Abstract
Extensively drug-resistant Klebsiella pneumoniae (XDR-KP) infections cause high mortality and are disseminating globally. Identifying the genetic basis underpinning resistance allows for rapid diagnosis and treatment. XDR isolates sourced from Greece and Brazil, including 19 polymyxin-resistant and five polymyxin-susceptible strains, were subjected to whole genome sequencing. Seventeen of the 19 polymyxin-resistant isolates harboured variations upstream or within mgrB. The most common mutation identified was an insertion at nucleotide position 75 in mgrB via an ISKpn26-like element in the ST258 lineage and ISKpn13 in one ST11 isolate. Three strains acquired an IS1 element upstream of mgrB and another strain had an ISKpn25 insertion at 133 bp. Other isolates had truncations (C28STOP, Q30STOP) or a missense mutation (D29E) affecting mgrB. Complementation assays revealed all mgrB perturbations contributed to resistance. Missense mutations in phoQ (T281M, G385C) were also found to facilitate resistance. Several variants in phoPQ co-segregating with the ISKpn26-like insertion were identified as potential partial suppressor mutations. Three ST258 samples were found to contain subpopulations with different resistance-conferring mutations, including the ISKpn26-like insertion colonizing with a novel mutation in pmrB (P158R), both confirmed via complementation assays. These findings highlight the broad spectrum of chromosomal modifications which can facilitate and regulate resistance against polymyxins in K. pneumoniae.
Keywords: Klebsiella pneumoniae, antibiotic resistance, polymyxin, chromosomal variants
Data Summary
Whole genome sequencing of the 24 clinical isolates has been deposited under BioProject PRJNA307517 (www.ncbi.nlm.nih.gov/bioproject/PRJNA307517).
Impact Statement.
Klebsiella pneumoniae contributes to a high abundance of nosocomial infections and the rapid emergence of antimicrobial resistance hinders treatment. Polymyxins are predominantly utilized to treat multidrug-resistant infections, but resistance to the polymyxins is arising. This increasing prevalence in polymyxin resistance is particularly evident in Greece and Brazil. Identifying the genomic variations conferring resistance in clinical isolates from these regions will help with potentially detecting novel variants and tracing the spread of particular strains. This study commonly found mutations in the gene mgrB, the negative regulator of PhoPQ, known to cause resistance in K. pneumoniae. In the remaining isolates, missense mutations in phoQ were accountable for resistance. Multiple novel mutations were detected to be segregating with mgrB perturbations. This was either due to a mixed heterogeneous sample of two polymyxin-resistant strains, or because of multiple mutations within the same strain. Of interest was the validation of novel mutations in phoPQ segregating with a previously known ISKpn26-like element in disrupted mgrB isolates. Complementation of these phoPQ mutations revealed a reduction in minimum inhibitory concentrations and suggests the first evidence of partial suppressor mutations in K. pneumoniae. This research builds upon our current understanding of heterogeneity, lineage-specific mutations and regulatory variations relating to polymyxin resistance.
Introduction
Klebsiella pneumoniae (KP) strains classified as extensively drug-resistant (XDR) are rapidly emerging due to the dissemination of plasmid-encoded resistance towards aminoglycosides, β-lactams, fluoroquinolones and carbapenems [1]. Notably, carbapenem-resistant KP have been linked to high morbidity and an overall mortality of 48 % in infected patients [2]. Polymyxin B and colistin (polymyxin E) are now one of the last viable therapeutic options [3]. Unfortunately, resistance to this last-line antibiotic class is an increasing global burden, with countries particularly impacted including Asia (South Korea [4, 5], India [6, 7]), Europe (Greece [8–10]), Italy [10, 11]) and Latin America (Brazil [12, 13]). Mortality is influenced by polymyxin resistance typically occurring on a multidrug-resistant (MDR) or XDR background; nephrotoxicity leads to suboptimal dosing as well as inadequacies in detection of heteroresistance [10, 14]. As a result, there is considerable uncertainty regarding the mortality associated with polymyxin-resistant infections. Combining several clinical cohorts has provided overall mortality estimates ranging from 20 to 100 % [10].
Polymyxins infiltrate Gram-negative bacteria via initial binding to the basal component of lipopolysaccharide, lipid A. This causes the displacement of Mg2+ and Ca2+, disrupting the outer and inner membrane integrity, resulting in leakage of cytoplasmic contents and subsequent cell death, but the exact mechanism involved remains elusive [15, 16]. Inhibition of an intracellular target, the type II NADH-quinolone oxidoreductases, embedded in the inner membrane has also been identified [17]. An exposure in KP leads to the emergence of polymyxin resistance by triggering the activation of the two-component regulatory systems, PmrAB and PhoPQ [18–20]. These systems regulate a pathway that modulates pmrC and the pmrHFIJKLM operon, facilitating the addition of phosphoethanolamine (pEtN) and/or 4-amino-4-deoxy-l-arabinose to lipid A phosphate groups, resulting in impaired polymyxin binding interactions [21–23]. Disruption of mgrB, the negative regulator of PhoPQ, has been commonly observed in isolates of clinical origin [8, 24]. The constitutive up-regulation of pmrC and the pmrHFIJKLM operon incurs a minimal fitness cost and appears to be stable, with few reports of reversions [25, 26]. Heteroresistant populations, in which only a subset of bacteria are resistant in a phenotypically susceptible sample, have been reported in KP, which complicates diagnosis [27]. The emergence of pandrug-resistant KP is of grave concern [28] and this acquisition of resistance is further exacerbated by the recently reported plasmid-encoded colistin resistance gene mcr-1, which encodes a pEtN transferase enzyme [29, 30]. The presence of mcr genes in KP is currently a rarity with only a few reports of mcr-1, -1.2 and -3 [31–33]. Göttig et al. recently established a fitness cost associated with KP harbouring mcr-1, in contrast to Escherichia coli, which may explain the lack of isolates acquiring this gene [34]. This study aimed to investigate XDR-KP clinical isolates arising in Greece and Brazil during 2012–2014 to identify and validate genetic variants contributing to resistance. These variants were compared to prior clinical isolates to ascertain if these mutations have been previously detected globally.
Methods
Bacterial isolates
Polymyxin-resistant XDR-KP clinical isolates were acquired from the Hygeia General Hospital, Athens, Greece, and Instituto Dante Pazzanese de Cardiologia, Brazil, from patients in 2012–2014. These isolates were sampled at random (non-sequential). We also obtained five polymyxin-susceptible strains which were utilized as a genomic background reference and a negative control for complementation assays. Cultures were supplied as stabs/slants or on agar, and were subsequently cultured in nutrient broth. Cultures were made to 20 % (v/v) glycerol and stored at −80 °C. When required for assay or extraction, glycerol stocks were struck out to obtain single colonies on either nutrient agar or tryptic soy agar with 5 % defibrinated sheep blood. Reference strains included E. coli (ATCC 25922) and Klebsiella spp. (ATCC 13883, ATCC 700603, ATCC BAA-2146), which were obtained from the American Type Culture Collection (ATCC).
Antimicrobial susceptibility assays
Species identification and susceptibility profiles of clinical isolates from Greece and Brazil were evaluated in the clinic using the VITEK2 system (bioMérieux). Strains were further validated at the Institute for Molecular Bioscience (IMB) (The University of Queensland, Australia) using the standard Clinical and Laboratory Standards Institute (CLSI) approved broth microdilution (BMD) methods with cation-adjusted Mueller-Hinton broth (caMHB). Resistance was determined as per CLSI guidelines [35] except for tigecycline and fosfomycin for which The European Committee on Antimicrobial Susceptibility Testing (EUCAST) (Version 7.1, 2017) (see http://www.eucast.org) guidelines were implemented. Classification of isolates as MDR or XDR was determined through guidelines previously outlined [36].
DNA extraction
Isolates selected for sequencing exhibited a polymyxin-resistant XDR profile with five polymyxin-susceptible strains included to discern the mutations in mgrB, pmrAB and phoPQ segregating with susceptible isolates. DNA was extracted from overnight cultures using the DNeasy Blood and Tissue Kit (Qiagen) with the additional enzymatic lysis buffer pre-treatment as per the manufacturer’s instructions. DNA was quantified with Qubit3.0 (ThermoFisher Scientific).
DNA library preparation and sequencing
Library preparation was performed using the Nextera XT kit (Illumina) with 1 ng input of DNA as per the manufacturer’s instructions. Quality of libraries was checked using a 2100 Bioanalyzer (Agilent Technologies). Libraries were prepared using the 2×300 v3 MiSeq kit and sequenced on an Illumina MiSeq with 300 bp paired-end sequencing reads and >100× coverage per sample.
Sequencing analysis
Paired-end reads were trimmed with Trimmomatic [37] and assembled using SPAdes [38]. The Rapid Annotation using Subsystem Technology (RAST) was utilized to annotate assembled genomes [39]. Assemblies were also uploaded to the Centre for Genomic Epidemiology (CGE) to identify sequence types (STs) (MultiLocus Sequence Typing Server 1.8 [40]) and acquired antibiotic resistance genes (ResFinder 3.0 [41]). A neighbour-joining tree was reconstructed using the 2358 Klebsiella pneumoniae/quasipneumoniae/variicola genes known to form the core genome multi-locus sequence type (cgMLST) using Ridom SeqSphere +v4.0.1 software [42]. Complete assemblies of publicly available reference genomes were obtained from www.ncbi.nlm.nih.gov/gen ome/?term=klebsiella. ST references included HS11286 (ST11), MS6671 (ST147), and NJST258_1 and NJST258_2 (ST258). Species references were K. quasipneumoniae (ATCC 700603, HKUOPA4) and K. variicola (At-22, GJ1).
Variant detection
Variants both in and flanking the genes pmrAB, phoPQ and mgrB were examined and sequence reads of all strains were aligned to the assembly of 20_GR_12, a polymyxin-susceptible ST258 strain with the least number of contigs, using BWA-MEM [43]. The alignment was analysed through FreeBayes [44] to identify single nucleotide and small indel variation, using a diploid analysis. The diploid analysis displays reads mapping to the predominant variant in the isolate and if a variant in lower abundance (≥20 % of reads) was identified, this was classified as heterogeneity. The effects of variations were determined by snpEff [45]. The impact on protein sequence was further confirmed by the Protein Variation Effect Analyzer (PROVEAN) [46]. For the analysis of large chromosome changes, the gene sequences including 300 bp flanking were extracted from the assemblies. A multiple alignment of each gene was reconstructed from the pairwise alignment to the longest gene sequence. Furthermore, assemblies of the five genes were aligned to the reference polymyxin-susceptible isolate ATCC 700603 to discern lineage- and species-specific variation.
Insertion sequence element validation
ISFinder [47] was used for the identification of insertion sequence (IS) elements. To confirm disruptive IS elements, mgrB was amplified with the primers displayed in Table S1 (available in the online version of this article) via 2× Phusion HF master mix (Invitrogen) under the following cycling conditions: 98 °C for 10 s, 50 °C for 30 s and 72 °C for 60 s (35×). Amplicon identity was validated via Sanger sequencing.
Complementation assays
The contribution of variants to resistance was validated through complementation assays as previously described [48]. Briefly, genes (Table S1) were amplified from a polymyxin-susceptible isolate, 20_GR_12, and cloned into the pCR-Blunt II-TOPO vector via the Zero Blunt TOPO PCR cloning kit (Invitrogen). Chemically competent E. coli TOP10 cells were transformed and selected by the addition of 50 mg l−1 kanamycin in Mueller-Hinton agar (MHA). Isolation of plasmids was done via the PureLink Quick Plasmid Miniprep Kit (Invitrogen). The methodology for preparing electrocompetent cells and complementation assays was kindly provided by Dr Aurélie Jayol and Professor Patrice Nordmann. Briefly, overnight cultures were subcultured into 200 ml Luria-Bertani broth (1 : 100 dilution) and grown to an OD600 of 0.6±0.05. Cells were chilled on ice before centrifugation (10 000 r.p.m., 10 min, 4 °C), washed twice with ice cold 10 % glycerol and concentrated to 500 µl. KP isolates were transformed via electroporation (25 µF, 200 Ω, 1.25 kV cm−1) with a Gene Pulser (Bio-Rad Laboratories). Selection was accomplished through supplementation of ≥500 mg l−1 zeocin on MHA plates. Transformed colonies (n≥2) were acquired and placed in MHB containing 1500 mg l−1 zeocin and 1 mM isopropyl β-d-1-thiogalactopyranoside (Sigma Aldrich). If polymyxin susceptibility was not restored upon complementation, genes harbouring mutations were further amplified and introduced into 20_GR_12. To discern the impact of additional mutations in phoPQ and pmrB segregating with disrupted mgrB, mutant genes were introduced into a polymyxin-resistant isolate only harbouring an IS element mgrB disruption, 7_GR_13. Controls included transformation of wild-type (WT) genes into 20_GR_12, sequencing of amplicons prior to introduction in the vector and in KP-transformed strains undergoing a plasmid extraction, and further PCR of the multiple cloning site. Antimicrobial testing against colistin and polymyxin B were conducted as described above.
Results
Characterization of clinical isolates
KP isolates were all characterized in the hospital microbiology facility using VITEK2 cards. Several discrepancies were detected between VITEK2 and broth microdilution (BMD) results (Tables 1 and S2), predominantly the level of resistance towards aminoglycosides, tetracyclines, fosfomycin and tigecycline. A major dissimilarity was polymyxin susceptibility in 6_GR_12 (sensitive in BMD, resistant in VITEK2) and resistance in 23_GR_13 (resistant in BMD, sensitive in VITEK2). Polymyxin resistance was identified in 19 of the isolates and minimum inhibitory concentrations (MICs) ranged from 8 to >64 mg l−1. An abundance of acquired resistance genes (Table 2) was detected and this presence corresponded to the antimicrobial testing phenotype. This analysis did not identify mcr genes (mcr-1, -2, -3, -4, -5) in these strains. Only 18_GR_14 and 19_GR_14 were not identified as extended-spectrum beta-lactamase producers amongst the polymyxin-resistant strains. Consequently, all polymyxin-resistant strains that harboured non-susceptibility to at least one antibiotic in 15 or more of the 17 antimicrobial categories were defined as XDR.
Table 1. BMD and VITEK2 antimicrobial testing for the 24 clinical isolates.
| Strain* | Source† | Resistance profile‡ | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||||||||
| AMK | GEN | TOB | CPT | TZP | IPM | MEM | CFZ | FEP | CTX | CAZ | FOX | CIP | SXT | TGC | ATM | AMP | SAM | CHL | FOF | CST | MIN | TET | ||
| 1_GR_13 | St | R | R | R | RN | R | R | R | RN | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| 2_GR_12 | U | R | R | R | RN | R | R | R | RN | R | R | R | R | R | R | IR | R | R | R | R | RS | R | R | R |
| 3_GR_13 | S | R | R | R | RN | R | R | R | RN | RI | R | R | R | R | R | I | R | R | R | R | R | RI | IR | R |
| 4_GR_12 | B | R | R | R | RN | R | R | R | RN | R | R | R | R | R | R | IR | R | R | R | R | R | R | IR | R |
| 5_GR_13 | St | S | S | I | RN | R | R | R | RN | R | R | R | R | R | R | IR | R | R | R | R | R | R | R | R |
| 6_GR_12 | St | S | S | IR | RN | R | R | R | RN | R | R | R | R | R | R | R | R | R | R | R | R | SR | IR | R |
| 7_GR_13 | St | R | S | R | RN | R | R | R | RN | R | R | R | R | R | R | R | R | R | R | R | R | R | SR | SI |
| 8_GR_13 | St | R | R | R | RN | R | R | R | RN | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| 9_GR_12 | Br | IR | S | R | RN | R | R | R | RN | R | R | R | R | R | R | IR | R | R | R | R | R | R | IR | SI |
| 10_GR_13 | B | SR | S | R | RN | R | R | R | RN | R | R | R | R | R | R | I | R | R | R | R | R | R | SR | SI |
| 11_BR_13 | U | S | S | RN | RN | R | R | R | RN | R | R | R | R | R | RN | I | R | R | R | SN | RN | R | SN | SN |
| 12_BR_13 | Br | S | RS | IN | RN | R | R | R | RN | RI | R | R | R | R | RN | IR | R | R | R | RN | RN | R | IN | SN |
| 13_GR_14 | Br | IR | S | R | RN | R | R | R | RN | R | RN | R | R | R | R | SR | R | R | R | R | R | R | SI | SI |
| 14_GR_14 | U | IR | SR | R | RN | R | R | R | RN | R | RN | R | R | R | R | SR | R | R | R | R | R | R | SR | SR |
| 15_GR_13 | St | IR | S | R | RN | R | R | R | RN | RI | RN | R | R | R | R | IR | R | R | R | R | R | R | SI | S |
| 16_GR_13 | St | R | R | R | RN | R | R | R | RN | R | RN | R | R | R | R | RI | R | R | R | R | R | R | IR | R |
| 17_GR_14 | St | R | R | R | RN | R | R | R | RN | R | RN | R | R | R | R | I | R | R | R | R | R | R | SR | R |
| 18_GR_14 | St | IR | S | R | RN | R | R | R | RN | R | R | R | R | R | R | I | R | R | R | R | R | R | SI | S |
| 19_GR_14 | St | IR | S | R | RN | R | R | R | RN | R | R | R | R | R | R | R | R | R | R | R | R | R | IR | IR |
| 20_GR_12 | St | R | S | R | RN | R | R | R | RN | RI | R | R | R | R | R | R | R | R | R | R | R | S | R | R |
| 21_GR_13 | U | S | SR | I | RN | R | R | IR | RN | RI | R | R | R | S | R | S | S | R | R | S | S | S | S | S |
| 22_GR_12 | S | IR | S | R | RN | R | R | R | RN | RI | R | R | R | R | R | I | R | R | R | R | RS | S | SI | S |
| 23_GR_12 | St | R | R | R | RN | R | R | R | RN | R | R | R | R | R | R | R | R | R | R | R | RS | RS | R | R |
| 24_GR_13 | St | IR | S | R | RN | R | R | R | RN | RI | R | R | R | R | R | IR | R | R | R | R | R | S | IR | S |
*Strain identification: numerical order catalogued at IMB_Country (GR, Greece; BR, Brazil)_last two digits of isolation year.
†Source represented as B, blood; Br; bronchial secretion; U, urine; S, sputum; St, stool.
‡Antibiotic resistance as determined by BMD according to CLSI guidelines [EUCAST for fosfomycin (disc diffusion) and tigecycline] and in superscript, any discrepancies identified in VITEK2 results. Antibiotic classes tested include: 1, aminoglycosides (amikacin, AMK; gentamicin, GEN; tobramycin, TOB); 2, anti-methicillin-resistant Staphylococcus aureus (MRSA) cephalosporins (ceftaroline, CPT); 3, anti-pseudomonal penicillins + β-lactamase inhibitors (piperacillin-tazobactam, TZP); 4, carbapenems (imipenem, IPM; meropenem, MEM); 5, non-extended spectrum cephalosporins (1st and 2nd generation) (cefazolin, CFZ); 6, extended-spectrum cephalosporins (3rd and 4th generation) (cefepime, FEP; cefotaxime, CTX, ceftazidime, CAZ); 7, cephamycins (cefoxitin, FOX); 8, fluoroquinolones (ciprofloxacin, CIP); 9, folate pathway inhibitors (trimethoprim-sulfamethoxazole, SXT); 10, glycylcyclines (tigecycline, TGC); 11, monobactams (aztreonam, ATM); 12, penicillins (ampicillin, AMP); 13, penicillins + β-lactamase inhibitors (amipicillin-sulbactam, SAM); 14, phenicols (chloramphenicol, CHL); 15, phosphonic acids (fosfomycin, FOF); 16, polymyxins (colistin, CST); 17, tetracyclines (minocycline, MIN; tetracycline, TET). R, resistant; I, intermediate; S, susceptible; N, not tested.
Table 2. Potential mutations contributing to polymyxin resistance and acquired resistance genes.
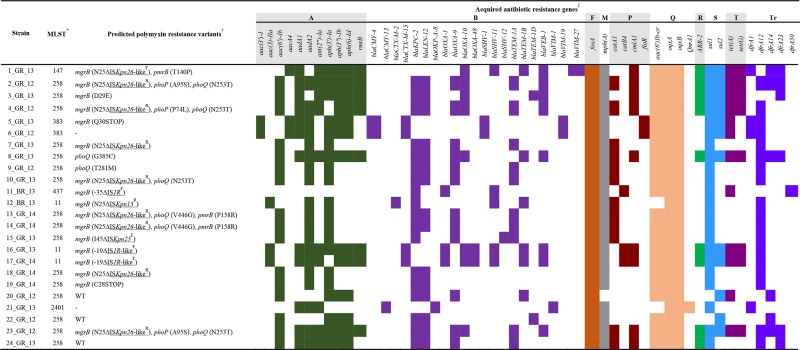 |
*Multilocus sequence type as identified through MultiLocus Sequence Typing Server 1.8.
†Variations detected in mgrB, phoPQ and pmrAB potentially causing polymyxin resistance. Significant non-synonymous changes determined by PROVEAN analysis. WT (wild-type) alleles in comparison to 20_GR_12. Displayed as gene impacted, initial amino acid, position and new amino acid. If a dash (–) is shown in front of the position, variant is encoded upstream and if a dash (–) is only displayed, no significant non-synonymous changes were detected in these loci. Insertion sequences (underlined) classified as Δ, identity as per ISFinder and orientation in superscript. Orientation determined as forward, F, if transposase is in the same direction as mgrB and conversely, reverse, R, if in the opposite direction to mgrB.
‡Acquired antibiotic resistance genes detected via ResFinder 3.0. Classes of antibiotics impacted are displayed as: A, aminoglycoside; B, beta-lactam; F; fosfomycin; M, macrolide; P, phenicol; Q, quinolone; R, rifampicin; S, sulphonamide; T, tetracycline; Tr, trimethoprim. Shading indicates detection of a gene (≥90 % homology, ≥60 % sequence length).
Sequence type determination
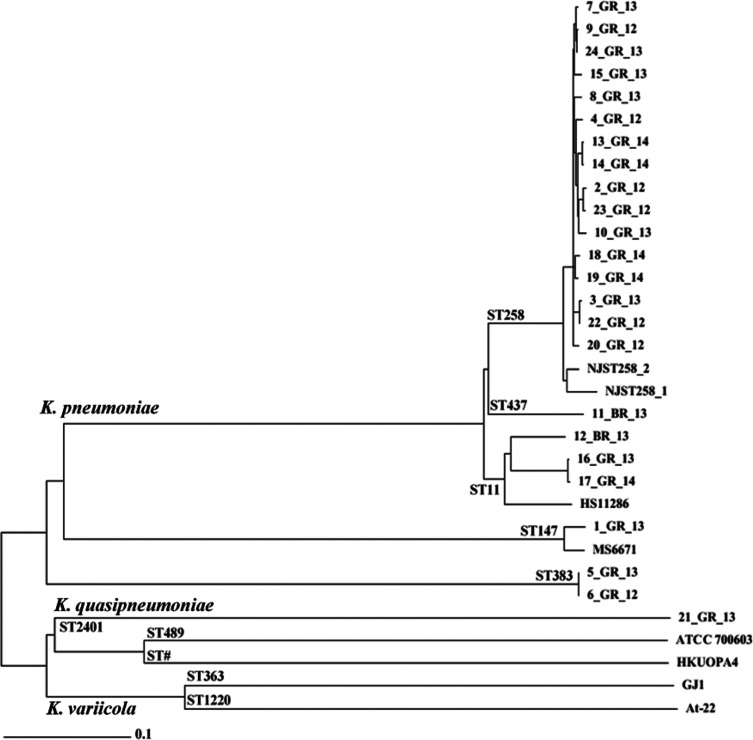
Sixteen of the 22 Greek clinical strains were found to belong to ST258 and the remaining were ST11, ST147 or ST383 (Table 1). While 5_GR_13 and 6_GR_12 were both ST383, only 5_GR_13 was resistant to polymyxin. Among the two strains from Brazil, 11_BR_13 was ST437 and 12_BR_13 was ST11. Strain 21_GR_13 had a profile previously undefined and has been newly designated ST2401. Further cgMLST studies were conducted on the isolates using complete assemblies of reference genomes for ST11 (HS11286), ST147 (MS6671), ST258 (NJST258_1, NJST258_2), K. quasipneumoniae (KQ) (ATCC 700603, HKUOPA4) and K. variicola (KV) (At-22, GJ1) (Fig. 1). For the ST258 isolates, these were more similar to NJST258_2 than to NJST258_1. Within this cluster, 7_GR_13, 9_GR_12 and 24_GR_13 were closely related (≤15 allelic changes). Similarly grouped together were 2_GR_12 and 23_GR_12; 3_GR_13 and 22_GR_12; 13_GR_14 and 14_GR_14; and 18_GR_14 and 19_GR_14. In ST11, 16_GR_13 and 17_GR_14 harboured only three allele differences and the Brazilian isolate, 12_BR_13, had 206 variants apparent. ST383 isolates 5_GR_13 and 6_GR_12 only exhibited one allele change. ST147 1_GR_13 was not clonal to the previous pandrug-resistant KP, MS6671. Clustering analysis revealed 21_GR_13 as KQ rather than KV.
Fig. 1.
Neighbour-joining tree of core genome MLST of 24 Klebsiella clinical isolates. Clustering of STs is indicated at the base of diverging branches. ST# indicates an uncharacterized MLST according to MLST server 1.8. cgMLST was used to compare completed assemblies including HS11286 (ST11), MS6671 (ST147), and NJST258_1 and NJST258_2 (ST258). Assemblies were also compared against K. quasipneumoniae (ATCC 700603, HKUOPA4) and K. variicola (At-22, GJ1) genomes.
MgrB disruption
Seventeen of the 19 polymyxin-resistant strains exhibited either missense mutations, nonsense mutations or IS elements in mgrB (Table 2). Both 5_GR_13 and 19_GR_14 harboured a truncation while an amino acid change, D29E, was apparent in 3_GR_13. IS element disruptions in mgrB were present in 14 strains and in nine isolates, and an IS5-like element was integrated at nucleotide position 75 (Fig. S1). Sanger sequencing revealed this element was closely related to ISKpn26, herein known as ISKpn26-like, except for 12_BR_13 which matched ISKpn13. IS1R was detected upstream of mgrB in 11_BR_13 and an IS1R-like (A>C, 393 bp; C>T, 396 bp) element in 16_GR_13 and 17_GR_14. Strain 15_GR_13 had a deletion of the mgrB locus from nucleotide position 133 onwards. The 127 bp flanking region mapped to ISKpn25 with the transposase in the same orientation as mgrB. All three of the IS1 element insertions, but only one of the eight ISKpn26-like element insertions had the transposase in the same orientation as mgrB.
Single, multiple and heterogeneous mutations
Mutations in genes commonly identified to confer polymyxin resistance in KP include mgrB, phoPQ and pmrAB (Table 2). Several non-synonymous mutations were identified across the isolates (Table S3). These mutations were analysed through the prediction tool, PROVEAN, and not all were identified to be deleterious, although this does not negate a functional purpose for these variants. ST383 contained several mutations in pmrAB although only Q30STOP in polymyxin-resistant 5_GR_13 was predicted to have an impact. Similarly, neutral changes in all four of these genes were detected in polymyxin-susceptible KQ strains ATCC 700603 and 21_GR_13. Strains 8_GR_13 and 9_GR_12 harboured a single detrimental missense mutation in phoQ. Seven of the 17 isolates containing an mgrB variant were accompanied by one or more missense mutations in phoPQ and/or pmrB. Predicted deleterious variants segregating with disrupted mgrB included pmrB (T140P, P158R), phoP (P74L, A95S) and phoQ (N253T, V446G), which were commonly found in the ST258 lineage. V446G (phoQ) and P158R (pmrB) were heterogeneous in 13_GR_14 [mutation allele frequency of 65 % (V446G) and 66 % (P158R)] and 14_GR_14 [mutation allele frequency of 52 % (V446G) and 57 % (P158R)]. Assembly revealed 23_GR_12 harboured an ISKpn26-like disrupted mgrB alongside the intact version with mutations in phoP and phoQ in 57 % of the samples. Furthermore, assemblies for mgrB, pmrAB and phoPQ were aligned to ATCC 700603 (Table S4). Several non-synonymous mutations were detected, but the majority were not predicted to be deleterious. Various mutations were unique to KP compared to KQ. ST11, 147, 258 and 437 remained conserved across these genes with the exception of mutations predicted to be deleterious. ST383 harboured several dissimilarities including the lack of pmrA (D131N) and pmrB (S105N) and gain of pmrA (G144D, D149E) and pmrB (A5V, M175V). Only subtle differences were observed in KQ isolate 21_GR_ 13, which included pmrA (I220N, D221E) and pmrB (G358A). Predicted deleterious mutations detected both in polymyxin-susceptible and in polymyxin-resistant isolates included pmrA (Q140L) and pmrB (R256G).
Role of mgrB disruptions and presence of heterogeneity via complementation assays
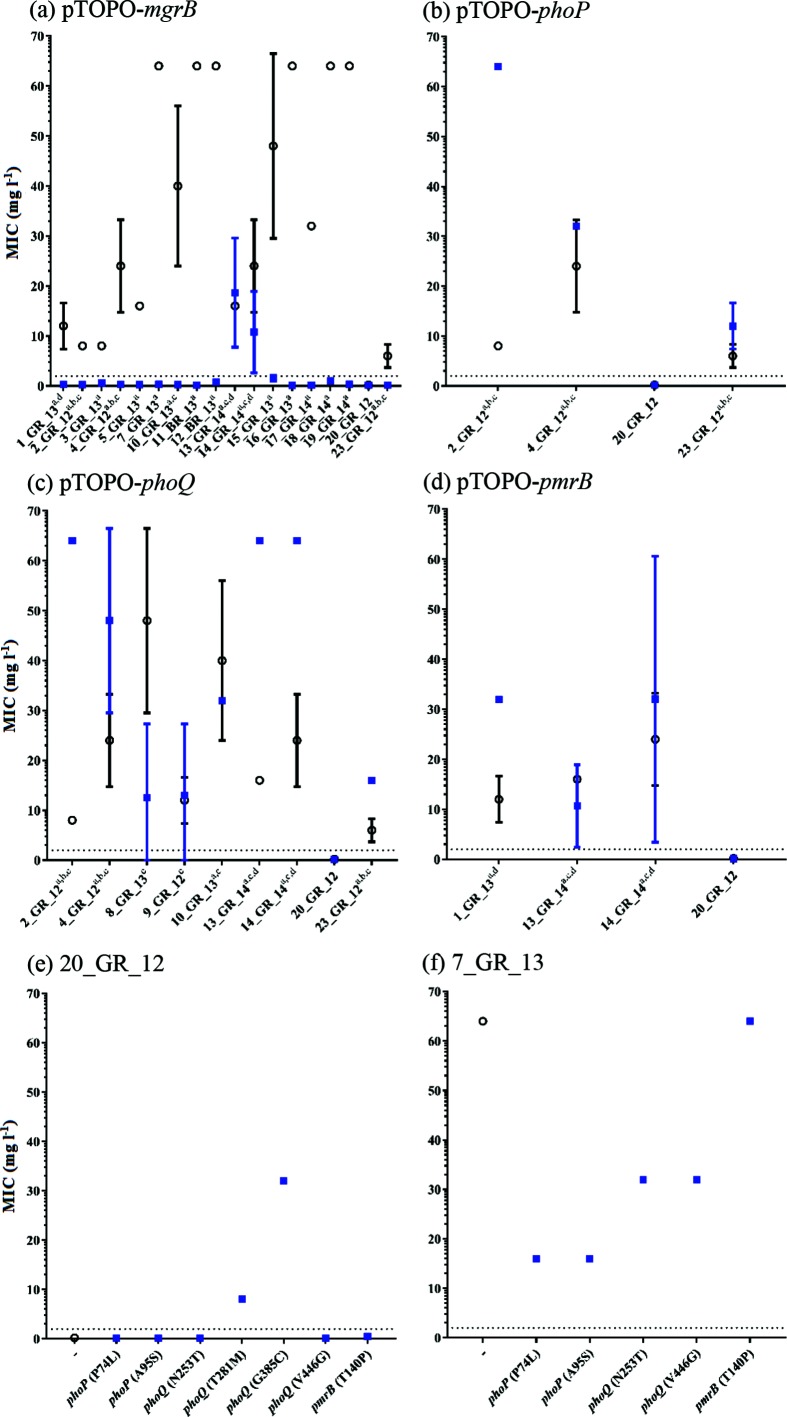
Complementation of the WT gene elucidated the role of these mutations in resistance (Fig. 2). MICs were determined against polymyxin B and colistin, but no difference was observed. Introduction of pTOPO-mgrB restored susceptibility in all resistant isolates with mgrB coding mutations or upstream disruptions, with the exception of two strains heterogeneous for the mgrB disruption and a pmrB coding mutation (13_GR_14 and 14_GR_14) (Fig. 2a). For these two strains, pTOPO-mgrB restored susceptibility in none of three 13_GR_14 colonies and one of three 14_GR_14 colonies. Transformation of one out of three colonies for both 13_GR_14 and 14_GR_14 strains with pTOPO-pmrB restored susceptibility (Fig. 2d) and mgrB amplification of these colonies revealed an intact mgrB locus (Fig. S2). Colonies which were reverted on complementation were further passaged three times with no antibiotic pressure in order to remove the plasmid and discern if these mutations were contributing to resistance. After passaging, pTOPO-mgrB isolates harboured an MIC of ≥64 mg l−1 whilst pTOPO-pmrB colonies had an MIC of 16 mg l−1, confirming two resistant populations in these samples. 23_GR_12 was also observed to have a heterogeneous mgrB disruption but did not carry a corresponding pmrB mutation, although it harboured similar mutations to 2_GR_12 in phoPQ. Amplification of mgrB identified two of three 23_GR_12 transformed colonies contained the IS element disruption and reverted to being susceptible upon complementation with pTOPO-mgrB.
Fig. 2.
Complementation assays and influence of gene on polymyxin resistance. Polymyxin B MICs measured before (○) and after (■) complementation of the wild-type gene (a) pTOPO-mgrB, (b) pTOPO-phoP, (c) pTOPO-phoQ or (d) pTOPO-pmrB in the indicated resistant isolates. (e) Mutated genes complemented into 20_GR_12 (polymyxin-susceptible isolate) to determine if the variant induces polymyxin resistance. (f) Complementation of 7_GR_13 (IS element disrupted mgrB control) to detect potential suppressor mutations. Strains shown on the x-axis for (a)–(d) and superscript indicates variants in genes including mgrB (a), phoP (b), phoQ (c) and pmrB (d) that differ from 20_GR_12. For (e) and (f), the x-axis shows the gene complemented with amino acid variation in parentheses. The dotted line at 2 mg l−1 represents the breakpoint for polymyxin B. Values are mean±sd, where no error bar indicates no fluctuation in MIC (n≥2 colonies).
Validation of resistance-conferring mutations in phoQ
Strains 8_GR_13 and 9_GR_12 harboured a single mutation in phoQ potentially conferring resistance (Table 2). When these isolates were transformed with pTOPO-phoQ, results remained variable where a lack of growth was present in a susceptible range (MIC ≤2 mg l−1), although several wells containing high polymyxin B concentrations exhibited growth (Fig. 2c). This result was reproducible (n≥4) and therefore the mutated gene was introduced into a polymyxin-susceptible isolate, 20_GR_12 (Fig. 2e). This complementation resulted in a consistent polymyxin-resistant phenotype.
Potential suppressor mutations in phoPQ
Several mutations co-segregating with the IS element-disrupted mgrB were detected, including phoP (P74L, A95S), phoQ (N253T, V446G) and pmrB (T140P). Complementation of WT genes in these isolates facilitated a ≥2-fold increase in MIC with the exception of 10_GR_13, which had an additional predicted neutral mutation in phoQ (A225T) (Table S3, Fig. 2b–d). To evaluate the potential influence of these mutations on polymyxin resistance, mutated genes were introduced into a strain only containing the mgrB IS element disruption, 7_GR_13 (Fig. 2f). Complementation of mutant pmrB (T140P) into 7_GR_13 did not lead to an observable corresponding reduction in MIC, but once transformed into 20_GR_12, a twofold increase in MIC was apparent (Fig. 2e). Variants in phoQ (N253T and V446G) exhibited a twofold reduction in MIC (Fig. 2e). Initially, the phoQ (V446G) mutation was anticipated to segregate with the mgrB-disrupted population in 13_GR_14 and 14_GR_14, but when phoQ was amplified from a colony reverted to susceptible via pTOPO-mgrB complementation, the WT phoQ was observed (Fig. S3). The phoQ (V446G) mutation was successfully amplified from a 14_GR_14 colony containing the pmrB (T158R) mutation. Although this mutation did not segregate with disrupted mgrB, it may act as a partial suppressor mutation when a resistance-conferring mutation is present in pmrB. Interestingly, a ≥4-fold reduction in MIC was witnessed for phoP mutations P47L and A95S, indicating partial suppressor mutations (Fig. 2e).
Discussion
Polymyxin resistance in XDR-KP is of grave concern given that this is a last-line antibiotic, and resistance is increasingly prevalent in countries such as Greece and Brazil [10, 12–14, 49]. We evaluated the genetic basis of polymyxin resistance in a series of Greek and Brazilian clinical isolates from patients in 2012–2014 and found variants in genes mgrB, phoPQ and pmrAB. Causative mutations attributed to polymyxin resistance were identified in these loci, but the contribution of other genes warrants further investigation.
Inactivation of mgrB was highly prevalent in these strains with an ISKpn26-like element being the predominant cause of resistance, as confirmed by complementation restoring susceptibility in all isolates. Several other studies have observed an IS5-like element integration in the same position, including reports from Greece, Italy, France, Turkey and Colombia [8, 9, 50, 51]. The ISKpn26-like element resembled the same sequence from Greek isolates previously described [51]. We identified that this mutation still persisted in 2014, after being first detected in 2012 [9]. Disruptions in mgrB including the ISKpn26-like forward insertion at nucleotide 75 in ST147, ISKpn13 integration at nucleotide 75 in ST11 and ISKpn25 in the ST258 lineage have yet to be reported. We identified IS1R or IS1R-like elements positioned upstream of mgrB in three isolates (11_BR_13, 16_GR_13, 17_GR_14) which were reverted upon complementation indicating an impact on the promoter region.
Truncations identified at positions 28 and 30 of mgrB have been previously detected, although these were identified in differing STs, indicating mutations potentially have arisen independently in Greece [24, 52]. Complementation restored susceptibility to polymyxins for these mutations and this study further revealed the amino acid change D29E in 3_GR_13 to be a resistance-conferring mutation. These findings support the notion that intact MgrB is required to confer negative feedback on PhoPQ [8]. The inactivation of mgrB is prevalent in polymyxin-resistant KP and may arise owing to its capacity to promote virulence and further attenuate the early host defence response, with little or no fitness cost [53].
Single predicted detrimental mutations were observed in the phoQ histidine kinase region, critical for phosphorylation and interaction with phoP, in 8_GR_13 (G385C) and 9_GR_12 (T281M). The G385C mutation had previously been reported, [24], but in a different ST. Complementation revealed an inconsistent MIC for these strains, although when a polymyxin-susceptible isolate was transformed with the mutated gene, full resistance was restored. Dominance of mutated phoQ has recently been highlighted and these results may imply the inability of pTOPO-phoQ to override the resistance caused by these mutations [54]. Furthermore, the inconsistencies in MIC may be attributed to the heightened expression of WT phoQ in the pCR-Blunt II-TOPO vector and warrants further investigation.
Several non-synonymous changes were identified to be not deleterious according to PROVEAN analysis. Notably, these were abundant in KQ strains, including 21_GR_13 and KP ST383 isolates. When these clinical isolates were aligned to ATCC 700603, multiple coding changes were identified, with the majority detected as neural changes with the exception of pmrA (Q140L) and pmrB (R256G). These mutations represent lineage-specific mutations, but this does not negate the possibility of previously resistance-conferring variants being acquired in these loci with subsequent reversion mutations to give rise to a susceptible phenotype.
Heterogeneity was apparent in several isolates. In near equal ratios, 13_GR_14 and 14_GR_14 possessed the ISKpn26-like mgrB disruption and a new mutation conferring resistance in pmrB, P158R, as determined by complementation. 23_GR_12 contained approximately half the reads mapping to the undisrupted genes and the other to the ISKpn26-like strain, with several additional predicted deleterious mutations. This heterogeneity may explain the initial clinical detection for this isolate to be polymyxin-susceptible.
Several isolates harbouring ISKpn26-like element-disrupted mgrB were accompanied by mutations in phoPQ and/or pmrB. These changes were present in ≥98 % of reads, making the involvement of heterogeneity unlikely. Once complemented, an increase in resistance was commonly recorded. This potentially reflects partial suppressor mutations as strains which solely possessed this IS element disruption commonly exhibited a heightened MIC of ≥64 mg l−1. One variant segregating with this disruption included pmrB T140P. This had formerly been identified in an ST258 lineage but even when the resistant gene was complemented, the MIC increased by twofold but was not defined as clinically resistant [24, 55].
When mutated phoP or phoQ were introduced into the mgrB-disrupted isolate, a reduction in MIC was apparent. The involvement of additional mutations in PhoPQ causing a suppressing effect on the level of resistance in a background where the disrupted mgrB is lacking has yet to be reported in KP. Previous research by Miller et al. [56] determined additional mutations in PhoPQ altered polymyxin resistance in Pseudomonas aeruginosa. Their study describes phoP mutations with the capacity to partially or fully suppress resistance-causing mutations in phoQ. These mutations in phoP were near or within the DNA binding site, which differs from our results, where the mutations are impacting the response regulatory region that interacts with PhoQ. Conversely, all mutations partially suppressing the MIC were identified to be targeting the HAMP (present in Histidine kinases, Adenylate cyclases, Methyl-accepting proteins and Phosphatases) domain and histidine kinase component of PhoQ. These were in regions similar to revertant P. aeruginosa strains identified by Lee and Ko [57]. We postulate these mutations are perturbing the critical transfer of phosphoryl groups from the histadine kinase of PhoQ to PhoP and subsequent pmrD expression. Whether these mutations constitute a fitness advantage due to the reduction of metabolism required for the production of lipopolysaccharide modifications is yet to be discerned. Furthermore, due to variability in some of the complementation data, a knockout phoPQ background and introduction of genes that are potential suppressor mutations is required.
Rapid and accurate detection of mutations attributed to polymyxin resistance remains a long-standing problem. Our research has contributed to the current understanding of the dissemination and evolution of this resistance in KP. Although our sample size is limited, this study highlights several issues arising from solely interrogating genomes for resistance detection, including ST-specific non-synonymous changes, and heterogeneity. Our study reveals several mutations causing polymyxin resistance across various STs in comparison with prior literature. These include the mgrB ISKpn26-like disruption (nucleotide 75), truncations in mgrB (nucleotides 28 and 30) and a missense mutation in phoQ (G385C). The study provides the first potential report of suppressor mutations for polymyxin resistance. Through complementation assays, we have discerned the role of these modifications and have identified resistance-causing variants that can be monitored in future genome-based diagnostics.
Data bibliography
NCBI Bioproject PRJNA307517 (2016).
Liu P, Li P, Jiang X, Bi D, Xie Y, Tai C, et al. Complete genome sequence of Klebsiella pneumoniae subsp. pneumoniae HS11286, a multidrug-resistant strain isolated from human sputum. J Bacteriol 2012;194:1841–1842. NCBI BioProject PRJNA78789.
Zowawi HM, Forde BM, Alfaresi M, Alzarouni A, Farahat Y, et al. Stepwise evolution of pandrug-resistance in Klebsiella pneumoniae. Sci Rep 2015;5:15082. European Nucleotide PRJEB7538.
Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, et al. Molecular dissection of the evolution of carbapenemresistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci USA 2014;111:4988–4993. European Nucleotide PRJNA237670.
Elliott AG, Ganesamoorthy D, Coin L, Cooper MA, Cao MD. Complete Genome Sequence of Klebsiella quasipneumoniae subsp. similipneumoniae Strain ATCC 700603. Genome Announc 2016;4:e00438-16.
Liu L, Ahmad AH, Leung FC. Klebsiella quasipneumoniae strain HKUOPA4, complete genome. NCBI Bioproject PRJNA224116 (2017).
Pinto-Tomás AA, Anderson MA, Suen G, Stevenson DM, Chu FS, et al. Symbiotic nitrogen fixation in the fungus gardens of leaf-cutter ants. Science 2009;326:1120–1123.
Di DY, Jang J, Unno T, Hur HG. Emergence of Klebsiella variicola positive for NDM-9, a variant of New Delhi metallo-β-lactamase, in an urban river in South Korea. J Antimicrob Chemother 2017;72:1063–1067.
Funding information
LC is an ARC Future Fellow (FT110100972). MAC is an NHMRC Principal Research Fellow (APP1059354) and currently holds a fractional Professorial Research Fellow appointment at the University of Queensland with his remaining time as CEO of Inflazome Ltd, a company headquartered in Dublin, Ireland, that is developing drugs to address clinical unmet needs in inflammatory disease by targeting the inflammasome. MEP is an Australian Postgraduate Award scholar. AGE and MATB are supported in part by a Wellcome Trust Strategic Award 104797/Z/14/Z. Research was supported by NHMRC grants (APP1005350, APP 1045326), an NIH grant (R21AI098731/R33AI098731) and an AID sequencing Grant (2013) as well as funding from the Institute for Molecular Bioscience Centre for Superbug Solutions (610246).
Acknowledgements
We thank Dr Aurélie Jayol and Professor Patrice Nordmann for providing their complementation assay methodology. The authors thank Maite Amado and Angela M. Kavanagh for technical support with susceptibility assays and M. Rhia L. Stone for the quality control of antibiotics. We acknowledge the sequencing services provided by the Australian Genome Research Facility. We thank the team of the curators of the Institut Pasteur MLST system (Paris, France) for importing novel alleles, profiles and/or isolates at http://bigsdb.web.pasteur.fr.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
There is no human or animal work in this study.
Supplementary Data
Footnotes
Abbreviations: ATCC, American Type Culture Collection; BMD, broth microdilution; caMHB, cation-adjusted Mueller-Hinton broth; cgMLST, core genome multi-locus sequence type; CLSI, Clinical and Laboratory Standards Institute; EUCAST, The European Committee on Antimicrobial Susceptibility Testing; HAMP, present in Histidine kinases, Adenylate cyclases, Methyl-accepting proteins and Phosphatases; IS, insertion sequence; KP, Klebsiella pneumoniae; KQ, Klebsiella quasipneumoniae; KV, Klebsiella variicola; MDR, multidrug-resistant; MHA, Mueller-Hinton agar; MHB, Mueller-Hinton broth; MIC, minimum inhibitory concentration; MLST, multi-locus sequence type; pEtN, phosphoethanolamine; PROVEAN, Protein Variation Effect Analyzer; ST, sequence type; WT, wild-type; XDR, extensively drug-resistant; XDR-KP, extensively drug-resistant Klebsiella pneumoniae.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Four supplementary tables and three supplementary figures are available with the online version of this article.
References
- 1.Ramirez MS, Traglia GM, Lin DL, Tran T, Tolmasky ME. Plasmid-mediated antibiotic resistance and virulence in Gram-negatives: the Klebsiella pneumoniae paradigm. Microbiol Spectr. 2014;2:1–15. doi: 10.1128/microbiolspec.PLAS-0016-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 3.Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther. 2012;10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 4.Suh JY, Son JS, Chung DR, Peck KR, Ko KS, et al. Nonclonal emergence of colistin-resistant Klebsiella pneumoniae isolates from blood samples in South Korea. Antimicrob Agents Chemother. 2010;54:560–562. doi: 10.1128/AAC.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SY, Shin J, Shin SY, Ko KS. Characteristics of carbapenem-resistant Enterobacteriaceae isolates from Korea. Diagn Microbiol Infect Dis. 2013;76:486–490. doi: 10.1016/j.diagmicrobio.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Mohamudha PR, Harish BN, Parija SC. Emerging carbapenem resistance among nosocomial isolates of Klebsiella pneumoniae in South India. Int J Pharma Bio Sci. 2010;1:1–11. [Google Scholar]
- 7.Goel G, Hmar L, Sarkar de M, Bhattacharya S, Chandy M. Colistin-resistant Klebsiella pneumoniae: report of a cluster of 24 cases from a new oncology center in eastern India. Infect Control Hosp Epidemiol. 2014;35:1076–1077. doi: 10.1086/677170. [DOI] [PubMed] [Google Scholar]
- 8.Cannatelli A, Giani T, D'Andrea MM, Di Pilato V, Arena F, et al. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother. 2014;58:5696–5703. doi: 10.1128/AAC.03110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavroidi A, Katsiari M, Likousi S, Palla E, Roussou Z, et al. Characterization of ST258 colistin-resistant, blaKPC-producing Klebsiella pneumoniae in a Greek Hospital. Microb Drug Resist. 2016;22:392–398. doi: 10.1089/mdr.2015.0282. [DOI] [PubMed] [Google Scholar]
- 10.Giamarellou H. Epidemiology of infections caused by polymyxin-resistant pathogens. Int J Antimicrob Agents. 2016;48:614–621. doi: 10.1016/j.ijantimicag.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Monaco M, Giani T, Raffone M, Arena F, Garcia-Fernandez A, et al. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: a rapidly evolving problem in Italy, November 2013 to April 2014. Euro Surveill. 2014;19:pii=20939. doi: 10.2807/1560-7917.ES2014.19.42.20939. [DOI] [PubMed] [Google Scholar]
- 12.Pereira GH, Garcia DO, Mostardeiro M, Fanti KS, Levin AS. Outbreak of carbapenem-resistant Klebsiella pneumoniae: two-year epidemiologic follow-up in a tertiary hospital. Mem Inst Oswaldo Cruz. 2013;108:113–115. doi: 10.1590/S0074-02762013000100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaspar GG, Bellissimo-Rodrigues F, Andrade LN, Darini AL, Martinez R. Induction and nosocomial dissemination of carbapenem and polymyxin-resistant Klebsiella pneumoniae. Rev Soc Bras Med Trop. 2015;48:483–487. doi: 10.1590/0037-8682-0041-2015. [DOI] [PubMed] [Google Scholar]
- 14.Ah YM, Kim AJ, Lee JY. Colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents. 2014;44:8–15. doi: 10.1016/j.ijantimicag.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 15.Koike M, Iida K, Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969;97:448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon RA, Chopra I. Leakage of periplasmic proteins from Escherichia coli mediated by polymyxin B nonapeptide. Antimicrob Agents Chemother. 1986;29:781–788. doi: 10.1128/AAC.29.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deris ZZ, Akter J, Sivanesan S, Roberts KD, Thompson PE, et al. A secondary mode of action of polymyxins against Gram-negative bacteria involves the inhibition of NADH-quinone oxidoreductase activity. J Antibiot. 2014;67:147–151. doi: 10.1038/ja.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groisman EA, Kayser J, Soncini FC. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol. 1997;179:7040–7045. doi: 10.1128/jb.179.22.7040-7045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velkov T, Thompson PE, Nation RL, Li J. Structure- activity relationships of polymyxin antibiotics. J Med Chem. 2010;53:1898–1916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng HY, Chen YF, Peng HL. Molecular characterization of the PhoPQ-PmrD-PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J Biomed Sci. 2010;17:60. doi: 10.1186/1423-0127-17-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helander IM, Kato Y, Kilpeläinen I, Kostiainen R, Lindner B, et al. Characterization of lipopolysaccharides of polymyxin-resistant and polymyxin-sensitive Klebsiella pneumoniae O3. Eur J Biochem. 1996;237:272–278. doi: 10.1111/j.1432-1033.1996.0272n.x. [DOI] [PubMed] [Google Scholar]
- 22.Velkov T, Deris ZZ, Huang JX, Azad MA, Butler M, et al. Surface changes and polymyxin interactions with a resistant strain of Klebsiella pneumoniae. Innate Immun. 2014;20:350–363. doi: 10.1177/1753425913493337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright MS, Suzuki Y, Jones MB, Marshall SH, Rudin SD, et al. Genomic and transcriptomic analyses of colistin-resistant clinical isolates of Klebsiella pneumoniae reveal multiple pathways of resistance. Antimicrob Agents Chemother. 2015;59:536–543. doi: 10.1128/AAC.04037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olaitan AO, Diene SM, Kempf M, Berrazeg M, Bakour S, et al. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int J Antimicrob Agents. 2014;44:500–507. doi: 10.1016/j.ijantimicag.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Arena F, Henrici de Angelis L, Cannatelli A, di Pilato V, Amorese M, et al. Colistin resistance caused by inactivation of the MgrB regulator is not associated with decreased virulence of sequence type 258 KPC carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2016;60:2509–2512. doi: 10.1128/AAC.02981-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JY, Choi MJ, Choi HJ, Ko KS. Preservation of acquired colistin resistance in Gram-negative bacteria. Antimicrob Agents Chemother. 2015;60:609–612. doi: 10.1128/AAC.01574-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meletis G, Tzampaz E, Sianou E, Tzavaras I, Sofianou D. Colistin heteroresistance in carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2011;66:946–947. doi: 10.1093/jac/dkr007. [DOI] [PubMed] [Google Scholar]
- 28.Zowawi HM, Forde BM, Alfaresi M, Alzarouni A, Farahat Y, et al. Stepwise evolution of pandrug-resistance in Klebsiella pneumoniae. Sci Rep. 2015;5:15082. doi: 10.1038/srep15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 30.Stoesser N, Mathers AJ, Moore CE, Day NP, Crook DW. Colistin resistance gene mcr-1 and pHNSHP45 plasmid in human isolates of Escherichia coli and Klebsiella pneumoniae. Lancet Infect Dis. 2016;16:285–286. doi: 10.1016/S1473-3099(16)00010-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Tian GB, Zhang R, Shen Y, Tyrrell JM, et al. Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: an epidemiological and clinical study. Lancet Infect Dis. 2017;17:390–399. doi: 10.1016/S1473-3099(16)30527-8. [DOI] [PubMed] [Google Scholar]
- 32.di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici de Angelis L, et al. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother. 2016;60:5612–5615. doi: 10.1128/AAC.01075-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin W, Li H, Shen Y, Liu Z, Wang S, et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio. 2017;8:e00543-17. doi: 10.1128/mBio.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tietgen M, Semmler T, Riedel-Christ S, Kempf VAJ, Molinaro A, et al. Impact of the colistin resistance gene mcr-1 on bacterial fitness. Int J Antimicrob Agents. 2017:pii: S0924-8579. doi: 10.1016/j.ijantimicag.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing– Twenty-sixth Edition: Approved standard M100S. Wayne, PA: CLSI; 2016. [Google Scholar]
- 36.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 37.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST) Nucleic Acids Res. 2014;42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, et al. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol. 2012;50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jünemann S, Sedlazeck FJ, Prior K, Albersmeier A, John U, et al. Updating benchtop sequencing performance comparison. Nat Biotechnol. 2013;31:294–296. doi: 10.1038/nbt.2522. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv. 2012:12073907. [Google Scholar]
- 45.Cingolani P, Platts A, Wang Lel, Coon M, Nguyen T, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi Y, Chan AP. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31:2745–2747. doi: 10.1093/bioinformatics/btv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jayol A, Poirel L, Brink A, Villegas MV, Yilmaz M, et al. Resistance to colistin associated with a single amino acid change in protein PmrB among Klebsiella pneumoniae isolates of worldwide origin. Antimicrob Agents Chemother. 2014;58:4762–4766. doi: 10.1128/AAC.00084-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neonakis IK, Samonis G, Messaritakis H, Baritaki S, Georgiladakis A, et al. Resistance status and evolution trends of Klebsiella pneumoniae isolates in a university hospital in Greece: ineffectiveness of carbapenems and increasing resistance to colistin. Chemotherapy. 2010;56:448–452. doi: 10.1159/000320943. [DOI] [PubMed] [Google Scholar]
- 50.Poirel L, Jayol A, Bontron S, Villegas MV, Ozdamar M, et al. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70:75–80. doi: 10.1093/jac/dku323. [DOI] [PubMed] [Google Scholar]
- 51.Cannatelli A, D'Andrea MM, Giani T, di Pilato V, Arena F, et al. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob Agents Chemother. 2013;57:5521–5526. doi: 10.1128/AAC.01480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aires CA, Pereira PS, Asensi MD, Carvalho-Assef AP. mgrB mutations mediating polymyxin B resistance in Klebsiella pneumoniae isolates from rectal surveillance swabs in Brazil. Antimicrob Agents Chemother. 2016;60:6969–6972. doi: 10.1128/AAC.01456-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kidd TJ, Mills G, Sá-Pessoa J, Dumigan A, Frank CG, et al. A Klebsiella pneumoniae antibiotic resistance mechanism that subdues host defences and promotes virulence. EMBO Mol Med. 2017;9:430–447. doi: 10.15252/emmm.201607336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halaby T, Kucukkose E, Janssen AB, Rogers MR, Doorduijn DJ, et al. Genomic characterization of colistin heteroresistance in Klebsiella pneumoniae during a nosocomial outbreak. Antimicrob Agents Chemother. 2016;60:6837–6843. doi: 10.1128/AAC.01344-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, et al. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci USA. 2014;111:4988–4993. doi: 10.1073/pnas.1321364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller AK, Brannon MK, Stevens L, Johansen HK, Selgrade SE, et al. PhoQ mutations promote lipid A modification and polymyxin resistance of Pseudomonas aeruginosa found in colistin-treated cystic fibrosis patients. Antimicrob Agents Chemother. 2011;55:5761–5769. doi: 10.1128/AAC.05391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JY, Ko KS. Mutations and expression of PmrAB and PhoPQ related with colistin resistance in Pseudomonas aeruginosa clinical isolates. Diagn Microbiol Infect Dis. 2014;78:271–276. doi: 10.1016/j.diagmicrobio.2013.11.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.