial logistic regression of growth variables and SBP trajectory classes adjusted for gestational age, parity, SES in infancy, maternal age (time-invariant covariates-intrauterine environment and maternal factors).
Model 3: Multinomial logistic regression of growth variables and SBP trajectory classes with height as time-varying covariate (a marker of childhood environment) and gestational age as a time-invariant covariate.
Model 4: Multinomial logistic regression of growth variables and SBP trajectory classes with height as time varying covariate and gestational age, parity, SES in infancy, and maternal age as a time-invariant covariates.
Rapid weight gain in infancy and mid-childhood and linear growth were associated with higher odds of being in the middle versus lower DBP trajectory (Table 4). However, in height-adjusted models, an association only remained for relative weight gain in infancy (OR: 1.43; 1.06–1.95; p=0.0214). The upper versus lower DBP trajectory group in boys was positively associated with relative weight gain in infancy and linear growth in mid-childhood, but these associations were diminished or reversed in height-adjusted models, leaving linear growth in infancy as the only significant predictor of upper versus lower DBP trajectory (OR: 0.47; 0.29–0.76; p=0.0021). In girls, relative weight gain and linear growth in mid-childhood were positively associated with being in the middle versus lower DBP trajectory, while weight gain in infancy was a predictor of upper vs lower DBP trajectory (OR: 2.53; 1.14–5.57; p=0.0219).
Table 4.
Association of birth weight relative weight gain and linear growth in infancy and mid-childhood, and DBP trajectory groups between 5 and 18 years (OR, 95%CI, p value)*
| A. BOYS | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Middle vs Lower trajectory group | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value |
| Birth weight(z score) | 0.97 | 0.72–1.33 | 0.8688 | 0.99 | 0.71–1.38 | 0.9371 | 0.82 | 0.64–1.06 | 0.1359 | 0.83 | 0.63–1.10 | 0.2019 |
| Relative weight gain in infancy | 1.73 | 1.23–2.42 | 0.0015 | 1.76 | 1.22–2.56 | 0.0028 | 1.37 | 1.04–1.8 | 0.0251 | 1.43 | 1.06–1.95 | 0.0214 |
| Relative weight gain in mid-childhood | 1.37 | 1.01–1.85 | 0.0447 | 1.44 | 1.03–2.01 | 0.0351 | 1.22 | 0.96–1.56 | 0.1029 | 1.26 | 0.96–1.64 | 0.0926 |
| Relative linear growth in infancy | 1.02 | 0.71–1.46 | 0.9100 | 1.13 | 0.76–1.67 | 0.5598 | 0.72 | 0.55–0.94 | 0.0171 | 0.78 | 0.58–1.05 | 0.1001 |
| Relative linear growth in mid-childhood | 1.51 | 1.06–2.15 | 0.0228 | 1.68 | 1.19–2.38 | 0.0034 | 0.97 | 0.75–1.24 | 0.8032 | 1.14 | 0.86–1.52 | 0.3633 |
| Upper vs Lower trajectory group | ||||||||||||
| Birth weight(z score) | 0.93 | 0.65–1.34 | 0.7133 | 0.93 | 0.63–1.36 | 0.7017 | 0.81 | 0.46–1.42 | 0.4609 | 0.68 | 0.45–1.03 | 0.0658 |
| Relative weight gain in infancy | 1.57 | 0.98–2.52 | 0.0614 | 1.77 | 1.16–2.72 | 0.0087 | 1.03 | 0.55–1.90 | 0.9359 | 1.38 | 0.89–2.15 | 0.1519 |
| Relative weight gain in mid-childhood | 1.12 | 0.72–1.72 | 0.6190 | 1.40 | 0.94–2.08 | 0.0976 | 0.93 | 0.56–1.54 | 0.7739 | 1.37 | 0.88–2.13 | 0.1627 |
| Relative linear growth in infancy | 1.21 | 0.81–1.81 | 0.3535 | 1.17 | 0.76–1.78 | 0.4787 | 0.75 | 0.36–1.56 | 0.4392 | 0.47 | 0.29–0.76 | 0.0021 |
| Relative linear growth in mid-childhood | 1.75 | 1.20–2.54 | 0.0036 | 1.83 | 1.23–2.71 | 0.0028 | 1.05 | 0.63–1.76 | 0.8473 | 0.80 | 0.53–1.21 | 0.2848 |
| B.GIRLS | ||||||||||||
| Middle vs Lower trajectory group | ||||||||||||
| Birth weight(z score) | 1.07 | 0.86–1.35 | 0.5310 | 1.02 | 0.80–1.30 | 0.8553 | 1.03 | 0.82–1.30 | 0.7790 | 0.99 | 0.78–1.26 | 0.9339 |
| Relative weight gain in infancy | 1.36 | 1.07–1.73 | 0.0108 | 1.27 | 0.99–1.63 | 0.0611 | 1.31 | 1.03–1.67 | 0.0265 | 1.27 | 0.98–1.63 | 0.0677 |
| Relative weight gain in mid-childhood | 1.23 | 1.00–1.51 | 0.0511 | 1.30 | 1.04–1.63 | 0.0234 | 1.20 | 0.97–1.47 | 0.0956 | 1.28 | 1.02–1.61 | 0.0317 |
| Relative linear growth in infancy | 1.27 | 1.01–1.59 | 0.0392 | 1.24 | 0.97–1.58 | 0.0809 | 1.15 | 0.89–1.47 | 0.2858 | 1.08 | 0.83–1.42 | 0.5544 |
| Relative linear growth in mid-childhood | 1.28 | 1.03–1.58 | 0.0234 | 1.27 | 1.00–1.60 | 0.0460 | 1.18 | 0.94–1.48 | 0.1502 | 1.17 | 0.91–1.49 | 0.2205 |
| Upper vs Lower trajectory group | ||||||||||||
| Birth weight(z score) | 1.16 | 0.44–3.06 | 0.7615 | 1.47 | 0.73–2.97 | 0.2830 | 1.46 | 0.72–2.95 | 0.2944 | 1.44 | 0.68–3.03 | 0.3379 |
| Relative weight gain in infancy | 4.25 | 1.51–11.94 | 0.0061 | 2.90 | 1.36–6.18 | 0.0057 | 2.65 | 1.25–5.58 | 0.0106 | 2.53 | 1.14–5.57 | 0.0219 |
| Relative weight gain in mid-childhood | 1.08 | 0.47–2.48 | 0.8640 | 1.58 | 0.81–3.10 | 0.1835 | 1.47 | 0.77–2.82 | 0.2468 | 1.52 | 0.80–2.90 | 0.2047 |
| Relative linear growth in infancy | 0.88 | 0.37–2.14 | 0.7856 | 1.07 | 0.55–2.10 | 0.8396 | 0.88 | 0.44–1.74 | 0.7039 | 1.00 | 0.49–2.02 | 0.9942 |
| Relative linear growth in mid-childhood | 2.20 | 0.90–5.36 | 0.0840 | 1.56 | 0.80–3.04 | 0.1950 | 1.52 | 0.80–2.90 | 0.1996 | 1.48 | 0.74–2.96 | 0.2715 |
Multinomial logistic regression models showing the odds ratios, 95% confidence intervals and p values of the association between growth and BP trajectories:
Model 1: Multinomial logistic regression of growth variables and DBP trajectory classes adjusted for gestational age (time-invariant covariate).
Model 2: Multinomial logistic regression of growth variables and DBP trajectory classes adjusted for gestational age, parity, SES in infancy, maternal age (time-invariant covariates).
Model 3: Multinomial logistic regression of growth variables and DBP trajectory classes with height as time-varying covariate and gestational age as a time-invariant covariate.
Model 4: Multinomial logistic regression of growth variables and DBP trajectory classes with height as time varying covariate and gestational age, parity, SES in infancy and maternal age as a time-invariant covariates.
Discussion
Using GBTM, we identified three sex-specific trajectories for SBP and DBP; each showing gradual increases in BP with age in Black South African children. In boys, upper and middle BP height-adjusted trajectory membership was inversely associated with birth weight and positively associated with relative weight gain and linear growth in infancy. Girls who experienced accelerated growth (both in weight and height) in infancy and childhood tended to be in the upper vs lower SBP trajectory, while rapid growth in infancy positively predicted being in the middle vs lower class. With regard to DBP in girls, rapid relative weight gain in infancy and mid-childhood were predictors for the upper and middle trajectories (in comparison to the lower trajectory). These findings support the hypothesis that accelerated relative weight gain in early life may program higher long-term disease risks.23
A major feature of our study is that we confirmed heterogeneity in BP over time in children and adolescents by reporting three latent classes for SBP and DBP for each sex. Previous longitudinal cohort data analyses of BP trajectories focused on adult populations 24,25 or growth curve modelling using one BP trajectory.26 Consistent with the findings from the Framingham Heart study of 1060 males aged 25+ with longitudinal BP measurements at four time points, we identified three trajectory classes for SBP and DBP.12 However, other studies found different numbers of classes, including a UK study which reported 2 classes each for SBP and DBP in midlife in men.15 The CARDIA cohort in USA examined adults aged 18 to 30 years at baseline and reported five distinct trajectories of mid-BP (mean of SBP and DBP). However, this study did not disentangle the age-related changes in SBP and DBP, which are physiologically distinct measures.27
In the present study, height between 5 and 18 years of age was closely associated with trajectory groups, except for the girls’ upper SBP and DBP trajectories. These findings might imply that stature may have a greater impact on BP in boys than girls. With regards to sex differences, a study of BP trajectories in a Caribbean adolescent population aged 11 to 18 years reported that rate of change of both SBP and DBP was greater in boys than in girls.28 Sex discrepancies in BP trajectories and their associations with early growth might be attributed to influence of androgens on the regulation of long term BP in response to early environmental factors. 29 Although the specific mechanisms underpinning sex differences in the programming of life course BP are not fully known, testosterone is reportedly positively associated with renin angiotensin activation and oxidative stress, which in turn contribute to elevated BP differently in males 30 and females.31
This study confirms that BP increases with age and height between ages 5 and 18 years, except in the upper BP trajectories for girls. Unlike in adults, height is a recognized major determinant of childhood BP and is routinely considered to evaluate childhood BP centiles and hypertension status.32 Childhood height is an indicator of growth of the arterial tree, which influences hemodynamic BP control mechanisms especially vasculature structure and function.33 These findings are consistent with a number of cross sectional studies34 and confirm the importance of inclusion of age and height in the definition of elevated BP in children and adolescents. Since there are physiologic increases in childhood BP with height, it is important to consider the effect of height as a covariate when identifying trajectory groups, so as to avoid simply categorizing tall children with normal BP for their height.
For height-adjusted BP trajectories, the association between infant and mid-childhood linear growth and BP, especially for boys and for DBP, implies that change in BP may be more susceptible to later stature in boys than girls. In support of the fetal origins hypothesis, we reported that higher birth weight was associated with lower odds of being in the middle versus the lower boys’ SBP trajectory in the final models. This finding may indicate that children who are born low weight but who go on to increase their stature after mid-childhood may be at risk of having moderately increasing BP.
In support of the growing evidence on BP tracking in childhood, 35 we have shown that hypertensive status at age 5 years was highly predictive of BP trajectory between 5–18 years. This finding validates the importance of early detection through routine BP screening in childhood to identify children who are most likely to follow elevated BP trajectories over their life course. Early onset of elevated BP may indicate that physiological mechanisms influencing variation in BP and risk of elevated BP might start in early life and may amplify with age in response to genetic and environmental factors.
A major strength of this study is its prospective nature. In addition, there were no major differences between those children excluded from the study and the analytical sample with regards to key study characteristics and any differences were adjusted in the multivariate models. We employed GBTM to identify sex-specific BP trajectories. This is an extension of traditional growth modelling which takes into account time varying and invariant covariates in characterizing the change in BP over time and heterogeneity within BP data. GBTM is suitable for examining physiological traits like BP which are highly variable in children and adolescents and provides better model fit and parsimony compared to linear mixed models.22 However, inferences regarding latent class membership and their association with relative weight gain in early life might not extend to the entire South African population since the analyses were confined to the black ethnic sample. Use of ambulatory blood pressure monitoring measure may provide more insight into BP trajectories compared to an average BP measurement on one occasion. Future research studies need to replicate group based trajectory modeling in other cohort studies using other time varying covariates like physical activity and dietary patterns.
In the context of an emerging epidemic of hypertension in black LMIC populations, this study suggests that BP trajectories may be set early in childhood, with BP in early childhood BP highly predictive of BP trajectories. Early life growth, in particular high relative weight gain, may be a crucial target for interventions to interrupt elevated BP trajectories and to prevent the early onset of raised BP. Further research needs to identify individuals in the middle and upper trajectories, to assess other cardiovascular disease indicators such as left ventricular hypertrophy, arterial stiffness, and to consider appropriate interventions in early life to interrupt the trajectories and benefit long-term health.
Supplementary Material
Perspectives.
In summary, this study highlights the potential for early interventions aimed at optimizing growth in early life may play an important role in prevention of elevated BP progression in children and adolescents. This calls for a concerted effort among global healthcare practitioners, researchers, and other related organizations to prioritize pediatric hypertension on the global health agenda especially in Low to Middle Income countries (LMICs) where the burden of disease attributed to hypertension is already high in midlife and exerting pressure on the health care system. Future research needs to explore the clinical relevance of the elevated BP trajectories, the key risk factors and progression to and hypertension and cardiovascular disease in adulthood.
Novelty and Significance.
What is new?
This is the first study to show distinct BP trajectories from early childhood to late adolescence in African children
Rapid early growth predicts higher longitudinal BP trajectories in childhood
What is relevant?
Age related changes in SBP in children and adolescents are height dependent hence longitudinal profiles of pediatric BP should account for stature.
Summary
Distinct trajectories of age-, sex- and height- dependent BP are detectable in children from 5 years of age. Rapid growth (both in weight and linear) in infancy and mid-childhood predict the progression of BP between mid-childhood and late adolescence
Acknowledgments
We are grateful for the support from the data collection team and the Birth to Twenty participants for taking part in the study.
Sources of Funding
This study was funded by the Wellcome Trust and the UK MRC/DfID African Research Leader Scheme, the South African Medical Research Council, University of Witwatersrand, Johannesburg, South Africa and the DST-NRF Centre of Excellence in Human Development
Footnotes
Disclosures
None.
References
- 1.Forouzanfar MH, Alexander L, Anderson HR, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Flynn J. The changing face of pediatric hypertension in the era of the childhood obesity epidemic. Pediatr. Nephrol. 2013;28(7):1059–1066. doi: 10.1007/s00467-012-2344-0. [DOI] [PubMed] [Google Scholar]
- 4.Kagura J, Adair LS, Musa MG, Pettifor JM, Norris SA. Blood pressure tracking in urban black South African children: birth to twenty cohort. BMC pediatrics. 2015;15:78. doi: 10.1186/s12887-015-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels SR, Meyer RA, Strife CF, Lipman M, Loggie JM. Distribution of target-organ abnormalities by race and sex in children with essential hypertension. J. Hum. Hypertens. 1990;4(2):103–104. [PubMed] [Google Scholar]
- 6.Lambrechtsen J, Rasmussen F, Hansen HS, Jacobsen IA. Tracking and factors predicting rising in 'tracking quartile' in blood pressure from childhood to adulthood: Odense Schoolchild Study. J. Hum. Hypertens. 1999;13(6):385–391. doi: 10.1038/sj.jhh.1000836. [DOI] [PubMed] [Google Scholar]
- 7.Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension. 2003;41(3):451–456. doi: 10.1161/01.HYP.0000054212.23528.B2. [DOI] [PubMed] [Google Scholar]
- 8.Law CM, Shiell AW, Newsome CA, et al. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105(9):1088–1092. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 9.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J. Hypertens. 2000;18(7):815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 10.Su S, Wang X, Pollock JS, et al. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation. 2015;131(19):1674–1681. doi: 10.1161/CIRCULATIONAHA.114.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voors AW, Webber LS, Frerichs RR, Berenson GS. Body height and body mass as determinants of basal blood pressure in children--The Bogalusa Heart Study. American journal of epidemiology. 1977;106(2):101–108. doi: 10.1093/oxfordjournals.aje.a112439. [DOI] [PubMed] [Google Scholar]
- 12.Kerner B, Muthen BO. Growth mixture modelling in families of the Framingham Heart Study. BMC Proc. 2009;3 Suppl 7:S114. doi: 10.1186/1753-6561-3-s7-s114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tielemans SM, Geleijnse JM, Menotti A, et al. Ten-year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: the Minnesota Business and Professional Men Study and the Zutphen Study. Journal of the American Heart Association. 2015;4(3):e001378. doi: 10.1161/JAHA.114.001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theodore RF, Broadbent J, Nagin D, et al. Childhood to Early-Midlife Systolic Blood Pressure Trajectories: Early-Life Predictors, Effect Modifiers, and Adult Cardiovascular Outcomes. Hypertension. 2015;66(6):1108–1115. doi: 10.1161/HYPERTENSIONAHA.115.05831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wills AK, Lawlor DA, Muniz-Terrera G, et al. Population heterogeneity in trajectories of midlife blood pressure. Epidemiology. 2012;23(2):203–211. doi: 10.1097/EDE.0b013e3182456567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter L, Norris S, Pettifor J, Yach D, Cameron N. Cohort Profile: Mandela's children: the 1990 Birth to Twenty study in South Africa. Int. J. Epidemiol. 2007;36(3):504–511. doi: 10.1093/ije/dym016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National High Blood Pressure Education Program Working Group on High Blood Pressure in C, Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 18.W.H.O. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 19.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J. Clin. Epidemiol. 2005;58(12):1320–1324. doi: 10.1016/j.jclinepi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Jones BL, Nagin DS. A note on a Stata plugin for estimating group-based trajectory models. Sociological Methods & Research. 2013;42:608–613. [Google Scholar]
- 22.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 23.Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol. Rev. 2014;94(4):1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wills AK, Lawlor DA, Matthews FE, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8(6):e1000440. doi: 10.1371/journal.pmed.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joas E, Backman K, Gustafson D, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59(4):796–801. doi: 10.1161/HYPERTENSIONAHA.111.182204. [DOI] [PubMed] [Google Scholar]
- 26.Hlaing WM, Prineas RJ, Zhu Y. Trajectory of systolic blood pressure in children and adolescents. Ann. Epidemiol. 2006;16(1):11–18. doi: 10.1016/j.annepidem.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311(5):490–497. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols S, Cadogan F. Anthropometry and blood pressure changes in a Caribbean adolescent population of African ancestry: an evaluation of longitudinal data using a multilevel mixed regression approach. West Indian Med. J. 2012;61(7):674–683. [PubMed] [Google Scholar]
- 29.Ojeda NB, Intapad S, Alexander BT. Sex differences in the developmental programming of hypertension. Acta physiologica. 2014;210(2):307–316. doi: 10.1111/apha.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ojeda NB, Grigore D, Yanes LL, et al. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;292(2):R758–763. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 31.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50(4):679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98(4 Pt 1):649–658. [PubMed] [Google Scholar]
- 33.Montgomery SM, Berney LR, Blane D. Prepubertal stature and blood pressure in early old age. Archives of disease in childhood. 2000;82(5):358–363. doi: 10.1136/adc.82.5.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita Y, Kouda K, Nakamura H, Nishio N, Takeuchi H, Iki M. Growth-related disappearance of the childhood relationship between height and blood pressure levels. Ann. Hum. Biol. 2014;41(1):91–93. doi: 10.3109/03014460.2013.822558. [DOI] [PubMed] [Google Scholar]
- 35.Toschke AM, Kohl L, Mansmann U, von Kries R. Meta-analysis of blood pressure tracking from childhood to adulthood and implications for the design of intervention trials. Acta paediatrica. 2010;99(1):24–29. doi: 10.1111/j.1651-2227.2009.01544.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.