ABSTRACT
Objectives: Notch1 regulates tumor biology in a complex, context-dependent manner. The roles of Notch1 in tongue cancer are still controversial. The aim of this study is to investigate the roles of Notch1 in tongue cancer.
Materials and Methods: The expression of Notch1 was tested between tongue cancer and normal samples by using immunohistochemistry. Tongue cancer cells were transfected with siRNA or plasmid, respectively. Cell proliferation, apoptosis, migration and invasion ability were tested in appropriate ways. The subcutaneous tumor model was established to observe the tumor growth.
Results: Notch1 was upregulated in tongue carcinoma tissues and the expression of Notch1 was related with tumor stage and differentiation. Overexpression of Notch1 could increase tongue cancer cells proliferation, invasion and migration. But inhibited the expression of Notch1 could decrease cells proliferation, invasion and migration and promote cell apoptosis in vitro and in vivo.
Conclusion: Our results prove that the oncogenic role of Notch1 in tongue cancer and provide the direction of targeted therapy of tongue cancer.
KEYWORDS: Notch1, tongue cancer, metastasis, proliferation, apoptosis
Introduction
Oral cavity cancer is one of the most prevalent cancers in the world; the incidence rate and mortality rate is 4.3% and 2.1%, respectively, worldwide based on GLOBOCAN 2012 data (http://gco.iarc.fr/today/). Tongue cancer is the most commonly occurring oral cavity cancers and is generally caused by squamous cell carcinoma. A study showed that the incidence of tongue squamous cell carcinoma in general has increased worldwide [1]. Tongue cancer migrate rapidly to the adjacent structures and travels through the bloodstream and invades other organs and will seriously affect the patient's quality of life and facial appearance. Therefore, it is of great significance to find an effective way to inhibit the development of tongue cancer from the molecular level and to improve the prognosis of patients.
Notch is an ancient signaling pathway that includes four Notch receptors (Notch 1, 2, 3, and 4) and five Notch ligands (Delta-like 1, 3, and 4 and Jagged1 and 2) in the mammal. When Notch receptors and ligands interact, the pathway is activated. The process involves two key proteolytic enzymes. The first is a tumor necrosis factor-α-converting enzyme that cleaves the extracellular portion of the receptor. The second hydrolysis process is regulated by γ-secretase to release the Notch intracellular domain (NICD) located in the nucleus, which binds to the transcription factor CSL and so on, triggering downstream genes [2].
Numerous studies have reported both the tumor-suppressive and oncogenic roles of the Notch pathway, such as in the non-small cell lung carcinoma cell [3], breast cancer [4], and salivary gland adenoid cystic carcinoma in which Notch has oncogenic roles [5], while it is reported as a cancer suppressive gene in bladder cancer [6], endometrial cancer [7], and myeloid leukemia [8]. It is indicated that Notch activity regulates tumor biology in a complex, context-dependent manner. The roles of Notch signaling pathways in the carcinogenesis and progress of the tongue are still conflicted. The aim of this study is to investigate the roles of Notch1 in tongue cancer. We assessed the expression level of Notch1 in clinical tongue cancer specimen and adjacent tissues. We altered the expression of Notch1 in tongue cancer cells, then tested the proliferation, invasion, and migration ability variation in vitro and in the nude mice xenograft tumor model.
Results
Notch1 is upregulated in tongue carcinoma
To investigate the role of Notch receptors in tongue carcinoma, we firstly tested the expression of the four Notch receptors in three different tongue cancer cell lines, and the RT-PCR results shown that the expression of Notch1 is much higher than the other Notch receptors in tongue cancer cells (Fig.S1). Moreover, the immunohistochemistry assay was used to explore the expression level of Notch1 in tongue carcinoma. The protein of Notch1 is expressed in the cytoplasm and the images showed that the level of Notch1 expression in tongue carcinoma tissues were higher than the level in the adjacent normal tissues (Figure 1). In 50 pairs of tongue squamous cell carcinoma tissues and adjacent tissues, as shown in Table 1, there are 32 cases with high expression and 18 cases with low expression of Notch1 in tongue squamous cell carcinoma, while there are 23 cases of negative and 27 cases with low expression of Notch1 in adjacent tissue. Further analysis of clinical information revealed that the expression of Notch1 is higher in moderately or poorly differentiation group than that in well differentiation tissues (P<0.05) and the expression of Notch1 in the tumor of early stage (stage I) is lower than that with middle or late stage (stage II+III+IV, P<0.05, Table 1). All above indicated that Notch1 is likely to play a carcinogenic role in tongue cancer and it's worth digging into it.
Figure 1.
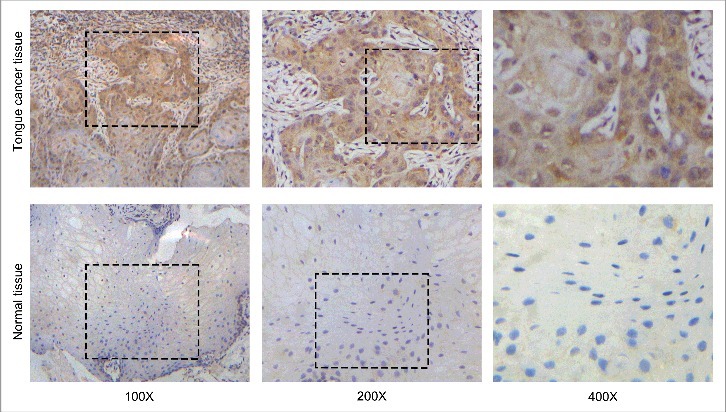
Notch1 is upregulated in tongue squamous cell carcinoma. Representative images for the expression of Notch1 by immunohistochemistry in tongue cancer tissues and normal tissues.
Table 1.
Expression of Notch1 in tongue cancer samples and normal samples.
| Grade | ||||||
|---|---|---|---|---|---|---|
| Parameter | Total | Negative | Low Notch1 | High Notch1 | X2 | P |
| Cancer or not | ||||||
| Tongue cancer | 50 | 0 | 18 | 32 | 56.800 | 4.6349 × 10−13 |
| Normal tissue | 50 | 23 | 27 | 0 | ||
| Gendera | ||||||
| Female | 23 | 0 | 10 | 13 | 1.034 | 0.309 |
| Male | 27 | 0 | 8 | 19 | ||
| Agea | ||||||
| ≤55 | 33 | 0 | 12 | 21 | 0.006 | 0.941 |
| >55 | 17 | 0 | 6 | 11 | ||
| Invasiona | ||||||
| Yes | 44 | 0 | 16 | 28 | 0.000 | 0.999 |
| No | 6 | 0 | 2 | 4 | ||
| Metastasis(Lymph node)a | ||||||
| Yes | 7 | 0 | 2 | 5 | 0.000 | 0.986 |
| No | 43 | 0 | 16 | 27 | ||
| Differentiationa | ||||||
| Well | 32 | 0 | 15 | 17 | 4.563 | 0.033 |
| Moderately or poorly | 18 | 0 | 3 | 15 | ||
| Tumor Stagea | ||||||
| Early | 23 | 0 | 12 | 11 | 4.836 | 0.028 |
| Middle or late | 27 | 0 | 6 | 21 | ||
Analysis in the tongue cancer tissues.
Notch1 promotes the proliferation of tongue cancer cells in vitro and in vivo
To explore the role of Notch1 in the function of proliferation of cancer cells, siRNA-mediated knockdown of Notch1 was firstly used in CAL-27 cells. The results of real-time PCR (Figure 2A) and western blotting (Figure 2B) showed a lower expression level of Notch1 in siRNA-1166 and siRNA-2010 groups than in the negative control group. Our CCK8 assays (Figure 2C) and colony-formation assays (Figure 2D) data showed that the growth of CAL-27 cells was significantly inhibited after transfection with siRNA. We got the same trend when silenced the expression of Notch1 in SCC-9 cells and TCA-8113 cells (Fig. S2). From the results, we believe that silencing Notch1 expression can inhibit tongue cancer cells proliferation. Next, to explore the relationship between Notch1 and CAL-27 cells apoptosis, the flow cytometry analysis was applied, and the results showed that upon knockdown of Notch1 both early apoptosis cells and late apoptosis cells distinctly increased compared with the negative control (Figure 2E). So Notch1 contributes to proliferation by anti-apoptosis of CAL-27 cells.
Figure 2.
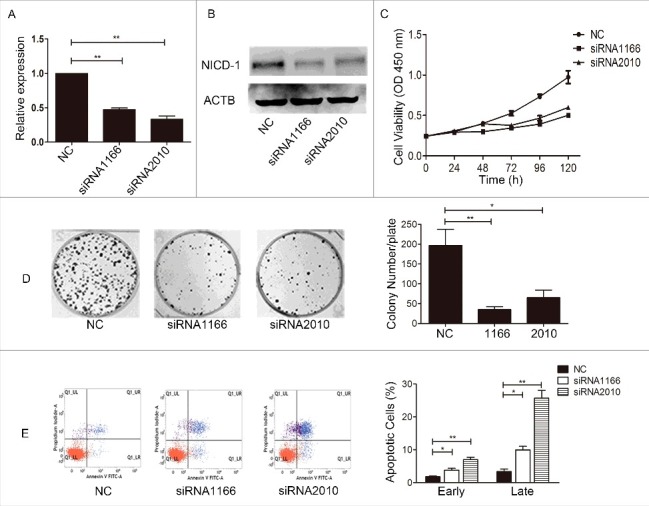
Inhibited the expression of Notch1 influenced the proliferation and apoptosis of CAL-27 cells in vitro. After siRNA transfection in CAL-27 cells, the expression of Notch1 was detected by real-time PCR (A) and Western blotting (B), the cell proliferation was measured by CCK8 assay (C, P < 0.01 from 72 h to 120 h) and colony-formation assay (D), and the cell apoptosis was measured by flow cytometry with Annexin V and PI staining (E). It was labelled * when P < 0.05, ** when P < 0.01.
To validate the results of in vitro studies, we performed an in-depth observation of the change of tumor growth in vivo after downregulation of Notch1. The siRNA-transfected CAL-27 cells were subcutaneously injected into the axilla of the forelimbs of nude mice. Every 3 days after injection, we measured the body weight and tumor volume, and the animals were sacrificed 32 days later. The results showed that there was basically no variation in the body weight (Figure 3A) of the nude mice, while the tumor volume (Figure 3B) and tumor weight (Figure 3C-D) of the siRNA transfection group were suppressed when compared with the negative control. We tested the expression of Notch1 in tumor tissues by Real-time PCR, and the results showed that the expression of Notch1 was lower in the siRNA transfection group than the negative control (Figure 3E). After Notch1 were knocked down, the expression of Ki-67 were decreased, but the expression of Caspase 9 was increased in the xenograft tumors (Figure 3F-G). The data implied that downregulation of Notch1 in tongue cancer cells can inhibit cell growth by inducing cellular apoptosis.
Figure 3.
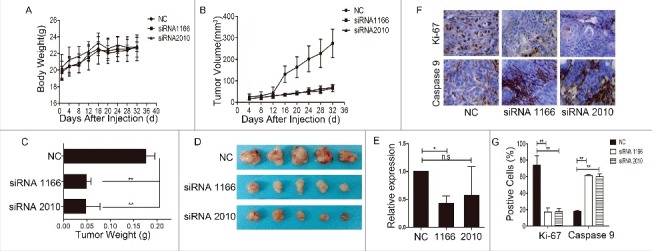
Knockdown of Notch1 inhibited the growth of tumors in vivo. The changes of body weight (A) and tumor volume (B) of nude mice were monitored during the experiment; the graph shows that there were no significant changes in body weight among the groups, while the tumor volume of the siRNA interference group was smaller than the negative control; C-D, the animals were sacrificed and the tumors were removed for weighing (C) and photographing (D); the column graph shows that after knockdown of Notch1, the tumor weights were lighter than the negative control, and the photograph of the tumors had a similar trend; E, real-time PCR was used to verified the expression of Notch1 in tumors; G-F, using immunohistochemistry detected the expression of Ki-67 (upper panel) and cleaved Caspase-9 (lower panel) in the transplantation tumors (DAB, 400 ×). Data represents means ± S.D. of three independent experiments. It was labelled * when P < 0.05, ** when P < 0.01.
Secondly, we attempted to perform a series of experimental observations to investigate the effects of Notch1 overexpression. After transfection of plasmid (pcDNA3.1-NICD1), the real-time PCR test (Figure 4A) and western blotting (Figure 4B) showed that Notch1 was overexpressed in CAL-27 cells. The results of CCK8 assay (Figure 4C) showed the effect of Notch1 on short-term cell growth of CAL-27 cells was gentle, but the colony-formation assays (Figure 4D) demonstrated that cell proliferation increased greatly after overexpression of Notch1. We got the same trend when enhanced the expression of Notch1 in SCC-9 cells and TCA-8113 cells (Fig. S3). Thus, Notch1 is intimately related to tongue cancer cells proliferation in vitro. We also tested the change of tumorigenicity after transfection of plasmid (pcDNA3.1-NICD1). Every 3 days after injection, we measured the body weight and tumor volume, and the animals were sacrificed 30 days later. The results showed that there was basically no variation in the body weight (Figure 5A) in each group, while the tumor volume (Figure 5B) and tumor weight (Figure 5C-D) of the Notch1 overexpression group were larger and heavier compared with the vector group. And the expression of Notch1 of tumor tissues were verified by western blotting, the results showed that the protein expression of Notch1 was up-regulated in overexpression group (Figure 5E). Taken together, our data indicate that Notch1 plays an oncogenic role in tongue carcinoma that contributes to the proliferation and anti-apoptosis of tongue cancer cells. So, it may serve as a target gene for the treatment of tongue cancer.
Figure 4.
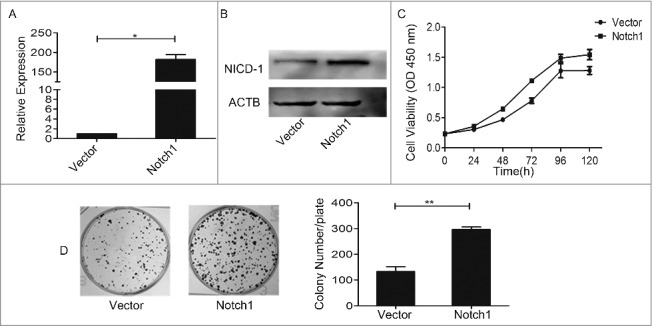
Overexpression of Notch1 enhanced the growth ability of CAL-27 cells in vitro. After overexpression of Notch1 in CAL-27 cells by plasmid, the expression of Notch1 was detected by real-time PCR (A) and Western blotting (B), the cell proliferation was measured by CCK8 assay (C, P < 0.05 from 48 h to 120 h) and colony-formation assay (D). It was labelled * when P < 0.05, ** when P < 0.01.
Figure 5.
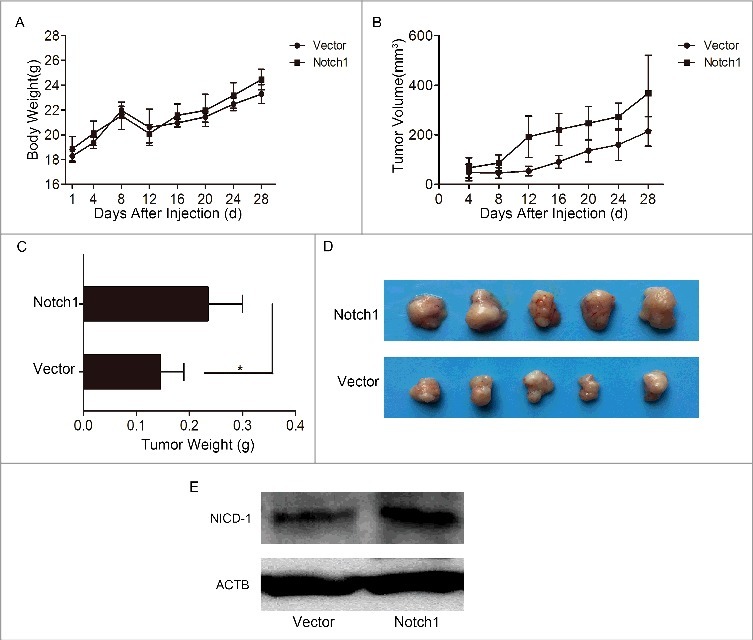
Overexpression of Notch1 enhanced the growth of tumors in vivo. The changes of body weight (A) and tumor volume (B) of nude mice were monitored during the experiment; C-D, the animals were sacrificed and the tumors were removed for weighing (C) and photographing (D); E, the expression of Notch1 of tumor tissues were detected by western bolting.
Notch1 is associated with metastatic potential of tongue cancer cells
We did further studies to determine whether Notch1 is associated with the metastatic potential of tongue cancer cells. The results of wound-healing assay (Figure 6A) showed that cultured 36 h after the scratched monolayer, the negative control CAL-27 cells almost healed, while there were wide areas in the siRNA transfection cells. At the same time, the transwell assay showed that both without (upper panel) and with (lower panel) Matrigel, the numbers of cell-permeated septum of the negative control were more than the experimental groups (Figure 6B). The results demonstrated that after knockdown of Notch1, the migration and invasion of CAL-27 cells decreased. But when overexpression of Notch1 in the wound-healing assay (Figure 7A), the transwell assay (Figure 7B) both without (upper panel) and with (lower panel) Matrigel-coating showed the inverse results. These data showed that Notch1 increases cell mobility of tongue cancer cells.
Figure 6.
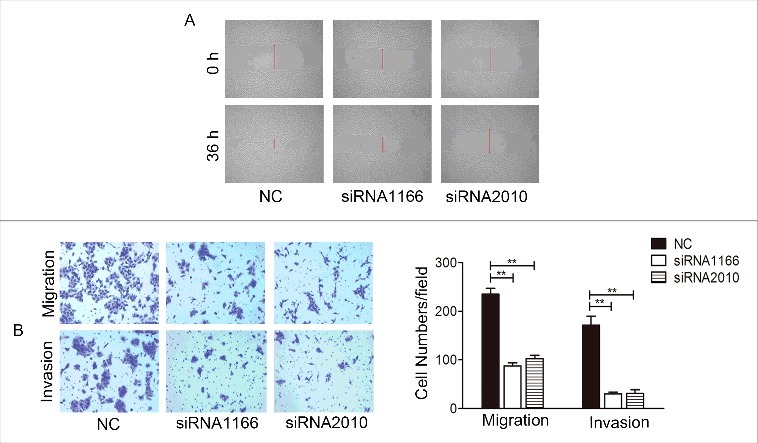
Knockdown of Notch1 suppressed the invasion and migration of CAL-27 cells in vitro. The effect of Notch1 knock down on the cells migration and invasion was measured by wound healing assay (A) and transwell assay with or without Matrigel coating (B), representative images shown the transwell assay without (upper panel) or with (lower panel) coated Matrigel. It was labelled * when P < 0.05, ** when P < 0.01.
Figure 7.
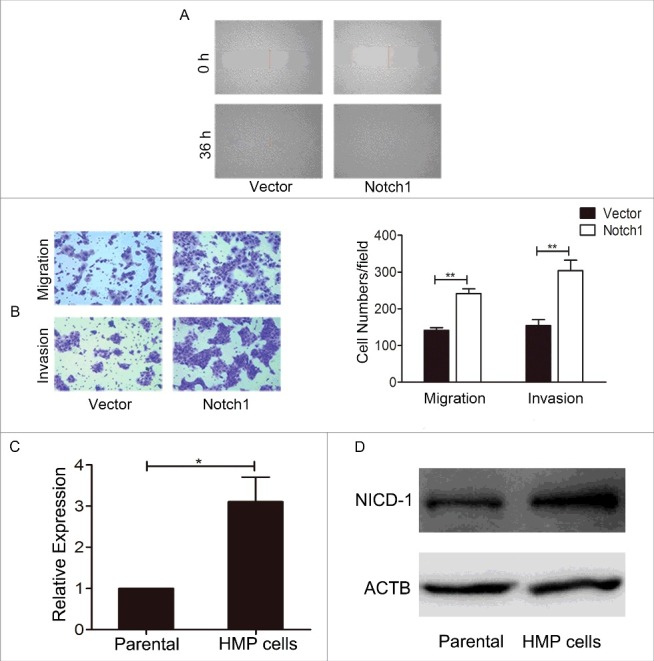
Overexpression of Notch1 enhanced the metastasis ability of CAL-27 cells in vitro. After overexpression of Notch1 in CAL-27 cells, the cells migration and invasion were measured by wound healing assay (A) and transwell assay with or without Matrigel coating (B). Data represents means ± S.D. of three independent experiments. The expression level of Notch1 was tested between CAL-27 cells (parental cells) and HMP cells, the real-time PCR (C) and Western blotting (D) result showed that Notch1 express higher in HMP cells than parental cells. It was labelled * when P < 0.05, ** when P < 0.01.
Orthotopic metastatic tongue cancer mouse model was established in our lab. CAL-27 cells were injected into tongue of nude mice and the lymph nodes metastatic lesions were harvested and nude mice for the next round of in vivo selection. After three rounds of in vivo selection, a high metastatic potential cell line (HMP) was established and the lymph nodes metastatic rate increased from 5% (Parental, 1/20) to 55% (11/21). To avoid the contamination of cells from nude mice, cell line authentication is achieved by genetic profiling using polymorphic short tandem repeat (STR) loci after each round selection and the results showed consistency with CAL-27 (Fig. S4) and 100% match with CAL-27 using STR database search. The results of real-time PCR (Figure 7C, p < 0.05) and western blotting (Figure 7D) found that the expression of Notch1 in the HMP cells is higher than that in parental cells. In conclusion, all the data above indicated a positive correlation between the expression of Notch1 and the metastasis ability of tongue cancer cells.
Discussion
Surgery combined with radiotherapy and chemotherapy is generally employed comprehensive therapy in tongue cancer treatment. Even so, the 5-year survival rate is not ideal [9]. So it is very important to find new therapies or genes as treatment targets to improve patients’ prognosis.
Notch is a conserved and evolved signaling pathway that regulates cell-to-cell communication and participates in organ development and intracellular balance [10]. However, the role of Notch signaling pathway in tumor development is still controversial, especially the role of Notch1 in head and neck squamous cell carcinoma. There are two major opposing views: is Notch1 a cancerogenic gene or suppressor gene? On the one hand, a few researchers have reported that Notch1 mutations were detected in the head and neck squamous cell carcinoma of Caucasians, suggesting that Notch1 plays a major role in suppressing cancer [11,12]. While interestingly the same research team in another study showed that two types exist in Caucasians, the wild type and the mutant type. Significantly, most mutants in the Caucasian population are inactivated mutations, and the expression level of the downstream genes of Notch were detected close to normal tissue; therefore the researchers thought it is insufficient to demonstrate that Notch1 is a tumor suppression gene. On the contrary, they found the expression of a part of Notch receptors and ligands in wild type cancer samples higher than in normal tissue [13]. Successively, a study of Chinese oral squamous cell carcinoma also tested a high mutation rate in Notch1, but unlike Caucasians, most of the mutations detected in Chinese are activating mutations, and it was found that the survival rate and disease-free survival rate of patients with Notch1 mutations was significantly lower than non-mutated patients [14,15]. Some research also found that Notch1 is somatically amplified or overexpressed in early tongue cancer in Indian and indicated Notch1 is an oncogenic role in tongue cancer [16]. Our data demonstrated that the expression of Notch1 in tongue squamous cell carcinoma was higher than in peritumoral tissue. Many other studies involving oral squamous cell carcinoma or tongue cancer also have shown that the expression of Notch genes in cancer tissues is higher than in the adjacent normal tissues [15,17,18]. Samantha et al. [19] thought whether Notch1 will be either oncogenic or tumor suppressive depend on the expression of p21Waf1/Cip1. We thought the main reasons for this controversial outcome are due to the mutation type of Notch, various ethnic, geographical, and environmental factors, as well as the number of samples collected. But this might be a result of the complexity of the Notch signaling pathway. Apostolos et al. [8] also found Notch in the same tumor existing in two different functions. Recent studies have shown an oncogenic or tumor suppressive role of Notch, depending on the context [20]. However the results of our present studies showed that Notch1 plays an oncogenic role in tongue cancer.
To further verify the tumor promotion role of Notch1 in tongue cancer, we observed the changes of cell proliferation, migration, and invasion in vitro and in vivo by altering the expression of Notch1 in tongue cancer cells. Our CCK-8 assay, colony-formation assay, and nude mice implantation model data shows that the growth ability of tongue cancer cells decreased after Notch1 siRNA was transfected, while overexpression of Notch1 had the reverse result. There are many other researches supported our results such as: Rose et al. [21] indicated that downregulating Notch1 with siRNA could inhibit the proliferation of ovarian cancer cells. Kim and colleagues [22] reported that after transfection with Notch1 siRNA, the growth of anaplastic thyroid cancer cells decreased. Meanwhile we observed that when Notch1 was suppressed, the percentage of apoptotic cells increased both in the flow cytometry test and the immunohistochemical staining which analyzed the expression of Caspase 9 in the xenograft tumors in nude mice. It's similar to the studies by Dai et al. [23]. They provided evidence that inhibited Notch1 could induce increased laryngeal squamous cell carcinoma cell apoptosis. Ai and colleagues [24] found that knockdown Notch1 could raise the apoptosis rate of bladder cancer cells, too.
Tumor invasion and metastasis will largely affect the prognosis of patients. Many studies showed that ectopic expression of Notch1 has a close relationship with cancer cells migration and invasion. And a lot of studies reported that the expression of Notch1 has a negative correlation with the expression of E-cadherin and implied that Notch1 is related to epithelial-to-mesenchymal transition (EMT) in breast cancer [25], non-small cell lung cancer [26], endometrial cancer [27], and intrahepatic cholangiocarcinoma [28]. Our results also indicate that Notch1 increased the invasion and migration ability of CAL-27 cells and found the high expression of Notch1 in HMP cells. What if suppressing the expression of Notch1 could get the opposite results? According to Yan et al. [29] research has shown that downregulation of Notch1 could reduce gastric signet ring cell carcinoma cell migration. Some research has indicated that Notch1 facilitates invasion and motility of breast cancer cells [30]. These are consistent with our results. Ahn et al. [31] found that autophagy plays a tumor suppression function in breast cancer, because it inhibits Notch1 signaling pathway by promoting NICD degradation. Wang et al. [32] found that liver cancer stem cells initiate tumor growth and self-renewal through Notch and Wnt/β-catenin up-regulation. The above studies can provide directions for our future experiments and our future studies will detected the deep mechanism of Notch1 in tongue cancer.
Above all, we deem that the Notch signaling pathway is a meaningful target for treating malignant cancer. Our results prove that Notch1 is an oncogene in tongue cancer and provide valuable experimental data on Notch1 as a potential target in the clinical treatment of tongue cancer.
Materials and methods
Clinical samples
Tissue samples were obtained from the Fuzhou General Hospital of Nanjing Military Command. A total of 50 pairs of tongue carcinoma tissues and adjacent normal tissues were included. This study was approved by the Institutional Review Board of Fujian Medical University, and written informed consent was obtained from each participant.
Immunohistochemistry
The expression of Notch1 in clinical samples was measured by immunohistochemical staining as described previously [5]. The Notch1 expression levels were divided to three groups according to the final score of each slice: negative (0 score), low expression (1-4 scores) and high expression (5-9 scores).
Cell lines and cell cultures
The CAL-27, SCC-9 cell lines were bought from ATCC (American Type Culture Collection) and TCA-8113 cell line was a gift from Dr. Chen (College of Stomatology, Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine). The cells were maintained in the medium as suggested and incubated at 37℃ in a humidified atmosphere of 95% air and 5% CO2. All cell lines were STR-authenticated annually by Shanghai Biowing Applied Biotechnology Co. LTD, Shanghai, China.
siRNA transfection
The siRNA was designed and synthesized (Gene Pharma, Shanghai, China). The specific sequences of siRNA are shown in Table S1. We transfected the CAL-27 cells, SCC-9 cells and TCA-8113 cells according to the instruction manual of Lipofectamine RNAiMAX (Invitrogen, Catalog # 13778150).
Plasmid amplification and transfection
The pcDNA3.1-NICD1 plasmid was a kind gift from Dr. Glenn Doughty, Harvard Medical School [33]. Plasmid amplification was accomplished through transformation and pumping. We transfected the cells following the instruction of Lipofectamine 3000 (Invitrogen, Catalog # L3000015).
Quantitative real-time PCR
The expression of Notch relevant mRNA was tested by quantitative real-time PCR assay as previously described [5]. The sequences of primers are listed in Table S2. Data were analyzed according to the 2−∆∆Ct method.
Western blotting
The expression of Notch relevant protein was tested by western blotting assay as previously described [5]. The primary and secondary antibodies were shown in Table S3.
Cell proliferation assay
Cell proliferation was detected by Cell Counting Kit-8 (CCK8) reagent and colony formation assay as previously described [5].
Wound healing assay
The CAL-27 cells that were transfected with siRNA or pcDNA3.1-NICD1 plasmid were seeded in a 6-well plate. The cells grew to monolayers covering the bottom of the plate, with 20 μL of tips cross-scratched on the cells, and replaced with 0.1% fetal bovine serum DMEM (Dulbecco's Modified Eagle's Medium), after being scratched at 0 h and 36 h for a fixed-point to take pictures.
Cell migration and invasion assay
Cell migration and invasion ability were measured using twenty-four-well transwell chambers (8-μm pore size) without or with Matrigel respectively as previously described [5].
Apoptosis assay
Apoptotic cells were detected by FITC Annexin V Apoptosis Detection Kit (BD Pharmingen, Catalog # 556547 followed Flow cytometer analysis.
Establish xenograft tumor model
Female BALB/c nude mice 6–8 weeks of age were purchased from the Center for Animal Experiments of Fujian Medical University. Cells (2 × 106) were suspended in 0.2 ml serum-free DMEM and injected into the right axillary fossa of each mouse. Tumor size was calculated using the formula V = width2 × length/2. At the end of the experiment, the tumors were harvested and weighed. The experimental animal protocols were approved by the Animal Care and Use Committee of Fujian Medical University.
Statistical analysis
The chi-square test was used for Notch1 immunoreactivity statistical analysis. The statistical analyses of the other experiments in this study were determined by one-way analysis of variance. It was indicated in the figures as n.s when P > 0.05, P < 0.05 was considered statistically significant, and * when P < 0.05, ** when P < 0.01.
Supplementary Material
Funding Statement
This work was supported by the National Natural Sciences Foundation of China [grant number 81641105], [grant number 81172583]; Natural Sciences Foundation of Fujian province [grant number 2017J01520]; Fujian Medical Innovation Grant [grant number 2014-cx-26]; Scientific Funding for leading research at FMUSS [grant number 2015-KQYY-LJ].
Acknowledgments
We thank Dr. Glenn Doughty for sending us a kind gift of the pcDNA3.1-NICD1 plasmid. We thank Dr. Chen for sending us a kind gift of the tongue cancer cell line TCA-8113.
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- [1].Ng JH, Iyer NG, Tan MH, et al. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck. 2017;39(2):297–304. doi: 10.1002/hed.24589. PMID:27696557 [DOI] [PubMed] [Google Scholar]
- [2].Gordon WR, Arnett KL, Blacklow SC. The molecular logic of Notch signaling–a structural and biochemical perspective. J Cell Sci. 2008;121(Pt 19):3109–3119. doi: 10.1242/jcs.035683. PMID:18799787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang JX, Han YP, Bai C, et al. Notch1/3 and p53/p21 are a potential therapeutic target for APS-induced apoptosis in non-small cell lung carcinoma cell lines. Int J Clin Exp Med. 2015;8(8):12539–12547. PMID:26550164 [PMC free article] [PubMed] [Google Scholar]
- [4].Pei J, Wang B. Notch-1 promotes breast cancer cells proliferation by regulating LncRNA GAS5. Int J Clin Exp Med. 2015;8(8):14464–14471. PMID:2514954126550436 [PMC free article] [PubMed] [Google Scholar]
- [5].Su BH, Qu J, Song M, et al. NOTCH1 signaling contributes to cell growth, anti-apoptosis and metastasis in salivary adenoid cystic carcinoma. Oncotarget. 2014;5(16):6885–6895. doi: 10.18632/oncotarget.2321. PMID:25149541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rampias T, Vgenopoulou P, Avgeris M, et al. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat Med. 2014;20(10):1199–1205. doi: 10.1038/nm.3678. PMID:25194568 [DOI] [PubMed] [Google Scholar]
- [7].Sasnauskiene A, Jonusiene V, Krikstaponiene A, et al. NOTCH1, NOTCH3, NOTCH4, and JAG2 protein levels in human endometrial cancer. Medicina (Kaunas). 2014;50(1):14–18. doi: 10.1016/j.medici.2014.05.002. PMID:25060200 [DOI] [PubMed] [Google Scholar]
- [8].Klinakis A, Lobry C, Abdel-Wahab O, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473(7346):230–233. doi: 10.1038/nature09999. PMID:21562564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taghavi N, Yazdi I. Prognostic factors of survival rate in oral squamous cell carcinoma: clinical, histologic, genetic and molecular concepts. Arch Iran Med. 2015;18(5):314–319. doi:0151805/AIM.0010. PMID:25959914 [PubMed] [Google Scholar]
- [10].Koch U, Radtke F. Notch signaling in solid tumors. Curr Top Dev Biol. 2010;92:411–455. doi: 10.1016/S0070-2153(10)92013-9. PMID:20816403 [DOI] [PubMed] [Google Scholar]
- [11].Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. PMID:21798893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi: 10.1126/science.1206923. PMID:21798897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sun W, Gaykalova DA, Ochs MF, et al. Activation of the NOTCH pathway in head and neck cancer. Cancer Res. 2014;74(4):1091–1104. doi: 10.1158/0008-5472.CAN-13-1259. PMID:24351288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mao L. NOTCH mutations: multiple faces in human malignancies. Cancer Prev Res (Phila). 2015;8(4):259–261. doi: 10.1158/1940-6207.CAPR-15-0063. PMID:25712049 [DOI] [PubMed] [Google Scholar]
- [15].Song X, Xia R, Li J, et al. Common and complex Notch1 mutations in Chinese oral squamous cell carcinoma. Clin Cancer Res. 2014;20(3):701–710. doi: 10.1158/1078-0432.CCR-13-1050. PMID:24277457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Upadhyay P, Nair S, Kaur E, et al. Notch pathway activation is essential for maintenance of stem-like cells in early tongue cancer. Oncotarget. 2016;7(31):50437–50449. doi: 10.18632/oncotarget.10419. PMID:27391340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang TH, Liu HC, Zhu LJ, et al. Activation of Notch signaling in human tongue carcinoma. J Oral Pathol Med. 2011;40(1):37–45. doi: 10.1111/j.1600-0714.2010.00931.x. PMID:20819128 [DOI] [PubMed] [Google Scholar]
- [18].Yoshida R, Nagata M, Nakayama H, et al. The pathological significance of Notch1 in oral squamous cell carcinoma. Lab Invest. 2013;93(10):1068–1081. doi: 10.1038/labinvest.2013.95. PMID:23938602 [DOI] [PubMed] [Google Scholar]
- [19].Cialfi S, Palermo R, Manca S, et al. Loss of Notch1-dependent p21(Waf1/Cip1) expression influences the Notch1 outcome in tumorigenesis. Cell Cycle. 2014;13(13):2046–2055. doi: 10.4161/cc.29079. PMID:24801890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ntziachristos P, Lim JS, Sage J, et al. From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell. 2014;25(3):318–334. doi: 10.1016/j.ccr.2014.02.018. PMID:24651013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rose SL, Kunnimalaiyaan M, Drenzek J, et al. Notch 1 signaling is active in ovarian cancer. Gynecol Oncol. 2010;117(1):130–133. doi: 10.1016/j.ygyno.2009.12.003. PMID:20060575 [DOI] [PubMed] [Google Scholar]
- [22].Kim HJ, Kim MJ, Kim A, et al. The role of Notch1 signaling in anaplastic Thyroid Carcinoma. Cancer Res Treat. 2017;49(2):509–517. doi: 10.4143/crt.2016.214. PMID:27586674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dai MY, Fang F, Zou Y, et al. Downregulation of Notch1 induces apoptosis and inhibits cell proliferation and metastasis in laryngeal squamous cell carcinoma. Oncol Rep. 2015;34(6):3111–3119. doi: 10.3892/or.2015.4274. PMID:26398771 [DOI] [PubMed] [Google Scholar]
- [24].Ai X, Jia Z, Liu S, et al. Notch-1 regulates proliferation and differentiation of human bladder cancer cell lines by inhibiting expression of Kruppel-like factor 4. Oncol Rep. 2014;32(4):1459–1464. doi: 10.3892/or.2014.3350. PMID:25109409 [DOI] [PubMed] [Google Scholar]
- [25].Bolos V, Mira E, Martinez-Poveda B, et al. Notch activation stimulates migration of breast cancer cells and promotes tumor growth. Breast Cancer Res. 2013;15(4):R54. doi: 10.1186/bcr3447. PMID:23826634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim A, Kim EY, Cho EN, et al. Notch1 destabilizes the adherens junction complex through upregulation of the Snail family of E-cadherin repressors in non-small cell lung cancer. Oncol Rep. 2013;30(3):1423–1429. doi: 10.3892/or.2013.2565. PMID:23807483 [DOI] [PubMed] [Google Scholar]
- [27].Liao Y, He X, Qiu H, et al. Suppression of the epithelial-mesenchymal transition by SHARP1 is linked to the NOTCH1 signaling pathway in metastasis of endometrial cancer. BMC Cancer. 2014;14:487. doi: 10.1186/1471-2407-14-487. PMID:24997474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhou Q, Wang Y, Peng B, et al. The roles of Notch1 expression in the migration of intrahepatic cholangiocarcinoma. BMC Cancer. 2013;13:244. doi: 10.1186/1471-2407-13-244. PMID:23688168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yan C, Yu J, Kang W, et al. miR-935 suppresses gastric signet ring cell carcinoma tumorigenesis by targeting Notch1 expression. Biochem Biophys Res Commun. 2016;470(1):68–74. doi: 10.1016/j.bbrc.2015.12.116. PMID:26742429 [DOI] [PubMed] [Google Scholar]
- [30].Li L, Zhao F, Lu J, et al. Notch-1 signaling promotes the malignant features of human breast cancer through NF-kappaB activation. PLoS One. 2014;9(4):e95912. doi: 10.1371/journal.pone.0095912. PMID:24760075 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [31].Ahn JS, Ann EJ, Kim MY, et al. Autophagy negatively regulates tumor cell proliferation through phosphorylation dependent degradation of the Notch1 intracellular domain. Oncotarget. 2016;7(48):79047–79063. doi: 10.18632/oncotarget.12986. PMID:27806347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang R, Sun Q, Wang P, et al. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7(5):5754–5768. doi: 10.18632/oncotarget.6805. PMID:26735577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stifani S, Blaumueller CM, Redhead NJ, et al. Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nat Genet. 1992;2(4):343. doi: 10.1038/ng1292-343a. PMID:1303292 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


