Key Points
Question
Does 12-week treatment with vaginal 10-μg estradiol tablet or vaginal moisturizer improve postmenopausal vulvovaginal symptoms more than placebo?
Findings
In a randomized clinical trial of 302 postmenopausal women with moderate-to-severe vulvovaginal symptoms, vaginal 10-μg estradiol tablet plus placebo gel and vaginal moisturizer plus placebo tablet were not more efficacious than dual placebo at reducing symptom severity or improving sexual function.
Meaning
Shared decision making for treatment of postmenopausal vulvovaginal symptoms can be based on cost and patient formulation preference; vaginal estradiol tablets appear not to add benefit beyond vaginal gel or moisturizer.
This randomized clinical trial compares the efficacy of a low-dose vaginal estradiol tablet and a vaginal moisturizer, each vs placebo, for treatment of moderate-to-severe postmenopausal vulvovaginal symptoms.
Abstract
Importance
Nearly half of postmenopausal women report bothersome vulvovaginal symptoms, but few data support the efficacy of 2 commonly recommended treatments.
Objective
To compare the efficacy of a low-dose vaginal estradiol tablet and a vaginal moisturizer, each vs placebo, for treatment of moderate-to-severe postmenopausal vulvovaginal symptoms.
Design, Setting, and Participants
This 12-week multicenter randomized clinical trial enrolled postmenopausal women with moderate to severe symptoms of vulvovaginal itching, pain, dryness, irritation, or pain with penetration.
Interventions
Vaginal 10-μg estradiol tablet (daily for 2 weeks, then twice weekly) plus placebo gel (3 times a week) (n = 102) vs placebo tablet plus vaginal moisturizer (n = 100) vs dual placebo (n = 100).
Main Outcomes and Measures
The main outcome was decrease in severity (0-3) of most bothersome symptom (MBS) between enrollment and 12 weeks. Additional measures included a composite vaginal symptom score, Female Sexual Function Index (FSFI) score (2-36), modified Female Sexual Distress Score–Revised item 1, treatment satisfaction and meaningful benefit, Vaginal Maturation Index, and vaginal pH.
Results
The 302 women had a mean (SD) age of 61 (4) years and were primarily white (267 [88%]), college educated (200 [66%]), and sexually active (245 [81%]). Most women (294 [97%]) provided data for the primary analysis. The most commonly reported MBS was pain with vaginal penetration (182 [60%]), followed by vulvovaginal dryness (63 [21%]). Mean baseline MBS severity was similar between treatment groups: estradiol, 2.4 (95% CI, 2.3 to 2.6); moisturizer, 2.5 (95% CI, 2.3 to 2.6); placebo, 2.5 (95% CI, 2.4 to 2.6). All treatment groups had similar mean reductions in MBS severity over 12 weeks: estradiol, −1.4 (95% CI, −1.6 to −1.2); moisturizer, −1.2 (95% CI, −1.4 to −1.0); and placebo, −1.3 (95% CI, −1.5 to −1.1). No significant differences were seen between estradiol (P = .25) or moisturizer (P = .31) compared with placebo. Mean total FSFI improvement was similar between estradiol (5.4; 95% CI, 4.0 to 6.9) and placebo (4.5; 95% CI, 2.8 to 6.1) (P = .64), and between moisturizer (3.1; 95% CI, 1.7 to 4.5) and placebo (P = .17).
Conclusions and Relevance
Our results suggest that neither prescribed vaginal estradiol tablet nor over-the-counter vaginal moisturizer provides additional benefit over placebo vaginal tablet and gel in reducing postmenopausal vulvovaginal symptoms.
Trial Registration
clinicaltrials.gov Identifier: NCT02516202
Introduction
An estimated 40% to 54% of postmenopausal women report bothersome vulvovaginal symptoms,1,2,3 including vaginal dryness (up to 75%) and pain with intercourse (40%).2,4,5 In 2014, the North American Menopause Society coined the term genitourinary syndrome of menopause (GSM) to reflect the multifaceted nature of this prevalent problem, replacing genitourinary and vulvovaginal atrophy.6 Recent evidence shows decrements in quality of life from moderate to severe vulvovaginal symptoms in women aged 40 to 75 years comparable to those caused by other chronic conditions such as arthritis, chronic obstructive pulmonary disease, and irritable bowel syndrome.1 Yet more than half of symptomatic women are not using any medication to treat their symptoms.7,8
Recommendations for treatment of GSM focus primarily on vaginal products.9,10 However, issues with recommended vaginal treatments include messiness, expense, safety concerns, and lack of symptom relief.11 In postmenopausal women with vaginal dryness, itching, pain, or burning, meta-analyses of randomized trials conclude that vaginal estrogen cream use reduces symptoms in the majority of women,12,13 but few women use them beyond 6 months.14 Four randomized clinical trials have assessed vaginal estrogen tablet efficacy for GSM,15,16,17,18 but only 2 industry-sponsored trials have evaluated the current 10-μg product.15,17 Although clinicians often recommend vaginal moisturizers,7 few studies exist to support this recommendation.19,20,21
Surveys of postmenopausal women demonstrate a preference for effective, nonhormonal therapies, often due to safety concerns.22 We aimed to evaluate the efficacy of low-risk therapies: vaginal estradiol tablets and a vaginal moisturizer in women with moderate to severe vulvovaginal symptoms. We hypothesized that the vaginal estradiol tablet is more efficacious than placebo tablet and that a vaginal moisturizer is more efficacious than placebo gel, in the relief of postmenopausal vaginal symptoms.
Methods
Study Design
This randomized, double-blind, placebo-controlled 12-week trial was conducted at 2 centers: Kaiser Permanente Washington Health Research Institute in Seattle and University of Minnesota in Minneapolis. We compared treatment efficacy for moderate to severe vulvovaginal symptoms between 10-μg vaginal estradiol tablets, a vaginal moisturizer, and matching placebos for each. The study was approved by institutional review boards at participating institutions. Participants provided written informed consent. Enrollment began in April 2016, targets were achieved by February 2017, and final follow-up visits occurred in April 2017.
Patient Selection
Women were recruited through direct mailings and Facebook ads targeted to women aged 50 to 70 years within 20 miles of the clinical sites. Inclusion criteria were as follows: age 45 to 70 years, at least 2 years since last menses, report of at least 1 moderate to severe symptom of vulvovaginal itching, pain, irritation, or dryness experienced at least weekly within the past 30 days; or pain with penetration at least once monthly. Exclusion criteria included current vaginal infection, use of hormonal medication in past 2 months, use of antibiotics or vaginal moisturizer in past month, and chronic premenopausal vulvovaginal symptoms. The study protocol (Supplement 1) provides additional details of study procedures.
Randomization by permuted blocks of 9 and stratified by site was conducted via secure web-based database, and implemented by a computerized inventory system for dispensing identical-appearing tablets in bottles and gel in tubes. Participants, study personnel, and clinicians were blinded to treatment assignments.
Interventions
Women were randomly assigned 1:1:1 to Vagifem 10-μg tablet + placebo vaginal gel, placebo vaginal tablet + Replens vaginal moisturizer, or placebo tablet + placebo gel. The active ingredient for tablets is 10.3 μg of estradiol hemihydrate, equivalent to 10 μg of estradiol. Placebo tablets contained inactive ingredients identical to Vagifem. The ingredients in Replens are purified water, glycerin, mineral oil, polycarbophil, carbomer homopolymer type B, hydrogenated palm oil glyceride, sorbic acid, and sodium hydroxide. The placebo was hydroxyethylcellulose gel, shown to have minimal effect on vaginal microbiota and inflammation.23,24 Placebo gel varied slightly from Replens in viscosity, 13 800 centipoise, and pH, 4.5 (Replens, 13 000 centipoise and pH 3.0). Study medications were formulated and/or packaged by Sharp Clinical Services.
Women were instructed to use the vaginal tablet daily for 2 weeks, then twice weekly for the remaining 10 weeks, and the vaginal moisturizer every 3 days throughout the trial. During the first 2 weeks, participants were advised to use the tablet in the morning and gel in the evening. After that, participants were instructed to use products on alternate days.
Data Collection
Telephone contact at 1, 3, and 11 weeks after randomization assessed protocol adherence and adverse events. Follow-up visits were conducted 4 and 12 weeks after randomization. At each visit, women completed questionnaires and underwent vaginal sample collection for wet mount evaluation, pH measurement, and vaginal maturation index (VMI, a measure of vaginal mucosal cell maturation due to estrogen effects)25 (at baseline and 12 weeks). At follow-up visits, women were asked to bring medications; remaining pills were counted and gel tubes weighed to provide medication adherence estimates.
Measurements
The primary outcome was severity of the most bothersome symptom (MBS) defined by the participant at trial enrollment as vulvovaginal itching, pain, dryness, irritation, or pain with penetration. Severity was rated 0 to 3, signifying none, mild, moderate, or severe.26 At each visit, women completed a questionnaire about presence and severity of vulvovaginal symptoms.
Prespecified secondary outcomes were composite Vaginal Symptom Index (VSI),15 satisfaction with treatment received (Likert scale: 0 = not satisfied to 10 = completely satisfied), meaningful benefit from the study medications (yes or no), Female Sexual Function Index (FSFI),27 Female Sexual Distress Scale–Revised Item 1,28 VMI,10,26 and pH.26 Vaginal Symptom Index was the mean severity score of the 5 vulvovaginal symptoms listed as MBS choices. Post hoc secondary outcomes were severity of pain with penetration, and vaginal dryness.29 Additional questionnaires included Menopausal Quality of Life,30 Generalized Anxiety Disorder 7,31 and Patient Health Questionnaire 8.32
Adverse events were assessed at each visit by a questionnaire listing symptoms potentially related to active agents (increased vaginal secretions, vaginal itching, breast tenderness, vaginal bleeding, vulvovaginal skin rash). Any new complaints reported at visits or by telephone were evaluated and classified according to the Medical Dictionary for Regulatory Activities.33
Statistical Analysis
Ninety-five women per group provided 89% power to detect an effect size of 0.5 standard deviation (SD) units change from baseline to week 12 in MBS severity between intervention group and placebo,19 based on a t test with a 2-sided α = .025 to account for 2 treatment group comparisons. The planned enrollment of 318 participants allowed for 10% loss to follow-up. The modified intent-to-treat analysis included all randomized participants who provided a baseline MBS score and corresponding vulvovaginal symptom severity at week 4 or 12, regardless of adherence to treatment assignment.
The primary outcome was change in severity of the MBS between enrollment and weeks 4 and 12. Treatment group differences were assessed by repeated-measures linear regression models of the 4- and 12-week continuous outcome measures (MBS, VSI, pain with penetration, vaginal dryness, VMI as percent superficial cells, FSFI) as a function of randomization assignment, baseline value of the outcome measure, visit, and clinical site. Robust standard errors were estimated via generalized estimating equations to adjust for correlation between repeated outcome measures. To facilitate comparisons, models of MBS severity were reanalyzed among participants meeting eligibility criteria for previously published trials: baseline pH greater than 5 and VMI with no more than 5% superficial cells.15,16,17 Additional analysis evaluated intervention effects in models including only women adherent to treatment, defined as using at least 80% of medication. Two variables were hypothesized a priori to modify treatment response on the primary outcome: age and years since menopause. Tests for interaction between these variables and treatment assignment were performed within the linear regression models.
Treatment group differences at week 12 in proportions of women with medication adherence, at least 2-point drop in MBS severity, at least 50% decrease in MBS severity, vaginal pH of 5 or less, VMI greater than 5% superficial cells, sexual distress, and meaningful benefit from study medication use were assessed via χ2 tests. Adverse events were compared between treatment groups via Fisher exact tests. Week 12 differences in treatment satisfaction were evaluated by t tests. Analyses were conducted using SAS, version 9.4 (SAS Institute), with 2-sided P ≤ .025 considered statistically significant for the primary outcome and P ≤ .05 for secondary outcomes.
Results
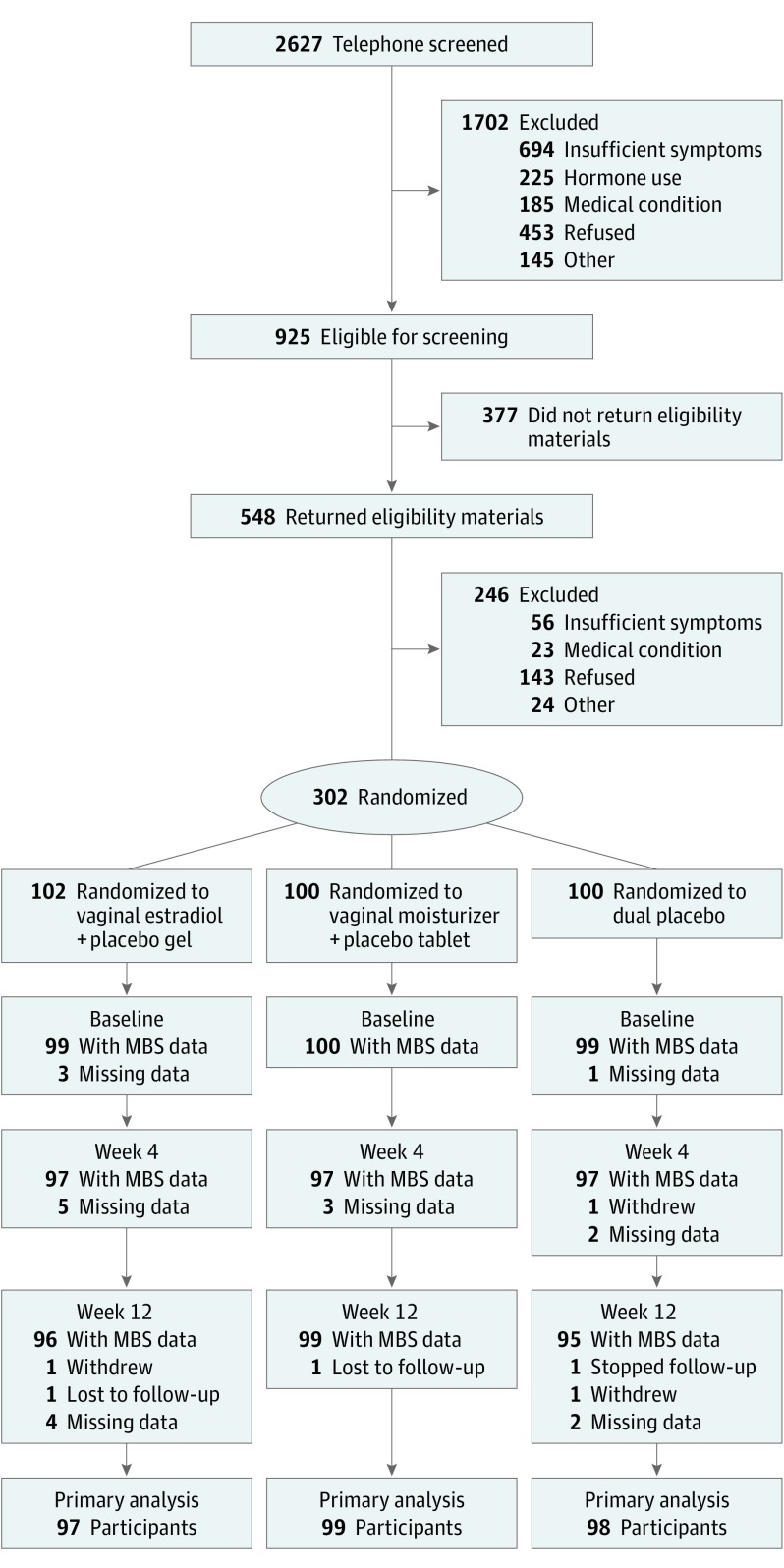
Three hundred two women were randomized to receive vaginal estradiol tablet plus placebo gel (n = 102), placebo tablet plus vaginal moisturizer (n = 100), or dual placebo (n = 100). Study retention was high: 294 of 302 (97%) women provided primary analysis data (Figure 1). Most women were between 55 and 64 years old (235 [78%]), white (267 [88%]), and married or partnered (257 [85%]). Baseline characteristics were comparable between treatment groups (Table 1).
Figure 1. Recruitment, Enrollment, Randomization, and Follow-up of Participants.
MBS indicates most bothersome symptom.
Table 1. Baseline Characteristics of Trial Participantsa.
| Characteristic | Vaginal Estradiol (n = 102) |
Vaginal Moisturizer (n = 100) |
Dual Placebo (n = 100) |
|---|---|---|---|
| Age at screening, mean (SD), y | 61 (4) | 61 (4) | 61 (4) |
| Race, No. (%) | |||
| White | 87 (85) | 90 (90) | 90 (90) |
| African American | 7 (7) | 3 (3) | 2 (2) |
| Other/unknown | 8 (8) | 7 (7) | 8 (8) |
| BMI, mean (SD) | 27 (5) | 26 (4) | 26 (6) |
| Education, No. (%) | |||
| High school diploma/GED or less | 2 (2) | 3 (3) | 6 (6) |
| School after high school | 31 (30) | 27 (27) | 31 (31) |
| College graduate | 67 (66) | 70 (70) | 63 (63) |
| Marital status, No. (%) | |||
| Never married | 8 (8) | 2 (2) | 4 (4) |
| Divorced or widowed | 10 (10) | 8 (8) | 12 (12) |
| Married or like relationship | 83 (81) | 90 (90) | 84 (84) |
| Smoking, No. (%) | |||
| Never | 66 (65) | 67 (67) | 66 (66) |
| Past | 31 (30) | 33 (33) | 32 (32) |
| Current | 4 (4) | 0 | 2 (2) |
| Alcohol use, drinks/wk, No. (%) | |||
| 0 | 30 (29) | 31 (31) | 28 (28) |
| 1-6 | 50 (49) | 46 (46) | 53 (53) |
| ≥7 | 21 (21) | 23 (23) | 19 (19) |
| Menopause Quality of Life Questionnaire total, mean (SD) | 3.3 (1.2) | 3.2 (1.1) | 3.3 (1.0) |
| Patient Health Questionnaire 8 depression, No. (%) | |||
| None (0-4) | 69 (68) | 75 (75) | 69 (69) |
| Mild (5-9) | 25 (25) | 22 (22) | 23 (23) |
| Moderate/severe (≥10) | 7 (7) | 3 (3) | 8 (8) |
| Generalized Anxiety Disorder Questionnaire 7 anxiety, No. (%) | |||
| None (0-4) | 64 (63) | 75 (75) | 64 (64) |
| Mild (5-9) | 25 (25) | 21 (21) | 24 (24) |
| Moderate/severe (≥10) | 12 (12) | 4 (4) | 12 (12) |
| Sexually active, No. (%) | |||
| Yes | 81 (79) | 80 (80) | 84 (84) |
| No | 20 (20) | 20 (20) | 16 (16) |
| Female Sexual Function Index total, mean (SD) | 15.2 (5.9) | 15.2 (6.5) | 16.1 (6.6) |
| Female Sexual Distress Scale–Revised, item 1, distressed about sex life, No. (%) | |||
| Never/rarely | 15 (15) | 12 (12) | 18 (18) |
| Occasionally | 33 (32) | 33 (33) | 33 (33) |
| Frequently/always | 53 (52) | 54 (54) | 49 (49) |
| Vaginal pH, No. (%) | |||
| ≤5 | 18 (18) | 12 (12) | 9 (9) |
| >5 | 81 (79) | 87 (87) | 90 (90) |
| Vaginal maturation index, No. (%) | |||
| ≤5% Superficial cells | 86 (84) | 78 (78) | 81 (81) |
| >5% Superficial cells | 6 (6) | 11 (11) | 7 (7) |
| Missing | 10 (10) | 11 (11) | 12 (12) |
| Most bothersome symptom, No. (%) | |||
| Vulvar and/or vaginal itching | 10 (10) | 4 (4) | 6 (6) |
| Vulvar and/or vaginal pain | 5 (5) | 7 (7) | 2 (2) |
| Vaginal dryness | 23 (23) | 17 (17) | 23 (23) |
| Vulvar and/or vaginal irritation | 7 (7) | 4 (4) | 8 (8) |
| Pain with vaginal penetration | 54 (53) | 68 (68) | 60 (60) |
| Self-reported health, No. (%) | |||
| Excellent | 26 (26) | 27 (27) | 20 (20) |
| Very good | 41 (40) | 55 (55) | 47 (47) |
| Good | 33 (32) | 15 (15) | 30 (30) |
| Fair/poor | 1 (1) | 3 (3) | 3 (3) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Women were randomized 1:1:1 to vaginal estradiol 10-μg tablet + placebo gel, over-the-counter vaginal moisturizer + placebo tablet, or placebo tablet + placebo gel. There were no significant differences in demographic characteristics between groups.
A total of 182 (60%) women endorsed MBS as pain with vaginal penetration, 63 (21%) dryness, 20 (7%) itching, 19 (6%) irritation, and 14 (5%) pain. Baseline mean (SD) MBS severity was 2.5 (0.6). The majority of women (245 [81%]) were sexually active: 202 (67%) with a male partner, 3 (1%) with a female partner, and 136 (45%) self-stimulation. Baseline median vaginal pH was 7 (interquartile range, 6.0-7.5). Of 269 (89%) baseline VMI samples with adequate cellularity for analysis, 91% had no more than 5% superficial cells.
At completion, of participants who returned tablets for counting (263 [87%]) and gel for weighing (259 [86%]), 94% were tablet adherent and 90% were gel adherent (ie, used >80% of medication doses). Adherence did not vary significantly across treatment groups (estradiol tablets, 84 of 102 [82%] vs placebo tablets, 80 of 100 [80%]; P = .67; vaginal moisturizer, 75 of 100 [75%] vs placebo gel, 78 of 100 [78%]; P = .62).
Neither treatment reduced MBS severity between baseline and 4 or 12 weeks more than placebo (Table 2 and Figure 2). All groups had a mean 1.2- to 1.4-point decrease from baseline MBS score by 12 weeks. A decrease of 2 points signifies a clinically meaningful change from moderate to severe symptoms to mild to none. There was no difference between intervention vs placebo groups in proportion of women with a decrease of at least 2 points in MBS severity between 0 and 12 weeks (estradiol, 47 [49%] vs placebo, 43 [45%]; P = .61; moisturizer, 35 [35%] vs placebo, 43 [47%]; P = .16). Most women had a decrease of at least 50% in symptom severity (estradiol, 67 [70%] vs placebo, 62 [65%]; P = .50; moisturizer, 53 [54%] vs placebo, 62 [65%]; P = .10). Mean VSI decreased less than 1 point from a mean of 1.6 in all treatment groups.
Table 2. Most Bothersome Symptom (MBS) Severity Over 4 and 12 Weeks of Treatment for 302 Postmenopausal Women.
| Parameter | Vaginal Estradiol Tablet + Placebo Gel | Vaginal Moisturizer + Placebo Tablet | Dual Placebo | Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol vs Placebo | Moisturizer vs Placebo | |||||||||
| No. | Mean (95% CI) | No. | Mean (95% CI) | No. | Mean (95% CI) | Mean (95% CI) | P Valuea | Mean (95% CI) | P Valuea | |
| MBS severityb | ||||||||||
| Baseline | 99 | 2.4 (2.3 to 2.6) | 100 | 2.5 (2.3 to 2.6) | 99 | 2.5 (2.4 to 2.6) | 0.0 (−0.2 to 0.1) | .25 | 0.0 (−0.2 to 0.2) | .31 |
| Week 4 minus baseline | 97 | −1.2 (−1.4 to −1.0) | 97 | −1.0 (−1.2 to −0.8) | 97 | −1.1 (−1.3 to −0.9) | −0.2 (−0.5 to 0.1) | 0.1 (−0.2 to 0.4) | ||
| Week 12 minus baseline | 96 | −1.4 (−1.6 to −1.2) | 99 | −1.2 (−1.4 to −1.0) | 95 | −1.3 (−1.5 to −1.1) | −0.1 (−0.4 to 0.2) | 0.2 (−0.1 to 0.4) | ||
| Vaginal Symptom Indexc | ||||||||||
| Baseline | 102 | 1.6 (1.5 to 1.7) | 100 | 1.6 (1.5 to 1.7) | 100 | 1.6 (1.5 to 1.7) | 0.1 (−0.1 to 0.2) | .99 | 0.0 (−0.1 to 0.2) | .05 |
| Week 4 minus baseline | 100 | −0.7 (−0.8 to −0.5) | 97 | −0.5 (−0.7 to −0.4) | 98 | −0.6 (−0.8 to −0.5) | 0.0 (−0.2 to 0.2) | 0.1 (−0.1 to 0.3) | ||
| Week 12 minus baseline | 99 | −0.9 (−1.1 to −0.8) | 99 | −0.7 (−0.9 to −0.6) | 96 | −0.9 (−1.0 to −0.7) | −0.1 (−0.3 to 0.1) | 0.1 (−0.1 to 0.3) | ||
| Pain with penetrationd | ||||||||||
| Baseline | 75 | 2.5 (2.3 to 2.6) | 84 | 2.5 (2.4 to 2.6) | 87 | 2.5 (2.4 to 2.6) | −0.1 (−0.2 to 0.1) | .21 | −0.1 (−0.2 to 0.1) | .08 |
| Week 4 minus baseline | 74 | −1.4 (−1.7 to −1.2) | 81 | −1.0 (−1.3 to −0.8) | 85 | −1.2 (−1.4 to −0.9) | −0.3 (−0.6 to 0.1) | 0.1 (−0.2 to 0.5) | ||
| Week 12 minus baseline | 73 | −1.5 (−1.7 to −1.2) | 83 | −1.1 (−1.4 to −0.9) | 83 | −1.5 (−1.8 to −1.3) | 0.1 (−0.3 to 0.4) | 0.4 (0.1 to 0.7) | ||
| Drynessd | ||||||||||
| Baseline | 89 | 2.3 (2.2 to 2.4) | 81 | 2.4 (2.3 to 2.5) | 78 | 2.4 (2.3 to 2.6) | −0.1 (−0.3 to 0.0) | .95 | −0.1 (−0.2 to 0.1) | .36 |
| Week 4 minus baseline | 87 | −1.1 (−1.3 to −0.9) | 78 | −1.0 (−1.2 to −0.8) | 76 | −1.2 (−1.4 to −1.0) | 0.2 (−0.1 to 0.5) | 0.2 (−0.1 to 0.5) | ||
| Week 12 minus baseline | 86 | −1.4 (−1.6 to −1.2) | 80 | −1.3 (−1.5 to −1.1) | 74 | −1.4 (−1.6 to −1.2) | 0.0 (−0.3 to 0.3) | 0.1 (−0.2 to 0.4) | ||
P values from comparison of each treatment vs placebo in a repeated-measures linear model of outcome as a function of randomization assignment, baseline value of the outcome measure, visit week (categorical), and clinical site.
Participants scored vulvovaginal itch, pain, dryness, irritation, or pain with penetration on a scale from none (0) to severe (3) and identified 1 of these as their MBS for the trial outcome.
Vaginal Symptom Index = mean severity score of 5 vulvovaginal symptoms.
Among participants with a moderate or severe score at baseline.
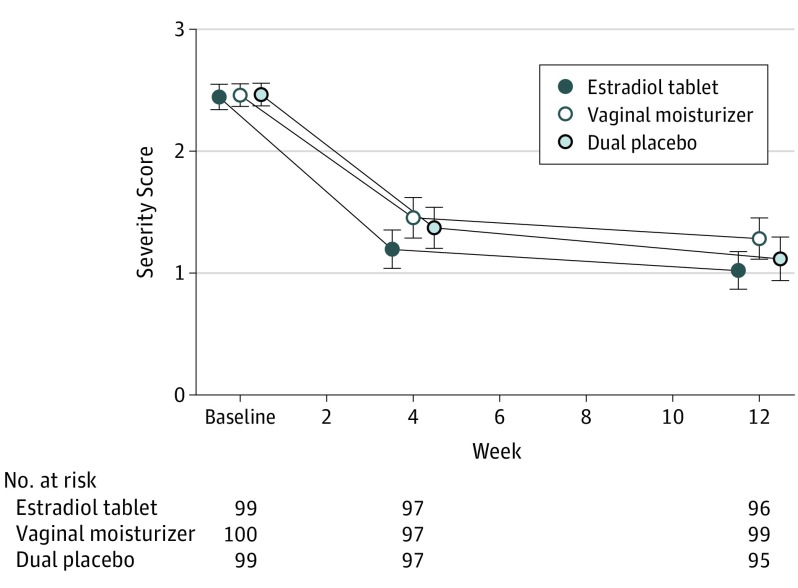
Figure 2. Most Bothersome Symptom Severity Score Change Over 12 Weeks.
Most bothersome symptom severity scores (1 indicates mild; 2, moderate; 3, severe) at 0, 4, and 12 weeks of treatment in women randomized to vaginal estradiol tablet (10 μg) + placebo gel, vaginal moisturizer + placebo tablet, or placebo gel + placebo tablet. Women chose vulvovaginal itching, pain, dryness, irritation, or pain with vaginal penetration as their most bothersome symptom at enrollment. There was no significant difference in the change from baseline in severity scores between treatment groups at 4 or 12 weeks (estradiol vs dual placebo, P = .25; moisturizer vs dual placebo, P = .31).
A higher proportion of women in the estradiol group had a pH change from greater than 5 at baseline to 5 or less at week 12 compared with placebo (36 [46%] vs 10 [12%]; P < .001); no difference was observed between moisturizer and placebo (8 [9%] vs 10 [12%]; P = .60). More women in the estradiol tablet group increased VMI superficial cells from 5% or less at baseline to greater than 5% at week 12 compared with placebo (45 [57%] vs 8 [11%]; P < .001). The same proportion of women in the moisturizer and placebo groups had an increase (8 [11%]; P = .95).
Change in FSFI did not significantly vary between treatment groups, either total score or any of the 6 domains (Table 3). The FSFI domain with the greatest improvement at 12 weeks was Lubrication, increasing by a mean of 1.4 (95% CI, 1.1-1.8) points in the estradiol + placebo gel group, 1.2 (95% CI, 0.8-1.6) points in dual placebo, and 0.9 (95% CI, 0.6-1.3) points in moisturizer + placebo tablet. Most women were “frequently” or “always” distressed about sex life at enrollment (Table 1). At 12 weeks, nearly half of women in the estradiol and placebo groups endorsed “rarely” or “never” distressed (47 [47%] estradiol, 29 [29%] moisturizer, 41 [43%] placebo; estradiol vs placebo, P = .50; moisturizer vs placebo, P = .05). Mean (SD) treatment satisfaction was similar between groups: 8.6 (2.6) for estradiol tablet, 7.7 (3.2) for moisturizer, and 8.1 (3.0) for placebo. More women in the estradiol tablet group reported “meaningful benefit” from treatment than placebo (79 [80%] vs 62 [65%]; P = .02), but no difference was observed between moisturizer and placebo (57 [58%] vs 62 [65%]; P = .39).
Table 3. Female Sexual Function Index (FSFI) Scores Over 4 and 12 Weeks of Treatment for 302 Postmenopausal Women.
| FSFI Domaina | Vaginal Estradiol Tablet + Placebo Gel | Vaginal Moisturizer + Placebo Tablet | Dual Placebo | Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol vs Placebo | Moisturizer vs Placebo | |||||||||
| No. | Mean (95% CI) | No. | Mean (95% CI) | No. | Mean (95% CI) | Mean (95% CI) | P Valueb | Mean (95% CI) | P Valueb | |
| Total | ||||||||||
| Baseline | 81 | 15.2 (13.9 to 16.5) | 86 | 15.1 (13.7 to 16.5) | 85 | 16.1 (14.6 to 17.5) | −0.8 (−2.8 to 1.1) | .64 | −0.9 (−2.9 to 1.1) | .17 |
| Week 4 minus baseline | 62 | 3.1 (1.5 to 4.7) | 73 | 2.9 (1.7 to 4.1) | 73 | 3.2 (1.8 to 4.7) | −0.1 (−2.3 to 2.1) | −0.3 (−2.2 to 1.5) | ||
| Week 12 minus baseline | 64 | 5.4 (4.0 to 6.9) | 80 | 3.1 (1.7 to 4.5) | 70 | 4.5 (2.8 to 6.1) | 1.0 (−1.2 to 3.2) | −1.3 (−3.5 to 0.8) | ||
| Desire | ||||||||||
| Baseline | 98 | 2.4 (2.2 to 2.6) | 98 | 2.4 (2.2 to 2.6) | 96 | 2.5 (2.3 to 2.7) | −0.1 (−0.4 to 0.2) | .58 | −0.1 (−0.4 to 0.2) | .55 |
| Week 4 minus baseline | 96 | 0.1 (−0.1 to 0.3) | 95 | 0.2 (0.0 to 0.4) | 93 | 0.2 (0.0 to 0.4) | −0.1 (−0.3 to 0.2) | 0.0 (−0.3 to 0.2) | ||
| Week 12 minus baseline | 94 | 0.1 (0.0 to 0.3) | 97 | 0.1 (−0.1 to 0.3) | 91 | 0.1 (−0.1 to 0.4) | 0.0 (−0.3 to 0.3) | 0.0 (−0.3 to 0.3) | ||
| Arousal | ||||||||||
| Baseline | 91 | 2.8 (2.5 to 3.1) | 89 | 2.9 (2.6 to 3.2) | 93 | 2.8 (2.5 to 3.2) | 0.0 (−0.5 to 0.4) | .36 | 0.1 (−0.4 to 0.5) | .13 |
| Week 4 minus baseline | 71 | 0.3 (0.0 to 0.6) | 81 | 0.4 (0.1 to 0.6) | 82 | 0.4 (0.1 to 0.7) | −0.1 (−0.5 to 0.3) | 0.0 (−0.4 to 0.3) | ||
| Week 12 minus baseline | 74 | 0.5 (0.2 to 0.8) | 86 | 0.4 (0.1 to 0.7) | 79 | 0.8 (0.4 to 1.1) | −0.3 (−0.7 to 0.2) | −0.4 (−0.8 to 0.1) | ||
| Lubrication | ||||||||||
| Baseline | 89 | 2.0 (1.7 to 2.2) | 89 | 2.2 (1.9 to 2.4) | 92 | 2.2 (2.0 to 2.5) | −0.2 (−0.6 to 0.1) | .54 | 0.0 (−0.4 to 0.3) | .32 |
| Week 4 minus baseline | 68 | 0.7 (0.3 to 1.0) | 80 | 0.6 (0.3 to 0.9) | 80 | 0.7 (0.3 to 1.1) | 0.0 (−0.5 to 0.5) | −0.1 (−0.6 to 0.4) | ||
| Week 12 minus baseline | 72 | 1.4 (1.1 to 1.8) | 86 | 0.9 (0.6 to 1.3) | 77 | 1.2 (0.8 to 1.6) | 0.2 (−0.3 to 0.8) | −0.2 (−0.8 to 0.3) | ||
| Orgasm | ||||||||||
| Baseline | 91 | 3.1 (2.7 to 3.5) | 90 | 3.2 (2.8 to 3.6) | 92 | 3.1 (2.8 to 3.5) | −0.1 (−0.6 to 0.5) | .14 | 0.0 (−0.5 to 0.6) | .37 |
| Week 4 minus baseline | 71 | 0.2 (−0.2 to 0.5) | 82 | 0.6 (0.2 to 0.9) | 82 | 0.6 (0.2 to 1.0) | −0.5 (−1.0 to 0.1) | 0.0 (−0.5 to 0.5) | ||
| Week 12 minus baseline | 73 | 0.6 (0.2 to 0.9) | 86 | 0.6 (0.2 to 0.9) | 79 | 0.8 (0.4 to 1.1) | −0.2 (−0.7 to 0.3) | −0.2 (−0.7 to 0.3) | ||
| Satisfaction | ||||||||||
| Baseline | 86 | 3.0 (2.6 to 3.3) | 88 | 3.0 (2.7 to 3.3) | 89 | 3.2 (2.9 to 3.5) | −0.2 (−0.7 to 0.2) | .29 | −0.2 (−0.6 to 0.3) | .29 |
| Week 4 minus baseline | 68 | 0.6 (0.3 to 0.9) | 76 | 0.4 (0.1 to 0.7) | 79 | 0.5 (0.3 to 0.8) | 0.1 (−0.4 to 0.5) | −0.1 (−0.5 to 0.3) | ||
| Week 12 minus baseline | 71 | 0.9 (0.6 to 1.3) | 82 | 0.4 (0.1 to 0.7) | 76 | 0.5 (0.2 to 0.8) | 0.4 (0.0 to 0.8) | −0.1 (−0.5 to 0.3) | ||
| Pain | ||||||||||
| Baseline | 90 | 1.6 (1.3 to 1.9) | 90 | 1.4 (1.2 to 1.7) | 93 | 1.8 (1.5 to 2.1) | −0.2 (−0.6 to 0.2) | .47 | −0.4 (−0.8 to 0.0) | .76 |
| Week 4 minus baseline | 70 | 0.8 (0.3 to 1.3) | 82 | 0.9 (0.5 to 1.2) | 81 | 0.6 (0.2 to 1.1) | 0.1 (−0.6 to 0.8) | 0.2 (−0.3 to 0.8) | ||
| Week 12 minus baseline | 73 | 1.4 (0.9 to 1.8) | 86 | 1.0 (0.7 to 1.4) | 79 | 0.9 (0.5 to 1.4) | 0.4 (−0.2 to 1.0) | 0.1 (−0.4 to 0.7) | ||
The FSFI is a 19-item questionnaire with a maximum score of 36, which is calculated by adding weighted scores of 6 domains. Higher scores are better, and a score of less than 26 is consistent with sexual dysfunction. Each domain has a maximum score of 6, calculated by multiplying the total score of all questions by a domain factor.
P values from comparison of each treatment vs placebo in a repeated-measures linear model of outcome as a function of randomization assignment, baseline value of the outcome measure, visit week (categorical), and clinical site.
In regression models including only medication-adherent women, changes in MBS severity and FSFI did not differ from the intent-to-treat analysis (eTables 1 and 2 in Supplement 2). In analysis limited to women meeting enrollment criteria for previous trials of pH greater than 5 and no more than 5% superficial cells on VMI (n = 205), we saw no difference in results. Neither age nor years since menopause modified response to estradiol, although women younger than 60 years demonstrated greater MBS improvement with placebo than with moisturizer (eTable 3 in Supplement 2).
Vaginal candidiasis was diagnosed by microscopy in 5 (5%) participants randomized to estradiol, 2 (2%) to moisturizer, and 2 (2%) to dual placebo. Two additional women reported a yeast infection diagnosed elsewhere. Adverse events were not different between the treatment groups (eTable 4 in Supplement 2). Three women randomized to estradiol received a diagnosis of cancer (all judged unrelated to study medication): 1 with lymphoma at 4 weeks withdrew, 1 with breast cancer at 4 weeks stopped therapy but continued other procedures, and 1 breast cancer diagnosis was made after study completion.
Discussion
In this randomized clinical trial of 302 women with moderate to severe postmenopausal vulvovaginal symptoms, no treatment group differences in symptom reduction were observed for vaginal estradiol tablet plus placebo gel vs dual placebo, or vaginal moisturizer plus placebo tablet vs dual placebo. The lack of efficacy of the active treatment groups over dual placebo was similar whether women chose pain with vaginal penetration, vaginal dryness, or other symptoms as their MBS. We demonstrated similar improvement in symptoms and sexual function in all 3 treatment groups.
The North American Menopause Society recommends nonhormonal vaginal therapies as first-line treatment for GSM,9 while recommendations in Europe support vaginal estrogen therapy.10 Many women report substantial concerns about the long-term safety of hormonal products, and prefer to use nonhormonal products.22 Vaginal moisturizers such as Replens, containing mucoadhesives such as polycarbophil to extend benefit from intermittent dosing,34 have been evaluated in small, open-label studies using the same dosing strategy as in our trial. Two studies, including 1 randomized crossover trial in breast cancer survivors, demonstrated no significant difference between placebo and moisturizer products, although both decreased symptom severity.21,35 A recent study comparing dehydroepiandrosterone (DHEA) in a mucoadhesive base vs base alone demonstrated no difference in symptom improvement, but better sexual function in women using DHEA.21 We chose our placebo gel because it was shown not to alter vaginal microbiota or inflammation23,24 and is a formulation with less mucoadhesive properties than Replens,34 although of similar viscosity and pH.23 The effectiveness of our placebo in decreasing symptom severity suggests that the mucoadhesive properties lauded by vaginal moisturizer manufacturers may not be necessary to achieve symptom relief.
Two of 3 randomized clinical trials of low-dose vaginal estrogen therapy demonstrated greater decrease in symptom severity vs placebo, although in all trials symptoms diminished in both groups.17,36,37 Symptom reduction with estradiol tablets in our study was comparable to the existing literature for vaginal estrogen, newly approved vaginal DHEA, and oral ospemifene, a selective estrogen receptor modulator.38,39 Our participants using vaginal estradiol tablets had a mean decrease in symptom severity from baseline of 1.4 points, similar to the 1.2 to 1.3 seen in prior studies of the vaginal tablet,15,17 and the 1.4 points seen with oral ospemifene38 and vaginal DHEA,39 but slightly less than that seen with a softgel formulation (mean [SD], 1.69 [0.07]).37 While 10 μg is a low dose, 2 studies comparing 10- and 25-μg doses did not show a difference in efficacy.15,37 The differential change in VMI in our estradiol group demonstrates the biologic effect of estrogen vs placebo but was not linked to differences in symptom improvement. Overall, the largest difference between our trial and others is the magnitude of symptom improvement in our placebo group. Our placebo was quite different from placebo creams and tablets used in other trials of vaginal estrogen, and meets many of the criteria outlined in a recent review as optimal for vaginal moisturizing products.40
The placebo effect in treatment trials for postmenopausal vaginal symptoms is substantial. In previous trials, placebo tablets were associated with a mean decrease in symptom severity of 0.8 to 0.87 points,15,17 while placebo softgel was associated with a mean decrease of 1.28 points in dyspareunia severity.37 In 2 studies of vaginal estrogen cream, dyspareunia severity decreased a mean of 0.7 to 0.9 points in placebo groups.36,41 The 1.3-point mean symptom severity decrease in our dual placebo group is larger than other trials’ placebo effects, with the exception of the softgel study (REJOICE).37 The symptoms chosen as outcomes for trials—dryness, pain with intercourse, itching, and irritation—are the most commonly reported vulvovaginal symptoms among postmenopausal women,3,4,5 and severity is correlated with sexual function scores, suggesting that they are relevant patient outcomes.42 However, the profound placebo response seen in our trial and others, not linked to changes in pH or VMI, suggests that many factors in addition to the local vaginal environment contribute to symptoms.
Many women prescribed vaginal therapy for vulvovaginal symptoms seem unsatisfied with treatment, as continuation rates are low.44 In previous studies, adherence to tablets was better than vaginal creams.45,46,47 Other reports suggest that women prefer tablet formulation to products that increase discharge or are messy, and would be willing to pay more for a tablet formulation.48 However, medication cost is a substantial barrier for many women, and hormone-based therapies are expensive. A 1-month supply of vaginal estrogen cream, vaginal estrogen tablets, or newer US Food and Drug Administration–approved products marketed specifically for dyspareunia (ospemifene and vaginal DHEA) can cost between $82 and $200,49,50 while Replens costs approximately $20. Our results suggest that most women can achieve greater than 50% reduction in symptom severity with regular, consistent use of a vaginal gel with lubricant properties and do not see added symptom improvement with vaginal estradiol.
Limitations
To our knowledge, this is the first randomized clinical trial evaluating short-term (12 weeks) efficacy of recommended nonhormonal and hormonal vaginal therapies for postmenopausal vulvovaginal symptoms, and the only one with a dual placebo arm. We enrolled a large cohort of women with moderate to severe symptoms, with excellent participant retention and medication adherence. While our study was not designed to compare active interventions head to head, efficacy comparisons of vaginal estradiol and moisturizer with dual placebo in the same population provide insight into the relative benefit of each. The generalizability of our trial results is limited by the relatively homogenous population, despite enrolling at 2 geographically distinct sites and using 2 recruitment strategies. In contrast to studies limiting the population to women with high vaginal pH and/or 5% or less superficial cells,15,17,36,43 we included all postmenopausal women reporting moderate to severe vulvovaginal symptoms, making our population more consistent with women presenting to primary care settings.
Conclusions
Our study demonstrates that a better understanding of the underlying mechanism of GSM is needed to guide efforts to improve vaginal treatment options. Many postmenopausal women with moderate to severe vulvovaginal symptoms can be treated with a nonprescription vaginal lubricating gel. However, not all gel formulations may have the same effects, and some women may prefer nongel formulations. Treatment choice should be based on individual patient preferences regarding cost and formulation.
Trial Protocol
eTable 1. Sensitivity Analyses of Most Bothersome Symptom Severity
eTable 2. Sensitivity Analyses of Female Sexual Function Index Total Score
eTable 3. Most Bothersome Symptom Severity at Weeks 4 and 12 by Subgroups
eTable 4. Newly Emergent Moderate or Severe Adverse Events During Intervention
References
- 1.DiBonaventura M, Luo X, Moffatt M, Bushmakin AG, Kumar M, Bobula J. The association between vulvovaginal atrophy symptoms and quality of life among postmenopausal women in the United States and western Europe. J Womens Health (Larchmt). 2015;24(9):713-722. [DOI] [PubMed] [Google Scholar]
- 2.Minkin MJ, Reiter S, Maamari R. Prevalence of postmenopausal symptoms in North America and Europe. Menopause. 2015;22(11):1231-1238. [DOI] [PubMed] [Google Scholar]
- 3.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133-2142. [DOI] [PubMed] [Google Scholar]
- 4.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (Real Women’s Views of Treatment Options for Menopausal Vaginal Changes) survey. J Sex Med. 2013;10(7):1790-1799. [DOI] [PubMed] [Google Scholar]
- 5.Nappi RE, Kokot-Kierepa M. Vaginal health: Insights, Views & Attitudes (VIVA)—results from an international survey. Climacteric. 2012;15(1):36-44. [DOI] [PubMed] [Google Scholar]
- 6.Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel . Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063-1068. [DOI] [PubMed] [Google Scholar]
- 7.Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14(3):413-424. [DOI] [PubMed] [Google Scholar]
- 8.Nappi RE, Kokot-Kierepa M. Women’s voices in the menopause: results from an international survey on vaginal atrophy. Maturitas. 2010;67(3):233-238. [DOI] [PubMed] [Google Scholar]
- 9.Management of symptomatic vulvovaginal atrophy: 2013 position statement of the North American Menopause Society. Menopause. 2013;20(9):888-902. [DOI] [PubMed] [Google Scholar]
- 10.Sarri G, Davies M, Lumsden MA; Guideline Development Group . Diagnosis and management of menopause: summary of NICE guidance. BMJ. 2015;351:h5746. [DOI] [PubMed] [Google Scholar]
- 11.Freedman MA. Perceptions of dyspareunia in postmenopausal women with vulvar and vaginal atrophy: findings from the REVIVE survey. Womens Health (Lond). 2014;10(4):445-454. [DOI] [PubMed] [Google Scholar]
- 12.Cardozo L, Bachmann G, McClish D, Fonda D, Birgerson L. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: second report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol. 1998;92(4, pt 2):722-727. [DOI] [PubMed] [Google Scholar]
- 13.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;(4):CD001500. [DOI] [PubMed] [Google Scholar]
- 14.Shulman LP, Portman DJ, Lee WC, et al. A retrospective managed care claims data analysis of medication adherence to vaginal estrogen therapy: implications for clinical practice. J Womens Health (Larchmt). 2008;17(4):569-578. [DOI] [PubMed] [Google Scholar]
- 15.Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol. 2008;111(1):67-76. [DOI] [PubMed] [Google Scholar]
- 16.Eriksen PS, Rasmussen H. Low-dose 17 beta-estradiol vaginal tablets in the treatment of atrophic vaginitis: a double-blind placebo controlled study. Eur J Obstet Gynecol Reprod Biol. 1992;44(2):137-144. [DOI] [PubMed] [Google Scholar]
- 17.Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet. Obstet Gynecol. 2008;112(5):1053-1060. [DOI] [PubMed] [Google Scholar]
- 18.Simunić V, Banović I, Ciglar S, Jeren L, Pavicić Baldani D, Sprem M. Local estrogen treatment in patients with urogenital symptoms. Int J Gynaecol Obstet. 2003;82(2):187-197. [DOI] [PubMed] [Google Scholar]
- 19.Biglia N, Peano E, Sgandurra P, et al. Low-dose vaginal estrogens or vaginal moisturizer in breast cancer survivors with urogenital atrophy: a preliminary study. Gynecol Endocrinol. 2010;26(6):404-412. [DOI] [PubMed] [Google Scholar]
- 20.Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23(3):259-263. [DOI] [PubMed] [Google Scholar]
- 21.Loprinzi CL, Abu-Ghazaleh S, Sloan JA, et al. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol. 1997;15(3):969-973. [DOI] [PubMed] [Google Scholar]
- 22.Wysocki S, Kingsberg S, Krychman M. Management of vaginal atrophy: implications from the REVIVE survey. Clin Med Insights Reprod Health. 2014;8:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson BA, Kelly C, Ramjee G, et al. ; HPTN 035 Study Team . Appropriateness of hydroxyethylcellulose gel as a placebo control in vaginal microbicide trials: a comparison of the two control arms of HPTN 035. J Acquir Immune Defic Syndr. 2013;63(1):120-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tien D, Schnaare RL, Kang F, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21(10):845-853. [DOI] [PubMed] [Google Scholar]
- 25.Lindau ST, Dude A, Gavrilova N, Hoffmann JN, Schumm LP, McClintock MK. Prevalence and correlates of vaginal estrogenization in postmenopausal women in the United States. Menopause. 2017;24(5):536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration Guidance for industry: estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms—recommendations for clinical evaluation. Rockville, MD: US Food and Drug Administration; 2003.
- 27.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191-208. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter JS, Reed SD, Guthrie KA, et al. Using an FSDS-R item to screen for sexually related distress: a MsFLASH analysis. Sex Med. 2015;3(1):7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Ng M-J, van der Vlugt TH, Price PH, Orencia A. Statistical considerations for the efficacy assessment of clinical studies of vulvar and vaginal atrophy. Drug Inf J. 2010;44(5):581-588. [Google Scholar]
- 30.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 1996;24(3):161-175. [DOI] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. [DOI] [PubMed] [Google Scholar]
- 32.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22(11):1596-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown EG, Wood L, Wood S. The Medical Dictionary for Regulatory Activities (MedDRA). Drug Saf. 1999;20(2):109-117. [DOI] [PubMed] [Google Scholar]
- 34.Andrews GP, Donnelly L, Jones DS, et al. Characterization of the rheological, mucoadhesive, and drug release properties of highly structured gel platforms for intravaginal drug delivery. Biomacromolecules. 2009;10(9):2427-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nachtigall LE. Comparative study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61(1):178-180. [DOI] [PubMed] [Google Scholar]
- 36.Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause. 2009;16(4):719-727. [DOI] [PubMed] [Google Scholar]
- 37.Constantine GD, Simon JA, Pickar JH, et al. ; REJOICE Study Group . The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol soft-gel capsule for symptomatic vulvar and vaginal atrophy. Menopause. 2017;24(4):409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachmann GA, Komi JO; Ospemifene Study Group . Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480-486. [DOI] [PubMed] [Google Scholar]
- 39.Labrie F, Archer DF, Koltun W, et al. ; VVA Prasterone Research Group . Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243-256. [DOI] [PubMed] [Google Scholar]
- 40.Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19(2):151-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause. 2009;16(4):735-741. [DOI] [PubMed] [Google Scholar]
- 42.Pinkerton JV, Bushmakin AG, Komm BS, Abraham L. Relationship between changes in vulvar-vaginal atrophy and changes in sexual functioning. Maturitas. 2017;100:57-63. [DOI] [PubMed] [Google Scholar]
- 43.Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58. [DOI] [PubMed] [Google Scholar]
- 44.Palma F, Xholli A, Cagnacci A; Writing Group of the AGATA Study . Management of vaginal atrophy: a real mess. results from the AGATA Study. Gynecol Endocrinol. 2017;33(9):702-707. [DOI] [PubMed] [Google Scholar]
- 45.Portman D, Shulman L, Yeaw J, et al. One-year treatment persistence with local estrogen therapy in postmenopausal women diagnosed as having vaginal atrophy. Menopause. 2015;22(11):1197-1203. [DOI] [PubMed] [Google Scholar]
- 46.Weissmann-Brenner A, Bayevsky T, Yoles I. Compliance to vaginal treatment-tablets versus cream: a retrospective 9 years study. Menopause. 2017;24(1):73-76. [DOI] [PubMed] [Google Scholar]
- 47.Minkin MJ, Maamari R, Reiter S. Improved compliance and patient satisfaction with estradiol vaginal tablets in postmenopausal women previously treated with another local estrogen therapy. Int J Womens Health. 2013;5:133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattsson LA, Ericsson Å, Bøgelund M, Maamari R. Women’s preferences toward attributes of local estrogen therapy for the treatment of vaginal atrophy. Maturitas. 2013;74(3):259-263. [DOI] [PubMed] [Google Scholar]
- 49.Barenberg BJ, Smith T, Nihira MA. Compounded estradiol cream: a cost conscious alternative. J Okla State Med Assoc. 2014;107(4):155-156. [PubMed] [Google Scholar]
- 50.GoodRx website. https://www.goodrx.com/. Accessed August 1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Sensitivity Analyses of Most Bothersome Symptom Severity
eTable 2. Sensitivity Analyses of Female Sexual Function Index Total Score
eTable 3. Most Bothersome Symptom Severity at Weeks 4 and 12 by Subgroups
eTable 4. Newly Emergent Moderate or Severe Adverse Events During Intervention