Abstract
Many wildlife species shift their diets to use novel resources in urban areas. The consequences of these shifts are not well known, and consumption of reliable—but low quality—anthropogenic food may present important trade-offs for wildlife health. This may be especially true for carnivorous species such as the American white ibis (Eudocimus albus), a nomadic wading bird which has been increasingly observed in urban parks in South Florida, USA. We tested the effects of anthropogenic provisioning on consumer nutrition (i.e. dietary protein), body condition and ectoparasite burdens along an urban gradient using stable isotope analysis, scaled mass index values and GPS transmitter data. Ibises that assimilated more provisioned food were captured at more urban sites, used more urban habitat, had lower mass–length residuals, lower ectoparasite scores, assimilated less δ15N and had smaller dietary isotopic ellipses. Our results suggest that ibises in urban areas are heavily provisioned with anthropogenic food, which appears to offer a trade-off by providing low-quality, but easily accessible, calories that may not support high mass but may increase time available for anti-parasite behaviours such as preening. Understanding such trade-offs is important for investigating the effects of provisioning on infection risk and the conservation of wildlife in human-modified habitats.
This article is part of the theme issue ‘Anthropogenic resource subsidies and host–parasite dynamics in wildlife’.
Keywords: provisioning, urban wildlife, disease ecology, body condition, ectoparasite, stable isotope analysis
1. Introduction
Urbanization has important consequences for the abundance and types of resources available to wildlife. Urban areas typically contain novel foods, including non-native species of flora and fauna and anthropogenic (i.e. human-sourced) food provided intentionally or unintentionally by people as provisioned food (e.g. bread in parks) and food waste (e.g. garbage in landfills). As a result, many wildlife species in urban areas shift their diets to incorporate, for example, more domestic animals [1], ornamental plants [2] and provisioned food [3,4]. These shifts in diet in part reflect the ubiquity of human feeding of wildlife; for example, between 43% and 78% of households regularly feed urban birds [5].
The consequences of these urbanization-associated dietary shifts for wildlife health and ecology are varied. Some studies have detected increased body condition in urban wildlife, potentially due to higher and more reliable caloric intake (e.g. [6,7]). Others, however, have found no difference (e.g. [8]) or that wildlife in urban areas have lower indices of body condition relative to their wildland counterparts (e.g. [9–11]). This may stem from the relatively poor quality of provisioned food (e.g. [12]) and anthropogenic food waste (e.g. [13]), and from the relatively lower abundance of high-quality natural food items in urban areas (e.g. [14]). For example, diets deficient in protein can impair host immune function [15]. Further, resource provisioning in urban areas, either intentional or unintentional, can reduce wildlife movement rates [16], promote locally high animal densities which can lead to overcrowding and increased competition [17,18], and increase novel interspecific interactions [19]. All of these changes can promote pathogen transmission for wildlife (reviewed in [20–22]). Conversely, provisioning may reduce exposure to parasites transmitted through the consumption of intermediate hosts [23,24] or reduce the time and energy required for foraging and increase time spent grooming or preening [25]. Thus, when considering the impacts of provisioned food on wildlife health and condition, there may be important trade-offs that depend on food quantity and quality, and on host and pathogen life history [26].
The effects of food provisioning on wildlife condition may be more apparent in recently urbanizing species and are especially important for species of conservation concern. Although many species studied in urban areas are relatively abundant and considered urban-associated (e.g. house sparrows (Passer domesticus)), declining species may use zoos, residential yards, urban parks and landfills as alternative habitat following the loss or degradation of more ‘natural’ habitat [27]. This is especially true for species with strict habitat requirements and in places where urbanization is rapid. Such a process is currently occurring in South Florida, where the American white ibis (Eudocimus albus) is increasingly observed in urban zoos, lawns, parks and landfills [28].
The American white ibis is a wading bird that ranges over coastal areas in the southeastern USA. White ibises are nomadic and travel long distances to find appropriately shallow wetlands for their main prey items—aquatic invertebrates (i.e. crayfish (Astacoidea) and fiddler crabs (Uca spp.)), as well as other aquatic animals [29]. Ibises will also, however, forage for terrestrial invertebrates and at landfills [30,31]. Likely due to extensive degradation of the Everglades ecosystem, white ibis populations declined by 80% in South Florida over the past century [32,33], and over the past 20 years ibis have been increasingly observed in urban areas [28]. Urban areas may provide alternative wetland habitat for ibises at ponds and canal edges where ibises can forage for aquatic and terrestrial invertebrates. Additionally, urban ibises are also regularly fed bread and chips by people in parks [28]. We know that some individual ibises routinely use urban parks and ibises in more urban areas more likely shed Salmonella spp. [28]. However, it is unclear to what extent ibises are provisioned in urban parks and what effects these potential diet shifts have on ibis condition and health. For example, provisioned bread, chips and other simple carbohydrates may offer inadequate dietary protein relative to aquatic invertebrates, but may be easier to acquire, requiring less time and energy for foraging.
The effects of urban habitat use and consumption of provisioned food on wildlife health can be elucidated by measuring the habitat use, diet and health of individual animals along a gradient of urban development. Health is the outcome of many components and here we will focus on host condition, which we define as body condition and ectoparasite infestation; two factors likely to be affected by provisioning. We measured ibis diets using stable isotope analysis, a technique used to estimate the assimilated diets of individual animals by quantifying δ13C (ratio of 13C : 12C) and δ15N (15N : 14N) in consumer tissues. These isotopes reveal underlying carbon sources because the relative amount of 13C differs for marine and terrestrial communities and C4 versus C3 plants [34], and consumer trophic level, because 14N is preferentially excreted by consumers [35]. Stable isotope analysis is especially useful in studies of anthropogenic provisioning and infection risk because 13C is enriched in C4 plants such as corn, which is prevalent in processed foods [36].
In this study, we tested the hypothesis that white ibises consume provisioned anthropogenic food in urban parks and this dietary shift impacts host condition by altering host nutrition (i.e. dietary protein), body condition and risk of ectoparasite infestation as measures of health. We predicted that ibises using areas with more urban development would have diets higher in provisioned anthropogenic food and lower in protein. We further predicted that ibises in urban areas would have larger dietary niches and higher body condition scores if provisioned food supplements natural diets. Alternatively, we predicted that ibises would have smaller dietary niches and poorer body condition if they primarily consume provisioned food. Lastly, we hypothesized that ibises with diets higher in provisioned food would have higher ectoparasite scores because of detrimental effects of low-protein diets on immune function but may, alternatively, have lower ectoparasite burdens because they have more time and energy for parasite defense.
2. Material and methods
(a). Study area
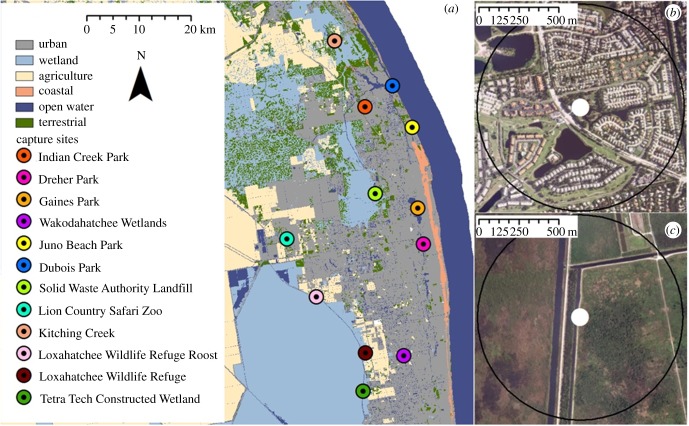
We captured American white ibises in Palm Beach and Martin Counties in South Florida between October 2015 and July 2016. To address changes in foraging behaviour and condition based on urbanization, we captured ibises at 12 capture sites at which foraging ibises had been observed or reported. These sites were located along a gradient of urban land use (see Land Cover Analysis section 2c below) and included five urban parks, a zoo, a landfill, three restored or constructed wetlands and two natural wetlands (figure 1 and table 1). South Florida has a tropical climate that is delineated into a rainy season (May–October) and dry season (November–April). These fluctuations in precipitation create hydrological changes throughout the area and drive the nomadic movements of white ibises as they search for shallow and ephemeral wetlands with high prey densities [32]. We designed seasonal capture periods to collect samples during the pre-breeding (February and March), breeding (June and July) and post-breeding (October and November) seasons [37] to capture and control for any variability in ibis behaviour or physiology linked to hydrology.
Figure 1.
(a) Map of ibis capture sites in Palm Beach and Martin Counties, Florida. Raster layer shows land cover categories reclassified from the Cooperative Land Cover (CLC) layer. Circles show capture locations ordered from most to least urban land cover within a 650 m radius (black circles in panels (b) and (c)). Our capture sites ranged from 91% urban land cover (b) to 0% urban land cover (c).
Table 1.
Summary of sites at which white ibises were captured in South Florida. Land cover data were calculated as a proportion of a 650 m buffer around capture sites (n = 12) using the CLC dataset for the state of Florida. N refers to sample size of captured ibises.
| proportion surrounding land cover |
|||||||
|---|---|---|---|---|---|---|---|
| site | N | urban | wetland | agriculture | coast | other water | other terrestrial |
| Indian Creek Park | 23 | 0.91 | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 |
| Dreher Park | 22 | 0.90 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 |
| Gaines Park | 21 | 0.84 | 0.00 | 0.00 | 0.00 | 0.16 | 0.00 |
| Wakodahatchee Wetlands | 19 | 0.65 | 0.27 | 0.01 | 0.00 | 0.07 | 0.00 |
| Juno Beach Park | 17 | 0.61 | 0.04 | 0.00 | 0.03 | 0.23 | 0.08 |
| Dubois Park | 11 | 0.53 | 0.00 | 0.00 | 0.06 | 0.39 | 0.02 |
| Solid Waste Authority Landfill | 18 | 0.49 | 0.11 | 0.00 | 0.00 | 0.07 | 0.32 |
| Lion Country Safari Zoo | 15 | 0.47 | 0.09 | 0.00 | 0.00 | 0.03 | 0.41 |
| Kitching Creek | 15 | 0.25 | 0.15 | 0.25 | 0.00 | 0.19 | 0.16 |
| Loxahatchee Wildlife Refuge Roost | 17 | 0.20 | 0.45 | 0.22 | 0.00 | 0.12 | 0.00 |
| Loxahatchee Wildlife Refuge | 9 | 0.04 | 0.74 | 0.18 | 0.00 | 0.02 | 0.02 |
| TetraTech Constructed Wetland | 10 | 0.00 | 0.92 | 0.00 | 0.00 | 0.08 | 0.00 |
(b). Ibis capture
We captured ibises using a combination of nylon slip-knot leg lassos [28], modified manually operated flip traps [38] and mist nets with decoys [39]. Once ibises were restrained, we assessed ibis age (adults ≥3 years had all white feathers, subadults less than 3 years had some brown plumage [40]) and collected standard morphometric measurements (culmen length, wing chord length, tarsus length and width). We then assessed ectoparasite loads of lice (Phthiraptera: Ardeicola spp., Colpocephalum spp., Ibidoecus spp. and Plegadiphilus spp.) on head and neck, and mite (Acari) eggs on flight feathers. Ectoparasite burdens were scored on an ordinal scale: 1 (no parasites observed), 2 (less than 50 mite eggs or less than 5 lice), 3 (between 50 and 100 mite eggs and/or less than 10 lice), 4 (between 100 and 150 mite eggs and/or less than 20 lice) and 5 (greater than 150 mite eggs and/or greater than 20 lice). We measured ibis body condition in three ways. Firstly, at capture, on an ordinal scale from 1 to 5 based on the palpation of the thickness of the pectoral muscle and presence of subcutaneous fat on and around the sternum. Ibises with little muscle over the sternum received a score of 1 or 2, ibises with noticeable pectoral muscle around the sternum received a 3, and ibises with a broad U-shaped chest wherein the muscle and subcutaneous fat either extends to or past the sternum received a 4 or 5 [41]. We also measured ibis mass at capture, which has been successfully used as a measure of condition in birds [42]. Lastly, we quantified body condition using the scaled mass index, which is a measure of relative size using mass–length data and can be corrected for differences in the relationship between mass and length for different demographics [43]. We calculated the scaled mass index separately for males and females because males are typically larger than females and we confirmed ibis sex using standard molecular techniques [44]. To calculate the scaled mass index, we used tarsus length because it was the length measure most strongly correlated with mass in this and other ibis studies [45]. The ordinal body condition scores and scaled mass index values were correlated (R2 = 0.14, F1,192 = 26.3, p < 0.0001) and so we used the scaled mass index values as the condition measure because it was less subjective.
We collected ≤1% of the bird's body weight of blood from the jugular or metatarsal vein for stable isotope analysis. We then chilled and centrifuged blood samples in the field within 5 h of collection to separate plasma from whole blood cells. We extracted the plasma and froze the red blood cells at ≤−80°C until analysis. We fitted a subset of ibises with EcoTone Kite GPS-GSM trackers (http://www.ecotone-telemetry.com; North Star Science and Technology, Oakton, VA, USA) if the combined mass of the transmitter and Teflon harness attachment was ≤3% of the bird's body mass [46]. These solar-powered transmitters collected locations every two hours between sunrise and sunset (i.e. when the bird was not roosting) and remotely delivered locations over the GSM cellular networks.
While capturing ibises, we scored the level of habituation of the overall ibis flock to human presence on an ordinal scale from 1 to 5. Ibises that actively approached humans and begged for food were scored a 5; birds that tolerated humans within 3 m and begged for food were scored a 4; ibises that tolerated humans between 3 and 10 m were scored a 3; ibises that tolerated humans within 10 m but did not consume anthropogenic food were scored a 2; ibises intolerant of human presence that flushed at 10 m were scored a 1.
(c). Land cover analysis
To quantify ibis use of urban habitats, we assessed urban land cover with the 2016 Cooperative Land Cover (CLC) map for the state of Florida (CLC v. 3.2, Florida Fish and Wildlife Conservation Commission, 2016) in ArcGIS. We used the 10 m raster geospatial layer with 234 land cover classes which we reclassified into urban, wetland, agriculture, coastal, open water and all other terrestrial land cover types (land cover classifications listed in electronic supplementary material, table S1). A small number (0.58%) of ibis locations fell outside of Florida state boundaries and for these locations we used the C-CAP coastal land cover dataset from NOAA (NOAA Office for Coastal Management, 2010).
For ibises fitted with GPS transmitters we calculated their habitat use by measuring the proportion of each land cover class within a 650 m radius around each GPS location. This window size was determined using a first passage time analysis [47] of ibis 2-h locations. First passage time is the time it takes an animal to leave a circle of a fixed radius and can thus indicate the spatial scale at which different types of movements occur. We chose the median smallest optimal radius size to correspond with the scale at which ibises exhibited localized movements of area-restricted search (i.e. foraging). We selected the median value due to right-skewed distribution of individual first passage time radii. In addition to identifying the scale at which ibises may select foraging areas, this approach enabled us to account for uncertainty in ibis locations from the high mobility of the birds in a 2-h window, the use of edges between multiple land cover types, and GPS error. For ibises that were not fitted with GPS transmitters, we used the surrounding land cover at their capture location as a proxy for habitat use. To do so, we calculated the proportion of the aforementioned land cover classes within a 650 m radius around each capture site (table 1).
(d). Stable isotope analysis
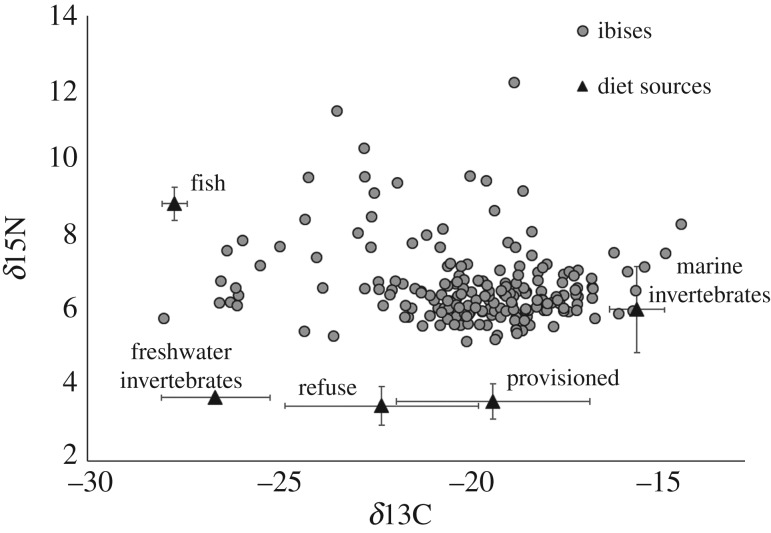
We quantified ibis diet using δ13C and δ13N stable isotope analysis of red blood cells. We chose red blood cells because they have a relatively slow turnover rate relative to plasma and store assimilated diet information for approximately the past 60 days [48]. We also analysed the isotopic composition of food items known to be important in white ibis diet based on previous studies [30]. We compiled these food items into five categories based on ecological or isotopic similarity: freshwater and terrestrial invertebrates (crayfish (Family: Astacoidea), American cockroach (Periplaneta americana)), marine invertebrates (fiddler crabs (Uca spp.)), provisioned anthropogenic food which we observed being provided to ibises by park visitors during capture sessions (bread and corn chips), refuse (food regurgitated by a captured ibis at a landfill, chicken) and values for small fish from the Everglades (flagfish (Jordanella floridae), eastern mosquitofish (Gambusia holbrooki), golden topminnow (Fundulus chrysotus)) [49] (figure 2).
Figure 2.
Stable isotope signatures of 193 white ibises captured in South Florida and five diet sources included in an R package SIAR mixing model.
Prior to analysis, we lyophilized red blood cell samples for at least 48 h and weighed approximately 1.2 mg of material into tin cups. The carbon and nitrogen isotopic ratios in samples were then determined with a continuous-flow isotope mass spectrometer (Thermo Finnigan Delta V, Bremen, Germany) coupled to a CHN analyzer (Carlo Erba NA1500, Milan, Italy) with a Thermo Finnigan Conflo III interface (Bremen, Germany). Control samples were processed every 10 samples to control for isotopic drift during analysis and isotopic ratios were calculated in reference to PeeDee Belemnite for 13C and atmospheric air for 15N. All analyses were completed at the UGA Stable Isotope Ecology Laboratory (Center for Applied Isotope Studies, University of Georgia, Athens, Georgia).
(e). Statistical analysis
We quantified ibis consumption of provisioned food, refuse, fish, freshwater and terrestrial invertebrates, and marine invertebrates by calculating five-source mixing models for individual ibises using the R package SIAR [50]. SIAR relates the stable isotope signatures of consumers with diet sources and uses a Bayesian approach to calculate posterior probabilities for the proportion of consumer diet composed of each diet source (i.e. the probability that diet source X makes up Y% of consumer diet). We accounted for enrichment from diet items to consumer blood cells using experimentally derived trophic enrichment factors for piscivorous gulls from the literature (ΔCdiet–blood = −0.3 ± 0.8‰, ΔNdiet–blood = 3.1 ± 0.2‰; [51]) because these values have not yet been calculated for ibis whole blood nor other wading bird species. For each individual ibis, we calculated the average proportion of each diet source across the 500 000 Markov chain Monte Carlo (MCMC) iterations run by SIAR (burn in = 5000).
We used generalized linear mixed models (GLMMs) with capture site as a random effect to quantify the relationship between the assimilation of provisioned food (response variable) and urban land cover, age, season, scaled mass index values as a measure of body condition, ectoparasite score, flock habituation and all two-way interactions as covariates. We generated models for all possible combinations of these covariates using all-subsets modelling [52] and quantified the importance of each covariate by model averaging their parameter estimates in the top set of models (Akaike information criterion: AICc less than 4 from the top model). We also ran separate all-subsets models for ibis body condition (GLMM) and ectoparasite score (Poisson GLMM) as response variables and the assimilation of provisioned food, season, urban land cover, habituation, age and all two-way interactions as covariates. Prior to running models, we calculated covariance matrices and variables that were highly correlated (r² > 0.5) were not included together.
We tested whether ibises captured in more urban areas had larger or smaller dietary niches using the R package SIBER [53]. To do so, we calculated the standard ellipse area for each capture site, which contains the central 40% of the data for ibises at each capture site. Large ellipse areas indicate that a group of consumers assimilates diet sources with more varied isotope values and thus a broader diet and larger dietary niche. We then regressed the land cover values surrounding each capture site with the ellipse area of ibises at that site.
We further tested whether differences in diet observed across capture sites related to urban habitat use with the data from ibises fitted with GPS transmitters. We used linear regression to quantify the association between the proportion of GPS locations in urban and wetland habitat types for individual birds and their estimated assimilation of provisioned food or freshwater invertebrates from the SIAR mixing models.
3. Results
We captured 193 white ibises at 12 sites (average number of ibises per site = 16; range = 9–23; table 1) and fitted 36 ibises with GPS transmitters. Most birds (69%) were adults at least three years of age and we captured more juveniles in wetlands than in urban parks, landfills and zoos (juveniles: urban: 33/127, wetland: 32/66). Our sample size was highest in the pre-breeding season (n = 91) relative to the breeding (n = 45) and post-breeding (n = 57) seasons. Only one ibis was recaptured and we only included its initial sample in the analysis.
Based on the first passage time analysis, the smallest optimal radius (i.e. the area within which small-scale movements occurred) was 650 m. Based on this radius, the amount of urban land cover surrounding a capture site ranged from 0 to 91% (mean = 49 ± 31%; table 1). The proportion of urban land cover surrounding a site was negatively correlated with the proportion of wetland (marginal R² = 0.70, F1,11 = 23.7, p < 0.001) and agriculture (marginal R² = 0.35, F1,11 = 5.46, p = 0.04) and so we only included urban land cover in the models. Ibises fitted with GPS transmitters that were captured at sites surrounded by more urban land cover also used more urban habitat (R² = 0.73, F1,35 = 99.3, p < 0.0001). Greater flock habituation to humans was associated with more urban land cover (marginal R2 = 0.53, F1,11 = 11.1, p = 0.008) and was thus not included in models with urban land cover.
Using individual five-source mixing models, we estimated that provisioned anthropogenic food comprised between 4% and 70% of ibis diet (figure 2 and figure 3). In order of importance, ibises that consumed a higher proportion of provisioned food were captured at sites with greater urban land cover, had lower body condition as measured by scaled mass index values, were captured during the post-breeding season, had lower ectoparasite scores, and were slightly more likely to be sub-adults (table 2).
Figure 3.
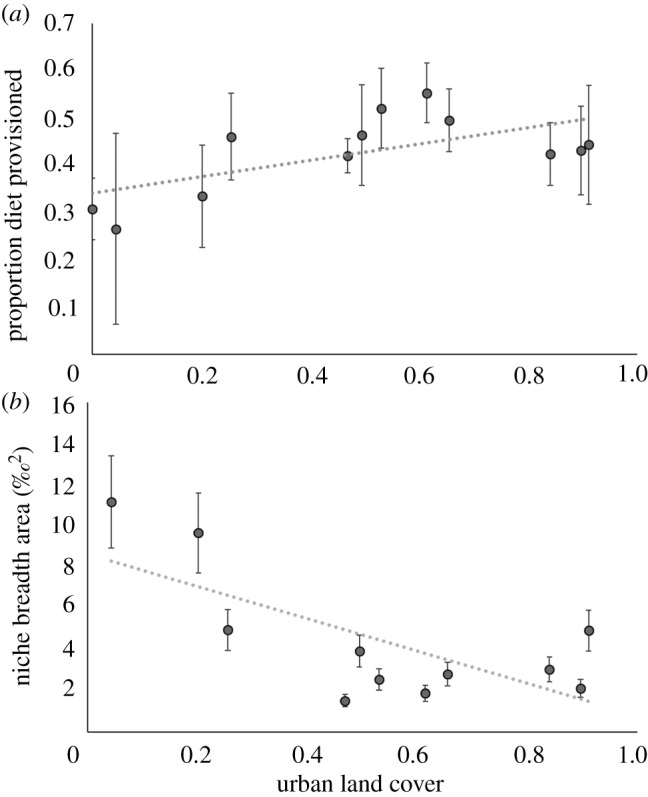
Relationships between ibis diet and surrounding land cover at capture site. Circles indicate average values of each capture site for (a) proportion of ibis diet comprised of provisioned food, (b) isotopic area as a measure of niche breadth. Error bars show standard deviation. Urban land cover was calculated as a proportion of the 650 m buffer around capture sites (n = 12) using the Cooperative Land Cover (v. 3.2) dataset for the state of Florida.
Table 2.
Model-averaged parameter estimates in the top model set (less than four AICc) for generalized linear mixed models with proportion of diet comprised of provisioned food or freshwater invertebrates as response variables with capture site as a random effect. Importance refers to the sum of all model Akaike weights in which the explanatory variable appears.
| response variable | covariate | β | s.e. | importance |
|---|---|---|---|---|
| proportion diet provisioned food | intercept | −0.51 | 0.45 | |
| urban land cover | 1.85 | 0.62 | 0.98 | |
| body condition | −0.45 | 0.18 | 0.89 | |
| season (pre-breeding) | −0.52 | 0.36 | 0.72 | |
| season (breeding) | −0.18 | 0.41 | 0.72 | |
| ectoparasite score | −0.18 | 0.07 | 0.71 | |
| season × body condition (pre-breeding) | 0.42 | 0.15 | 0.42 | |
| urban cover × season (pre-breeding) | 0.42 | 0.63 | 0.34 | |
| age (subadult) | 0.07 | 0.14 | 0.12 | |
| proportion diet invertebrates | intercept | 0.350 | 0.016 | |
| body condition | 0.019 | 0.0029 | 0.93 | |
| wetland land cover | 0.252 | 0.057 | 0.89 | |
| season: pre-breeding | −0.014 | 0.021 | 0.75 | |
| season: breeding | 0.0013 | 0.0255 | 0.75 | |
| wetland cover × pre-breeding | 0.376 | 0.067 | 0.62 | |
| wetland cover × breeding | 0.0042 | 0.168 | 0.62 |
Ibises captured at sites surrounded by more urban land cover assimilated more provisioned food (conditional R² = 0.19, F1,11 = 22.5, p < 0.001; figure 3a) and had smaller standard area ellipses (conditional R² = 0.46, F1,11 = 8.4, p = 0.02; figure 3b). Ibises captured at sites surrounded by more urban land cover also assimilated less δ15N (proxy for dietary protein; β = 0.1, s.e. = 0.04, p < 0.0001). Ibises fitted with GPS transmitters that spent more time in urban areas had diets higher in provisioned food, though this relationship was logarithmic rather than linear (loglinear regression: marginal R² = 0.48, F1,35 = 27.2, p < 0.001; linear regression: marginal R² = 0.28, F1,35 = 14.9, p < 0.001).
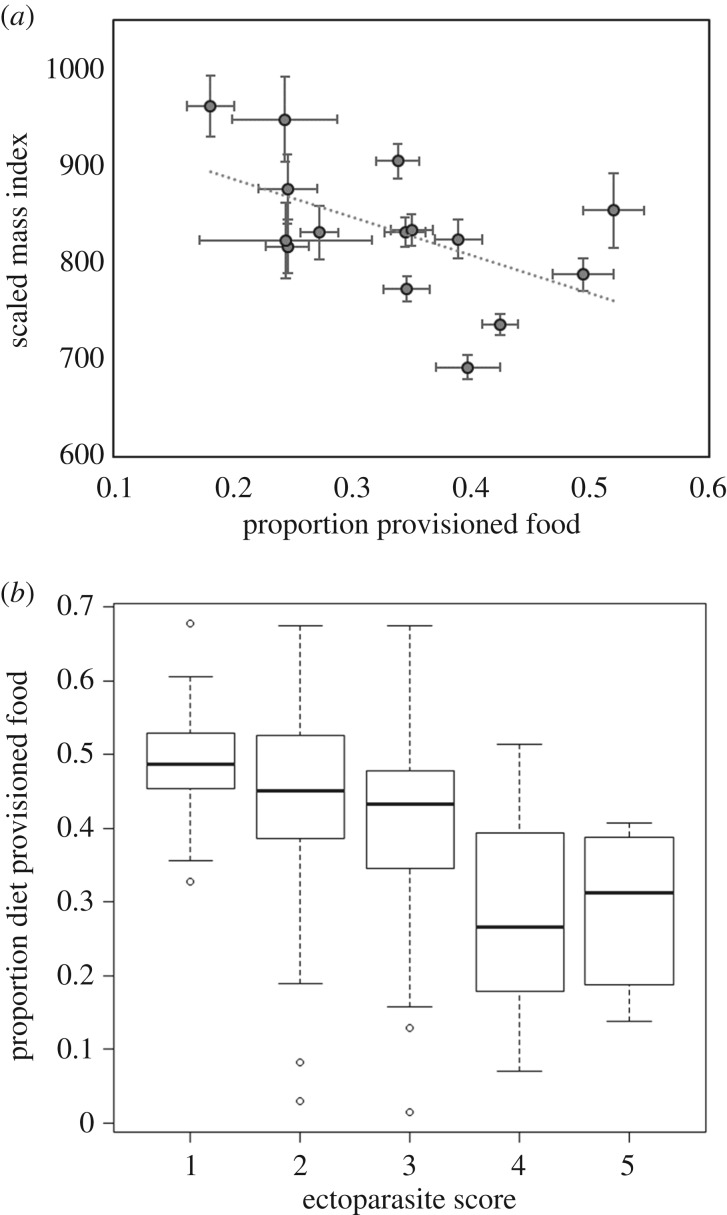
Ibises with diets higher in provisioned food were lower in mass (conditional R² = 0.15, F1,11 = 18.8, p < 0.0001) and had lower scaled mass index values (conditional R² = 0.29, F1,11 = 5.3, p = 0.03; figure 4a and table 3). Diets higher in provisioned food were also associated with lower ectoparasite scores (conditional R² = 0.12, F1,11 = 26.2, p < 0.0001; figure 4b and table 3).
Figure 4.
Association between ibis condition and assimilation of provisioned food. (a) Proportion of ibis diet composed of provisioned food as estimated using a five-source Bayesian mixing model and average ibis body condition at each capture site, measured using the scaled mass index. Error bars indicate standard error. (b) Proportion of ibis diet composed of provisioned food and ectoparasite score of individual ibises, assessed on an ordinal scale ranging from 1 (no lice and mites observed) to 5 (heavy burdens of lice and mites on head, neck, and flight feathers).
Table 3.
Model-averaged parameter estimates in the top model set (less than four AICc) for generalized linear mixed models with ibis body condition (scaled mass index) or ectoparasite intensity (ordinal score) as response variables with capture site as a random effect. Importance refers to the sum of all model Akaike weights in which the explanatory variable appears.
| response variable | covariate | β | s.e. | importance |
|---|---|---|---|---|
| body condition | intercept | 0.96 | 0.32 | |
| provisioned food | −1.02 | 0.43 | 1.00 | |
| age | −0.06 | 0.38 | 1.00 | |
| season (pre-breeding) | 0.63 | 0.59 | 1.00 | |
| season (breeding) | −1.33 | 0.96 | 1.00 | |
| provisioned food × season (pre-breeding) | 0.72 | 1.4 | 0.91 | |
| provisioned food × season (breeding) | 3.07 | 1.92 | 0.91 | |
| age × season (pre-breeding) | −0.79 | 0.51 | 0.82 | |
| age × season (breeding) | −0.45 | 0.29 | 0.82 | |
| mass | intercept | 0.84 | 0.48 | |
| provisioned food | −1.81 | 0.86 | 0.95 | |
| age | −0.15 | 0.19 | 0.23 | |
| season (pre-breeding) | −0.72 | 0.64 | 0.21 | |
| season (breeding) | −1.24 | 0.92 | 0.21 | |
| provisioned food × season (pre-breeding) | 1.70 | 1.23 | 0.18 | |
| provisioned food × season (breeding) | 2.80 | 1.86 | 0.18 | |
| age (subadult) × season (pre-breeding) | −0.54 | 0.36 | 0.01 | |
| age (subadult) × season (breeding) | −0.55 | 0.40 | 0.01 | |
| ectoparasite intensity | intercept | 3.13 | 0.38 | |
| provisioned food | −2.2 | 0.72 | 1.00 | |
| season (pre-breeding) | −0.15 | 0.76 | 0.77 | |
| season (breeding) | 0.22 | 0.54 | 0.77 | |
| provisioned food × season (pre-breeding) | −0.15 | 0.78 | 0.47 | |
| provisioned food × season (breeding) | −0.19 | 1.20 | 0.47 | |
| age (subadult) | −0.01 | 0.09 | 0.18 |
Individual mixing models estimated that freshwater and terrestrial invertebrates composed between 1% and 67% of ibis diet. Assimilation of freshwater and terrestrial invertebrates was especially high in the pre-breeding season and was associated with greater wetland cover surrounding capture sites and increased scaled mass index values (table 2). Other land cover types, however, were not significantly associated with diet. Ibises fitted with GPS transmitters that spent more time in wetland areas assimilated more freshwater and terrestrial invertebrates (marginal R2 = 0.13, F1,35 = 5.1, p = 0.03).
4. Discussion
In this study, we tested to what extent a recently urbanized wading bird consumed provisioned anthropogenic food across an urban gradient and the consequences of these diet shifts for several aspects of host condition including nutrition, body condition and ectoparasite burdens. We found that greater urban habitat use, inferred from land cover surrounding either capture sites or GPS transmitter locations, was associated with increased assimilation of provisioned food (bread, chips) and diets lower in protein. Similarly, greater use of wetlands was associated with increased assimilation of aquatic and terrestrial invertebrates. These shifts in diet with habitat use appear to have both positive and negative consequences for ibis condition. Ibises with diets higher in provisioned food had poorer body condition (i.e. lower mass than expected based on size) but had lower ectoparasite burdens.
The increase we observed in the assimilation of provisioned food by ibises in urban areas indicates that ibises are likely routinely fed bread and chips by people in parks. This is consistent with our observations during ibis captures of park visitors feeding large flocks of ibises with bread and chips (MH Murray 2016, personal observation). The changes in diet we observed are likely chronic in nature as we analysed the isotopic composition of red blood cells with a relatively long turnover (i.e. approximately 2 months, [48]). Thus, these birds may be habitually foraging for anthropogenic food at parks and other urban areas over the span of several months. Indeed, several of the ibises tracked in this study spent over 80% of their foraging hours in urban parks. The related Australian white ibis (Threskiornis molucca) also consistently uses urban habitats over the span of months to years, and some individuals have become reliant on provisioned food and garbage [54].
Provisioned food appeared to be most important during the post-breeding season in the autumn relative to pre-breeding (spring) and breeding (summer) seasons. Seasonal changes in the consumption of provisioned food might arise from seasonal changes in energy demands. For example, the skin on the face and legs of white ibises changes from pink to red prior to the breeding season to attract a mate, which is likely energetically costly and dependent on β-carotene. Similarly, long-distance movements for nest building and mate attraction may drive the observed relative increase of aquatic protein-rich prey [31]. This seasonal fluctuation in diet also suggests that urban habitat use is not consistent in all seasons. Similar to other species (e.g. European white storks, Ciconia ciconia [55]), ibises may make more use of anthropogenic food when appropriate foraging conditions for natural prey are less available [30]. Similarly, flying foxes can exhibit longer residence times in urban habitat patches when natural food resources are less available [56]. Little is known about urban habitat use by nomadic species, including seasonal shifts therein. One notable example is the recent establishment of resident year-round populations of Australian white ibises in urban areas [57].
Many other species have more diverse diets in urban areas by supplementing their natural diets with anthropogenic food and by exhibiting more individual variation in diet (e.g. coyotes [3,58]). Unlike these species, we found that the use of provisioned food by ibises homogenized their diets as they had smaller dietary niches. This implies that urban ibises may be transitioning from foraging primarily on the most abundant aquatic organisms available during different hydrological periods [30], to foraging predominantly on isotopically similar novel foods in urban areas. The effects of urbanization on diet diversity may be species-specific and more studies are needed to better understand the relationships between diet diversity and urbanization more generally.
In urban parks, we observed that the public routinely provides bread and other carbohydrate-rich foods to ibises and waterfowl. This increased consumption of anthropogenic food will likely negatively impact ibis health as bread and chips are notoriously poor in protein or key micronutrients relative to invertebrate prey. As part of a concurrent study on ibis diet and immune function, nutritional analyses revealed that a diet containing white bread in the proportions we observed would offer 48% less protein, 59% less calcium and 48% less phosphorous (L Hoopes 2017, personal communication). This relatively protein-poor diet may explain why urban ibises had lower mass than expected based on their body size, and perhaps more deposition of fat over muscle mass [59]. Among provisioned wildlife, a shift toward protein-poor foods is common [60–62]. For example, suburban American crow (Corvus brachyrhynchos) nestlings were smaller and had lower serum protein levels than rural crows, and supplementation with a high-protein diet increased urban nestling size, indicating they were protein-limited [9]. The reduced protein content of provisioned bread and chips relative to invertebrates may also impair immune function and increase ibis susceptibility to pathogen infection [15].
Although provisioned food can be of lower nutritional quality and compromise body condition, its availability and reliability may reduce the time and energy required for foraging. This shift may allow for the redistribution of energy for immune defense against pathogens and parasites, preening and resting, all of which can improve host condition. For example, provisioned rhesus macaques (Macaca mulatta) in urban Dhaka, Bangladesh spent less time foraging and more time resting and grooming than rural macaques [25]. Preening, wherein birds pull their feathers between their mandibles, has been shown to decrease ectoparasite load in many bird species [63,64]. This may explain why ibis that assimilated more provisioned food also exhibited lower ectoparasite loads. While capturing ibis, we routinely observed ibises resting and preening for extended periods of time in urban parks, but we typically observed ibises either foraging or flying in wetlands rather than resting or preening (MH Murray 2016, personal observation). The differences we observed in ectoparasite loads with urbanization and provisioning may also be due to changes in contact rates as avian lice are primarily transmitted through direct contact [65]. Chewing feather mites (Phthiraptera: Ischnocera) are mainly transmitted vertically in nests and are thus unlikely to represent adult exposure but rather resistance [65]. While the burdens of feather mites we observed would likely have little overall effect on host functioning, the high burdens of head lice (greater than 50 of Ibidoecus bimaculatus) we observed on some ibis could result in considerable losses in energy reserves (MJ Yabsley 2017, personal communication) and chewing lice infestations have been shown to decrease the long-term survival of avian hosts [66]. The opposing impacts of provisioning on ibis body condition and ectoparasite loads emphasize the trade-offs involved in provisioning for many other species; for example, the coupling of higher overwinter survival and higher pathogen prevalence in ungulates [20,67]. To evaluate the net effects of provisioning for wildlife health, more information is needed on the physiological costs of parasite burdens, pathogenicity of infections and impacts of body condition on immune function and survival.
The potential for urban areas to act as alternative wildlife habitat may depend on human behaviour change if provisioned food negatively affects consumer nutrition or body condition. Unlike many other urban birds, American white ibises are not yet known to breed in urban areas consistently and will travel to mixed-species rookeries in freshwater wetlands [32]. This may buffer ibis nestling development somewhat against the effects of anthropogenic food; however, white ibises are known to travel to landfills and provide chicken and other protein-rich foods to nestlings during resource-poor periods [30]. Other species, such as Australian magpies (Gymnorhina tibicen), consume anthropogenic foods as adults but do not provide it to their nestlings [68]. If the American white ibis begins to breed regularly in urban parks or becomes dependent on anthropogenic food for offspring provisioning, it may be especially damaging to nestlings who are most susceptible to pathogens known to be carried by ibis (e.g. Salmonella), especially in urban areas [28] or acquired from landfills (e.g. Clostridium botulinum) and may also promote the spread of zoonotic pathogens for which ibis are reservoirs [69].
While we detected significant relationships between urban habitat use, the assimilation of provisioned food and ibis condition, we cannot ascertain the direction of causality. Increased consumption of provisioned bread may cause decreased body condition and ectoparasite burdens via the mechanisms described above. Provisioning sites in urban areas may, however, instead be preferentially attractive to birds in poor condition as provisioned food is reliable and easily accessible. These relationships may also be confounded because white ibises show strong conspecific attraction [70] and may be attracted to flocks in urban parks prior to being attracted to provisioned food. Further, the long-term changes in diet we measured may obscure individual differences in the use of provisioned food. For example, some individuals may intensely use provisioned food during times of very high or very low water levels unsuitable for ibis foraging while others consume small amounts daily. Combining both short- and long-term measures of diet and habitat selection from GPS locations could elucidate these differences. Further, due to the logistical constraints, we captured ibises by baiting them with food to lassos and flip nets in urban parks and using mist nets in wetlands. By baiting ibises with food in urban areas, we may have underrepresented ibises that were unwilling to approach humans or were less interested in provisioned food. However, we are confident our estimates are representative of birds caught using other methods because we captured ibises using mist nets at one wetland site surrounded by an urban neighbourhood (Wakodahatchee) consuming diets high in anthropogenic foods.
In this study, we show that increased urban habitat use by a recently urbanized wading bird is associated with higher assimilation of anthropogenic provisioned food, lower dietary protein, reduced body condition and lower ectoparasite burdens. Provisioned food may thus offer trade-offs for host condition. The net effects of provisioning on wildlife health and condition may determine whether urban areas serve as refugia or population sinks as pristine habitat patches decline for the white ibis and many other species. Further studies are needed to assess the consequences of these changes for disease susceptibility and pathogen transmission in urban areas.
Supplementary Material
Supplementary Material
Acknowledgements
We thank our collaborators Sonia Altizer, Richard Hall, Erin Lipp and Kristen Navara as well as volunteers and undergraduate researchers for their help with study design and data collection. We also thank many government biologists and managers who provided access to capture sites.
Ethics
All capture and handling procedures were reviewed and approved by the University of Georgia's Institutional Animal Care and Use Committee (A2016 11-019-Y1-A0), a Florida Wildlife Conservation Commission permit (LSSC-11-00119F) and a USFWS permit (MB779238-0).
Data accessibility
All associated data on ibis condition, diet and capture sites are available as electronic supplementary material. The transmitter data are part of a larger study but are available upon request from the author.
Authors' contributions
M.H.M. carried out the data analysis and statistical analysis, participated in data collection, participated in the design of the study and drafted the manuscript; A.D.K. participated in data analysis and data collection; J.W.C. and S.E.C. participated in study design; M.J.Y. performed molecular work; H.C.A., T.E. and C.N.W. collected field data; S.M.H. conceived of the study, designed the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests but are research collaborators with Daniel Becker, Sonia Altizer and Richard Hall.
Funding
All authors were supported by an NSF EEID grant no. (DEB-1518611).
References
- 1.Athreya V, Odden M, Linnell JDC, Krishnaswamy J, Karanth U. 2013. Big cats in our backyards: persistence of large carnivores in a human dominated landscape in India. PLoS ONE 8, 2–9. ( 10.1371/journal.pone.0057872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson HE, Breck SW, Baruch-Mordo S, Lewis DL, Lackey CW, Wilson KR, Broderick J, Mao JS, Beckmann JP. 2015. Shifting perceptions of risk and reward: dynamic selection for human development by black bears in the western United States. Biol. Conserv. 187, 164–172. ( 10.1016/j.biocon.2015.04.014) [DOI] [Google Scholar]
- 3.Murray MH, Cembrowski A, Latham ADM, Lukasik VM, Pruss S, St Clair CC. 2015. Greater consumption of protein-poor anthropogenic food by urban relative to rural coyotes increases diet breadth and potential for human-wildlife conflict. Ecography 38, 1235–1242. ( 10.1111/ecog.01128) [DOI] [Google Scholar]
- 4.Plummer KE, Siriwardena GM, Conway GJ, Risely K, Toms MP. 2015. Is supplementary feeding in gardens a driver of evolutionary change in a migratory bird species? Glob. Chang. Biol. 21, 4353–4363. ( 10.1111/gcb.13070) [DOI] [PubMed] [Google Scholar]
- 5.Robb GN, McDonald RA, Chamberlain DE, Bearhop S. 2008. Food for thought: supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 6, 476–484. ( 10.1890/060152) [DOI] [Google Scholar]
- 6.Wilcoxen TE, et al. 2015. Effects of bird-feeding activities on the health of wild birds. Conserv. Physiol. 3, 1–13. ( 10.1093/conphys/cov058.Introduction) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko Y, Maruyama N. 2005. Changes in Japanese badger (Meles meles anakuma) body weight and condition caused by the provision of food by local people in a Tokyo suburb. Mamm. Sci. 45, 157–164. [Google Scholar]
- 8.Bókony V, Seress G, Nagy S, Lendvai ÁZ, Liker A. 2012. Multiple indices of body condition reveal no negative effect of urbanization in adult house sparrows. Landsc. Urban Plan. 104, 75–84. ( 10.1016/j.landurbplan.2011.10.006) [DOI] [Google Scholar]
- 9.Heiss RS, Clark AB, McGowan KJ. 2009. Growth and nutritional state of American Crow nestlings vary between urban and rural habitats. Ecol. Appl. 19, 829–839. ( 10.1890/08-0140.1) [DOI] [PubMed] [Google Scholar]
- 10.Prange S, Gehrt SD, Wiggers EP. 2003. Demographic factors contributing to high raccoon densities in urban landscapes. J. Wildl. Manage. 67, 324–333. ( 10.2307/3802774) [DOI] [Google Scholar]
- 11.Liker A, Papp Z, Bókony V, Lendvai ÁZ. 2008. Lean birds in the city: body size and condition of house sparrows along the urbanization gradient. J. Anim. Ecol. 77, 789–795. ( 10.1111/j.1365-2656.2008.01402.x) [DOI] [PubMed] [Google Scholar]
- 12.Knapp CR, Hines KN, Zachariah TT, Perez-Heydrich C, Iverson JB, Sandra D, Halach SC, Lattin CR, Romero LM. 2013. Physiological effects of tourism and associated food provisioning in an endangered iguana. Conserv. Physiol. 1, 1–12. ( 10.1093/conphys/cot032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray MH, Hill J, Whyte P, St. Clair CC. 2016. Urban compost attracts coyotes, contains toxins, and may promote disease in urban-adapted wildlife. Ecohealth 13, 285–298. ( 10.1007/s10393-016-1105-0) [DOI] [PubMed] [Google Scholar]
- 14.Isaksson C, Andersson S. 2007. Carotenoid diet and nestling provisioning in urban and rural great tits Parus major. J. Avian Biol. 38, 564–572. ( 10.1111/j.2007.0908-8857.04030.x) [DOI] [Google Scholar]
- 15.Taylor AK, Cao W, Vora KP, De La Cruz J, Shieh W-J, Zaki SR, Katz JM, Sambhara S, Gangappa S. 2013. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J. Infect. Dis. 207, 501–510. ( 10.1093/infdis/jis527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert NI, Correia RA, Silva JP, Pacheco C, Catry I, Atkinson PW, Gill JA, Franco AMA. 2016. Are white storks addicted to junk food? Impacts of landfill use on the movement and behaviour of resident white storks (Ciconia ciconia) from a partially migratory population. Mov. Ecol. 4, 1–13. ( 10.1186/s40462-016-0070-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semeniuk CA, Rothley KD. 2008. Costs of group-living for a normally solitary forager: effects of provisioning tourism on southern stingrays Dasyatis americana. Mar. Ecol. Prog. Ser. 357, 271–282. ( 10.3354/meps07299) [DOI] [Google Scholar]
- 18.Semeniuk CAD, Bourgeon S, Smith SL, Rothley KD. 2009. Hematological differences between stingrays at tourist and non-visited sites suggest physiological costs of wildlife tourism. Biol. Conserv. 142, 1818–1829. ( 10.1016/j.biocon.2009.03.022) [DOI] [Google Scholar]
- 19.Galbraith JA, Beggs JR, Jones DN, Stanley MC. 2015. Supplementary feeding restructures urban bird communities. Proc. Natl Acad. Sci. USA 112, E2648–E2657. ( 10.1073/pnas.1501489112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray MH, Becker DJ, Hall RJ, Hernandez SM. 2016. Wildlife health and supplemental feeding: a review and management recommendations. Biol. Conserv. 204, 163–174. ( 10.1016/j.biocon.2016.10.034) [DOI] [Google Scholar]
- 21.Becker DJ, Hall RJ. 2014. Too much of a good thing: resource provisioning alters infectious disease dynamics in wildlife. Biol. Lett. 10, 1–5. ( 10.1098/rsbl.2014.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall SR, Sivars-Becker L, Becker C, Duffy MA, Tessier AJ, Caceres CE. 2007. Eating yourself sick: transmission of disease as a function of foraging ecology. Ecology 10, 207–218. ( 10.1111/j.1461-0248.2006.01011.x) [DOI] [PubMed] [Google Scholar]
- 23.Lane KE, Holley C, Hollocher H, Fuentes A. 2011. The anthropogenic environment lessens the intensity and prevalence of gastrointestinal parasites in Balinese long-tailed macaques (Macaca fascicularis). Primates 52, 117–128. ( 10.1007/s10329-010-0230-6) [DOI] [PubMed] [Google Scholar]
- 24.Hegglin D, Bontadina F, Contesse P, Gloor S, Deplazes P. 2007. Plasticity of predation behaviour as a putative driving force for parasite life-cycle dynamics: the case of urban foxes and Echinococcus multilocularis tapeworm. Funct. Ecol. 21, 552–560. ( 10.1111/j.1365-2435.2007.01257.x) [DOI] [Google Scholar]
- 25.Jaman MF, Huffman MA. 2013. The effect of urban and rural habitats and resource type on activity budgets of commensal rhesus macaques (Macaca mulatta) in Bangladesh. Primates 54, 49–59. ( 10.1007/s10329-012-0330-6) [DOI] [PubMed] [Google Scholar]
- 26.Galbraith JA, Stanley MC, Jones DN, Beggs JR. 2017. Experimental feeding regime influences urban bird disease dynamics. J. Avian Biol. 48, 700–713. ( 10.1111/jav.01076) [DOI] [Google Scholar]
- 27.Goddard MA, Dougill AJ, Benton TG. 2010. Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol. Evol. 25, 90–98. ( 10.1016/j.tree.2009.07.016) [DOI] [PubMed] [Google Scholar]
- 28.Hernandez SM, et al. 2016. Urbanized White Ibises (Eudocimus albus) as carriers of Salmonella enterica of significance to public health and wildlife. PLoS ONE 11, 1–22. ( 10.1371/journal.pone.0164402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kushlan JA, Kushlan MS. 1975. Food of the White Ibis in southern Florida. Fla Field Nat. 3, 1. [Google Scholar]
- 30.Dorn NJ, Cook MI, Herring G, Boyle RA, Nelson J, Gawlik DE. 2011. Aquatic prey switching and urban foraging by the White Ibis Eudocimus albus are determined by wetland hydrological conditions. Ibis 153, 323–335. ( 10.1111/j.1474-919X.2011.01101.x) [DOI] [Google Scholar]
- 31.Heath JA, Frederick P, Kushlan JA, Bildstein KL.2009. White Ibis (Eudocimus albus) Ithaca: Cornell Lab of Ornithology. Birds North Am. Online. See http://bna.birds.co .
- 32.Frederick P, Gawlik DE, Ogden JC, Cook MI, Lusk M. 2009. The White Ibis and wood stork as indicators for restoration of the everglades ecosystem. Ecol. Indic. 9, S83–S95. ( 10.1016/j.ecolind.2008.10.012) [DOI] [Google Scholar]
- 33.Crozier GE, Gawlik DE. 2003. Wading bird nesting effort as an index to wetland ecosystem integrity. Waterbirds 26, 303–324. ( 10.1675/1524-4695(2003)026%5B0303:WBNEAA%5D2.0.CO;2) [DOI] [Google Scholar]
- 34.West JB, Bowen GJ, Cerling TE, Ehleringer JR. 2006. Stable isotopes as one of nature's ecological recorders. Trends Ecol. Evol. 21, 408–414. ( 10.1016/j.tree.2006.04.002) [DOI] [PubMed] [Google Scholar]
- 35.DeNiro MJ, Epstein S. 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 45, 341–351. ( 10.1016/0016-7037(81)90244-1) [DOI] [Google Scholar]
- 36.Jahren AH, Kraft RA. 2008. Carbon and nitrogen stable isotopes in fast food: signatures of corn and confinement. Proc. Natl Acad. Sci. USA 105, 17 855–17 860. ( 10.1073/pnas.0809870105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kushlan JA. 1986. Responses of wading birds to seasonally fluctuating water levels: strategies and their limits. Colon. Waterbirds 9, 155–162. ( 10.2307/1521208) [DOI] [Google Scholar]
- 38.Parker JM, Folk MJ, Baynes SB, Candelora KL. 2008. Use of clap traps in capturing nonmigratory whooping cranes in Florida. Proc. Tenth North Am. Crane Work. 10, 141–146. (http://digitalcommons.unl.edu/nacwgproc/196) [Google Scholar]
- 39.Heath JA, Frederick PC. 2003. Trapping white ibises with rocket nets and mist nets in the Florida Everglades. J. Field Ornithol. 74, 187–192. ( 10.1648/0273-8570-74.2.187) [DOI] [Google Scholar]
- 40.De Santo TL, Mcdowell SG, Bildstein KL. 1990. Plumage and behavioral development of nestling white ibises. Wilson Bull. 102, 226–238. [Google Scholar]
- 41.Doneley B, Harrison G, Lightfoot T. 2006. Maximizing information from the physical examination. In Clinical avian medicine (eds Harrison G, Lightfoot T), pp. 153–211. Palm Beach, FL: Spix Publishing. [Google Scholar]
- 42.Schamber JL, Esler D, Flint PL, Schamber JL, Esler D, Flint PL. 2009. Evaluating the validity of using unverified indices of body condition. J. Avian Biol. 40, 49–56. ( 10.1111/j.1600-048X.2008.04462.x) [DOI] [Google Scholar]
- 43.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 44.Fridolfsson A-K, Ellegren H. 1999. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30, 116–121. [Google Scholar]
- 45.Herring G, Eagles-Smith CA, Gawlik DE, Beerens JM, Ackerman JT. 2014. Physiological condition of juvenile wading birds in relation to multiple landscape stressors in the Florida everglades: effects of hydrology, prey availability, and mercury bioaccumulation. PLoS ONE 9, e106447 ( 10.1371/journal.pone.0106447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casper RM. 2009. Guidelines for the instrumentation of wild birds and mammals. Anim. Behav. 78, 1477–1483. ( 10.1016/j.anbehav.2009.09.023) [DOI] [Google Scholar]
- 47.Bailey H, Thompson P. 2006. Quantitative analysis of bottlenose dolphin movement patterns and their relationship with foraging. J. Anim. Ecol. 75, 456–465. ( 10.1111/j.1365-2656.2006.01066.x) [DOI] [PubMed] [Google Scholar]
- 48.Hobson KA, Clark RG. 1993. Turnover of 13 C in cellular and plasma fractions of blood: implications for nondestructive sampling in avian dietary studies. Auk 110, 638–641. ( 10.2307/4088430) [DOI] [Google Scholar]
- 49.Sargeant BL, Gaiser EE, Trexler JC. 2010. Biotic and abiotic determinants of intermediate-consumer trophic diversity in the Florida everglades. Mar. Freshw. Res. 61, 11–22. ( 10.1071/MF08322) [DOI] [Google Scholar]
- 50.Parnell AC, Inger R, Bearhop S, Jackson AL. 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5, e9672 ( 10.1371/journal.pone.0009672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hobson KA, Clark RG. 1992. Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor 94, 189–197. ( 10.2307/1368808) [DOI] [Google Scholar]
- 52.Barton K.2015. MuMIn: multi-model inference. R package version 1.13.4.
- 53.Jackson AL, Inger R, Parnell AC, Bearhop S. 2011. Comparing isotopic niche widths among and within communities: SIBER - Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602. ( 10.1111/j.1365-2656.2011.01806.x) [DOI] [PubMed] [Google Scholar]
- 54.Martin J, French K, Major R. 2012. Behavioural adaptation of a bird from transient wetland specialist to an urban resident. PLoS ONE 7, e50006 ( 10.1371/journal.pone.0050006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciach M, Kruszyk R. 2010. Foraging of white storks Ciconia ciconia on rubbish dumps on non-breeding grounds. Waterbirds 33, 101–104. ( 10.1675/063.033.0112) [DOI] [Google Scholar]
- 56.Páez DJ, Restif O, Eby P, Plowright RK. 2018. Optimal foraging in seasonal environments: implications for residency of Australian flying foxes in food-subsidized urban landscapes. Phil. Trans. R. Soc. B 373, 20170097 ( 10.1098/rstb.2017.0097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin JM, French K, Major RE. 2007. The pest status of Australian white ibis (Threskiornis molucca) in urban situations and the effectiveness of egg-oil in reproductive control. Wildl. Res. 34, 319–324. ( 10.1071/WR07005) [DOI] [Google Scholar]
- 58.Fedriani JM, Fuller TK, Sauvajot RM. 2001. Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography 24, 325–331. ( 10.1111/j.1600-0587.2001.tb00205.x) [DOI] [Google Scholar]
- 59.Chan-McLeod ACA, White RG, Holleman DF. 1994. Effects of protein and energy intake, body condition, and season on nutrient partitioning and milk production in caribou and reindeer. Can. J. Zool. 72, 938–947. ( 10.1139/z94-127) [DOI] [Google Scholar]
- 60.Newsome SD, Ralls K, Job CVH, Fogel ML, Cypher BL. 2010. Stable isotopes evaluate exploitation of anthropogenic foods by the endangered San Joaquin kit fox (Vulpes macrotis mutica). J. Mammal. 91, 1313–1321. ( 10.1644/09-MAMM-A-362.1) [DOI] [Google Scholar]
- 61.Van Hemert C, Handel C, O'Brien DM. 2012. Stable isotopes identify dietary changes associated with beak deformities in black-capped chickadees (Poecile atricapillus). Auk 129, 460–466. ( 10.1525/auk.2012.12037) [DOI] [Google Scholar]
- 62.Weiser EL, Powell AN. 2011. Evaluating gull diets: a comparison of conventional methods and stable isotope analysis. J. Field. Ornithol. 82, 297–310. ( 10.1111/j.1557-9263.2011.00333.x) [DOI] [Google Scholar]
- 63.Clayton DH, Koop JAH, Harbison CW, Moyer BR, Bush SE. 2010. How birds combat ectoparasites. Open Ornithol. J. 3, 41–71. ( 10.2174/1874453201003010041) [DOI] [Google Scholar]
- 64.Clayton DH, Moyer BR, Bush SE, Jones TG, Gardiner DW, Rhodes BB, Goller F. 2005. Adaptive significance of avian beak morphology for ectoparasite control. Proc. R. Soc. B 272, 811–817. ( 10.1098/rspb.2004.3036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clayton DH, Tompkins DM. 1994. Ectoparasite virulence is linked to mode of transmission. Proc. R. Soc. Lond. B 256, 211–217. ( 10.1098/rspb.1994.0072) [DOI] [PubMed] [Google Scholar]
- 66.Brown CR, Brown MB, Rannala B. 1995. Ectoparasites reduce long-term survival of their avian host. Proc. R. Soc. Lond. B 262, 313–319. ( 10.1098/rspb.1995.0211) [DOI] [Google Scholar]
- 67.Sorensen A, van Beest FM, Brook RK. 2014. Impacts of wildlife baiting and supplemental feeding on infectious disease transmission risk: a synthesis of knowledge. Prev. Vet. Med. 113, 356–363. ( 10.1016/j.prevetmed.2013.11.010) [DOI] [PubMed] [Google Scholar]
- 68.O'Leary R, Jones DN. 2006. The use of supplementary foods by Australian magpies Gymnorhina tibicen: implications for wildlife feeding in suburban environments. Austral. Ecol. 31, 208–216. ( 10.1111/j.1442-9993.2006.01583.x) [DOI] [Google Scholar]
- 69.Epstein JH, Mckee J, Shaw P, Hicks V, Micalizzi G, Daszak P, Kilpatrick M, Kaufman G. 2007. The Australian White Ibis (Threskiornis molucca) as a reservoir of zoonotic and livestock pathogens. Ecohealth 3, 290–298. ( 10.1007/s10393-006-0064-2) [DOI] [Google Scholar]
- 70.Bildstein KL. 1993. White ibis: wetland wanderer. Washington, DC: Smithsonian Institute Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All associated data on ibis condition, diet and capture sites are available as electronic supplementary material. The transmitter data are part of a larger study but are available upon request from the author.