This randomized clinical trial evaluates the efficacy of oral mixed tocotrienols for patients with diabetic peripheral neuropathy.
Key Points
Question
Are oral mixed tocotrienols efficacious in reducing neuropathic symptoms in patients with diabetic peripheral neuropathy?
Findings
In this randomized clinical trial of 300 adults with diabetic peripheral neuropathy, tocotrienols administered for 1 year did not reduce the overall neuropathic Total Symptom Score. Post hoc subgroup analyses revealed that tocotrienols might improve lancinating pain in those with uncontrolled diabetes and normal homocysteine levels.
Meaning
Supplementation of oral mixed tocotrienols, 400 mg/d for 1 year, did not improve neuropathic symptoms for diabetic peripheral neuropathy; the effects on lancinating pain require further investigation.
Abstract
Importance
Management of painful diabetic peripheral neuropathy remains challenging. Most therapies provide symptomatic relief with varying degrees of efficacy. Tocotrienols have modulatory effects on the neuropathy pathway and may reduce neuropathic symptoms with their antioxidative and anti-inflammatory activities.
Objective
To evaluate the efficacy of oral mixed tocotrienols for patients with diabetic peripheral neuropathy.
Design, Setting, and Participants
The Vitamin E in Neuroprotection Study (VENUS) was a parallel, double-blind, placebo-controlled trial that recruited participants from January 30, 2011, to December 7, 2014, with 12 months of follow-up. This trial screened 14 289 patients with diabetes from 6 health clinics and ambulatory care units from 5 public hospitals in Malaysia. A total of 391 patients who reported neuropathic symptoms were further assessed with Total Symptom Score (TSS) and Neuropathy Impairment Score (NIS). Patients 20 years or older with a TSS of 3 or higher and an NIS of 2 or higher were recruited.
Interventions
Patients were randomized to receive 200 mg of mixed tocotrienols twice daily or matching placebo for 12 months. Patients with hyperhomocysteinemia (homocysteine level ≥2.03 mg/L) received oral folic acid, 5 mg once daily, and methylcobalamin, 500 μg thrice daily, in both groups.
Main Outcomes and Measures
The primary outcome was patient-reported neuropathy TSS (lancinating pain, burning pain, paresthesia, and asleep numbness) changes at 12 months. The secondary outcomes were NIS and sensory nerve conduction test result.
Results
Of 391 eligible patients, 300 were recruited (130 [43.3%] male; mean [SD] age, 57.6 [8.9] years; mean [SD] duration of diabetes, 11.4 [7.8] years) and 229 (76.3%) completed the trial. The TSS changes between the tocotrienols and placebo groups at 12 months (−0.30; 95% CI, −1.16 to 0.56; P = .49) were similar. No significant differences in NIS (0.60; 95% CI, −1.37 to 2.65; P = .53) and sensory nerve conduction test assessments were found between both groups. In post hoc subgroup analyses, tocotrienols reduced lancinating pain among patients with hemoglobin A1C levels greater than 8% (P = .03) and normohomocysteinemia (homocysteine level <2.03 mg/L; P = .008) at 1 year. Serious adverse events in both groups were similar, except more infections were observed in the tocotrienols group (6.7% vs 0.7%, P = .04). Results reported were of modified intention-to-treat analyses.
Conclusions and Relevance
Supplementation of oral mixed tocotrienols, 400 mg/d for 1 year, did not improve overall neuropathic symptoms. The preliminary observations on lancinating pain among subsets of patients require further exploration.
Trial Registration
National Medical Research Registry Identifier: NMRR-10-948-7327 and clinicaltrials.gov Identifier: NCT01973400
Introduction
Diabetic peripheral neuropathy (DPN) remains underdiagnosed and thus undertreated. It affects 26% to 53% of patients with diabetes, imposing a significant public health burden worldwide. Neuropathic pain occurs in 21% to 53% of patients with DPN and impairs quality of life. Neuropathy has been associated with a quarter of the total cost of diabetes care, whereas direct medical cost for those with neuropathic pain was reported to be 4.2-fold higher than for those without.
Improved glycemic control and pain management are the mainstay strategies to manage DPN. Various pharmacologic therapies are available to treat DPN. Used alone or in combination, these therapies achieve a varying degree of satisfactory outcomes but are partly limited by their adverse effects. They mainly provide symptomatic relief and do not affect the underlying neuropathic process.
Oxidative stress exerts an important role for the pathogenesis of DPN. Efficacy of alternative agents, such as α-lipoic acid (ALA), benfotiamine, acetyle-l-carnitine, vitamin E, vitamin D2, and actovegin, have been studied with variable results.
Tocotrienols are naturally occurring subtypes of vitamin E that modulate glutamate-induced neuronal apoptosis via inhibition of the 12-lipoxygenase pathway in in vitro studies. Tocotrienols reverse neuropathic pain via modulation of oxidative-nitrosative stress, inflammatory cytokine release, and caspase-3 in rats with diabetes. Oral administration of 400 mg/d of tocotrienols for 2 years among 121 volunteers attenuated the progression of brain white matter lesions without notable adverse reactions, suggestive of neuroprotective properties. These observations have led to the inception and conduct of this study, which evaluated the efficacy of oral mixed tocotrienols in patients with DPN during 12 months.
Methods
Study Design and Participants
The Vitamin E Neuroprotection Study (VENUS) was a multicenter, randomized, double-blind, placebo-controlled trial conducted from November 30, 2011, to August 7, 2015. The trial protocol can be found in Supplement 1. Patients 20 years or older with diabetes were prescreened by face-to-face interview at outpatient units in 6 public primary care clinics and medical outpatient clinics in 5 public hospitals located in the Penang and Kedah states of Malaysia. Potential participants with symptomatic DPN were invited to attend screening visits at respective study clinics. This study was approved by the Medical Research Ethics Committee, Ministry of Health. Written informed consents were obtained from all participants.
During the screening visit, patients were assessed with Total Symptom Score (TSS) and Neuropathy Impairment Score (NIS), and venous blood samples were obtained for measurement of fasting blood glucose level, hemoglobin A1c (HbA1c) level, and renal and liver profiles. Patients were eligible for recruitment with a TSS of 3 or higher and an NIS of 2 or higher. These cutoffs were selected to allow detection of the least clinically significant improvements in scores. We excluded individuals with uncontrolled diabetes (HbA1c>12% [to convert to proportion of total hemoglobin, multiply by 0.01]), drug and/or alcohol dependency, psychiatric disorders, and impaired renal (serum creatinine level ≥1.7 mg/dL [to convert to micromoles per liter, multiply by 88.4]) and/or liver (level of hepatic transaminases ≥5-fold upper limit of normal) functions. We also excluded pregnant or lactating women, those who were immunocompromised, and those with symptomatic peripheral vascular disease or other diseases that might interfere with the administration of the investigational products or interpretation of the results.
Randomization and Masking
A total of 300 participants were recruited and randomly assigned to 2 groups with a 1:1 allocation ratio using permuted block randomization (block size of 10). The patients received 1 capsule of 200 mg of mixed tocotrienols or 1 capsule of identical-looking placebo twice daily for 12 months. Both active (containing 200 mg of tocotrienols in a 1-g capsule) and placebo (containing 1 g of tocotrienols-free palm oil in a capsule) capsules were manufactured by Hovid Bhd. The randomization allocation sequence was generated by an independent pharmacist using an offsite computer who also prepared the investigational products with allocated coding and unique identification numbers. Randomization and allocation were fully concealed from the principal investigators, all participating clinicians, research officers, and participants until the study was completed.
Procedures
The participants were interviewed for their clinical histories and underwent anthropometric examinations for body mass index, TSS and NIS assessments, and blood sampling for fasting blood glucose, HbA1c, serum homocysteine, folate, vitamin B12, and total tocotrienol levels. The TSS and NIS were assessed again at 6 months and at the end of the study at 12 months. Blood samples used to test for the aforementioned biomarkers were obtained during the follow-up visits.
Serum homocysteine level was measured using a chemiluminescent microparticle immunoassay (Architect homocysteine assay, Abbott Laboratories) for the quantitative determination of total l-homocysteine in human serum on the Architect i System. Serum folate and vitamin B12 levels were measured using competitive immunoassays with direct chemiluminescent technology (Advia Centaur Folate and Advia Centaur VB12 assays, Bayer Diagnostics). Plasma total tocotrienol levels were determined using a simple, validated high-performance liquid chromatographic method with fluorescence detection.
Sensory nerve conduction tests (NCTs) were performed at the baseline visit and the end of the study. The NCTs were conducted by a single operator at Seberang Jaya Hospital and interpreted by a clinical neurologist (I.L.). Patients were selected if they were able to travel to the center and consented to undergo NCTs.
All patients attended follow-up visits every 3 months to obtain the investigational products. They were advised to contact research officers immediately if they suspected a reaction toward the investigational products. All the adverse events (AEs) and serious adverse events (SAEs) were reported to the Medical Research Ethics Committee according to the local protocol. All events in relation to the study intervention were examined by subinvestigators at respective clinical sites followed by a detailed discussion at study update meetings.
Outcomes
Our primary outcome of the study was the mean difference in TSS changes at 12 months of supplementation compared with baseline between the tocotrienols and placebo groups. The secondary outcome was a change in NIS evaluated at 12 months compared with baseline. A minimal reduction of 1.83 (a half-range of a maximal single symptom) in the TSS and 2 in the NIS are considered to be clinically meaningful responses to treatment.
For TSS assessment, participants were asked to grade the severity and frequency of the neuropathic symptoms that they experienced over peripheral limbs (eTable 1 in Supplement 2). The TSS ranges from 0 to 14.64. The NIS assessment was performed by the study clinicians on participants to assess their neuropathy impairment. All study clinicians underwent standardized training with a neurologist (I.L.). The NCT assessed the latency, amplitude, and velocity conduction of sensory components of median, radial, and sural nerves.
Adherence
Adherence to study treatment was monitored by returned capsule count. Participants were required to return all remaining capsules at each follow-up visit, which were counted and documented. Plasma total tocotrienol levels were measured to ensure that participants in both groups were adherent to investigational product use at baseline and 3-month intervals up to 12 months. The levels were analyzed at the end of study to maintain masking.
Baseline Serum Homocysteine Level Stratification
Hyperhomocysteinemia exerts neurotoxic effects and correlates with development of neuropathy. Although the cutoff for hyperhomocysteinemia is arbitrary, the upper reference limit for total homocysteine level in adults younger than 65 years was set at 2.03 mg/L (to convert to micromoles per liter, multiply by 7.397). We adopted the conservative cutoff of 2.03 mg/L to initiate treatment for reduction of hyperhomocysteinemia. All participants with hyperhomocysteinemia in both groups received concurrent supplementation of oral folic acid, 5 mg/d, and methylcobalamin, 500 μg thrice daily, as part of the treatment proposed to reduce hyperhomocysteinemia, with evidence from the Heart Outcomes Prevention Evaluation (HOPE) 2 study. In the stratified analysis, participants were further categorized to an A subgroup (homocysteine level <2.03 mg/L) and an H subgroup (homocysteine level ≥2.03 mg/L) at baseline.
Statistical Analysis
Sample size for this study was calculated using PS: Power and Sample Size Calculation software, version 3.1.2, based on the SYDNEY 2 trial (Assessment of Efficacy and Safety of Oral Alpha-Lipoic Acid for Symptomatic Diabetic Neuropathy), which investigated the effects of ALA on DPN. Reductions in TSS were computed after treatment, with a corresponding SD of 3.37. Our study was designed for a minimum of 272 participants (136 in each arm) to achieve 90% power to detect a difference between groups of 1.33 of the primary end point after 12 months of supplementation, which reflects a minimum 1-level reduction in frequency and pain severity. To accommodate a dropout rate of 10%, we increased the sample size to 150 per arm for a total of 300 participants.
All the data were hosted at Universiti Sains Malaysia. The data were deidentified before their use in analyses. All data were analyzed according to a modified intention-to-treat protocol. All normally distributed continuous variables were reported as mean (SD) except for serum total tocotrienol level, which was reported as mean (SEM). Comparison for categorical variables between the tocotrienol and placebo groups was performed using χ2 tests or Fisher exact tests if the assumptions for the χ2 test were unmet. Paired t tests were used to detect differences in clinical and biochemical markers before and after supplementation in both groups at 6 and 12 months, whereas independent t tests were performed to compare these markers and AE rates across both groups. P < .05 was considered to be statistically significant for all analyses. Statistical analyses were performed using SPSS, version 23.0. Adverse events were reported in the full trial population, with reference to the Medical Dictionary for Regulatory Activities, version 12.0.
Results
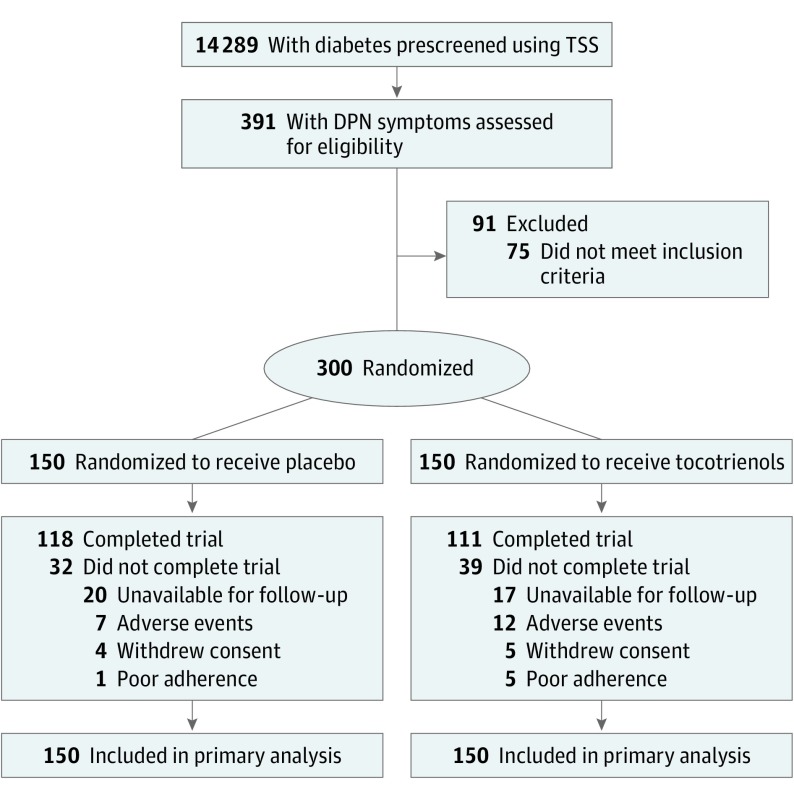
From November 30, 2011, to July 4, 2014, a total of 14 289 patients with diabetes were prescreened, and 391 who reported neuropathic symptoms were assessed with TSS and NIS. The last follow-up for the last patient was August 7, 2015. A total of 300 patients who fulfilled the study criteria were recruited for this study, and 229 (76.3%) completed the trial during 12 months (130 [43.3%] male; mean [SD] age, 57.6 [8.9] years; mean [SD] duration of diabetes, 11.4 [7.8] years). Among those who completed the study, 111 participants (74.0%) received tocotrienols and 118 (78.7%) received placebo (Figure 1). Seventy-one participants discontinued the study, among whom 37 were lost to follow-up, 19 because of AEs, 9 because of personal reasons, and 6 because of poor adherence. The baseline characteristics were similar between the groups (eTable 2 in Supplement 2).
Figure 1. Trial Profile.
DPN indicates diabetic peripheral neuropathy; TSS, Total Symptom Score.
Baseline characteristics were similar between the groups except for distribution of antidiabetic therapies (Table 1). More patients in the placebo arm were treated solely with oral antidiabetics, whereas more in the tocotrienols arm received insulin therapy, although the HbA1c levels were comparable between the groups. Two patients in each group had type 1 diabetes, with the remaining having type 2 diabetes. The patients had diabetes with a mean duration of more than 10 years, with a mean HbA1c level exceeding 8%. Most were overweight and had multiple comorbidities. The TSSs and NISs were similar between the groups except for the TSS subscore for paresthesia.
Table 1. Baseline Characteristics by Study Groupa.
| Characteristic | Placebo (n = 150) |
Tocotrienols (n = 150) |
P Value |
|---|---|---|---|
| Male | 63 (42.0) | 67 (44.7) | .64 |
| Age, mean (SD), y | 57.2 (8.9) | 58.0 (8.9) | .45 |
| Ethnicity | |||
| Malay | 81 (54.0) | 86 (57.3) | .53 |
| Chinese | 16 (10.7) | 9 (6.0) | |
| Indian | 52 (34.7) | 54 (36.0) | |
| Others | 1 (0.7) | 1 (0.7) | |
| Duration of diabetes, mean (SD), y | 11.1 (8.0) | 11.8 (7.6) | .46 |
| Treatment | |||
| Oral OADs only | 87 (58.0) | 64 (42.7) | .008 |
| Insulin only | 4 (2.7) | 12 (8.1) | .04 |
| Combined insulin and OADs | 58 (38.7) | 73 (48.7) | .08 |
| Comorbidities | |||
| Hypertension | 106 (70.7) | 103 (68.7) | .71 |
| Dyslipidemia | 119 (79.3) | 130 (86.7) | .09 |
| Ischemic heart disease | 33 (22.0) | 23 (15.3) | .14 |
| BMI, mean (SD) | 28.2 (5.06) | 27.6 (5.36) | .29 |
| HbA1c level, mean (SD), % | 8.7 (1.82) | 9.2 (1.97) | .06 |
| Fasting blood glucose level, mean (SD), mg/dL | 151 (60) | 164 (76) | .15 |
| Serum homocysteine level, mean (SD), mg/L | 1.80 (0.70) | 1.84 (0.91) | .66 |
| Serum folate level, mean (SD), ng/mL | 9.4 (5.3) | 9.2 (5.4) | .79 |
| Serum vitamin B12 level, mean (SD), pg/mL | 594.5 (307.6) | 606.2 (317.1) | .74 |
| TSS, mean (SD) | 7.6 (2.5) | 8.2 (2.9) | .07 |
| Component TSS, mean (SD) | |||
| Lancinating pain | 0.8 (1.3) | 1.1 (1.4) | .10 |
| Burning pain | 1.7 (1.3) | 1.7 (1.4) | .75 |
| Paresthesia | 2.4 (0.9) | 2.6 (0.8) | .009 |
| Asleep numbness | 2.8 (0.6) | 2.8 (0.8) | .89 |
| Neuropathy Impairment Score, mean (SD) | 15.8 (8.4) | 16.2 (9.0) | .73 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HbA1c, hemoglobin A1c; OADs, oral antidiabetics; TSS, Total Symptom Score.
SI conversion factors: To convert fasting blood glucose to millimoles per liter, multiply by 0.0555; homocysteine to micromoles per liter, multiply by 7.397; folate to nanomoles per liter, multiply by 2.266; and B12 to picomoles per liter, multiply by 0.7378.
Data are presented as number (percentage) of patients unless otherwise indicated.
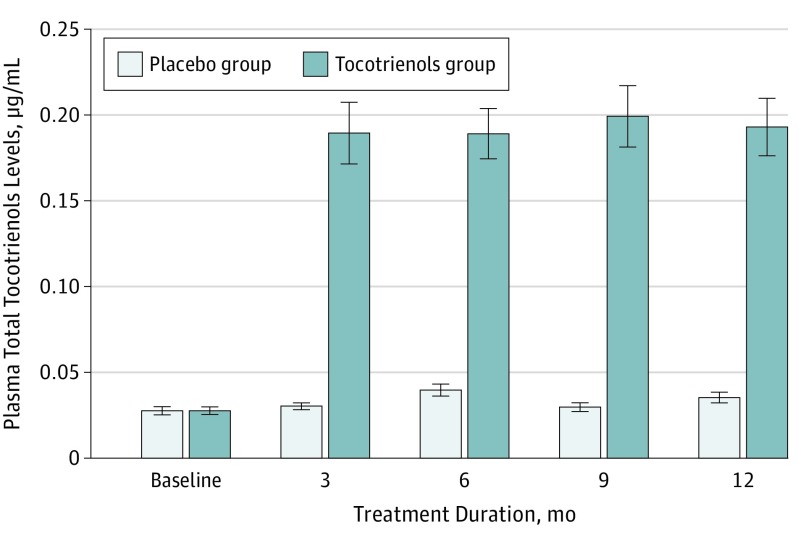
The mean investigational product adherence during 12 months, reported by pill counting, was 81.7% in the tocotrienols group and 86.1% in the placebo group (P = .16). The mean level of tocotrienol at baseline was 0.03 (0.003) μg/mL in the tocotrienol group and 0.03 (0.002) μg/mL in the placebo group. The mean level of plasma total tocotrienols increased significantly by 5.3-fold to 0.19 (0.018) μg/mL in the intervention group after 3-month supplementation and plateaued afterward (Figure 2).
Figure 2. Mean Plasma Total Tocotrienol Levels During 12 Months by Study Group.
Error bars indicate SEM.
The treatment and placebo groups achieved clinically meaningful responses of reductions greater than 1.83 in TSS at 12 months (Table 2). No significant differences were found in TSS change between the tocotrienol and placebo groups at 12 months (−0.30; 95% CI, −1.16 to 0.56; P = .49).
Table 2. Total and Component TSS Changes During 12 Months by Study Group.
| Assessment | Placebo (n = 150) |
Tocotrienols (n = 150) |
P Value (Between Groups) | ||
|---|---|---|---|---|---|
| Mean (SD) | P Value (Within Group) | Mean (SD) | P Value (Within Group) | ||
| TSS | |||||
| Baseline | 7.6 (2.5) | NA | 8.1 (2.9) | NA | NA |
| 6 mo | 5.6 (3.2) | <.001 | 5.7 (3.2) | <.001 | NA |
| 12 mo | 4.9 (3.3) | <.001 | 5.0 (3.3) | <.001 | NA |
| Change (6 mo minus baseline) | −2.2 (3.1) | NA | −2.6 (3.3) | NA | .24 |
| Change (12 mo minus baseline) | −3.0 (3.3) | NA | −3.3 (3.3) | NA | .49 |
| Lancinating pain | |||||
| Baseline | 0.8 (1.3) | NA | 1.0 (1.4) | NA | NA |
| 6 mo | 0.6 (1.2) | .08 | 0.5 (1.0) | <.001 | NA |
| 12 mo | 0.6 (1.2) | .02 | 0.4 (1.0) | <.001 | NA |
| Change (6 mo minus baseline) | −0.2 (1.4) | NA | −0.6 (1.4) | NA | .04 |
| Change (12 mo minus baseline) | −0.3 (1.4) | NA | −0.6 (1.3) | NA | .07 |
| Burning pain | |||||
| Baseline | 1.7 (1.3) | NA | 1.7 (1.4) | NA | NA |
| 6 mo | 1.1 (1.3) | <.001 | 1.2 (1.3) | <.001 | NA |
| 12 mo | 0.9 (1.2) | <.001 | 0.9 (1.2) | <.001 | NA |
| Change (6 mo minus baseline) | −0.6 (1.5) | NA | −0.6 (1.4) | NA | .93 |
| Change (12 mo minus baseline) | −0.8 (1.4) | NA | −0.9 (1.5) | NA | .83 |
| Paresthesia | |||||
| Baseline | 2.4 (0.9) | NA | 2.6 (0.8) | NA | NA |
| 6 mo | 1.6 (1.2) | <.001 | 1.7 (1.3) | <.001 | NA |
| 12 mo | 1.3 (1.3) | <.001 | 1.5 (1.3) | <.001 | NA |
| Change (6 mo minus baseline) | −0.9 (1.4) | NA | −0.9 (1.2) | NA | .66 |
| Change (12 mo minus baseline) | −1.1 (1.4) | NA | −1.1 (1.3) | NA | .82 |
| Asleep numbness | |||||
| Baseline | 2.8 (0.6) | NA | 2.8 (0.8) | NA | NA |
| 6 mo | 2.3 (1.1) | <.001 | 2.3 (1.0) | <.001 | NA |
| 12 mo | 2.1 (1.0) | <.001 | 2.2 (1.1) | <.001 | NA |
| Change (6 mo minus baseline) | −0.5 (1.1) | NA | −0.5 (1.0) | NA | .87 |
| Change (12 mo minus baseline) | −0.7 (1.1) | NA | −0.6 (1.2) | NA | .43 |
Abbreviations: NA, not applicable; TSS, Total Symptom Score.
Slight improvements in total NIS were observed in both groups, without significant between-group differences by 12 months (0.6; 95% CI, −1.37 to 2.65; P = .53) (eTable 3 in Supplement 2). Sensory scores for the upper and lower limbs and tendon reflex score improved in the placebo group but not in the tocotrienol group. Muscle strength over the peripheral upper and lower limbs was unaffected by either intervention. Latency, amplitude, and velocity conduction for sensory median, radial, and sural nerves was similar between the groups (eTable 4 in Supplement 2).
Glycemic control between the groups was similar (eTable 5 in Supplement 2). However, the placebo group had a progressive significant increase of 0.4% in HbA1c level (P = .01) and of 23 mg/dL (to convert to millimoles per liter, multiply by 0.0555) in fasting blood glucose level (P = .002) during 1 year. In contrast, glycemic control in the tocotrienol group remained stable.
Most patients had adequate folate (293 patients [97.7%]; >2.8 ng/mL [to convert to nanomoles per liter, multiply by 2.266]) and vitamin B12 (296 [98.7%]; >211 pg/mL [to convert to picomoles per liter, multiply by 0.7378]). A total of 108 (72.0%) in the placebo group and 101 (67.3%) in the tocotrienols group had normal homocysteine levels (<2.03 mg/L). Four patients had vitamin B12 levels that ranged from 184 to 205 pg/mL with hyperhomocysteinemia (homocysteine level ≥2.03 mg/L). Patients with normohomocysteinemia in both groups had progressive increment in their serum homocysteine levels, although their folate and vitamin B12 levels remained within the normal range (eTable 6 in Supplement 2). In contrast, patients with hyperhomocysteinemia had lower folate and vitamin B12 levels at baseline. After oral folic acid and methylcobalamine supplementation, their homocysteine levels had decreased significantly, whereas their serum folate and vitamin B12 levels were increased.
In post hoc subgroup analyses, the tocotrienols group experienced a significant reduction in lancinating pain score at 6 months compared with the placebo group (−0.6; 95% CI, −0.71 to −0.01; P = .04). A further reduction occurred at 12 months, but it did not achieve statistical significance (−0.64; 95% CI, −0.70 to 0.02; P = .07). Reduction in other components of TSS were similar between the groups during the study period. Compared with patients with baseline HbA1c levels of 8.0% or less, those with HbA1c levels greater than 8% in the tocotrienols group had their lancinating pain score reduced by 0.7 at 12 months (95% CI, −0.92 to −0.05; P = .03) (Table 3). Patients with normohomocysteinemia in the tocotrienols group experienced reduction in their lancinating pain at the end of the study (−0.9; 95% CI, −1.06 to −0.16; P = .008) but not among those with hyperhomocysteinemia despite treatment with homocysteine-lowering agents.
Table 3. Lancinating Pain Score Changes According to Baseline Antidiabetic Regimen, HbA1c Level, and Serum Homocysteine Level by Study Group.
| Score Change From Baseline | By Month 6 | By Month 12 | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 150) |
Tocotrienols (n = 150) |
P Value | Placebo (n = 150) |
Tocotrienols (n = 150) |
P Value | |
| Baseline HbA1c | ||||||
| HBA1c ≤8.0% | −0.4 (1.6) | −0.4 (1.2) | .97 | −0.4 (1.7) | −0.6 (1.1) | .71 |
| HBA1c >8.0% | −0.1 (1.3) | −0.7 (1.4) | .008 | −0.2 (1.2) | −0.7 (1.4) | .03 |
| Baseline homocysteinea | ||||||
| A subgroup (<2.03 mg/L) | −0.2 (1.5) | −0.7 (1.5) | .01 | −0.3 (1.5) | −0.9 (1.3) | .008 |
| H subgroup (≥2.03 mg/L) | −0.4 (1.4) | −0.3 (1.0) | .71 | −0.4 (1.1) | −0.2 (1.2) | .39 |
Abbreviation: HbA1c, hemoglobin A1c.
SI conversion factors: To convert homocysteine to micromoles per liter, multiply by 7.397; folate to nanomoles per liter, multiply by 2.266.
In the stratified analysis, participants were further categorized to an A subgroup (<2.03 mg/L) and an H subgroup (≥2.03 mg/L) at baseline.
Nineteen patients withdrew from the study because of AEs (7 from the placebo group and 12 from the tocotrienol group) (eTable 2 in Supplement 2). All events occurred after 1 month of supplementation of the investigational products.
Sixty patients reported at least 1 AE (eTable 7 in Supplement 2). In a total of 69 AEs reported, 34 events (49.3%) occurred among 30 patients in the placebo group and 35 events (50.7%) among 30 patients in the tocotrienols group (χ21 < 0.001, P > .99). Skin and soft-tissues disorders, minor traumatic injuries, and infections were among the most common AEs reported. More infectious events occurred in the placebo group, whereas more unintended traumatic injuries were observed in the tocotrienol groups. Most AEs occurred after 3 months of study enrollment.
A total of 48 SAEs that involved hospitalization for preexisting conditions or other causes (eTable 8 in Supplement 2) were reported. Of these, 21 events occurred among 15 patients in the placebo group and 27 events among 24 patients in the tocotrienols group (χ21 = 1.926, P = .16). Most patients had concomitant comorbidities and macrovascular and microvascular complications of diabetes. Three patients with diabetic foot ulcers underwent lower limb amputations. The incidence of SAEs was similar between the groups, with the exception that more infections were observed in the tocotrienols group (6.7% vs 0.7%, P = .04). One patient in the tocotrienols group died of nosocomial sepsis complicated with renal failure and severe metabolic acidosis after 11 months of supplementation. Most of the events occurred after 3 months of enrollment in the study. All reported SAEs were unlikely to be related to the study treatments.
Discussion
This is a novel study on the efficacy and safety of oral administration of mixed tocotrienols for the treatment of DPN in a multiethnic Asian population. In this study, supplementation of tocotrienols for 1 year did not influence the TSS, NIS, and sensory NCT outcomes for patients with DPN.
To date, evidence on the clinical efficacies of nutraceuticals on DPN is limited. Although previous studies have reported on the efficacy of DPN after ALA injection, oral ALA had conflicting results, depending on the measured outcomes and treatment duration. Our study findings were similar to those of the NATHAN 1 (Assessment of Efficacy and Safety of Oral Alpha-Lipoic Acid for Diabetic Neuropathy [Stage 1 or 2]) study, which also did not meet its primary end point using a composite score after daily supplementation of 600 mg of ALA for 4 years. The pilot BEDIP (Benfotiamine in Diabetic Polyneuropathy) trial found that 200 mg/d of benfotiamine for 3 weeks improved neuropathic symptoms, but higher dosages up to 600 mg/d did not improve the Neuropathy Symptom Score or TSS as demonstrated in the BENDIP (Benfotiamine in Diabetic Polyneuropathy) study.
The prominent placebo effects observed in this trial are relatively common. This phenomenon has increased in recent trials on the treatment of various types of neuropathic pain and might lead to an underestimation of drug effects. The close monitoring might have partially contributed to the placebo responses, especially in patient-reported outcome such as TSS. In addition, pain threshold can vary among individuals. A Cochrane review reported that a moderate placebo effect was detected in self-reported continuous outcomes across interventional trials on any clinical condition, and such an effect was greater for trials that involved self-reported pain outcomes.
The NIS is a more objective assessment that provides direct measures of nerve dysfunction. It assesses predominantly evoked pain using pinprick, sensory, and vibration loss but does not detect changes in spontaneous pain experienced by patients with painful DPN, which may partially explain the absence of treatment effects on NIS in this study. There was no apparent impairment in sensory nerve conduction for patients from both groups. Patients with painful DPN had predominantly small fiber neuropathy, and abnormal electrophysiologic findings may be absent in the presence of symptoms, as observed in this study.
Post hoc subgroup analyses found that tocotrienols might alleviate lancinating pain in patients with DPN who had poorly controlled diabetes (HbA1c level >8%) and normohomocysteinemia (homocysteine level <2.03 mg/L) compared with placebo. Neuropathic pain is a complex group of positive sensory phenomena attributable to neuronal dysfunction at different levels, with each attributable to discrete pathophysiologic mechanisms that operate at a defined level. Nonetheless, whether tocotrienols might modulate the mechanisms that lead to lancinating pain but not the other neuropathic symptoms with different underlying pathophysiologic mechanisms remains unclear. Therefore, we interpret these preliminary observations with caution because the cutoff to define a clinically meaningful change in a TSS subscale is lacking.
Homocysteine level is independently associated with the development and severity of DPN. A total of 30.3% of our study population had hyperhomocysteinemia. Patients with normohomocysteinemia at baseline responded better to tocotrienols in reducing their lancinating pain. For those with hyperhomocysteinemia, additional supplementation of oral folic acid and methylcobalamin significantly decreased homocysteine levels by 26.3% in the placebo group and 24.7% in the tocotrienols group. Despite normalization of hyperhomocysteine levels, we did not observe any effect of tocotrienols on lancinating pain similar to that in patients with normohomocysteinemia at the baseline visit. Because of the complex DPN development and progression, a possible interaction between variable serum homocysteine and effect of pharmacologic interventions on DPN require further investigation. Such an investigation might provide insights into explaining why previous alternative therapies produced variable results.
Oral mixed tocotrienols are well tolerated and relatively safe. The high number of AEs and SAEs in both groups reflected the increased risk of complications associated with long-standing diabetes.
Limitations
The main limitation in our study was the lack of an objective test, such as a quantitative sensory test and/or nerve biopsy, in assessing the peripheral nerve dysfunction. Nonetheless, patient-reported outcome based on TSS was acceptable for patients. Moreover, the diagnosis and assessment of painful DPN rely on patients’ perception and description of pain, whereas external observers’ impressions are noted to have limited value in the assessment of patients’ responses toward new therapies for neuropathic pain. In addition, the study population, which was composed of patients with multiple comorbidities, duration of diabetes of more than 10 years, and a mean suboptimal HbA1c level greater than 8.5%, was reflective of real-life practice.
A lower cutoff TSS of 3 or higher was selected to include patients with mild DPN compared with 4 or higher in the ALADIN 3 (α-Lipoic Acid in Diabetic Neuropathy) trial and 5 or higher in the SYDNEY 2 trial. We acknowledged a dropout rate of 23.7% from this trial. However, our study had 83.4% power to detect a mean difference of 1.33 for the TSS after a 12-month period in both groups. In addition, the placebo group might have less severe disease, with a higher proportion taking only oral antidiabetics, whereas more in the tocotrienols group received insulin. We recognized inclusion of patients with vitamin B12 deficiency as a drawback. Two patients in each group were found to have vitamin B12 levels of 211 pg/mL or lower with concomitant hyperhomocysteinemia. After supplementation of mecobalamin and folic acid, their serum vitamin B12 and homocysteine levels were restored to normal levels by 6 months.
Conclusions
Oral supplementation of 400 mg/d of mixed tocotrienols for 1 year did not reduce overall neuropathic symptoms of DPN. Tocotrienols were relatively well tolerated and had a safety profile comparable to that of placebo. The preliminary observations of the effect of tocotrienols on lancinating pain among subsets of patients generate new hypotheses and need further evaluation. We propose future trials on the effects of longer-term mixed tocotrienols on DPN progression and patient quality of life.
Trial Protocol
eTable 1. Symptoms and Scoring of TSS for DPN
eTable 2. Baseline Characteristics for Withdrawn Subjects by Study Group
eTable 3. Mean NIS and Score Changes Over 12 Months by Study Group
eTable 4. Sensory Nerve Conduction Test by Study Group
eTable 5. Glycemic Control Over 12 Months by Study Group
eTable 6. Serum Homocysteine, Vitamins B9 and B12 Over 12 Months by Baseline Homocysteine
eTable 7. Common Adverse Events Reported by Study Group
eTable 8. Serious Adverse Events Reported by Study Group
References
- 1.Herman WH, Kennedy L. Underdiagnosis of peripheral neuropathy in type 2 diabetes. Diabetes Care. 2005;28(6):1480-1481. [DOI] [PubMed] [Google Scholar]
- 2.Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med. 2007;8(suppl 2):S50-S62. [DOI] [PubMed] [Google Scholar]
- 3.Sobhani S, Asayesh H, Sharifi F, et al. Prevalence of diabetic peripheral neuropathy in Iran: a systematic review and meta-analysis. J Diabetes Metab Disord. 2014;13(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. 2015;29(2):212-217. [DOI] [PubMed] [Google Scholar]
- 6.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attal N, Cruccu G, Baron R, et al. ; European Federation of Neurological Societies . EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. [DOI] [PubMed] [Google Scholar]
- 8.Vinik AI, Strotmeyer ES, Nakave AA, Patel CV. Diabetic neuropathy in older adults. Clin Geriatr Med. 2008;24(3):407-435, v. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association 9. Microvascular complications and foot care. Diabetes Care. 2016;39(suppl 1):S72-S80. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34(9):2054-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44(11):1973-1988. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004;21(2):114-121. [DOI] [PubMed] [Google Scholar]
- 13.Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur J Endocrinol. 2012;167(4):465-471. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler D, Hanefeld M, Ruhnau KJ, et al. ; ALADIN III Study Group. . Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). Diabetes Care. 1999;22(8):1296-1301. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler D, Ametov A, Barinov A, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29(11):2365-2370. [DOI] [PubMed] [Google Scholar]
- 16.Haupt E, Ledermann H, Köpcke W. Benfotiamine in the treatment of diabetic polyneuropathy–a three-week randomized, controlled pilot study (BEDIP study). Int J Clin Pharmacol Ther. 2005;43(2):71-77. [DOI] [PubMed] [Google Scholar]
- 17.Stracke H, Gaus W, Achenbach U, Federlin K, Bretzel RG. Benfotiamine in diabetic polyneuropathy (BENDIP): results of a randomised, double blind, placebo-controlled clinical study. Exp Clin Endocrinol Diabetes. 2008;116(10):600-605. [DOI] [PubMed] [Google Scholar]
- 18.Sima AA, Calvani M, Mehra M, Amato A; Acetyl-L-Carnitine Study Group . Acetyl-l-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28(1):89-94. [DOI] [PubMed] [Google Scholar]
- 19.Tütüncü NB, Bayraktar M, Varli K. Reversal of defective nerve conduction with vitamin E supplementation in type 2 diabetes: a preliminary study. Diabetes Care. 1998;21(11):1915-1918. [DOI] [PubMed] [Google Scholar]
- 20.Yueyue Z, Xiaoxia S, Dexue L, Ruige L. Efficacy and safety of vitamin D2 supplementation on diabetic peripheral neuropathy: a multicentre, randomised, double-blind trial. Lancet Diabetes Endocrinol. 2016;4:S29. [Google Scholar]
- 21.Ziegler D, Movsesyan L, Mankovsky B, Gurieva I, Abylaiuly Z, Strokov I. Treatment of symptomatic polyneuropathy with actovegin in type 2 diabetic patients. Diabetes Care. 2009;32(8):1479-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna S, Roy S, Ryu H, et al. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem. 2003;278(44):43508-43515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanna S, Roy S, Slivka A, et al. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005;36(10):2258-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhad A, Chopra K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology. 2009;57(4):456-462. [DOI] [PubMed] [Google Scholar]
- 25.Gopalan Y, Shuaib IL, Magosso E, et al. Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke. 2014;45(5):1422-1428. [DOI] [PubMed] [Google Scholar]
- 26.Yap SP, Julianto T, Wong JW, Yuen KH. Simple high-performance liquid chromatographic method for the determination of tocotrienols in human plasma. J Chromatogr B Biomed Sci Appl. 1999;735(2):279-283. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H, Reichel G. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients: a 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Care. 1997;20(3):369-373. [DOI] [PubMed] [Google Scholar]
- 28.Ametov AS, Barinov A, Dyck PJ, et al. ; SYDNEY Trial Study Group . The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: the SYDNEY trial. Diabetes Care. 2003;26(3):770-776. [DOI] [PubMed] [Google Scholar]
- 29.González R, Pedro T, Martinez-Hervas S, et al. Plasma homocysteine levels are independently associated with the severity of peripheral polyneuropathy in type 2 diabetic subjects. J Peripher Nerv Syst. 2012;17(2):191-196. [DOI] [PubMed] [Google Scholar]
- 30.Brazionis L, Rowley K Sr, Itsiopoulos C, Harper CA, O’Dea K. Homocysteine and diabetic retinopathy. Diabetes Care. 2008;31(1):50-56. [DOI] [PubMed] [Google Scholar]
- 31.Refsum H, Smith AD, Ueland PM, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50(1):3-32. [DOI] [PubMed] [Google Scholar]
- 32.Lonn E, Yusuf S, Arnold MJ, et al. ; Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators . Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567-1577. [DOI] [PubMed] [Google Scholar]
- 33.Dupont WD, Plummer WD Jr. Power and sample size calculations: a review and computer program. Control Clin Trials. 1990;11(2):116-128. [DOI] [PubMed] [Google Scholar]
- 34.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010;(1):CD003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9):2456-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serra J. Targeting membrane hyperexcitability: a promising pharmacological approach to the management of neuropathic pain. Eur Neurol Dis. 2006;2:15-19. [Google Scholar]
- 38.Tesfaye S, Boulton AJ, Dyck PJ, et al. ; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Symptoms and Scoring of TSS for DPN
eTable 2. Baseline Characteristics for Withdrawn Subjects by Study Group
eTable 3. Mean NIS and Score Changes Over 12 Months by Study Group
eTable 4. Sensory Nerve Conduction Test by Study Group
eTable 5. Glycemic Control Over 12 Months by Study Group
eTable 6. Serum Homocysteine, Vitamins B9 and B12 Over 12 Months by Baseline Homocysteine
eTable 7. Common Adverse Events Reported by Study Group
eTable 8. Serious Adverse Events Reported by Study Group