Abstract
Background
To investigative the association of triglycerides (TG) and total cholesterol (TC) concentrations with impaired fasting glucose/ impaired glucose tolerance (IFG/IGT) in Chinese adults.
Methods
The population-based cross-sectional diabetes survey was conducted in 2006 and 2009 in Qingdao, separately. 4400 participants (1 793 men and 2607 women) were include in current analysis. IFG/IGT was defined according to fasting plasma glucose (FPG) and/or 2 h post-load plasma glucose (2 h PG). Logistic regression models and areas under receiver operating characteristic curves (AUROC) were performed to estimate the associations between TG, TC levels and IFG/IGT.
Results
Spearman analysis showed that serum TG and TC was independently and positively associated with FPG and 2 h PG. As compared with normoglycaemia, the odds ratio[(95% confidence intervals), OR(95%CI)] for IFG/IGT corresponding to hypertriglyceridemia (HTG) were 1.61 (1. 17, 2. 22) in men and 1.57(1.15, 2.14) in women for TG and accompany with Hypercholesterolemia (HTC) 1.56 (1.15, 2.13) and 1. 20 (0.93, 1.54) for TC, when adjusting for confounding factor. The AUROCs of TG, TC for IFG/IGT were relatively smaller (0.50 < AUROC< 0. 7) in both gender. The optimal cut-offs for TG and TC was 1.61, 4.91 in men and 1. 24, 5. 32 in women, respectively.
Conclusions
Evaluated TG in both gender and TC in men were independently associated with the present of the IFG/IGT, yet, could not be an authentic predictors of IFG/IGT in both men and women in current Chinese population.
Keywords: Triglycerides, Total cholesterol, Adult onset IFG/IGT
Background
Pre-diabetes refers to a condition in elevating plasm glucose, and that is consider normal levels but is not high enough to be classified as diabetes. Pre-diabetes has been defined by impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) according to the 2006 World Health Organization (WHO)/International Diabetes Federation (IDF) criteria [1]. Individuals with IFG/IGT were at about 5–10% high risk of developing diabetes worldwide [2, 3]. In China, the prevalence of IFG/IGT is surprisingly high and continues to increase. In a cross-sectional study reported in 2007–2008 involving a nationally representative sample of 46,239 adults, 20 years of age or older, the prevalence of IFG/IGT15.5% [4], and which is far higher than previously reported figures [5]. In additional, with the high epidemiological status of IFG/IGT, they also have a high risk of dyslipidemia [6].
Dyslipidemia can be defined by abnormal level of triglycerides (TG) and total cholesterol (TC). However, the association of TG, TC and IFG/IGT is still not clear. Existing researches demonstrated that hyperglycaemiawas in concert with the potential risk factors of cardiovascular diseases [7–9], for instance elevated TG [10] and TC [11]. Previously cohort study indicated that elevated TG was a high risk for diabetes in adults [12, 13]. Akehi et al. [14] also found the strong association between TC and the plasma glucose levels at 60 min. Chakarova et al. [15] examined associations of serum lipid with IFG/IGT in 1240 adults in the population-based cross-sectional study, the results showed that IFG presents with significant differences in the levels of TG and TC are significantly higher compared to the group with normoglycaemia, while TG of IGT group are increased as compared to normoglycaemia, and the differences in TC between IGT and normoglycaemia are not statistically significant.
There are some studies about the association of TG, TC with pathoglycemia in China. Previously two cross-sectional studies [16, 17] examined that elevated TG and TC are risk factors for diabetes in Chinese adults. Multivariate logisticregression analyses of a cross-sectional study involving 4 583Chinese adults reportedhigher TG was therisk factors for developing pre-diabetes [18]. However, so we have known, the association between TG, TC and IFG/IGT is still not fully elucidated at present. Therefore, we designed this study to assess the association between TG, TC and IFG/IGT in general population based on a large population-based cross-sectional survey in Qingdao, China.
Methods
Study population
The current study utilized data form Qingdao Diabetes Prevention Program (QDDPP). The general individuals aged 35–74 years who had lived in Qingdao city for at least 5 years were valid in 2006 and 2009, respectively.This study applied strict exclusion criteria: 1) previously diagnosed diabetes; 2) fasting plasma glucose (FPG) and 2 h post-load plasma glucose (2 h PG) based newly diagnosed diabetes; 3) missing for glycated haemoglobin (HbA1c), TG, TC, waist circumference (WC), body mass index (BMI).
Setting
This study was conducted in three rural districts (Jiaonan, Jimo and Huangdao) and three urban districts (Sifang, Shibei, and Shinan) and in 2006 and 2009. All collected data from six districts were combined to QDDPP, andinputted by uniform staff in the study.
Sampling methods and size
The QDDPP survey included both stratified random samples. The data included 10 465 individuals (5355 in 2006 and 5110 in 2009) of 12,100 citizens (6100 in 2006 and 6000 in 2009) with a response rate of 87. 8% in 2006 and 85.2% in 2009, respectively (Fig. 1). Following the exclusion criteria, 4400 participants (1793 men and 2607women) were included in this current analysis.
Fig. 1.
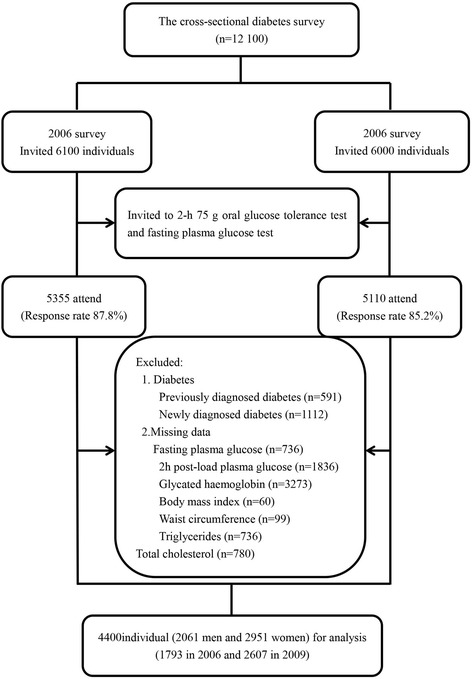
Flow chart of the Participants included in the cross sectional analysis
Ethical considerations
All the participants voluntarily signed the informed consent before their participation, and the security, anonymity and privacy of participants was respected rigorously in this study. The ethics committee of the Qingdao Municipal Center for Disease Control and Prevention formally reviewed and approved our study in 2008 and 2014, and the ethical approval number was Document No. 1 and Document No. 7, respectively.
Data collection
The standardized questionnaire was used in face-to-face interview by trained doctors and nurses. Age (years), gender (men/women), marital status, smoking status, alcohol-drinking status, educational attainment, personal monthly income, family history of diabetes were collected through questionnaire. The criteria of marital status were recorded into unmarried or married. The criteria for smoking habit and alcohol-drinking habit were the following: 1) non-smokers or current smokers, 2) non-drinkers or current drinkers. The criteria for educational attainment were defined into school years ≤9y or > 9y. The criteria of personal socio-economic clusters were categorized into personal monthly income≤599 Chinese Yuan (CNY), 600–1999 CNY, or ≥ 2000 CNY. Family history of diabetes was defined; at least one family member diagnosed with diabetes (including parents, sibling and/or off-springs, etc). Height and weight of the participants were measured in only light clothes and barefoot by trained doctors and nurses, and BMI was computed as weight (kilograms) dividing by height squared m2). WC was measured to the nearest 0. 1 cm at the narrowest point between the rib cage and the iliac crest. Blood pressure of participants was measured on the upper right arm in siting position.
After an overnight fast of at least 10 hours, blood samples were collected from the antecubital vein into a vacuum tube containing sodium fluoride, and transported in a dark box with ice to the laboratory and stored at − 80 °C freezer within no more than 6 hours. A standard 2-h 75 g oral glucose tolerance tests (OGTT) was performed in all subjectswith no history of diabetes. All detection index of blood samples were analyzed in the central laboratory of Qingdao Endocrinology and Diabetes Hospital: 1) FPG; 2) 2 h PG; 3) fasting serum TG; 4) fasting serum TC; 5) Serum alanine aminotransferase (ALT) 6) serum gamma-glutamyltransferase (GGT)
Variable definitions
Categories of glucose tolerance were defined according to the 2006 World Health Organization (WHO)/International Diabetes Federation (IDF) criteria [1]. Newly diagnosed diabetes was defined as FPG ≥ 7.0 mmol/l and/or 2 h PG ≥ 11. 1 mmol/l.IFG/IGT was defined as IFG (FPG 6. 1–6. 9 mmol/l), and/or IGT (2 h PG 7. 8–11.0 mmol/l). Normoglycaemia was defined as FPG < 6. 1 mmol/l and 2 h PG < 7. 8 mmol/l. Participants with previously diagnosed diabetes and/or newly diagnosed diabetes were excluded from the current analysis.
TG and TC were categorized according to the most recent guideline from Chinese Heart Association [19]. Participants were divided into three TG categorical groups as follows: normal TG (TG < 150 mg/dl), borderline high TG (150 ≤ TG < 200 mg/dl), hypertriglyceridemia (HTG) (TG ≥ 200 mg/dl). Participants were also subdivided as follows: normal TC (TC < 200 mg/dl), borderline high TC (200 ≤ TC < 240 mg/dl), and hypercholesterolemia (HTC) (TC ≥ 240 mg/dl).
Statistical analysis
Continuous variables and categorical variables are presented as means [95% confidence intervals (95% CI)] and number (percentage).The general linear model for continuous variable and the Chi-squared test for categorical variable were used to compare the difference between different glucose categories. Correlations between TG, TC and plasma glucose were assessed by spearman correlation coefficient. Logistic regression analysis was used to estimate the odd ratio (OR) and 95% CI for the association of serum TG, TC with IFG/IGT in men and women. Area under receiver operating characteristic (AUROC) was investigate the association of TG, TC and IFG/IGT. According to AUROC, the diagnostic values of TG, TC were assessed: an AUROC of ≤0.5 was considered a chance result; 0.5–0. 7, low accuracy; 0. 7–0. 9, moderate accuracy;≥0. 9, high accuracy. The optimal cut-offs for TG and TC was selected based on the sensitivity and specificity. Statistical analyses were performed using IBM SPSS Statistics 18.0. A P value less than 0.05 was considered to be statistically significant.
Results
In this study, 35.4% (634/1793) men and 35.0% (912/2607) had IFG/IGT. The baseline characteristics of the study population were presented in Table 1. Compared with normal glycaemia, those individuals with IFG/IGT were older, more obese, and have higher levels of systolic blood pressure (SBP), ALT, GGT, TG and TC. Family history of diabetes, unban living and higher income level in men and less school years, lower income level and higher level diastolic blood pressure (DBP) in women with pre-diabetes was more common than in those with normoglycaemia.
Table 1.
Baseline characteristics of participants according to glucose (N = 4400)
| Men | Women | |||
|---|---|---|---|---|
| Normoglycaemia | IFG/IGT | Normoglycaemia | IFG/IGT | |
| Number (%) | 1159 (64.6) | 634 (35.4)ǂ | 1695 (65.0) | 912 (35.0)ǂ |
| Age (years) | 49. 1 (48.4, 49.7) | 52.5 (51.7, 53.4)ǂ | 48. 1 (47.6, 48.5) | 52.7 (52.0, 53.3)ǂ |
| Urban living | 353 (30.5) | 234 (36.9)* | 766 (45.2) | 405 (44.4) |
| Married, n (%) | 1104 (96.8) | 602 (95.6) | 1609 (95.7) | 839 (93.6)* |
| School years > 9 (%) | 427 (37.0) | 246 (38.9) | 629 (37.2) | 207 (22.8)ǂ |
| Current Smoking (yes, %) | 611 (52.9) | 313 (49.5) | 19 (1.1) | 11 (1.2) |
| Alcohol-drinking (yes, %) | 503 (43.5) | 285 (45.1) | 27 (1.6) | 13 (1.4) |
| Family history of diabetes (yes, %) | 144 (13.3) | 101 (16.9)* | 301 (18.7) | 172 (20.0) |
| Income (CNY/month), n (%) | ||||
| ≤ 599 | 413 (36.8) | 182 (29.4)* | 720 (44.2) | 468 (53.2)ǂ |
| 600–1999 | 527 (47.0) | 320 (51.8) | 803 (49.3) | 376 (42.7) |
| ≥ 2000 | 181 (16.1) | 116 (18.8) | 105 (6.4) | 36 (4.1) |
| Body mass index (kg/m2) | 24.9 (24.7, 25.0) | 25.7 (25.5, 26.0)ǂ | 25.0 (24.8, 25.1) | 26.1 (25.8, 26.3)ǂ |
| Waist circumference (cm) | 85.2 (84.7, 85.8) | 88.1 (87.3, 88.9)ǂ | 81.1 (80.7, 81.5) | 83.0 (82.4, 83.6)ǂ |
| Systolic blood pressure (mmHg) | 131.8 (130.8, 132.9) | 134.9 (133.5, 136.3)* | 127.5 (126.6, 128.4) | 135.3 (134.0, 136.6)ǂ |
| Diastolic blood pressure (mmHg) | 85.7 (85.1, 86.4) | 86.9 (85.9, 87.8) | 81.9 (81.3, 82.4) | 85.1 (84.4, 85.9)ǂ |
| Fasting plasma glucose (mmol/L) | 5.20 (5.16, 5.24) | 5.98 (5.93, 6.02)ǂ | 5.24 (5.21, 5.26) | 5.85 (5.81, 5.89)ǂ |
| 2 h post-load plasma glucose (mmol/L) | 5.69 (5.61, 5.77) | 7.67 (7.56, 7.78)ǂ | 6.02 (5.96, 6.08) | 8.02 (7.95, 8.10)ǂ |
| Glycated haemoglobin (%) | 4.34 (4.29, 4.39) | 4.45 (4.39, 4.52)* | 4.33 (4.29, 4.38) | 4.39 (4.33, 4.45) |
| Alanine amino transferase (U/L) | 15.6 (14.9, 16.4) | 19.6 (18.5, 20.6)ǂ | 13.6 (13.1, 14.2) | 15.4 (14.7, 16.3)ǂ |
| Gamma-glutamyl transferase (U/L) | 32.9 (30.0, 35.7) | 43.3 (39.4, 47.2)ǂ | 17.5 (16.6, 18.3) | 20.1 (19.0, 21.3)ǂ |
| Triglycerides (mmol/L) | 1.31 (1.1, 26.37) | 1.59 (1.52, 1.67)ǂ | 1. 22 (1.1, 18.26) | 1.41 (1.36, 1.46)ǂ |
| Normal TG | 924 (79.7) | 441 (69.6)ǂ | 1421 (83.8) | 649 (71.2)ǂ |
| Borderline high TG | 123 (10.6) | 86 (13.6) | 169 (10.0) | 157 (17.2) |
| Hypertriglyceridemia | 112 (9.7) | 107 (16.9) | 105 (6.2) | 106 (11.6) |
| Total cholesterol (mmol/L) | 5.14 (5.08, 5.19) | 5.37 (5.29, 5.45)ǂ | 5.22 (5.18, 5.27) | 5.33 (5.27, 5.39)* |
| Normal TC | 647 (55.8) | 278 (43.8)ǂ | 906 (53.5) | 382 (41.9)ǂ |
| Borderline high TC | 373 (32.2) | 246 (38.8) | 539 (31.8) | 336 (36.8) |
| Hypercholesterolemia | 139 (12.0) | 110 (17.4) | 250 (14.7) | 194 (17.0) |
Data are age-adjusted mean (95% confidence interval) or n (%) as indicated. *P < 0.05, ǂ< 0.001, normoglycaemia versus pre-diabetes within the same gender
In both gender, the level of serum TG was independently and positively associated with FPG and 2 h PG (P < 0.05), and the similar association was between serum TC and FPG, 2 h PG (P < 0.05). The spearman association was shown in Fig. 2.
Fig. 2.
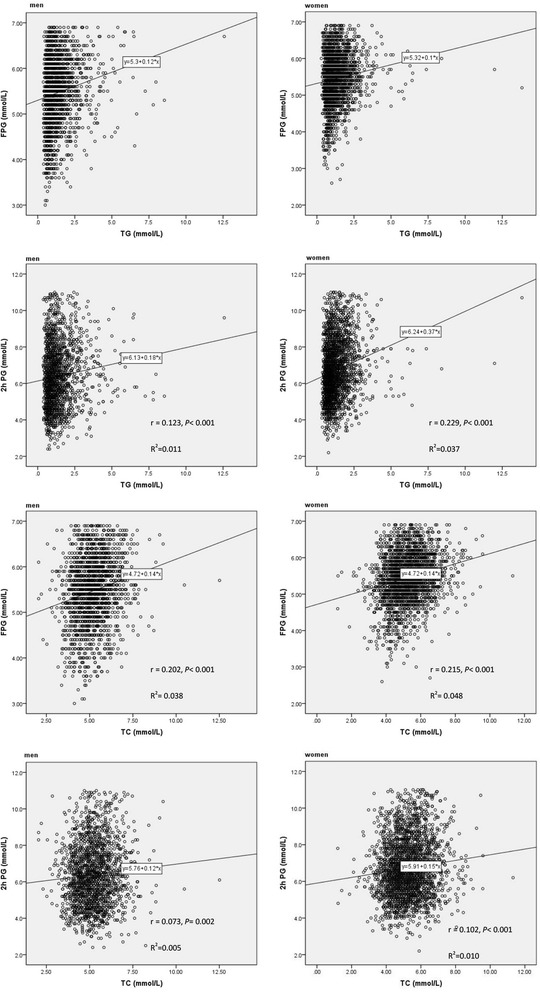
Spearman correlation between triglycerides, total cholesterol and plasma glucose
Table 2 showed OR for IFG/IGT in association to triglycerides and total cholesterol concentration by univariate logistic regression. In both gender, age, BMI, WC, SBP, DBP, ALT and GGT were significantly associated with a higher risk for IFG/IGT (P < 0.05). Unban living and higher income level were significantly associated with a higher risk for IFG/IGT in men(P < 0.05), while married, more school years and higher income level were significantly associated with a lower risk for IFG/IGT in men(P < 0.05). Borderline high TG, HTG and borderline high TC, HTC had a higher OR for IFG/IGT in both men and women (P < 0.05).
Table 2.
Odds ratio (95% confidence interval) for IFG/IGT in association to triglycerides and total cholesterol concentration by univariate logistic regression
| Men | Women | |||
|---|---|---|---|---|
| OR | P value | OR | P value | |
| Age (years) | 1.03 (1.02, 1.04) | <0.001 | 1.05 (1.04, 1.06) | <0.001 |
| Urban living | 1.34 (1.09, 1.64) | 0.005 | 0.97 (0.82, 1.14) | 0.701 |
| Married, n (%) | 0.70 (0.42, 1.16) | 0.167 | 0.67 (0.47, 0.95) | 0.026 |
| School years >9 (%) | 1.09 (0.89, 1.33) | 0.423 | 0.50 (0.41, 0.60) | <0.001 |
| Current Smoking (yes, %) | 0.87 (0.72, 1.06) | 0.172 | 1.08 (0.51, 2.27) | 0.847 |
| Alcohol-drinking (yes, %) | 1.07 (0.88, 1.30) | 0.519 | 0.89 (0.46, 1.74) | 0.742 |
| Family history of diabetes (yes, %) | 1.32 (1.00, 1.74) | 0.048 | 1.08 (0.88, 1.33) | 0.457 |
| Income (CNY/month), n (%) | 0.007 | <0.001 | ||
| ≤599 | 1.00 | 1.00 | ||
| 600–1999 | 1.38 (1.10, 1.72) | 0.005 | 0.72 (0.61, 0.85) | <0.001 |
| ≥2000 | 1.45 (1.09, 1.95) | 0.012 | 0.53 (0.36, 0.78) | 0.002 |
| Body mass index (kg/m2) | 1.07 (1.04, 1.10) | <0.001 | 1.11 (1.08, 1.13) | <0.001 |
| Waist circumference (cm) | 1.03 (1.02, 1.04) | <0.001 | 1.04 (1.03, 1.05) | <0.001 |
| Systolic blood pressure (mmHg) | 1.01 (1.01, 1.02) | <0.001 | 1.03 (1.02, 1.03) | <0.001 |
| Diastolic blood pressure (mmHg) | 1.01 (1.00, 1.02) | 0.041 | 1.03 (1.02, 1.04) | <0.001 |
| Alanine amino transferase (U/L) | 1.02 (1.01, 1.03) | <0.001 | 1.02 (1.01, 1.03) | <0.001 |
| Gamma-glutamyltransferase (U/L) | 1.00 (1.00, 1.01) | 0.001 | 1.02 (1.01, 1.02) | <0.001 |
| Triglycerides (mmol/L) | <0.001 | <0.001 | ||
| Normal TG | 1.00 | 1.00 | ||
| Borderline high TG | 1.47 (1.09, 1.97) | 0.012 | 2.03 (1.61, 2.58) | <0.001 |
| Hypertriglyceridemia | 2.00 (1.50, 2.67) | <0.001 | 2.21 (1.66, 2.94) | <0.001 |
| Total cholesterol (mmol/L) | <0.001 | <0.001 | ||
| Normal TC | 1.00 | |||
| Borderline high TC | 1.54 (1.24, 1.90) | <0.001 | 1.48 (1.23, 1.77) | <0.001 |
| Hypercholesterolemia | 1.84 (1.38, 2.45) | <0.001 | 1.84 (1.47, 2.30) | <0.001 |
Bold type indicated statistically significant values
Table 3 showed the multivariate adjusted OR between TG, TC and IFG/IGT employing backward method. The multivariate adjusted OR of having IFG/IGT was significantly higher in men with HTG,borderline high TC, HTC, and in women with borderline high TG, HTGthan in those with the normal TG, TC. The association between borderline high TG in men, borderline high TC, HTC in women and IFG/IGT was no significant, but increased risk.Concomitantly, age, SBP, ALT were significant positive association with an increased risk of IFG/IGT in both gender in multivariate logistic model. While, a significant positive association wasdiscovered between IFG/IGT and higher income level, WC in men and urban living in women as well as a significant invert association with more school years, middle income level in women. The results of the multivariable logistic analysis were not changed substantively when BMI taken a place of WC was entered into the models.
Table 3.
Odds ratio (95% confidence interval) for IFG/IGT in association to triglycerides and total cholesterol concentration by multivariable logistic regression
| Men | Women | |||
|---|---|---|---|---|
| TG | ||||
| Normal TG | 1.00 | 1.00 | ||
| Borderline high TG | 1. 28(0.93, 1.77) | 1.67(1. 28,2. 16)* | ||
| Hypertriglyceridemia | 1.61(1.17, 2.22)* | 1.57(1.15, 2.14)* | ||
| TC | ||||
| Normal TC | 1.00 | 1.00 | ||
| Borderline high TC | 1. 31(1.04, 1.64)* | 1. 17(0.96, 1.43) | ||
| Hypercholesterolemia | 1.56(1.15, 2.13)* | 1.2(0.93, 1.54) | ||
| Age (years) | 1.04(1.03, 1.05)* | 1.04(1.03, 1.05)* | 1.02(1.01, 1.03)* | 1.02(1.01, 1.03)* |
| Urban living | 1. 29(1.04, 1.59)* | 1. 26(1.02, 1.56)* | ||
| School years > 9 (%) | 0.65(0.52, 0.82)* | 0.66(0.53, 0.83)* | ||
| Income (CNY/month), n (%) | ||||
| ≤599 | 1.00 | 1.00 | 1.00 | 1.00 |
| 600–1999 | 1.52(1.1, 20.94)* | 1.52(1.1, 19.93)* | 0.73(0.60, 0.89)* | 0.73(0.60, 0.89)* |
| ≥2000 | 1.58(1.15, 2.17)* | 1.60 (1.16, 2.2)* | 0.67(0.43, 1.05) | 0.71(0.45, 1. 10) |
| Waist circumference (cm) | 1.02(1.01, 1.03)* | 1.02(1.01, 1.03)* | 1.01(1.00, 1.02) | |
| Systolic blood pressure (mmHg) | 1.01(1.00, 1.01)* | 1.01(1.00, 1.01)* | 1.02(1.01, 1.02)* | 1.02(1.01, 1.02)* |
| Alanine amino transferase (U/L) | 1.02(1.01, 1.03)* | 1.02(1.01, 1.03)* | 1.01(1.00, 1.02)* | 1.01(1.00, 1.02)* |
| Gamma-glutamyl transferase (U/L) | 1.00 (1.00, 1.00) | |||
The logistic regression models were employed backward. All variables of table were listed in the final models.TG category and TC category entered models separately. * P < 0.05 for factors associated with depression
The AUROCs of TG, TC for IFG/IGT were relatively smaller (0.50 < AUROC< 0. 7) in both gender. The optimal cut-offs for TG and TC were 1.61 (sensitivity: 0. 34, specificity: 0.77), 4.91 (sensitivity: 0.70, specificity: 0.43) in men and 1. 24 (sensitivity: 0.52, specificity: 0.67), 5. 32 (sensitivity: 0.53, specificity: 0.59) in women, respectively. The AUROCs of TG, TC for pre-diabetes was presented in Table 4 and Fig. 3.
Table 4.
Area under the receiver operating characteristics curves of TG, TC for IFG/IGT
| AUROC (95%CI) | P value | |
|---|---|---|
| Men | ||
| TG | 0.58 (0.55, 0.60) | < 0.001 |
| TC | 0.59 (0.56, 0.62) | < 0.001 |
| Women | ||
| TG | 0.61 (0.59, 0.63) | < 0.001 |
| TC | 0.57 (0.55, 0.60) | < 0.001 |
Fig. 3.
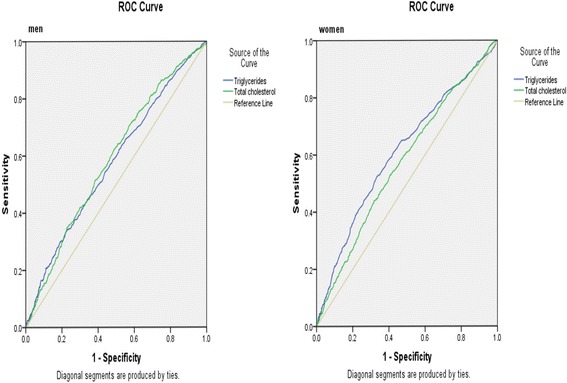
Area under the receiver operating characteristics curves of triglycerides and total cholesterol for IFG/IGT
Discussion
In the current population-based cross-sectional study, spearman analysis revealed that serum TG and TC were independently and positively associated with FPG, 2 h-PG in both men and women. Multivariate logistic analysis showed HTG in both gender,borderline high TG in women, and borderline high TC, HTC in men were positively associated with IFG/IGT independent of other known risk factors. However, AUROC results showed TG and TC as predictor of IFG/IGT was low accuracy.
To the best of our knowledge, serum TG was associated with glucose. Akehi et al. [14] demonstrated that the strong association between TG and plasma glucose levels at 60 min, which hinted TG could be as a predictor for futurediabetes in young males. The cross-sectional and cohort studies of adults all indicated that elevated TG level was a positively associated with diabetic subjects after adjusted for confounding factor [13, 16, 17]. Furthermore, Rasmussen et al. reported that hypertriacylglycerolaemia was significant predictor of progression in IFG/IGT subjects, and decrease of hypertriacylglycerolaemia markedly reduced the risk of diabetes in these high-risk subjects [20]. These findings also agree with our results. In our present multivariable analysis, the associations between TG and IFG/IGT remained constantly considering the confounding factors. Furthermore, previous research demonstrated that evaluated fasting TG could predict future diabetes [21, 22]. However, TG predicting IFG/IGT TG was low accuracy, and the first in Chinese population. The previously follow-up study indicated evaluated TG increase the risk of evaluated glucose [13]. Meanwhile, TG level changes were associated with initiation of changes in lifestyle parameters [23]. The patho-physiological mechanisms of abnormal TC and glucose are intricate and hard to fully understand. TG is responsible for the bidirectional transference of adipose fat and blood glucose from the liver. Previously reported research has confirmed that excessive TG are restructured into adipose fat in liver and stored into hepatocytes [24]. While, superfluous adipose fat can reduce response to insulin signaling [25].
Yet, less research on TC and IFG/IGT has been done at present. Akehi et al. [14] found that TC was significantly associated with plasma glucose levels at 60 min and 120 min in young males. Previously cross-sectional studies [16, 17] revealed that elevated TC increased the risk for diabetes in multivariable logistic models. Meanwhile, the population-based studies [26, 27] reported that TC was a significant association with pre-diabetes after adjusting for multiple covariates. Our present analysiswassimilar but not identical to previous researches. Evaluated TC was significant positive associationin men and insignificant positive association in women with IFG/IGT, yet, couldn’t predict IFG/IGT accurately. Published study has indicated that excess TC may alter the potassium channels of beta-cells, which reduce the activity of glucokinase, and then affect insulin signaling reducing the metabolism of glucose [28].
The present study demonstrated that IFG/IGT was significant positively associated with age, urban living, WC and SBP, which were similar to published studies [18, 29, 30]. Abnormal glucose, which brings on diabetic microangiopathy, is more likely to occur as a consequence of ageing, elevated WC, and urbanization. Microangiopathy causes other series of physiological dysfunction, such as hypertension. Of note, the inverse association between and abnormal glucose had been revealed in previous and present studies [31, 32]. The individual with higher educational attainment are more interested in health. Nevertheless, the effect of personal monthly income on glucose is the controversial issues in present study, and previously studies also showeda mixed opinion on income [33, 34]. Perhaps,men with higher income have greater living and mental pressure and unhealthy lifestyle. However, women with higher income pay more attention to health in China.So, educational initiatives for individuals with abnormal glucose are necessary by present results.
As far as we know, this is the first study to investigate the association between TG, TC level and IFG/IGT in China. In addition, the present study has some strengths. Firstly, this is the relatively large number of subjects included in this population-based study to provide the association between TG, TC and IFG/IGT with high statistical power for data analyses. Secondly, all IFG/IGT subjects were defined according to FPG and/or 2 h PG, which was accepted standard criteria in current. Thirdly, we have fully captured multiple statistical models to investigate the strength of the association between TG, TC level and IFG/IGT in different populations and different types of variables. However, our study suffered from a few potential weaknesses that merit comment. The current study was from two cross-sectional studies, it is enormous challenges to evaluate the association between TG, TC and IFG/IGT. Secondly, only PFC, 2 h PG, TG and TC were performed once in the current study.
Conclusion
Evaluated TG and TC has emphasized significantly positively associated with IFG/IGT. HTG, borderline high TC, HTC in men and borderline high TG, HTG in women had anextremelyadverse impacton IFG/IGT, however, TG and TC could not be anauthenticpredictors of IFG/IGT in this Chinese population. Additionally, further research about the association between TC, TG concentration and hyperglycemia should be conduct large-scale, multi-population-based study to confirm the conclusion in Chinese population.
Acknowledgements
We also thank the participants, primary care doctors, and nurses who participated in this survey.
Funding
This work was supported by grants from Qingdao Diabetes Prevention Program and World Diabetes Foundation [WDF05–108 and WDF07–308]. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The aggregate data supporting findings contained within this manuscript will be shared upon request submitted to the corresponding author. Identifying patient data will not be shared.
Abbreviations
- 2 h PG
2-h plasma glucose
- ALT
Alanine amino transferase
- AUROC
Area under receiver operating characteristic
- BMI
Body mass index
- CI
Confidence intervals
- DBP
Diastolic blood pressure
- FPG
Fasting plasma glucose
- GGT
Gamma-glutamyltransferase
- HbA1c
Glycatedhaemoglobin
- HTC
Hypercholesterolemia
- HTG
Hypertriglyceridemia
- IDF
International Diabetes Federation
- IFCC
International Federation of Clinical Chemistry
- IFG
Impaired fasting glucose
- IGT
Impaired glucose tolerance
- OGTT
2 h 75 g oral glucose tolerance tests
- OR
Odds ratio
- SBP
Systolic blood pressure
- TC
Total Cholesterol
- TG
Triglycerides
- WC
waist circumference
- WHO
World Health Organization
Authors’ contributions
ZB, JC, JS and AM were the primary authors and leading investigators. NY, JC, MK, ZB, WW, HX and QQcarried out the experiments, analyzed experimental results. ZB, JC and JS wrote the manuscript. All authors read and approved the final manuscript.
Ethics and consent to participate
All the participants voluntarily signed the informed consent before their participation and the consent of ethics, including three urban districts (Shinan, Shibei and Sifang) and three rural districts (Huangdao, Jiaonan, Jimo), was obtained from ethics committee in Qingdao Municipal Center for Disease Control and Prevention. This study was approved by the local ethics committee at Qingdao Municipal Health and Family planning commission.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jing Cui, Email: Cuijing_0623@163.com.
Jianping Sun, Email: qdcdcsjp@126.com.
Wei Wang, Email: 8657536523@qq.com.
Nafeesa Yasmeen, Email: zeshanfeed@gmail.com.
Ma Ke, Email: 871667400@qq.com.
Hualei Xin, Email: Xinhualei1987@126.com.
Qing Qiao, Email: 116661036@qq.com.
Aiguo Ma, Phone: +86053282991518, Email: magfood@126.com.
Zulqarnain Baloch, Phone: +8618344564625, Email: znablooch@yahoo.com.
References
- 1.WHO / IDF . Consultation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO /international diabetes federation consultation. Geneva: The World Health Organization Document Production Services; 2006. [Google Scholar]
- 2.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 3.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE. Diabetes prevention program research group. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the diabetes prevention program outcomes study. Lancet. 2012;379:2243–2251. doi: 10.1016/S0140-6736(12)60525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J, China National Diabetes and Metabolic Disorders Study Group Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 5.Gu D, Reynolds K, Duan X, Xin X, Chen J, Wu X, Mo J, Whelton PK, He J, InterASIA Collaborative Group Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International collaborative study of cardiovascular disease in Asia (InterASIA) Diabetologia. 2003;46:1190–1198. doi: 10.1007/s00125-003-1167-8. [DOI] [PubMed] [Google Scholar]
- 6.Meigs JB, Wilson PW, Nathan DM, D'Agostino RB, Williams K, Haffner SM. Prevalence and Characteristics of the metabolic syndrome in the San Antonio heart and Framingham offspring studies. Diabetes. 2003;52(8):2160–2167. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 7.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 8.Christian JB, Bourgeois N, Snipes R, Lowe KA. Prevalence of severe (500 to 2000mg/dl) hypertriglyceridemia in United States adults. Am J Cardiol. 2011;107:891–897. doi: 10.1016/j.amjcard.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Nagasawa S-y, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, Murakami Y, Miura K, Ueshima H. For the evidence for cardiovascular prevention from observational cohorts in Japan (EPOCH-JAPAN) research group. Relation between serum Total cholesterol level and cardiovascular disease stratified by sex and age group: a pooled analysis of 65 594 individuals from 10 cohort studies in Japan. J Am Heart Assoc. 2012;1(5):e001974. doi: 10.1161/JAHA.112.001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kompoti M, Mariolis A, Alevizos A, Kyrazis I, Protopsaltis I, Dimou E, Lentzas I, Levisianou D, Gova A, Melidonis A. Elevated serum triglycerides is the strongest single indicator for the presence of metabolic syndrome in patients with type 2 diabetes. Cardiovasc Diabetol. 2006;5:21. doi: 10.1186/1475-2840-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee EJ, Han K, Ko SH, Ko KS, Lee WY. Increased risk for diabetes development in subjects with large variation in total cholesterol levels in 2,827,950 Koreans: a nationwide population-based study. PLoS One. 2017;12(5):e0176615. doi: 10.1371/journal.pone.0176615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mykkänen L, Kuusisto J, Pyörälä K, Laakso M. Cardiovascular disease risk factors as predictors of type 2 (non-insulin-dependent) diabetes mellitus in elderly subjects. Diabetologia. 1993;36:553–559. doi: 10.1007/BF02743273. [DOI] [PubMed] [Google Scholar]
- 13.Tirosh A, Shai I, Bitzur R, Kochba I, Tekes-Manova D, Israeli E, Shochat T, Rudich A. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care. 2008;31:2032–2037. doi: 10.2337/dc08-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akehi Y, Tsutsumi Y, Tatsumoto A, Yoshida R, Ohkubo K, Takenoshita H, Kudo T, Ashida K, Anzai K, Yamashita T, Kawashima H. Serum γ-glutamyltransferase, triglyceride and total cholesterol are possible prediabetic risk markers in young Japanese men. Endocr J. 2010;57(11):981–989. doi: 10.1507/endocrj.K10E-174. [DOI] [PubMed] [Google Scholar]
- 15.Chakarova N, Tankova T, Atanassova I, Dakovska L. Serum lipid and hsCRP levels in prediabetes--impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) Diabetes Res ClinPract. 2009;86(1):56–60. doi: 10.1016/j.diabres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Cui J, Sun J, Wang W, Xin H, Qiao Q, Baloch Z, Aiguo MA. The association of triglycerides and total cholesterol concentrationswith newly diagnosed diabetes in adults in China. Oncotarget. 2017;8(61):103477–103485. doi: 10.18632/oncotarget.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J, Zha X, Li H, Guo R, Zhu Y, Wen Y. Analysis of blood glucose distribution characteristics and its risk factors among a health examination population in Wuhu (China) Int J Environ Res Public Health. 2016;13(4):392. doi: 10.3390/ijerph13040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M, Lin H, Yuan Y, Wang F, Xi Y, Wen LM, Shen P, Bu S. Prevalence of pre-diabetes and its associated risk factors in rural areas of Ningbo, China. Int J Environ Res Public Health. 2016;13(8):E808. doi: 10.3390/ijerph13080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joint Committee for Developing Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults. Guidelines on prevention and treatment of blood lipid abnormality in Chinese adults (Revised in 2016). Chinese Circulation Journal. 2016(31):937–53.
- 20.Rasmussen SS, Glumer C, Sandbaek A, Lauritzen T, Borch-Johnsen K. Determinants of progression from impaired fasting glucose and impaired glucose tolerance to diabetes in a high-risk screened population: 3-year follow-up in the ADDITION study, Denmark. Diabetologia. 2008;51:249–257. doi: 10.1007/s00125-007-0893-8. [DOI] [PubMed] [Google Scholar]
- 21.Dotevall A, Johansson S, Wilhelmsen L, Rosengren A. Increased levels of triglycerides, BMI and blood pressure and low physical activity increase the risk of diabetes in Swedish women. A prospective 18-year follow-up of the BEDA study. Diabet Med. 2004;21(6):615–622. doi: 10.1111/j.1464-5491.2004.01189.x. [DOI] [PubMed] [Google Scholar]
- 22.Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ. 1995;310(6979):560–564. doi: 10.1136/bmj.310.6979.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tirosh A, Rudich A, Shochat T, Tekes-Manova D, Israeli E, Henkin Y, Kochba I, Shai I. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med. 2007;147(6):377–385. doi: 10.7326/0003-4819-147-6-200709180-00007. [DOI] [PubMed] [Google Scholar]
- 24.Adiels M, Taskinen MR, Boren J. Fatty liver, insulin resistance, and dyslipidemia. Curr Diabetes Rep. 2008;8:60–64. doi: 10.1007/s11892-008-0011-4. [DOI] [PubMed] [Google Scholar]
- 25.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 26.Giannini S, Bardini G, Dicembrini I, Monami M, Rotella CM, Mannucci E. Lipid levels in obese and nonobese subjects as predictors of fasting and postload glucose metabolism. J Clin Lipidol. 2012;6:132–138. doi: 10.1016/j.jacl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Meikle PJ, Wong G, Barlow CK, Weir JM, Greeve MA, MacIntosh GL, Almasy L, Comuzzie AG, Mahaney MC, Kowalczyk A. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS One. 2013;8(9):e74341. doi: 10.1371/journal.pone.0074341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardini G, Rotella CM, Giannini S. Dyslipidemia and diabetes: reciprocal impact of impaired lipid metabolism and Beta-cell dysfunction on micro- and macrovascular complications. Rev Diabet Stud. 2012;9(2–3):82–93. doi: 10.1900/RDS.2012.9.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin Y, Han W, Wang Y, Zhang Y, Wu S, Zhang H, Jiang L, Wang R, Zhang P, Yu Y, Li B. Identification of risk factors affecting impaired fasting glucose and diabetes in adult patients from Northeast China. Int J Environ Res Public Health. 2015;12(10):12662–12678. doi: 10.3390/ijerph121012662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chhetri JK, Zheng Z, Xu X, Ma C, Chan P. The prevalence and incidence of frailty in pre-diabetic and diabetic community-dwelling older population: results from Beijing longitudinal study of aging II (BLSA-II) BMC Geriatr. 2017;17(1):47. doi: 10.1186/s12877-017-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiting D, Unwin N, Roglic G. Diabetes: equity and social determinants. In: Blas E, Kurup A, editors. Equity, social determinants and public health programmes. Geneva: World health Organization; 2010. p. 77–94. ISBN: 9789241563970.
- 32.Linetzky B, De Maio F, Ferrante D, Konfino J, Boissonnet C. Sex-stratified socio-economic gradients in physical inactivity, obesity, and diabetes: evidence of short-term changes in Argentina. Int J Public Health. 2013;58(2):277–284. doi: 10.1007/s00038-012-0371-z. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham J, O'Dea K, Dunbar T, Weeramanthri T, Shaw J, Zimmet P. Socioeconomic status and diabetes among urban indigenous Australians aged 15-64 years in the DRUID study. Ethn Health. 2008;13(1):23–37. doi: 10.1080/13557850701803130. [DOI] [PubMed] [Google Scholar]
- 34.Walatara KN, Athiththan LV, Hettiaratchi UK, Perera PR. Effect of demographic status and lifestyle habits on Glycaemic levels in apparently healthy subjects: a cross-sectional study. J Diabetes Res. 2016;2016:5240503. doi: 10.1155/2016/5240503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The aggregate data supporting findings contained within this manuscript will be shared upon request submitted to the corresponding author. Identifying patient data will not be shared.


