Key Points
Question
Can long-term survival be predicted by a simple scoring system for patients with myotonic dystrophy type 1, the most frequent neuromuscular disease?
Findings
In this cohort study including 1296 adults with myotonic dystrophy type 1, a practical risk score to predict the probability of 10-year survival was developed and validated internally and externally based on a set of common patient characteristics, including age, diabetes, need for support when walking, heart rate, systolic blood pressure, first-degree atrioventricular block, bundle-branch block, and lung vital capacity.
Meaning
The myotonic dystrophy type 1 prognostic score is associated with long-term survival.
This cohort study aims to develop and validate a prognostic score to predict 10-year survival in patients with myotonic dystrophy type 1.
Abstract
Importance
Life expectancy is greatly shortened in patients presenting with myotonic dystrophy type 1 (DM1), the most common neuromuscular disease. A reliable prediction of survival in patients with DM1 is critically important to plan personalized health supervision.
Objective
To develop and validate a prognostic score to predict 10-year survival in patients with DM1.
Design, Setting, and Participants
In this longitudinal cohort study, between January 2000 and November 2014, we enrolled 1296 adults referred to 4 tertiary neuromuscular centers in France for management of genetically proven DM1, including 1066 patients in the derivation cohort and 230 in the validation cohort. Data were analyzed from December 2016 to March 2017.
Main Outcomes and Measures
Factors associated with survival by multiple variable Cox modeling, including 95% confidence intervals, and development of a predictive score validated internally and externally. Mean values are reported with their standard deviations.
Results
Of the 1296 included patients, 670 (51.7%) were women, and the mean (SD) age was 39.8 (13.7) years. Among the 1066 patients (82.3%) in the derivation cohort, 241 (22.6%) died over a median (interquartile range) follow-up of 11.7 (7.7-14.3) years. Age, diabetes, need for support when walking, heart rate, systolic blood pressure, first-degree atrioventricular block, bundle-branch block, and lung vital capacity were associated with death. Simplified score points were attributed to each predictor, and adding these points yielded scores between 0 and 20, with 0 indicating the lowest and 20 the highest risk of death. The 10-year survival rate was 96.6% (95% CI, 94.4-98.9) in the group with 0 to 4 points, 92.2% (95% CI, 88.8-95.6) in the group with 5 to 7 points, 80.7% (95% CI, 75.4-86.1) in the group with 8 to 10 points, 57.9% (95% CI, 49.2-66.6) in the group with 11 to 13 points, and 19.4% (95% CI, 8.6-30.1) in the group with 14 points or more. In 230 patients (17.7%) included in the validation cohort, the 10-year survival rates for the groups with 0 to 4, 5 to 7, 8 to 10, 11 to 13, and 14 points or more were 99.3% (95% CI, 95.0-100), 80.6% (95% CI, 67.1-96.7), 79.3% (95% CI, 66.2-95.1), 43.2% (95% CI, 28.2-66.1), and 21.6% (95% CI, 10.0-46.8), respectively. The calibration curves did not deviate from the reference line. The C index was 0.753 (95% CI, 0.722-0.785) in the derivation cohort and 0.806 (95% CI, 0.758-0.855) in the validation cohort.
Conclusions and Relevance
The DM1 prognostic score is associated with long-term survival.
Introduction
Steinert disease, also known as myotonic dystrophy type 1 (DM1), is the most common inherited neuromuscular disease in adults, with a 1:8000 prevalence worldwide.1 It is an autosomal dominant disorder caused by the expansion of a (CTG)n triplet repeat in the 3′ untranslated region of the DMPK gene.2 Aside from muscle weakness and myotonia, the disease involves the respiratory, cardiac, endocrine, ocular, and central nervous systems.3,4
Life expectancy is greatly shortened in patients with DM1, mainly because of an increased risk of respiratory complications and sudden cardiac death.5,6 Despite the known correlation between overall disease severity and size of the CTG amplification in populations with DM1,7 the wide individual variability in vital prognosis complicates the management of the disorder.8 A reliable prediction of long-term mortality in this progressive disease is of major importance to plan a personalized health surveillance program based on appropriate screenings, monitoring of manageable complications, and specific preventive measures to improve its prognosis.
Several prognostic factors have been identified in DM1, such as age, severity of muscle weakness,5,6 or cardiac conduction defects,9 although, to our knowledge, a reliable prognostic risk score has yet to be created. Our objective was to develop a model to predict the survival of patients presenting with DM1 based on a set of common patient characteristics.
Methods
Derivation and Validation Cohorts
Our derivation cohort consisted of 1066 consecutive patients 18 years and older admitted to our medical institutions between January 2000 and November 2014 for the management of DM1, diagnosed by the presence of 50 or more CTG triplets in the 3′ untranslated region of the DMPK gene on blood leukocytes and included in the DM1 Heart Registry (clinicaltrials.gov identifier, NCT01136330), regardless of their underlying cardiovascular status. At the time of initial referral to the Cardiology Department, Cochin Hospital, Paris, France, or the Neurology Department, Pitié-Salpêtrière Hospital, Paris, France, patients presenting with DM1 underwent neurological, respiratory, and cardiac examinations. From June 2010 to November 2014, we prospectively entered observations from these examinations and results from genetic tests into a dedicated database; data from January 2000 to May 2010 were entered retrospectively. We based the development of the DM1 prognostic score on the observations made during the first hospitalization of these patients between January 2000 and November 2014. Because referral medical centers dedicated to the management of neuromuscular diseases are in charge of the genetic testing and follow-up of patients with DM1 in the French health care system, the vast majority of patients diagnosed in our geographic area (Paris region) during the study period were included in the registry. Therefore, our study sample is representative of the overall DM1 population in that area and subject to a minimal selection bias. This study, in compliance with the ethical principles formulated in the Declaration of Helsinki, was approved by the Comité de Protection des Personnes Ile de France III, and all patients granted written informed consent to participate in the registry.
Our validation cohort included 230 consecutive adult patients referred to the neurology or cardiology departments of Centre Hospitalier Universitaire de Nantes, Nantes, France, and Centre Hospitalier Régional Universitaire de Tours, Tours, France, between January 2000 and November 2014 for the management of DM1, diagnosed by the presence of 50 or more CTG triplets in the DMPK gene on blood leukocytes. The vast majority of patients with DM1 diagnosed in the catchment area of these medical centers during the study period were included in the study. Patients in the validation and derivation cohorts underwent the same genetic, neurological, respiratory, and cardiac investigations.
Determination of Vital Status and Causes of Death
The primary end point of the study was overall survival. We consulted the National Death Registry for all patients included in the derivation and validation cohorts at the end of the observation period in November 2014 to ascertain their vital status and determine the primary cause of death. The primary physicians, the hospital departments tracking all fatal outcomes, or both were contacted to collect information relative to the circumstances of deaths. Two study investigators (P.L. and D.D.) uninvolved in the data collection and unaware of the patients’ clinical statuses and medical managements reviewed these circumstances and classified all deaths according to their primary cause, based on the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision.
Predictors of Clinical Events
Based on a comprehensive literature review and clinical relevance, we identified 10 variables previously collected in the DM1 Heart Registry and potentially associated with all-cause mortality in DM1: age; mutation size; need for support when walking (defined as permanent use of a cane or a wheelchair); cardiovascular mortality, including atrial fibrillation, first-degree atrioventricular block on electrocardiogram, left or right bundle-branch block, and left ventricular ejection fraction; and respiratory mortality, including lung vital capacity and swallowing disorders. We also selected characteristics from our database that had a possible influence on the vital prognosis in the general population, including sex, diabetes, heart rate, systolic and diastolic blood pressure, dyslipidemia, hypertension, and smoking status. Heart rate and blood pressure were measured routinely during examinations by a cardiologist, according to American Heart Association guidelines10 after appropriate patient preparation, including a delay of 5 or more minutes before the first measurements were made.
Statistical Analysis
Categorical data are reported as counts and percentages and quantitative data as means and standard deviations or medians and interquartile ranges. Missing baseline data were handled with multiple imputations by chained equations using the baseline cumulative hazard of death in the imputation model.11,12 All predictors considered in the model development were used in the imputation model. Because roughly 35% of patients had 1 or more missing predictors, 35 independent imputed data sets were generated and analyzed separately.13 Convergence of the multiple imputation algorithms was assessed by visual inspection of the mean and variance of the imputations streams. Estimates were then pooled over the 35 imputations according to the standard rules by Rubin14 to provide point estimates and confidence intervals for each parameter.
Overall survival was estimated using the Kaplan-Meier product-limit method. Variables previously shown or suspected to influence survival in individuals with DM1 were analyzed using a Cox multiple variable model. The proportional hazards assumption was checked by examination of Schoenfeld residuals and the Grambsch-Therneau lack-of-fit test.15 Restricted cubic splines were used to determine whether continuous predictors could be analyzed as linear terms.16 Continuous variables were then grouped into 3 categories according to predefined cutoffs to simplify the presentation of the model and calculation of scores. For age, CTG expansion size, heart rate, and systolic blood pressure, in absence of widely accepted thresholds, we used sample tertiles to avoid overoptimistic data-driven cutoff points in the prognostic value of the variables. For vital capacity, we used thresholds that are commonly used in clinical practice.17 Two strategies were used to develop the model with the imputed data.18,19 First, we used Wald tests for the pooled regression coefficients to simplify the model with a backward selection procedure, with P value cutoffs mimicking the use of Akaike information criterion. This rule corresponds to a P value of .157 for a variable with 1 df. We used a similar backward elimination procedure in each imputed data set and retained the model comprising the variables selected in most imputed data sets. Both strategies selected the same variables. Two-by-two interactions between each of the selected variables were then examined using Wald tests for the pooled regression coefficients. No higher-order interactions were considered.
The model performance was evaluated by the concordance (C) index as a measure of discrimination and by the calibration curve. The C index measures how well the model discriminates between survivors and nonsurvivors and can be viewed as the extension of the area under the receiver operating characteristics curve for survival data.20 It varies theoretically between 0.5 and 1.0, with a value of 1.0 indicating perfect discrimination. The calibration curve contrasts observed vs predicted probabilities of events and evaluates the accuracy of the predictions. The slope of the calibration curve is a measure of overoptimism of the model predictions.
Because prognostic models derived from multiple variable regression with variable selection are prone to overestimate regression coefficients, our model was validated internally using bootstrapping.20 The second model selection strategy was repeated in 200 bootstrap samples, and the model estimated in each sample was evaluated in the original sample. The differences between the bootstrap and the original sample performances were taken as a measure of the overoptimism of the selected model. The C index corrected for overoptimism was then estimated, and the corrected calibration slope was used as a shrinkage factor for the regression coefficients of the selected model. In each case, the predictions and the estimation of model performance were estimated within the imputed data sets and then pooled (pool-last method), as recommended.21
The final model was presented with hazard ratios obtained after shrinkage with their 95% confidence intervals. In addition, a simplified scoring system was obtained by rounding the shrunken regression coefficients multiplied by 4 to the nearest integer. This facilitated the calculation of the prediction score. Predicted probabilities of survival at 10 years were then presented for each single score value, except for extreme values, which were grouped into scores of 0 or 1 and scores of 15 or greater, given the small samples sizes.
The DM1 prognostic score was externally validated according to principles and methods of Royston and Altman.22 Briefly, we first estimated the regression coefficient of the DM1 prognostic score in a Cox model fit to the validation cohort data set to estimate the calibration slope. A slope of 1 is ideal, while a slope less than 1 indicates a lower discrimination and a slope greater than 1 a higher discrimination. Second, we computed the discrimination of the score and the simplified score in the validation data by the C index. We then contrasted the observed survival estimated by the Kaplan-Meier method in the external validation cohort and the model-based predictions derived from the original model for grouped values of the simplified score. Finally, we contrasted the regression coefficients of the Cox model (log[hazard ratio]) for these categories in the derivation and the external validation cohorts. Missing data were handled by multiple imputation for the score development. Because data from 45 of 230 patients (20.0%) in the validation cohort were missing on the covariates needed to compute the score, 20 imputed data sets were created. All results presented correspond to pooled results over these 20 imputed data sets.
In all analyses, tests were 2-sided, and statistical significance was set at P < .05, except when otherwise stated. All analyses were performed using R statistical software version 3.2.0 (The R Foundation).23 More precisely, the survival package was used for survival analyses, the rms package was used for model building and internal validation, the boot package for bootstrap, and the mice package for multiple imputation.
Results
Characteristics and Outcome of the Derivation Cohort
At the time of initial presentation between January 2000 and November 2014, the mean (SD) age of the 1066 patients included in the derivation cohort was 39.3 (13.7) years, and 548 (51.4%) were women. Other baseline characteristics are presented in Table 1. Over a median (interquartile range) follow-up of 11.7 (7.7-14.3) years, 241 patients (22.6%) died at a mean (SD) age of 56.0 (11.6) years, representing a 2.4% (95% CI, 2.1-2.7) annual mortality (eFigure 1 in the Supplement). The primary causes of death are listed in the eTable in the Supplement. The most common primary causes of death were respiratory failure in 44 patients (18.3%), sudden cardiac death in 33 (13.7%), and pneumonia in 31 (12.9%).
Table 1. Characteristics of the Derivation and Validation Cohorts.
| Characteristic | Derivation Cohort (n = 1066) | Validation Cohort (n = 230) | ||||
|---|---|---|---|---|---|---|
| Original Dataa | Imputed Data Value | Original Dataa | Imputed Data Value | |||
| No. (%) | Missing, No. | No. (%) | Missing, No. | |||
| Age, mean (SD), y | 39.3 (13.7) | 0 | 39.3 (13.7) | 41.9 (13.8) | 0 | 41.9 (13.8) |
| Sex | ||||||
| Female | 548 (51.4) | 0 | 548 (51.4) | 122 (53.0) | 0 | 122 (53.0) |
| Male | 518 (48.6) | 0 | 518 (48.6) | 108 (47.0) | 0 | 108 (47.0) |
| Log(No. of CTG repeats), mean (SD) | 2.65 (0.36) | 117 | 2.66 (0.36) | NA | NA | NA |
| Diabetes | 72 (6.8) | 0 | 72 (6.8) | 15 (6.8) | 9 | 17 (7.5) |
| Dyslipidemia | 165 (15.5) | 0 | 165 (15.5) | NA | NA | NA |
| Hypertension | 67 (6.3) | 0 | 67 (6.3) | NA | NA | NA |
| Smoker | 181 (17.0) | 0 | 181 (17.0) | NA | NA | NA |
| Dysphagia | 193 (20.0) | 101 | 214 (20.1) | NA | NA | NA |
| Need for walking support | 43 (4.0) | 22 | 45 (4.2) | 34 (14.8) | 0 | 34 (14.8) |
| Heart rate, median (IQR), beats per min | 68 (60-77) | 33 | 68 (60-77) | 66 (60-75) | 10 | 66 (60-75) |
| Blood pressure, median (IQR), mm Hg | ||||||
| Systolic | 117 (109-127) | 62 | 117 (109-128) | 120 (110-130) | 18 | 120 (110-130) |
| Diastolic | 70 (63-75) | 63 | 70 (63-75) | NA | NA | NA |
| Atrial fibrillation | 56 (5.3) | 1 | 56 (5.3) | NA | NA | NA |
| First-degree atrioventricular block | 325 (31.3) | 29 | 335 (31.4) | 105 (46.1) | 2 | 106 (45.9) |
| Bundle-branch block | 186 (17.4) | 35 | 198 (18.5) | 71 (31.1) | 2 | 72 (31.3) |
| Left ventricular ejection fraction, median (IQR), % | 65 (60-67) | 66 | 65 (60-67) | NA | NA | NA |
| Predicted vital capacity, median (IQR), % | 83 (68-95) | 139 | 83 (68-95) | 78 (63-94) | 21 | 78 (63-94) |
Abbreviations: IQR, interquartile range; NA, not applicable.
Values presented are for nonmissing data for the original data set and averaged over all complete data sets for imputed data.
Prognostic Score
The model selection procedure retained age, diabetes, smoking status, need for support when walking, heart rate, systolic blood pressure, first-degree atrioventricular, bundle-branch block, and vital capacity when based on the standard rules by Rubin14 for pooling the model results across imputed data sets and on each imputed data set separately. In addition, the interactions between age and systolic blood pressure, diabetes and vital capacity, diabetes and heart rate, and need for support when walking and first-degree atrioventricular block exceeded the threshold for inclusion in the model. Examination of the models with and without interactions and with and without smoking status revealed a higher calibration in the models without interactions and without smoking status at inclusion, which were finally retained.
The regression coefficients for the full multiple variable and the retained models (corrected for overoptimism) are presented in Table 2. The model was well calibrated (eFigure 2 in the Supplement), and its optimism-corrected C index was 0.756 (95% CI, 0.725-0.787) for the original score and 0.753 (95% CI, 0.722-0.785) for the simplified score.
Table 2. Associations Between Prediction Variables and Survival.
| Characteristic | Hazard Ratio (95% CI)a | Simplified Score Points | |||
|---|---|---|---|---|---|
| Full Multiple Variable | P Value | Final | P Value | ||
| Age, y | |||||
| 30-45 | 2.64 (1.51-4.61) | <.001 | 2.42 (1.47-4.00) | <.001 | 4 |
| >45 | 8.50 (4.92-14.7) | <.001 | 6.82 (4.20-11.1) | <.001 | 8 |
| Men | 1.20 (0.91-1.58) | .20 | NA | NA | NA |
| Log(No. of CTG repeats) | |||||
| (2 × 55) − (2 × 86) | 1.17 (0.80-1.71) | .43 | NA | NA | NA |
| (2 × 87) − (3 × 52) | 1.24 (0.86-1.79) | .26 | NA | NA | NA |
| Diabetes | 1.78 (1.20-2.63) | .004 | 1.73 (1.25-2.39) | .001 | 2 |
| Dyslipidemia | 0.90 (0.64-1.25) | .52 | NA | NA | NA |
| Smoking | 1.25 (0.91-1.71) | .17 | NA | NA | NA |
| Dysphagia | 1.10 (0.79-1.52) | .59 | NA | NA | NA |
| Need for walking support | 2.04 (1.29-3.20) | .002 | 1.88 (1.27-2.79) | .002 | 3 |
| Heart rate, beats per min | |||||
| 63-73 | 1.36 (0.97-1.90) | .07 | 1.32 (0.98-1.78) | .07 | 1 |
| ≥74 | 1.45 (1.04-2.02) | .03 | 1.41 (1.05-1.90) | .02 | 1 |
| Systolic blood pressure, mm Hg | |||||
| ≤110 | 1.42 (1.04-1.93) | .03 | 1.36 (1.03-1.79) | .03 | 1 |
| 111-121 | 1.00 (0.71-1.40) | .99 | 1.00 (0.74-1.35) | .99 | 0 |
| Atrial fibrillation | 1.04 (0.66-1.64) | .85 | NA | NA | NA |
| First-degree atrioventricular block | 1.32 (0.99-1.75) | .06 | 1.34 (1.04-1.72) | .02 | 1 |
| Bundle-branch block | 1.43 (1.07-1.91) | .02 | 1.45 (1.12-1.87) | .005 | 1 |
| Left ventricular ejection fraction, % | |||||
| 60-66 | 0.86 (0.62-1.19) | .36 | NA | NA | NA |
| >66 | 0.82 (0.57-1.18) | .29 | NA | NA | NA |
| Vital capacity, % predicted value | |||||
| 60-90 | 1.25 (0.87-1.78) | .22 | 1.29 (0.94-1.76) | .12 | 1 |
| ≤60 | 2.27 (1.48-3.46) | <.001 | 2.32 (1.63-3.30) | <.001 | 3 |
Abbreviation: NA, not applicable.
The hazard ratios were pooled over the 35 imputed data sets. Hazard ratios in the final model are shrunk by the calibration slope (0.904).
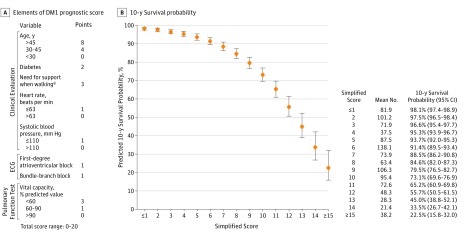
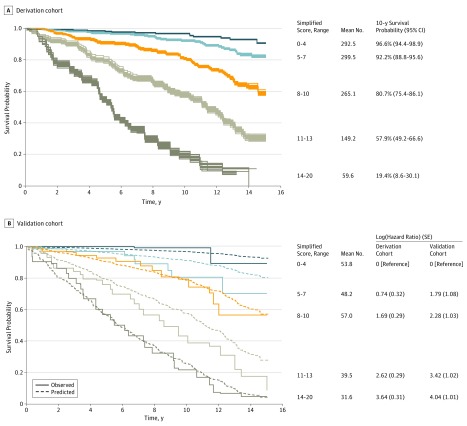
Simplified score points were attributed to the variables of the final model by rounding the regression coefficients multiplied by 4. Adding the score points of each relevant characteristic of individual patients yielded scores between 0 and 20, with 0 indicating the lowest and 20 the highest risk of death. The probability of 10-year survival predicted by the score value is presented in Figure 1. Survival curves for categories of the simplified score for the 35 imputed data sets revealed a clear separation of the curves and small between-imputation variations (Figure 2A).
Figure 1. Elements of the Prediction Score and Predicted 10-Year Survival Probability According to the Myotonic Dystrophy Type 1 (DM1) Prognostic Score.
The error bars indicate 95% CIs. ECG indicates electrocardiography.
aDefined as permanent use of a cane or wheelchair.
Figure 2. Overall Survival According to Simplified Score Categories.
A, The curves are plotted for each of the 35 imputed data sets. B, The solid lines indicate Kaplan-Meier estimates pooled over the imputed data sets. The dashed lines indicate predictions using the score and baseline hazard estimated in the derivation cohort.
External Validation Cohort
At the time of initial presentation, the mean (SD) age of the 230 patients in the validation cohort was 41.9 (13.8) years, and 122 (53.0%) were women. Their other characteristics are presented in Table 1. Of these 230 patients, 71 (30.9%) died over a median (interquartile range) follow-up of 8.4 (5.9-12.0) years. The slope of the score in the Cox model was 1.07 (95% CI, 0.82-1.33; P = .22), indicating slightly better discrimination than in the derivation cohort. This was confirmed by discrimination indexes that were somewhat higher than in the derivation cohort, with a C index of 0.804 (95% CI, 0.754-0.853) for the original score and 0.806 (95% CI, 0.758-0.855) for the simplified score. Figure 2B shows observed and predicted survivals for the same score categories as in Figure 2A, with an excellent calibration of the score for the 2 extreme categories as well as for simplified scores between 8 and 10. The predicted survival was slightly more optimistic for the 2 other categories, especially beyond 6 to 7 years. Nonetheless, the groups remained clearly separated over a longer follow-up. Table 3 shows the survival probability in the validation cohort at 5, 10, and 15 years according to the DM1 prognostic score values.
Table 3. Survival Probability in the Validation Cohort According to the Myotonic Dystrophy Type 1 Prognostic Score.
| Simplified Score Range | Years of Follow-up, Survival Probability (95% CI), % | ||
|---|---|---|---|
| 5 | 10 | 15 | |
| 0-4 | 100 (ND) | 99.3 (95.0-100) | 89.3 (72.3-100) |
| 5-7 | 97.0 (91.5-100) | 80.6 (67.1-96.7) | 70.3 (50.8-97.2) |
| 8-10 | 92.8 (85.4-100) | 79.3 (66.2-95.1) | 56.5 (37.4-85.3) |
| 11-13 | 75.8 (62.7-91.7) | 43.2 (28.2-66.1) | 8.6 (0-28.4) |
| 14-20 | 56.8 (41.5-77.8) | 21.6 (10.0-46.8) | 4.8 (0.7-31.5) |
Abbreviation: ND, not defined.
Discussion
We have developed a practical risk score that predicts the probability of long-term survival of patients presenting with DM1 based on a set of common patient characteristics. We found that age, diabetes, need for support when walking, heart rate, systolic blood pressure, first-degree atrioventricular, bundle-branch block, and vital capacity contained the largest amount of prognostic information.
To our knowledge, the development of this score is the first attempt to reliably predict survival in patients with DM1. Based on a simple scoring method, the DM1 prognostic score accurately discriminated survivors from nonsurvivors in both the derivation and the validation cohorts and showed well-calibrated predictions of overall survival at 10 years. A comparison of our score with existing disease-agnostic risk prediction models might have added to its clinical contribution, although we were unable to find such risk score applicable to our DM1 population based on a set of variables present in our database. However, compared with prognostic scores developed for other long-term disorders, the power of our model in the prediction of 10-year survival in patients with DM1, a complex disease involving multiple systems, was particularly high.24
The use of this score in routine clinical care is simple because it only requires 8 patient characteristics obtained by clinical examinations, electrocardiography, and pulmonary function tests. Its ability to predict patient survival over the long term may help caregivers plan a personalized supervision of the disease. The multidisciplinary management of DM1 by centers dedicated to the treatment of muscular diseases includes appropriate screening, surveillance of manageable complications, and specialized therapies. This multidisciplinary management has improved patients’ prognosis by preventing respiratory and cardiac complications. The patients likely to benefit the most from our prognostic score remain to be determined, although we presume that those at highest risk would benefit from closer surveillance, more intensive health education and counseling, and earlier awareness and management of complications known to precipitate fatal events, such as deglutition disorders, infections of the airways, and atrial fibrillation. On the other hand, for patients at lower risk, particularly those in intermediate groups whose risk may be difficult to estimate, preventive measures seem preferable because they can be delivered over longer periods of time for a less advanced and more easily treatable disease.
Among the characteristics included in the DM1 prognostic score, age, limitation of ambulation, conduction defects on electrocardiography, and depressed vital capacity have previously been associated with survival.5,6,9 The inclusion of conduction defects on electrocardiography and impaired vital capacity in this score was not surprising because respiratory insufficiency, pneumonia, and sudden death have been, by far, the most frequent causes of death in this and several earlier studies.5,6 Other variables included in our analysis, such as CTG amplification size, dysphagia, atrial fibrillation, and left ventricular dysfunction, had no additional predictive value despite being associated with a higher mortality.
An arterial systolic blood pressure of 110 mm Hg or less and a heart rate greater than 63 beats per minute were also independently associated with overall survival and added prognostic value above and beyond the classical prognostic factors of the disease. Measurements of blood pressure and heart rate, which are also predictive of cardiovascular and all-cause mortality in the general population,25,26 may be separately associated with DM1 and its pathophysiology. Systolic hypotension has been previously described in patients presenting with DM1,27 although, to our knowledge, whether it has prognostic value has not been previously reported. It is not clear whether it is a specific complication of the disease related to the genetic mutation or a nonspecific marker of disease severity. A slow heart rate was associated with a better vital prognosis, which was unexpected because, in these patients, it was mostly caused by sinus node dysfunction, itself often present in the context of advanced heart disease.28
Limitations
Our study had limitations. Because data points were missing, we used the multiple imputation technique to borrow information from all potential predictors and from the outcome. There is growing theoretical and empirical support favoring multiple imputation to correct for missing data.19,29,30 Even after verification of the convergence of the algorithm, some assumptions underlying the use of multiple imputation, such as randomly missing data, could not be verified despite being clinically plausible. However, a risk persists that the prognostic value of variables with the highest proportion of missing information was underestimated. It is also noteworthy that data were not generally missing because of the absence of respiratory or cardiovascular investigations but because we had limited inclusion in our statistical model to data collected at the time of baseline hospitalization. Second, a higher proportion of patients presenting with conduction defects on electrocardiography underwent electrophysiological studies and prophylactic pacing in our study compared with other studies, which may have underestimated the weight of conduction defects in the evaluation of the risk of death.28 Similarly, a high proportion of patients with decreased vital capacity were treated with noninvasive ventilation, which may have underestimated the weight of restrictive respiratory insufficiency on vital prognosis. These preventive measures are in agreement with current recommendations regarding the cardiac and respiratory management of myotonic dystrophy.8 Caregivers using the DM1 prognostic score should take into consideration differences in their practices with regard to these preventive measures to not underestimate their patients’ risk of death. Another limitation of this score is that as follow-up duration increases, the survival estimates become less precise and the confidence intervals widen, especially by 15 years, when the clinical relevance of the score is probably lower than at 5 or 10 years. In addition, the confidence intervals appeared largest in patients whose scores were the highest, especially for follow-up durations above 10 years.
Conclusions
We developed and validated internally and externally the DM1 prognostic score, a reliable predictor of the probability long-term survival in patients with DM1 based on a set of common patient characteristics, including age, diabetes, need for support when walking, heart rate, systolic blood pressure, first-degree atrioventricular, bundle-branch block, and vital capacity.
eTable. Primary causes of 241 deaths in the derivation cohort classified according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision.
eFigure 1. Overall survival of the 1066 patients in the derivation cohort.
eFigure 2. Calibration curve of 10-year survival.
References
- 1.Ashizawa T, Epstein HF. Ethnic distribution of myotonic dystrophy gene. Lancet. 1991;338(8767):642-643. [DOI] [PubMed] [Google Scholar]
- 2.Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255(5049):1253-1255. [DOI] [PubMed] [Google Scholar]
- 3.Harper P. Myotonic Dystrophy. 3rd ed London, England: Harcourt Publishers; 2001. [Google Scholar]
- 4.Pelargonio G, Dello Russo A, Sanna T, De Martino G, Bellocci F. Myotonic dystrophy and the heart. Heart. 2002;88(6):665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Die-Smulders CE, Höweler CJ, Thijs C, et al. Age and causes of death in adult-onset myotonic dystrophy. Brain. 1998;121(pt 8):1557-1563. [DOI] [PubMed] [Google Scholar]
- 6.Mathieu J, Allard P, Potvin L, Prévost C, Bégin P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology. 1999;52(8):1658-1662. [DOI] [PubMed] [Google Scholar]
- 7.Tsilfidis C, MacKenzie AE, Mettler G, Barceló J, Korneluk RG. Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nat Genet. 1992;1(3):192-195. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon C, Chouinard MC, Laberge L, et al. ; DMI Expert Panel . Health supervision and anticipatory guidance in adult myotonic dystrophy type 1. Neuromuscul Disord. 2010;20(12):847-851. [DOI] [PubMed] [Google Scholar]
- 9.Groh WJ, Groh MR, Saha C, et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;358(25):2688-2697. [DOI] [PubMed] [Google Scholar]
- 10.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans. Circulation. 2005;111(5):697-716. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585-598. [DOI] [PubMed] [Google Scholar]
- 12.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28(15):1982-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley and Sons; 2004. [Google Scholar]
- 15.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(4):515-556. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 16.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. [DOI] [PubMed] [Google Scholar]
- 17.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation: a consensus conference report. Chest. 1999;116(2):521-534. [DOI] [PubMed] [Google Scholar]
- 18.Wood AM, White IR, Royston P. How should variable selection be performed with multiply imputed data? Stat Med. 2008;27(17):3227-3246. [DOI] [PubMed] [Google Scholar]
- 19.Vergouwe Y, Royston P, Moons KG, Altman DG. Development and validation of a prediction model with missing predictor data: a practical approach. J Clin Epidemiol. 2010;63(2):205-214. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361-387. [DOI] [PubMed] [Google Scholar]
- 21.Wood AM, Royston P, White IR. The estimation and use of predictions for the assessment of model performance using large samples with multiply imputed data. Biom J. 2015;57(4):614-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol. 2013;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The R Foundation The R project for statistical computing. http://www.R-project.org/. Accessed December 12, 2017.
- 24.Ferrero P, Iacovoni A, D’Elia E, Vaduganathan M, Gavazzi A, Senni M. Prognostic scores in heart failure: critical appraisal and practical use. Int J Cardiol. 2015;188:1-9. [DOI] [PubMed] [Google Scholar]
- 25.Mensink GB, Hoffmeister H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur Heart J. 1997;18(9):1404-1410. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113(6):1489-1494. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien T, Harper PS, Newcombe RG. Blood pressure and myotonic dystrophy. Clin Genet. 1983;23(6):422-426. [DOI] [PubMed] [Google Scholar]
- 28.Wahbi K, Meune C, Porcher R, et al. Electrophysiological study with prophylactic pacing and survival in adults with myotonic dystrophy and conduction system disease. JAMA. 2012;307(12):1292-1301. [DOI] [PubMed] [Google Scholar]
- 29.Moons KG, Donders RA, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59(10):1092-1101. [DOI] [PubMed] [Google Scholar]
- 30.Chevret S, Seaman S, Resche-Rigon M. Multiple imputation: a mature approach to dealing with missing data. Intensive Care Med. 2015;41(2):348-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Primary causes of 241 deaths in the derivation cohort classified according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision.
eFigure 1. Overall survival of the 1066 patients in the derivation cohort.
eFigure 2. Calibration curve of 10-year survival.