Abstract
Background
Management of patients with a suspected ACS and LBBB is a challenge to the clinician.
Aim
To detect the ability of IMA to exclude myocardial ischemia in suspected patients with ACS and LBBB.
Material and methods
A total of 68 patients with suspected ACS and LBBB (group I) and another twenty patients age and sex matched known to have LBBB with normal coronary angiography (group II) were included in this study and subjected to: routine laboratory tests, 12 lead ECG, echocardiography, and measurement of serum troponin I (TnI) and IMA (measured by ELISA). Diagnostic coronary angiography was performed on all patients and scored by severity and modified Gensini scores.
Results
IMA and TnI levels are significantly increased in group I compared to group II (P value <0.001). IMA with a cutoff value >95 could predict significant CAD (lesions >50%) with AUC of 0.923, sensitivity of 88%, specificity of 83.33%, PPV of 93.6%, NPV of 71.4% and accuracy 86.76%. Moreover, by using both simple and multiple logistic regression analyses IMA could also independently detect significant CAD. The combined use of IMA and TnI significantly improved the sensitivity and the negative predictive value to 98% and 90.9% respectively.
Conclusion
There was a distinct advantage of measuring IMA in patients presenting to the emergency department with acute chest pain and LBBB to rule out a final diagnosis of ACS.
Keywords: IMA, LBBB, ACS
1. Introduction
The accurate diagnosis of ACS seems to be very important as about 15–20 million patients per year present to the emergency department (ED) in with acute chest pain or other symptoms suggestive of ACS in Europe and USA.1, 2, 3
Management of patients with a suspected ACS and LBBB is a challenge to the clinician as they may be at higher risk for AMI, congestive heart failure, and death compared with patients without LBBB.4
It seems that the current treatment approach exposes a significant proportion of patients with new or presumed new LBBB to the risks of fibrinolytic therapy or increased rates of false-positive cardiac catheterization laboratory activation without the likelihood of significant benefit as data suggest that a significant proportion of these patients will not have an occluded culprit artery at cardiac catheterization.5 So, other diagnostic modalities are needed to guide selection of appropriate patients with suspected ACS and LBBB for reperfusion therapy.5
IMA is a form of human serum albumin in which the N-terminal amino acids have been modified by ischemia6 as the result of hypoxia, acidosis, free-radical injury and energy-dependent membrane disruption.7
Although most of the biomarkers are negative in acute myocardial ischemia, IMA is highly sensitive and detectable in the reversible early phase of ACS.8
The advantage of IMA assay over high sensitivity cardiac troponin T (hs-cTnT) is that the levels of IMA are positive within minutes of ischemia and remain elevated for up to several hours, allowing detection before the development of myocardial necrosis.6 Hence, a negative IMA result in addition to initial evaluation based on the clinical presentation may help in moving the patients into a low-risk category, thereby providing a major cost saving.9
So, the aim of this study was to detect the ability of IMA to exclude ischemia in patients presented with ACS and LBBB.
2. Patients and methods
This study was performed in the cardiovascular department at Al-Minya University Hospital between September 2014 and March 2016. All patients gave written consent.
This study included 68 patients admitted with suspected ACS and LBBB (group I). Another twenty patients age and sex matched known to have LBBB with normal coronary angiography (done as pre-operative assessment) were taken as a control group (group II).
Exclusion criteria: Recent cerebrovascular stroke, advanced peripheral vascular disease, acute limb ischemia, liver cirrhosis, renal impairment (serum creatinine more than 1.5 mg/dl), hypoalbuminemia (serum albumin level less than 3.5 mg/dl), acute heart failure, and skeletal muscle injury.
All patients were subjected to the following:
History taking, clinical examination, 12 lead ECG recording and transthoracic Echocardiography.
Laboratory Investigations include random blood sugar, renal function tests, liver enzymes (ALT and AST) and serum albumin, serum troponin I (after 6 h from the onset of chest pain) and IMA measured at presentation in samples by a commercially available enzyme linked immunosorbent assay.
Coronary angiography: Diagnostic coronary angiography was performed on all patients via femoral artery with standard Judkin’s technique. Coronary angiograms were interpreted visually and scored by two techniques, as follows:
With the severity score, the number of major vessels with luminal stenosis ⩾70% (lumen diameter reduction) is scored from 0 to 3 (for right, left anterior descending, and circumflex arteries). Left main stenosis ⩾70% was scored as one-vessel disease if there was no lesion ⩾70% in other vessels.10
The modified Gensini score11 in which the most severe stenosis in each of eight coronary segments (LMT, LAD, the main diagonal, the 1st septal perforator, LCX, the main OM/PL, RCA and PDA) was graded from 1 to 4 (1: 1% to 49% lumen diameter reduction, 2: 50% to 74% stenosis, 3: 75% to 99% stenosis, 4: 100% occlusion) to give a total score of between 0 and 32. Each point was multiplied with separate coefficients based on vessel segments.
This score therefore gives a measure of both severity and extent of coronary atherosclerosis.12
Statistical Methods: All data were tabulated, digitized, and fed into a personal computer program of high statistical capabilities (SPSS version 18) for statistical analysis. Parametric data were expressed as means ± standard deviations, while nonparametric data were expressed as percentages. Parametric quantitative data were analyzed using Independent sample test. Nonparametric quantitative data were analyzed by Mann Whitney test. Qualitative data were analyzed by Chi squared test. Parametric correlation was done by Pearson and nonparametric correlation was done by Spearman test. A p-value of <0.05 was chosen as the level of statistical significance.
3. Results
There is no statistically significant difference between the two groups either the demographic data or the clinical parameters (Table 1).
Table 1.
Demographic and clinical data of both study groups.
| Cases | Control | P value | |
|---|---|---|---|
| (n = 68) | (n = 20) | ||
| Age | |||
| Range | (34–75) | (48–70) | 0.356 |
| Mean ± SD | 60.32 ± 7.64 | 58.6 ± 5.91 | |
| Sex | |||
| Male | 42 (61.8%) | 13 (65%) | 0.793 |
| Female | 26 (38.2%) | 7 (35%) | |
| Diabetes mellitus | |||
| No | 39 (57.4%) | 10 (50%) | 0.561 |
| Yes | 29 (42.6%) | 10 (50%) | |
| Hypertension | |||
| No | 21 (30.9%) | 8 (40%) | 0.446 |
| Yes | 47 (69.1%) | 12 (60%) | |
| Smoking | |||
| No | 34 (50%) | 12 (60%) | 0.431 |
| Yes | 34 (50%) | 8 (40%) | |
| Family history | |||
| −Ve | 63 (92.6%) | 20 (100%) | 0.212 |
| +Ve | 5 (7.4%) | 0 (0%) | |
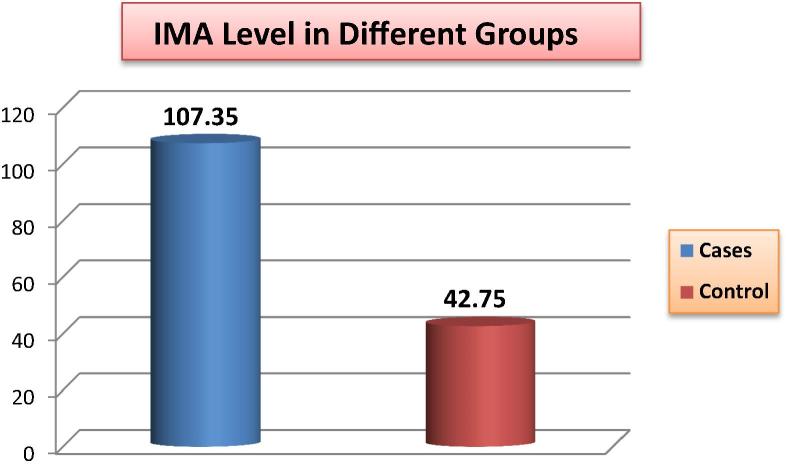
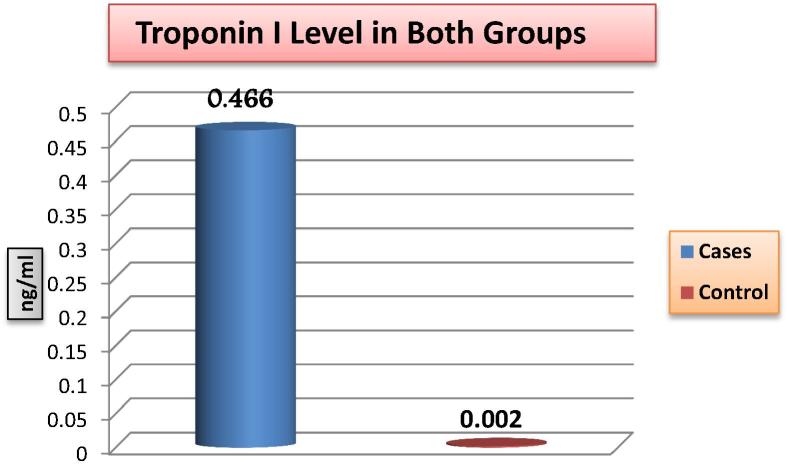
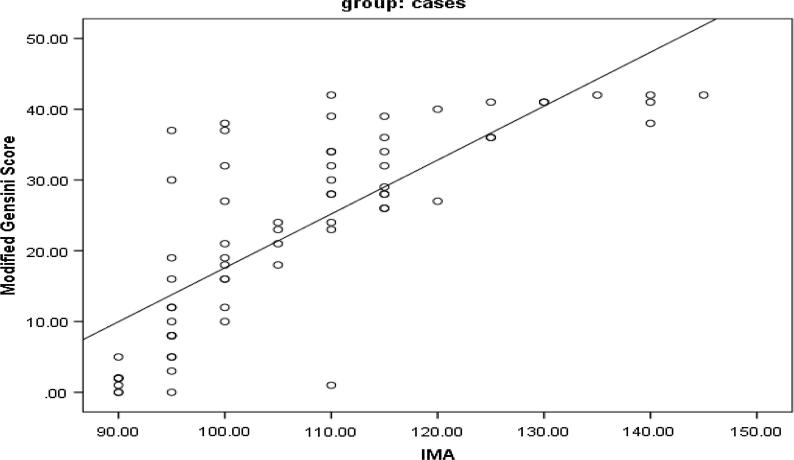
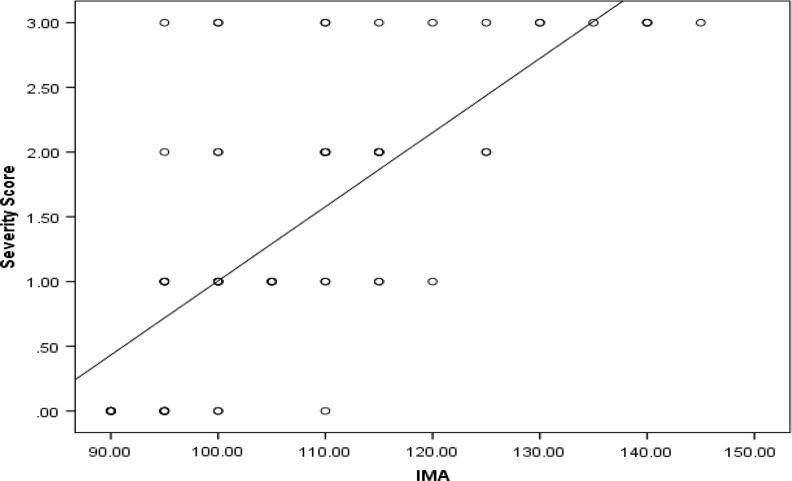
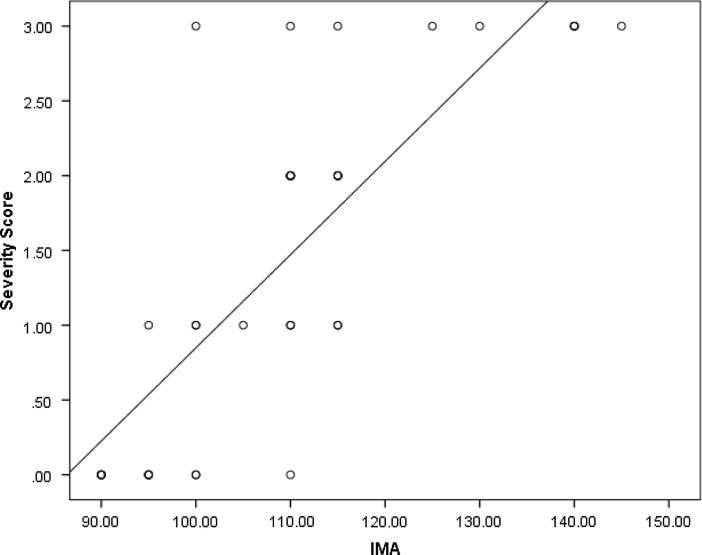
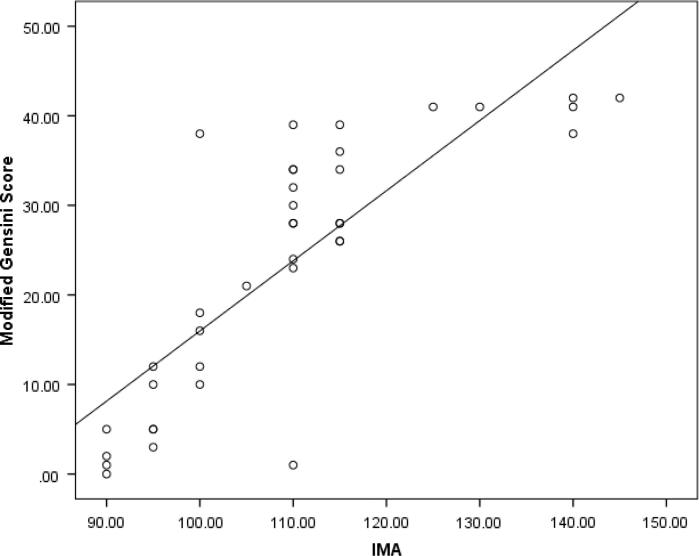
There is statistically significant difference between the two groups in the IMA level as well as in troponin I levels (P value <0.001) (Fig. 1, Fig. 2). Moreover, IMA is significantly positively correlated with troponin I (Table 2) as well as both the severity and Modified Gensini scores (P value <0.001) (Fig. 3, Fig. 4).
Fig. 1.
Comparison of IMA level between the two study groups.
Fig. 2.
Comparison of troponin I level between the two study groups.
Table 2.
Correlation of IMA level with Troponin I level in the patients.
| IMA |
|||
|---|---|---|---|
| r | P value | ||
| Troponin I | 0.200 | 0.102 | |
Fig. 3.
Correlation of IMA level with both severity and Modified Gensini scores.
Fig. 4.
Correlation of IMA level with both severity and Modified Gensini scores.
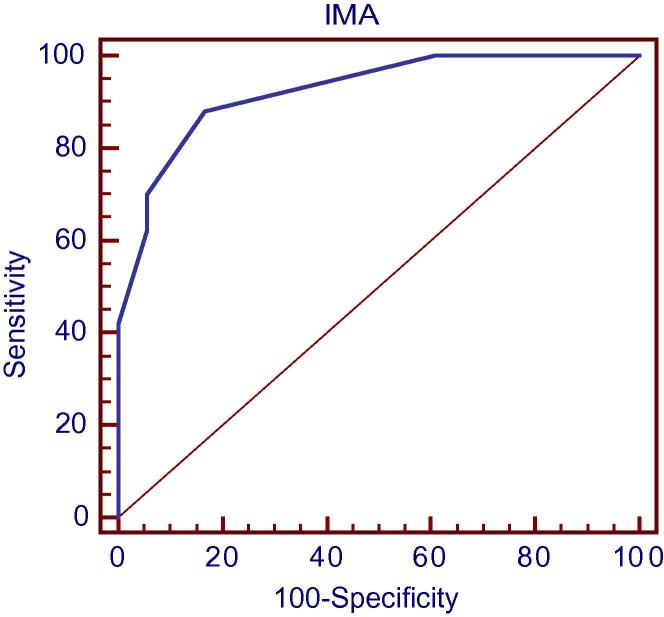
IMA with a cutoff value >95 could predict significant CAD (lesions >50%) in the patients with ACS and LBBB with AUC of 0.923, sensitivity of 88%, specificity of 83.33%, PPV of 93.6%, NPV of 71.4% and accuracy 86.76% (Fig. 5).
Fig. 5.
ROC analysis for prediction of significant CAD according to IMA in the patients group.
Moreover, by using both simple and multiple logistic regression analyses IMA could also independently detect significant CAD in the patients group (Table 3).
Table 3.
The regression analysis for diagnosis of significant CAD in the patient group.
| Simple logistic regression |
Multiple logistic regression |
|||
|---|---|---|---|---|
| OR | P value | AOR | P value | |
| 95% CI | 95% CI | |||
| Age | 1.07 | 0.053 | 1.23 | 0.052 |
| (0.99–1.15) | (0.99–1.53) | |||
| Albumin | 0.16 | 0.056 | 0.05 | 0.175 |
| (0.24–1.05) | (0.001–3.98) | |||
| IMA | 0.14 | 0.001* | 0.51 | 0.001* |
| (1.13–1.6) | (1.18–1.92) | |||
OR: Odds Ratio.
AOR: Adjusted odds ratio.
CI: Confidence Interval.
Bold significant represent the correlation between IMA and CAD.
A total of 38 patients had negative troponin assay out of 68 patients included in this study. By severity score, 27 patients with negative troponin assay had obstructive CAD. Even in troponin negative patients, IMA was significantly positively correlated with both severity and Modified Gensini scores (P value <0.001) (Fig. 6, Fig. 7).
Fig. 6.
Correlation of IMA level with both severity and Modified Gensini scores in Troponin negative patients.
Fig. 7.
Correlation of IMA level with both severity and Modified Gensini scores in Troponin negative patients.
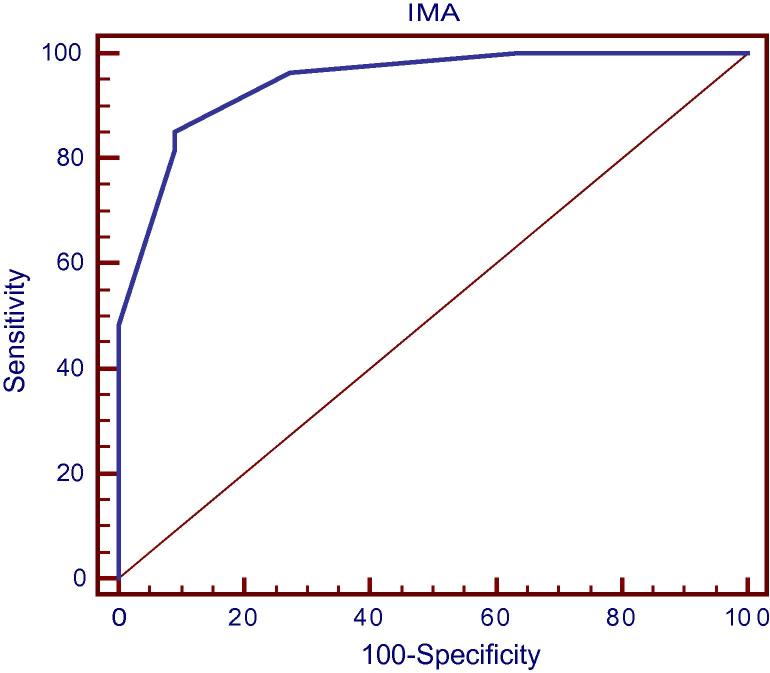
Furthermore, IMA with the same cutoff value (>95) could predict significant CAD (lesions >50%) in patients with ACS, and LBBB and have negative troponin assay with sensitivity 96.3%, specificity 72.73%, PPV of 8976%, NPV of 88.9%, AUC of 0.944 and accuracy 89.47% (Fig. 8).
Fig. 8.
ROC curve analysis for prediction of severity According to IMA (in Troponin −ve patients).
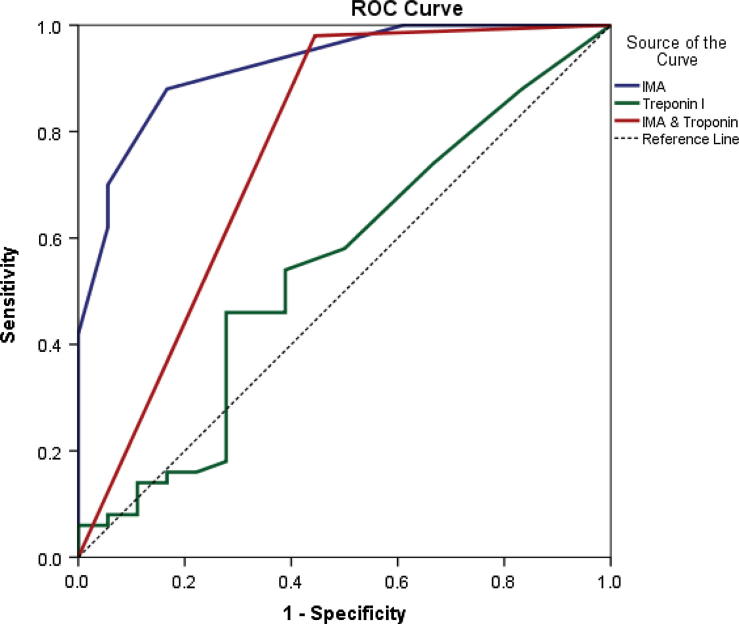
The performance of IMA and TnI alone and in combination for the diagnosis of ACS has been presented in Table 4 and Fig. 9. The sensitivity, specificity, positive predictive value and the negative predictive value were analyzed. The combined use of IMA and TnI significantly improved the sensitivity and the negative predictive value to 98% and 90.9% respectively.
Table 4.
The performance of IMA & TnI for diagnosis of ACS in the patients group.
| Variable | Optimal cutoff | AUC | P value | Sensitivity | Specificity | PPV | NPV | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| IMA | >95 | 0.923 | <0.001* | 88 | 83.33 | 93.6 | 71.4 | 86.76 |
| Troponin | >0.1 | 0.549 | 0.551 | 46 | 72.22 | 85.1 | 32.5 | 52.9 |
| IMA & Troponin | 0.768 | 0.001* | 98 | 55.6 | 86 | 90.9 | 86.76 | |
Bold significant represent the correlation between IMA and CAD.
Fig. 9.
ROC curve analysis for prediction of ACS according to IMA, TnI and combination.
4. Discussion
More than 50% of patients who present to the emergency department with acute chest pain are admitted to exclude ACS.13
The two components of the acute coronary syndrome (ACS), acute myocardial infarction (AMI) and unstable angina (USA), are the major causes of death and disability worldwide.1, 2, 14 The risk of death and the benefit from early reperfusion are highest within the first hours; therefore, early diagnosis is critical.1, 2, 3, 14
The current treatment approach (based on current guidelines) exposes a significant proportion of patients with a suspected ACS and LBBB to urgent reperfusion strategies without the likelihood of significant benefit, and leads to increased rates of false-positive cardiac catheterization activation.5 The prevalence of false-positive catheterization laboratory activation was 14% overall, but among patients presenting with LBBB, the rate of false activation was 39–44%.15, 16
Most of the biomarkers of AMI are the products of myocardial necrosis and thus are detected typically at a later stage of the myocardial damage.17 Therefore, rapidly detectable, highly sensitive markers would be desirable for myocardial ischemia, to identify the patients with only ischemia and those who are early in the course of an acute coronary syndrome without the evidence of any myocardial necrosis especially in early presenters.
IMA that has the advantages of being positive within minutes of ischemia and remains elevated for up to several hours allowing detection before the development of myocardial necrosis, has already been licensed by the US Food and Drug Administration for the diagnosis of suspected myocardial ischemia.18
The major strength of our study is that it evaluated the role of IMA in early detection of ischemia and excluding the diagnosis of ACS because the diagnosis of these patients is often difficult in this category of patients with LBBB. Moreover, we used an ELISA to measure serum IMA levels, which is faster, less expensive and comparably reliable with the albumin cobalt binding assay, which has been used in previous studies.19, 20
In the present study, IMA levels were significantly higher in group I (ischemic chest pain and LBBB) as compared to group II (LBBB without chest pain) (P < 0.001).
Our finding supported the results of the studies of others.18, 21, 22, 23 Several mechanisms have been postulated for the generation of IMA within minutes after the onset of acute myocardial ischemia such as hypoxia, acidosis, free radical damage, release of fatty acids, membrane energy-dependent sodium and calcium pump disruptions, and free iron and copper ion exposures.21, 24
The most important findings in the current work is that IMA with a cutoff value >95 could predict significant obstructive CAD (lesions >50%) in patients with ACS and LBBB with sensitivity of 88%, specificity of 83.3%, PPV of 93.6%, NPV of 71.4%, accuracy 86.7% and AUC of 0.923. Also, significant positive correlation was detected between IMA and the extent of CAD measured by the Severity score (P value <0.001) and the severity and complexity of the lesions measured by Modified Gensini score (P value <0.001).
Moreover, results of this study revealed that IMA is an independent factor for diagnosis of obstructive CAD in these patients using both Simple (OR 0.14, P value = 0.001) and Multiple Logistic Regressions (OR 0.51, P value = 0.001).
Furthermore, this study revealed that 71% of troponin negative patients have significant obstructive coronary artery disease while IMA could predict significant obstructive CAD in those troponin negative patients (with a cutoff value >95) with sensitivity of 96.3%, specificity of 72.7%, PPV of 89.7%, NPV of 88.9% and accuracy 89.5%.
There was a significant positive correlation between IMA and both the severity score (P value <0.001) and the Modified Gensini score (P value = 0.001) in troponin negative patients.
To the best of our knowledge, no previous study uses IMA for detection of ischemia in patients with LBBB and acute coronary syndrome.
Our results were in complete conformity to those of Zhong et al. who enrolled 129 patients and found that IMA levels and the number of diseased coronary arteries (detected by coronary angiography) were significantly correlated (P < 0.01). Logistic regression analysis showed that IMA was an independent predictor of CHD.25
Bhagwan et al. reported a sensitivity of 88% and a specificity of 94% for the IMA assay, which were quite close to our results. They also reported an AUC under an ROC plot of 0.95.26
Similarly, Gurumurthy et al. found that mean serum IMA levels in 540 patients with STEMI, NSTEMI, and UA were significantly higher than noncardiac chest pain and also healthy subjects (P < 0.001). ROC curve analysis revealed that the cutoff value above which IMA can be considered positive was 84.4 U/L. The area under the curve was found to be 0.933 with 95% CI (0.911–0.954) (P < 0.0001). The sensitivity and specificity were found to be 88% and 89%, respectively.27
Our results shared that previous study of IMA showed that it is possible to distinguish reliably between the ACS and non-ACS populations (area under the ROC curve 0.95).28
Our observations supported also Demirtas et al. who reported that IMA and BNP may predict the extension of coronary artery disease before performing coronary angiography in the early stage of ACS. The extension of coronary artery disease was calculated with Gensini score index and more than 50% stenosis was accepted as severe coronary artery stenosis.29
Moreover, Huang et al. showed that IMA level in NSTEMI patients was significantly higher than that of the healthy control group, and it had obviously positive correlation with coronary artery stenosis degree.30
Finally, the diagnostic performance of the IMA level in the ACS patients was greater as compared to that of the TnI assay. The sensitivity and the specificity of IMA were significantly greater than those of TnI. The combination of the IMA and the TnI results improved the sensitivity of the detection of ACS to 98% with a negative predictive value of 92%.
These results were in complete conformity to those of previous reports17 which had revealed that a combination of the IMA, ECG and the TnI results had improved the sensitivity to 96% for the detection of ACS. Saif et al., found that the combination of IMA, Myoglobin, CK-MB and TnI increased the sensitivity for detecting ischemia to 97%, with a negative predictive value of 92%.31
These results of IMA may improve our ability to make early and accurate decisions for exclusion of ACS in patients with LBBB.
5. Conclusion
Our findings suggest that IMA can be used as an independent biomarker or an additional parameter along with TnI, to augment the confidence for exclusion of cardiac ischemia in patients with LBBB. This combination seems to have clear potentials of saving unnecessary risks, and costs.
However, larger studies are needed to evaluate the value of IMA as an exclusion test in those patients and to compare its performance with other cardiac biomarkers and imaging tests.
6. Limitations
Our study has limitations. The study population was relatively small; therefore, we cannot draw concrete conclusions.
Another limitation is that exact determination of the age of LBBB as previous ECG is lacking in most of patients.
Finally, another limitation of our findings is that IMA is not specific for myocardial ischemia because acute infection, advanced cancer and brain ischemia are also associated with increased IMA.
Conflict of interest
No conflict of interest.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
References
- 1.Thygesen K., Mair J., Giannitsis E., Mueller C., Lindahl B., Blankenberg S. The Study Group on Biomarkers in Cardiology of the ESCWGoACC. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33:2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K., Alpert J.S., Jaffe A.S., Simoons M.L., Chaitman B.R., White H.D. Joint ESC/ACCF/AHA/WHF task force for the universal definition of myocardial. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 3.Safford M.M., Parmar G., Barasch C.S., Halanych J.H., Glasser S.P., Goff D.C. Hospital laboratory reporting may be a barrier to detection of ‘microsize’ myocardial infarction in the US: an observational study. BMC Health Serv Res. 2013;13:162. doi: 10.1186/1472-6963-13-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansilal S., Aneja A., Mathew V. Long-term cardiovascular outcomes in patients with angina pectoris presenting with bundle branch block. Am J Cardiol. 2011;107:1565–1570. doi: 10.1016/j.amjcard.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neeland I.J., Kontos M.C., de Lemos J.A., Reynolds D.W. Evolving considerations in the management of patients with left bundle branch block and suspected myocardial infarction. J Am Coll Cardiol. 2012;60(2):96–105. doi: 10.1016/j.jacc.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu A.H. 2nd ed. Totowa, NJ; Humana: 2003. Pathology and laboratory medicine. Clinical markers. [Google Scholar]
- 7.Sbarouni E., Georgiadou P., Kremastinos D.T., Voudris V. Ischemia modified albumin: Is this marker of ischemia ready for prime time use? Hellenic J Cardiol. 2008;49:260–266. [PubMed] [Google Scholar]
- 8.Apple F.S. Clinical and analytical review of ischemia-modified albumin measured by the albumin cobalt binding test. Adv Clin Chem. 2005;39:1–10. doi: 10.1016/s0065-2423(04)39001-3. [DOI] [PubMed] [Google Scholar]
- 9.Bar-Or D., Curtis G., Rao N., Bampos N., Lau E. Characterization of the Co(2+) and Ni(2+) binding amino-acid residues of the N-terminus of human albumin. An insight into the mechanism of a new assay for myocardial ischemia. Eur J Biochem. 2001;268:42–47. doi: 10.1046/j.1432-1327.2001.01846.x. [DOI] [PubMed] [Google Scholar]
- 10.Mark R.A., Akihiro N., Anthony K., Jacqui R., Robyn M., Brian P.B. Carotid intima-media thickness is only weakly correlated with the extent and severity of coronary artery disease. Circulation. 1995;92:2127. doi: 10.1161/01.cir.92.8.2127. 34. [DOI] [PubMed] [Google Scholar]
- 11.Gensini G.G. The pathological anatomy of the coronary arteries of man. In: Gensini G.G.M.D., editor. Coronary arteriography. Futura Publishing Co.; Mount Kisco, New York: 1975. pp. 271–274. [Google Scholar]
- 12.Mahmut A., Sadık S., Mustafa K., Fuat G., Şule K. Evaluation of ultrasonographic fatty liver and severity of coronary atherosclerosis, and obesity in patients undergoing coronary angiography. Anadolu Kardiyol Derg. 2009;9:273–279. [PubMed] [Google Scholar]
- 13.Ward R.P., Mansour I.N., Lemieux N., Gera N., Mehta R., Lang R.M. Prospective evaluation of the clinical application of the American College of Cardiology Foundation/American Society of Echocardiography Appropriateness Criteria for transthoracic echocardiography. JACC Cardiovasc Imag. 2008;1:663–671. doi: 10.1016/j.jcmg.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K., Mair J., Katus H., Plebani M., Venge P., Collinson P. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010;31:2197–2204. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- 15.Larson D.M., Menssen K.M., Sharkey S.W. “False-positive” cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA. 2007;298:2754–2760. doi: 10.1001/jama.298.23.2754. [DOI] [PubMed] [Google Scholar]
- 16.Lopes R.D., Siha H., Fu Y. Diagnosing acute myocardial infarction in patients with left bundle branch block. Am J Cardiol. 2011;108:782–788. doi: 10.1016/j.amjcard.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Takshid M., Kojuri, Tabei B., Tavasouli A., Heidary S., Tabanbeh M. Early diagnosis of acute coronary syndrome with sensitive troponin I and ischemia modified albumin. Iran Cardiovasc Res J. 2010;4(4):144–151. [Google Scholar]
- 18.Peacock F., Morris D., Anwaruddin S., Christenson R., Collinson P., Goodware S. Meta-analysis of Ischemia Modified Albumin to rule out acute coronary syndromes in the emergency department. Am Heart J. 2006;152:253–262. doi: 10.1016/j.ahj.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Peacock F., Morris D.L., Anwaruddin S. Meta-analysis of ischemia-modified albumin to rule out acute coronary syndromes in the emergency department. Am Heart J. 2006;152:253–262. doi: 10.1016/j.ahj.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Sinha M.K., Vazquez J.M., Calvino R., Gaze D.C., Collinson P.O., Kaski J.C. Effects of balloon occlusion during percutaneous coronary intervention on circulating ischemia modified albumin and transmyocardial lactate extraction. Heart. 2006;92:1852–1853. doi: 10.1136/hrt.2005.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui L., Zhang J., Yonghua Wu, Xiaozhou Hu. Assay of ischemia-modified albumin and C-reactive protein for early diagnosis of acute coronary syndromes. J. Clin. Lab. Anal. 2008;22:45–49. doi: 10.1002/jcla.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil S., Banker M., Padalkar R., Pathak A., Bhagat S., Ghone R. The clinical assessment of ischaemia modified albumin and troponin I in the early diagnosis of the acute coronary syndrome. J Clin Diagn Res. 2013 May;7(5):804–808. doi: 10.7860/JCDR/2013/5288.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhakthavatsala Reddy C., Cyriac Cijo, Desle Hrishikesh B. Role of “Ischemia Modified Albumin” (IMA) in acute coronary syndromes. Indian Heart J. 2014;66:656–662. doi: 10.1016/j.ihj.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez R.A., Abreu G., Garcia G., Samini F., Reiter R., Kaski J. Association of ischemia modified albumin and melatonin in patients with ST-elevation myocardial infarction. Atherosclerosis. 2008;99:73–78. doi: 10.1016/j.atherosclerosis.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Zhong Y., Wang N., Xu H., Hou X., Xu P., Zhou Z. Ischemia-modified albumin in stable coronary atherosclerotic heart disease: clinical diagnosis and risk stratification. Coron Artery Dis. 2012;23(8):538–541. doi: 10.1097/MCA.0b013e328358a5e9. [DOI] [PubMed] [Google Scholar]
- 26.Bhagwan N., Ernest M., Rios P., Yang J. Evolution of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem. 2003;49:581–585. doi: 10.1373/49.4.581. [DOI] [PubMed] [Google Scholar]
- 27.Gurumurthy P., Borra S., Krishna R., Yeruva R., Victor D., Babu S. Estimation of Ischemia Modified Albumin (IMA) levels in patients with acute coronary syndrome. Indian J Clin Biochem. 2014 Jul;29(3):367–371. doi: 10.1007/s12291-013-0367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhagavan N.V., Lai E.M., Rios P.A., Yang J., Ortega-Lopez A.M., Shinoda H. Evaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem. 2003 Apr;49(4):581–585. doi: 10.1373/49.4.581. [DOI] [PubMed] [Google Scholar]
- 29.Demirtas A., Karabag T., Sayin M., Akpinar I., Yavuz N., Aydin M. Can Ischemia modified albumin and brain natriuretic peptide levels predict the extension of coronary artery disease in low-intermediate risk unstable angina pectoris? J Am Coll Cardiol. 2013;62(18_S2) C121–C121. [Google Scholar]
- 30.Huang Y., Chen Z., Bai Y., Chen G., Peng Y., Yong-Kui Study on the relationship between serum Hcy, IMA, the levels of inflammatory factors and the severity of coronary artery disease with non ST segment elevation acute myocardial infarction patients. Hainan Med J. 2015:36–38. [Google Scholar]
- 31.Saif A., James L.J., Aaron L.B., Elizabeth L.L., Kent B.L. Ischemia –modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol. 2005;123:140–145. doi: 10.1309/4bctg5ucymqfwblr. [DOI] [PubMed] [Google Scholar]