Abstract
Purpose of review
β-hydroxy β-methylbutyrate (HMB) has been used for many years in athletes for muscle buildup and strength, and endurance enhancement. In recent years, its interest quickly expanded in older (diseased) populations and during (exercise) rehabilitation and recovery from hospitalization and surgery. We will discuss recent literature about HMB metabolism, its pharmacokinetics compared to the frequently used metabolite leucine, effectiveness of HMB to improve outcome in older diseased adults, and novel approaches for HMB use.
Recent findings
HMB supplementation resulted in positive outcomes on muscle mass and functionality, related to its anabolic and anticatabolic properties and prolonged half-life time in blood. Furthermore, it was able to increase the benefits of (exercise) rehabilitation programs to enhance recovery from illness or medical procedures. There is promising evidence that HMB might support bone density, improve cognitive function, and reduce abdominal obesity which is of importance particularly in the older (diseased) population.
Summary
The older diseased population might benefit from dietary HMB because of its established positive properties as well as its long lasting (pharmacological) effect. Besides evaluating its efficacy and application in various clinical conditions, more research is needed into the mechanisms of action, the optimal dosage, and its potential additional beneficial effects on outcome.
Keywords: HMB kinetics, anticatabolic and anabolic potential, pharmacokinetics, outcome, older (diseased) population
Introduction
β-hydroxy β-methylbutyrate (HMB), a metabolite of the amino acid leucine, has been studied for many decades. In humans, HMB has been widely used as ergogenic supplement by individuals usually combined with exercise training to increase muscle mass and strength. Most of this research has focused on healthy young individuals and particularly in athletes to induce muscle buildup and strength, enhance aerobic performance and resistance to fatigue from exercise, reduce muscle damage, and improve regenerative capacity (1–3). In the past decade, HMB use in older adults has gained in interest to reduce sarcopenia and improve muscle function. Positive effects of HMB, mainly used in combination with other nutritional substrates, were observed in various disease states and particularly when combined with exercise.
Brief overview of mechanisms of actions of HMB to increase muscle mass and function
In the past years, several studies, including animal models, have focused on unraveling the mechanisms of action of HMB (3–10). In brief, HMB supplementation is effective in increasing myofibrillar protein synthesis via the mTOR pathway and the growth hormone/IGF-1 axis, and in preventing muscle protein breakdown via the ubiquitin proteasome and the autophagy-lysosome systems. HMB might increase plasma growth hormone and IGF-1 concentrations (11), and particularly when provided prior to resistance exercise augments the growth hormone response (12). HMB, despite being a leucine metabolite, signals to mTORC1 through mechanisms distinct from those of leucine. HMB also decreases cell apoptosis, therefore improving cell survival and differentiation of muscle stem cell. Furthermore, HMB is involved in the downregulation of NFkB and FOXO transcription factors, improves mitochondrial biogenesis, enhanced sarcoplasmic reticulum calcium release during exercise, and has been shown to stabilize cell membranes via cholesterol synthesis HMG-coenzyme A reductase.
Measuring HMB kinetics to obtain endogenous HMB production rate using pulse isotope tracer approach
To evaluate the importance of HMB for human health and the body’s requirement in case of increased demand, insight is needed in the metabolic pathways and production rates of HMB in health and disease. HMB is an endogenous metabolite from the reversible transamination of leucine to α-ketoisocaproic acid (KIC) by branched chain amino acid (BCAA) transaminase which mainly occurs in the skeletal muscle. 5–10% of the KIC is converted to HMB by KIC dioxygenase, which is high in liver and low in the muscle compartment. Human plasma concentrations of leucine, KIC and HMB are shown in Table 1 (13). We observed that plasma HMB concentration, which is 3% of that of leucine, was lower in older than in younger adults, although this was not confirmed in another recent study (14). Measurement of plasma HMB concentration is important as a strong association between plasma HMB concentrations and muscle strength and mass was found in young and older adults and in chronic diseases (14, 15).
Table 1.
Plasma concentration in the postabsorptive condition in μmol/l (mean (SE))(13)
| Substrate | Younger Adults (n=10, 20–30y) | Older Adults (n=17, 60–70y) | P value |
|---|---|---|---|
| Leucine | 104.4 (10.7) | 99.4 (5.1) | 0.636 |
| α-ketoisocaproic acid (KIC) | 27.4 (2.5) | 20.7 (1.4) | 0.0206 |
| β-hydroxy β-methylbutyrate (HMB) | 3.5 (0.3) | 2.6 (0.2) | 0.0098 |
The pathways underlying differences in HMB plasma levels among individuals can be studied using stable isotope techniques. Previously it was assumed that whole body HMB production rate, estimated from urinary excretion that is about 34% of total production, is approximately 0.3 mg/kg BW/day in pigs (2.5 μmol/kg BW/day) (16). As stable isotope tracer methodology is a more accurate way to measure whole body production rate of HMB, we chose to use a small pulse of labeled HMB stable tracer (13) as the isotope approach. When combined with the stable tracer pulses of KIC and leucine, the whole body production rates of these metabolites as well as their conversion rates can simultaneously be determined (leucine > KIC > HMB pathway) (Table 2; unpublished).
Table 2.
Whole body production rates in μmol/kg lean body mass*h as measured by stable tracer methodology (mean (SE)) after a pulse with the respective tracers (Unpublished data).
| Substrate (stable tracer) | Older adults (n=11; 65–75y) |
|---|---|
| Leucine (L-[U-13C6]) | 154.1 (18.4) |
| α-ketoisocaproic acid ([1-13C]) | 23.7 (2.64) |
| β-hydroxy β-methylbutyrate ([3,4 methyl-13C3]) | 0.17 (0.02) |
The stable tracer study shows that whole body KIC production is about 15% of leucine turnover, while HMB production is less than 1% of KIC turnover and 0.1% of leucine turnover. The total HMB production is about 4 μmol/kg lean mass/day. We previously showed, using an incorporation tracer calculation, that whole body HMB production rate accounts for 0.66% of leucine turnover (17), also indicating that HMB production in the human body is very low.
Administration of an oral dose of 3.42 gram of free leucine resulted in a plasma HMB increase (from 3 to 10 μM) 2.5 hours after intake (4), followed by a further gradual increase in HMB concentration. In recent studies in middle aged adults, we noticed that plasma leucine concentration peaks at 60 min (from 96 to 220 μM) after intake of a high protein nutritional supplement, and that a return to baseline values was observed 3 hours after intake. Interestingly, the increase in plasma HMB concentration was much longer and peaked at 6 hours (from 1.8 to 2.3 μM) after intake, and plasma HMB concentration was back to baseline value at 12 hours. This indicates that a very large amount of leucine need to be taken in order to substantially increase plasma HMB concentration.
Pharmacokinetics of leucine and HMB
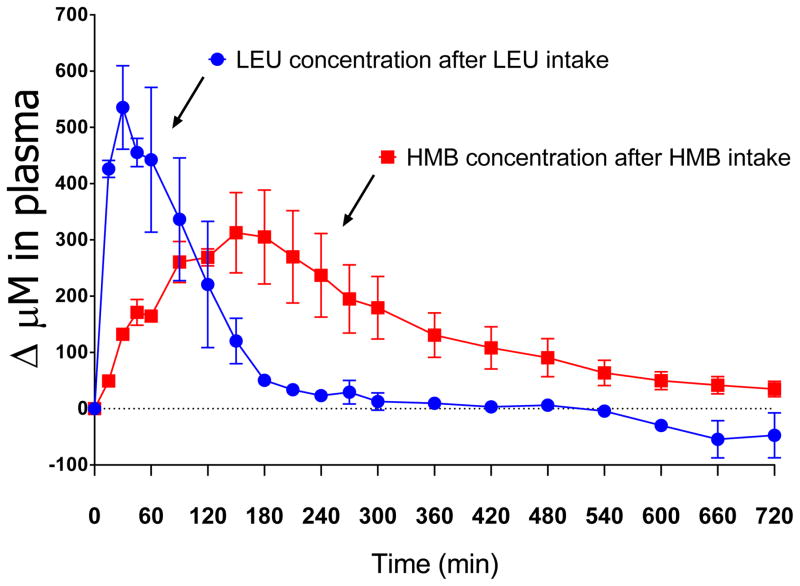
Two forms of HMB are currently being used in pharmacokinetic studies and the calcium salt form (Ca-HMB) is the most commonly form used. Providing 3.42 gram of Calcium HMB increases plasma HMB concentration within 60 min to about 480 μM, followed by a very slow decline (10). Intake of 3.42 gram of Free Acid HMB increases plasma HMB concentration to 400 μM, also followed by a slow decline (4). The difference in plasma HMB response suggests a slightly better systemic bioavailability after Calcium HMB intake (10), in line with previous data (18). We tested in a pilot study (Figure) also the effects of adding Ca-HMB to a high protein nutritional supplement and found the peak HMB concentration 3 hours after combined intake of the HMB and nutritional supplement. This peak HMB concentration was 80% of that obtained after intake of HMB alone but also remained elevated up to 12 hours after intake (19). This is likely caused by slow metabolism of HMB in the body and the urinary excretion pathway of HMB. In contrast, the pharmacokinetics of leucine are characterized by a very fast absorption and distribution (20–24). Intake of 3 gram of leucine, either as a single free amino amino acid or as part of a free amino acid mixture, will increase plasma leucine concentration rapidly by 500 μM followed by a fast return to baseline within 4 hours after intake (Figure), likely caused by incorporation of leucine into protein.
Figure.
The change in plasma concentration after intake of a high protein nutritional supplement (ONS) to which 3 gram leucine was added or 1.5 gram of Calcium HMB. The pharmacokinetics show a fast absorption and return to baseline when leucine was added to the nutritional supplement, while a slow absorption and slow return to baseline when HMB was added to the nutritional supplement.
How does the effects of HMB compare to Leucine?
Recent studies showed that acute intake of about 3 gram of HMB (either Ca-HMB or Free Acid-HMB) was able to increase muscle protein synthesis (MPS) two fold (4, 10) and reduce muscle protein breakdown by half (MPS) (10), leading to increased net protein synthesis. Intake of 3 grams of leucine increased muscle protein synthesis rate to the same extent as HMB (4), however, the mechanisms behind the reduction in muscle protein breakdown rate remained unclear. Most acute studies did not take in consideration the differences in pharmacokinetics between leucine (fast uptake and fast return to baseline levels) and HMB (fast uptake and slow return to baseline levels). As HMB has a more prolonged effect on muscle protein synthesis and breakdown rates than leucine, the favorable effects of HMB on protein anabolism would likely have been larger when longer observation periods were used. Although there are no studies directly comparing the effects of HMB and leucine interventions, the results from recent bedrest studies could give some insight. When HMB was given for a longer period, such as during 10 days of bedrest in older adults (25), it attenuated muscle loss. When an essential amino acid mixture high in leucine was provided to older adults in the same model of 10 days of bedrest, only a few muscle function tests improved but no attenuation in muscle loss was observed (26). Also in middle aged adults, leucine partially protected against muscle and functional loss after 14 days of bedrest (27). Our present working hypothesis is that both HMB and leucine are able to attenuate muscle loss in older adults during catabolic conditions, albeit HMB being more promising because of a longer half-life time in blood.
HMB intervention studies in older adults and in disease states
HMB given as a dietary supplement in humans has anticatabolic effects as it blunts age-related losses of strength and myofiber dimensions (28). Prolonged Ca-HMB supplementation was able to improve strength, body composition, functionality and muscle quality with and without resistance exercise in older adults (29). Oral nutritional supplement containing Ca-HMB improved leg muscle strength and quality in malnourished older adults with mild-moderate sarcopenia (30). We recently found no muscle loss after 10 days of bedrest in healthy older adults supplemented with HMB, and a muscle gain was observed after 8 weeks of exercise rehabilitation (25) (Table 3). Supplementation of 1.5 g Ca-HMB for 8 weeks during a mild fitness program in older women (31) resulted in increased muscle strength and endurance, and improved 6 minutes walking distance but no increase in muscle mass.
Table 3.
Published studies in past 2 years investigating effects of HMB intervention in healthy and diseased older adults
| Author | Study population | Design | Nutritional intervention | (Exercise) rehabilitation and assessments | Primary Results |
|---|---|---|---|---|---|
| Berton, 2015 (31) | Older women 65y and older (n=80) | Randomized, parallel group, open label | 1.5g/day of calcium HMB vs. control for 8 wks | Twice-weekly mild fitness program for 8 wks (ie resistance exercise) Assessments at baseline and after 8wks |
No difference in short physical performance battery, handgrip strength or body composition. HMB group: ↑ isokinetic flexion and extension, ↑isometric strength, ↑ 6MWT, ↑ handgrip endurance |
| Cramer 2016 (30) | Malnourished and sarcopenic men and women 65y and older (n=330) | Multicenter, randomized, double-blinded, controlled clinical trial | Experimental ONS (20g protein, 499 IU Vit D3, 1.5g Ca-HMB) vs. control (14g protein, 147 IU Vit D3) for 24 wks. 2 servings/day | Assessments at wk 0, 12 and 24 | Experimental ONS vs control: ↑ leg muscle strength and quality in mild moderate sarcopenia but not severe sarcopenia |
| Nishizaki, 2015 (36) | Knee osteo arthritis patients undergoing total knee arthroplasty (n=23) | Randomized controlled study | 2.4g HMB/14g ARG/14g GLN (n=13) vs. control (n=10) daily for 5 days before and 28 days after surgery | Strength training, range of motion exercise and walking training postop. Assessments 7 days prior and 14, 28 and 42 days post surgery | HMB: No loss of muscle strength between pre and post operative day 14. No difference in non-operative side, length of hospital stay, body weight changes |
| Stout 2015 (47) | Healthy elderly men (n=48) | Randomized, double blind, controlled study | 1.5g Ca-HMB + 4g CHO vs. 200 mg calcium + 4g CHO twice daily | Twelve weeks of resistance training (RT). Assessments pre and post RT | HMB+RT: ↓ adipose fat mass |
| Deutz, 2016 (33) | 65y and older, hospitalized for exacerbation COPD, CHF, acute myocardial infarction, pneumonia (n=652) | multicenter, randomized, placebo controlled double-blind | High Protein-HMB (20g protein, 11g fat, 44g CHO, 1.5g Ca-HMB, 160IU vitamin D) vs placebo, 2*/day from hospitalization until 90-day postdischarge | Inpatient and posthospital discharge Assessments at hospital admission and discharge, day 30, 60 and 90 postdischarge |
No difference in primary composite endpoint (90 d postdischarge incidence of death or nonelective readmission). High Protein-HMB: ↓ 90-day mortality, ↑ survival, ↑ odd of patients achieving better nutritional status at day 90, ↑ body weight day 30 |
| Ekinci, 2016 (35) | Older women with hip fracture admitted to orthopedic clinics (n=75) | Randomized controlled study | 3g CaHMB, 1000IU vitamin D, 36g protein nutritional supplement 2 servings/day postoperatively for 30 days vs. control | Assessment preoperatively and at postoperative day 15 and 30. | CaHMB/vitamin D/protein combination:Shorter wound healing period. ↑ ambulatory and mobilization, ↑ muscle strength. |
| Fitschen, 2016 (32) | Maintenance hemodialysis patients (n=33) | Double-blind, placebo controlled, randomized trial | 3g/d Ca-HMB vs. placebo 7d/w for 6 months | Assessment during 6 months of hemodialysis | No difference in body composition, strength, bone density, physical function, fall risk, quality of life. Compliance problems |
| Olveira, 2016 (34) | Bronchiectasis (n=30) | single center, randomized controlled trial, parallel treatment design | Oral nutritional supplement (ONS).18g protein, 1.5g HMB, 1.7g prebiotic fiber vs. no supplement once/day | 12 wks of pulmonary rehabilitation. Assessment at baseline, 12 and 24 wks | ONS: ↑ Bone density, handgrip strength, Mid-arm muscle circumference, physical functioning domain in quality of life. Non-sign ↑ myostatin |
Ca: calcium, CHO: carbohydrates, HMB: β-hydroxy β-methylbutyrate, 6MWT; 6 minute walk test, ONS: oral nutritional supplement, RT: resistance training
The anticatabolic effects of HMB has previously been found in patients with cancer, immunodeficiency syndrome, and COPD admitted to an intensive care unit. In the past year (Table 3), the clinical application of HMB has been reported in a wider group of diseased patients. HMB supplementation for 6 months showed no effect of body composition, muscle strength, quality of life in hemodialysis patients (32), although reduced compliance to intake might be a confounding factor. HMB as part of protein supplementation resulted in 50% lower mortality and gain in nutritional status and muscle strength at 90-day post-discharge in older malnourished hospitalized patients (33), and in improved muscle strength and mass, and physical functioning in patients with bronchiectasis when combined with pulmonary rehabilitation (34). Furthermore, HMB as part of protein or amino acid supplementation resulted in shorter wound healing, better ambulatory and mobilization status, and in increased muscle strength postoperatively in older women with hip fractures (35), and in preserved quadriceps muscle strength after surgery in knee osteoarthritis patients (36). The increased interest and generally positive outcomes observed in recent years of HMB supplementation in the older population with different diseases but also in relation to medical treatment, hospitalization, and surgical procedures, will likely further increase its use in research studies which is required to determine its applicability to improve health and well-being of various clinical populations as well as its optimal dosage.
Other new potential beneficial effects of HMB
Cognitive enhancement
Normal aging results in cognitive decline including deficits in attention, memory, decision making, visuospatial skills negatively affecting quality of life. Nutritional interventions of HMB is a potential approach for reducing these deficits as HMB is known to cross the blood–brain barrier (37). In mice, no effect were found of short-term HMB supplementation on cognition (38). However in aged rats, daily HMB supplementation has been shown to mitigate age-related declines in dendritic material and the total number of dendritic spines in the medial prefrontal cortex (39) and to prevent age-related decrement of water maze performance (40). Recently, treatment with HMB improved working memory performance in middle-age males and old age rats (41). In the cognitive flexibility task, there was an age-dependent deficit in acquisition of the visual strategy that was not present in old age males treated with HMB. HMB also reduced the deficit in visual strategy acquisition in middle age females. These data suggest that HMB supplementation may be an effective nutritional intervention for diminishing age-associated cognitive decline. The precise mechanism through which HMB induces anti-aging cognitive benefits are still unknown.
Improved Bone Health
Currently used approaches to treat osteoporosis include antiresorptive and anabolic agents influencing bone metabolism. As HMB has anabolic effects on muscle (see above), it has been suggested that HMB is also anabolic for bone as reflected by higher bone mineral density and improved morphometric and mechanical properties (42). Furthermore, HMB intake improved bone properties during simulated sustained military operations in mice (43). Although human studies are still limited, a recent case report showed that 61 weeks of oral administration with calcium-HMB improved volumetric bone mineral density of lumbar spine in the trabecular and cortical bone compartments (44). Positive effects of 2.5 years of HMB treatment on bone density of lumbar spine and femur in were also shown (44). Oral protein supplementation enriched with HMB during pulmonary rehabilitation improved bone density in patients with bronchiectasis (34). No effects of HMB supplementation for 6 months on bone density were found in hemodialysis patients, but 31% of the HMB group were noncompliant (32). The exact mechanisms positively influencing bone tissue metabolism by HMB, as well as the relationship between HMB dosage and the response of the skeletal system needs further investigation.
Reducing Fat mass in abdominal obesity
Recent studies showed that HMB improves metabolic capacity to utilize fat and increases fatty acid oxidation in adipocytes and muscle cells (8, 45). Growth hormone, which is increased after HMB intake (12), is known to stimulate lipolysis. Accumulation of abdominal fat mass has been correlated with increased risk of hypertension, diabetes and cardiovascular disease. HMB in combination with resistance exercise resulted in (relative) lower values for whole body fat (29, 34, 46) and in decreased adipose fat mass in older adult men (47). No positive effects of HMB supplementation on abdominal fat mass or fat area in muscle have however been found in other studies (31, 32), indicating the use of HMB to reduce fat mass particularly in the treatment of abdominal obesity is less certain (48).
Is HMB an effective anabolic agent to improve outcome in diseased populations?
It has become clearer that the anabolic properties of HMB in relation to muscle mass and function, and its potential to enhance the effects of and recovery from exercise are also present in older (diseased) populations. There is sufficient evidence that both leucine and HMB can stimulate protein synthesis and reduce protein breakdown, albeit with different efficacy (4, 7, 10). The fact that the half-life time in blood of HMB is longer than that of leucine, favors the use of HMB above leucine to achieve protein anabolism. Furthermore, there is promising evidence that HMB might support bone density, improve cognitive function, and reduce abdominal obesity in older adults. The exact mechanisms of action and the optimal dosage for HMB supplementation in diseased populations remain unclear and more research is needed in various clinical conditions.
Key Points.
Use of HMB has gained lately a considerable amount of attention in diseased populations to regain their muscle mass and function, improve their condition and well-being, and to improve their treatment response.
The advantages of HMB above other available dietary metabolites are its anticatabolic and long lasting (pharmacological) effect.
Evidence exists that HMB might support bone density, improve cognitive function, and reduce abdominal obesity which is of crucial importance in the older population.
A large amount of dietary leucine need to be taken in order to substantially increase plasma HMB concentration.
The optimal clinical dosage of HMB and its application in various clinical conditions needs to be established.
Acknowledgments
We would to thank all staff and researchers of the Center for Translational Research in Aging and Longevity for their continuous support.
Financial support and sponsorship
Research reported in this publication was supported by the National Institute of Environmental Health Sciences under grant number P30ES023512 and the National Heart, Lung, and Blood Institute under grant number R01HL132887 of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: ME and ND have received research funding and speaker honoraria from Abbott Nutrition.
Contributor Information
Mariëlle P. K. J. Engelen, Center for Translational Research in Aging & Longevity, Department of Health and Kinesiology, Texas A&M University, College Station, TX.
Nicolaas E. P. Deutz, Center for Translational Research in Aging & Longevity, Department of Health and Kinesiology, Texas A&M University, College Station, TX.
References
- 1.Molfino A, Gioia G, Fanelli FR, Muscaritoli M. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials. Amino Acids. 2013:1–20. doi: 10.1007/s00726-013-1592-z. [DOI] [PubMed]
- 2.Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J. International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB) J Int Soc Sports Nutr. 2013;10(1):6. doi: 10.1186/1550-2783-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Holecek M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. Journal of cachexia, sarcopenia and muscle. 2017;8(4):529–41. doi: 10.1002/jcsm.12208. Epub 2017/05/12. Excellent review paper that is a good start for new researchers in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. The Journal of physiology. 2013;591(Pt 11):2911–23. doi: 10.1113/jphysiol.2013.253203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirza KA, Pereira SL, Voss AC, Tisdale MJ. Comparison of the anticatabolic effects of leucine and Ca-β-hydroxy-β-methylbutyrate in experimental models of cancer cachexia. Nutrition. 2014;30(7–8):807–13. doi: 10.1016/j.nut.2013.11.012. doi: http://dx.doi.org/10.1016/j.nut.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Giron MD, Vilchez JD, Shreeram S, Salto R, Manzano M, Cabrera E, Campos N, Edens NK, Rueda R, Lopez-Pedrosa JM. beta-Hydroxy-beta-methylbutyrate (HMB) normalizes dexamethasone-induced autophagy-lysosomal pathway in skeletal muscle. PloS one. 2015;10(2):e0117520. doi: 10.1371/journal.pone.0117520.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giron MD, Vilchez JD, Salto R, Manzano M, Sevillano N, Campos N, Argiles JM, Rueda R, Lopez-Pedrosa JM. Conversion of leucine to beta-hydroxy-beta-methylbutyrate by alpha-keto isocaproate dioxygenase is required for a potent stimulation of protein synthesis in L6 rat myotubes. Journal of cachexia, sarcopenia and muscle. 2016;7(1):68–78. doi: 10.1002/jcsm.12032.. Epub 2016/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X, Duan Y, Yao K, Li F, Hou Y, Wu G, Yin Y. beta-Hydroxy-beta-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids. 2016;48(3):653–64. doi: 10.1007/s00726-015-2126-7. [DOI] [PubMed] [Google Scholar]
- 9.Vallejo J, Spence M, Cheng AL, Brotto L, Edens NK, Garvey SM, Brotto M. Cellular and Physiological Effects of Dietary Supplementation with beta-Hydroxy-beta-Methylbutyrate (HMB) and beta-Alanine in Late Middle-Aged Mice. PLoS One. 2016;11(3):e0150066. doi: 10.1371/journal.pone.0150066.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Wilkinson DJ, Hossain T, Limb MC, Phillips BE, Lund J, Williams JP, Brook MS, Cegielski J, Philp A, Ashcroft S, Rathmacher JA, Szewczyk NJ, Smith K, Atherton PJ. Impact of the calcium form of β-hydroxy-β-methylbutyrate upon human skeletal muscle protein metabolism. Clinical Nutrition. 2017 doi: 10.1016/j.clnu.2017.09.024. doi: https://doi.org/10.1016/j.clnu.2017.09.024. Interesting study that show the effects of protein synthesis and breakdown of HMB. [DOI] [PMC free article] [PubMed]
- 11.Portal S, Zadik Z, Rabinowitz J, Pilz-Burstein R, Adler-Portal D, Meckel Y, Cooper DM, Eliakim A, Nemet D. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: a prospective randomized, double-blind, placebo-controlled study. Eur J Appl Physiol. 2011;111(9):2261–9. doi: 10.1007/s00421-011-1855-x. Epub 2011/02/18. [DOI] [PubMed] [Google Scholar]
- 12.Townsend JR, Hoffman JR, Gonzalez AM, Jajtner AR, Boone CH, Robinson EH, Mangine GT, Wells AJ, Fragala MS, Fukuda DH, Stout JR. Effects of beta-Hydroxy-beta-methylbutyrate Free Acid Ingestion and Resistance Exercise on the Acute Endocrine Response. Int J Endocrinol. 2015;2015:856708. doi: 10.1155/2015/856708.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Deutz NEP, Thaden JJ, Ten Have GAM, Walker DK, Engelen M. Metabolic phenotyping using kinetic measurements in young and older healthy adults. Metabolism: clinical and experimental. 2018;78:167–78. doi: 10.1016/j.metabol.2017.09.015. Epub 2017/10/08. New approach to measure a large panel of metabolites with stable tracers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuriyan R, Lokesh DP, Selvam S, Jayakumar J, Philip MG, Shreeram S, Kurpad AV. The relationship of endogenous plasma concentrations of beta-Hydroxy beta-Methyl Butyrate (HMB) to age and total appendicular lean mass in humans. Experimental gerontology. 2016;81:13–8. doi: 10.1016/j.exger.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Engelen M, Walker D, Wierzchowska-Mcnew A, Jeon M, Deutz N. MON-P130: B-Hydroxy-B-Methylbutyrate (HMB) Plasma Levels are Strongly Related to Muscle Mass and Strength in Patients with Chronic Obstructive Pulmonary Disease. Clinical Nutrition. 2017;36:S227. [Google Scholar]
- 16.Van Koevering M, Nissen S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. The American journal of physiology. 1992;262(1 Pt 1):E27–31. doi: 10.1152/ajpendo.1992.262.1.E27. [DOI] [PubMed] [Google Scholar]
- 17*.Walker DK, Thaden JJ, Wierzchowska-McNew A, Engelen M, Deutz NEP. Determination of beta-hydroxy-beta-methylbutyrate concentration and enrichment in human plasma using chemical ionization gas chromatography tandem mass spectrometry. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2017;1040:233–8. doi: 10.1016/j.jchromb.2016.11.010. Epub 2016/11/20. Method to measure the HMB concentration and enrichment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shreeram S, Johns PW, Subramaniam S, Ramesh S, Vaidyanathan V, Puthan JK, Mandal S, Mamidi VK, Gelling RW. The Relative Bioavailability of the Calcium Salt of β-Hydroxy-β-Methylbutyrate Is Greater Than That of the Free Fatty Acid Form in Rats. The Journal of Nutrition. 2014 doi: 10.3945/jn.114.196527. [DOI] [PubMed] [Google Scholar]
- 19.Vukovich MD, Slater G, Macchi MB, Turner MJ, Fallon K, Boston T, Rathmacher J. beta-hydroxy-beta-methylbutyrate (HMB) kinetics and the influence of glucose ingestion in humans. J Nutr Biochem. 2001;12(11):631–9. doi: 10.1016/s0955-2863(01)00182-6. Epub 2002/05/29 S0955-2863(01)00182-6 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin Nutr. 2014;33(2):211–20. doi: 10.1016/j.clnu.2013.06.014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell WK, Phillips BE, Hill I, Greenhaff P, Lund JN, Williams JP, Rankin D, Wilkinson DJ, Smith K, Atherton PJ. Human skeletal muscle is refractory to the anabolic effects of leucine during the postprandial muscle-full period in older men. Clin Sci (Lond) 2017;131(21):2643–53. doi: 10.1042/CS20171230. Epub 2017/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Effectiveness of essential amino acid supplementation in stimulating whole body net protein anabolism is comparable between COPD patients and healthy older adults. Metabolism: clinical and experimental. 2017;69:120–9. doi: 10.1016/j.metabol.2016.12.010.. Epub 2017/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy CH, Saddler NI, Devries MC, McGlory C, Baker SK, Phillips SM. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. The American Journal of Clinical Nutrition. 2016 doi: 10.3945/ajcn.116.136424. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell WK, Phillips BE, Williams JP, Rankin D, Lund JN, Smith K, Atherton PJ. A Dose- rather than Delivery Profile–Dependent Mechanism Regulates the “Muscle-Full” Effect in Response to Oral Essential Amino Acid Intake in Young Men. The Journal of Nutrition. 2015 doi: 10.3945/jn.114.199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32(5):704–12. doi: 10.1016/j.clnu.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29(1):18–23. doi: 10.1016/j.clnu.2009.03.009. Epub 2009/05/08. [DOI] [PubMed] [Google Scholar]
- 27.English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. The American Journal of Clinical Nutrition. 2016;103(2):465–73. doi: 10.3945/ajcn.115.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson JM, Grant SC, Lee SR, Masad IS, Park YM, Henning PC, Stout JR, Loenneke JP, Arjmandi BH, Panton LB, Kim JS. Beta-hydroxy-beta-methyl-butyrate blunts negative age-related changes in body composition, functionality and myofiber dimensions in rats. Journal of the International Society of Sports Nutrition. 2012;9(1):18. doi: 10.1186/1550-2783-9-18. Epub 2012/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stout JR, Smith-Ryan AE, Fukuda DH, Kendall KL, Moon JR, Hoffman JR, Wilson JM, Oliver JS, Mustad VA. Effect of calcium beta-hydroxy-beta-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: A randomized, double-blind pilot trial. Experimental gerontology. 2013;48(11):1303–10. doi: 10.1016/j.exger.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 30*.Cramer JT, Cruz-Jentoft AJ, Landi F, Hickson M, Zamboni M, Pereira SL, Hustead DS, Mustad VA. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial. Journal of the American Medical Directors Association. 2016;17(11):1044–55. doi: 10.1016/j.jamda.2016.08.009. doi: http://dx.doi.org/10.1016/j.jamda.2016.08.009 Trial that shows that a high protein + HMB supplement can improve strength. [DOI] [PubMed] [Google Scholar]
- 31.Berton L, Bano G, Carraro S, Veronese N, Pizzato S, Bolzetta F, De Rui M, Valmorbida E, De Ronch I, Perissinotto E, Coin A, Manzato E, Sergi G. Effect of Oral Beta-Hydroxy-Beta-Methylbutyrate (HMB) Supplementation on Physical Performance in Healthy Old Women Over 65 Years: An Open Label Randomized Controlled Trial. PloS one. 2015;10(11):e0141757. doi: 10.1371/journal.pone.0141757.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitschen PJ, Biruete A, Jeong J, Wilund KR. Efficacy of beta-hydroxy-beta-methylbutyrate supplementation in maintenance hemodialysis patients. Hemodialysis International. 2016 doi: 10.1111/hdi.12440. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 33*.Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, Hegazi RA, Tappenden KA, Ziegler TR, Group NS. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin Nutr. 2016;35(1):18–26. doi: 10.1016/j.clnu.2015.12.010. Important paper that show that a nutritional supplement with high protein and HMB reduce mortality in malnourished hospitalized patients. [DOI] [PubMed] [Google Scholar]
- 34.Olveira G, Olveira C, Dona E, Palenque FJ, Porras N, Dorado A, Godoy AM, Rubio-Martinez E, Rojo-Martinez G, Martin-Valero R. Oral supplement enriched in HMB combined with pulmonary rehabilitation improves body composition and health related quality of life in patients with bronchiectasis (Prospective, Randomised Study) Clin Nutr. 2016;35(5):1015–22. doi: 10.1016/j.clnu.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Ekinci O, Yanik S, Terzioglu Bebitoglu B, Yilmaz Akyuz E, Dokuyucu A, Erdem S. Effect of Calcium beta-Hydroxy-beta-Methylbutyrate (CaHMB), Vitamin D, and Protein Supplementation on Postoperative Immobilization in Malnourished Older Adult Patients With Hip Fracture: A Randomized Controlled Study. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2016 doi: 10.1177/0884533616629628. [DOI] [PubMed] [Google Scholar]
- 36.Nishizaki K, Ikegami H, Tanaka Y, Imai R, Matsumura H. Effects of supplementation with a combination of beta-hydroxy-beta-methyl butyrate, L-arginine, and L-glutamine on postoperative recovery of quadriceps muscle strength after total knee arthroplasty. Asia Pacific journal of clinical nutrition. 2015;24(3):412–20. doi: 10.6133/apjcn.2015.24.3.01. [DOI] [PubMed] [Google Scholar]
- 37.Santos-Fandila A, Zafra-Gomez A, Barranco A, Navalon A, Rueda R, Ramirez M. Quantitative determination of beta-hydroxymethylbutyrate and leucine in culture media and microdialysates from rat brain by UHPLC-tandem mass spectrometry. Anal Bioanal Chem. 2014;406(12):2863–72. doi: 10.1007/s00216-014-7694-y. Epub 2014/03/05. [DOI] [PubMed] [Google Scholar]
- 38.Munroe M, Pincu Y, Merritt J, Cobert A, Brander R, Jensen T, Rhodes J, Boppart MD. Impact of beta-hydroxy beta-methylbutyrate (HMB) on age-related functional deficits in mice. Experimental gerontology. 2017;87(Pt A):57–66. doi: 10.1016/j.exger.2016.11.010. Epub 2016/11/27. [DOI] [PubMed] [Google Scholar]
- 39.Kougias DG, Nolan SO, Koss WA, Kim T, Hankosky ER, Gulley JM, Juraska JM. Beta-hydroxy-beta-methylbutyrate ameliorates aging effects in the dendritic tree of pyramidal neurons in the medial prefrontal cortex of both male and female rats. Neurobiol Aging. 2016;40:78–85. doi: 10.1016/j.neurobiolaging.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Kougias DG, Hankosky ER, Gulley JM, Juraska JM. Beta-hydroxy-beta-methylbutyrate (HMB) ameliorates age-related deficits in water maze performance, especially in male rats. Physiol Behav. 2017;170:93–9. doi: 10.1016/j.physbeh.2016.12.025. Epub 2016/12/31. [DOI] [PubMed] [Google Scholar]
- 41.Hankosky ER, Sherrill LK, Ruvola LA, Haake RM, Kim T, Hammerslag LR, Kougias DG, Juraska JM, Gulley JM. Effects of beta-hydroxy-beta-methyl butyrate on working memory and cognitive flexibility in an animal model of aging. Nutr Neurosci. 2017;20(7):379–87. doi: 10.1080/1028415X.2016.1145376. [DOI] [PubMed] [Google Scholar]
- 42.Tatara MR. Effect of beta-hydroxy-beta-methylbutyrate (HMB) administration on volumetric bone mineral density, and morphometric and mechanical properties of tibia in male turkeys. J Anim Physiol Anim Nutr (Berl) 2009;93(6):669–77. doi: 10.1111/j.1439-0396.2008.00854.x. [DOI] [PubMed] [Google Scholar]
- 43.Henning PC, Park BS, Kim JS. beta-Hydroxy-beta-methylbutyrate improves bone properties and attenuates the depression of protein synthesis during a simulated sustained operation. Mil Med. 2014;179(6):679–85. doi: 10.7205/MILMED-D-13-00421. [DOI] [PubMed] [Google Scholar]
- 44.Tatara MR, Krupski W, Majer-Dziedzic B. Bone mineral density changes of lumbar spine and femur in osteoporotic patient treated with bisphosphonates and beta-hydroxy-beta-methylbutyrate (HMB): Case report. Medicine (Baltimore) 2017;96(41):e8178. doi: 10.1097/MD.0000000000008178. Epub 2017/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruckbauer A, Zemel MB, Thorpe T, Akula MR, Stuckey AC, Osborne D, Martin EB, Kennel S, Wall JS. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutrition & metabolism. 2012;9(1):77. doi: 10.1186/1743-7075-9-77.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hector A, Phillips SM. Protein Recommendations for Weight Loss in Elite Athletes: A Focus on Body Composition and Performance. International journal of sport nutrition and exercise metabolism. 2017:1–26. doi: 10.1123/ijsnem.2017-0273. Epub 2017/11/29. [DOI] [PubMed] [Google Scholar]
- 47.Stout JR, Fukuda DH, Kendall KL, Smith-Ryan AE, Moon JR, Hoffman JR. beta-Hydroxy-beta-methylbutyrate (HMB) supplementation and resistance exercise significantly reduce abdominal adiposity in healthy elderly men. Experimental gerontology. 2015;64:33–4. doi: 10.1016/j.exger.2015.02.012. Epub 2015/02/24. [DOI] [PubMed] [Google Scholar]
- 48.Rossi AP, D’Introno A, Rubele S, Caliari C, Gattazzo S, Zoico E, Mazzali G, Fantin F, Zamboni M. The Potential of beta-Hydroxy-beta-Methylbutyrate as a New Strategy for the Management of Sarcopenia and Sarcopenic Obesity. Drugs & aging. 2017;34(11):833–40. doi: 10.1007/s40266-017-0496-0. [DOI] [PubMed] [Google Scholar]