Abstract
Subnanoscale zerovalent iron (ZVI) synthesized using smectite clay as a template was utilized to investigate reduction of decabromodiphenyl ether (DBDE). The results revealed that DBDE was rapidly debrominated by the prepared smectite-templated ZVI with a reaction rate 10 times greater than that by conventionally prepared nanoscale ZVI. This enhanced reduction is plausibly attributed to the smaller-sized smectite-templated ZVI clusters (~0.5 nm) vs that of the conventional nanoscale ZVI (~40 nm). The degradation of DBDE occurred in a stepwise debromination manner. Pentabromodiphenyl ethers were the terminal products in an alkaline suspension (pH 9.6) of smectite-templated ZVI, whereas di-, tri-, and tetrabromodiphenyl ethers formed at the neutral pH. The presence of tetrahydrofuran (THF) as a cosolvent at large volume fractions (e.g., >70%) in water reduced the debromination rates due to enhanced aggregation of clay particles and/or diminished adsorption of DBDE to smectite surfaces. Modification of clay surfaces with tetramethylammonium (TMA) attenuated the colsovent effect on the aggregation of clay particles, resulting in enhanced debromination rates. Smectite clay provides an ideal template to form subnanoscale ZVI, which demonstrated superior debromination reactivity with DBDE compared with other known forms of ZVIs. The ability to modify the nature of smectite clay surface by cation exchange reaction utilizing organic cations can be harnessed to create surface properties compatible with various contaminated sites.
Graphical Abstract
INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) have been widely used as flame retardants in plastic products to suppress combustion and formation of toxic fumes.1,2 These compounds are recalcitrant in the environment and, likewise, resist degradation in many engineered treatment processes. As a result, PBDEs are ubiquitous in the environment and found in human blood, milk, and tissues.3–6 Concerns about contamination in human tissues by PBDEs are growing because the results of animal studies have demonstrated that PBDEs disrupt the endocrine system, threaten reproductive health, and impair development of neural system.7–9 Among the PBDEs commonly used, decabromodiphenyl ether (DBDE) comprises 80% of the total consumption.10 Therefore, efficient and costeffective technologies are needed for remediation of PBDE-contaminated (especially DBDE) sites.
PBDEs are highly lipophilic compounds as evidenced by their high octanol/water partition coefficients (KOW). For instance, tetrabromodiphenyl ether exhibits log KOW of 5.9, and KOW values increase for diphenyl ethers containing more –Br substituents. The lipophilicity and recalcitrance cause PBDEs to accumulate in soil/sediment, rather than in water, after they are released to the environment.11,12 Soil washing with organic cosolvents is an effective approach to remove PBDEs from contaminated soils.13 Subsequent treatment using zerovalent iron (ZVI) to reduce PBDEs to less toxic products (e.g., diphenyl ether) is a promising strategy for remediation of PBDE contamination.14 This treatment strategy might also be applicable to PBDE-enriched sewage from wastewater treatment plants. In addition, the presence of organic cosolvent may diminish ZVI reaction with water and, hence, enhance its utilization efficiency. The reaction of PBDEs with ZVI generally manifests a stepwise debromination process leading to diminished hazard of PBDE contamination to the ecosystem quality.10
Reductive reactions with ZVI have been widely accepted as an attractive approach for remediation of halogenated organic compounds.15–17 Intensive efforts have been devoted to the development of efficient forms of ZVI for application in field trials because this technology is economically feasible and does not pose much risk of ancillary harm to the environment.10,18,19 ZVI particles with large surface area, especially nanoscale ZVIs, consistently demonstrate high reaction efficiencies with halogenated organic compounds.20,21 Nanoscale ZVIs exhibited approximately 7-fold greater reactivity with DBDE, relative to microscale ZVIs that had smaller surface area.2 A problem with nanoscale ZVI particles is their tendency to agglomerate in preparation and application, hence diminishing their reaction efficiencies. To minimize such aggregation effect on reactivity, anionic hydrophilic polymers, silica, starch, and resins have been utilized as matrixes to facilitate the dispersion of ZVI particles in the solution.19,22–26 Recently, Gu et al. utilized smectite clay as supporting template to synthesize smectite-intercalated ZVI with particle sizes of ~0.5 nm.27 This smectite-templated ZVI demonstrated a superior reductive reactivity with organic contaminants such as nitroaromatics.
In this study, we investigated the reductive debromination of DBDE by smectite-templated subnanoscale ZVI in water/ cosolvent solution. The reaction intermediates and products were identified to elucidate the operative reaction mechanisms. In addition, smectite with the templated ZVIs was modified by ion exchange reaction using organic cations to displace strongly hydrated inorganic cations; this modification lessens the hydrophilic nature of the clay surfaces and gallery regions. The goal of this surface modification was to enhance the dispersion of smectite-templated ZVI particles and, hence, the debromination of DBDE in water/cosolvent systems that might be used in soil-washing processes aimed to solublize soil-bound PBDEs.
EXPERIMENTAL SECTION
Chemicals
Decabromodiphenyl ether (>98%), sodium borohydride (>98%), silver nitrate (>99%) and tetramethy-lammonium (TMA) bromide (>98%) were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI). A standard mixture of 39 PBDE congeners was obtained from AccuStandard Inc. (New Haven, CT). Anhydrous ferric chloride (>99%), hydrochloric acid (~37%), hydrofluoric acid (~48%), and nitric acid (~70%) were obtained from Fisher Scientific (Fair Lawn, NJ). Smectite clay (Wyoming montmor-illonite SWy-2) was obtained from the Source Clays Repository of the Clay Minerals Society. Acetonitrile, tetrahydrofuran (THF), and toluene were purchased from Sigma-Aldrich Chemical Co..
Preparation and Characterization of Nanoscale and Subnanoscale ZVIs
The “conventional” nanoscale ZVI was prepared by dropwise addition of aqueous NaBH4 solution (1.6 M) to 1.0 M FeCl3 solution under continuous stirring.2 The morphology and particle size of the nanoscale ZVI thus formed was analyzed using transmission electron microscopy (TEM). Smectite-templated subnanoscale ZVI was prepared using the procedure reported by Gu et al.27 Specifically, smectite clay (50 g) was mixed with 1.0 L of 0.1 M FeCl3 under acidic conditions (pH ~4) for 6 h and centrifuged at 3295g for 15 min to separate the supernatant from clay. This procedure was repeated four times followed by several water-washing cycles until Cl− was absent from the supernatant tested by AgNO3. For TMA-modified Fe(III)-smectite, TMA bromide (0.052 g) was mixed with 2.0 g of Fe(III)-smectite in aqueous solution. The added TMA was equivalent to 15% of the cation exchange capacity of smectite. The Fe(III)- and TMA-modified Fe(III)-smectites were freeze-dried to obtain clay powders. The smectite powders were then weighed into glass centrifuge tubes and mixed with water to form suspensions in which pH was adjusted to ~2.5 using 1.0 M HCl and excess NaBH4 was added. The Fe(III) on mineral surfaces was immediately reduced to Fe0 as evidenced by changing to black color.27 The clay suspension was then rinsed with water to remove excess NaBH4. The freshly prepared smectite-templated ZVI was immediately used for reaction with DBDE.
Iron content in the prepared clay was determined by HCl–HNO3–HF digestion followed by analysis using a Perkin-Elmer atomic absorption spectrophotometer (AAS). Organic carbon content of the TMA-modified clay was determined using a Shimadzu total organic carbon analyzer coupled to a solid sample combustion module. X-ray diffraction (XRD) patterns were obtained using a PANalytical Empyrean X-ray diffractometer equipped with Co Kα radiation (λ = 1.79 Å). For the freeze-dried clay, XRD signals were collected with diffraction angle ranging from 2 to 13° at a scanning step of 0.03° s−1. For clay suspensions, the XRD signals were collected at a scanning step of 0.002° s−1 using a reflection-transmission spinning sample holder. TEM coupled to a JEOL 2200FS 200-kV field emission microscope was utilized to obtain the clay-templated ZVI image and measure the particle size. X-ray energy dispersive spectroscopy (EDS) was utilized to identify the element composition and distribution.
Debromination of DBDE
DBDE was dissolved in the water/THF mixture (5.0 mL) systematically varying from 10 to 90% THF (by volume) at a concentration of 2.5 mg/L. The prepared clay of 20 mg (containing 0.62 mg of ZVI) was mixed with the DBDE solution in a glass vial. Water used to prepare the solution was boiling for 0.5 h and then purged with N2 for another 0.5 h to remove dissolved oxygen. After the samples were prepared, the glass vials were immediately transported into an anaerobic chamber and rotated on a mechanical mixer. At a given reaction interval, several vials were taken from the anaerobic chamber and centrifuged at 3295g for 10 min to separate supernatant from clay particles. The supernatant was collected and analyzed for DBDE concentration using a PerkinElmer high-performance liquid chromatograph (HPLC) equipped with a UV–visible detector set at a wavelength of 235 nm. The mobile phase was acetonitrile/water mixture (v/v = 98/2) with a flow rate at 1.0 mL/min.
DBDE reaction intermediates and products were analyzed using an Agilent gas chromatograph/mass spectrometer (GC/ MS) system equipped with a DB-1HT column (30 m × 0.25 mm). The supernatant obtained after DBDE reacted with smectite-templated ZVI was exposed to flowing air in a fume hood overnight to allow THF to volatilize from the bulk solution. The remaining supernatant sample was extracted with toluene three times. The clay residue was also extracted with toluene and combined with the supernatant extract. The volume of the combined extracts was reduced to 0.5 mL by a gentle N2 stream. The average extraction recovery of DBDE in the control (in the presence of clay but absence of ZVI) was 70% with standard deviation of 14%. For GC/MS analysis, the oven temperature was held at 100 °C for 2 min, ramped to 320 °C at a rate of 5 °C/min, and then held at 320 °C for 3 min. The injection port temperature was set at 300 °C. The helium gas flow rate was 1.4 mL/min. Intermediate PBDEs were identified and quantified by comparing the retention time and mass spectra with those of authentic standards.
Corrosion of Smectite-Templated ZVI in Water/THF Solution
To evaluate the influence of THF on the corrosion of ZVI, smectite-templated ZVI (0.05 g of clay) was mixed with 15.0 mL of aqueous solutions containing 10, 30, 50, 70, and 90% THF (by volume). At a given sampling time, solution pH was acidified to ~3.5 using HCl, followed by immediate centrifugation at 3295g for 5 min to separate smectite-ZVI particles from solution. The THF in the supernatant was removed by a gentle N2 stream in a fume hood and then replenished to 15.0 mL with water. Na+ and Fe2+ concentrations in the solution were determined using AAS.
RESULTS AND DISCUSSION
DBDE Reaction with Smectite-Templated Subnano-scale vs Conventional Nanoscale ZVIs
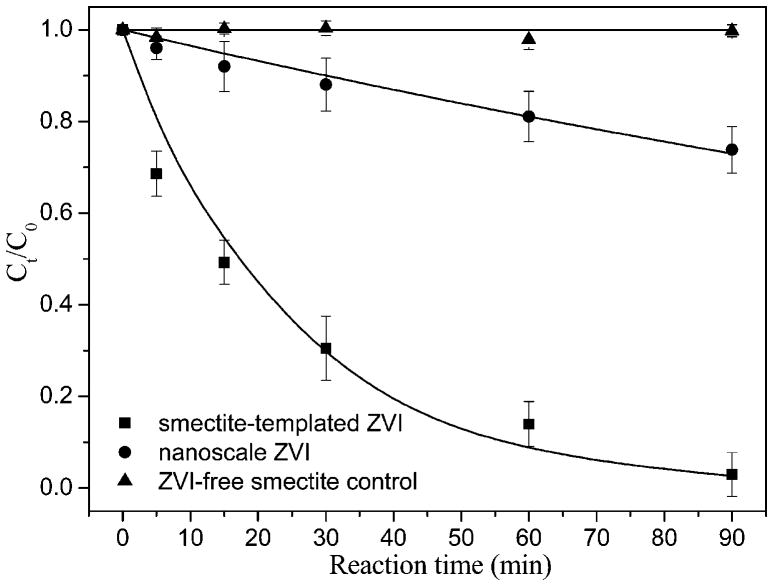
Reaction of DBDE (2.5 mg/L) with smectite-templated subnanoscale vs conventional nanoscale ZVIs, each containing the same amount of Fe0 (0.62 mg), was measured in THF/water solution (50% of THF) as a function of contact time (Figure 1). Nearly 100% of DBDE was degraded after a 90 min reaction with smectite-templated ZVI, whereas only 26% of DBDE was broken down from the solution by reaction with conventional nanoscale ZVI. No apparent change in DBDE concentration was observed for ZVI-free control during the study period, indicating that sorption of DBDE to clay surface was minimal. For the controls of Na+- or Fe2+-saturated smectites, there was little decrease of DBDE concentration indicating that neither structural nor exchanged iron is capable of reducing DBDE. The debromination of DBDE is thus attributed to the exchanged and then reduced ZVI.
Figure 1.
Comparison of DBDE reaction with smectite-templated and conventional nanoscale ZVIs in tetrahydrofuran(THF)/water solution (50% of THF by volume). DBDE initial concentration = 2.5 mg/L, and ZVI = 0.62 mg for both forms of ZVIs.
In the present studty, the amount of ZVIs was ~100 times the electrons needed to reduce DBDE to diphenyl ether. Therefore, it is reasonable to assume that the reaction follows the pseudo-first-order reaction with DBDE. The data fit well (Figure 1) to the pseudo-first-order kinetics model: Ct/C0 = exp(−kt), where Ct (mg/L) and C0 (mg/L) refer to DBDE concentration at a given reaction time t and initial time, and k (min−1) is the reaction rate constant. The estimated rate constant was 3.6 × 10−2 min−1 for DBDE degradation by the smectite-templated ZVI, >10 times greater than that by the conventional nanoscale ZVI (3.2 × 10−3 min−1). This is plausibly attributed to the smaller size of ZVI clusters (~0.5 nm) intercalated in smectite interlayers (described below) and to formation of nonreactive Fe oxides on the conventional nanoscale ZVI surfaces.28
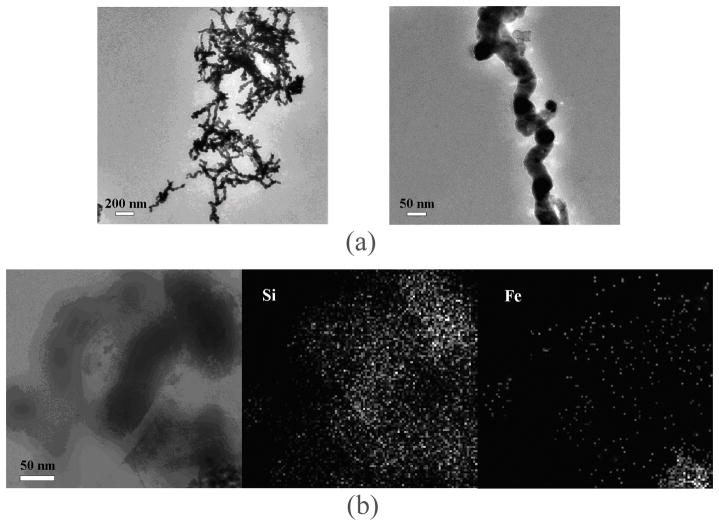
The morphology of the synthesized conventional nanoscale ZVI is shown in Figure 2a. These conventional ZVI particles appear to exist primarily as spherical structures with diameters averaging 30–50 nm and then form aggregates with lengths ranging from 200 nm to 2 μm. Similar morphologies have been observed in previous studies.23,29 A TEM image of the smectite-templated ZVI is shown in Figure 2b, along with the elemental Si and Fe distribution mapping of the same region. Since Si is the major framework element of layered smectite structures, the distribution of Fe was similar to Si (Figure 2b). This result implies that Fe is uniformly distributed on smectite surfaces. For clay structural Fe, no Fe signal was observed for Na+-saturated smectite indicating that the Fe mapping performed in this study is primarily the ZVI emanating from the reduction of exchanged Fe(III) by NaBH4. The formed ZVIs were intercalated in the clay interlayer gallery as well as resided on the external surfaces of clay tactoids.30 The relatively high intensity of signals at the lower right corner of the Fe mapping (Figure 2b right panel) could result from ZVI clusters on external surfaces of smectite, with the sizes ranging between 5 and 20 nm. The intercalated ZVIs apparently are much smaller with the size of 0.5 nm estimated from X-ray diffraction measurements of interlayer distance (see below).
Figure 2.
(a) TEM image of aggregated spherical particles of synthesized conventional nanoscale ZVI. (b) TEM image smectite-templated ZVI (left) and elemental distribution mapping of Si (middle) and Fe (right) of the same region.
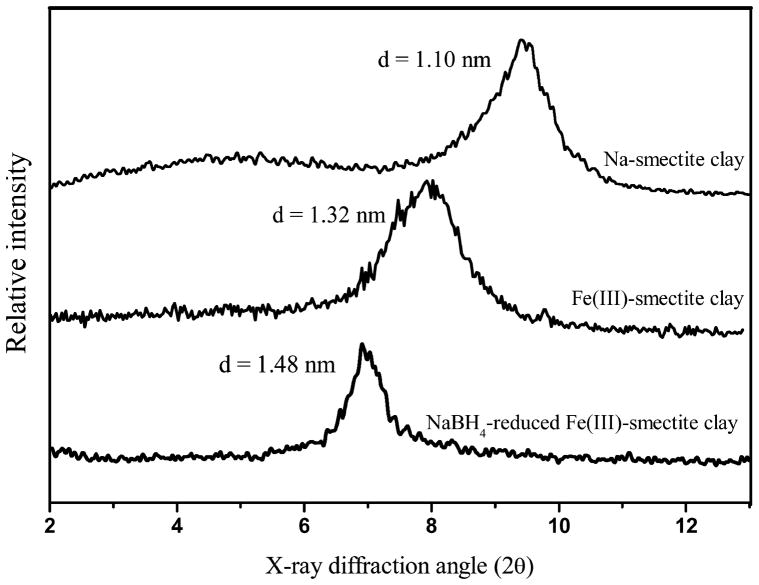
The added NaBH4 reduced the exchanged Fe(III) located either on the smectite external surfaces of stacked aggregates of clay platelets or in the interlayer gallery regions.30 XRD patterns for Na- and Fe(III)-smectite, as well as NaBH4-reduced Fe(III)-clay, are shown in Figure 3, along with estimated basal spacings. The negative charges of the NaBH4- reduced Fe(III)-clay are presumably compensated with Na+. However, the basal spacing of NaBH4-reduced Fe(III)-clay did not collapse to 1.10 nm (the basal spacing of Na-smectite), indicating that the formed ZVI clusters are intercalated between the layers and act as pillars that expand the interlayer distance (beyond 1.10 nm). It can be inferred that such interlayer ZVI particles have sizes of ~0.56 nm perpendicular to clay sheets, based on the measurement that observed basal spacing of 1.48 nm (Figure 3) is subtracted by the thickness of one smectite layer (0.92 nm). These ZVI clusters between clay interlayers are approximately 2 orders of magnitude smaller than the synthesized nanoscale ZVI (30–50 nm). The large surface area of these extremely small-sized ZVI particles could be the major factor for the enhanced reduction of DBDE by smectite-templated ZVI (Figure 1). In addition to the large surface area, smectite surfaces adsorbed Fe2+ ions which were produced by ZVI reducing PBDEs and occupied cation exchange sites and hydroxyl groups on siloxane surfaces. This could isolate the Fe2+ from the ZVI clusters and thereby prevent the formation of nonreactive Fe oxide layers that would inhibit further ZVI reactivity.
Figure 3.
X-ray diffraction patterns of Na- and Fe(III)-saturated smectite and NaBH4-reduced Fe(III)-smectite clay.
Debromination Reaction
Smectite-templated ZVI rapidly debrominated DBDE and converted it to lower –Br substituted congeners in a stepwise manner (Figure 4). At early stages of reaction (e.g., 20 min) in alkaline solution (pH 9.6), DBDE lost one or two –Br moieties and sequentially formed nona- and octa-BDEs. As the reaction proceeded, more –Br of PBDE was replaced with –H forming hepta-, hexa-, and penta-BDEs (Figure 4a,b). Multiple congeners were simultaneously formed as indicated by the increase of peak numbers in GC-MS chromatograms. This result suggests the formation of several PBDE isomers and no apparent selectivity for debromination at the position (ortho, meta, vs para). This is in contrast to most microbially mediated dehalogenation processes.31,32 Compared with previous studies, smectite-templated ZVI demonstrated a superior reactivity toward the debromination of DBDE. Li et al. reported that debromination of DBDE solution (0.1 mg/L in 8.0 mL) with 13.75 g/L of resin-bound nanoscale ZVI produced penta-BDE after a 2 day reaction.23 Keum and Li applied macroscale ZVI (5.0 g) to reduce 10.0 mL of 5 mg/L DBDE solution. After a 5 day reaction, >60% of the products were hexa- and lower-brominated diphenyl ethers.10 In the current study, only 0.62 mg Fe0 was used to reduce 5.0 mL of 2.5 mg/L of DBDE in water/THF solution. Penta-BDEs appeared as debrominated products within 5 h, and ~80% of products formed were penta- and hexa-BDE congeners after a 9 h reaction in alkaline solution (Figure 4a,b). Therefore, the smectite-templated ZVI synthesized and described herein demonstrated much faster and more extensive debromination, as well as a much greater Fe0 use efficiency, compared with macroscale or nanoscale ZVIs reported in previous studies.
Figure 4.
GC/MS chromatograms of DBDE and debrominated products formed at different reaction times in solution of (a) pH 9.6 and (c) pH 6.3. Distribution percentage of congeners formed from DBDE reduction by smectite-templated ZVI in the solution of (b) pH 9.6 and (d) pH 6.3.
It was observed that after 9 h reaction, the most abundant end products from the debromination of DBDE by smectite-templated ZVI were primarily penta- and hexa-BDEs in the alkaline (pH 9.6) solution (Figure 4a,b). In a slightly acidic solution (pH ~6.3), significant amounts of lower-brominated congeners, including di-, tri-, and tetrabromodiphenyl ethers, were formed indicating DBDE underwent more rapid and extensive debrominative reaction at lower pH (Figure 4c,d). These results suggest that acidic solution favors the debromination process. A previous study reported that for debromination of DBDE by nanoscale ZVI, mono-BDEs were formed in the solution of pH 5 and 7, while in alkaline solution (pH 10) penta- and higher Br-substituted diphenyl ethers were the major products.2 The alkaline solution (e.g., pH 9.6 in this study) was expected to favor the formation of relatively insoluble and nonreactive Fe-oxides/hydroxides which could attach to smectite surfaces and inhibit diffusion and interaction of PBDEs with intercalated ZVI clusters.
Cosolvent Effect
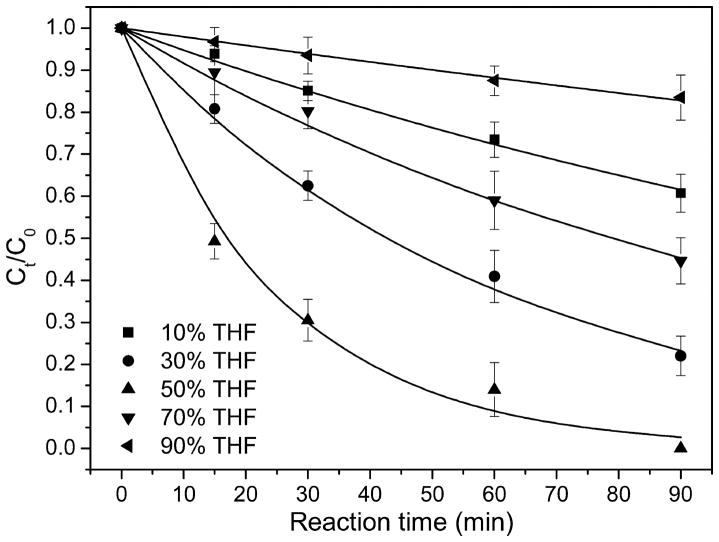
Synthetic ZVI particles in water could be corroded and produce hydrogen gas Fe0 + 2H2O → Fe2+ + H2 + 2OH−, which consumes ZVI and thus reduces its reaction efficiency.33,34 The addition of organic cosolvent decreases the potential contacts of ZVI with water, which could help maintain the reactivity of ZVI. This hypothesis was tested by measuring the degradation of DBDE as a function of reaction time in THF/water solution with THF volume percentage of 10, 30, 50, 70, and 90% (Figure 5). DBDE removal efficiency in THF/water systems decreased in the order of 50% > 30% > 70% > 10% > 90% THF. Correspondingly, the estimated pseudo-first-order reaction rate constants were 3.6 × 10−2, 1.6 × 10−2, 7.9 × 10−3, 4.9 × 10−3, and 2.1 × 10−3 min−1.
Figure 5.
Debromination of DBDE with smectite-templated ZVI in tetrahydrofuran (THF)/water solution with varying THF volume fractions. The initial DBDE concentration was 2.5 mg/L, and smectite-templated ZVI dosage was 0.62 mg per 5.0 mL of solution.
As hypothesized, the reaction efficiency of smectite-templated ZVI increased monotonically as the THF fraction increased from 10 to 50%, presumably due to the concomitant decrease in water activity. However, in the solution containing 70 and 90% of THF, the degradation rates of DBDE were reduced. The presence of THF enhances the dissolution of DBDE in bulk solution; this effect could be sufficient to diminish contact of DBDE to the ZVI reactive surfaces. In addition, clay platelets with relatively hydrophilic surfaces and strongly hydrated exchangeable cations tend to form larger aggregates as the solution becomes more hydrophobic, hence diminishing the contact and/or diffusion of DBDE to the reactive ZVI sites in smectite interlayers. Thus, the presence of an organic cosolvent apparently causes opposing impacts on the debromination of DBDE by smectite-templated ZVI, and we attempt to elucidate these effects below.
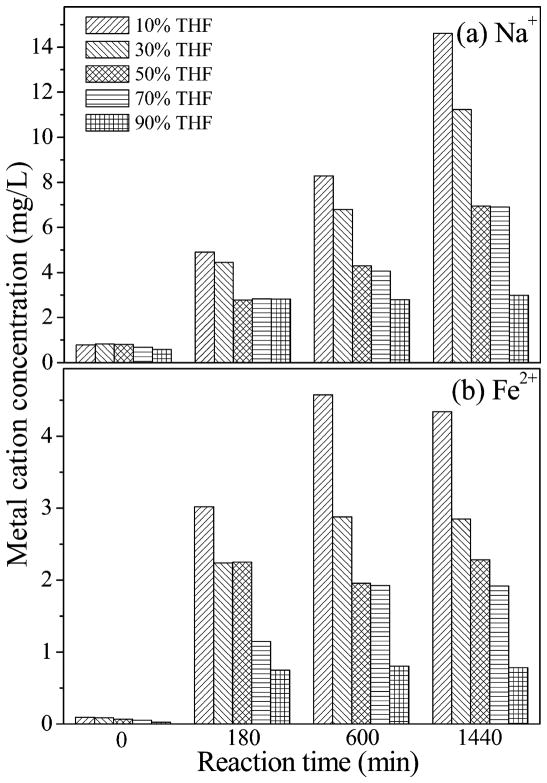
The corrosion (oxidation) of ZVI by water results in the release of Fe(II) to the solution which could displace Na+ on clay surfaces or associated with hydroxyl groups on the siloxane surfaces. The Na+ and Fe2+ concentrations in the solution were measured (Figure 6). Within the first 5 min, minimal amounts of Na+ and Fe2+ were released into solution from the freshly prepared smectite-ZVI slurries in all samples. As the reaction proceeded, more Na+ and Fe2+ were released into the solution due to the reaction of ZVI with water; it is likely that much of the Fe(II) released from water–ZVI reactions would displace Na+ that originally occupied the negatively charged sites on clay surfaces. Interestingly, smaller amounts of Fe2+ and Na+ were released to the solution containing larger volume percentage of THF (e.g., 70 and 90%) (Figure 6). As hypothesized, the decreased water activity diminishes the extent of water reaction with ZVI, supporting the concept that organic cosolvent can reduce the corrosion of ZVI and thereby enhance its reductive reaction efficiency.
Figure 6.
Concentration of (a) Na+ and (b) Fe2+ released from smectite-templated ZVI in tetrahydrofuran (THF)/water solution containing varying THF fractions.
Aqueous solution with THF volume percentage of 70 or 90%, however, manifested lower degradation efficiencies for DBDE (Figure 5). As discussed above, this inhibitory effect might result from diminished adsorption of DBDE on mineral surfaces and reduced diffusion of DBDE into smectite interlayer regions of larger clay aggregates formed in a THF/water mixture. Dehalogenation by ZVI is a surface-mediated reaction in which organic contaminants must closely attach to ZVI surfaces to enable the electron transfer.21,33,35 Smectite surfaces are generally regarded as hydrophilic in nature because the cation exchange sites are compensated by the strongly hydrated Na+.36 These hydrophilic clay particles tended to aggregate rather than disperse as THF was added, and the bulk solution became more hydrophobic. Indeed, the formation of larger clay aggregates was visually observed in the solution containing high fractions of THF.
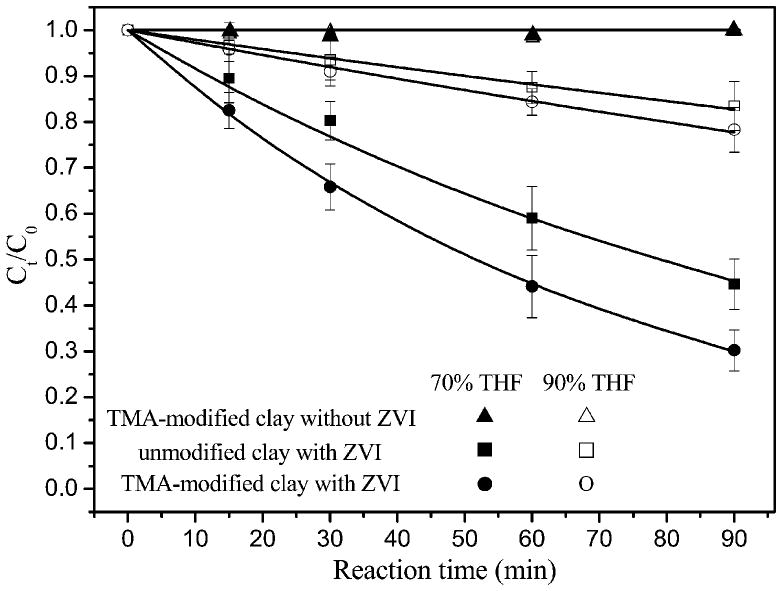
In an effort to enhance the dispersion of smectite-templated ZVI in THF/water solution, TMA was added at the amount equivalent to 15% of the clay cation exchange capacity to Fe(III)-smectite before reduction with NaBH4. We hypothesized that this modification could diminish the hydrophilicity of clay surfaces and increase the compatibility of clays with THF/ water solution. The TMA-modification of smectite-templated ZVI significantly increased the DBDE degradation rates in the solutions containing 70 and 90% of THF (Figure 7). In the control experiment with TMA-smectite (lack of ZVI), DBDE concentration remained constant indicating DBDE was not adsorbed by TMA-modified clay. Note that these degradation rates increased despite the reduced amount of Fe0 in the TMA-modified systems. The estimated debromination rate constants were 1.2 × 10−2 and 2.8 × 10−3 min−1 for TMA-modified clays in 70% and 90% THF/water solution, which are 51.9% and 33.3% greater than the degradation rates with the unmodified smectite-templated ZVI. TMA-modification of smectite surfaces leads to a decrease in the hydrophilic nature of clay surfaces and the interlayer environment,37,38 which helps clay particles better disperse in THF/water solution and plausibly enhances contact of DBDE with ZVI reaction sites.20,39 Smectite modified with organic ammonium cations typically results in increased interlayer spacings,37,40 which could facilitate the diffusion of DBDE to clay interlayer regions hence increasing its contact with ZVI. However, in this study, the relatively small organic cation (TMA) and low loading rate (15% of cation exchange capacity) did not render significant increase of basal spacing (<0.4 Å) compared with the unmodified smectite-ZVI particles. This suggests that the enhanced ZVI reactivity of TMA-modified clay is attributed primarily to the reduced hydrophilic nature of clay surfaces. Such modification allows clay particles to better disperse in THF/water solution hence increasing DBDE diffusion into the clay interlayer regions where the reaction with ZVI occurs.
Figure 7.
Reduction of DBDE with unmodified and TMA-modified smectite-templated subnanoscale ZVI in tetrahydrofuran(THF)/water solution. The same amount of Fe0 (0.62 mg) was applied in both experiments.
Environmental Implications
This study indicates that smectite-templated subnanoscale ZVI is a promising functional material that can be utilized to treat a wide variety of pollutants including PBDEs which are otherwise recalcitrant to natural attenuation or treatments in engineered systems. Sequential combination with biodegradation could become a more efficient and effective treatment for PBDE contaminated sites.41 Use of clays as templates to secure subnanoscale ZVI not only provides a more reactive and efficient form of ZVI but also may avoid the potential problems and toxicities associated with application of conventional nanoscale ZVI particles.
Acknowledgments
This project was supported in part by Grant P42 ES004911 from National Institute of Environmental Health Sciences (NIEHS), National Institute of Health, and Michigan AgBioResearch.
Footnotes
Notes
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS.
The authors declare no competing financial interest.
References
- 1.La Guardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol. 2006;40:6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- 2.Shih YH, Tai YT. Reaction of decabrominated diphenyl ether by zerovalent iron nanoplarticles. Chemosphere. 2010;78:1200–1206. doi: 10.1016/j.chemosphere.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 3.Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mother’s milk. Environ Health Perspect. 2003;111:1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.She J, Petreas M, Winkler J, Visita P, McKinney M, Kopec D. PBDEs in the San Francisco Bay Area: Measurements in harbor seal blubber and human breast adipose tissue. Chemosphere. 2002;46:697–707. doi: 10.1016/s0045-6535(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 6.Hites RA. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 7.COT Statement on Brominated Flame Retardants in Fish from the Skerne-Tees Rivers System. Committee on Toxicity of Chemicals in Food Consumer Products and the Environment; London: 2004. [Google Scholar]
- 8.Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109:49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meerts I, Van Zanden JJ, Luijks EAC, Van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- 10.Keum YS, Li QX. Reductive debromination of polybrominated diphenyl ethers by zerovalent iron. Environ Sci Technol. 2005;39:2280–2286. doi: 10.1021/es048846g. [DOI] [PubMed] [Google Scholar]
- 11.Kastanek F, Maleterova Y, Kastanek P, Rott J, Jiricny V, Jiratova K. Complex treatment of wastewater and groundwater contaminated by halogenated organic compounds. Desalination. 2007;211:261–271. [Google Scholar]
- 12.Vonderheide AP, Mueller KE, Meija J, Welsh GL. Polybrominated diphenyl ethers: Causes for concern and knowledge gaps regarding environmental distribution, fate and toxicity. Sci Total Environ. 2008;400:425–436. doi: 10.1016/j.scitotenv.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Loraine GA. Effects of alcohols, anionic and nonionic surfactants on the reduction of PCE and TCE by zero-valent iron. Water Res. 2001;35:1453–1460. doi: 10.1016/s0043-1354(00)00422-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang Y, Ahn S, Luthy R. Debromination of polybrominated diphenyl ethers by nanoscale zerovalent iron: pathways, kinetics, and reactivity. Environ Sci Technol. 2010;44:8236–8242. doi: 10.1021/es101601s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillham RW, Ohannesin SF. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water. 1994;32:958–967. [Google Scholar]
- 16.Kim YH, Carraway ER. Dechlorination of pentachlorophenol by zero valent iron and modified zero valent irons. Environ Sci Technol. 2000;34:2014–2017. [Google Scholar]
- 17.Xiong Z, Zhao D, Pan G. Rapid and complete destruction of perchlorate in water and ion-exchange brine using stabilized zero-valent iron nanoparticles. Water Res. 2007;41:3497–3505. doi: 10.1016/j.watres.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Cho HH, Park JW. Sorption and reduction of tetrachloroethylene with zero valent iron and amphiphilic molecules. Chemosphere. 2006;64:1047–1052. doi: 10.1016/j.chemosphere.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 19.He F, Zhao D, Paul C. Field assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones. Water Res. 2010;44:2360–2370. doi: 10.1016/j.watres.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Alessi DS, Li ZH. Synergistic effect of cationic surfactants on perchloroethylene degradation by zero-valent iron. Environ Sci Technol. 2001;35:3713–3717. doi: 10.1021/es010564i. [DOI] [PubMed] [Google Scholar]
- 21.Weber EJ. Iron-mediated reductive transformations: Investigation of reaction mechanism. Environ Sci Technol. 1996;30:716–719. [Google Scholar]
- 22.He F, Zhao D. Preparation and characterization of a new class of starch-stabilized bimetallic nonaparticles for degradation of chlorinated hydrocarbons in water. Environ Sci Technol. 2005;39:3314–3320. doi: 10.1021/es048743y. [DOI] [PubMed] [Google Scholar]
- 23.Li A, Tai C, Zhao ZS, Wang YW, Zhang QH, Jiang GB, Hu JT. Debromination of decabrominated diphenyl ether by resin-bound iron nanoparticles. Environ Sci Technol. 2007;41:6841–6846. doi: 10.1021/es070769c. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Dozier A, Bhattacharyya D. Synthesis of nanoscale bimetallic particles in polyelectrolyte membrane matrix for reductive transformation of halogenated organic compounds. J Nanopart Res. 2005;7:449–467. [Google Scholar]
- 25.Zhu BW, Lim TT, Feng J. Reductive dechlorination of 1,2,4-trichlorobenzene with palladized nanoscale Fe0 particles supported on chitosan and silica. Chemosphere. 2006;65:1137–1145. doi: 10.1016/j.chemosphere.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Qiu XH, Fang ZQ, Liang B, Gu FL, Xu ZC. Degradation of decabromodiphenyl ether by nano zero-valent iron immobilized in mesoporous silica microspheres. J Hazard Mater. 2011;193:70–81. doi: 10.1016/j.jhazmat.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Gu C, Jia HZ, Li H, Teppen BJ, Boyd SA. Synthesis of highly reactive subnano-sized zero-valent iron using smectite clay templates. Environ Sci Technol. 2010;44:4258–4263. doi: 10.1021/es903801r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang GCC, Lee HL. Chemical reduction of nitrate by nanosized iron: Kinetics and pathways. Water Res. 2005;39:884–894. doi: 10.1016/j.watres.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Lowry GV, Johnson KM. Congener-specific dechlorination of dissolved PCBs by microscale and nanoscale zerovalent iron in a water/methanol solution. Environ Sci Technol. 2004;38:5208–5216. doi: 10.1021/es049835q. [DOI] [PubMed] [Google Scholar]
- 30.Jia HZ, Gu C, Boyd SA, Teppen BJ, Johnston CT, Song CY, Li H. Comparison of reactivity of nanoscale zero-valent iron formed on clay surfaces. Soil Sci Soc Am J. 2011;75:357–364. [Google Scholar]
- 31.Gerecke AC, Hartmann PC, Heeb NV, Kohler HPE, Giger W, Schmid P, Zennegg M, Kohler M. Anaerobic degradation of decabromodiphenyl ether. Environ Sci Technol. 2005;39:1078–1083. doi: 10.1021/es048634j. [DOI] [PubMed] [Google Scholar]
- 32.Borja J, Taleon DM, Auresenia J, Gallardo S. Polychlorinated biphenyls and their biodegradation. Process Biochem. 2005;40:1999–2013. [Google Scholar]
- 33.Lee CL, Jou CJG. Reduced degradation of chlorobenzene in cosolvent solution using nanoscale zerovalent iron with microwave irradiation. Environ Eng Sci. 2010;28:191–195. [Google Scholar]
- 34.Wang Q, Snyder S, Kim J, Choi H. Aqueous ethanol modified nanoscale zerovalent iron in bromate reduction: Synthesis, characterization, and reactivity. Environ Sci Technol. 2009;43:3292–3299. doi: 10.1021/es803540b. [DOI] [PubMed] [Google Scholar]
- 35.Nurmi JT, Tratnyek PG, Sarathy V, Baer DR, Amonette JE, Pecher K, Wang CM, Linehan JC, Matson DW, Penn RL, Driessen MD. Characterization and properties of metallic iron nanoparticles: Spectroscopy, electrochemistry, and kinetics. Environ Sci Technol. 2005;39:1221–1230. doi: 10.1021/es049190u. [DOI] [PubMed] [Google Scholar]
- 36.Laird DA, Fleming PD. Mechanisms for adsorption of organic bases on hydrated smectite surfaces. Environ Toxicol Chem. 1999;18:1668–1672. [Google Scholar]
- 37.Boyd SA, Mortland MM, Chiou CT. Sorption characteristics of organic-compounds on hexadecyltrimethylammonium-smectite. Soil Sci Soc Am J. 1988;52:652–657. [Google Scholar]
- 38.Xu SH, Boyd SA. Cationic surfactant adsorption by swelling and nonswelling layer silicates. Langmuir. 1995;11:2508–2514. [Google Scholar]
- 39.Sheng GY, Xu SH, Boyd SA. Mechanism(s) controlling sorption of neutral organic contaminants by surfactant-derived and natural organic matter. Environ Sci Technol. 1996;30:1553–1557. [Google Scholar]
- 40.Boyd SA, Lee JF, Mortland MM. Attenuating organic contaminant mobility by soil modification. Nature. 1998;333:345–347. [Google Scholar]
- 41.Kim YM, Murugesan K, Chang YY, Kim EJ, Chang YS. Degradation of polybrominated diphenyl ethers by a sequential treatment with nanoscale zero valent iron and aerobic biodegradation. J Chem Technol Biotechnol. 2012;87:216–224. [Google Scholar]