Abstract
Collective decision-making is a daily occurrence in the lives of many group-living animals, and can have critical consequences for the fitness of individuals. Understanding how decisions are reached, including who has influence and the mechanisms by which information and preferences are integrated, has posed a fundamental challenge. Here, we provide a methodological framework for studying influence and leadership in groups. We propose that individuals have influence if their actions result in some behavioural change among their group-mates, and are leaders if they consistently influence others. We highlight three components of influence (influence instances, total influence and consistency of influence), which can be assessed at two levels (individual-to-individual and individual-to-group). We then review different methods, ranging from individual positioning within groups to information-theoretic approaches, by which influence has been operationally defined in empirical studies, as well as how such observations can be aggregated to give insight into the underlying decision-making process. We focus on the domain of collective movement, with a particular emphasis on methods that have recently been, or are being, developed to take advantage of simultaneous tracking data. We aim to provide a resource bringing together methodological tools currently available for studying leadership in moving animal groups, as well as to discuss the limitations of current methodologies and suggest productive avenues for future research.
This article is part of the theme issue ‘Collective movement ecology’.
Keywords: decision-making, leadership, social behaviour, collective movement, methods
1. Introduction
As animals move through their habitat, they are confronted with repeated choices about how to react to environmental stimuli. Was that a falcon or a shadow? Should I remain here or travel elsewhere to find food? Because these decisions can have life or death implications, and are often made with imperfect information, selection should strongly favour behavioural and cognitive adaptations that improve individual decision-making ability [1]. In social species that move in cohesive groups, the quality of a group's decisions, and thus the ability of group members to effectively exploit their environment, depends on how individual information and preferences are integrated to reach consensus. Pooling information can improve the accuracy of group decisions—a fundamental principle of human justice systems [2]. In fact, quorum processes, where most or all group members contribute equally to collective decisions, are widespread in the natural world [3–5]. Under certain conditions, however, leadership by one or a few individuals can be beneficial for all [6], for example, when individuals with relevant information lead [7–9]. Importantly, when the interests of group members diverge, leadership can be a strategy that some individuals pursue to selfishly influence collective decisions in their favour [10]. In such cases, other group members face a choice: remain with their group and accept an individually sub-optimal outcome (i.e. consensus cost [5]), or leave and lose the advantages of group life. Given the impact that collective decision-making processes have on the ability of group-living animals to respond to environmental challenges (e.g. finding food, evading predators) [7,8], as well as on the costs individuals pay and benefits they gain from group living, research on leadership in animal groups has widespread importance. However, studying leadership is complicated by technological, analytical and conceptual challenges that have impeded understanding of this central aspect of social living.
(a). A historical perspective on leadership
Descriptions of leadership in animal societies stretch back at least to Aristotle [11], but in the classical synthesis Sociobiology [12], Wilson highlighted widespread variation in leadership roles across the animal kingdom. Importantly, in characterizing the behaviour of groups of individuals acting in concert, he clearly distinguished the process of leadership (leading other members of the society when the group progresses from one place to another) from coordination (where joint action is achieved but no leadership is assumed). In part, this distinction arose from Wilson's and contemporary authors' [13–15] view of leadership as inherently linked to the social hierarchies of structured animal societies, at the time primarily studied in primates and other social mammals. As a consequence, research on collective movement diverged along taxonomic lines, with scientists working on mammals, and in particular on primates (including humans), framing their research to address the question ‘who leads?’ [16], while work on fish, birds and insects instead focused on understanding how group coordination is achieved [17–21].
Recently, a key insight from the field of collective behaviour has bridged the divide between the perspective of ‘inherent leaders’ (leaders are pre-determined according to traits or social status) versus ‘emergent leaders’ (leaders emerge from simple coordination mechanisms [9,22,23]) by demonstrating that interaction rules can vary according to traits (or motivation) [24,25] and such variation can have consequences on outcomes at the group level ([9,22,26]). Thus, leadership and collective decision-making are fundamentally linked. All organisms that move together must follow a set of basic rules, such as attraction, repulsion and/or alignment, that structure their interactions and allow group members to maintain cohesion. In such systems, leadership can be completely emergent. Experiments in fish [27,28] and humans [29] have confirmed that small numbers of individuals can influence large groups without any active signalling, or any explicit information about the identity of leaders if they simply have a bias to move in a particular direction. Thus, variation in interaction rules can produce the entire range of decision-making processes, from fully democratic to despotic. However, the process of leadership is not necessarily completely emergent [30]. For example, individuals can attempt to influence others by engaging in movements and behaviours that are independent of this rule set (such behaviours are often called ‘initiations’ [31]), which can have impacts on the behaviour of potential followers that are obeying more general coordination rules.
Variation in the movement rules that individuals employ can allow certain individuals to wield more or less influence over the group's trajectory [22,24]. At the same time, for an individual to influence others, it must be followed [32,33]. Kummer [34] first highlighted that, in many instances, group decisions are ultimately dictated not by the ‘leader’ but by their followers. Initiators make suggestions about the timing or direction of travel, but it is followers who ultimately decide when and where the group goes (see also [35]). For example, Rowell [15] observed that her study troop of olive baboons would only depart when a specific old female set off, suggesting that, while other troop members could attempt to influence the group by making initiation movements, this individual ultimately controlled the group decision by choosing to follow, or not. Thus, leadership is not only the study of who initiates actions, but also more fundamentally the study of the mechanisms of collective behaviour and how the behavioural interactions among individuals scale up to the patterns we observe at group level.
Plato's quote: ‘The wise shall lead and rule, and the ignorant shall follow’ [36, p. 120] is an axiom that exemplifies the definitional problems that have plagued studies of leadership both in humans and in non-human animals. The assumption that characteristics such as wisdom or ignorance are consistent traits of individuals led Galton, for example, to argue that leadership in humans is inherited [37]. We suggest that first asking how groups make decisions (i.e. focusing on the mechanism), instead of solely aiming to identify who leads (i.e. focusing on the outcomes), is key to avoiding such problematic conclusions. This mechanistic approach is more in line with the notions of optimality that form the foundation of behavioural ecology [1] and, with its focus on individuals' behavioural strategies, will prove more useful for addressing evolutionary questions about leadership.
(b). Defining influence and leadership
How groups make decisions is a multi-faceted problem, and it is not enough to determine where, on a spectrum from equally shared (i.e. democratic) to completely unshared (i.e. despotic) [38], the decision-making process falls. It is also key to understand how groups make decisions, when decisions are made, who has influence, and why particular group members come to disproportionately affect group behaviour. To address these questions, studies will benefit from partitioning behaviours and actions across time and contexts. A starting point is to have clear definitions. We propose that the field will be helped by explicitly differentiating between influence and leadership. We suggest that an individual has influence if its actions result in some behavioural change among other individuals in its group. Here, we focus on behaviours related to group movement, though an individual can also have influence in other domains [39]. An individual leads if it has repeated influence, either directly or hierarchically, on the behaviour of others.
(c). Hypotheses about who leads
There are a number of hypotheses about why some individuals are frequently influential while others that attempt to initiate movement or change the group's travel direction ultimately fail to recruit followers. Variation in leadership ability may stem from inter-individual differences in information [7,28] or motivation [24,40], in traits like dominance or age [10,41], or in individuals' phenotypic fit to their group (e.g. relative speed [25]). However, social characteristics can also play a particularly important role in determining which individuals emerge as leaders. The history of prior interactions that defines a social relationship may modulate rules of attraction, avoidance and/or alignment, shaping how group-mates respond to one another's movements. For example, the strength of their social connection predicts whether one baboon will follow the movement initiation attempt of another [31]. Thus, it may be easier for a socially well-connected individual to drive group decisions. By contrast, in some instances, influence may be a by-product, rather than a direct consequence, of social relationships. Visibility is often key to coordination [27,42], and dominance relationships affect not only where individuals tend to be positioned with respect to the rest of their group but also how much social monitoring they receive [43]. High-ranking animals may thus emerge as leaders not because of their social status per se, but simply because they are highly visible. Theoretical [44,45] and empirical [46,47] studies have also suggested that the structure of social relationships has important implications for how collective movement decisions emerge, as well as an important impact on key performance parameters such as how long it takes to make decisions. Our understanding of how the structure of animal societies promotes or impedes the emergence of leadership remains incomplete. However, the potential for social relationships to affect the influence that individuals have on their groups highlights why studying the mechanics of decision-making (how decisions are reached by the entire group) is important for understanding leadership.
(d). Outline of paper
The plethora of ways in which influence is achieved within animal groups, combined with the different types of data that have been collected by researchers, has led to a wide variety of different methods for inferring influence and leadership in groups. Here, we suggest a broad methodological framework with the aim of helping to structure studies of leadership. We review the different ways in which influence has been defined and operationalized in past studies of group movement, with a particular emphasis on more recent methods that take advantage of simultaneous movement data to draw conclusions. We then discuss ways in which methods for inferring influence can be scaled up to draw broader conclusions about the process of decision-making within animal groups. Finally, we highlight outstanding issues that are still limiting progress in this area, and outline some future directions that we anticipate will facilitate more robust evaluation of how animal groups make movement decisions.
2. A methodological framework for studying leadership in groups
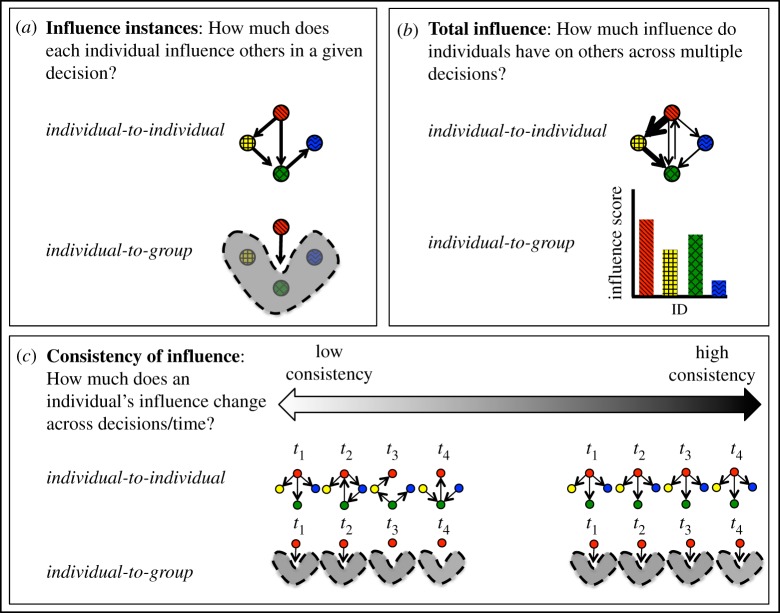
Studies of leadership will benefit from having a clear methodological framework and terminology to guide the acquisition and interpretation of data. Here, we suggest that studying leadership within groups requires several steps (figure 1). In step 1, data on influence instances (i.e. situations in which individuals exert influence on others, figure 1a) can be collected in several ways, partly determined by the method of data collection used. Such influence instances can be studied on two levels. First, we can study who influences whom during a given group decision, and potentially the strength of these influences, which we here term the individual-to-individual level. Alternatively (or additionally), we can quantify the overall contribution of an individual to a group-level outcome, which we here term the individual-to-group level. In step 2, either of these types of influence can be summarized over repeated events to quantify how much influence each individual has had across multiple group decisions (or across time, in the case of continuous movement), which we term total influence (figure 1b). Finally, a key to understanding leadership is to determine whether the influence of individuals on others in their group changes over time, or across decision-making events. We term this third step consistency of influence (figure 1c). Both total influence and consistency of influence can be measured using data at the individual-to-individual level and at the individual-to-group level.
Figure 1.
A conceptual framework for studying leadership in animal groups. Studies typically observe influence instances (a), instances in which a given individual influences the group (in the case of individual-to-group observations) or another individual (in the case of individual-to-individual observations) during a given decision, or at a particular moment in time. Coloured dots indicate individuals, with arrows representing influences between them. (b) Influence instances are then aggregated over multiple decisions, or across time, to estimate an individual's total influence over other individuals in its group (individual-to-individual level), or over the group as a whole (individual-to-group level). Such estimates often take the form of weighted network edges in the case of dyadic influence relationships (top of panel) or an aggregate influence ‘score’ or ‘rank’ in the case of the individual-to-group level (bottom of panel). (c) Finally, it is important to assess the consistency of influence across multiple events (here shown labelled as t1–t4) to determine the extent to which individuals influence one another (or the group) the same amount during each group decision, or across time.
(a). Influence at the individual-to-individual level (Who influences whom within the group?)
Group-level outcomes arise from the interactions among individuals within a group [9]. That is, for a given movement or group decision, individuals exert influence on their group-mates (in the sense of causally affecting their behaviour), rather than on the entire group directly. Thus, at the lowest level, one can measure or infer these individual-to-individual instances of influence to understand the dynamics of how a particular decision was made. Such relationships could be dyadic (e.g. A influenced B) or higher-order (e.g. A and B together influenced C). Moreover, these relationships can be characterized as binary (whether they happened or not) or quantified based on differing strengths of the influence. Quantifying individual-to-individual influence instances represents a bottom-up approach to studying leadership and is increasingly facilitated by the ability to monitor all or most individuals within a group simultaneously [48].
(b). Influence at the individual-to-group level (Who influences overall group decisions?)
Individual-to-individual influences within a group ultimately give rise to collective (i.e. group-level) outcomes. We refer to the overall influence of an individual over a group decision as individual-to-group influence. For example, an individual could have sole influence over a group decision such as the direction the group moves in (in which case its individual-to-group influence would be high) or could contribute only a small amount to this decision (in which case its individual-to-group influence would be low). It is possible to characterize individual-to-group influence without necessarily having information on the underlying individual-to-individual relationships that give rise to it (a strategy taken by several of the methods we outline below). However, this approach cannot capture the details of the within-group dynamics leading to collective outcomes. This point is important, as multiple configurations of individual-to-individual influence could give rise to the same pattern of individual-to-group influence. For example, if A influences both B and C to move towards a particular foraging patch, A might have the same individual-to-group influence as if it influences B to move towards the patch and then B subsequently influences C. A method that only captures individual-to-group influence might fail to distinguish between these two scenarios, which could have important consequences on our ability to predict how the group would behave if, for example, individual B left the group.
(c). Total influence (How much influence does each individual have over repeated observations?)
Over a set of repeated decisions, one can determine which individuals have had the highest overall influence by calculating a score for each individual that summarizes its combined influence over all observations. When assessed at the individual-to-individual level, total influence can take the form of weighted edges in an influence network (figure 1b). If assessed at the individual-to-group level, total influence can take the form of an influence ‘score’, such as a percentage or a rank (figure 1c). Scores can then be regressed against other attributes, such as dominance, age, body size or social relationships to test hypotheses about the characteristics associated with leadership ability.
(d). Consistency of influence (Are individuals' roles in group decisions stable?)
Key to understanding the dynamics of collective decision-making is the question of whether the influence of specific individuals is consistent over multiple group decisions, or across time. Consistency can be measured at the individual-to-individual level as well as the individual-to-group level. For example, one might find that individual A consistently influences individual B (a consistent individual-to-individual influence), or that A is the most influential individual in the group across all group decisions (consistent individual-to-group influence). It is important to assess consistency independently of total influence because, for example, individual A could have intermediate influence either by consistently showing an intermediate amount of influence or by being extremely influential sometimes and not at all influential at other times.
(e). Caveats and considerations
Regardless of the level of analysis, studies of leadership must define behaviours that are believed to be important indicators of influence. Without an understanding of the neurological mechanisms that underpin decision-making (but see [49,50]), the behaviours that we measure will always represent proxies for influence. For example, one might define A as influencing B if A makes a turn before B does. Of course, without knowing why these individuals moved where they did, it is not possible to say with certainty that A's movement caused B to move. However such an observation might nonetheless be useful as a proxy for influence at the individual-to-individual level.
It is also important to note that many systems will exhibit multiple types of group decisions, and that the process of how influence yields collective outcomes may differ across contexts. For example, in the case of movement, groups may need to make consensus decisions about both the timing (e.g. when to leave a foraging patch) and direction (e.g. which patch to move to next) of travel. It is possible to study individual-to-individual and individual-to-group influence in relation to both timing and directional decisions (or a combination of both), and in fact theoretical predictions about the level of decision-sharing expected in these two contexts differ [51]. Thus, influence should, in most cases, not be thought of as a catch-all concept, but should be defined in relation to the specific process under study.
Assumptions about what defines influence will necessarily have consequences for the conclusions drawn about the structure of leadership in animal groups, and thus should be chosen with care and explicitly described. In the following section, we elaborate on some of the methods that have been used for inferring influence across a range of different systems with different dynamics, at both the individual-to-individual and individual-to-group levels. We focus specifically on the case of group movement because many of the methods are applicable specifically to this domain. However, we suggest that the general framework described above should be applicable beyond movement decisions to other domains of group decision-making [39].
3. Methods for inferring influence
Conflicts of interest are a nearly unavoidable consequence of group living, and because some individuals must compromise their preferred patterns of behaviour to maintain group cohesion, who has influence and how such influence is acquired is key to understanding the costs and benefits of sociality. The earliest studies of leadership in animal groups based their assessments of influence on direct field observations. They therefore inferred influence from properties of individuals and groups that humans can observe directly, and mostly were restricted to assessing individual-to-group influence. Recent advances in GPS tracking and computer vision have opened up the possibility to collect detailed spatial data on the movements of all animals within groups, thus expanding the opportunity to study individual-to-individual influence. A number of methods have been developed in the field of collective behaviour, and these are set apart from previous work by their focus on the dynamics of movement rather than discrete decisions. In §3a, we outline approaches that have been used, or are being developed, to infer influence directly from observations of moving animal groups. In §3b, we describe methods for aggregating across repeated observations to measure total influence. In §3c, we describe methods for calculating consistency of influence.
(a). Methods for inferring influence instances from observational data
(i). Spatial position within a group
Perhaps the most straightforward way of estimating which individuals have influence over group movement is by assessing which individuals occupy the front-most positions in the group. A strength of this method is that it can usually be documented via direct observation, without needing data on the fine-scale movements of all individuals within a group. Because of its simplicity, determining who is in front is perhaps the most commonly-used method for inferring influence (see the electronic supplementary material, table S1). For example, in a study of spotted hyenas [52], the individual at the front of a progression prior to a reunion between subgroups was identified as ‘leading’ (individual-to-group influence), and the study assessed how often each individual in the population took this role (a proxy for total influence). Spatial position can also be used to study influence at an individual-to-individual level, for example, by assessing which individuals are ahead of which other individuals in group progressions [53]. A disadvantage of this approach is its assumption that a front position implies influence, which is not necessarily the case, and behavioural dynamics other than leadership may drive patterns of within-group spatial positioning [54].
(ii). Initiating and being followed
A second commonly employed method for inferring influence at an individual-to-group level is to identify individuals that successfully initiate group movements (see the electronic supplementary material, table S1). Typically, this method has been applied to cases where a group changes state from stationary to moving, e.g. in the cases of departures from sleeping sites [31,55] or foraging locations [56–58]. Initiators are typically defined by a set of behavioural characteristics, often involving moving a threshold distance away from the centre or periphery of the group, and being followed by a set number or percentage of group members within a defined time-frame. This approach has the advantage that it is often possible to carry out from direct observations in the field, as well as from fine-scale movement data, and it has been widely applied because of our a priori belief that such initiations of group departures are important in determining group movements. The fact that such initiations are observed across a variety of taxa also speaks to the wide applicability of this approach.
A recent generalization of this method applied the concept of initiators and followers to infer influence at the individual-to-individual level in a troop of wild baboons, and developed an algorithm to automatically extract such interactions [59]. Here, sequences of movement are extracted in which one individual moved away from another and either was followed (characterized as a ‘successful pull’) or was not followed and subsequently returned (characterized as a ‘failed pull’). The method proceeds by measuring the distance between pairs of individuals over time, defining potential ‘pull’ events as instances when this distance first increases and then decreases (extracted by identifying sequences where the dyadic distance goes from a local minimum, to a local maximum and back to a local minimum). The individual that moves more during the first part of an event (when the distance between the individuals is increasing) is defined as the ‘initiator’. An event is classified as a ‘successful pull’ if the other (potential follower) individual moves more during the second part of the event (when the distance between individuals is decreasing), effectively closing the gap by approaching the initiator. If instead the initiator moved more during this time (thus returning toward the potential follower), then the event is characterized as an ‘unsuccessful pull’. A threshold is also applied to filter out very weak or unclear events, in which either distances did not change much, or both individuals moved approximately the same amount. After extracting these dyadic ‘pull’ interactions, Strandburg-Peshkin et al. [59] then identified events where multiple initiators interacted with the same potential follower at the same time to assess the dynamics of collective decision-making within the baboon troop, revealing patterns consistent with shared decision-making and with previous simulation models of collective motion. Influence was defined as having been successful in initiating, and this was verified by correlating the direction of successful initiations with the direction of the subsequent group movement over a longer time scale.
The benefit of the generalized approach described above is that it is applicable both to instances when the group is initially stationary, and to instances when it is already moving. Which of these two phases is more important remains an empirical (and, likely, system-specific) question, and future research should investigate whether a group's destination is decided during or prior to the initiation of movement, or if negotiation continues among group-mates throughout the navigational phase of movement. This approach can also extract successful and failed initiations across a range of different time scales at once (as the time scale of each interaction is determined by the times at which maxima and minima in the dyadic distance occur). However, a spatial scale must be set to specify the minimum change in dyadic distance required to be considered a departure from the current maximum or minimum—thus, the method still requires assumptions about what spatial scales are relevant to a given system. Additionally, the method assumes that influence occurs through individuals moving away and being followed; if the distances between individuals do not change very much, the method is unlikely to be appropriate.
In some cases, initiations are accompanied by, or consist entirely of, acoustic or visual signals by the initiator other than movement. For example, meerkats use specific ‘move’ calls to initiate group departure from a foraging patch [60], and in white-faced capuchins, ‘trill’ vocalizations and back-glances were found to increase the probability of a successful initiation [61,62]. Such behaviours can be incorporated into analyses either at the level of identifying initiations (e.g. a vocalization in itself can be considered an ‘initiation’), or as an additional factor that may affect initiation success (e.g. if some initiation movements are also accompanied by vocalizations). More generally, incorporating signalling across different modalities (e.g. acoustic and visual) into studies of collective behaviour is an area that merits further work.
(iii). Time-lagged directional correlations
An alternative method of inferring influence in moving animal groups focuses on changes in direction rather than initiations per se. Here, the time delay between changes in direction of pairs of individuals is used to infer which animal influenced the other (i.e. individual-to-individual influence). Nagy et al. [63] used this method to assess the group dynamics of pigeon flocks. They computed the cross-correlation between the headings of each pair of birds for a variety of different time lags, then determined which time lag maximized this correlation for each pair, and used these to construct a directed leader–follower network based on the time delays. They showed that the resulting network had a hierarchical structure: in other words, if bird A influenced bird B, and bird B influenced bird C, bird A also influenced bird C, and with a delay that is approximately equal to the sum of the delays between A and B, and B and C. Thus, using this method it is possible to scale up from these individual-to-individual relationships to construct a ranking of individuals, where an individual's rank serves as a measure of its individual-to-group influence during flight.
Time-lagged directional correlation methods have been extended to give an instantaneous measure of influence (rather than being summarized over an entire movement sequence) [64], and to assess influence in other species including dogs [65], bats [66] and storks [67]. Implicit in this approach is the requirement that animals must be moving continuously, so that their directional headings have meaning. If individuals are not strongly coordinated in their movements, correlations in their travel directions cannot reliably be measured or distinguished from noise. The approach also assumes that following occurs after a constant time lag within each dyad (however, spatial relationships can vary).
(iv). Information-theoretic approaches
Information-theoretic approaches are a model-free way to infer causal influences from trajectory data. Here, entropy-based measures are used to infer influence relationships between individuals in a group. Entropy is a measure of the uncertainty in a distribution of values. For example, if individual A's heading distribution has high entropy, this means that we have very little ability to predict which direction individual A is moving in at any given time. Information-theoretic approaches use measures based on the reduction in entropy about one individual (e.g. individual A) when we know something about another individual (e.g. individual B)—a concept referred to as mutual information. Most commonly, the transfer entropy (TE) between two individuals' positions, headings, or some other characteristic of their trajectories, is computed. The TE is an asymmetric measure that captures the flow of information from one time series to another. In our heading example, it can be understood as the answer to the question: if we know individual A's heading in the past, how much more information (reduction in uncertainty) can we get about individual A's current heading if we know the heading of individual B? Mathematically, TE is defined as
| 2.10 |
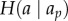 represents the entropy of the distribution of a value a (e.g. the heading of individual A at time t) conditioned on all of its values in the past, ap.
represents the entropy of the distribution of a value a (e.g. the heading of individual A at time t) conditioned on all of its values in the past, ap. 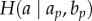 represents the entropy of the distribution of value a conditioned on both its own values in the past (ap) and the values of a second variable b (e.g. the heading of individual B), at all times in the past. Intuitively, the first term in the equation represents how much uncertainty we would have in predicting the value of a at time t (which, in our example, represents the heading of individual A), given that we know all of the previous values of a. The second term represents how much uncertainty we would have about this same value if we also knew all of the previous values of b (in our example, the heading of individual B). Therefore, their difference intuitively represents how much the uncertainty of predicting a is reduced by knowing the previous values of b, and therefore can be thought of as the ‘information flow’ from B to A. In practice, conditioning these distributions on long sequences of past values is usually not possible owing to the finite amount of data available; thus in most cases a Markov assumption is made so that only the values of a and b at the previous time step are used in the computation.
represents the entropy of the distribution of value a conditioned on both its own values in the past (ap) and the values of a second variable b (e.g. the heading of individual B), at all times in the past. Intuitively, the first term in the equation represents how much uncertainty we would have in predicting the value of a at time t (which, in our example, represents the heading of individual A), given that we know all of the previous values of a. The second term represents how much uncertainty we would have about this same value if we also knew all of the previous values of b (in our example, the heading of individual B). Therefore, their difference intuitively represents how much the uncertainty of predicting a is reduced by knowing the previous values of b, and therefore can be thought of as the ‘information flow’ from B to A. In practice, conditioning these distributions on long sequences of past values is usually not possible owing to the finite amount of data available; thus in most cases a Markov assumption is made so that only the values of a and b at the previous time step are used in the computation.
Because A and B can influence one another, the net TE is sometimes computed as a measure of how much more B influences A than vice versa. This is defined as
This approach has recently been used to identify leader–follower relationships in simulated data, and was found to be more accurate at correctly classifying these relationships than time-lagged directional correlation methods [68]. Experiments in which the TE was measured between a real and a robotic fish also demonstrated a proof-of-concept for this method [69]. One shortcoming of the TE approach (which is also a limitation of other approaches) is that it incorporates both direct and indirect influences. In other words, if individual A influences individual B, which influences individual C, the TE from A to C would be positive, even if A does not influence C directly. To avoid this problem, a generalization of this approach has also recently been proposed based on the principle of optimal causation entropy [70], which allows such indirect influences to be computationally removed [71].
Overall, strengths of these methods include their solid theoretical basis in information theory and their ability to capture relationships among the movements or decisions of individuals that are nonlinear, without assuming any specific underlying model of influence. Additionally, the methods are highly general and can apply across a wide range of domains and types of data, beyond collective movement data. For example, TE has been used to quantify causal relationships in systems ranging from neural [72] and gene regulatory [73] networks to financial markets [74] and social media [75]. The main requirement is time-series data from multiple individuals within a group (or, more generally, multiple interacting parts of a system). However, practical challenges remain, as computing the entropy of these distributions is computationally challenging, and can require more data than are often available from empirical studies of animal groups (though a recently developed algorithm for inferring causal links could alleviate this problem; see [70]). The method is thus most likely to be suitable in studies that have many repeated observations of individual movements or decisions. Another challenge is that the lack of an underlying model can make interpretation difficult, as positive information flow can represent a multitude of possible relationships among individual movements or decisions. Despite these challenges, the general nature of these approaches, coupled with advances increasing the practicality of their use [70], suggests that they will be widely applicable, and may be especially useful for comparisons across disparate systems.
(v). Inferring influence from the outcomes of decisions
An entirely different, and complementary, approach to assessing influence is by observing where groups go, and relating these group decisions to the preferences of individual group members. In other words, if groups more often move to locations preferred by a certain individual or set of individuals, it can be inferred that these individuals were able to exert influence over the decision-making process. For example, King et al. [10] used the movements of a troop of baboons to a location with a food distribution beneficial to dominant troop members to infer that these individuals strongly influence group decisions. In a laboratory study, Couzin et al. [76] used fish trained to approach different colours to reveal an important role of uninformed individuals in favouring democratic decision-making. A drawback of this approach is that inferring such ‘destination-based’ influence requires manipulation, or at least knowledge, of the preferences of group members, which is frequently not possible. Moreover, even if all preferences are known, inferring the relative influence of all individuals in a group may still be quite challenging owing to complicating factors such as correlated preferences. A simple example of this issue is if two individuals within the group both prefer the same destination. If the group is then observed to travel to this destination, it cannot be determined how much each of these individuals contributed to the group decision.
(b). Methods for determining total influence
The ability to regularly drive collective decisions in one's favour can, presumably, have important fitness consequences. It is thus of interest to assess the total influence of each individual over repeated group decisions (or over another individual, across repeated instances). However, doing so immediately raises one key decision that has to be made: what units of aggregation are meaningful? The definition used to summarize repeated observations of influence can have important consequences for later interpretation. For example, using an initiation-based method of inferring influence, one could reasonably define the total influence of individual A on B (or on the group as a whole) either as the raw number of times B followed A, or as the fraction of times that B followed A when A initiated. The latter case is linked to the question of ‘leader quality’ by explicitly taking into account failed initiations, which may or may not be relevant depending on the biological question under consideration. However, it would fail to capture the impact of an individual who was often unsuccessful in its initiation attempts but also made many successful initiations. If A initiated 100 times, and was followed by B 40 times, is it more or less influential than B who initiated 30 times and was always followed by A? Having information about the context in which initiations are made could help resolve this question (see §5).
When data are collected at the individual-to-individual level, quantifying the total influence of each individual on the group as a whole (i.e. total influence at the individual-to-group level) may still be of interest. One approach for doing this is to generate an index of influence for each individual, much in the same way as studies have generated indices for dominance rank [77]. For example, each initiator–follower interaction can be quantified as a win–loss relationship, and entered directly into methods such as Elo scores [78]. The Elo method generates scores that can be used to rank individuals. This method may be particularly useful for studying leadership because it generates meaningful indices that facilitate comparisons of relative ability (e.g. is there one notable outlier that would represent a despot?).
In cases where dyadic influence relationships have been quantified, another natural way to assimilate information about repeated initiations is to use a network approach. This approach involves either calculating a summary of the leader–follower relationship between each pair of individuals (e.g. how many times has A influenced B, out of the total number of times A and B were involved in influence behaviours), or generating a directed network (e.g. how many times A influenced B, and how many times B influenced A). Summarizing the position of individuals within these influence networks can then be done using the wealth of tools available from the social network toolbox (see [76,79] for an introduction). One useful network metric is eigenvector centrality, which quantifies, in a recursive fashion, the extent to which an individual has influence over others who are themselves influential [80]. Thus, individuals with the highest values of eigenvector centrality are those that have most influence on the entire network. More generally, there are a range of other statistical methods available for constructing and analysing networks, and these are likely to apply to the domain of leadership as well (e.g. [81]).
One challenge that has not been dealt with particularly well methodologically is that such a dyadic perspective will fail to be realistic if there are significant non-dyadic influence relationships. For example, if individual C only follows when both A and B initiate, but not when either initiates alone, this triadic relationship would be difficult to capture using standard network tools. Such non-dyadic interactions may in fact be key to the decision-making process, for example, through the well-documented use of quorum rules in animal group decisions [82–85]. One approach that could prove useful here is maximum entropy modelling. This is an information-theoretic approach (distinct from the approaches described above) which can quantify how much of the information contained in a joint distribution (e.g. of group states) is accounted for by pairwise relationships alone, as opposed to higher-order interactions. Maximum entropy modelling, which has already been successfully employed to describe the dynamics of bird flocks [86], could give insight into whether pairwise interactions represent a good approximation of the system dynamics or whether higher-order interactions are essential, particularly for systems in which large amounts of data from multiple individuals are available.
When an underlying movement or decision-making model is known, or can be reasonably posited for a given system, a model-fitting approach to inferring total influence becomes possible. Such an approach could operate either at the individual-to-individual level, for example, by fitting parameters to represent different interaction strengths for each pair of individuals, or at the individual-to-group level, such as by fitting an overall ‘attraction strength’ for each individual in the group [87,88]. While such model-fitting approaches hold promise, they rely on specifying an underlying movement decision-making model (which is often unknown or poorly known for natural systems), or network model, and can become computationally intractable when the number of individuals in a group is large. This second problem can be partially mediated by additional assumptions, such as grouping individuals into different classes and assuming that all individuals within a given class can be described by the same model coefficient [89]; however, such an approach can come at the cost of biological validity.
(c). Methods for assessing consistency of influence
In addition to quantifying their total influence, it is also important to understand the consistency with which individuals influence one another or their groups. For example, an individual could achieve the same total influence either by influencing each group decision by a small amount, or by strongly driving a small subset of group decisions. Consistency of influence can be assessed at both the individual-to-individual and the individual-to-group levels. One important aspect to note is that consistent individual-to-group influence does not necessarily imply consistent individual-to-individual influence relationships. For example, individual A could always exert high influence over group decisions, but could do so via influencing different individuals each time. Therefore, where possible, consistency of influence should be assessed at both of these levels.
When individuals’ individual-to-group level influence is represented by an influence ‘score’ or rank, standard metrics for measuring repeatability can be employed [86,90]. However, care should be taken when performing hypothesis testing owing to the non-independence of data. For example, if one individual wields most of the influence, by definition others have little or no influence. Thus, one individual's influence during one group action cannot be independent of the behaviour of others in its group. To address this issue, we suggest randomization-based procedures to construct null models and test significance (see [91] for a guide). In the case above, to determine whether individual A's influence is more consistent across group decisions than expected by chance, one could first measure the repeatability of individual A's influence in the real data, then compare this value to the values computed for randomized datasets where the identities of all individuals within the group have been shuffled for each decision.
Assessing the repeatability of individual-to-individual influence requires considering a larger and more complex set of measurements, but the general strategy remains the same. In the case where dyadic influence relationships have been quantified, Mantel tests can be used to quantify the correlation between influence networks across multiple group decisions, or across time. Again, owing to non-independence, randomizations such as node permutations should be employed when performing hypothesis testing. An advantage of using randomizations is that they allow alternative hypotheses to be tested, and other underlying factors to be quantified, such as the effect of spatial distribution of individuals in the repeatability of songbirds in a woodland social network [92]. Similar strategies can also be employed for higher-order influence relationships (such as triadic relationships).
An additional complication arises if group composition is not stable over time. In such cases, consistency becomes harder to assess because an individual's influence over a group decision likely depends on the identities of the other group members present. It may be possible in these cases to perform coarser-grained consistency measurements, such as quantifying how often A has more influence than B (rather than A's total amount of influence), as inequality-type measurements may be more robust to changes in group composition. However, assessing the consistency of influence in groups that change membership is an important area that warrants further investigation.
4. From individual influence to decision-making process
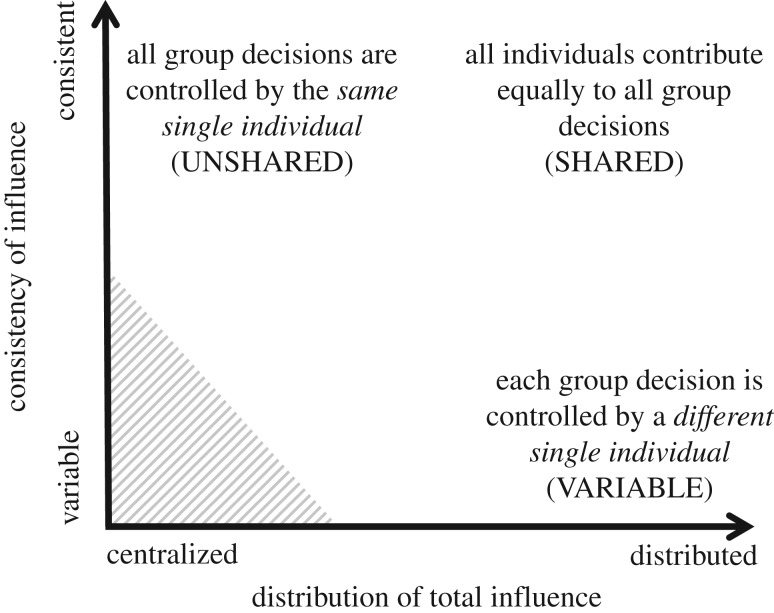
A major take-home message from our proposed framework is that the distribution of total influence over group decisions should be considered separately from the consistency of influence across decisions or over time. Recent conceptualizations of leadership in animal groups have focused primarily on the distribution of total influence [3], highlighting a continuum of consensus decision-making processes ranging from completely shared (all individuals contribute equally) to completely unshared (one individual always dominates). However, considering the consistency dimension is also critical to understanding what type of group decision-making is exhibited in a given study system. We therefore suggest that the overall decision-making process of a given system can be characterized along two axes (figure 2), representing the distribution of influence (ranging from centralized to distributed) and the consistency of influence (ranging from variable to consistent). In this plane, shared decision-making naturally falls in the upper right corner (distributed and consistent), despotic or unshared decision-making in the upper left (centralized and consistent) and variable influence by a different individual each time in the bottom right (distributed and variable).
Figure 2.
Assessing overall decision-making patterns based on the distribution and consistency of influence. We suggest that decision-making systems can be usefully categorized along two axes: the distribution of total influence and the consistency of influence. The distribution of total influence (x-axis) can range from centralized (one individual has high total influence, and the rest have little or none) to distributed (all individuals have approximately equal total influence). The consistency (y-axis) can range from variable (individuals are not consistent in their influence across multiple events) to consistent (individuals have the same influence across all events). Placing decision-making systems along these two axes clearly divides the space into sections corresponding to different decision-making types that have previously been discussed in the literature, including despotic/unshared decision-making (centralized, consistent), shared decision-making (distributed, consistent) and variable influence (distributed, variable). The bottom left corner is shaded because it is not possible to have a completely centralized distribution of total influence that is also highly variable. This visualization also highlights the importance of considering consistency of influence in addition to total influence, as it is only along this dimension that variable influence and shared decision-making can be distinguished.
Characterizing the decision-making process along both of these axes helps to better draw connections among the diversity of decision-making systems seen in nature, and to clarify the distinctions among them. For example, one might have a system where each individual contributes equally at an aggregate level (decision-making is highly distributed; figure 2, x-axis), but this could take the form of each individual controlling a different group decision (i.e. variable influence [3]), or all individuals contributing partially to each group decision (i.e. shared decision-making). This distinction, which can only be recognized by also considering the consistency dimension, has important ecological and evolutionary implications: groups with true shared decision-making, in the sense of all individuals contributing to each group decision, can take advantage of the ‘wisdom of crowds’ [93,94] and other types of collective information processing [3,23], whereas a system in which a different individual controls the decision each time would be more likely to represent ‘leading according to need’ [24] or leadership by informed individuals [7]. Ultimately, characterizing the group decision-making type along both of these axes may help to account for the diversity of decision-making systems seen in nature.
5. Outstanding issues and future directions
A major issue with studying leadership is how to account for extrinsic factors that influence movement or decision-making. In some cases, by influencing all individuals simultaneously (or with very short time delays), such external factors can generate patterns of collective behaviour that could be misinterpreted as arising from influence among group members. For example, when a group of animals flees in the face of a predator's approach, they may each be responding independently to the looming threat, or the flight of one or a few individuals may drive the response of the rest. While the issue of disentangling correlation from causation is an inevitable challenge in observational studies, it is becoming increasingly possible to measure and attempt to account for environmental features that are likely to shape how animals move and make decisions [7,95,96]. However, establishing what extrinsic factors are important within a given system, and when and how they affect collective decision-making, remains a challenge that must be tackled to draw robust conclusions about individual influence and leadership.
As animals move through the landscape, or as the environment changes, the context in which they make decisions also changes. For example, recent evidence in killer whales [7] and elephants [8] shows that matriarchs are important repositories of knowledge, and lead their groups to rarely visited resources when food becomes scarce. More broadly, as animal groups move through the landscape, how they reach consensus could vary depending on the context—for example, when navigating to food versus migrating versus escaping from predators. Because influence can vary based on context, being able to identify the relevant contexts, and to determine the current context of each decision, will be important when measuring the consistency of influence. Moreover, determining how flexible group decision-making processes are, and what mechanisms allow for such flexibility, remain important and unanswered questions.
Many of the methods for inferring influence that we outline above require a significant amount of data and, importantly, rely on collecting data on individuals simultaneously. At present, there is little guidance about how sensitive methods for studying leadership are to having an incomplete sample of the population (i.e. missing individuals). For guidance, we can turn to the literature on animal social network analysis, which suggests that the social network position of individuals can be reasonably estimated with as few as 30% of the individuals observed [97], and that sampling should prioritize greater resolution in terms of events occurring between known individuals rather than maximizing the number of individuals observed [98]. Given that initiations and followership are inherently structured as networks, these conclusions are promising for studies that struggle to achieve complete coverage of groups. However, we recommend that further research tackles this problem, and that studies with incomplete data try to quantify the uncertainty associated with their data [99].
Finally, a major limitation in the study of leadership has been the difficulty in performing experimental manipulations. Significant insight has been gained through controlled experimental manipulations in laboratory settings. Translating these into wild populations, particularly in combination with advanced analytical methods for extracting influence, should be a research priority. Studies will be able to build on several notable recent efforts. One successful approach has been to manipulate the incentives of individuals within groups by providing experimental feeding patches. For example, King et al. [10] provided a high value food patch that could be monopolized by the dominant baboon in a troop, thus increasing the dominant individual's incentive to move to that patch. A more flexible approach is to create multiple patches, and to manipulate which individuals have access to which patch. For example, Firth et al. [100] provided feeders that gave access only to specific individuals (via their unique microchip ID), using these to experimentally split where members of a mated pair of songbirds could feed and thus determine how they resolve this conflict. Such an approach could be scaled up to larger social groups, and used to create a variety of different conflict scenarios—such as between a dominant individual and the majority of individuals. Such experiments would enable inferences about leadership based on the outcomes of group decisions, while applying the methods outlined in this paper will help to elucidate the mechanisms by which these outcomes arise.
Supplementary Material
Acknowledgements
We thank Martin Wikelski for support and Gabriella Gall, Harry Marshall, and one anonymous reviewer for comments on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
A.S.-P., M.C.C. and D.R.F. conceived the article, and all authors contributed to design and writing of the manuscript.
Competing interests
We have no competing interests.
Funding
A.S.-P., D.P. and D.R.F. received funding from the Max Planck Institute for Ornithology. A.S.P. received additional funding from a Human Frontiers Science Program Long-Term Fellowship (LT000492/2017) and the Gips-Schüle Foundation. D.R.F. received additional funding from the Daimler und Benz Stiftung (32-03/16). M.C.C. acknowledges support from the National Science Foundation (IOS-1250895, III-1514174, SMA 1620391) and the David & Lucile Packard Foundation (no. 2016-65130).
References
- 1.Krebs JR, Davies NB. 1987. An introduction to behavioural ecology. New York, NY: Wiley-Blackwell. [Google Scholar]
- 2.Conradt L, List C. 2009. Group decisions in humans and animals: a survey. Phil. Trans. R. Soc. B 364, 719–742. ( 10.1098/rstb.2008.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conradt L, Roper TJ. 2005. Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456. ( 10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- 4.Conradt L. 2012. Models in animal collective decision-making: information uncertainty and conflicting preferences. Interface Focus 2, 226–240. ( 10.1098/rsfs.2011.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conradt L, Roper TJ. 2003. Group decision-making in animals. Nature 421, 155–158. ( 10.1038/nature01294) [DOI] [PubMed] [Google Scholar]
- 6.Gavrilets S, Auerbach J, van Vugt M. 2016. Convergence to consensus in heterogeneous groups and the emergence of informal leadership. Sci. Rep. 6, 29704 ( 10.1038/srep29704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brent LJN, Franks DW, Foster EA, Balcomb KC, Cant MA, Croft DP. 2015. Ecological knowledge, leadership, and the evolution of menopause in killer whales. Curr. Biol. 25, 746–750. ( 10.1016/j.cub.2015.01.037) [DOI] [PubMed] [Google Scholar]
- 8.McComb K, Moss C, Durant SM, Baker L, Sayialel S. 2001. Matriarchs as repositories of social knowledge in African elephants. Science 292, 491–494. ( 10.1126/science.1057895) [DOI] [PubMed] [Google Scholar]
- 9.Couzin ID, Krause J, Franks NR, Levin SA. 2005. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516. ( 10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- 10.King AJ, Douglas CMS, Huchard E, Isaac NJB, Cowlishaw G. 2008. Dominance and affiliation mediate despotism in a social primate. Curr. Biol. 18, 1833–1838. ( 10.1016/j.cub.2008.10.048) [DOI] [PubMed] [Google Scholar]
- 11.Aristotle, Schneider JG. 1862. Aristotle's history of animals: in ten books, p. 4. London, UK: Henry G. Bohn. [Google Scholar]
- 12.Wilson EO. 2000. Sociobiology: the new synthesis, 25th anniversary edn. Cambridge, MA: Harvard University Press. [Google Scholar]
- 13.Chance MRA. 1967. Attention structure as the basis of primate rank orders. Man 2, 503 ( 10.2307/2799336) [DOI] [Google Scholar]
- 14.DeVore I, Washburn SL. 2004. Baboon ecology and human evolution. In African ecology and human evolution, 2nd edn (eds Howell C, Bourlière F), pp. 335–367. London, UK: Routledge. [Google Scholar]
- 15.Rowell TE. 1972. Female reproduction cycles and social behavior in primates. In Adv. Stud. Behav. Primates 4, 69–105. [Google Scholar]
- 16.King AJ, Sueur C. 2011. Where next? Group coordination and collective decision making by primates. Int. J. Primatol. 32, 1245–1267. ( 10.1007/s10764-011-9526-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon DM. 2010. Ant encounters: interaction networks and colony behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 18.Hemelrijk CK, Hildenbrandt H. 2008. Self-organized shape and frontal density of fish schools. Ethology 114, 245–254. ( 10.1111/j.1439-0310.2007.01459.x) [DOI] [Google Scholar]
- 19.Herbert-Read JE, Perna A, Mann RP, Schaerf TM, Sumpter DJT, Ward AJW. 2011. Inferring the rules of interaction of shoaling fish. Proc. Natl Acad. Sci. USA 108, 18 726–18 731. ( 10.1073/pnas.1109355108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballerini M, et al. 2008. Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl Acad. Sci. USA 105, 1232–1237. ( 10.1073/pnas.0711437105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vicsek T, CziroK A, Ben-Jacob E, Cohen I, Shochet O. 1995. Novel type of phase transition in a system of self-driven particles. Phys. Rev. Lett. 75, 1226–1229. ( 10.1103/PhysRevLett.75.1226) [DOI] [PubMed] [Google Scholar]
- 22.Couzin ID, Krause J, James R, Ruxton GD, Franks NR, 2002. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11. ( 10.1006/jtbi.2002.3065) [DOI] [PubMed] [Google Scholar]
- 23.Berdahl A, Torney CJ, Ioannou CC, Faria JJ, Couzin ID. 2013. Emergent sensing of complex environments by mobile animal groups. Science 339, 574–576. ( 10.1126/science.1225883) [DOI] [PubMed] [Google Scholar]
- 24.Conradt L, Krause J, Couzin ID, Roper TJ, 2009. ‘Leading according to need’ in self-organizing groups. Am. Nat. 173, 304–312. ( 10.1086/596532) [DOI] [PubMed] [Google Scholar]
- 25.Pettit B, Ákos Z, Vicsek T, Biro D. 2015. Speed determines leadership and leadership determines learning during pigeon flocking. Curr. Biol. 25, 3132–3137. ( 10.1016/j.cub.2015.10.044) [DOI] [PubMed] [Google Scholar]
- 26.del M Delgado M, Miranda M, Alvarez SJ, Gurarie E, Fagan WF, Penteriani V, di Virgilio A, Morales JM. 2018. The importance of individual variation in the dynamics of animal collective movements. Phil. Trans. R. Soc. B 373, 20170008 ( 10.1098/rstb.2017.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strandburg-Peshkin A, et al. 2013. Visual sensory networks and effective information transfer in animal groups. Curr. Biol. 23, R709–R711. ( 10.1016/j.cub.2013.07.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reebs SG. 2000. Can a minority of informed leaders determine the foraging movements of a fish shoal? Anim. Behav. 59, 403–409. ( 10.1006/anbe.1999.1314) [DOI] [PubMed] [Google Scholar]
- 29.Dyer JRG, Ioannou CC, Morrell LJ, Croft DP, Couzin ID, Waters DA, Krause J. 2008. Consensus decision making in human crowds. Anim. Behav. 75, 461–470. ( 10.1016/j.anbehav.2007.05.010) [DOI] [Google Scholar]
- 30.Petit O, Bon R. 2010. Decision-making processes: the case of collective movements. Behav. Processes 84, 635–647. ( 10.1016/j.beproc.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 31.King AJ, Sueur C, Huchard E, Cowlishaw G. 2011. A rule-of-thumb based on social affiliation explains collective movements in desert baboons. Anim. Behav. 82, 1337–1345. ( 10.1016/j.anbehav.2011.09.017) [DOI] [Google Scholar]
- 32.Gautrais J. 2010. The hidden variables of leadership. Behav. Processes 84, 664–667. ( 10.1016/j.beproc.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 33.King AJ. 2010. Follow me! I'm a leader if you do; I'm a failed initiator if you don't? Behav. Processes 84, 671–674. ( 10.1016/j.beproc.2010.03.006) [DOI] [PubMed] [Google Scholar]
- 34.Kummer H. 1968. Social organization of hamadryas baboons: a field study. Chicago, IL: University of Chicago Press. [Google Scholar]
- 35.Byrne RW. 2000. How monkeys find their way: leadership, coordination, and cognitive maps of African baboons. In On the move (ed. Paul BSG.), pp. 491–518. Chicago, IL: University of Chicago Press. [Google Scholar]
- 36.Burns JM. 1978. Leadership. New York, NY: Harper Collins. [Google Scholar]
- 37.Galton F. 1869. Hereditary genius: an inquiry into its laws and consequences. London, UK: R. Clay, Sons, and Taylor (London). [Google Scholar]
- 38.Conradt L, Roper TJ. 2009. Conflicts of interest and the evolution of decision sharing. Phil. Trans. R. Soc. B 364, 807–819. ( 10.1098/rstb.2008.0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith JE, et al. 2016. Leadership in mammalian societies: emergence, distribution, power, and payoff. Trends Ecol. Evol. 31, 54–66. ( 10.1016/j.tree.2015.09.013) [DOI] [PubMed] [Google Scholar]
- 40.Fischhoff IR, Sundaresan SR, Cordingley J, Larkin HM, Sellier M-J, Rubenstein DI. 2007. Social relationships and reproductive state influence leadership roles in movements of plains zebra, Equus burchellii. Anim. Behav. 73, 825–831. ( 10.1016/j.anbehav.2006.10.012) [DOI] [Google Scholar]
- 41.Sueur C, Petit O. 2008. Shared or unshared consensus decision in macaques? Behav. Processes 78, 84–92. ( 10.1016/j.beproc.2008.01.004) [DOI] [PubMed] [Google Scholar]
- 42.Rosenthal SB, Twomey CR, Hartnett AT, Wu HS, Couzin ID. 2015. Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. Proc. Natl Acad. Sci. USA 112, 4690–4695. ( 10.1073/pnas.1420068112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treves A. 2000. Theory and method in studies of vigilance and aggregation. Anim. Behav. 60, 711–722. ( 10.1006/anbe.2000.1528) [DOI] [PubMed] [Google Scholar]
- 44.Bode NWF, Franks DW, Wood AJ. 2012. Leading from the front? Social networks in navigating groups. Behav. Ecol. Sociobiol. 66, 835–843. ( 10.1007/s00265-012-1331-6) [DOI] [Google Scholar]
- 45.Bode NWF, Wood AJ, Franks DW. 2011. The impact of social networks on animal collective motion. Anim. Behav. 82, 29–38. ( 10.1016/j.anbehav.2011.04.011) [DOI] [Google Scholar]
- 46.Moussaïd M, Perozo N, Garnier S, Helbing D, Theraulaz G. 2010. The walking behaviour of pedestrian social groups and its impact on crowd dynamics. PLoS ONE 5, e10047 ( 10.1371/journal.pone.0010047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bode NWF, Holl S, Mehner W, Seyfried A. 2015. Disentangling the impact of social groups on response times and movement dynamics in evacuations. PLoS ONE 10, e0121227 ( 10.1371/journal.pone.0121227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughey LF, Hein AM, Strandburg-Peshkin A, Jensen FH. 2018. Challenges and solutions for studying collective animal behaviour in the wild. Phil. Trans. R. Soc. B 373, 20170005 ( 10.1098/rstb.2017.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasson U, Ghazanfar AA, Galantucci B, Garrod S, Keysers C. 2012. Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends Cogn. Sci. 16, 114–121. ( 10.1016/j.tics.2011.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang J, Chen C, Dai B, Shi G, Ding G, Liu L, Lu C. 2015. Leader emergence through interpersonal neural synchronization. Proc. Natl Acad. Sci. USA 112, 4274–4279. ( 10.1073/pnas.1422930112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conradt L, Roper TJ. 2010. Deciding group movements: where and when to go. Behav. Processes 84, 675–677. ( 10.1016/j.beproc.2010.03.005) [DOI] [PubMed] [Google Scholar]
- 52.Smith JE, Estrada JR, Richards HR, Dawes SE, Mitsos K, Holekamp KE. 2015. Collective movements, leadership and consensus costs at reunions in spotted hyaenas. Anim. Behav. 105, 187–200. ( 10.1016/j.anbehav.2015.04.023) [DOI] [Google Scholar]
- 53.Altmann SA. 1979. Baboon progressions: order or chaos? A study of one-dimensional group geometry. Anim. Behav. 27, 46–80. ( 10.1016/0003-3472(79)90128-3) [DOI] [Google Scholar]
- 54.Krause J. 1994. Differential fitness returns in relation to spatial position in groups. Biol. Rev. Camb. Phil. Soc. 69, 187–206. ( 10.1111/j.1469-185X.1994.tb01505.x) [DOI] [PubMed] [Google Scholar]
- 55.Stueckle S, Zinner D. 2008. To follow or not to follow: decision making and leadership during the morning departure in chacma baboons. Anim. Behav. 75, 1995–2004. ( 10.1016/j.anbehav.2007.12.012) [DOI] [Google Scholar]
- 56.Tokuyama N, Furuichi T. 2017. Leadership of old females in collective departures in wild bonobos (Pan paniscus) at Wamba. Behav. Ecol. Sociobiol. 71, 55 ( 10.1007/s00265-017-2277-5) [DOI] [Google Scholar]
- 57.Barelli C, Barelli C, Heistermann M, Boesch C. 2008. Female white-handed gibbons (Hylobates lar) lead group movements and have priority of access to food resources. Behaviour 145, 965–981. ( 10.1163/156853908784089243) [DOI] [Google Scholar]
- 58.Lee HC, Teichroeb JA. 2016. Partially shared consensus decision making and distributed leadership in vervet monkeys: older females lead the group to forage. Am. J. Phys. Anthropol. 161, 580–590. ( 10.1002/ajpa.23058) [DOI] [PubMed] [Google Scholar]
- 59.Strandburg-Peshkin A, Farine DR, Couzin ID, Crofoot MC. 2015. Group decisions. Shared decision-making drives collective movement in wild baboons. Science 348, 1358–1361. ( 10.1126/science.aaa5099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bousquet CAH, Manser MB. 2011. Resolution of experimentally induced symmetrical conflicts of interest in meerkats. Anim. Behav. 81, 1101–1107. ( 10.1016/j.anbehav.2011.02.030) [DOI] [Google Scholar]
- 61.Boinski S. 1993. Vocal coordination of troop movement among white-faced capuchin monkeys, Cebus capucinus. Am. J. Primatol. 30, 85–100. ( 10.1002/ajp.1350300202) [DOI] [PubMed] [Google Scholar]
- 62.Boinski S, Campbell AF. 1995. Use of trill vocalizations to coordinate troop movement among white-faced capuchins: a second field test. Behaviour 132, 875–901. ( 10.1163/156853995X00054) [DOI] [Google Scholar]
- 63.Nagy M, Akos Z, Biro D, Vicsek T. 2010. Hierarchical group dynamics in pigeon flocks. Nature 464, 890–893. ( 10.1038/nature08891) [DOI] [PubMed] [Google Scholar]
- 64.Nagy M, Vasarhelyi G, Pettit B, Roberts-Mariani I, Vicsek T, Biro D. 2013. Context-dependent hierarchies in pigeons. Proc. Natl Acad. Sci. USA 110, 13 049–13 054. ( 10.1073/pnas.1305552110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ákos Z, Beck R, Nagy M, Vicsek T, Kubinyi E. 2014. Leadership and path characteristics during walks are linked to dominance order and individual traits in dogs. PLoS Comput. Biol. 10, e1003446 ( 10.1371/journal.pcbi.1003446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giuggioli L, McKetterick TJ, Holderied M. 2015. Delayed response and biosonar perception explain movement coordination in trawling bats. PLoS Comput. Biol. 11, e1004089 ( 10.1371/journal.pcbi.1004089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagy M, Couzin ID, Fiedler W, Wikelski M, Flack A. 2018. Synchronization, coordination and collective sensing during thermalling flight of freely migrating white storks. Phil. Trans. R. Soc. B 373, 20170011 ( 10.1098/rstb.2017.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butail S, Mwaffo V, Porfiri M. 2016. Model-free information-theoretic approach to infer leadership in pairs of zebrafish. Phys. Rev. E 93, 042411 ( 10.1103/PhysRevE.93.042411) [DOI] [PubMed] [Google Scholar]
- 69.Butail S, Ladu F, Spinello D, Porfiri M. 2014. Information flow in animal-robot interactions. Entropy 16, 1315–1330. ( 10.3390/e16031315) [DOI] [Google Scholar]
- 70.Sun J, Taylor D, Bollt EM. 2015. Causal network inference by optimal causation entropy. SIAM J. Appl. Dyn. Syst. 14, 73–106. ( 10.1137/140956166) [DOI] [Google Scholar]
- 71.Lord WM, Sun J, Ouellette NT, Bollt EM. 2016. Inference of causal information flow in collective animal behavior. arχiv 1606.01932 [q-bio.QM]. ( 10.1109/TMBMC.2016.2632099) [DOI]
- 72.Vicente R, Wibral M, Lindner M, Pipa G. 2011. Transfer entropy—a model-free measure of effective connectivity for the neurosciences. J. Comput. Neurosci. 30, 45–67. ( 10.1007/s10827-010-0262-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tung TQ, Ryu T, Lee KH, Lee D. et al. 2007. Inferring gene regulatory networks from microarray time series data using transfer entropy. In Proc. 20th IEEE Int. Symp. on Computer-Based Medical Systems (CBMS'07), Maribor, Slovenia (eds Kokol P, Podgorelec V, Mičetič-Turk D, Zorman M, Verlič M), pp. 383–388. Los Alamitos, CA: IEEE. [Google Scholar]
- 74.Leonidas SJ. 2014. Structure of a global network of financial companies based on transfer entropy. Entropy 16, 4443–4482. ( 10.3390/e16084443) [DOI] [Google Scholar]
- 75.Borge-Holthoefer J, Perra N, Goncalves B, Gonzalez-Bailon S, Arenas A, Moreno Y, Vespignani A. 2016. The dynamics of information-driven coordination phenomena: a transfer entropy analysis. Sci. Adv. 2, e1501158 ( 10.1126/sciadv.1501158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Couzin ID, Ioannou CC, Demirel G, Gross T, Torney CJ, Hartnett A, Conradt L, Levin SA, Leonard NE. 2011. Uninformed individuals promote democratic consensus in animal groups. Science 334, 1578–1580. ( 10.1126/science.1210280) [DOI] [PubMed] [Google Scholar]
- 77.Sánchez-Tójar A, Schroeder J, Farine DR. In press. A practical guide for inferring reliable dominance hierarchies and estimating their uncertainty. J. Anim. Ecol. [DOI] [PubMed] [Google Scholar]
- 78.Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A. 2011. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 82, 911–921. ( 10.1016/j.anbehav.2011.07.016) [DOI] [Google Scholar]
- 79.Farine DR, Whitehead H. 2015. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144–1163. ( 10.1111/1365-2656.12418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newman MEJ. 2010. Networks: an introduction. Oxford, UK: Oxford University Press. [Google Scholar]
- 81.Scharf HR, Hooten MB, Fosdick BK, Johnson DS, London JM, Durban JW.2016. Dynamic social networks based on movement. arχiv 1512.07607 [stat.AP]. See https://arxiv.org/pdf/1512.07607 .
- 82.Ward AJW, Sumpter DJT, Couzin ID, Hart PJB, Krause J. 2008. Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953. ( 10.1073/pnas.0710344105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sueur C, Deneubourg J-L, Petit O. 2010. Sequence of quorums during collective decision making in macaques. Behav. Ecol. Sociobiol. 64, 1875–1885. ( 10.1007/s00265-010-0999-8) [DOI] [Google Scholar]
- 84.Bousquet CAH, Sumpter DJT, Manser MB. 2011. Moving calls: a vocal mechanism underlying quorum decisions in cohesive groups. Proc. R. Soc. B 278, 1482–1488. ( 10.1098/rspb.2010.1739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petit O, Gautrais J, Leca J-B, Theraulaz G, Deneubourg J-L. 2009. Collective decision-making in white-faced capuchin monkeys. Proc. R. Soc. B 276, 3495–3503. ( 10.1098/rspb.2009.0983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bialek W, Cavagna A, Giardina I, Mora T, Silvestri E, Viale M, Walczak AM. 2012. Statistical mechanics for natural flocks of birds. Proc. Natl Acad. Sci. USA 109, 4786–4791. ( 10.1073/pnas.1118633109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonnell TR, Henzi SP, Barrett L. 2016. Direction matching for sparse movement data sets: determining interaction rules in social groups. Behav. Ecol. 28, 193–203. ( 10.1093/beheco/arw145) [DOI] [Google Scholar]
- 88.Bonnell TR, Clarke PM, Henzi SP, Barrett L. 2017. Individual-level movement bias leads to the formation of higher-order social structure in a mobile group of baboons. R. Soc. Open Sci. 4, 170148 ( 10.1098/rsos.170148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torney CJ, Lamont M, Debell L, Angohiatok RJ, Leclerc L-M, Berdahl AM. 2018. Inferring the rules of social interaction in migrating caribou. Phil. Trans. R. Soc. B 373, 20170385 ( 10.1098/rstb.2017.0385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. Camb. Phil. Soc. 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 91.Farine DR. 2017. A guide to null models for animal social network analysis. Methods Ecol. Evol. 10, 1309–1320. ( 10.1111/2041-210X.12772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aplin LM, et al. 2015. Consistent individual differences in the social phenotypes of wild great tits, Parus major. Anim. Behav. 108, 117–127. ( 10.1016/j.anbehav.2015.07.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galton F. 1907. Vox populi. Nature 75, 450–451. ( 10.1038/075450a0) [DOI] [Google Scholar]
- 94.Couzin ID. 2009. Collective cognition in animal groups. Trends Cogn. Sci. 13, 36–43. ( 10.1016/j.tics.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 95.Strandburg-Peshkin A, Farine DR, Crofoot MC, Couzin ID. 2017. Habitat and social factors shape individual decisions and emergent group structure during baboon collective movement. eLife 6, e19505 ( 10.7554/eLife.19505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Calabrese JM, Fleming CH, Fagan WF, Rimmler M, Kaczensky P, Bewick S, Leimgruber P, Mueller T. 2018. Disentangling social interactions and environmental drivers in multi-individual wildlife tracking data. Phil. Trans. R. Soc. B 373, 20170007 ( 10.1098/rstb.2017.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silk MJ, Jackson AL, Croft DP, Colhoun K, Bearhop S. 2015. The consequences of unidentifiable individuals for the analysis of an animal social network. Anim. Behav. 104, 1–11. ( 10.1016/j.anbehav.2015.03.005) [DOI] [Google Scholar]
- 98.Franks DW, Ruxton GD, James R. 2010. Sampling animal association networks with the gambit of the group. Behav. Ecol. Sociobiol. 64, 493–503. ( 10.1007/s00265-009-0865-8) [DOI] [Google Scholar]
- 99.Farine DR, Strandburg-Peshkin A. 2015. Estimating uncertainty and reliability of social network data using Bayesian inference. R. Soc. Open Sci. 2, 150367 ( 10.1098/rsos.150367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Firth JA, Voelkl B, Farine DR, Sheldon BC. 2015. Experimental evidence that social relationships determine individual foraging behavior. Curr. Biol. 25, 3138–3143. ( 10.1016/j.cub.2015.09.075) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.